Abstract
Herpes simplex virus type 1 (HSV-1), a neurotropic herpes virus, is able to establish a lifelong latent infection in the human host. Following primary replication in mucosal epithelial cells, the virus can enter sensory neurons innervating peripheral tissues via nerve termini. The viral genome is then transported to the nucleus where it can be maintained without producing infectious progeny, and thus latency is established in the cell. Yin–Yang balance is an essential concept in traditional Chinese medicine (TCM) theory. Yin represents stable and inhibitory factors, and Yang represents the active and aggressive factors. When the organism is exposed to stress, especially psychological stress caused by emotional stimulation, the Yin–Yang balance is disturbed and the virus can re-engage in productive replication, resulting in recurrent diseases. Therefore, a better understanding of the stress-induced susceptibility to HSV-1 primary infection and reactivation is needed and will provide helpful insights into the effective control and treatment of HSV-1. Here we reviewed the recent advances in the studies of HSV-1 susceptibility, latency and reactivation. We included mechanisms involved in primary infection and the regulation of latency and described how stress-induced changes increase the susceptibility to primary and recurrent infections.
Key words: Herpes simplex virus type 1, HSV-1, Susceptibility, Latency, Reactivation, Stress
Abbreviations: 4E-BP, eIF4E-binding protein; AD, Alzheimer's disease; AKT, protein kinase B; AMPK, AMP-dependent kinase; BCL-2, B-cell lymphoma 2; cGAS, cyclic GMP-AMP synthase; CNS, central nervous system; CoREST, REST corepressor 1; CORT, corticosterone; CPE, cytopathic effect; CTCF, CCCTC-binding factor; CTL, cytotoxic T lymphocyte; DAMPs, damage-associated molecular patterns; DCs, dendritic cells; DEX, dexamethasone; GREs, GR response elements; GRs, glucocorticoid receptors; H3K9, histone H3 on lysines 9; HCF-1, host cell factor 1; HDACs, histone deacetylases; HPA axis, hypothalamo–pituitary–adrenal axis; HPK, herpetic simplex keratitis; HPT axis, hypothalamic–pituitary–thyroid axis; HSV-1, herpes simplex virus type 1; ICP, infected cell polypeptide; IRF3, interferon regulatory factor 3; KLF15, Krüppel-like transcription factor 15; LAT, latency-associated transcripts; LRF, Luman/CREB3 recruitment factor; LSD1, lysine-specific demethylase 1; MAVS, mitochondrial antiviral-signaling protein; MOI, multiplicity of infection; mTOR, mammalian target of rapamycin; ND10, nuclear domains 10; NGF, nerve growth factor; NK cells, natural killer cells; OCT-1, octamer binding protein 1; ORFs, open reading frames; PAMPs, pathogen-associated molecular patterns; PDK1, pyruvate dehydrogenase lipoamide kinase isozyme 1; PI3K, phosphoinositide 3-kinases; PML, promyelocytic leukemia protein; PNS, peripheral nervous system; PRC1, protein regulator of cytokinesis 1; PRRs, pattern-recognition receptors; PTMs, post-translational modifications; RANKL, receptor activator of NF-κB ligands; REST, RE1-silencing transcription factor; ROS, reactive oxygen species; SGKs, serum and glucocorticoid-regulated protein kinases; SIRT1, sirtuin 1; sncRNAs, small non-coding RNAs; T3, thyroid hormone; TCM, traditional Chinese medicine; TG, trigeminal ganglia; TK, thymidine kinase; TRIM14, tripartite motif-containing 14; TRKA, tropomyosin receptor kinase A; TRM, tissue resident memory T cells
Graphical abstract
Following primary infection, HSV-1 establishes latency and reactivates under stress. Stress disturbs inner immune Yin–Yang balance, increasing the susceptibility to HSV-1 infection. In latent infection, stress induces changes in hormone level, chromosomal modification and oxidation, thereby disturbing the Yin–Yang balance between HSV-1 and the host defense, leading to recurrent diseases.
1. Introduction
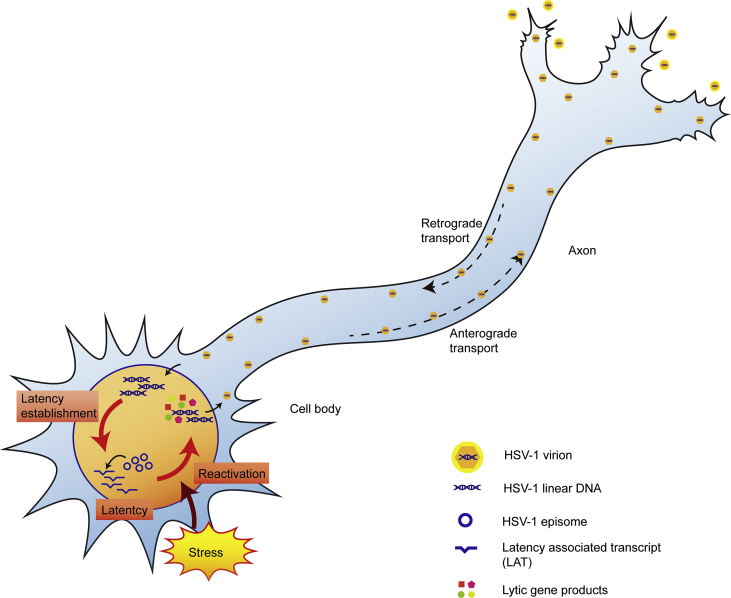
Herpes simplex virus type 1 (HSV-1), a ubiquitous human pathogen, is a neurotropic virus with a linear double-stranded DNA genome that contains more than 80 open reading frames (ORFs) and is about 152 kilo base-pairs (kbp) long. Upon primary infection, the virus replicates within epithelial cells and undergoes its typical lytic life cycle with a cascade of immediate early (IE), early (E), and late (L) genes. Then, it enters axon terminals of sensory neurons and is retrogradely transported to the corresponding sensory ganglia, usually trigeminal ganglia (TG), where a latent infection can be established. In response to a variety of stressors, the latent virus can be reactivated periodically to resume productive replication followed by the formation of infectious virus, which is anterogradely transported back to peripheral tissues or infects further neuronal cells to remain in host1, 2. During anterograde transport, both enveloped capsids and non-enveloped capsids are detected3. Primary infection, latency and reactivation complete the life cycle of the overall HSV-1 infection (Fig. 1). It is also noteworthy that HSV-1 gene expression during latency and reactivation might differ between rodent and mammal models4.
Figure 1.
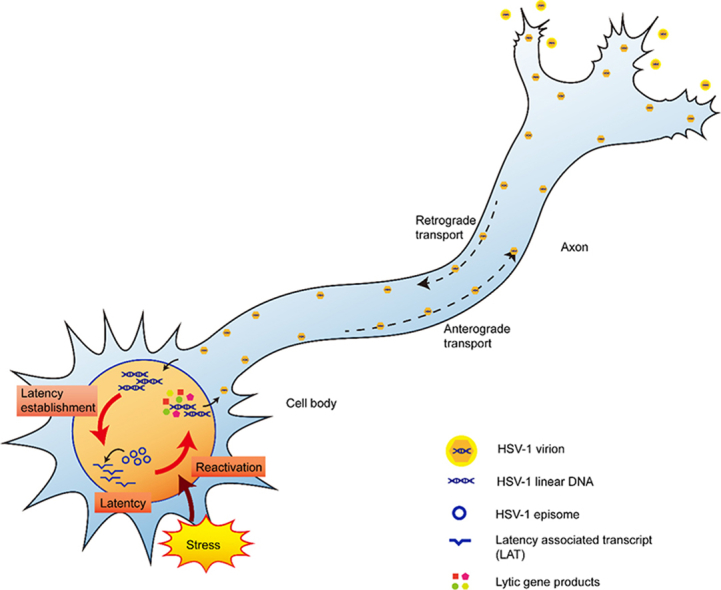
The life cycle of HSV-1 in neurons. During the establishment of latency, the virus invades the axonal termini through virion fusion and travels to the nucleus through retrograde transport. Then the virus enters its latency. During latency, the virus maintains itself in the neuron as an episome while silencing most of its genome and transcribing only a series of mRNAs, especially LAT. When stimulated by stress factors, the virus reactivates and starts to massively replicate its lytic gene. The proliferated virus then travels to the axon termini through anterograde transport. The complete assembly of the virion is finished in the process of egression. After the egression, the virus re-infects epithelial cells and causes recurrent lesion.
HSV-1 is commonly acquired during early childhood, primarily through oral secretions; while sexual transmission is an increasingly common cause of infection in some countries5. Worldwide, the global prevalence of HSV-1 is approximately 90%, and in some rural areas, the seroprevalence is even higher, up to 100%6, 7, 8, 9, 10, 11. The success of HSV-1 infection can be attributed to its ability to establish lifelong persistent infection of the host, termed latency. In latent state, HSV-1 maintains the episomal viral genome in neuronal nuclei without producing infectious progeny for long period of time. Eventually, the virus can re-enter the lytic replication program for productive replication, a process known as reactivation. Reactivation ensures long-term persistence and dissemination of the virus to further host cells or new hosts. Furthermore, the lifespan of the latent infected cell is extended, and thus the virus is able to escape immune surveillance.
HSV-1 has raised concerns because it can cause many diseases of various severity. Acute HSV-1 infection can cause herpes labialis (cold sores)12, gingivostomatitis13 and eczema herpeticum14. It should be noted that, currently, there is no treatment to completely remove HSV-1 once an individual is infected. Reactivation of latently infected HSV-1 can cause recrudescent lesions and is the main reason for herpes viral encephalitis15, 16, 17 and herpetic keratitis18. Recurrent ocular infection can lead to irreversible corneal scarring and blindness19. Increasing number of studies20, 21 have confirmed HSV-1 as pathogen directly related to nervous system degenerative diseases like Alzheimer's disease (AD). Reactivation of HSV-1 will increase the risk of developing AD22. Importantly, an amplified concentration of HSV-1 antibody and the use of antivirals can antagonize the nerve damage of AD, which has also proven that HSV-1 in latent status leads to long term damage to central nervous system23, 24. It might likewise be the cause of Meniere's disease, an inner ear disease with spinning sensation, loss of hearing, and pressured feelings in the ear25. Hence, all these findings have emphasized the importance on the study of HSV-1 latent infection.
As a rapidly developing systematical medical science, traditional Chinese medicine (TCM) is a systematical medicinal science with an application history of over 2000 years in China, widely spread in Asia. At present, a broad range of research in the field of TCM is proceeding. As a treasured resource accumulated by the continuous practice of Chinese people, it has inspired many new discoveries in drug and therapeutic developments26, 27, 28, 29. One of the basic theories in the TCM field is the theory of Yin–Yang balance, which is also applied as a philosophical term. Therein, Yin represents the repressive and inhibitory factors, while Yang stands for the active and aggressive factors. The confrontation, homeostasis, and transformation between Yin and Yang compose the Yin–Yang balance. In different contexts and situations, the components of Yin and Yang refer to different elements. For instance, in “Shang-Huo” syndrome, the hyperactivity of Yang, in this case is “Huo” (fire), causes increased neuroendocrine activation, hence breaking the Yin–Yang balance30, 31. In TCM theory, the disturbance of Yin–Yang balance is an essential cause of diseases.
Stress has been defined as a non-specific reaction of an organism, which fails to respond appropriately to abnormal environmental stimuli or emotional/physical threats. Disturbing the Yin–Yang balance, stress can increase the susceptibility to infectious agents, influence the severity of infectious disease and reactivate latent herpesviruses by significantly modulating the central nervous (CNS), endocrine and immune systems. TCM theory suggests that internal injury caused by excess of seven emotions, named “Qi-Qing Nei-Shang”, also known as emotional stress in modern medical science, disturbs Yin–Yang balance, “Qi-Xue” and viscera balance, inducing the stagnation of Qi32. Chronically, the stagnation transforms into “Shang-Huo” syndrome, where Yang dominates Yin31. One of the typical symptoms of “Shang-Huo” is heat sore on the face, which is related to the reactivation of latent HSV-1 infection by modern medicine. Here we review the latest insights into the mechanism of stress-induced susceptibility to HSV-1 and its reactivation from latency in order to shed light to future researches on HSV-1 latency and the possible solutions to the effective control of latent HSV-1 infection reactivation.
2. Stress increases the susceptibility of HSV-1 infection by disturbing host inner Yin–Yang balance
It has been widely accepted that stress during HSV-1 infections can influence the susceptibility, infection severity and infection types33, 34, 35, 36. The host defense against HSV-1 consists of three main parts: innate immunity, adaptive immunity and intrinsic immunity37. Therein, innate and adaptive immunity are the main defense strategies for most mitotic cells and have been investigated more thoroughly38. Under normal condition, the host innate and adaptive immunities perform in a cooperative and mutually restrictive Yin–Yang balance, ensuring adequate level of defense against pathogens. However, when the balance is disturbed by stimulations, the immunity balance begins to wander, which consequently leads to immune compromise.
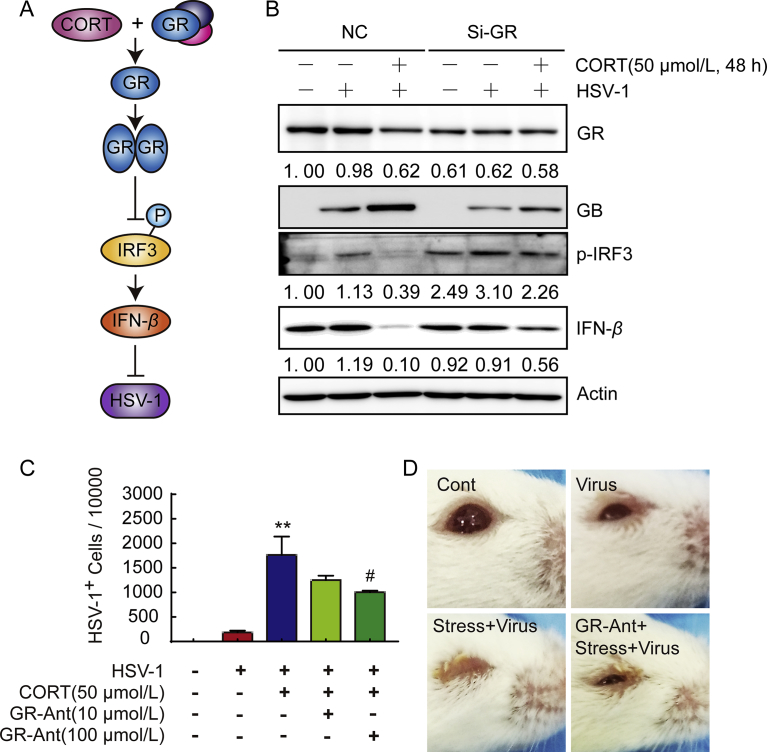
Our previous studies39, 40, 41 have already demonstrated that restraint stress is able to induce immune compromise, thereby increasing the susceptibility to viral infection and the severity of the infection, such as influenza; and that anti-stress treatments can reverse such changes. Under normal conditions, the morbidity of mice inoculated with H1N1 virus was about 30%, while the mice pretreated with restraint stress for 22 h showed a morbidity of 100%42. Survival curves, lung index, virus nucleoprotein level and immunohistochemistry results showed significantly increased disease severity42, 43, 44, 45, 46. Further investigation showed decreased mitochondrial antiviral-signaling protein (MAVS) level, natural killer (NK) cell activity and T cell activity in stressed mice, indicating significant impairment of both innate and adaptive immunity42, 45, 46. TCM formulas, such as KangBingDu Oral Liqid, are able to reverse the stress effects and reduce host susceptibility to virus, furtherly proving the feasibility to apply TCM in viral infection42, 44. Chronic psychological stress is able to inhibit innate and adaptive immune response towards HSV-1 including NK cell activity, HSV-specific CD8+ T cell number and activity, immunity related cytokine level and lymphocyte infiltration35. Restraint stress prolongs the length of HSV-1 infection and increases the number of infected neurons, resulting in longer viral progeny and more severe recurrent lesions47. Based on the unpublished data we obtained, using corticosterone (CORT) stress model in normal and glucocorticoid receptor (GR) knocked-down SH-SY5Y cells, as well as restraint-stressed mice models, we have confirmed that stress hormone CORT can enhance HSV-1 susceptibility, and that such increase is largely dependent on GR. Our results further demonstrated that the GR-dependent effect of CORT on HSV-1 susceptibility is related to the inhibition of interferon regulatory factor 3 (IRF3) phosphorylation and the decrease of IFN-β, indicating an inhibitory effect of GR on innate immunity (Fig. 2). Interestingly, it has been found that, different from chronic psychological stress and restraint stress, social disruption stress can enhance the innate immune responsed to a primary HSV-1 infection in both cornea and TG of mice by increased expression of anti-viral cytokines and infiltration of macrophages, ultimately reducing the severity and frequency of future recurrences48. Under HSV-1 infection, tripartite motif-containing 14 (TRIM14) is likely to cleave the ubiquitin chain of cyclic GMP-AMP synthase (cGAS) and prevent it from being degraded through autophagy, ultimately enhancing IFN signaling, thus improving immune responses49.
Figure 2.
CORT enhances HSV-1 susceptibility, inhibits innate immunity and GR knock-down attenuates the effect. (A) The schematic illustration of the effect of CORT on HSV-1 susceptibility. (B) SH-SY5Y cells were transfected with vectors (NC groups) or GR siRNA (Si-GR groups). One day after the transfection, the cells were pretreated with CORT for 48 h and then inoculated with HSV-1 F strain (MOI = 1) for 24 h. In the NC groups, CORT induced significant increase in viral protein GB and decrease in IFN-β and phosphorylated IRF3, while in the Si-GR groups, the effect of CORT was attenuated. These results indicate that stress hormone CORT is able to enhance HSV-1 susceptibility. GR is indispensable for its effect, and such effect is related to innate immunity. (C) Flow cytometry results show that pretreatment of CORT significantly increased the susceptibility of SH-SY5Y to HSV-1. High dose of GR antagonist RU486 significantly attenuated the effect of CORT. GR-Ant, GR antagonist RU486. Significances were marked as **P < 0.01 vs. virus group and #P < 0.05 vs. CORT+virus group. (D) Mice loaded with restraint stress showed more serious HSK phenotype than the unstressed group. RU486 alleviated the symptom. GR-Ant, GR antagonist RU486. Data shown in this figure are unpublished data of our group.
Besides innate and adaptive immunity, another currently under researched immunity, intrinsic immunity is closely related to the defense against HSV-1 invasion. To some extent, intrinsic immunity might play a more important role in neurons than in other cells50. Evidence also shown that neuronal cells are less responsive to IFN signaling than other types of cells51. As far as we know, there are three components for host intrinsic defense against HSV-1: autophagy, HSV-1 repressive complex and nuclear domains 10 (ND10) nuclear bodies50. As the virus enter the neuron, virions are degraded through xenophagy activated by pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs) and pattern-recognition receptors (PRRs). During virus replication, viral DNA and proteins are recognized and degraded through autophagy51, 52. Autophagy may also be induced by IFN-β during HSV-1 replication53. The increased cGAS mentioned earlier is reported to interact with beclin-1, contributing to the autophagy of viral DNA54. HSV-1 has evolved a confrontational mechanism to counter host autophagic defense through a viral protein, infected cell polypeptide 34.5 (ICP34.5)55, 56, 57, 58, 59, 60. It is well understood that autophagy is enhanced under stress61, 62. On the one hand, enhanced autophagy may improve the intrinsic defense against HSV-157; on the other hand, however, the increased autophagy may also prolong host cell survival and provide a more advantageous environment for HSV-1 replication63. Besides, whether stress-induced autophagy has the same virus clearance effect as xenophagy, a selective autophagy, remains unknown. Moreover, stress-induced autophagy upregulation might increase the degradation of cGAS, casing a loss of IFN signaling49. Therefore, the exact fate of HSV-1 susceptibility under stress-induced autophagy enhancement requires further investigation. The conflict between the facts that stress increases HSV-1 susceptibility and that stress enhances autophagy suggests more complicated mechanisms for stress-induced susceptibility. One possible explanation is that stress-induced autophagy increases the degradation of intrinsic defense components, such as promyelocytic leukemia protein (PML) in ND10 nuclear bodies, and hence defecting the intrinsic immune response, which is especially essential for the defense against HSV-1 infection64. Therefore, the stress-induced autophagy of intrinsic immune components may be a possible research direction in the future.
3. The Yin–Yang balance between HSV-1 and host cell defense: the establishment and maintenance of latency
HSV-1 is characterized by establishing latency as a non-integrated, nucleosome-associated episome in neuronal nuclei. In the process of latency establishment, new sets of Yin factors and Yang factors counteract, transform, and ultimately reach a new Yin–Yang balance between the virus and the host. When the new homeostatic Yin–Yang balance is created, the virus enters its latent state in which it resides relatively silently in the nucleus of the infected cells without producing infectious viral progeny. It is hypothesized that neuronal latency is the result of a failure to initiate the lytic cascade, which might be determined by the distinctive architecture of neurons. Therefore, here we introduce the molecular process of normal HSV-1 lytic infection process and illustrate how latency is established. The initiation of IE genes, specifically, ICP0, is the essential trigger for the following expression of E and L genes, indispensable for HSV-1 lytic infection65. Therefore, triggering IE gene expression would be crucial for entering lytic infection. With the cooperation of lysine-specific demethylase 1 (LSD1), the viral protein VP16 recruit octamer binding protein 1 (OCT1) and host cell factor 1 (HCF1) in order to form enough OCT-1/HCF-1/VP16 triplets. The triplets then bind to IE gene promoters and activate IE gene transcription66. ICP0 can then replace the histone deacetylases (HDACs) in the HDAC/RE1-silencing transcription factor (REST)/REST corepressor 1 (CoREST)/LSD1 repressor complex, after which the previously suppressed E and L gene expression can be triggered67. Only with enough VP16 entering the nucleus can HSV-1 successfully enter its lytic phase. However, specific characteristics of neurons make it especially suitable for HSV-1 to establish latency. The other two ingredients in the triplet tend to distribute differently in neurons, compared with other cells. HCF1 is more abundant in the cytoplasm than in the nucleus in unstressed neurons, while at the same time, OCT-1 is downregulated in neurons; both lead to less VP16 in the nucleus68. The HSV-1 genome is also closely associated with ND10 nuclear bodies, which consists of main components like PML, death-domain associated protein (Daxx) and SP100 nuclear antigen69. Under the stimulation of IFNs, they are able to bind closely to HSV-1 genome and inhibit its lytic replication70. However, such defense is also countered by viral protein ICP0, which possesses the activity of E3 ubiquitin ligase64.
In fact, the number of viral particles that infect axons is another factor to determine whether the virus enter latency or not. The viral genome was silenced below a threshold multiplicity of infection (MOI) of 0.1; while high MOI infection resulted in productive infections71. To sum up, the special characteristics of neurons which are able to prevent the initiation of the viral lytic cascade and reduce the number of virions from the axons are the two important factors for the establishment of neuronal latency.
In contrast, immune surveillance seems to be dispensable for the establishment of viral latency, though the system is also critical for the early control of viral distribution as well as elimination of the replication of virus. Mice that lack the innate and adaptive immune system are still able to establish HSV-1 latency in TG72. Furthermore, HSV-1 latency can be established in the absence of neuronal IFN signaling73. Evidence in rabbit model showed that latency-associated transcripts (LATs) are able to enhance latency establishment74, which indicates that LAT participate in the establishment of the latent state. However, since latency is still able to establish in the absence of it, and that it also accumulates in productively infected cells post-infection, LAT may not be crucial for latency establishment75.
Whether HSV-1 can establish non-neuronal latency is unclear. However, there are several publications on cornea latent infection, suggesting that the cornea might be a possible site of latency76. There were evidences indicating the potential of HSV-1 latent infection in the human cornea early in the 90s77, 78. Further studies have shown that HSV-1 DNA can be present in human corneas even though ocular herpetic disease has not been found in the corneas from animal models including mice79, 80, 81, 82 and nonhuman primates83. These results are consistent with the findings in rabbits, i.e., the retrograde migration of HSV-1 DNA occurs from the transplanted corneas of rabbits latently infected with LAT positive HSV-1 to the uninfected rabbits following transcorneal epinephrine iontophoresis75. In addition, LAT was found in the human cornea80, but no other transcriptional products (RNA) or expression products (protein) were observed. Given the lack of such observations, HSV-1 latency in the cornea is still disputed, i.e., if the virus is truly latent in the cornea or only at a transition point along the exit path from a sensory ganglion. So far, there are three hypotheses for the presence of this DNA in human corneas: (i) operational latency; (ii) a low persistent infection in the cornea and (iii) reactivation from neuronal sites. Hence, operational latency in the cornea is very likely to be dependent on novel detection methods, which is more sensitive than present virus detection methods84.
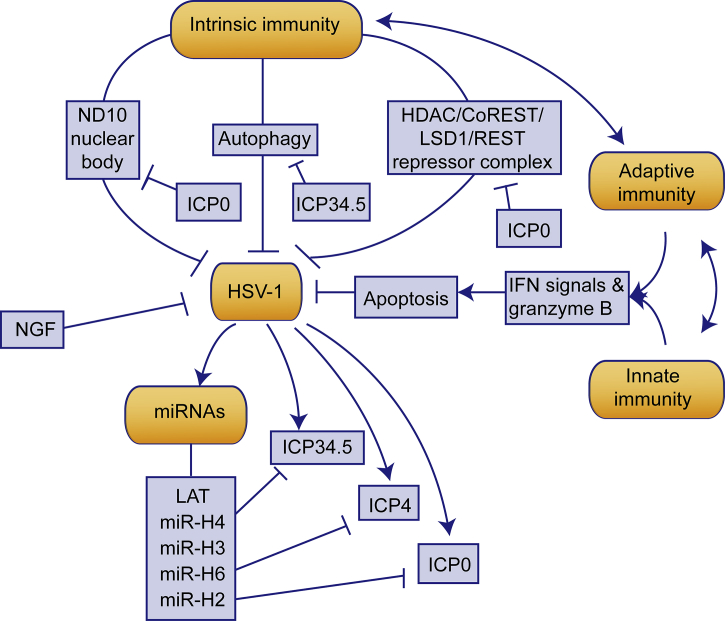
Maintenance of viral latency is dependent on the delicate Yin–Yang balance maintained between HSV-1 and the synergistic effects of several host factors: immunity, HSV-1 microRNAs and other factors (Fig. 3). In this context, Yang is the virus-stimulating factors, including HSV-1 virus itself, promoted glucocorticoid level, oxidational damages, etc.; Yin is the virus-inhibiting factors, such as immunity, thyroid hormone level, etc. The currently most frequently used antiviral treatments for HSV-1 are DNA polymerase inhibitors including acyclovir, famciclovir, and valaciclovir85, 86. Attenuated mutations of HSV-1 lower the virulence by deletion of ICP087, deletion of ICP34.588, expression of ICP34.5 complementary miRNAs89, etc. They both act as interventions of the Yang, which attenuate the Yang factors in the Yin–Yang balance and facilitate the maintenance of latency. When the organism experiences stress stimulation, the balance will be interrupted, ultimately leading to reactivation.
Figure 3.
The interaction between HSV-1 and host cell defense during latency. During latency, HSV-1 activity is inhibited by multiple factors. Intrinsic, innate and adaptive immunity supervise HSV-1 replication while modulating each other. Intrinsic immunity inhibits HSV-1 activity mainly through ND10 nuclear body, autophagy and HDAC/CoREST/LSD1/REST repressor complex, and HSV-1 acts against them through the effect of ICP0 and ICP34.5. Adaptive and innate immunity inhibit the viral activity through granzyme B and IFN-γ secretion, leading to host cell apoptosis. Excretion of NGF by neurons inhibits HSV-1 activity. During latency, HSV-1 only expresses LAT-derived miRNAs, which are able to inhibit lytic gene expressions.
During latency, viral gene expression is largely suppressed except for the abundant expression of LATs and other non-coding RNAs. LAT and its associated miRNA species have been found to influence viral maintenance by suppressing HSV-1 gene expression in vivo and in vitro90, 91, 92. In murine ganglia latently infected by LAT-mutant HSV-1, both the neurovirulence and reactivation frequency are significantly increased93 and the latency is impaired94, providing further clues for the role of LAT in latency maintenance.
In addition to repressing virus-encoded gene expression, LAT can promote neuronal cell survival by inhibiting apoptosis95, which may contribute to maintaining the latency and increasing the survival of the virus in the host. LAT's anti-apoptotic function is mediated through multiple ways. On one hand, cells are protected against cold-shock-induced apoptosis mainly by preventing the dephosphorylation of protein kinase B (AKT), a serine/threonine protein kinase promoting cell survival96. On the other hand, LAT expressing plasmids are able to inhibit the two major apoptosis pathways in mammals induced by caspase-8, caspase-9, and caspase-397, 98, 99. LAT can prevent infected neurons from being killed by CD8+ T cells through anti-apoptosis activity98.
A set of miRNAs derived from LAT are able to target viral transcripts and inhibit HSV-1 gene expression100. HSV-1-miR-H6 can target ICP4 mRNA, inhibiting the expression of the transcription factor crucial to E and L gene transcription, blocking virus lytic infection and decreasing IL-6 expression, hence promoting HSV-1 latency101, 102. HSV-1-miR-H2, which has been associated with the regulation of latency, binds to viral ICP0 mRNA and inhibits its expression. ICP0 plays a major role in both primary and recurrent HSV-1 infections. Its expression triggers the entry of HSV-1 into the replication cycle93, 103. HSV1-miR-H4 inhibits the expression of the viral ICP34.5 gene, an important lytic neurovirulence factor indispensable for promoting viral spread from TG cells to non-neuronal cells89, 104, 105. Two small non-coding RNAs (sncRNAs) derived from LAT also contribute to the decrease of lytic gene expression and apoptosis inhibition91, 106.
Another strategy employed by HSV-1 is to activate cellular glucose synthesis and glycolysis, in order to increase available energy for virus survival107. The activation of AMP-dependent kinase (AMPK) and sirtuin 1 (SIRT1) pathways is effective in inhibiting productive infection and protecting cells from virus related damage108. The modulation of AMPK/SIRT1 axis is modulated over time to suit the HSV-1 infection process109. An over-expression of Luman/CREB3 recruitment factor (LRF), acts as a repressor in a direct or indirect manner to inhibit essential genes of HSV-1 lytic infection110.
Furthermore, different cytokines have different abilities to support latency and suppress lytic HSV-1 replication, providing a fundamental-level control based on neuron–virus interaction111. IFN-β can achieve control of the infection by enhancing the restriction of HSV-1 replication in neuronal cells104. IFN-β regulates LAT expression, which has a positive effect on neuron survival73. Nerve growth factor (NGF)-responsive receptors and signal transduction pathways are necessary to maintain latency and prevent reactivation. This is consistent with the ability of HSV-1 to establish latency in primary sympathetic neurons and Nd-PC12 cells cultured in the presence of NGF112, 113. NGF ablation can induce HSV-1 reactivation in sensory and sympathetic neurons in vitro or after anti-NGF treatment in vivo114. Moreover, neurons infected with latent HSV-1 experience reactivation in the presence of antibodies to NGF115. The ability of NGF to maintain latency has also been proven when herpetic keratitis was topically treated with NGF116. It turns out that the activation of pyruvate dehydrogenase lipoamide kinase isozyme 1 (PDK1) caused by the reaction of NGF with tropomyosin receptor kinase A (TRKA) is the dominant pathway of NGF to suppress reactivation. Suppression of phosphoinositide 3-kinases (PI3Ks) induces HSV-1 reactivation and activation of AKT, the key component for PI3K pathway, which is particularly critical for the maintenance of latency111, 117. This effect of NGF is closely related to mammalian target of rapamycin (mTOR), an important factor in the PI3K/AKT pathway, which controls the population of mRNAs that are actively translated into proteins. These proteins suppress the lytic cycle by sustaining the repressive chromatin state of the viral genome through activating eIF4E-binding protein (4E-BP)118.
During latency, the viral genome associates with nucleosomes by chromatin remodeling. Histone post-translational modifications (PTMs) representative of euchromatin and heterochromatin are found on HSV-1 genes during latency. As a result, the regulation of latent gene expression exists at the level of epigenetic modification. Two activating euchromatin-like modifications that commonly define areas of euchromatin are acetylation of histone H3 on lysines 9 and 14 (H3K9, K14) and dimethylation of histone H3 on lysine 4 (H3K4). Indeed, during lytic infection, acetylation of H3K9 and H3K14 are enriched upon lytic gene promoters; while repressive heterochromatin-like modifications are under-represented119. In contrast, during latency, the actively transcribed LAT locus become enriched in acetylated H3K9 and H3K14 at the LAT promoter and enhancer, while these modified histones are absent at the ICP0 promoter or DNA polymerase gene120. Meanwhile, viral lytic genes are enriched with repressive heterochromatin-like modifications such as methylated H3K27 and methylated H3K9. These observations correspond with latency feature that the LAT is abundant whilst lytic genes are silent. Furthermore, while HDAC inhibitors induce reactivation in latently infected mice121, inhibitors of LSD1 activities that can specifically block demethylation of the repressive markers, such as H3K9me3, H3K9me2 and H3K27me3, will enhance the epigenetic suppression of the viral genome and reduce the reactivation in cultured neurons122. In order to prevent the spread of heterochromatin into areas of euchromatin, there must be barriers in place to keep these domains separate. CCCTC-binding factor (CTCF), a transcriptional repressor, can bind to the sites in HSV-1 genome as boundary elements123. Thus, the transcriptionally active LAT promoter regions will be segregated from the repressed regions of the genome and the LAT enhancer will be prevented from acting on the surrounding lytic genes124. These findings have suggested that epigenetic regulation may control the switch between latency and reactivation.
Systemically, HSV-1 latent infection is surveyed by the immune system through the cooperation of tissue cells and immune cells including CD4+ and CD8+ T lymphocytes. These HSV-1 specific T cells belong to tissue resident memory T (TRM) cells. They have a longer lifespan than normal T cells, and establish a long-term residence in TG125. During latency, the lytic genome of HSV-1 is not completely silenced. Instead, limited lytic gene expression is frequently recognized by MHC class I molecules expressing cells, CD4+ and CD8+ T cells126. For example, local expression of viral proteins such as ICP6 and VP16 is recognized by TG-resident CD4+ and CD8+ T cells127. These facts have indicated that HSV-1 latency maintenance involves active identification and response for viral gene by host immune system. In TG, both HSV-1 specific and non-specific CD8+ T cells exist and cooperate with each other, contributing to the control of latency128. When HSV-1 lytic gene expresses at a relatively low level, non-specific CD8+ T cells can inhibit the reactivation through inhibiting ICP0 by IFN-γ. A novel autophagic response has been discovered, which is related to the IFN signaling in TG53. For neurons that are refractory to IFN-γ, the HSV-1 specific CD8+ T cells can excrete granzyme B to degrade ICP4, and then block viral gene expression129.
The research on HSV-1-specific CD8+ T cells is particularly comprehensive. These cells have been shown important for latency maintenance130, and the reactivation can be reduced by broadening the repertoire of HSV-1 specific T cell that is resident in TG131. The receptor activator of NF-κB ligands (RANKL) has the control over the induction of TG-resident CD8+ T cells. The activation of RANKL also prevents cell apoptosis132. The balance between the survey of TG-resident HSV-1 specific CD8+ T cells and their exhaustion monitored by HSV-1 LAT gene is important for the maintenance of latent status133. Though CD4+ T cells do not have direct effect on latency maintenance, they can perform an indirect function to prevent partial CD8+ T cell exhaustion134. Recently, it has been discovered that CD8α+ dendritic cells (DCs) play a more crucial role in latency maintenance than CD8+ T cells135. Moreover, CD8α+ DCs are influential in T cell exhaustion, which contributes to latency maintenance136.
4. Stress disrupts the Yin–Yang balance and causes reactivation
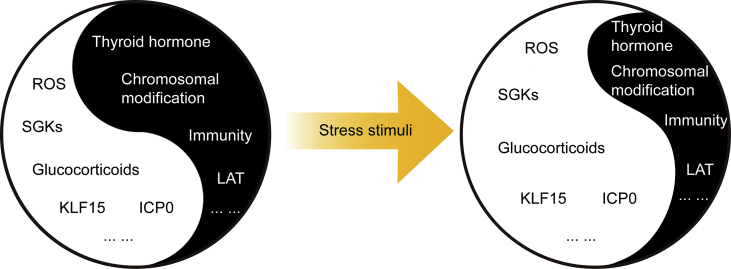
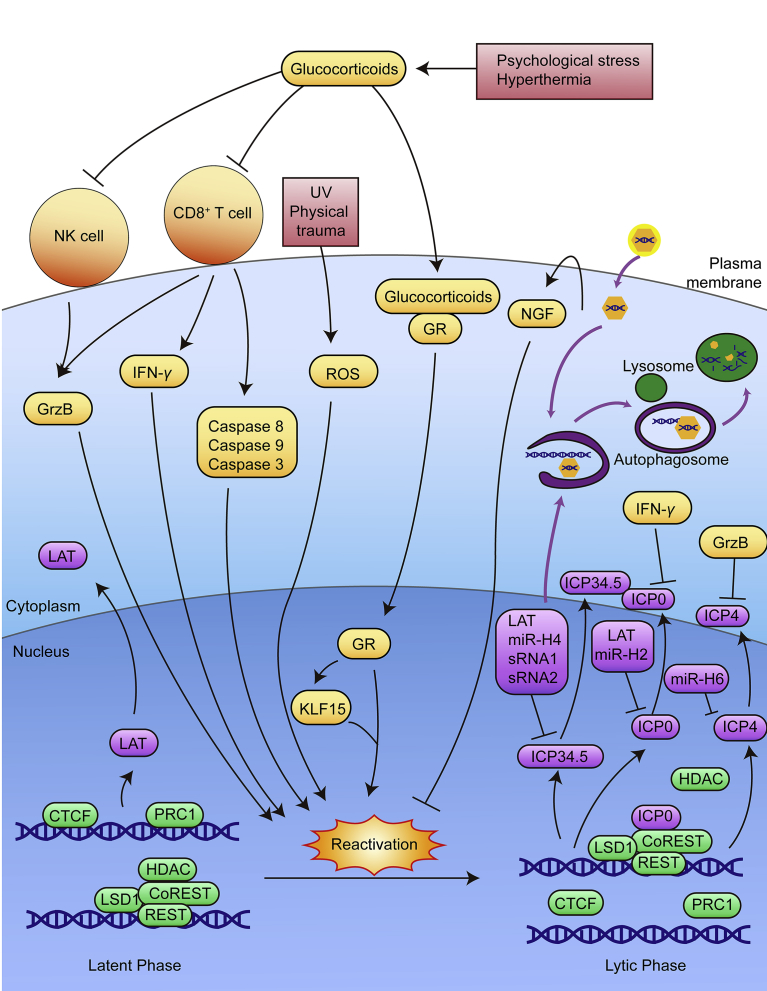
When the Yin–Yang balance maintained in latency is disrupted by stress, HSV-1 enters its reactivation cycle and causes recurrent diseases (Fig. 4). Stress induces the reactivation of latent HSV-1 through multiple mechanisms. When the stress is removed, the latency will be re-established due to the re-silenced viral genome137. Through investigations on previous researches, we summarized the molecular mechanisms in HSV-1 latency establishment, maintenance and reactivation in Fig. 5. Getting through the barriers of host immunity, HSV-1 virions enter the cell and some of them are degraded via xenophagy. During latency, the lytic genes are inhibited by chromosomal modifications, LAT-derived miRNAs, intrinsic, adaptive and innate immunity. Under psychological stress or hyperthermia, the increased glucocorticoids trigger reactivation by either directly activating the virus through GR activation or indirectly affecting host immunity. Under UV stress or physical trauma, ROS level increases, causing oxidational damage, hence inducing the reactivation. As we previously described in our publications, we have developed a successful disease susceptibility model, restraint-stressed mice model, to simulate the effects of stress on virus infection39, 40, 41, 42, 43, 44, 45, 46, 138, 139. Hence, restraint-stress mice model can be a feasible model for stress-induced HSV-1 reactivation, as illustrated in Fig. 6.
Figure 4.
Stress disturbs the Yin–Yang balance between HSV-1 stimulating and inhibiting factors. During HSV-1 latent infection, the HSV-1 stimulating and inhibiting factors form a delicate Yin–Yang balance. Yin factors include virus inhibiting factors such as thyroid hormone, chromosomal modification, host immunity and LAT; Yang factors are the virus stimulating factors like ROS, glucocorticoids, SGKs, KLF15, and ICP0. When the host experiences stress stimulation, for instance, psychological stress, the Yin factors are inhibited and the Yang factors are promoted. Hence the balance is disturbed, which ultimately leads to recurrent lesions.
Figure 5.
Molecular mechanism in an HSV-1-infected neuron during latency establishment, maintenance and reactivation. When HSV-1 virus enters the cell, some virions are degraded through xenophagy. HSV-1 tends to enter latency in unstressed neurons. During latency, HSV-1 DNA remains in its latent phase, CTCFs are attached to the insulators, and the major transcription product of IRL gene is LAT. LAT-derived miRNAs are able to inhibit lytic gene expression. PRC1 complexes are also attached to the genome, suggesting possible inhibitory effect. The HDAC/CoREST/LSD1/REST repressor complex is in its default state to suppress the expression of E and L genes. CD8+ T cells release IFN-γ, granzyme B and caspases into the neuron, and NK cells release granzyme B. IFN-γ inhibits the expression of ICP0, granzyme B inhibits the expression of ICP4, and miRNAs are able to inhibit the expressions of ICP0, ICP4 and ICP34.5. Neuron itself induces NGF which also inhibits reactivation. Psychological stress and hyperthermia increase the level of glucocorticoids, inhibiting the activity of immune cells. Glucocorticoids are also able to bind to glucocorticoid receptors and activate HSV-1 by inducing and cooperating with KLF15, thereby activating ICP0 transcription. UV and physical trauma increase ROS level. All these stress factors wake up the HSV-1 genome and lead to reactivation. During reactivation, PRC1 complexes are replaced, all CTCFs are evicted from the insulators. Consequently, the lytic genes are able to transcribe and the transcripts of IRL gene yield ICP0 and ICP4. ICP0 then removes HDAC from the HDAC/CoREST/LSD1/REST repressor complex, stimulating the expression of all lytic genes, leading to the complete reactivation of viral genome. Some of the newly synthesized viral DNA and protein components are degraded through autophagy.
Figure 6.
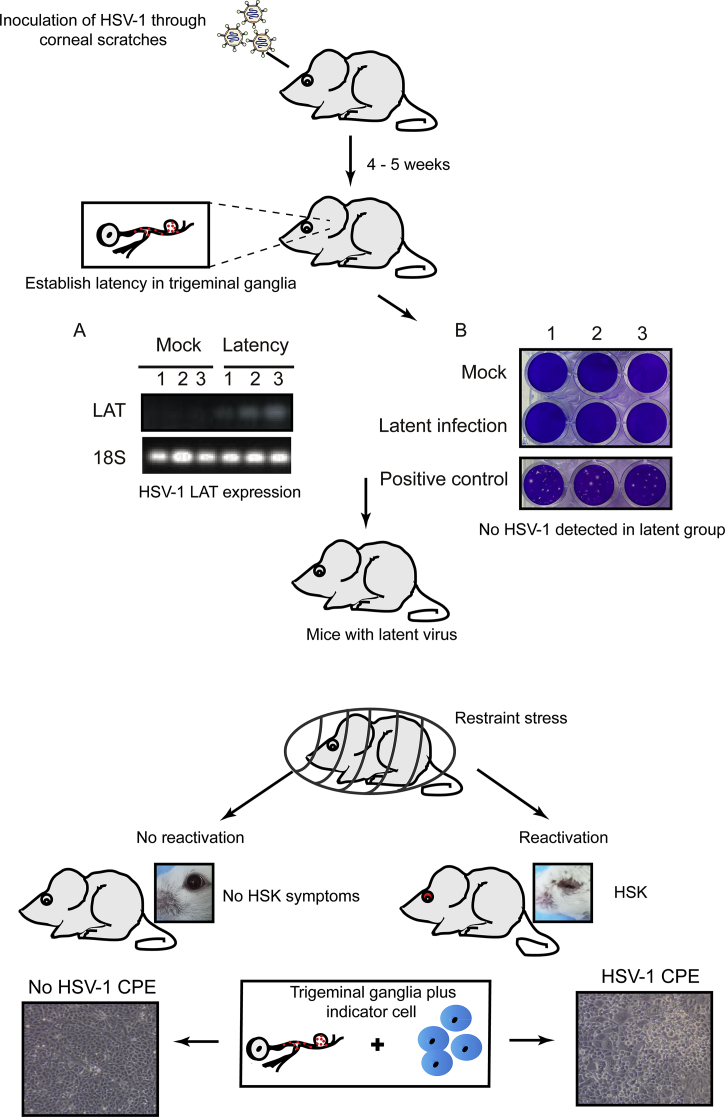
Illustration of restraint stress causing recurrent lesions in mice latently infected with HSV-1. Inoculated with HSV-1 through corneal scratches, the mice were kept under adequate condition for 4–5 weeks to establish latency. When the establishment of latency was confirmed in trigeminal ganglia via virus titration measurement, the LAT expression of uninfected and infected groups were tested. Plaque assay showed no detectable productive HSV-1 progeny. The latently infected mice were then loaded with restraint stress, and the phenotype was assessed afterward. Data shown in this figure are unpublished data of our group.
4.1. Stress induces hormonal changes
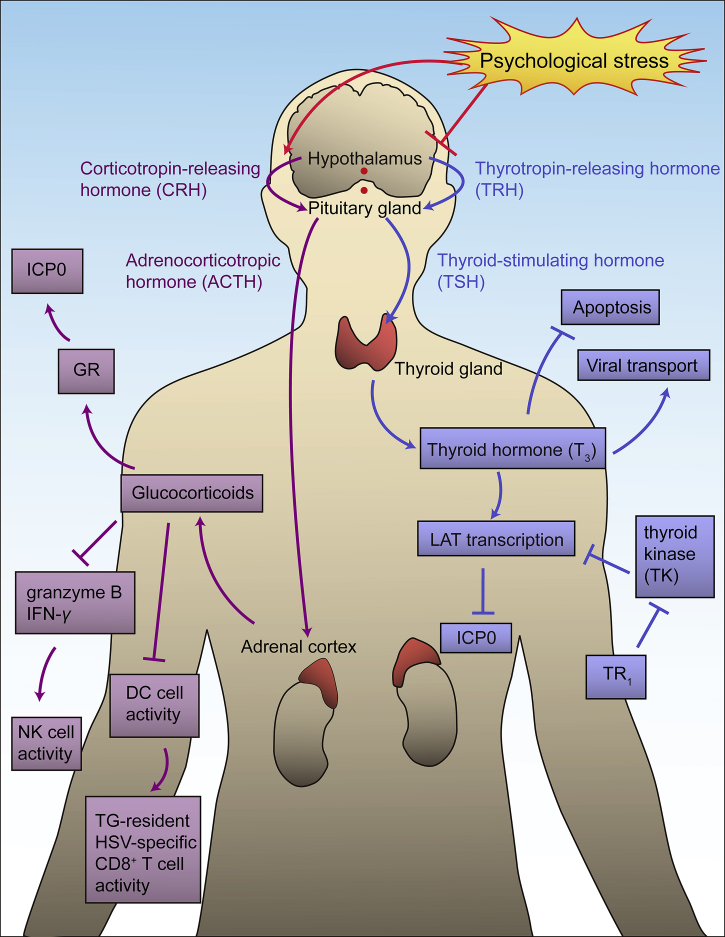
It is well established that stress-induced changes of hormones such as glucocorticoids and thyroid hormone can stimulate HSV-1140, 141 and drive reactivation from latency (Fig. 7). Stress, e.g., psychological stress and hyperthermia, can activate the hypothalamo–pituitary–adrenal (HPA) axis142 and increase the concentration of glucocorticoids in the blood143. Glucocorticoids induce HSV-1 reactivation mainly through two distinct processes: directly affecting HSV-1 virus and indirectly promoting reactivation. Glucocorticoids activate GRs and directly activate HSV-1 transcription by interacting with the GR response elements (GREs) on virus genome. A recent study showed that when cells are treated with synthetic corticosteroid dexamethasone (DEX), GR and Krüppel-like transcription factor 15 (KLF15) cooperate to stimulate reactivation through the transactivation of ICP0144. Serum and glucocorticoid-regulated protein kinases (SGKs) has been reported to increase after stress stimulation and participate in HSV-1 reactivation from latency145. The increased glucocorticoid level also affects both intrinsic and adaptive immunities. TG-resident HSV-1-specific CD8+ T cells are reduced and those survived CD8+ T cells compromise functionally, which induces HSV-1 reactivation for compromising CD8+ T cell surveillance. The impairment of CD8+ T cells may occur via affecting GRs on DCs that are responsible for priming these HSV-1 specific T cells36, 146. Interestingly, however, psychological stress might not diminish CD8+ T cells via impairing DCs. Instead, it could be due to the decrease of T cell stimulative cytokines such as IFN-γ and IL-2149. Further effects of glucocorticoid upregulation by stress are significantly reduced concentrations of granzyme B and IFN-γ142, 146, 147. Also, stress has been proven to cause mitochondrial damage and to decrease MAVS expression45. It can decrease NK cell activity in individuals that are latently infected with HSV-1, which might be due to the reduction of IFN-γ and IL-2148.
Figure 7.
Stress-induced hormone imbalance which leads to reactivation. Psychological stress acts on hypothalamus, stimulates HPA axis (purple arrows) and suppresses HPT axis (blue arrows). In HPA axis, CRH released by hypothalamus increases, enhancing the ACTH released by pituitary gland. As the ACTH level increases, glucocorticoid excreted by adrenal gland also increases. The increased glucocorticoid then activates GRs, activating the transcription of lytic gene ICP0. It also inhibits DC activity, causing the reduction of TG-resident HSV-specific CD8+ T cell activity, affecting adaptive immunity towards HSV-1. Since glucocorticoid is able to inhibit granzyme B and IFN-γ secretion, the granzyme B and IFN-γ level are reduced significantly, which causes the decrease of NK cell activity, affecting innate immunity towards HSV-1. In HPT axis, TRH released by hypothalamus decreases, reducing TSH released by pituitary gland. Thyroid gland then reduces the excretion of T3, which decreases the LAT transcription activated by T3. The suppressive effect of LAT to ICP0 transcription is weakened, and the increased ICP0 level enhances lytic gene transcription. The suppressive effect of T3 towards TK gene, an important viral gene for HSV-1 reactivation, is also decreased. Overexpressed TR1 receptor is able to increase the effect of T3, reversing the changes caused by the reduction of T3.
Thyroid hormone (T3) is able to cause strong suppression of HSV-1 replication149. T3 can activate LAT and consequently repress ICP0 expression150. It also has repressive effect on the HSV-1 thymidine kinase (TK) gene, which is important for viral reactivation149. Psychological stress inhibits the hypothalamic–pituitary–thyroid (HPT) axis and leads to the reduction in T3 secretion. Hence, with the decrease of T3 and the reduction in its suppression effects, there is an increase of ICP0 expression. It has been shown that the overexpression of thyroid receptor β1 can enhance LAT transcription and recruit H3K9me3 and H3K9me2 to repress the TK gene, leading to an increased virus suppression efficiency. In addition, TG neurons overexpressed with thyroid receptor β1 were less susceptive to the reactivation induced by T3 decrease151. However, it should be noted that the suppression effect of T3 only works on neuronal cells, and differential condensation of chromosome may be important in this process152. T3 also regulates the expression of dynein and modify neuronal outgrowth, suggesting the specific role T3 plays in viral transport and anti-apoptosis153.
4.2. Stress reverses chromosomal modification
Under stress, the chromosomal modifications on HSV-1 lytic DNA might be reversed and viral DNA expression might be modified, which lead to induction of lytic replication107. This hypothesis has been supported by a number of studies, which have found that chromatin remodeling around the LAT region and surrounding lytic genes is likely to occurs after a stress stimulus154, 155. Loss of CTCF proteins from chromosomal insulators through stress-induced phosphorylation increased the accessibility of viral genes for transcriptional activation. Replacement of demethylation of H3K27 on lytic genes with euchromatin that is triggered by displacing protein regulator of cytokinesis 1 (PRC1) complexes has been reported to have the similar function124. As a result, the lytic genes would productively express and eventually reactivate.
In fact, a number of transient chromatin modification have been discussed. It has been suggested that histone H3 at serine 10 undergoes a methyl/phospho switch during the first phase of reactivation, and activation of lytic genes is achieved without the removal of H3K9me3156. It is then followed by VP16 synthesis in the second phase157. When the VP16 promotor is activated, in the absence of other lytic viral gene expressions, the expression of VP16 leads to the exit of latency and the entry of lytic cycle158. Therefore, modification of VP16 is required for reactivation of HSV-1 in neuronal cells159. Administration of an inhibitor for helicase-primase is able to suppress such reactivation, which suggests the chromatin modification mentioned above to be essential160.
4.3. Stress causes oxidational damage and induces apoptosis
Under UV or physical trauma, dendrite mitochondria produce reactive oxygen species (ROS) to inhibit mTOR activity and decrease the expression of B-cell lymphoma 2 (BCL-2), inducing apoptosis161. In order to escape from the soon to be apoptotic cells, HSV-1 spreads among host cells in an attempt to infect new individuals. All viral gene classes will be expressed at the same time and enter full reactivation, followed by the infection of peripheral epithelial and neuronal cells to remain the survival and spread of HSV-1162. HSV-1 itself may also be a stimulant to apoptosis. HSV-1 protein ICP27 can increase host cell susceptibility to apoptosis, which is also probably through the production of higher level of ROS163.
5. Discussion and perspective
With the astonishingly rapid development of industrialization and economics in modern society, people have suffered much more stress caused by the environmental, psychological and physical factors. Stress, especially psychological stress caused by emotional stimulation, is able to increase the susceptibility and severity of infectious diseases and to cause the reactivation from latency, leading to disturbance on life quality. Therefore, reducing the effect of stress on diseases is becoming an urgent and widely concerned topic. Emotional stress causes internal damage called “Qi-Qing Nei-Shang” and consequently disturbs the Yin–Yang balance in the organism, leading to increased susceptibility to HSV-1 and the recurrent lesions. Nevertheless, how emotional stimulation affects the biological pattern and the nature of HSV-1 reactivation susceptibility still needs further systematic study. Moreover, considering that once an individual is infected, no treatment exists to remove the HSV-1 virus completely from the host, an approach to control latent infection and reduce reactivation becomes the crux of the issue.
In conclusion, it has been well-known that followed by the invasion of HSV-1 into the neurons, the virus travels through microtubules into the nucleus, where most of the HSV-1 genome is silenced due to the specific characteristics of neurons. However, the virus can still function properly to avoid the detection of immune system. Neuronal factors have contributed to the anti-apoptosis effect and facilitated the survival of infected cells. Epigenetic modification and the direct effect of latent gene can reduce lytic gene expression, and thus the virus cannot be detected by the immune system. The virus can also deplete T cells to reduce the survey intensity. The Yin–Yang balance between the virus-stimulating and virus-inhibiting factors maintains the latency. Under stress, oxidational damage, increased glucocorticoids and decreased thyroid hormones promote the Yang factors and inhibit the Yin factors, consequently disturbing the Yin–Yang balance, leading to productive viral replication.
However, several obstacles remain in the way towards more specific, accurate and coherent understanding of HSV-1 latency and reactivation. Current major animal and cellular models are not sufficient to unravel the mechanistic details. Models of closer genetic similarity with human, e.g., Rhesus macaques, and newly developed models like tree shrews may be more adequate models to study HSV-1 latency and reactivation4, 164. In addition, we still know very little about the molecular mechanism of other stress hormones such as epinephrine, growth hormone and prolactin33, 165. More investigations on other stress hormones are required for a better overall understanding on stress and HSV-1 reactivation. Additionally, according to a research by Edgar et al166, herpes virus infection is enhanced under circadian clock disruption stress, suggesting a new possible direction on how stress influences the susceptibility of HSV-1 infection. According to our current understanding on the role of stress in HSV-1 infection and the instruction of TCM theory, many small molecules with potential anti-HSV-1 activity have been discovered from TCMs. We have recently published a review specifically focusing on the anti-HSV-1 small molecules originated from different TCMs, and their pharmacodynamics mechanisms167. Therein, three main mechanisms were discussed, including autophagy regulation, immunity enhancement, and inhibition of HSV-1 virus replication and infection processes. Numerous sources, e.g., Lychee flower, Houttuynia cordata, and Curcuma longa L., and their effect corresponding mechanisms were discussed in detail. They are promising drug candidates for novel treatments to prevent stress-induced susceptibility to HSV-1 and the following recurrent diseases. Furthermore, the combination of Yin–Yang theory and modern molecular biology may create novel perspectives and approaches in new drug discovery and treatment development. Both Yin and Yang factors we demonstrated above are possible targets for anti-HSV-1 drug and treatment development, e.g., the enhancement of Yin factors including HSV-1-specific miRNAs, thyroid receptor β1, and ND10 nuclear bodies, and the inhibition of Yang factors including the expression of glucocorticoids, the cooperation of GR and KLF15, the activity of SGKs, the expression of ICP0 and ICP34.5. More systematic researches based on Yin–Yang theory and other TCM theories, combined with genomics, proteomics, metabolomics, high throughput in silico screening, etc., may be available approaches to unlock the treasure house of TCM therapy against HSV-1168, 169, 170. Further detailed explorations are required for a more thorough understanding of stress-induced HSV-1 susceptibility and reactivation and feasible treatments.
Acknowledgments
This work was supported, in part, by the National Key Research and Development Program of China, China (grant number 2017YFC1700404); Natural Science Foundation of China, China (grant numbers 81622050, 81573675, 81673709, 81560661 and 81873209); the Young Top-notch Talent Support Program of Guangdong Province, China (grant numbers 2014TQ01R229 and 2016TQ03R586); Guangdong Province Ocean and Fisheries Bureau-Key Technology Research and Development Program, China (grant number A201701A02); Guangdong Science and Technology Foundation for Distinguished Young Scholars, China (grant number 2017A030306004) and the Science and Technology Program of Guangzhou, China (grant numbers 201604046016 and 201610010182).
Rong-Rong He conceived the concept and the framework. Chang Yan collected and analyzed all the data. Chang Yan and Luo Zuo drafted the manuscript. Wen Li contributed to the original data. Xue Li, Robert Dallmann, Yi-Fang Li revised the manuscript. Hiroshi Kurihara and Rong-Rong He revised and approved the manuscript.
The authors declare no conflicts of interest.
Contributor Information
Hiroshi Kurihara, Email: hiroshi_kurihara@163.com.
Yi-Fang Li, Email: liyifang706@jnu.edu.cn.
Rong-Rong He, Email: rongronghe@jnu.edu.cn.
References
- 1.Kumar S.P., Chandy M.L., Shanavas M., Khan S., Suresh K.V. Pathogenesis and life cycle of herpes simplex virus infection-stages of primary, latency and recurrence. J Oral Maxillofac Surg Med Pathol. 2016;28:350–353. [Google Scholar]
- 2.Bigley N.J. Complexity of interferon-gamma interactions with HSV-1. Front Immunol. 2014;5:15. doi: 10.3389/fimmu.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinone S.E., Zaichick S.V., Smith G.A. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J Virol. 2010;84:13019–13030. doi: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L., Li Z., Wang E., Yang R., Xiao Y., Han H. Herpes simplex virus 1 infection of tree shrews differs from that of mice in the severity of acute infection and viral transcription in the peripheral nervous system. J Virol. 2015;90:790–804. doi: 10.1128/JVI.02258-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein D.I., Bellamy A.R., Hook E.W., 3rd, Levin M.J., Wald A., Ewell M.G. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56:344–351. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J.H., Huang K.Y.A., Chen C.Y., Chen C.J., Lin T.Y., Huang Y.C. Seroprevalence of herpes simplex virus type 1 and 2 in Taiwan and risk factor analysis, 2007. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134178. e0134178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley H., Markowitz L.E., Gibson T., McQuillan G.M. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999-2010. J Infect Dis. 2014;209:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 8.Vilibic-Cavlek T., Kolaric B., Ljubin-Sternak S., Mlinaric-Galinovic G. Herpes simplex virus infection in the Croatian population. Scand J Infect Dis. 2011;43:918–922. doi: 10.3109/00365548.2011.588611. [DOI] [PubMed] [Google Scholar]
- 9.Lin H., He N., Su M., Feng J., Chen L., Gao M. Herpes simplex virus infections among rural residents in eastern China. BMC Infect Dis. 2011;11:69. doi: 10.1186/1471-2334-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham A.L., Taylor R., Taylor J., Marks C., Shaw J., Mindel A. Prevalence of infection with herpes simplex virus types 1 and 2 in Australia: a nationwide population based survey. Sex Transm Infect. 2006;82:164–168. doi: 10.1136/sti.2005.016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levett P.N. Seroprevalence of HSV-1 and HSV-2 in Barbados. Med Microbiol Immunol. 2005;194:105–107. doi: 10.1007/s00430-004-0222-5. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham A., Griffiths P., Leone P., Mindel A., Patel R., Stanberry L. Current management and recommendations for access to antiviral therapy of herpes labialis. J Clin Virol. 2012;53:6–11. doi: 10.1016/j.jcv.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolokotronis A., Doumas S. Herpes simplex virus infection, with particular reference to the progression and complications of primary herpetic gingivostomatitis. Clin Microbiol Infect. 2006;12:202–211. doi: 10.1111/j.1469-0691.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- 14.Bussmann C., Peng W.M., Bieber T., Novak N. Molecular pathogenesis and clinical implications of eczema herpeticum. Expert Rev Mol Med. 2008;10:e21. doi: 10.1017/S1462399408000756. [DOI] [PubMed] [Google Scholar]
- 15.Whitley R.J. Herpes simplex encephalitis: adolescents and adults. Antivir Res. 2006;71:141–148. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Steiner I., Kennedy P.G., Pachner A.R. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 17.Perlejewski K., Popiel M., Laskus T., Nakamura S., Motooka D., Stokowy T. Next-generation sequencing (NGS) in the identification of encephalitis-causing viruses: unexpected detection of human herpesvirus 1 while searching for RNA pathogens. J Virol Methods. 2015;226:1–6. doi: 10.1016/j.jviromet.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Shimomura Y. Herpes simplex virus latency, reactivation, and a new antiviral therapy for herpetic keratitis. Nippon Ganka Gakkai Zasshi. 2008;112:247–264. [PubMed] [Google Scholar]
- 19.Toma H.S., Murina A.T., Areaux R.G., Jr., Neumann D.M., Bhattacharjee P.S., Foster T.P. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23:249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 20.Harris S.A., Harris E.A. Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic Alzheimer's disease. J Alzheimer's Dis. 2015;48:319–353. doi: 10.3233/JAD-142853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lövheim H., Gilthorpe J., Johansson A., Eriksson S., Hallmans G., Elgh F. Herpes simplex infection and the risk of Alzheimer's disease: a nested case-control study. Alzheimers Dement. 2015;11:587–592. doi: 10.1016/j.jalz.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 22.Lövheim H., Gilthorpe J., Adolfsson R., Nilsson L.G., Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer's disease. Alzheimers Dement. 2015;11:593–599. doi: 10.1016/j.jalz.2014.04.522. [DOI] [PubMed] [Google Scholar]
- 23.Agostini S., Mancuso R., Baglio F., Cabinio M., Hernis A., Costa A.S. High avidity HSV-1 antibodies correlate with absence of amnestic mild cognitive impairment conversion to Alzheimer's disease. Brain Behav Immun. 2016;58:254–260. doi: 10.1016/j.bbi.2016.07.153. [DOI] [PubMed] [Google Scholar]
- 24.Wozniak M.A., Frost A.L., Preston C.M., Itzhaki R.F. Antivirals reduce the formation of key Alzheimer's disease molecules in cell cultures acutely infected with herpes simplex virus type 1. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025152. e25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartels L.J., Danner C.J., Allen K.P. Office-based Meniere's disease management. Oper Tech Otolayngol Head Neck Surg. 2016;27:225–234. [Google Scholar]
- 26.Wang C.Y., Bai X.Y., Wang C.H. Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42:543–559. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Sun H., Zhang A., Sun W., Wang P., Wang Z. Potential role of metabolomics apporoaches in the area of traditional Chinese medicine: as pillars of the bridge between Chinese and Western medicine. J Pharm Biomed Anal. 2011;55:859–868. doi: 10.1016/j.jpba.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 28.Li S., Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11:110–120. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 29.Shen C.Y., Jiang J.G., Yang L., Wang D.W., Zhu W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br J Pharmacol. 2017;174:1395–1425. doi: 10.1111/bph.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S.R., Luo X., Li Y.F., Hiroshi K., He R.R. Emotional stress-induced Shanghuo syndrome increases disease susceptibility. China J Chin Mater Med. 2018;43:1529–1535. doi: 10.19540/j.cnki.cjcmm.20180104.021. [DOI] [PubMed] [Google Scholar]
- 31.He R.R., Kurihara H. Shanghuo syndrome in traditional Chinese medicine. World Sci Technol. 2008;10:37–41. [Google Scholar]
- 32.Li Y., Qin L., Jiao Y., Zhou Y., Xu L. Relationship of seven emotions and excessive internal heat. J Tradit Complement Med. 2017;32:443–445. [Google Scholar]
- 33.Ives A.M., Bertke A.S. Stress hormones epinephrine and corticosterone selectively modulate herpes simplex virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory, neurons. J Virol. 2017;91 doi: 10.1128/JVI.00582-17. e00582-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perng G.C., Osorio N., Jiang X., Geertsema R., Hsiang C., Brown D. Large amounts of reactivated virus in tears precedes recurrent herpes stromal keratitis in stressed rabbits latently infected with herpes simplex virus. Curr Eye Res. 2016;41:284–291. doi: 10.3109/02713683.2015.1020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashcraft K.A., Bonneau R.H. Psychological stress exacerbates primary vaginal herpes simplex virus type 1 (HSV-1) infection by impairing both innate and adaptive immune responses. Brain Behav Immun. 2008;22:1231–1240. doi: 10.1016/j.bbi.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashcraft K.A., Hunzeker J., Bonneau R.H. Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology. 2008;33:951–963. doi: 10.1016/j.psyneuen.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bieniasz P.D. Intrinsic immunity: a front-line defense against viral attack. Nat Immunol. 2004;5:1109–1115. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]
- 38.Yordy B., Iwasaki A. Cell type-dependent requirement of autophagy in HSV-1 antiviral defense. Autophagy. 2013;9:236–238. doi: 10.4161/auto.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He R.R., Yao X.S., Li H.Y., Dai Y., Duan Y.H., Li Y.F. The anti-stress effects of Sarcandra glabra extract on restraint-evoked immunocompromise. Biol Pharm Bull. 2009;32:247–252. doi: 10.1248/bpb.32.247. [DOI] [PubMed] [Google Scholar]
- 40.He R.R., Tsoi B., Li Y.F., Yao X.S., Kurihara H. The anti-stress effects of Guangdong herbal tea on immunocompromise in mice loaded with restraint stress. J Health Sci. 2011;57:255–263. [Google Scholar]
- 41.He R.R., Wang M., Wang C.Z., Chen B.T., Lu C.N., Yao X.S. Protective effect of apple polyphenols against stress-provoked influenza viral infection in restraint mice. J Agric Food Chem. 2011;59:3730–3737. doi: 10.1021/jf104982y. [DOI] [PubMed] [Google Scholar]
- 42.Chen H., Jie C., Tang L.P., Meng H., Li X.B., Li Y.B. New insights into the effects and mechanism of a classic traditional Chinese medicinal formula on influenza prevention. Phytomedicine. 2017;27:52–62. doi: 10.1016/j.phymed.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Cao H.J., Tan R.R., He R.R., Tang L.P., Wang X.L., Yao N. Sarcandra glabra extract reduces the susceptibility and severity of influenza in restraint-stressed mice. Evid Based Complement Alternat Med. 2012;2012:236539. doi: 10.1155/2012/236539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang L.P., Mao Z.F., Li X.X., Chen M., Li S.B., Tsoi B. ReDuNing, a patented Chinese medicine, reduces the susceptibility to H1N1 influenza of mice loaded with restraint stress. Eur J Integr Med. 2014;6:637–645. [Google Scholar]
- 45.Cai Y., Li Y.F., Tang L.P., Tsoi B., Chen M., Chen H. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. BioMed Res Int. 2015;2015:675149. doi: 10.1155/2015/675149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jie C., Luo Z., Chen H., Wang M., Yan C., Mao Z.F. Indirubin, a bisindole alkaloid from Isatis indigotica, reduces H1N1 susceptibility in stressed mice by regulating MAVS signaling. Oncotarget. 2017;8:105615–105629. doi: 10.18632/oncotarget.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz G.C., Sheridan J.F., Marucha P.T. Stress-induced changes in pathophysiology and interferon gene expression during primary HSV-1 infection. Brain Behav Immun. 2003;17:329–338. doi: 10.1016/s0889-1591(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 48.Dong-Newsom P., Powell N.D., Bailey M.T., Padgett D.A., Sheridan J.F. Repeated social stress enhances the innate immune response to a primary HSV-1 infection in the cornea and trigeminal ganglia of Balb/c mice. Brain Behav Immun. 2010;24:273–280. doi: 10.1016/j.bbi.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M., Meng Q., Qin Y., Liang P., Tan P., He L. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol Cell. 2016;64:105–119. doi: 10.1016/j.molcel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 50.Enquist L.W., Leib D.A. Intrinsic and innate defenses of neurons: détente with the herpesviruses. J Virol. 2017;91:e01200–e01216. doi: 10.1128/JVI.01200-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yordy B., Iijima N., Huttner A., Leib D., Iwasaki A. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe. 2012;12:334–345. doi: 10.1016/j.chom.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y., Galluzzi L., Zitvogel L., Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Katzenell S., Leib D.A. Herpes simplex virus and interferon signaling induce novel autophagic clusters in sensory neurons. J Virol. 2016;90:4706–4719. doi: 10.1128/JVI.02908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang Q., Seo G.J., Choi Y.J., Kwak M.J., Ge J., Rodgers M.A. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15:228–238. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su C., Zhan G., Zheng C. Evasion of host antiviral innate immunity by HSV-1, an update. Virol J. 2016;13:38. doi: 10.1186/s12985-016-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paludan S.R., Bowie A.G., Horan K.A., Fitzgerald K.A. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol. 2011;11:143–154. doi: 10.1038/nri2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Connell D., Liang C. Autophagy interaction with herpes simplex virus type-1 infection. Autophagy. 2016;12:451–459. doi: 10.1080/15548627.2016.1139262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosato P.C., Leib D.A. Neurons versus herpes simplex virus: the innate immune interactions that contribute to a host-pathogen standoff. Future Virol. 2015;10:699–714. doi: 10.2217/fvl.15.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leib D.A., Alexander D.E., Cox D., Yin J., Ferguson T.A. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol. 2009;83:12164–12171. doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orvedahl A., Alexander D., Talloczy Z., Sun Q., Wei Y., Zhang W. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Ogata M., Hino S.I., Saito A., Morikawa K., Kondo S., Kanemoto S. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murrow L., Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shintani T., Klionsky D.J. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang S., Long J., Zheng C.F. The potential link between PML NBs and ICP0 in regulating lytic and latent infection of HSV-1. Protein Cell. 2012;3:372–382. doi: 10.1007/s13238-012-2021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kukhanova M.K., Korovina A.N., Kochetkov S.N. Human herpes simplex virus: life cycle and development of inhibitors. Biochemistry (Mosc) 2014;79:1635–1652. doi: 10.1134/S0006297914130124. [DOI] [PubMed] [Google Scholar]
- 66.Zhou G., Du T., Roizman B. The role of the CoREST/REST repressor complex in herpes simplex virus 1 productive infection and in latency. Viruses. 2013;5:1208–1218. doi: 10.3390/v5051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roizman B. The checkpoints of viral gene expression in productive and latent infection: the role of the HDAC/CoREST/LSD1/REST repressor complex. J Virol. 2011;85:7474–7482. doi: 10.1128/JVI.00180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hafezi W., Lorentzen E.U., Eing B.R., Müller M., King N.J., Klupp B. Entry of herpes simplex virus type 1 (HSV-1) into the distal axons of trigeminal neurons favors the onset of nonproductive, silent infection. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu P., Mallon S., Roizman B. PML plays both inimical and beneficial roles in HSV-1 replication. Proc Natl Acad Sci U S A. 2016;113:E3022–E3028. doi: 10.1073/pnas.1605513113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu P., Roizman B. The SP100 component of ND10 enhances accumulation of PML and suppresses replication and the assembly of HSV replication compartments. Proc Natl Acad Sci U S A. 2017;114:E3823–E3829. doi: 10.1073/pnas.1703395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koyuncu O.O., Song R., Greco T.M., Cristea I.M., Enquist L.W. The number of alphaherpesvirus particles infecting axons and the axonal protein repertoire determines the outcome of neuronal infection. mBio. 2015;6 doi: 10.1128/mBio.00276-15. e00276-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellison A.R., Yang L., Voytek C., Margolis T.P. Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology. 2000;268:17–28. doi: 10.1006/viro.1999.0158. [DOI] [PubMed] [Google Scholar]
- 73.Rosato P.C., Katzenell S., Pesola J.M., North B., Coen D.M., Leib D.A. Neuronal IFN signaling is dispensable for the establishment of HSV-1 latency. Virology. 2016;497:323–327. doi: 10.1016/j.virol.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perng G.C., Slanina S.M., Yukht A., Ghiasi H., Nesburn A.B., Wechsler S.L. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J Virol. 2000;74:1885–1891. doi: 10.1128/jvi.74.4.1885-1891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng X., Marquart M.E., Loustch J.M., Shah P., Sainz B., Ray A. HSV-1 migration in latently infected and naive rabbits after penetrating keratoplasty. Investig Ophthalmol Vis Sci. 1999;40:2490–2497. [PubMed] [Google Scholar]
- 76.Al-Dujaili L.J., Clerkin P.P., Clement C., McFerrin H.E., Bhattacharjee P.S., Varnell E.D. Ocular herpes simplex virus: how are latency, reactivation, recurrent disease and therapy interrelated?. Future Microbiol. 2011;6:877–907. doi: 10.2217/fmb.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimomura Y., Mori Y., Inoue Y., Kiritooshi A., Ohashi Y., Manabe R. Herpes simplex virus latency in human cornea. Jpn J Ophthalmol. 1993;37:318–324. [PubMed] [Google Scholar]
- 78.Pavan-Langston D., Rong B.L., Dunkel E.C. Extraneuronal herpetic latency: animal and human corneal studies. Acta Ophthalmol Suppl. 1989;192:135–141. doi: 10.1111/j.1755-3768.1989.tb07104.x. [DOI] [PubMed] [Google Scholar]
- 79.Kaye S.B., Baker K., Bonshek R., Maseruka H., Grinfeld E., Tullo A. Human herpesviruses in the cornea. Br J Ophthalmol. 2000;84:563–571. doi: 10.1136/bjo.84.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Higaki S., Fukuda M., Shimomura Y. Virological and molecular biological evidence supporting herpes simplex virus type 1 corneal latency. Jpn J Ophthalmol. 2015;59:131–134. doi: 10.1007/s10384-014-0369-6. [DOI] [PubMed] [Google Scholar]
- 81.Kaufman H.E., Azcuy A.M., Varnell E.D., Sloop G.D., Thompson H.W., Hill J.M. HSV-1 DNA in tears and saliva of normal adults. Investig Ophthalmol Vis Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukuda M., Deai T., Higaki S., Hayashi K., Shimomura Y. Presence of a large amount of herpes simplex virus genome in tear fluid of herpetic stromal keratitis and persistent epithelial defect patients. Semin Ophthalmol. 2008;23:217–220. doi: 10.1080/08820530802111366. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy D.P., Clement C., Arceneaux R.L., Bhattacharjee P.S., Huq T.S., Hill J.M. Ocular herpes simplex virus type 1: is the cornea a reservoir for viral latency or a fast pit stop?. Cornea. 2011;30:251–259. doi: 10.1097/ICO.0b013e3181ef241d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennedy P.G., Cohrs R.J. Varicella-zoster virus human ganglionic latency: a current summary. J Neurovirol. 2010;16:411–418. doi: 10.1007/BF03210846. [DOI] [PubMed] [Google Scholar]
- 85.Kimberlin D.W., Whitley R.J. Antiviral therapy of HSV-1 and-2. In: Arvin A.C.-F.G., Mocarski E., editors. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press; Cambridge: 2007. pp. 1153–1174. [Google Scholar]
- 86.Snoeck R. Antiviral therapy of herpes simplex. Int J Antimicrob Agents. 2000;16:157–159. doi: 10.1016/s0924-8579(00)00233-8. [DOI] [PubMed] [Google Scholar]
- 87.Stow N.D., Stow E.C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 88.Valyi-Nagy T., Fareed M.U., O'Keefe J.S., Gesser R.M., MacLean A.R., Brown S.M. The herpes simplex virus type 1 strain 17+ gamma 34.5 deletion mutant 1716 is avirulent in SCID mice. J Gen Virol. 1994;75:2059–2063. doi: 10.1099/0022-1317-75-8-2059. [DOI] [PubMed] [Google Scholar]
- 89.Flores O., Nakayama S., Whisnant A.W., Javanbakht H., Cullen B.R., Bloom D.C. Mutational inactivation of herpes simplex virus 1 microRNAs identifies viral mRNA targets and reveals phenotypic effects in culture. J Virol. 2013;87:6589–6603. doi: 10.1128/JVI.00504-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Umbach J.L., Kramer M.F., Jurak I., Karnowski H.W., Coen D.M., Cullen B.R. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen W., Sa e Silva M., Jaber T., Vitvitskaia O., Li S., Henderson G. Two small RNAs encoded within the first 1.5 kilobases of the herpes simplex virus type 1 latency-associated transcript can inhibit productive infection and cooperate to inhibit apoptosis. J Virol. 2009;83:9131–9139. doi: 10.1128/JVI.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicoll M.P., Hann W., Shivkumar M., Harman L.E., Connor V., Coleman H.M. The HSV-1 latency-associated transcript functions to repress latent phase lytic gene expression and suppress virus reactivation from latently infected neurons. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005539. e1005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang X., Brown D., Osorio N., Hsiang C., BenMohamed L., Wechsler S.L. Increased neurovirulence and reactivation of the herpes simplex virus type 1 latency-associated transcript (LAT)-negative mutant dLAT2903 with a disrupted LAT miR-H2. J Neurovirol. 2016;22:38–49. doi: 10.1007/s13365-015-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicoll M.P., Proenca J.T., Connor V., Efstathiou S. Influence of herpes simplex virus 1 latency-associated transcripts on the establishment and maintenance of latency in the ROSA26R reporter mouse model. J Virol. 2012;86:8848–8858. doi: 10.1128/JVI.00652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.You Y., Cheng A.C., Wang M.S., Jia R.Y., Sun K.F., Yang Q. The suppression of apoptosis by alpha-herpesvirus. Cell Death Dis. 2017;8:e2749. doi: 10.1038/cddis.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carpenter D., Hsiang C., Jiang X., Osorio N., BenMohamed L., Jones C. The herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) protects cells against cold-shock-induced apoptosis by maintaining phosphorylation of protein kinase B (AKT) J Neurovirol. 2015;21:568–575. doi: 10.1007/s13365-015-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones C. Bovine herpes virus 1 (BHV-1) and herpes simplex virus type 1 (HSV-1) promote survival of latently infected sensory neurons, in part by inhibiting apoptosis. J Cell Death. 2013;6:1–16. doi: 10.4137/JCD.S10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang X.Z., Chentoufi A.A., Hsiang C.H., Carpenter D., Osorio N., BenMohamed L. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J Virol. 2011;85:2325–2332. doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carpenter D., Hsiang C., Brown D.J., Jin L., Osorio N., BenMohamed L. Stable cell lines expressing high levels of the herpes simplex virus type 1 LAT are refractory to caspase 3 activation and DNA laddering following cold shock induced apoptosis. Virology. 2007;369:12–18. doi: 10.1016/j.virol.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piedade D., Azevedo-Pereira J.M. The role of microRNAs in the pathogenesis of herpesvirus infection. Viruses. 2016;8:156. doi: 10.3390/v8060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duan F., Ni S., Nie Y., Huang Q., Wu K. Small interfering RNA targeting for infected-cell polypeptide 4 inhibits herpes simplex virus type 1 replication in retinal pigment epithelial cells. Clin Exp Ophthalmol. 2012;40:195–204. doi: 10.1111/j.1442-9071.2011.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duan F., Liao J., Huang Q., Nie Y., Wu K. HSV-1 miR-H6 inhibits HSV-1 replication and IL-6 expression in human corneal epithelial cells in vitro. Clin Dev Immunol. 2012;2012:192791. doi: 10.1155/2012/192791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang X., Brown D., Osorio N., Hsiang C., Li L., Chan L. A herpes simplex virus type 1 mutant disrupted for microRNA H2 with increased neurovirulence and rate of reactivation. J Neurovirol. 2015;21:199–209. doi: 10.1007/s13365-015-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosato P.C., Leib D.A. Neuronal interferon signaling is required for protection against herpes simplex virus replication and pathogenesis. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005028. e1005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mattila R.K., Harila K., Kangas S.M., Paavilainen H., Heape A.M., Mohr I.J. An investigation of herpes simplex virus type 1 latency in a novel mouse dorsal root ganglion model suggests a role for ICP34.5 in reactivation. J Gen Virol. 2015;96:2304–2313. doi: 10.1099/vir.0.000138. [DOI] [PubMed] [Google Scholar]
- 106.Gupta A., Gartner J.J., Sethupathy P., Hatzigeorgiou A.G., Fraser N.W. Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature. 2006;442:82–85. doi: 10.1038/nature04836. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479–480:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leyton L., Hott M., Acuña F., Caroca J., Nuñez M., Martin C. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV-1 infection. Virus Res. 2015;205:63–72. doi: 10.1016/j.virusres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 109.Martin C., Leyton L., Arancibia Y., Cuevas A., Zambrano A., Concha M.I. Modulation of the AMPK/Sirt1 axis during neuronal infection by herpes simplex virus type 1. J Alzheimer's Dis. 2014;42:301–312. doi: 10.3233/JAD-140237. [DOI] [PubMed] [Google Scholar]
- 110.Audas T.E., Hardy-Smith P.W., Penney J., Taylor T., Lu R. Characterization of nuclear foci-targeting of Luman/CREB3 recruitment factor (LRF/CREBRF) and its potential role in inhibition of herpes simplex virus-1 replication. Eur J Cell Biol. 2016;95:611–622. doi: 10.1016/j.ejcb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Camarena V., Kobayashi M., Kim J.Y., Roehm P., Perez R., Gardner J. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe. 2010;8:320–330. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wilcox C.L., Smith R.L., Freed C.R., Johnson E.M., Jr. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci. 1990;10:1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Danaher R.J., Jacob R.J., Miller C.S. Establishment of a quiescent herpes simplex virus type 1 infection in neurally-differentiated PC12 cells. J Neurovirol. 1999;5:258–267. doi: 10.3109/13550289909015812. [DOI] [PubMed] [Google Scholar]
- 114.Hill J.M., Garza H.H., Jr., Helmy M.F., Cook S.D., Osborne P.A., Johnson E.M., Jr. Nerve growth factor antibody stimulates reactivation of ocular herpes simplex virus type 1 in latently infected rabbits. J Neurovirol. 1997;3:206–211. doi: 10.3109/13550289709018295. [DOI] [PubMed] [Google Scholar]
- 115.Zhou G., Du T., Roizman B. HSV carrying WT REST establishes latency but reactivates only if the synthesis of REST is suppressed. Proc Natl Acad Sci U S A. 2013;110:E498–E506. doi: 10.1073/pnas.1222497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lambiase A., Coassin M., Costa N., Lauretti P., Micera A., Ghinelli E. Topical treatment with nerve growth factor in an animal model of herpetic keratitis. Graefes Arch Clin Exp Ophthalmol. 2008;246:121–127. doi: 10.1007/s00417-007-0593-6. [DOI] [PubMed] [Google Scholar]
- 117.Liu X., Cohen J.I. The role of PI3K/Akt in human herpesvirus infection: from the bench to the bedside. Virology. 2015;479–480:568–577. doi: 10.1016/j.virol.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kobayashi M., Wilson A.C., Chao M.V., Mohr I. Control of viral latency in neurons by axonal mTOR signaling and the 4E-BP translation repressor. Genes Dev. 2012;26:1527–1532. doi: 10.1101/gad.190157.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang J., Kent J.R., Placek B., Whelan K.A., Hollow C.M., Zeng P.Y. Trimethylation of histone H3 lysine 4 by Set1 in the lytic infection of human herpes simplex virus 1. J Virol. 2006;80:5740–5746. doi: 10.1128/JVI.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kubat N.J., Amelio A.L., Giordani N.V., Bloom D.C. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J Virol. 2004;78:12508–12518. doi: 10.1128/JVI.78.22.12508-12518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clement C., Bhattacharjee P.S., Kumar M., Foster T.P., Thompson H.W., Hill J.M. Upregulation of mouse genes in HSV-1 latent TG after butyrate treatment implicates the multiple roles of the LAT-ICP0 locus. Investig Ophthalmol Vis Sci. 2011;52:1770–1779. doi: 10.1167/iovs.09-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hill J.M., Quenelle D.C., Cardin R.D., Vogel J.L., Clement C., Bravo F.J. Inhibition of LSD1 reduces herpesvirus infection, shedding, and recurrence by promoting epigenetic suppression of viral genomes. Sci Transl Med. 2014;6:265ra169. doi: 10.1126/scitranslmed.3010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guo J., Li N., Han J., Pei F., Wang T., Lu D. DNA recognition patterns of the multi-zinc-finger protein CTCF: a mutagenesis study. Acta Pharm Sin B. 2018;8:900–908. doi: 10.1016/j.apsb.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bloom D.C., Giordani N.V., Kwiatkowski D.L. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta. 2010;1799:246–256. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Egan K.P., Wu S., Wigdahl B., Jennings S.R. Immunological control of herpes simplex virus infections. J Neurovirol. 2013;19:328–345. doi: 10.1007/s13365-013-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma J.Z., Russell T.A., Spelman T., Carbone F.R., Tscharke D.C. Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004237. e1004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Velzen M., Jing L., Osterhaus A.D., Sette A., Koelle D.M., Verjans G.M. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003547. e1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sheridan B.S., Cherpes T.L., Urban J., Kalinski P., Hendricks R.L. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Knickelbein J.E., Khanna K.M., Yee M.B., Baty C.J., Kinchington P.R., Hendricks R.L. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Held K., Derfuss T. Control of HSV-1 latency in human trigeminal ganglia-current overview. J Neurovirol. 2011;17:518–527. doi: 10.1007/s13365-011-0063-0. [DOI] [PubMed] [Google Scholar]
- 131.Ghiasi H., St Leger A.J., Jeon S., Hendricks R.L. Broadening the repertoire of functional herpes simplex virus type 1-specific CD8+ T cells reduces viral reactivation from latency in sensory ganglia. J Virol. 2013;191:2258–2265. doi: 10.4049/jimmunol.1300585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Finsterbusch K., Piguet V. Down-RANKing the threat of HSV-1: RANKL upregulates MHC-Class-I-restricted anti-viral immunity in herpes simplex virus infection. J Investig Dermatol. 2015;135:2565–2567. doi: 10.1038/jid.2015.293. [DOI] [PubMed] [Google Scholar]
- 133.Srivastava R., Dervillez X., Khan A.A., Chentoufi A.A., Chilukuri S., Shukr N. The herpes simplex virus latency-associated transcript gene is associated with a broader repertoire of virus-specific exhausted CD8+ T cells retained within the trigeminal ganglia of latently infected HLA transgenic rabbits. J Virol. 2016;90:3913–3928. doi: 10.1128/JVI.02450-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Frank G.M., Lepisto A.J., Freeman M.L., Sheridan B.S., Cherpes T.L., Hendricks R.L. Early CD4+ T cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J Immunol. 2010;184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mott K.R., Gate D., Matundan H.H., Ghiasi Y.N., Town T., Ghiasi H. CD8+ T cells play a bystander role in mice latently infected with herpes simplex virus 1. J Virol. 2016;90:5059–5067. doi: 10.1128/JVI.00255-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mott K.R., Allen S.J., Zandian M., Ghiasi H. Coregulatory interactions among CD8alpha dendritic cells, the latency-associated transcript, and programmed death 1 contribute to higher levels of herpes simplex virus 1 latency. J Virol. 2014;88:6599–6610. doi: 10.1128/JVI.00590-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kennedy P.G., Rovnak J., Badani H., Cohrs R.J. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96:1581–1602. doi: 10.1099/vir.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang C.Y., Huang J.W., Jie C., Yan C., Li Y.T., Kurihara H. Wanglaoji herbal tea protects against influenza-induced pneumonia in restraint-stressed mice via its anti-inflammatory effects. Int J Pharmacol. 2018;14:342–351. [Google Scholar]
- 139.Li Y.F., He R.R., Tsoi B., Li X.D., Li W.X., Abe K. Anti-stress effects of carnosine on restraint-evoked immunocompromise in mice through spleen lymphocyte number maintenance. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033190. e33190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sinani D., Cordes E., Workman A., Thunuguntia P., Jones C. Stress-induced cellular transcription factors expressed in trigeminal ganglionic neurons stimulate the herpes simplex virus 1 ICP0 promoter. J Virol. 2013;87:13042–13047. doi: 10.1128/JVI.02476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kushnir A.S., Davido D.J., Schaffer P.A. Role of nuclear factor Y in stress-induced activation of the herpes simplex virus type 1 ICP0 promoter. J Virol. 2010;84:188–200. doi: 10.1128/JVI.01377-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Curtin N.M., Boyle N.T., Mills K.H., Connor T.J. Psychological stress suppresses innate IFN-gamma production via glucocorticoid receptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun. 2009;23:535–547. doi: 10.1016/j.bbi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 143.Noisakran S., Halford W.P., Veress L., Carr D.J. Role of the hypothalamic pituitary adrenal axis and IL-6 in stress-induced reactivation of latent herpes simplex virus type 1. J Immunol. 1998;160:5441–5447. [PubMed] [Google Scholar]
- 144.Ostler J.B., Harrison K.S., Schroeder K., Thunuguntla P., Jones C. The glucocorticoid receptor (GR) stimulates herpes simplex virus 1 productive infection, in part because the infected cell protein 0 (ICP0) promoter is cooperatively transactivated by the GR and Krüppel-like transcription factor 15. J Virol. 2019;93 doi: 10.1128/JVI.02063-18. e02063-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kook I., Jones C. The serum and glucocorticoid-regulated protein kinases (SGK) stimulate bovine herpesvirus 1 and herpes simplex virus 1 productive infection. Virus Res. 2016;222:106–112. doi: 10.1016/j.virusres.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 146.Elftman M.D., Hunzeker J.T., Mellinger J.C., Bonneau R.H., Norbury C.C., Truckenmiller M.E. Stress-induced glucocorticoids at the earliest stages of herpes simplex virus-1 infection suppress subsequent antiviral immunity, implicating impaired dendritic cell function. J Immunol. 2010;184:1867–1875. doi: 10.4049/jimmunol.0902469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sommershof A., Basler M., Riether C., Engler H., Groettrup M. Attenuation of the cytotoxic T lymphocyte response to lymphocytic choriomeningitis virus in mice subjected to chronic social stress. Brain Behav Immun. 2011;25:340–348. doi: 10.1016/j.bbi.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 148.Marketon J.I.W., Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 149.Figliozzi R.W., Chen F., Balish M., Ajavon A., Hsia S.V. Thyroid hormone-dependent epigenetic suppression of herpes simplex virus-1 gene expression and viral replication in differentiated neuroendocrine cells. J Neurol Sci. 2014;346:164–173. doi: 10.1016/j.jns.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bedadala G.R., Pinnoji R.C., Palem J.R., Hsia S.C. Thyroid hormone controls the gene expression of HSV-1 LAT and ICP0 in neuronal cells. Cell Res. 2010;20:587–598. doi: 10.1038/cr.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen F., Figliozzi R.W., Bedadala G., Palem J., Hsia S.V. Overexpression of thyroid hormone receptor beta1 altered thyroid hormone-mediated regulation of herpes simplex virus-1 replication in differentiated cells. J Neurovirol. 2016;22:555–563. doi: 10.1007/s13365-016-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen F., Palem J., Balish M., Figliozzi R., Ajavon A., Hsia S.V. A novel thyroid hormone mediated regulation of HSV-1 gene expression and replication is specific to neuronal cells and associated with disruption of chromatin condensation. SOJ Pharm Pharm Sci. 2014;1:7. doi: 10.15226/2374-6866/1/1/00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hsia S.C., Bedadala G.R., Balish M.D. Effects of thyroid hormone on HSV-1 gene regulation: implications in the control of viral latency and reactivation. Cell Biosci. 2011;1:24. doi: 10.1186/2045-3701-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Neumann D.M., Bhattacharjee P.S., Giordani N.V., Bloom D.C., Hill J.M. In vivo changes in the patterns of chromatin structure associated with the latent herpes simplex virus type 1 genome in mouse trigeminal ganglia can be detected at early times after butyrate treatment. J Virol. 2007;81:13248–13253. doi: 10.1128/JVI.01569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Q.Y., Zhou C., Johnson K.E., Colgrove R.C., Coen D.M., Knipe D.M. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc Natl Acad Sci U S A. 2005;102:16055–16059. doi: 10.1073/pnas.0505850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Avgousti D.C., Weitzman M.D. Stress flips a chromatin switch to wake up latent virus. Cell Host Microbe. 2015;18:639–641. doi: 10.1016/j.chom.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim J.Y., Mandarino A., Chao M.V., Mohr I., Wilson A.C. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thompson R.L., Preston C.M., Sawtell N.M. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000352. e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Danaher R.J., Cook R.K., Wang C., Triezenberg S.J., Jacob R.J., Miller C.S. C-terminal trans-activation sub-region of VP16 is uniquely required for forskolin-induced herpes simplex virus type 1 reactivation from quiescently infected-PC12 cells but not for replication in neuronally differentiated-PC12 cells. J Neurovirol. 2013;19:32–41. doi: 10.1007/s13365-012-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kaufman H.E., Varnell E.D., Gebhardt B.M., Thompson H.W., Atwal E., Rubsamen-Waigmann H. Efficacy of a helicase-primase inhibitor in animal models of ocular herpes simplex virus type 1 infection. J Ocul Pharmacol Ther. 2008;24:34–42. doi: 10.1089/jop.2007.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alexander A., Cai S.L., Kim J., Nanez A., Sahin M., MacLean K.H. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Du T., Zhou G., Roizman B. Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc Natl Acad Sci U S A. 2012;109:14616–14621. doi: 10.1073/pnas.1212661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim J.C., Choi S.H., Kim J.K., Kim Y., Kim H.J., Im J.S. Herpes simplex virus type 1 ICP27 induces apoptotic cell death by increasing intracellular reactive oxygen species. Mol Biol (Mosk) 2008;42:470–477. [PubMed] [Google Scholar]
- 164.Aravantinou M., Frank I., Arrode-Bruses G., Szpara M., Grasperge B., Blanchard J. A model of genital herpes simplex virus type 1 infection in rhesus macaques. J Med Primatol. 2017;46:121–128. doi: 10.1111/jmp.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 166.Edgar R.S., Stangherlin A., Nagy A.D., Nicoll M.P., Efstathiou S., O'Neill J.S. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A. 2016;113:10085–10090. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li W., Wang X.-H., Luo Z., Liu L.-F., Yan C., Yan C.-Y. Traditional Chinese medicine as a potential source for HSV-1 therapy by acting on virus or the susceptibility of host. Int J Mol Sci. 2018;19:3266. doi: 10.3390/ijms19103266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gu P., Chen H. Modern bioinformatics meets traditional Chinese medicine. Briefings Bioinf. 2014;15:984–1003. doi: 10.1093/bib/bbt063. [DOI] [PubMed] [Google Scholar]
- 169.Zhao J., Jiang P., Zhang W. Molecular networks for the study of TCM pharmacology. Briefings Bioinf. 2010;11:417–430. doi: 10.1093/bib/bbp063. [DOI] [PubMed] [Google Scholar]
- 170.Li P., Yang L.P., Gong Y.W. Application of systems biology technology in research of traditional Chinese medicine. J Tradit Chin Med. 2009;29:153–157. doi: 10.1016/s0254-6272(09)60054-6. [DOI] [PubMed] [Google Scholar]