Abstract
Investigating how individuals adjust their investment into distinct components of the immune system under natural conditions necessitates to develop immune phenotyping tools that reflect the activation of specific immune components that can be measured directly in the field. Here, we examined individual variation of plasma neopterin, a biomarker of Th1 immunity in wild mandrills (Mandrillus sphinx), who are naturally exposed to a suite of parasites, including simian retroviruses and malaria agents. We analyzed a total of 201 plasma samples from 99 individuals and examined the effect of sex, age, social rank, reproductive state and disease status on neopterin levels. We found higher neopterin concentrations in males than females, but were unable to disentangle this effect from possible confounding effects of retroviral infections, which affect nearly all adult males, but hardly any females. We further detected a non-linear age effect with heightened neopterin levels in early and late life. In addition, adult males that harbored very high parasitaemia for Plasmodium gonderi also showed high neopterin levels. There was no effect of social rank in either male or female mandrills, and no effect of female reproductive state. Taken together, these results indicate that plasma neopterin may prove useful to investigate individual variation in investment into specific immune components, as well as to monitor the dynamics of immune responses to naturally occurring diseases that elicit a Th1 immune response.
Keywords: Immune system, Plasma neopterin, Mandrillus sphinx, Plasmodium gonderi, SIV
Graphical abstract

Highlights
-
•
We measured plasma neopterin, a promising immune marker, in wild mandrills.
-
•
We found a U-shaped age effect, with high neopterin levels in early and late life.
-
•
Adult males had more neopterin than females, possibly due to retroviruses.
-
•
Adult males with high Plasmodium gonderi parasitaemia had high neopterin levels.
-
•
Neopterin can help monitoring Th1 immunity and immunosenescence in primates.
1. Introduction
Understanding the selective pressures shaping the immune system requires an integrative approach acknowledging the complexity of immune responses in their natural environment. However, immunity has been primarily studied in the laboratory on a limited number of model species and in highly controlled environments with limited exposure to infection (Gardner and Luciw, 2008; Maizels and Nussey, 2013; Pedersen and Babayan, 2011). In contrast, natural environments are characterized by a great diversity of pathogenic communities, by intense competition between individuals due to resource limitation, as well as by variation in abiotic conditions such as humidity and/or temperature (Lazzaro and Little, 2009; Martin et al., 2008).
In addition, evolutionary models may often fail to integrate the plurality of immune responses. For example, adaptive immunity has two main components: the Th1 and the Th2 immune responses (Janeway et al., 2005). The Th1 component is triggered in response to intracellular viruses and bacteria and is relayed by cell effectors such as CD8+ cytotoxic T cells, macrophages, monocytes and dendritic cells (Pamer, 2009; Welsh et al., 2010). In contrast, the Th2 component is effective against extracellular pathogens, including most bacteria and macroparasites, and is mediated by antibodies produced by activated CD4+ T cells, IgE, and eosinophils (Anthony et al., 2007). The activation of one component generally down-regulates the other (Janeway et al., 2005), and increasing empirical evidence indicates a negative correlation across individuals in the ability to mount a Th1 versus a Th2 response (Ezenwa et al., 2010; Lienhardt et al., 2002), which may be, at least party, genetically determined (Else et al., 1990, 1993; Ezenwa et al., 2010). In rodents, helminthic parasitism is generally characterized by an up-regulation of the Th2 response and a down-regulation of the Th1 pathway (Bleay et al., 2007), in buffaloes, parasite clearance increased Th1-activity in treated individuals (Ezenwa and Jolles, 2015; Ezenwa et al., 2010). In addition, sexual differences in the Th1/Th2 balance have been demonstrated in several vertebrates (Alexander and Stimson, 1988): females generally show a predominant Th2 response compared to males (Schuurs and Verheul, 1990), and are therefore generally more resistant to a diversity of macroparasites (Klein, 2000; Zuk and McKean, 1996). It is therefore possible that trade-offs between immune function and reproduction or other functions may have operated differentially on distinct components of the immune response (Long and Nanthakumar, 2004) and it becomes important to investigate individual variation in specific markers of the Th1 versus Th2 immunity.
Here we investigate variation in plasma neopterin concentrations in a natural population of mandrills (Mandrillus sphinx) from Southern Gabon, for which detailed information on life-history, behaviour, physiology and health is available. Indeed, neopterin may represent a useful candidate marker of the activation of the Th1 response which can be relatively easily quantified from wild individuals. Neopterin is a pteridine released by specific immune cells, primarily macrophages and monocytes, during the activation of a Th1 specific immune response (Mosser and Edwards, 2008; Widner et al., 2000) upon their stimulation by IFNγ, and to a much lesser extent IFNα and other cytokines such as IL-2 (Murr et al., 2002). Neopterin production is thus typically associated to the synthesis of IFNγ, which can be released by innate or adaptive immune cells; in particular natural killer (NK) cells are an important early and transient innate source of IFNγ. The production of IFNγ is subsequently relayed by Th1 cells during the specific adaptive immune response, resulting in the continuous synthesis of neopterin (Mosser and Edwards, 2008). Neopterin is thus considered as a marker of the activation of innate or specific Th1 immunity (Murr et al., 2002).
Neopterin levels from blood and urine are highly correlated (Heistermann and Higham, 2015), due to renal clearance and relatively long half-life of circulating neopterin (90min in humans: Fuchs et al., 1994), and neopterin is highly stable in samples obtained and stored under field conditions (Heistermann and Higham, 2015). Nevertheless, neopterin levels are also highly variable within individuals, since they reflect the dynamics of immune response that vary with the nature and the course of an infection. Increases in neopterin levels are typically observed in human patients suffering from diseases involving Th1 immunity such as viral infections, for example HIV-1 infection (Denz et al., 1990; Fuchs et al., 1990; Murr et al., 2002; Mildvan et al., 2005; De Rosa et al., 2011; Arshadi et al., 2013) but also acute viral hepatitis, influenza, mumps, or measles (Murr et al., 2002). In addition, autoimmune diseases (Balázs et al., 2012; Nasonov et al., 2000; Sucher et al., 2010) including Crohn's disease (Husain et al., 2013; Prior et al., 1986), some parasitic diseases such as malaria (Reibnegger et al., 1984, 1987), and some bacterial diseases involving intracellular bacteria such as Mycobacterium tuberculosis may also result in elevated neopterin production (Murr et al., 2002). In nonhuman primates, heightened plasma neopterin is associated with increased activation of T-cells in mandrills that are co-infected by SIV (simian immunodeficiency virus) and STLV (simian T-cell leukemia virus) (Greenwood et al., 2014; Souquière et al., 2014). In captive macaques, SIV infection is associated to elevated neopterin concentrations (Higham et al., 2015), and urinary neopterin levels increase during a period of respiratory infection in bonobos and chimpanzees (Behringer et al., 2017; Löhrich et al., 2018; Wu et al., 2018). Finally, in Barbary macaques, urinary neopterin levels increase with age, which has been interpreted as a marker of immunosenescence (Müller et al., 2017).
Wild non-human primates represent interesting models in eco-immunology because they are phylogenetically close to humans, and therefore represent a potential source of zoonoses (Burgos-Rodriguez, 2011). Such proximity also facilitates the application of methods developed for humans to non-human primates. In addition, detailed field studies on wild non-human primates have gathered valuable datasets on the ecology, life-history and health of many individuals, and thus provide key opportunities to understand individual variation in immune phenotypes, as is the case of our field study of mandrills. Mandrills live in large multimale–multifemale social groups, and are characterized by intense male-male competition and pronounced sexual size dimorphism (Setchell et al., 2001). Dominant male mandrills generally produce higher testosterone levels than subordinates, in response to high levels of reproductive competition (Wickings and Dixson, 1992) and face high risks of injuries during the mating season (Abernethy et al., 2002; Bercovitch, 1993; Brockmeyer et al., 2015). Consequently, we may expect variation across sexes and individuals in physiological and immune performances. Finally, wild mandrills are natural carriers of simian retroviruses, such as SIV and STLV (Onanga et al., 2006; Souquière et al., 2012, 2001). They also carry two species of Plasmodium: Plasmodium gonderi and P. mandrilli (Charpentier et al., 2019). An earlier study in this population investigated variations in fecal neopterin levels and failed to detect any effects of injuries, gut parasitism or any other individual traits, suggesting that fecal neopterin levels may not represent a relevant phenotyping tool to monitor immune activity in wild non-human primates (Dibakou et al., 2019).
In this follow-up study, we first investigate individual differences in plasma neopterin levels in relation to sex, age, and social rank to test whether plasma neopterin may serve as a specific Th1 immune phenotyping tool. Additionally, we investigate the links between plasma neopterin and individual disease status. We focus here on the retrovirus SIV, which is known to elicit a Th1 immune response in mandrills (Souquière et al., 2014), and two species of Plasmodium which might be expected to trigger an activation of Th1 immunity (Reibnegger et al., 1987, 1984).
2. Materiel and methods
2.1. Study population
The study was conducted from April 2012 to April 2016 on a free-ranging non-provisioned population of ca. 160 habituated mandrills (in April 2016) living in a private park (Lékédi Park) and its vicinity, in Southern Gabon (see for details on the study population: Poirotte et al., 2016). This population is monitored daily within the framework of a long-term field project (“Mandrillus Project”). Every day, we collect detailed data on life-history, behaviour, physiology and health of individually-recognized individuals (Brockmeyer et al., 2015; Poirotte et al., 2017).
2.2. Individual characteristics
In the statistical models detailed below, we considered the effect of different individual traits such as sex, age, dominance rank and female reproductive status on plasma neopterin concentrations.
2.2.1. Age
Age in years was determined for all studied individuals. Individual's date of birth was exactly known or approximated to a few days for 42 animals and estimated based on body size and condition and patterns of tooth eruption and wear for the 57 other individuals (Galbany et al., 2014). The error made for these latter animals was, however, estimated to be less than a year for 40 of them. Other individuals were fully adult.
2.2.2. Dominance rank
Hierarchies were determined from patterns of approach-avoidance interactions obtained from ad libitum and focal data pooled together and using the corrected David's score to rank individuals (David, 1987; as per: Charpentier et al., 2018). Ranks are not comparable in males and females, we therefore studied their impact on neopterin concentrations separately. Females were classified in three categories of rank with a balanced sample size across these categories (high-ranking, medium-ranking, low-ranking females). Males were classified as either alpha (5 males representing 9 samples) or subordinates (12 males representing 20 samples). Indeed, the alpha male shows clear behavioral characteristics, distinct from all other males: he is generally frequently associated with females and monopolizes a large proportion of matings (Charpentier et al., 2005).
2.2.3. Female reproductive status
We determined the menstrual cycle of sexually mature females by visual inspection of their perineal skin. Females were considered cycling when they presented a visible sexual swelling of any size around the ano-genital area (Dixson, 1983). Gestation period was further deduced from patterns of birth and by the presence of a particular pink swelling (Setchell et al., 2002). Finally, the lactation period encompassed the six first months of an infant's life (Charpentier et al., 2018).
2.2.4. Ecological and reproductive season
In Gabon, there are four climatic seasons characterized by a marked long rainy season (Feb–May) and a long dry season (Jun–Sept), in addition to a short rainy season (Oct–Nov), and a short dry season (Dec–Jan). The ecological seasonality was described by the cumulated rainfall (mm) within 30 days preceding sample collection. Daily rainfall records were downloaded from the NASA website (https://disc.gsfc.nasa.gov/) for the geographic region of interest (GPS coordinates: 1°45′27″S 12°57′22″E for the top left corner, and 1°50′35″S 13°01′19″E for the bottom right corner) during the study period.
In addition, in the studied mandrills, most females are sexually receptive from April to September, leading to an overall increase in male-male aggression (See Fig. 1 in: Dibakou et al., 2019). We further considered the reproductive season (Apr–Sept) vs. the non-reproductive season (Oct–Mar) as a discrete proxy to describe reproductive seasonality.
2.3. Trapping and blood collection
Across the study period, five main trapping sessions occurred (Apr, 2012; Sep 2012, Apr, 2013; Jul 2014, Dec 2015). Individuals were darted and anesthetized with an intramuscular injection of a mixture of ketamine and xylazine (see for details: Charpentier et al., 2018). During these sessions, we gathered a variety of biological samples which have been used to document various aspects of individual health and immunity. In particular, we collected a total of 201 blood samples from the 99 trapped individuals (mean number of samples per individual ± SD: 2.03 ± 1.21; range: 1–7) of both sexes and all ages, from which we obtained neopterin concentrations. Plasma samples obtained after centrifugation (3000 rpm for 15 min) were immediately stored frozen at −20 °C before analyses that occurred in June 2017.
2.4. Plasma neopterin concentrations
We assessed plasma neopterin concentrations using commercial neopterin ELISA kits (#RE59321; IBL International GmbH, Germany) and following the manufacturer's protocol. We deposited, in duplicates, 20 μL of standards, controls (with known concentrations) and plasma samples into wells of the microtiter plate coated with a goat anti-rabbit antibody. We added 100 μL of enzyme conjugate and 50 μL of neopterin antiserum into each well. We covered the microtiter plate with a black adhesive foil and incubated it by gently vortexing the plate in the dark for 90 min. After incubation, we removed the adhesive foil and washed the plate four times with 300 μL of diluted wash buffer. We then added 150 μL of substrate solution into each well and incubated the plate for 10 min. We stopped the reaction by adding 150 μL of stop solution into each well. Finally, optical densities (OD) of the assayed samples were read using a microplate photometer with a 450 nm filter (Multiskan FC). Neopterin concentrations (in nmol/L) were deduced by fitting averaged OD on standard curves and were reliably obtained for 201 plasma samples for which duplicates showed a coefficient of variation < 10% (mean ± SD: 4.0% ± 2.8%). These samples were collected on a total of 53 males and 46 females aged 0.1–21.3 yrs.
2.5. Analytical validation
For each plate, we verified that the two internal quality controls yielded concentrations in acceptable ranges (control 1: 3.3–7.6 nmol/L; control 2: 13.1–27.1 nmol/L). We calculated inter-assay coefficients of variation using repeated concentrations of each of these two internal quality controls of low and high concentrations, run in each assay. CV were 29.9% and 23.3%, respectively. We then calculated parallelism using four serial dilutions of five mandrill blood samples (Supplementary Table S1). The slope of the standard curve was compared to the five slopes of the antibody binding using an ANCOVA (SAS Studio). Slopes were found parallel for all five samples (Supplementary Table S1). Finally, we measured spike recovery accuracy by adding standards of known concentration to each of the five blood samples (Supplementary Table S2). When comparing the expected concentrations of mixes with the observed concentrations, we found a correlation of 95.1% and a mean accuracy of 105.5 ± 9.4 (Supplementary Table S2).
2.6. Plasmodium determination
We evaluated the impact of Plasmodium parasitaemia on neopterin concentrations and performed qPCRs designed to amplify two different fragments located in the cytochrome b gene, one specific of P. mandrilli (106bp) and the other of P. gonderi (188bp), as previously described (Charpentier et al., 2019). In the studied mandrills, average prevalences were 33.0–41.1% (female-male, resp.) for P. mandrilli and 37.6–43.9% (female-male, resp.) for P. gonderi (and see: Charpentier et al., 2019). In our sample, parasitaemia ranged from 1 to 1,267,280 copies in females per μl of blood (median: 0; mean ± SD: 61 ± 216 after excluding one outlier - i.e. > 130,000 copies) and 1–273,424 copies in males (median: 0; mean ± SD: 540 ± 3182 after excluding two outliers) for P. mandrilli and from 1 to 2,551,395 copies in females (median: 0; mean ± SD: 1296 ± 8247 after excluding one outlier) and from 1 to 100,182 copies in males (median: 0; mean ± SD: 1614 ± 10,154) for P. gonderi. Both parasitaemia were uncorrelated in the three data sets (Pearson correlations: all individuals r = −0.007, P = 0.92; adult females: r = −0.015, P = 0.90; adult males: r = −0.068, P = 0.73).
2.7. SIV status
Like in HIV-infected human populations, the diagnosis of the SIV status is a combination of serological and molecular assays in the initial phase of the infection. We first screened sera samples for specific antibodies to SIVmnd-1 and SIVmnd-2 as previously reported (Souquière et al., 2001). Briefly, we determined the antibody reactivity against the SIV V3 loop Env protein by a specific SIVmnd peptide-based immunoassay. This initial screening is highly sensitive and primary infection can therefore be detected as early as 2–3 days following infection. Positive samples were then tested by PCR to quantify viral load and pol sequenced for phylogenetic analyses to differentiate between the two SIV types (Souquière et al., 2001). The combination between immunoassays and quantitative PCR allowed to identify the early stages of a primary infection characterized by the presence of viruses in large quantities and the absence (or very low quantities) of antibodies. The late stages of a primary infection (1–6 months following infection) were then inferred from Western Blot profiles. They are characterized by the absence (or low quantity) of viruses with the persistence of a particular pattern of reactivity against viral proteins (no reactivity against viral enzymes) in Western Blot. Primo-infection is followed by a chronic phase, also determined by Western Blot, associated to a slight decrease in CD4+ T cells count, and a lower proportion of naïve CD8+ T cells (Pandrea et al., 2003; Souquière et al., 2014).
In the study population, 13 males (aged 8.9–20.4) and 2 females (aged 10.6–19.5) were positive to SIV infection. We considered two stages of SIV infection because each of them represents an important phase of the virus cycle: primo-infection (high viremia) and chronic infection (Onanga et al., 2006, 2002).
2.8. Statistical analyses
We performed three sets of Linear Mixed Models (LMM) using the GLIMMIX procedure (SAS Studio) to study the relationship between plasma neopterin concentration (response variable) and different sets of predictors in three groups of individuals who may elicit different strategies of immune investment: (1) individuals of all sexes and ages, (2) sexually mature females, and (3) adult males. In all models, plasma neopterin concentrations were log-transformed to obtain normally distributed residuals and we standardized all continuous explanatory variables. Model fit was evaluated by checking the Gaussian distribution of the residuals and by performing a Shapiro-Wilk test (p > 0.10 in all instances). All three sets of LMMs included individual identity as a random effect. In all three models, we checked for possible multicollinearities between continuous predictors by calculating variances of inflation (VIF; REG procedure, SAS Studio). All VIF were < 4 (not shown) suggesting the absence of hazardous multicollinearities.
In a first model, we considered all samples for which all information below was available (N = 200 samples collected from 98 individuals of all sexes and ages; mean number of samples per individual ± SD: 2.04 ± 1.21; range: 1–7), in order to compare neopterin concentrations across ages and sexes. Fixed effects included (1) individual sex and (2) age along with its quadratic term age2; (3) cumulated rainfall and (4) the reproductive season (reproductive vs. non-reproductive); (5) two measures of parasitaemia (number of copies of each parasite species/μl of blood) of the two blood parasites P. gonderi and P. mandrilli at the time of sampling (one data-point was missing for this effect); and (6) storage time (i.e. time elapsed between collection and extraction of plasma samples, in days) and its quadratic term to control for potentially confounding effects of storage conditions on neopterin concentrations. SIV status was not fitted in this first model because it was confounded both with individual age and sex (SIV infected mostly adult males). Following this first model, we restricted the analysis to juveniles only (<4 yrs, N = 63 collected from 43 juveniles; mean number of samples per juvenile ± SD: 1.5 ± 0.7; range: 1–3) to investigate whether the sex effect found when considering all individuals (see Results) might already be detectable before the seroconversion to SIV of most males, which occurs in early adulthood.
The second model included adult females only (> 4 years, N = 70 samples collected from 32 females; mean number of samples per female ± SD: 2.2 ± 1.1; range: 1–4) to test whether neopterin levels vary with female social rank and reproductive state. We included as fixed effects: (1) female age, (2) dominance rank (class variable with three modalities: high, middle or low rank) and (3) reproductive state (swollen, non-swollen, pregnant, lactating); (4) cumulated rainfall; and (5) two measures of Plasmodium parasitaemia. We omitted storage time terms given that they were found to be non-significant in the first model based on all individuals. We also checked that cumulated rainfall did not confound any possible effect of female's reproductive state by removing the former. Indeed, swollen females are more numerous during the long dry season characterized by low rainfall. Removing cumulated rainfall from the model did not change our results (Supplementary Table S3). Finally, only two females were SIV positive, the corresponding effect was therefore impossible to test. We, however, kept these females into the final dataset because omitting them did not change the results as well (Supplementary Table S3).
In a last set of models, we restricted our analysis to adult males only (> 9 years old, N = 29 samples collected from 13 males; mean number of samples per males ± SD: 2.2 ± 1.2; range: 1–5) to test whether neopterin concentrations vary with male social rank and SIV status. Because of the limited sample size, we performed six univariate models. We studied the following fixed effects: (1) male age, (2) dominance rank (alpha vs. subordinate); (3) cumulated rainfall, (4) reproductive season; (5) two measures of Plasmodium parasitaemia, and (6) SIV infection status as a class variable with three modalities: negative (n = 2 samples from 2 males), primo-infection (n = 7 samples from 6 males), and chronic infection (n = 20 samples from 11 males). For SIV, the variance was not equal across SIV classes (heteroscedasticity) because of a bimodal distribution of neopterin concentrations in males in primo-infection. We therefore studied this particular predictor using a non-parametric analysis of variance (the NPAR1WAY procedure; SAS Studio). Finally, we checked that the only significant effect found in these males (see Results) remained significant when considering a full model including all predictors mentioned above (Supplementary Table S4). As above, we did not study storage time terms.
3. Results
3.1. Variation in plasma neopterin concentrations and individual traits
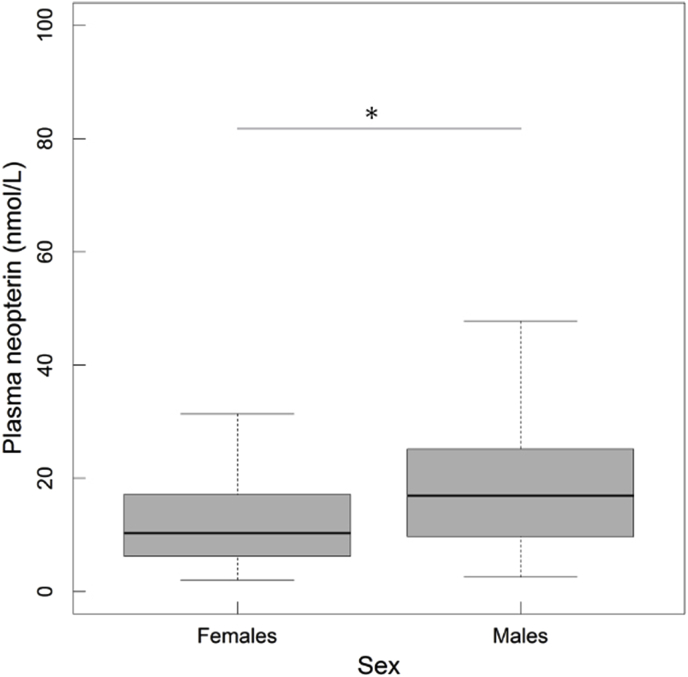
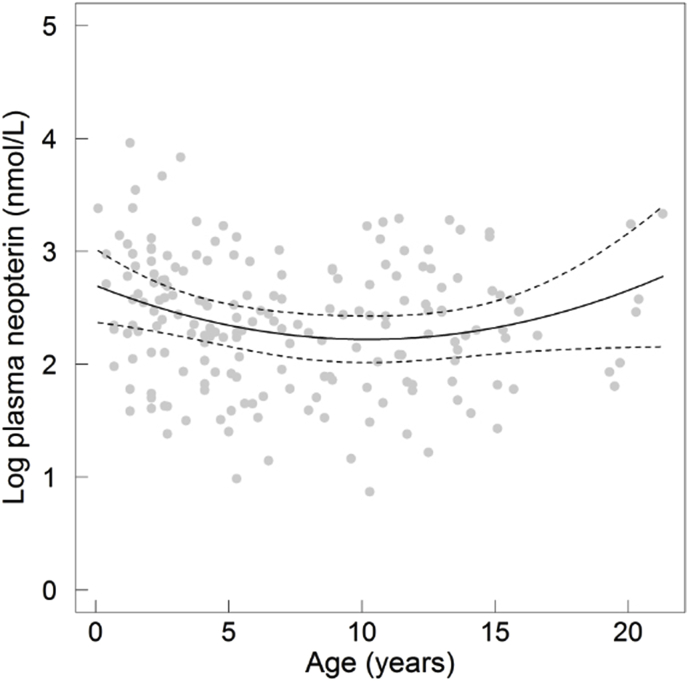
Neopterin concentrations in plasma varied between 2.0 and 98.8 nmol/L across all individuals (n = 201; mean ± SD: 16.7 ± 13.0). Neopterin was significantly different between sexes with males (n = 107; 19.5 ± 14.2) producing more neopterin than females (n = 93; one female was excluded because of the absence of data for P. gonderi; 13.4 ± 10.6; Table 1; Fig. 1). Age showed also a non-linear, U-shaped effect on neopterin, with heightened concentrations in early and late life (Table 1, Fig. 2). However, the effects of sex and age were potentially partially confounded with the positive retroviral status of adult males, as almost all of them were SIV-positive. In juveniles, we found no sex effect (Table 1), suggesting either that this effect was detectable only in adults, or that it reflected a retroviral effect.
Table 1.
Statistical results of multivariate models on the fixed effects predicting variation in plasma neopterin concentrations across male and female mandrills of all ages as well as across adult females only and juveniles only. Significant predictors (P < 0.05) are shown in bold.
| All animals (N = 200) |
Adult females (N = 70) |
Juveniles (N = 63) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | Estimate | Standard error | F | p-value | Estimate | Standard error | F | p-value | Estimate | Standard error | F | p-value |
| Sexa | F: 0.3152 | 0.1103 | 8.17 | 0.0052 | – | – | – | – | F: −0.04339 | 0.1911 | 0.05 | 0.8242 |
| Age | −0.457 | 0.1893 | 5.83 | 0.0177 | 0.0289 | 0.09658 | 0.09 | 0.7668 | −1.7245 | 2.0732 | 0.69 | 0.4218 |
| Age x Age | 0.4101 | 0.1895 | 4.68 | 0.033 | – | – | – | – | 5.1559 | 9.2284 | 0.31 | 0.5866 |
| Rankb | – | – | – | – | HR: 0.1915, MR: 0.1964 | HR: 0.2274, MR: −0.2541 | 0.44 | 0.6481 | – | – | – | – |
| Reproductive statusc | – | – | – | – | P: −0.3438, L: −0.2054 NC: −0.2683 |
P: 0.2423, L: 0.3454 NC: 0.2594 |
0.68 | 0.5688 | – | – | – | – |
| Cumulated rainfall | 0.01626 | 0.1391 | 0.01 | 0.9072 | 0.09471 | 0.0874 | 1.17 | 0.2869 | −1.1676 | 0.6916 | 2.85 | 0.1172 |
| Reproductive seasond | NR: 0.172 | 0.2657 | 0.42 | 0.519 | – | – | – | – | NR: 2.0165 | 1.0431 | 3.74 | 0.0771 |
| P. gonderi | −0.02922 | 0.0467 | 0.39 | 0.5331 | −0.00421 | 0.08348 | 0 | 0.9601 | −0.01037 | 0.2737 | 0.02 | 0.8801 |
| P. mandrilli | 0.009779 | 0.04742 | 0.04 | 0.8371 | 0.03278 | 0.08448 | 0.15 | 0.7006 | 4.3206 | 28.0363 | 0 | 0.9704 |
| Storage time | 0.2297 | 0.2668 | 0.74 | 0.3913 | – | – | – | – | −1.5944 | 1.3134 | 1.47 | 0.2481 |
| Storage time x storage time | −0.4154 | 0.26 | 2.55 | 0.1135 | – | – | – | – | 1.2149 | 1.2362 | 0.97 | 0.3451 |
Reference: males (F: females).
Reference: low rank (HR: high rank; MR: mid rank).
Reference: cycling females (P: pregnant females, L: lactating females, NC: non-cycling females).
Reference: reproductive season (NR: non-reproductive season).
Fig. 1.
Plasma neopterin concentrations (raw values) in relation to individual sex. The bottom and top of the box respectively represent the 25th and 75th quartiles, and the bold horizontal line the median. Whiskers show the interquartile range. Open squares indicate the mean of the distribution. Comparisons are denoted by “*” if significant.
Fig. 2.
Plasma neopterin concentrations (log-transformed) in relation to age in all individuals. The grey dots represent the raw values and the solid line represents the predicted values of the corresponding model. The dashed lines represent the 95% confidence intervals of the predicted values.
In adult males and females, we did not detect any effect of individual traits such as age, rank or reproductive state (in females) on neopterin concentrations. Neither monthly cumulated rainfalls nor storage time ever impacted neopterin concentrations in any of our models (Table 1, Table 2).
Table 2.
Statistical results of six univariate models on the fixed effects predicting variation in plasma neopterin concentrations across adult male only (except for SIV). The significant predictor (P < 0.05) is shown in bold.
| Adult males (N = 29) | ||||
|---|---|---|---|---|
| Fixed effects | Estimate | Standard error | F | p-value |
| Age | −0.1676 | 0.1556 | 1.16 | 0.2983 |
| Ranka | 0.2796 | 0.2462 | 1.29 | 0.27 |
| Cumulated rainfall | 0.07613 | 0.1013 | 0.56 | 0.464 |
| Reproductive seasonb | NR: 0.1358 | 0.213 | 0.41 | 0.53 |
| P. gonderi | 0.225 | 0.09288 | 5.87 | 0.0285 |
| P. mandrilli | 0.01939 | 0.1026 | 0.04 | 0.8527 |
Reference: subordinates (HR: high rank).
Reference: reproductive season (NR: non-reproductive season).
3.2. Variation in plasma neopterin concentrations and natural infections
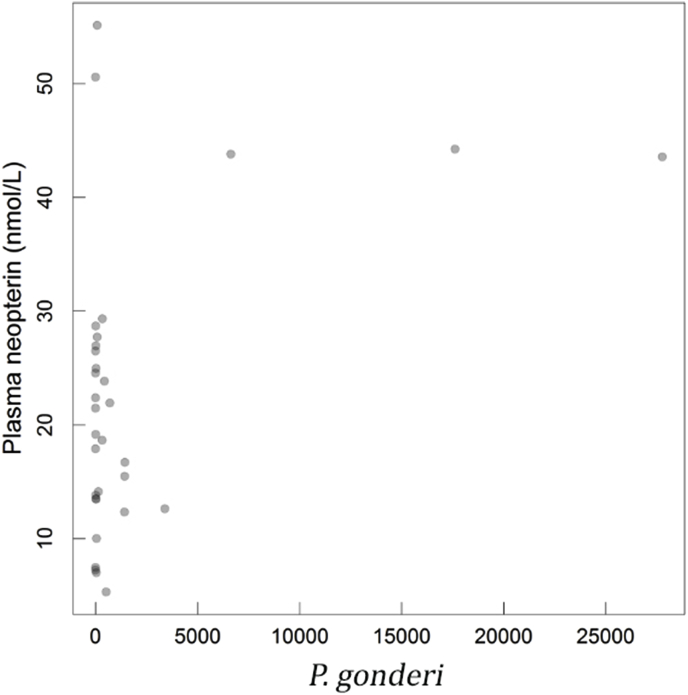
In adult males only, we found a positive association between neopterin concentrations and parasitaemia for one species of Plasmodium (Table 2; Fig. 3), although this association disappears when excluding the three males with high levels of parasitaemia (Supplementary Table S4). We did not find an effect of SIV status in these adult males (non-parametric analysis of variance: χ2 = 0.10, P = 0.95; Supplementary Fig. S1).
Fig. 3.
Plasma neopterin concentrations (raw values) in relation to P. Gonderi parasitaemia in adult males.
4. Discussion
In this study, we examined the links between individual, reproductive and ecological characteristics and plasma neopterin concentrations in a natural population of free-ranging mandrills. We also investigated relationships between naturally-occurring pathogens (simian retrovirus, malaria agents) and plasma neopterin concentrations.
First, we showed heightened neopterin concentrations in early and late life – though the latter effect may be weak as it failed to reach significance in adult males and females, likely because few individuals were older than 16 years old. This non-linear, moderate effect of age on plasma neopterin mirrors age-related variation of plasma or urinary neopterin reported by studies in humans (Berdowska and Zwirska-Korczala, 2002; Werner et al., 1987) especially for the age-related neopterin increase in older individuals (Capuron et al., 2014; Diamondstone et al., 1994; Frick et al., 2004; Reibnegger et al., 1988; Schennach et al., 2002; Spencer et al., 2010). Although the immune mechanisms leading to age-related increases in adult neopterin levels are less well documented in non-human primates than in humans, they seem relatively consistent across species and involve an associated increase with age in levels of IFNγ (Messaoudi et al., 2011; Meyer et al., 2012; Müller et al., 2017). The size of the age effect we observe in our study involves an increase of ca. 10% between the lowest levels (around 10 years old) and the highest levels (at 15–20 years old) of log-transformed neopterin levels, following patterns reported by Müller et al. (2017) in macaques, and of the same order of magnitude in humans (Diamondstone et al., 1994). Despite the fact that neopterin levels are considered as one of the best markers of immune senescence, which is further predictive of disease-specific mortality (Capuron et al., 2014), the causes underlying such age-related increase remain debated (Capuron et al., 2014; Schennach et al., 2002). It is unclear whether higher reference values for neopterin levels in the elderly reflects the normal course of immune ageing in a healthy organism, or may be due to the higher incidence of patients suffering from undiagnosed affections associated with the production of neopterin in such population. A longitudinal study focusing on a large cohort of healthy adults aged 18–87 years using strict exclusion criteria that may represent confounding effects (i.e., smoking, hypertension, pulmonary disease, and other chronic inflammatory disease) detected a weak and linear increase in neopterin levels with age, modulated by ethnic origin and body mass index, which started between the third and fourth decade, suggesting that it may be part of the physiological process of ageing – although the alternative explanation of late age-onset diseases cannot be excluded (Spencer et al., 2010). Although our sample of ageing individuals is fairly small given our focus on wild non-human primates, our results add to the growing body of evidence identifying urinary or plasma neopterin as a promising marker of immunosenescence in humans and non-human primates.
Elevated levels of neopterin in young individuals have been described in human populations (Berdowska and Zwirska-Korczala, 2002; Werner et al., 1987), though in fewer details than in adults, and the reasons behind elevated levels of neopterin in healthy children are rarely discussed in the clinical literature. Such increase starts as early as birth, as human newborns exhibit neopterin levels that are three times higher than maternal levels (and uncorrelated to maternal levels; Radunovic et al., 1999). This study documents a similar increase in the neopterin levels of juveniles for the first time in non-human primates, and therefore suggests that similar physiological processes may underlie the age-related dynamics of neopterin levels across human and non-human primates.
In addition, we found a sexual dimorphism in overall neopterin levels, with males producing on average 44% higher neopterin levels than females. This sexual effect disappears, however, when considering juveniles only. This pattern may be due to two different interpretative scenarios. First, the dimorphic response may only emerge in adults as a consequence of physiological changes associated with sexual maturation (Bouman et al., 2005; Klein, 2004). Testosterone has been found to polarize immune components: it generally plays an inhibiting role on Th2 response (Hepworth et al., 2010; Kissick et al., 2014; Muehlenbein and Watts, 2010; Yao et al., 2003) and possibly favors a Th1 response (Daynes et al., 1991; Giltay et al., 2000), which may explain the higher levels of neopterin in adult males compared to adult females or juveniles. However, studies on human and non-human primates usually report no sex difference in neopterin levels of healthy adults, meaning that such an explanation is not the most parsimonious interpretation (humans: Werner et al., 1987; Diamondstone et al., 1994 but see: Spencer et al., 2010 for a sex by ethnic group interaction; non-human primates: Higham et al., 2015; Müller et al., 2017; Behringer et al., 2017). Second, and alternatively, this sexually dimorphic response in adults may reflect a sex difference in retroviral infection status as almost all adult males were SIV positive. SIV is essentially thought to be transmitted via bites involving blood-salivary contacts (Apetrei et al., 2004; Phillips-Conroy et al., 1994), so males presumably acquire SIV infection during fights, which are common during the mating season and may explain the biased age- and sex-distribution of SIV infections observed in our study population. Results obtained on captive mandrills (Greenwood et al., 2014; Souquière et al., 2014), on captive macaques (Higham et al., 2015) as well as on humans (Fuchs et al., 1988; Mildvan et al., 2005; Murr et al., 2002) report increases in neopterin levels in individuals affected by SIV/HIV infections. The neopterin increase associated with primo-infection in experimentally infected macaques appears pronounced but transient, and peaks in days 10–20 following infection, before going back to much lower, near-baseline levels (Higham et al., 2015). In this study, we did not find any significant difference across males with different SIV status. However, among the 7 individuals in primo-infection, three of them showed values among the highest neopterin concentrations (> 43 nmol/L), and four others showed values among the lowest concentrations (< 13 nmol/L). It is therefore very possible that only three individuals were still in the critical post-infection time window of elevated neopterin values, whereas others were beyond that stage. The origins of the sex effect observed in plasma neopterin levels in this mandrill population will thus have to be addressed by future longitudinal studies, investigating the levels of neopterin before and after SIV infection, as well as in healthy, SIV-negative adults of both sexes, in order to understand if increases in neopterin levels reported in adult males in this sample represent a genuine sex difference in healthy adults, or a consequence of the sex difference in the prevalence of SIV infection.
In these adult males, we further found an association between plasma neopterin concentrations and Plasmodium gonderi infection. This relationship was essentially due to three heavily infected individuals showing elevated neopterin and the exclusion of these three individuals resulted in the disappearance of any relationship. Elevated levels of blood neopterin have also been reported in humans infected by P. falciparum (Reibnegger et al., 1987, 1984). This effect may be related to the immune activation in response to pathophysiological changes induced by Plasmodium, including a stimulation of both CD4+ and CD8+ T cells and an increased production of cytokines by the sporozoites that infect the hepatocytes and the asexual merozoites that reside in the red cells (Wikel, 1996). In these mandrills, we have previously shown that adult males were more susceptible to P. gonderi than females and were also more likely to be infected by this parasite at the beginning of a primo-infection by SIV (Charpentier et al., 2019). In line with this observation, the three individuals that exhibited high levels of P. gonderi were in a SIV-primo-infection. In addition, P. gonderi was found to influence several aspects of mandrill physiology, including skin temperatures and neutrophil/lymphocyte (N/L) ratio generally depending on individual age and sex (Charpentier et al., 2019). In particular, infected males showed decreased N/L ratio. Consequently, co-infection patterns, here observed between Plasmodium and SIV infections, seem to generate a suite of physiological and immunological modifications in male mandrills. A fruitful next step would now be to study the behavioral and fitness effects of infections by these two diseases, as well as related patterns of neopterin variations, and whether neopterin levels at a given stage of infection may prove predictive of disease outcomes in this population.
In line with previous studies on urinary or plasma neopterin in non-human primates (Löhrich et al., 2018; Müller et al., 2017; Wu et al., 2018), our study confirms the relevance of plasma neopterin as a promising marker capable of monitoring the dynamics of immune responses to naturally occurring diseases that elicit an inflammatory stage or a Th1 immune response, and can prove a useful tool to follow wildlife health.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank past and present field assistants of the Mandrillus Project for their help in data collection. We are also grateful to the SODEPAL-COMILOG society (ERAMET group) for their logistical contribution. This study was funded by a Station d’Etudes en Ecologie Globale (INEE-CNRS) and a Laboratoire International Associé (CIRMF and INEE-CNRS) to MC; a PEPS program (MI-CNRS) to EH; a Conservation Action Research Network—Congo Basin Grant Program and Bourses SUD (ISEM) to SD, EH, and MC. Data used in this manuscript are part of long-term data collected within the framework of the Mandrillus Project, funded by a grant of the Deutsche Forschungsgemeinschaft (DFG, KA 1082-20-1; 2012–2015) to MC and two grants from the Agence Nationale de la Recherche (ANR SLEEP 17-CE02-0002, 2017–2020 to MC; ANR ERS-17-CE02-0008, 2018–2021 to EH). This study was approved by an authorization from the CENAREST institute (permit number: AR0042/17/MESRS/CENAREST/CG/CST/CSAR). This is a Mandrillus Project publication number 21 and ISEM 2020-038-SUD.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2020.02.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Berdowska A., Zwirska‐Korczala K. Neopterin measurement in clinical diagnosis. J. Clin. Pharm. Therapeut. 2002;26:319–329. doi: 10.1046/j.1365-2710.2001.00358.x. [DOI] [PubMed] [Google Scholar]
- Abernethy K.A., White L.J.T., Wickings E.J. Hordes of mandrills (Mandrillus sphinx): extreme group size and seasonal male presence. J. Zool. 2002;258:131–137. [Google Scholar]
- Alexander J., Stimson W.H. Sex hormones and the course of parasitic infection. Parasitol. Today. 1988;4:189–193. [Google Scholar]
- Anthony R.M., Rutitzky L.I., Jr J.F.U., Stadecker M.J., Gause W.C. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C., Robertson D.L., Marx P.A. The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front. Biosci. 2004;9:225–254. doi: 10.2741/1154. [DOI] [PubMed] [Google Scholar]
- Arshadi D., Nikbin B., Shakiba Y., Kiani A., Jamshidi A.R., Boroushaki M.T. Plasma level of neopterin as a marker of disease activity in treated rheumatoid arthritis patients: association with gender, disease activity and anti-CCP antibody. Int. Immunopharm. 2013;17:763–767. doi: 10.1016/j.intimp.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Balázs C., Türke B., Vámos A. Determination of serum neopterin levels in patients with autoimmune thyroid diseases. Orv. Hetil. 2012;153:1127–1131. doi: 10.1556/OH.2012.29405. [DOI] [PubMed] [Google Scholar]
- Behringer V., Stevens J.M.G., Leendertz F.H., Hohmann G., Deschner T. Validation of a method for the assessment of urinary neopterin levels to monitor health status in non-human primate species. Front. Physiol. 2017;8:51. doi: 10.3389/fphys.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitch F.B. Dominance rank and reproductive maturation in male rhesus macaques (Macaca mulatta) J. Reprod. Fertil. 1993;99:113–120. doi: 10.1530/jrf.0.0990113. [DOI] [PubMed] [Google Scholar]
- Bleay C., Wilkes C.P., Paterson S., Viney M.E. Density-dependent immune responses against the gastrointestinal nematode Strongyloides ratti. Int. J. Parasitol. 2007;37:1501–1509. doi: 10.1016/j.ijpara.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman A., Heineman M.J., Faas M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- Brockmeyer T., Kappeler P.M., Willaume E., Benoit L., Mboumba S., Charpentier M.J.E. Social organization and space use of a wild mandrill (Mandrillus sphinx) group. Am. J. Primatol. 2015;77:1036–1048. doi: 10.1002/ajp.22439. [DOI] [PubMed] [Google Scholar]
- Burgos-Rodriguez A.G. Zoonotic diseases of primates. Vet. Clin. North. Am. Exot. Anim. Pract. 2011;14:557–575. doi: 10.1016/j.cvex.2011.05.006. viii. [DOI] [PubMed] [Google Scholar]
- Capuron L., Geisler S., Kurz K., Leblhuber F., Sperner-Unterweger B., Fuchs D. Activated immune system and inflammation in healthy ageing: relevance for tryptophan and neopterin metabolism. Curr. Pharmaceut. Des. 2014;20:6048–6057. doi: 10.2174/1381612820666140317110217. [DOI] [PubMed] [Google Scholar]
- Charpentier M., Peignot P., Hossaert-McKey M., Gimenez O., Setchell J.M., Wickings E.J. Constraints on control: factors influencing reproductive success in male mandrills (Mandrillus sphinx) Behav. Ecol. 2005;16:614–623. [Google Scholar]
- Charpentier M.J.E., Givalois L., Faurie C., Soghessa O., Simon F., Kappeler P.M. Seasonal glucocorticoid production correlates with a suite of small-magnitude environmental, demographic, and physiological effects in mandrills. Am. J. Phys. Anthropol. 2018;165:20–33. doi: 10.1002/ajpa.23329. [DOI] [PubMed] [Google Scholar]
- Charpentier M.J.E., Boundenga L., M B., Dibakou S.E., Arnathau C., Sidobre C., Willaume E., Mercier-Delarue S., Simon F., Rougeron V., Prugnolle F. A longitudinal molecular study of the ecology of malaria infections in free-ranging mandrills. Int. J. Parasitol.: Parasites. Wildl. 2019;10:241–250. doi: 10.1016/j.ijppaw.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H.A. Ranking from unbalanced paired-comparison data. Biometrika. 1987;74:432–436. [Google Scholar]
- Daynes R.A., Meikle A.W., Araneo B.A. Locally active steroid hormones may facilitate compartmentalization of immunity by regulating the types of lymphokines produced by helper T cells. Res. Immunol. 1991;142:40–45. doi: 10.1016/0923-2494(91)90010-g. [DOI] [PubMed] [Google Scholar]
- De Rosa S., Cirillo P., Pacileo M., Petrillo G., D'Ascoli G.-L., Maresca F., Ziviello F., Chiariello M. Neopterin: from forgotten biomarker to leading actor in cardiovascular pathophysiology. Curr. Vasc. Pharmacol. 2011;9:188–199. doi: 10.2174/157016111794519372. [DOI] [PubMed] [Google Scholar]
- Denz H., Fuchs D., Hausen A., Huber H., Nachbaur D., Reibnegger G., Thaler J., R Werner E., Wachter H. Value of urinary neopterin in the differential diagnosis of bacterial and viral infections. Klin. Wochenschr. 1990;68:218–222. doi: 10.1007/BF01662720. [DOI] [PubMed] [Google Scholar]
- Diamondstone L.S., Tollerud D.J., Fuchs D., Wachter H., Brown L.M., Maloney E., Kurman C.C., Nelson D.L., Blattner W.A. Factors influencing serum neopterin and 2-microglobulin levels in a healthy diverse population. J. Clin. Immunol. 1994;14:368–374. doi: 10.1007/BF01546321. [DOI] [PubMed] [Google Scholar]
- Dibakou S.E., Basset D., Souza A., Charpentier M., Huchard E. Determinants of variations in fecal neopterin in free-ranging mandrills. Front. Ecol. Evol. 2019;7:368. [Google Scholar]
- Dixson A.F. Observations on the evolution and behavioral significance of “sexual skin” in female primates. In: rosenblatt J.S., Hinde R.A., Beer C., Busnel M.-C., editors. Advances in the Study of Behavior. Academic Press; 1983. pp. 63–106. [Google Scholar]
- Else K.J., Wakelin D., Wassom D.L., Hauda K.M. The influence of genes mapping within the major histocompatibility complex on resistance to Trichuris muris infections in mice. Parasitology. 1990;101:61–67. doi: 10.1017/s0031182000079762. [DOI] [PubMed] [Google Scholar]
- Else K.J., Entwistle G.M., Grencis R.K. Correlations between worm burden and markers of Th1 and Th2 cell subset induction in an inbred strain of mouse infected with Trichuris muris. Parasite Immunol. 1993;15:595–600. doi: 10.1111/pim.1993.15.10.595. [DOI] [PubMed] [Google Scholar]
- Ezenwa V.O., Jolles A.E. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science. 2015;347:175–177. doi: 10.1126/science.1261714. [DOI] [PubMed] [Google Scholar]
- Ezenwa V.O., Etienne R.S., Luikart G., Beja‐Pereira A., Jolles A.E. Hidden consequences of living in a wormy world: nematode‐induced immune suppression facilitates tuberculosis invasion in African buffalo. Am. Nat. 2010;176:613–624. doi: 10.1086/656496. [DOI] [PubMed] [Google Scholar]
- Frick B., Schroecksnadel K., Neurauter G., Leblhuber F., Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin. Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Hausen A., Reibnegger G., Werner E.R., Dierich M.P., Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol. Today. 1988;9:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Artner-Dworzak E., Hausen A., Reibnegger G., Werner E.R., Werner-Felmayer G., Dierich M.P., Wachter H. Urinary excretion of porphyrins is increased in patients with HIV-1 infection. AIDS. 1990;4:341–344. doi: 10.1097/00002030-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Stahl-Hennig C., Gruber A., Murr C., Hunsmann G., Wachter H. Neopterin-its clinical use in urinalysis. Kidney Int. Suppl. 1994;47:S8–S11. [PubMed] [Google Scholar]
- Galbany J., Romero A., Mayo-Alesón M., Itsoma F., Gamarra B., Pérez-Pérez A., Willaume E., Kappeler P.M., Charpentier M.J.E. Age-related tooth wear differs between forest and savanna primates. PloS One. 2014;9 doi: 10.1371/journal.pone.0094938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M.B., Luciw P.A. Macaque models of human infectious disease. ILAR J. 2008;49:220–255. doi: 10.1093/ilar.49.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay E.J., Fonk J.C., von Blomberg B.M., Drexhage H.A., Schalkwijk C., Gooren L.J. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J. Clin. Endocrinol. Metab. 2000;85:1648–1657. doi: 10.1210/jcem.85.4.6562. [DOI] [PubMed] [Google Scholar]
- Greenwood E.J.D., Schmidt F., Liegeois F., Kondova I., Herbert A., Ngoubangoye B., Rouet F., Heeney J.L. Loss of memory CD4+ T-cells in semi-wild mandrills (Mandrillus sphinx) naturally infected with species-specific simian immunodeficiency virus SIVmnd-1. J. Gen. Virol. 2014;95:201–212. doi: 10.1099/vir.0.059808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistermann M., Higham J.P. Urinary neopterin, a non-invasive marker of mammalian cellular immune activation, is highly stable under field conditions. Sci. Rep. 2015;5:16308. doi: 10.1038/srep16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth M.R., Hardman M.J., Grencis R.K. The role of sex hormones in the development of Th2 immunity in a gender‐biased model of Trichuris muris infection. Eur. J. Immunol. 2010;40:406–416. doi: 10.1002/eji.200939589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham J.P., Kraus C., Stahl-Hennig C., Engelhardt A., Fuchs D., Heistermann M. Evaluating noninvasive markers of nonhuman primate immune activation and inflammation. Am. J. Phys. Anthropol. 2015;158:673–684. doi: 10.1002/ajpa.22821. [DOI] [PubMed] [Google Scholar]
- Husain N., Tokoro K., Popov J.M., Naides S.J., Kwasny M.J., Buchman A.L. Neopterin concentration as an index of disease activity in crohn's disease and ulcerative colitis. J. Clin. Gastroenterol. 2013;47:246. doi: 10.1097/MCG.0b013e3182582cdb. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Travers P., Walport M., Shlomchik M. sixth ed. 2005. Immunobiology: the Immune System in Health & Disease. (Garland, New York) [Google Scholar]
- Kissick H.T., Sanda M.G., Dunn L.K., Pellegrini K.L., On S.T., Noel J.K., Arredouani M.S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:9887–9892. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L. Hormones and mating system affect sex and species differences in immune function among vertebrates. Behav. Process. 2000;51:149–166. doi: 10.1016/s0376-6357(00)00125-x. [DOI] [PubMed] [Google Scholar]
- Klein S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Lazzaro B.P., Little T.J. Immunity in a variable world. Phil. Trans. R. Soc. B. 2009;364:15–26. doi: 10.1098/rstb.2008.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhardt C., Azzurri A., Amedei A., Fielding K., Sillah J., Sow O.Y., Bah B., Benagiano M., Diallo A., Manetti R., Manneh K., Gustafson P., Bennett S., D'Elios M.M., McAdam K., Prete G.D. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur. J. Immunol. 2002;32:1605. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Löhrich T., Behringer V., Wittig R.M., Deschner T., Leendertz F.H. The use of neopterin as a noninvasive marker in monitoring diseases in wild chimpanzees. EcoHealth. 2018;15:792–803. doi: 10.1007/s10393-018-1357-y. [DOI] [PubMed] [Google Scholar]
- Long K.Z., Nanthakumar N. Energetic and nutritional regulation of the adaptive immune response and trade‐offs in ecological immunology. Am. J. Hum. Biol. 2004;16:499–507. doi: 10.1002/ajhb.20064. [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Nussey D.H. Into the wild: digging at immunology's evolutionary roots. Nat. Immunol. 2013;14:879–883. doi: 10.1038/ni.2643. [DOI] [PubMed] [Google Scholar]
- Martin L.B., Weil Z.M., Nelson R.J. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc. B. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I., Estep R., Robinson B., Wong S.W. Nonhuman primate models of human immunology. Antioxidants Redox Signal. 2011;14:261–273. doi: 10.1089/ars.2010.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C., Kerns A., Haberthur K., Messaoudi I. Improving immunity in the elderly: current and future lessons from nonhuman primate models. AGE. 2012;34:1157–1168. doi: 10.1007/s11357-011-9353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildvan D., Spritzler J., Grossberg S.E., Fahey J.L., Johnston D.M., Schock B.R., Kagan J. Serum neopterin, an immune activation marker, independently predicts disease progression in advanced HIV-1 infection. Clin. Infect. Dis. 2005;40:853–858. doi: 10.1086/427877. [DOI] [PubMed] [Google Scholar]
- Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbein M.P., Watts D.P. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Biopsychosoc. Med. 2010;4:21. doi: 10.1186/1751-0759-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N., Heistermann M., Strube C., Schülke O., Ostner J. Age, but not anthelmintic treatment, is associated with urinary neopterin levels in semi-free ranging Barbary macaques. Sci. Rep. 2017;7:41973. doi: 10.1038/srep41973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr C., Widner B., Wirleitner B., Fuchs D. Neopterin as a marker for immune system activation. Curr. Drug Metabol. 2002;3:175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- Nasonov E.L., Samsonov M.I., Tilz G., Fuchs D. Neopterin: new immunological marker of autoimmune rheumatic disease. Klin. Med. (Mosc.) 2000;78:43–46. [PubMed] [Google Scholar]
- Onanga R., Kornfeld C., Pandrea I., Estaquier J., Souquière S., Rouquet P., Mavoungou V.P., Bourry O., M'Boup S., Barré-Sinoussi F., Simon F., Apetrei C., Roques P., Müller-Trutwin M.C. High levels of viral replication contrast with only transient changes in CD4 (+) and CD8 (+) cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J. Virol. 2002;76:10256–10263. doi: 10.1128/JVI.76.20.10256-10263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onanga R., Souquière S., Makuwa M., Mouinga-Ondeme A., Simon F., Apetrei C., Roques P. Primary simian immunodeficiency virus SIVmnd-2 Infection in mandrills (Mandrillus sphinx) J. Virol. 2006;80:3301–3309. doi: 10.1128/JVI.80.7.3301-3309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E.G. Tipping the balance in favor of protective immunity during influenza virus infection. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:4961–4962. doi: 10.1073/pnas.0901574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I., Onanga R., Kornfeld C., Rouquet P., Bourry O., Clifford S., Telfer P.T., Abernethy K., White L.T., Ngari P., Müller-Trutwin M., Roques P., Marx P.A., Simon F., Apetrei C. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virol. 2003;317:119–127. doi: 10.1016/j.virol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Pedersen A.B., Babayan S.A. Wild immunology. Mol. Ecol. 2011;20:872–880. doi: 10.1111/j.1365-294X.2010.04938.x. [DOI] [PubMed] [Google Scholar]
- Phillips-Conroy J.E., Jolly C.J., Petros B., Allan J.S., Desrosiers R.C. Sexual transmission of SIVagm in wild grivet monkeys. J. Med. Primatol. 1994;23:1–7. doi: 10.1111/j.1600-0684.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Poirotte C., Basset D., Willaume E., Makaba F., Kappeler P.M., Charpentier M.J.E. Environmental and individual determinants of parasite richness across seasons in a free-ranging population of mandrills (Mandrillus sphinx) Am. J. Phys. Anthropol. 2016;159:442–456. doi: 10.1002/ajpa.22888. [DOI] [PubMed] [Google Scholar]
- Poirotte C., Massol F., Herbert A., Willaume E., Bomo P.M., Kappeler P.M., Charpentier M.J.E. Mandrills use olfaction to socially avoid parasitized conspecifics. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1601721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior Ch, Bollbach R., Fuchs D., Hausen A., Judmaier G., Niederwieser D., Reibnegger G., Rotthauwe H.W., Werner E.R., Wachter H. Urinary neopterin, a marker of clinical activity in patients with crohn's disease. Clin. Chim. Acta. 1986;155:11–21. doi: 10.1016/0009-8981(86)90094-x. [DOI] [PubMed] [Google Scholar]
- Radunovic N., Kuczynski E., Rebarber A., Nastic D., Lockwood C.J. Neopterin concentrations in fetal and maternal blood: a marker of cell-mediated immune activation. Am. J. Obstet. Gynecol. 1999;181:170–173. doi: 10.1016/s0002-9378(99)70455-2. [DOI] [PubMed] [Google Scholar]
- Reibnegger G., Boonpucknavig V., Fuchs D., Hausen A., Schmutzhard E., Wachter H. Urinary neopterin is elevated in patients with malaria. Trans. R. Soc. Trop. Med. Hyg. 1984;78:545–546. doi: 10.1016/0035-9203(84)90080-4. [DOI] [PubMed] [Google Scholar]
- Reibnegger G., Fuchs D., Hausen A., Schmutzhard E., Werner E.R., Wachter H. The dependence of cell-mediated immune activation in malaria on age and endemicity. Trans. R. Soc. Trop. Med. Hyg. 1987;81:729–733. doi: 10.1016/0035-9203(87)90009-5. [DOI] [PubMed] [Google Scholar]
- Reibnegger G., Huber L.A., Jürgens G., Schönitzer D., Werner E.R., Wachter H., Wick G., Traill K.N. Approach to define “normal aging” in man. Immune function, serum lipids, lipoproteins and neopterin levels. Mech. Ageing Dev. 1988;46:67–82. doi: 10.1016/0047-6374(88)90115-7. [DOI] [PubMed] [Google Scholar]
- Schennach H., Murr C., Gächter E., Mayersbach P., Schönitzer D., Fuchs D. Factors influencing serum neopterin concentrations in a population of blood donors. Clin. Chem. 2002;48:643–645. [PubMed] [Google Scholar]
- Schuurs A.H.W.M., Verheul H.A.M. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Setchell J.M., Lee P.C., Wickings E.J., Dixson A.F. Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx) Am. J. Phys. Anthropol. 2001;115:349–360. doi: 10.1002/ajpa.1091. [DOI] [PubMed] [Google Scholar]
- Setchell J.M., Lee P.C., Wickings E.J., Dixson A.F. Reproductive parameters and maternal investment in mandrills (Mandrillus sphinx) Int. J. Primatol. 2002;23:51–68. [Google Scholar]
- Souquière S., Apetrei C., Chahroudi A., Pandrea I., Silvestri G., Roques P. Reply to “Control of simian immunodeficiency virus SIVmnd-1 RNA plasma viremia after coinfection or superinfection with SIVmnd-1 in SIVmnd-2-infected mandrills and vice versa. J. Virol. 2012;86:2387–2388. doi: 10.1128/JVI.06953-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquière S., Bibollet-Ruche F., Robertson D.L., Makuwa M., Apetrei C., Onanga R., Kornfeld C., Plantier J.-C., Gao F., Abernethy K., White L.J.T., Karesh W., Telfer P., Wickings E.J., Mauclere P., Marx P.A., Barre-Sinoussi F., Hahn B.H., Muller-Trutwin M.C., Simon F. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 2001;75:7086–7096. doi: 10.1128/JVI.75.15.7086-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquière S., Makuwa M., Sallé B., Lepelletier Y., Mortreux F., Hermine O., Kazanji M. Immunological alterations and associated diseases in mandrills (Mandrillus sphinx) naturally co-infected with SIV and STLV. Virol. 2014;454–455:184–196. doi: 10.1016/j.virol.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Spencer M.E., Jain A., Matteini A., Beamer B.A., Wang N.-Y., Leng S.X., Punjabi N.M., Walston J.D., Fedarko N.S. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. J. Gerontol. Ser. A. 2010;65A:858–865. doi: 10.1093/gerona/glq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher R., Schroecksnadel K., Weiss G., Margreiter R., Fuchs D., Brandacher G. Neopterin, a prognostic marker in human malignancies. Canc. Lett. 2010;287:13–22. doi: 10.1016/j.canlet.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Welsh R.M., Che Jenny W., Brehm Michael A., Selin Liisa K. Heterologous immunity between viruses. Immunol. Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E.R., Bichler A., Daxenbichler G., Fuchs D., Fuith L.C., Hausen A., Hetzel H., Reibnegger G., Wachter H. Determination of neopterin in serum and urine. Clin. Chem. 1987;33:62–66. [PubMed] [Google Scholar]
- Wickings E.J., Dixson A.F. Testicular function, secondary sexual development, and social status in male mandrills (Mandrillus sphinx) Physiol. Behav. 1992;52:909–916. doi: 10.1016/0031-9384(92)90370-h. [DOI] [PubMed] [Google Scholar]
- Widner B., Wirleitner B., Baier-Bitterlich G., Weiss Ü., Fuchs D. Cellular immune activation, neopterin production, tryptophan degradation and the development of immunodeficiency. Arch. Immunol. Ther. Exp. 2000:251–258. [PubMed] [Google Scholar]
- Wikel S.K., editor. The Immunology of Host-Ectoparasitic Arthropod Relationships. CAB International; Wallingford, Oxon, UK ; New York, NY, USA: 1996. [Google Scholar]
- Wu D.F., Behringer V., Wittig R.M., Leendertz F.H., Deschner T. Urinary neopterin levels increase and predict survival during a respiratory outbreak in wild chimpanzees (Taï National Park, Côte d'Ivoire) Sci. Rep. 2018;8:13346. doi: 10.1038/s41598-018-31563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao G., Liang J., Han X., Hou Y. In vivo modulation of the circulating lymphocyte subsets and monocytes by androgen. Int. Immunopharm. 2003;3:1853–1860. doi: 10.1016/j.intimp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zuk M., McKean K.A. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1024. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.