Abstract
IL-17 is produced by RAR-related orphan receptor gamma t (RORγt)-expressing cells including Th17 cells, subsets of γδT cells and innate lymphoid cells (ILCs). The biological significance of IL-17-producing cells is well-studied in contexts of inflammation, autoimmunity and host defense against infection. While most of available studies in tumor immunity mainly focused on the role of T-bet-expressing cells, including cytotoxic CD8+ T cells and NK cells, and their exhaustion status, the role of IL-17-producing cells remains poorly understood. While IL-17-producing T-cells were shown to be anti-tumorigenic in adoptive T-cell therapy settings, mice deficient in type 17 genes suggest a protumorigenic potential of IL-17-producing cells. This review discusses the features of IL-17-producing cells, of both lymphocytic and myeloid origins, as well as their suggested pro- and/or anti-tumorigenic functions in an organ-dependent context. Potential therapeutic approaches targeting these cells in the tumor microenvironment will also be discussed.
Keywords: Tumor microenvironment, Th17 cells, Interleukin-17, T-lymphocytes
INTRODUCTION
CD4+ T-cell subsets act as an essential arm of adaptive immunity-as helpers of CD8+ T- and B-cells, recruiters of immune cells to the inflammation site and initiators of immunological memory. CD4+ effector T-cells subsets can be divided into distinct lineages: Th1, Th2, Th17, Tfh, and Tregs-each with unique roles in host defense and immunity. In response against extracellular pathogens, Th17 cells function to recruit neutrophils and macrophages via the induction of chemokines such as CXCL1, CXCL2 and CXCL8 (1). Signals that drive Th17 differentiation include TGFβ, IL-6, IL-1β, or IL-21 (2,3). Although not required initially, IL-23 was also shown to be important for terminal differentiation and maintenance of Th17 cells (4). IL-17 production is a key feature of Th17, along with expression of the lineage-defining transcription factor RAR-related orphan receptor gamma t (RORγt) (5). Other cytokine production, such as IL-17F, IL-21, GM-CSF, and IL-22, has also been associated with Th17, and these effector cytokines allow Ag-specific Th17 cells to be pathogenic in autoimmunity (6,7,8,9).
As such, the differentiation requirements, effector functions, and the regulation of IL17-producing T-cells in the context of autoimmunity, allergic diseases, and host defense against bacterial infections and other opportunistic pathogens are well studied (10). However, almost 15 years after the discovery of Th17 as a distinct lineage of CD4+ T-cell subset, the scientific community is still debating its exact role in tumor immunity. This seems to be due to a couple of reasons: first of which is the wide diversity, and the relative plasticity of the IL-17 producing cells in the tumor microenvironment (Fig. 1). Confounding the matter is the tissue-specific niche that also diversifies the regulatory mechanism induced by IL-17 signaling. Lastly, the pleiotropic nature of IL-17, along with other related cytokines (i.e. IL-1β, IL-22, IL-23, TNF-α, IFN-γ) that are often secreted in conjunction with IL-17, can bring about drastically different net outcomes in a complex and dynamic disease such as cancer. As such, type 17 refers to the effector cellular program encompassing IL-17 and related cytokine signatures. The dominant “type 17”-driven mechanism at play seems to shift as the disease progresses. The pro-tumorigenic and anti-tumor properties of tumor-infiltrating type 17 compartments will be explored.
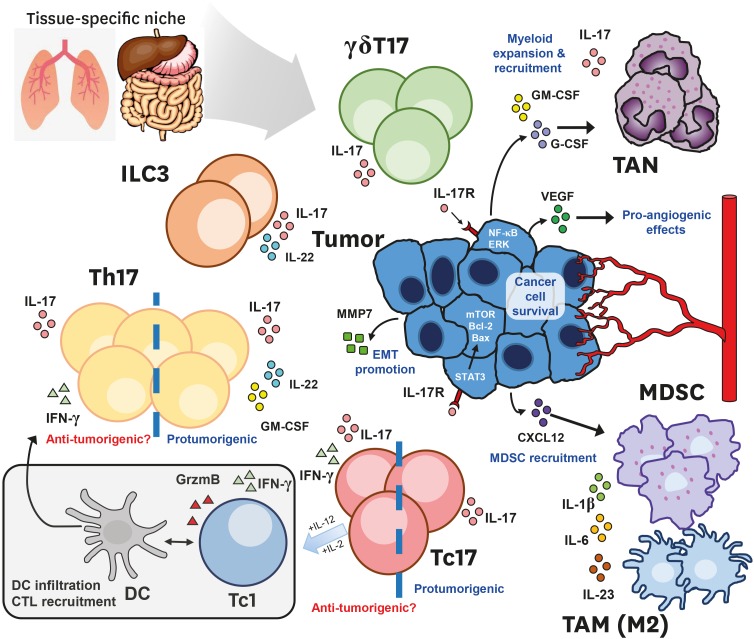
Figure 1. Type17 compartments in the tumor microenvironment.
The tissue-specific niche, and the stage of cancer progression dictate the heterogeneous composition of type 17 compartment in the tumor microenvironment. Both innate and adaptive arms of the immune system are capable of producing IL-17. Studies involving both CD4+ Th17 and CD8+ Tc17 T-cells reported protumorigenic or anti-tumorigenic roles. Anti-tumorigenic Th/c17 cells co-secrete IFN-γ, and exhibit type 1–17 hybrid phenotypes that lead to enhanced dendritic cell infiltration and tumor-Ag presentation, type 1-helper function or Tc1 conversion in the case of Tc17 cells under the appropriate cytokine stimuli. However, an increasing amount of evidence illustrate the protumorigenic mechanisms (highlighted in blue) of type 17 cells within the immunosuppressive endogenous microenvironment within the growing tumor. Largely, they work by two broad mechanisms: 1) the IL-17 mediated recruitment of immunosuppressive myeloid compartment, either directly by type 17 cells or indirectly by cancer cells in a chemokine dependent manner, 2) cancer intrinsic IL-17 signaling, leading to enhanced cancer cell survival, EMT and angiogenesis promotion.
EMT, epithelial–mesenchymal transition; TAM, tumor-associated macrophage.
This review first summarizes current understanding of the various type 17 compartments present in the tumor microenvironment, and the tissue-specific niche affecting the dominant type 17 mechanism at play. Additionally, the consequent protumorigenic or anti-tumor outcomes, as well as the prognostic factors for each cancer type are also discussed. Additionally, current type 17 mechanisms of resistance against conventional chemo-/radiotherapies, as well as current efforts to utilize type 17 axis in future cellular and immunotherapies will also be discussed. Although there are still many unanswered questions and missing pieces, the present review, along with others, can serve as a starting point for future discussions.
PRODUCERS OF IL-17 IN THE TUMOR MICROENVIRONMENT
Diverse cellular sources of IL-17 exist in the tumor microenvironment. Type 17 cellular compartments present in the tumor microenvironment are graphically outlined in Fig. 1.
Th17 cells
Tumor-infiltrating Th17 cells have been observed in both mouse (Table 1) and humans (Table 2) (11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64). Mechanistic studies add strength to the idea that chronic infection and inflammation are important environmental factors for tumorigenesis and such conditions also foster type 17 cell generation. For example, many have noted that Th17 frequency is higher in the tumor than in healthy tissues or PBMCs. Tumor cells, as well as tumor-derived fibroblasts and APCs in the tumor microenvironment have been demonstrated to produce the cytokine milieu that elicit Th17 generation and/or recruitment (58,65). Interestingly, tumor-derived TGFβ rather inhibited Th17 cell expansion, while promoting Treg generation. Others have suggested that tumor and/or tumor-associated myeloid cells can actively recruit Th17 cells via chemokine secretions (66,67).
Table 1. Summary of findings from mouse cancer model studies: function of type 17 lymphocytes.
| Cancer model | Sub-type | Function of type 17 cells (pro- or anti-tumorigenic?) | Ref. |
|---|---|---|---|
| Subcutaneous injection | EL4, CT26 | Pro-tumorigenic | (11) |
| B16 melanoma | Anti-tumorigenic | (12) | |
| MC38 | Anti-tumorigenic | (13) | |
| KPC, Braf-Pten, B16-melanoma | Pro-tumorigenic | (14) | |
| Hepa1-6 HCC | Pro-tumorigenic | (15) | |
| MA782 (orthotopic) | Pro-tumorigenic | (16) | |
| 4T1 (orthotopic) | Pro-tumorigenic | (16,17) | |
| Intraperitoneal injection | ID8 ovarian cancer | Pro-tumorigenic | (18,19) |
| Intravenous injection | B16 melanoma, LLC | Anti-tumorigenic | (20) |
| MC38 | Anti-tumorigenic | (13) | |
| Oncogene-activated | Constitutive MYC expression in early B-cells | Pro-tumorigenic | (21) |
| K14CrexCdh1f/fxTrp53f/f | Pro-tumorigenic | (22) | |
| (KEP) mice spontaneous breast cancer | |||
| KrasG12DxCCSPCre Lung Cancer | Pro-tumorigenic | (23) | |
| KrasLSL-G12Dxp53f/fxSftpcCre Lung Cancer | Pro-tumorigenic | (24) | |
| Ptenf/fxSMAD4f/fxCCSPCre Lung Cancer | Anti-tumorigenic | (25) | |
| Carcinogen-induced | DMBA/TPA-induced skin cancer/squamous cell carcinoma | Pro-tumorigenic | (26,27) |
| AOM DSS APCmin ETBF-induced colon cancer | Pro-tumorigenic | (28,29,30) | |
| Inflammation-accelerated | Helicobacter hepaticus-infected/AOM-treated129SvEvS6.Rag2−/− | Pro-tumorigenic | (31) |
AOM, azoxymethane; DMBA, 7,12-dimethylbenz[a]anthracene; DSS, dextran-sulfate sodium; ETBF, Enterotoxigenic Bacteroides fragilis; HCC, hepatocellular carcinoma; LLC, Lewis lung carcinoma; TPA, 12-O-tetradecanoylphorbol-13-acetate.
Table 2. Summary of type 17 T-cell types by human cancers and its associated prognosis.
| Cancer Type | Endogenous, tumor-infiltrating type 17 T-cells found | Overall survival | Ref. |
|---|---|---|---|
| Acute leukemia | Th17 | Improved | (32) |
| Acute myeloid leukemia | Tc17 | Poor | (33) |
| Breast | Th17 | Poor* | (34,35) |
| Colorectal cancer | gdT17, Th17 | Poor | (28,36,37,38) |
| Cervical carcinoma | Th17 | Improved† | (39) |
| Cervical squamous cell carcinoma | CD3+ IL17+ | Improved‡ | (40) |
| Esophageal squamous cell carcinoma | IL17+ cells | Improved | (41,42) |
| Gastric carcinoma | Th17 | Poor | (43,44) |
| Hepatocellular carcinoma | Tc17, Th17 | Poor | (45,46,47) |
| Lung cancer | Th17 | Improved | (48) |
| Lung carcinoma | Th17, gdT17 | Improved | (49,50) |
| Melanoma | Th17, Tc17 | Poor | (51) |
| Myeloma | Th17 | Poor | (52,53) |
| Nasopharyngeal | Th17, Tc17 | Improved? | (54) |
| Nasopharyngeal carcinoma | IL17+ cells | No correlation | (54,55) |
| Non-small cell lung | Th17 | Poor | (56,57) |
| Ovarian | Th17 | Improved | (58,59) |
| Pancreatic | Th17 | Poor | (60) |
| Prostate | Th17, NKT17 | Improved§ | (61,62) |
| Renal cell carcinoma | Th17 | Improved | (63) |
| Small cell lung | Th17 (peripheral) | Improved | (64) |
*Improved survival was observed in non-inflamed triple negative breast cancer. †In cervical carcinoma, IL-17 level as a whole was associated with poor outcome, due to the pro-tumorigenic contributions of IL-17 producing neutrophils. ‡Prognosis for IL-17+ cells in general was poor for cervical squamous cell carcinoma. §The prognosis for IL-17 producing cells in hormone-resistant prostate cancer.
Many have noted the positive correlation between the generation of Th17 cells in the growing tumor and cancer angiogenesis. The suggested direct link is thought to be IL-17 mediated stimulation of VEGF production from cancer cells (36,68). Similarly, microvascular density changes in mice with engrafted breast cancer cells treated with recombinant IL-17 demonstrated angiogenesis-promoting effects in vivo. Others have investigated whether IL-17 cytokine can affect early stages of tumorigenesis (28). IL-17 blockade significantly attenuated tumor formation in a Th17-driven colon tumorigenesis mouse model. In this model both Th17-cell and innate type 17-derived IL-17 production was suggested to induce bacteria-induced colon carcinogenesis.
Tc17 cells
Several groups have reported the presence of IL-17 producing CD8+ T (Tc17) cells in cancer patients. In human hepatocellular carcinomas, these cells were reported to accumulate in the invading tumor edges, where they co-localized with tumor-associated monocytes (15). These CD68+ monocytes secreted a key set of cytokines, notably IL-1β, IL-6, and IL-23, that allowed a superior induction and proliferation of Tc17 cells ex vivo. Tc17 cells found in the tumor expressed other signature type 17 molecules, such as RORγt, IL-22, and CCR6, while downregulating the conventional CTL program, such as granzyme B and perforin. Higher frequency of Tc17 cells have also been reported in nasopharyngeal carcinoma and gastric cancer patients (69,70). The biological function of these endogenous, tumor-infiltrating Tc17 population has not been elucidated. Few reports suggest a negative correlation between IL-17 producing T-cell frequency (including Tc17) and overall survival of cancer patients, suggesting protumorigenic properties (71). Mechanistic studies conducted ex vivo have suggested that tumor-infiltrating Tc17 cells induce the production of CXCL12 by tumor cells which in turn promote CXCR4-dependent migration of myeloid-derived suppressor cells (MDSCs) to the tumor microenvironment (70).
Due to the direct killing potential of CD8+ T-cells, many have attempted to take advantage of the plasticity of Tc17 cells as a cellular therapy alternative (72,73). Adoptive transfer of tumor-specific, in vitro differentiated Tc17 cells have shown considerable antitumor properties in certain mouse models of cancer, due to the enhanced survival capability of Tc17 cells and higher expression of stemness markers than Tc1 cells (74,75,76,77).
Innate cells of lymphoid origin: IL-17 secreting γδT (γδT17) cells, NKT, type 3 innate lymphoid cells (ILC3)
In mouse models of spontaneous breast cancer metastasis, γδT17 cells were shown to drive tumor-associated neutrophils (TAN) expansion, accumulation, phenotype in a G-CSF-dependent manner in mammary tumors (22). These TANs exert immunosuppressive functions by hindering effector CTL function, thereby facilitating cancer metastasis. Depletion of either γδT cells or neutrophils resulted in significant reduction of pulmonary and lymph node metastasis, thereby demonstrating the pro-metastatic role of γδT/IL-17/neutrophil axis in this breast cancer model (22). A mouse peritoneal/ovarian cancer model has demonstrated γδT17 accumulation in the peritoneal cavity in response to tumor challenge (18). γδT cells have been suggested to recruit macrophage subsets expressing high levels of IL-17 receptor, which have abilities to directly promote ovarian cancer cell proliferation in vitro. Collectively, γδT cell crosstalk with the tumor-associated myeloid compartment seems to occur via IL-17 in mouse cancer models (19). Such mechanisms of cellular crosstalk have also been demonstrated in human colorectal cancers (CRCs). Microbial products secreted by tumorous epithelial barriers induce preferential γδT17 polarization. γδT17-dervied GM-CSF recruit immunosuppressive neutrophils and polymorphonuclear myeloid-derived suppressor cells into the tumor (78). γδT17 infiltration positively correlated with clinical stage and other clinicopathological features of CRCs in cohorts, demonstrating the impact of γδT17 in potential prognosis prediction. Additionally, a high proportion of tumor-infiltrating γδT-cells were found to be functionally exhausted and created an ‘anergic’ immunosuppressive environment in which neighboring CD4+ or CD8+ T-cells were also affected in human pancreatic ductal adenocarcinomas (79).
IL-17 producing invariant NKT cells (NKT17), marked by CD24−CD44+NK1.1−, have been described to be endogenously enriched within certain tissues such as the lungs, intestines, and skin (80,81). Cancers arising in such tissue niches are expected to contain a higher frequency of NKT17. However, the precise role of NKT17 in tumorigenesis and tumor immunity so far remains elusive. ILC3s represent another innate lymphocyte compartment capable of producing IL-17 in the tumor microenvironment (82,83). Higher percentage of ILC3 was associated with IL-23 expression in non-small-cell lung carcinoma (NSCLC) cells. Pulmonary squamous cell carcinomas secreted functional levels of IL-23 that enabled ILC1 conversion into ILC3 ex vivo (84). IL-22 producing CCR6+ ILC3s have been suggested to increase the tumorigenic potential of colon cancer in mouse models (29,31). Ab-mediated depletion of natural cytotoxicity triggering receptor positive ILC3s led to decrease in metastasis in a mouse model of breast cancer (17). ILC3s recruited to the tumor microenvironment interact with stromal cells to create favorable conditions for cancer metastasis.
Innate sources of myeloid origin: macrophages, mast cells, neutrophils
Myeloid cells, most notably CD68+ macrophages (85,86), neutrophils (40), and mast cells (87,88) have also been shown to secrete IL-17. In fact, IL-17 secreted from myeloid cells (granulocytes and mast cells) was shown to constitute a larger portion of IL-17 secretion than those derived from T-cells in certain cancers (40,88,89). Neutrophils were primarily granulocytic in nature in squamous cervical cancers, and associated with poor survival. In addition, IL-17-expressing cells were independently associated with poor survival in early stage of the disease (40). IL-17 producing mast cells in esophageal squamous cell carcinoma were found to be densely located in the muscularis propria, and were suggested to function in the recruitment of effector CTLs and M1 macrophages to the site of tumor, thus acting as a favorable prognostic factor (41). However, in other cancer types opposite results were reported for IL-17+ mast cells (88).
Type 17 ‘package’ delivery: co-secretion of other effector cytokines
Confounding the matter, co-secretion of other effector cytokines, such as IL-21, IL-22, and GM-CSF, by type 17 cells in the tumor microenvironment adds another dimension of complexity. IL-21 has pleiotropic effects on both innate and adaptive immunity. IL-21 secretion has shown to enhance the cytotoxicity of CD8+ T-cells, and regulate NK cell maturation, while it can also hinder Ag presentation of dendritic cells and act as a pro-apoptotic signal. (90). As such, IL-21 has been tested in several phase II clinical trials for its potent anti-tumor effects either alone (91,92), or as a component of adoptive cellular therapy (93). However, little is known regarding the biological function of endogenous IL-21 derived from type 17 cells in the tumor.
IL-22 is known to be secreted by a special subset of Th17 cells residing in epidermis (94,95). In the context of cancer, IL-22 was suggested to favor tumor growth in several cancer models including nonmelanoma skin, colon and lung cancers (96,97). IL-22 receptor expression is limited to epithelial cells and IL-22 signaling can contribute to pro-survival signaling, angiogenesis and metastasis, part of which may be associated with its activation of STAT3 signaling pathway in cancer cells (29,98,99). As such, blockade of IL-22 significantly lowered tumor formation in a mouse model of colon cancer (31), and IL-22 expressing tumor-infiltrating cells correlated with more advanced tumor severity and reduced survival in human cancers (31,100). High levels of IL-22 have been detected in primary tumors, malignant pleural effusions (MPEs) and in sera of NSCLC patients (101).
IL-17 signaling can induce GM-CSF production in oncogene-driven cancer cells (102). CRC patients show higher blood GM-CSF levels than healthy control. Moreover, high GM-CSF expression in the tumor tissue correlated with local and distant metastasis, and poorer prognosis in various cancer types (102,103,104). GM-CSF can directly affect cancer cells and educate them to be more resistant to apoptosis and more prone to metastasis (105)
Despite the diverse cellular sources of IL-17, the protumorigenic type 17 axis can be organized into two types of mechanisms. First, IL-17 both expands and mobilizes immunosuppressive granulocytes, which can hinder effector cytotoxic T-cells or make the tumor microenvironment more ‘angiogenesis-friendly’. Secondly, other cytokines relevant in the type 17 context, such as GM-CSF, IL-6, and TNF can be produced to initiate a positive feedback loop in cellular crosstalk between cancer cells and immunosuppressive compartments in the environment. On the other hand, IL-17 mediated fostering of anti-tumor immunity was also demonstrated in several instances. For example, IFN-γ, TNF-α co-production from type 17 T-cells was often correlated with improved overall survival and better sensitivity to cancer therapy. Additionally, the presence of certain types of IL-17+ T-cells enhanced dendritic cell (DC) and CTL recruitment into the tumor bed, which boosts tumor-specific T-cell responses (25,106,107).
TISSUE-SPECIFIC NICHE DIVERSIFIES TYPE 17 RESPONSES
Cancer cells, tumor-derived fibroblasts, and Ag-presenting cells secrete several key cytokines for Th17 differentiation such as IL-1β, IL-6, IL-23, and TGF-β. In the tumor, IL-1β, probably produced by tumor-associated macrophages, was shown to be critical for the expansion of memory Th17 cells in ovarian and breast cancers (58,59). In mammary gland tumors, Prostaglandin E2 (PGE2)-induced IL-23 production led to Th17 cell expansion (108). In addition, IL-17 can signal epithelial cells and fibroblasts to produce a diverse array of chemokines and growth factors, which depending on the tissue-niche can either foster cancerous development, or assist in anti-tumor activities. For example, IL-17 was shown to induce the production of angiogenic factors such as VEGF, CXCL1, or CXCL8 in colorectal cancer and NSCLC patient samples (109,110), whereas the same cytokine induced secretion of angiostatic CXCL9 and CXCL10 chemokines in ovarian cancer, which in turn recruited effector CTL and NK cells (59). Therefore, the tissue-specific niche can be another factor that augments the diversity and function of type 17 cells. The following briefly summarizes studies that investigated the link between type 17 cells and cancers by tissue-type.
Gut: intestinal type 17 cells
Th17 cells act to support the integrity and homeostasis of the intestinal barrier in a non-inflammatory manner. These tissue-resident homeostatic Th17 cells are elicited by the commensal flora, and function to control their composition (111). Considering the close associations of certain commensals with intestinal cancers, it is possible to postulate homeostatic Th17 cells obtaining pathogenicity in inflammatory sites of precancerous lesions. The contributions of both adaptive (Th17) and innate (γδT17) type 17-driven mucosal immunity to oncogenic commensal-promoted colon carcinogenesis have been demonstrated in mouse models (14,28,112). Ab-blockade of IL-17 ameliorated tumorigenesis and growth, demonstrating a STAT3- and type 17-dependent pathway for commensal-induced colon carcinogenesis. IL-23, another type 17 cytokine, was shown to be secreted by tumor-associated myeloid cells activated by microbial products in colon cancer models (113). IL-23 has been suggested to be the driver of de novo gut carcinogenesis via the activation of IL-23R+ ILC3s (114). Similarly, it is well-known that Helicobacter pylori-associated gastritis is a major risk factor for gastric cancer. Such pathogenesis has shown to be correlated with a type 17-driven response (115). As such, the gut can foster a niche-specific type 17 response that is important in the initial stages of cancerous transformations.
Intestinal commensal-type 17 axis has been shown to exert pro-tumorigenic effect in distal tissues. In pancreatic cancer, the intestinal microbiome was shown to induce IL-17 expression from Th17 cells and promote pancreatic oncogenesis (14,116). Ablation of the microbiome with antibiotics reduces cancer growth in an IL-17–dependent fashion, which supports the protumoral role of bacteria and IL-17 in the early stages of oncogenesis in distal tissues (14,21). Whether the impact of dysbiosis on cancer growth is due to a specific gut microbe, or depletion of commensal flora in general remains to be studied.
Lung: pulmonary type 17 cells
In LSL-K-rasG12D murine lung cancer model, inflammatory macrophage–Th17 cell axis was critical for tumorigenesis (117), highlighting the tissue-specific context that shapes the cellular interactions of Type 17 cells. Tissue specific niche also can change the functional role of type 17 cells. Commensal bacteria have shown to shape the efficiency of immune surveillance in lung mucosal tissues via the induction of homeostatic γδT17 cells (20). The tissue-specific niche also seems to vary the degree to which IL-17 can affect early stages of cancer angiogenesis, especially tissues in which type 17 cells play a homeostatic role (36,68,118).
Murine models of lung cancer have demonstrated conflicting roles of IL-17. In a murine model of oncogenic mutation-driven lung cancer, Th17 cells accumulated in the tumor, and Th17-derived IL-17 regulated the recruitment of Gr-1+ CD11b+ myeloid cells to the tumor, thereby exerting a pro-tumorigenic role (23). In another recent study, lung commensal microbiota was shown to promote lung cancer development via induction of inflammatory tissue-resident γδT17 cells (24). Tumor burden was correlated with local bacterial load in the airway. Interestingly, bacterial intratracheal inoculation led to accelerated tumor growth and enhanced neutrophil expansion. A positive feedback loop seemed to be the important tumor-initiating mechanism, whereby oncogenic tumor initiating genes act to stimulate local microbiota. Anti-tumor properties of endogenous IL-17 have also been reported using IL-17-deficient mouse systems (13,106). Mechanistically, anti-tumor Th17 cells have been suggested to enhance the activation of tumor-specific CD8+ T-cells in the tumor in TCR-transgenic mouse models, but whether the same phenomenon occurs in human lung cancers remains to be determined.
Skin: cutaneous type 17 cells
Type 17 response in the skin acts to efficiently mediate wound healing and repair in cases of injury. A recent study illustrated how such intrinsic wound healing signaling can be hijacked by cancerous skin cells in tumorigenesis. Mechanistically, IL-17 receptor signaling can transactivate epidermal growth factor receptor (EGFR) in skin epithelial cells for tumorigenesis (119). IL-17 signaling seems to be important also in tumorigenesis via its direct capability to induce STAT3 activation in skin epithelial cancer cells. IL-17 deletion was associated with reduced tumorigenesis in a chemically-induced skin tumor model, with lower expression of STAT3-mediated chemokine expression, and lower infiltration of myeloid cells into the tumor (26).
Skin-commensal associated inflammatory Th17 response has shown to trigger skin cutaneous T-cell lymphoma progression. Markedly lower tumor burdens in STAT3-hyperactive mutant mouse group housed in germ-free conditions were observed compared to those in specific-pathogen free facilities (120).
Cancer-intrinsic IL-17 signaling: IL-17 acts as mitogenic stimulant
Both cancerous and healthy non-immune cells express functional IL-17 receptors (121,122). Whether cancer cells show elevated levels compared to healthy cells is unclear. IL-17 signaling directly activates the early stages of cancer cell proliferation via STAT3 signaling. Along with it, the downstream activation of transcription factors (NF-κB, STAT, and AP-1), kinases (MAPK), tissue remodeling matrix metalloproteinases (MMPs), and anti-apoptotic proteins (Akt, Erk, mTOR, Bcl-2, and Bax) are also stimulated in a myriad of cancers (123,124,125). For example, IL-17 ligation stimulates the proliferation and self-renewal of ovarian cancer stem cells in a dose-dependent fashion via the NF-κB and MAPK pathways (126). IL-17RA expression and engagement has shown to directly promote colonic tumorigenesis within transformed colonic epithelial cells (30).
Oncogenic mutations have shown to activate intrinsic IL-17 signaling in pancreatic neoplasia (116). During cancer angiogenesis, cancer cells become directly responsive to IL-17 signaling (68). IL-17 promoted tumor graft development and directly inhibited apoptosis in several breast cancer cell-lines in a TGF-β dependent manner (16). Furthermore, this anti-apoptotic effect was reversed after IL-17R suppression, indicating intrinsic signaling at play. IL-17-mediated prostate cancer promotion was demonstrated to occur through epithelial to mesenchymal transition as a result of enhanced MMP7 expression (127).
Cancer stage seems also change the dominant type-17 mechanism(s) at play. Regardless of the mechanism by which IL-17 promotes tumor growth, the protumoral properties of IL-17 via STAT3 signaling in cancer cells impact the early stages of oncogenesis rather than the later stages in an established tumor (27,128,129). Additionally, the extrinsic tumor microenvironment is a dynamic one that changes along with the progression of cancer. CCR4 expression among intratumoral Th17 cells has been reported to be higher than in blood Th17 in HCC patients (45). The frequency of CCR4+ intratumoral Th17 subset was shown to correlate positively with the stage of hepatocellular carcinoma patients (45).
TYPE 17 CELLS AS PROGNOSIS FACTORS AND THERAPEUTIC TARGETS
Despite the universal presence of tumor-infiltrating type 17 cells across many cancer types, the relative functions of these cells and the overall prognostic factors associated is an on-going effort that remains to be fully elucidated. Table 2 simply summarizes the current findings of the correlation between IL-17 producing T-cells and their overall prognosis of survival in various cancer types. Others have more comprehensively and systemically summarized the correlations between the presence of tumor-infiltrating type 17 cells and disease progression (130). It is important to highlight the distinction that needs to be made between IL-17 and type 17 cells. As demonstrated in lung adenocarcinoma, the presence of Th17 cells in the MPEs was associated with improved overall survival (48), while IL-17 levels in the same anatomical location were correlated with poor overall survival (56) Nevertheless, the following sections aim to outline the mechanistic insight of IL-17 producing T-cells in the tumor microenvironment in response to current therapy, and how current efforts aim to utilize type 17 axis for clinical remission (Fig. 2).
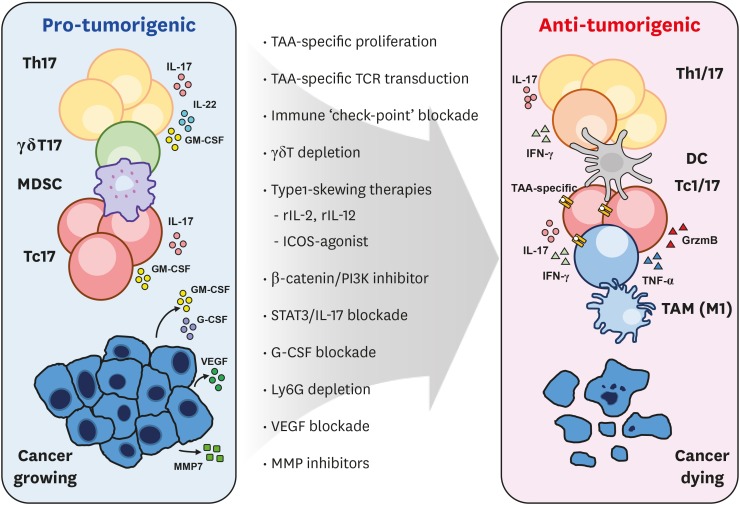
Figure 2. Developing strategies to utilize type 17 axis for anti-cancer therapy.
Currently developing strategies to transform the type 17 landscape within the tumor microenvironment for successful cancer elimination are illustrated.
TAM, tumor-associated macrophage.
Role of type17 cells in conventional therapy resistance
Resistance mechanisms against conventional chemo- or irradiation therapy are rather well-studied (131). Several reports have documented the involvement of IL-17 cytokine in the cancer-intrinsic acquisition of therapy resistance. Chemotherapy can trigger local inflammation, and such release of inflammatory cytokines could curtail the drug's anti-tumor effect. Indeed, treatment of 5-fluorouracil (5-FU) has shown to increase the production of IL-17 in the local tumor microenvironment (30). IL-17 secretion promotes the development of chemoresistance in breast and colon cancers by suppressing the expression of pro-apoptotic signals (132,133). Immunochemotherapy reagents and irradiation can also promote IL-17 secretion and induce resistance (11). In breast cancer patient samples, tumor-infiltrating lymphocytes (TILs) were demonstrated to be the main source of IL-17 (132), although in other cases autocrine IL-17 signaling seemed to be the more dominant mechanism. Tumor resistance mechanism to anti-angiogenic agents, such as anti-VEGF neutralizing Ab, has been shown to be triggered by IL-17 signaling as well. Tumor-infiltrating Th17 cells and IL-17 induced the expression of G-CSF from tumors through NF-κB and ERK signaling. This led to immature myeloid-cell mobilization and recruitment into the tumor microenvironment, thereby desensitizing the tumor microenvironment from the effects of anti-VEGF therapy (134). As such, current findings uniformly illustrate that in response to conventional cancer therapies, IL-17 secretion-whether it is secreted from cancer cells or tumor-infiltrating immune cells-contributes to the development of cancer resistance.
Therapeutic opportunities: tumor-specific Th17 adoptive cell transfer
Tumor-specific in vitro expanded Th17 cells have shown to eradicate cancer in melanoma models either by directly converting to Th1 cells (12) or assisting in Th1 recruitment into the tumor bed (135). In fact, tumor-specific Th17 cells have been reported to eradicate melanoma better than Th1 cells by recruiting DCs and CTLs to the tumor environment (106). This, along with a series of other proof of concept studies using mouse models of cancer, have demonstrated that type 17 cells can be potentiated to exhibit anti-tumor properties if the cells are exploited properly (136). Recent breakthroughs in chimeric Ag receptor (CAR) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 technologies have made cancer-targeting cellular therapies become a reality (137). The most practical cellular platform for these technologies are currently the patient's own PBMC-derived T-cells that are transduced, expanded ex vivo and then transfused back to the patient. However, the fundamental limitation to this method is that often times the patient's own T-cells exhibit phenotypic and/or functional exhaustion, and are not ‘fit’ enough to survive and function in vivo when transferred back into the patient (138). As a potential alternative, several research groups have attempted to take advantage of the plastic nature of type 17 cells to convert into IFN-γ-co producing hybrid-like cells in vivo after adoptive transfer (75). Compared to the type 1 counterpart, type 17-skewed cells display enhanced capacity for self-renewal and durable persistence, which can be potentiated to be less easily exhausted and more resistant to apoptosis than its Th1 or Tc1 counterparts (77).
Additionally, other type 17 cell compartments such as γδT, or iNKT cells, although less attractive than T-cell platforms due to the sheer quantity of cells that can be extracted from PBMC, can been suggested as alternatives for cellular therapies (139,140). As such, it will be important to understand how type 17 lymphocytes are regulated in the tumor microenvironments.
Immunotherapies targeting type 17 responses
Many immuno-modulating therapies, most notably immune check-point blockade Abs, have shown clinical efficacy in cancer therapy. Therapeutic agents that regulate the type 17 response within the tumor microenvironment such as RORγ agonists have also shown preclinical efficacy (77) and one candidate has already shown to be clinically safe (141). Currently, a phase Ib study is underway for assessing clinical and biological activity of the agonist in combination with anti-PD1 blockade (NCT03396497). Mechanisms and clinical efficacy remains to be determined. In a mouse model of melanoma, PI3K and β-catenin inhibition unleashed potent antitumor Th17 responses via conversion to an effector phenotype (142,143). Inducible T-cell co-stimulator (ICOS) agonistic signaling in a CAR-T construct also optimally expanded tumor-specific Th1/Th17 hybrid-like cells, with enhanced persistence in vivo compared to conventional CAR constructs (144). ICOS stimulation, PI3K inhibition, and other immunotherapeutic agents that can potentiate tumor-specific Th17 responses should be considered in the future.
On the other hand, treatment with an antagonistic IL-17 Ab has shown to block the development of pancreatic cancer metastasis in a murine xenograft model, indicating that IL-17 alone can be protumorigenic in the tumor microenvironment. In early stages of cancer development, anti–IL-17 Abs have shown to potentiate anti-VEGF therapy and prevent the progression of pancreatic intraepithelial neoplasia and pancreatic cancer metastasis. As such, consideration of protumorigenic and anti-tumor properties of type 17 cell types and developing therapeutic strategies to make the tumor environment more favorable for anti-tumor cell types seems to be an important focus point in future research.
Other small molecule agents that target STAT3 inhibition in cancer cells can also indirectly bring about the inhibition of type 17 responses in the tumor microenvironment (145). Several clinical trials have been conducted, at phase I/II levels, most notable of which are STAT3 inhibitors modulate STAT3 activity in cancer cells directly in advanced and solid cancers, as well as in cutaneous T-cell lymphomas (OPB-31121, OPB-51602, Otsuka Pharmaceutical, Tokyo, Japan; AZD9150, ISIS Pharmaceuticals Inc., Carlsbad, CA, USA). Clinical efficacy of these agents, and whether they work via the type 17 axis, remains to be determined.
CONCLUSIONS AND FUTURE PERSPECTIVES
Despite the complexity of the matter, therapeutic opportunities are being explored to either remove the immunosuppressive effects elicited by IL-17 signaling via Ab blockade, or drive type 17 cells towards cancer elimination via skewing towards the IFN-γ-producing cytotoxic type 1 subset. On-going efforts aim to better understand if and how the variously employed immunotherapeutic approaches and cancer resistant mechanisms are associated with type 17 responses. Seemingly contradictory results in the function of type 17 cells, even within the same cancer type, illustrate that the therapeutic approach targeting the type 17 axis should be individually adjusted according to cancer type, stage, and the resulting dominant type 17 mechanism at work.
ACKNOWLEDGEMENTS
This work was supported by the research grant (2017R1A2B3007392; to YC), the Basic Science Research Program (2016R1A6A3A11933284; to Kim BS), and the Global Ph.D. Fellowship Program (2017H1A2A1042662; to Kuen DS) from the National Research Foundation of Korea (NRF) funded by the Ministry of Education of Korea.
Abbreviations
- 5-FU
Fluorouracil
- CAR
chimeric Ag receptor
- CRC
colorectal cancer
- CRISPR
Clustered regularly interspaced short palindromic repeats
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- ICOS
inducible T-cell costimulatory
- ILC
innate lymphoid cell
- HCC
hepatocellular carcinoma
- MDSC
myeloid-derived suppressor cells
- MMP
matrix metalloproteinase
- MPE
malignant pleural effusions
- NKT17
IL-17 producing invariant NKT cells
- NSCLC
non-small cell lung carcinoma
- PGE2
prostaglandin E2
- RORγt
RAR-related orphan receptor gamma
- TAM
tumor-associated macrophage
- TAN
tumor-associated neutrophil
- Tc17
IL-17 producing CD8+ T
- TIL
tumor-infiltrating lymphocyte
- γδT17
IL-17 secreting γδT
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kuen DS, Chung Y.
- Data curation: Kuen DS.
- Funding acquisition: Kuen DS, Kim BS, Chung Y
- Supervision: Chung Y.
- Visualization: Kuen DS.
- Writing - original draft: Kuen DS.
- Writing - review & editing: Kim BS, Chung Y.
References
- 1.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 2.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 3.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo . Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 7.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 10.Kim BS, Park YJ, Chung Y. Targeting IL-17 in autoimmunity and inflammation. Arch Pharm Res. 2016;39:1537–1547. doi: 10.1007/s12272-016-0823-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhong W, Li Q. Rituximab or irradiation promotes IL-17 secretion and thereby induces resistance to rituximab or irradiation. Cell Mol Immunol. 2017;14:1020–1022. doi: 10.1038/cmi.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018;155:33–37.e6. doi: 10.1053/j.gastro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, Yin XY, Li L, Zheng L. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol. 2010;185:1544–1549. doi: 10.4049/jimmunol.0904094. [DOI] [PubMed] [Google Scholar]
- 16.Du JW, Xu KY, Fang LY, Qi XL. Interleukin-17, produced by lymphocytes, promotes tumor growth and angiogenesis in a mouse model of breast cancer. Mol Med Rep. 2012;6:1099–1102. doi: 10.3892/mmr.2012.1036. [DOI] [PubMed] [Google Scholar]
- 17.Irshad S, Flores-Borja F, Lawler K, Monypenny J, Evans R, Male V, Gordon P, Cheung A, Gazinska P, Noor F, et al. RORγt+ Innate Lymphoid Cells Promote Lymph Node Metastasis of Breast Cancers. Cancer Res. 2017;77:1083–1096. doi: 10.1158/0008-5472.CAN-16-0598. [DOI] [PubMed] [Google Scholar]
- 18.Rei M, Gonçalves-Sousa N, Lança T, Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ, Silva-Santos B. Murine CD27(−) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014;111:E3562–E3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, et al. The tumor-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng M, Qian L, Shen G, Bian G, Xu T, Xu W, Shen G, Hu S. Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism. Cancer Res. 2014;74:4030–4041. doi: 10.1158/0008-5472.CAN-13-2462. [DOI] [PubMed] [Google Scholar]
- 21.Calcinotto A, Brevi A, Chesi M, Ferrarese R, Garcia Perez L, Grioni M, Kumar S, Garbitt VM, Sharik ME, Henderson KJ, et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat Commun. 2018;9:4832. doi: 10.1038/s41467-018-07305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111:5664–5669. doi: 10.1073/pnas.1319051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell. 2019;176:998–1013.e16. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You R, DeMayo FJ, Liu J, Cho SN, Burt BM, Creighton CJ, Casal RF, Lazarus DR, Lu W, Tung HY, et al. IL17A regulates tumor latency and metastasis in lung adeno and squamous SQ.2b and AD.1 cancer. Cancer Immunol Res. 2018;6:645–657. doi: 10.1158/2326-6066.CIR-17-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Housseau F, Wu S, Wick EC, Fan H, Wu X, Llosa NJ, Smith KN, Tam A, Ganguly S, Wanyiri JW, et al. Redundant innate and adaptive sources of IL17 production drive colon tumorigenesis. Cancer Res. 2016;76:2115–2124. doi: 10.1158/0008-5472.CAN-15-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, Vatan L, Szeliga W, Dou Y, Owens S, et al. IL-22+CD4+ T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2014;40:772–784. doi: 10.1016/j.immuni.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052–1063. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–931. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abousamra NK, Salah El-Din M, Helal R. Prognostic value of Th17 cells in acute leukemia. Med Oncol. 2013;30:732. doi: 10.1007/s12032-013-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and interleukin-17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer Sci. 2014;105:933–942. doi: 10.1111/cas.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 35.Faucheux L, Grandclaudon M, Perrot-Dockès M, Sirven P, Berger F, Hamy AS, Fourchotte V, Vincent-Salomon A, Mechta-Grigoriou F, Reyal F, et al. A multivariate Th17 metagene for prognostic stratification in T cell non-inflamed triple negative breast cancer. OncoImmunology. 2019;8:e1624130. doi: 10.1080/2162402X.2019.1624130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol. 2014;31:82. doi: 10.1007/s12032-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 39.Alves JJ, De Medeiros Fernandes TA, De Araújo JM, Cobucci RN, Lanza DC, Bezerra FL, Andrade VS, Fernandes JV. Th17 response in patients with cervical cancer. Oncol Lett. 2018;16:6215–6227. doi: 10.3892/ol.2018.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Punt S, Fleuren GJ, Kritikou E, Lubberts E, Trimbos JB, Jordanova ES, Gorter A. Angels and demons: Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology. 2015;4:e984539. doi: 10.4161/2162402X.2014.984539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y, Xu J, Rao H, Chen S, Zhang L, et al. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2013;62:1575–1585. doi: 10.1007/s00262-013-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One. 2011;6:e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y, Saito H, Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res. 2012;178:685–691. doi: 10.1016/j.jss.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 46.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan J, Liu XL, Xiao G, Li NL, Deng YN, Han LZ, Yin LC, Ling LJ, Liu LX. Prevalence and clinical relevance of T-helper cells, Th17 and Th1, in hepatitis B virus-related hepatocellular carcinoma. PLoS One. 2014;9:e96080. doi: 10.1371/journal.pone.0096080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 49.Gong Y, Chen SX, Gao BA, Yao RC, Guan L. Cell origins and significance of IL-17 in malignant pleural effusion. Clin Transl Oncol. 2014;16:807–813. doi: 10.1007/s12094-013-1152-8. [DOI] [PubMed] [Google Scholar]
- 50.Bao Z, Lu G, Cui D, Yao Y, Yang G, Zhou J. IL-17A-producing T cells are associated with the progression of lung adenocarcinoma. Oncol Rep. 2016;36:641–650. doi: 10.3892/or.2016.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zelba H, Weide B, Martens A, Derhovanessian E, Bailur JK, Kyzirakos C, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, et al. Circulating CD4+ T cells that produce IL4 or IL17 when stimulated by Melan-A but not by NY-ESO-1 have negative impacts on survival of patients with stage IV melanoma. Clin Cancer Res. 2014;20:4390–4399. doi: 10.1158/1078-0432.CCR-14-1015. [DOI] [PubMed] [Google Scholar]
- 52.Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, Jagannath S, Dhodapkar MV. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen CJ, Yuan ZH, Liu YX, Hu GY. Increased numbers of T helper 17 cells and the correlation with clinicopathological characteristics in multiple myeloma. J Int Med Res. 2012;40:556–564. doi: 10.1177/147323001204000217. [DOI] [PubMed] [Google Scholar]
- 54.Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, Qian CN, Zeng YX. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Mo HY, Xiong G, Zhang L, He J, Huang ZF, Liu ZW, Chen QY, Du ZM, Zheng LM, et al. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. J Biol Chem. 2012;287:35484–35495. doi: 10.1074/jbc.M112.367532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu C, Yu L, Zhan P, Zhang Y. Elevated pleural effusion IL-17 is a diagnostic marker and outcome predictor in lung cancer patients. Eur J Med Res. 2014;19:23. doi: 10.1186/2047-783X-19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 58.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424–7437. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Derhovanessian E, Adams V, Hähnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 63.Huang Y, Wang J, Jia P, Li X, Pei G, Wang C, Fang X, Zhao Z, Cai Z, Yi X, et al. Clonal architectures predict clinical outcome in clear cell renal cell carcinoma. Nat Commun. 2019;10:1245. doi: 10.1038/s41467-019-09241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koyama K, Kagamu H, Miura S, Hiura T, Miyabayashi T, Itoh R, Kuriyama H, Tanaka H, Tanaka J, Yoshizawa H, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 65.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 66.Chen D, Jiang R, Mao C, Shi L, Wang S, Yu L, Hu Q, Dai D, Xu H. Chemokine/chemokine receptor interactions contribute to the accumulation of Th17 cells in patients with esophageal squamous cell carcinoma. Hum Immunol. 2012;73:1068–1072. doi: 10.1016/j.humimm.2012.07.333. [DOI] [PubMed] [Google Scholar]
- 67.Yu Q, Lou XM, He Y. Preferential recruitment of Th17 cells to cervical cancer via CCR6-CCL20 pathway. PLoS One. 2015;10:e0120855. doi: 10.1371/journal.pone.0120855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin S, Cao S, Che D, Liu F, Yu Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep. 2015;5:16053. doi: 10.1038/srep16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Huang ZF, Xiong G, Mo HY, Qiu F, Mai HQ, Chen QY, He J, Chen SP, Zheng LM, et al. Distribution, characterization, and induction of CD8+ regulatory T cells and IL-17-producing CD8+ T cells in nasopharyngeal carcinoma. J Transl Med. 2011;9:189. doi: 10.1186/1479-5876-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY, et al. CD8+ T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951–62.e8. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Lee MH, Tung-Chieh Chang J, Liao CT, Chen YS, Kuo ML, Shen CR. Interleukin 17 and peripheral IL-17-expressing T cells are negatively correlated with the overall survival of head and neck cancer patients. Oncotarget. 2018;9:9825–9837. doi: 10.18632/oncotarget.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tajima M, Wakita D, Satoh T, Kitamura H, Nishimura T. IL-17/IFN-γ double producing CD8+ T (Tc17/IFN-γ) cells: a novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. Int Immunol. 2011;23:751–759. doi: 10.1093/intimm/dxr086. [DOI] [PubMed] [Google Scholar]
- 74.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E, Yu XZ. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol. 2013;190:1873–1881. doi: 10.4049/jimmunol.1201989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bowers JS, Nelson MH, Majchrzak K, Bailey SR, Rohrer B, Kaiser AD, Atkinson C, Gattinoni L, Paulos CM. Th17 cells are refractory to senescence and retain robust antitumor activity after long-term ex vivo expansion. JCI Insight. 2017;2:e90772. doi: 10.1172/jci.insight.90772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu X, Majchrzak K, Liu X, Wyatt MM, Spooner CJ, Moisan J, Zou W, Carter LL, Paulos CM. In vitro priming of adoptively transferred T cells with a RORγ agonist confers durable memory and stemness in vivo . Cancer Res. 2018;78:3888–3898. doi: 10.1158/0008-5472.CAN-17-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VR, et al. γδ T cells support pancreatic oncogenesis by restraining αβ T cell activation. Cell. 2016;166:1485–1499.e15. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwon DI, Lee YJ. Lineage differentiation program of invariant natural killer T cells. Immune Netw. 2017;17:365–377. doi: 10.4110/in.2017.17.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18:559–574. doi: 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim CH. Control of innate and adaptive lymphocytes by the RAR-retinoic acid axis. Immune Netw. 2018;18:e1. doi: 10.4110/in.2018.18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruchard M, Ghiringhelli F. Deciphering the roles of innate lymphoid cells in cancer. Front Immunol. 2019;10:656. doi: 10.3389/fimmu.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koh J, Kim HY, Lee Y, Park IK, Kang CH, Kim YT, Kim JE, Choi M, Lee WW, Jeon YK, et al. IL23-producing human lung cancer cells promote tumor growth via conversion of innate lymphoid cell 1 (ILC1) into ILC3. Clin Cancer Res. 2019;25:4026–4037. doi: 10.1158/1078-0432.CCR-18-3458. [DOI] [PubMed] [Google Scholar]
- 85.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vykhovanets EV, Maclennan GT, Vykhovanets OV, Gupta S. IL-17 Expression by macrophages is associated with proliferative inflammatory atrophy lesions in prostate cancer patients. Int J Clin Exp Pathol. 2011;4:552–565. [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X, Churchill MJ, Nagar KK, Tailor YH, Chu T, Rush BS, Jiang Z, Wang EB, Renz BW, Wang H, et al. IL-17 producing mast cells promote the expansion of myeloid-derived suppressor cells in a mouse allergy model of colorectal cancer. Oncotarget. 2015;6:32966–32979. doi: 10.18632/oncotarget.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tu JF, Pan HY, Ying XH, Lou J, Ji JS, Zou H. Mast cells comprise the major of interleukin 17-producing cells and predict a poor prognosis in hepatocellular carcinoma. Medicine (Baltimore) 2016;95:e3220. doi: 10.1097/MD.0000000000003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X, Jin H, Zhang G, Lin X, Chen C, Sun J, Zhang Y, Zhang Q, Yu J. Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients. PLoS One. 2014;9:e106834. doi: 10.1371/journal.pone.0106834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 91.Petrella TM, Tozer R, Belanger K, Savage KJ, Wong R, Smylie M, Kamel-Reid S, Tron V, Chen BE, Hunder NN, et al. Interleukin-21 has activity in patients with metastatic melanoma: a phase II study. J Clin Oncol. 2012;30:3396–3401. doi: 10.1200/JCO.2011.40.0655. [DOI] [PubMed] [Google Scholar]
- 92.Petrella TM, Mihalcioiu CL, McWhirter E, Belanger K, Savage KJ, Song X, Hamid O, Cheng T, Davis ML, Lee CW, et al. Final efficacy results of NCIC CTG IND.202: a randomized phase II study of recombinant interleukin-21 (rIL21) in patients with recurrent or metastatic melanoma (MM) J Clin Oncol. 2013;31:9032. [Google Scholar]
- 93.Chapuis AG, Lee SM, Thompson JA, Roberts IM, Margolin KA, Bhatia S, Sloan HL, Lai I, Wagener F, Shibuya K, et al. Combined IL-21-primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient. J Exp Med. 2016;213:1133–1139. doi: 10.1084/jem.20152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 95.Jin M, Yoon J. From bench to clinic: the potential of therapeutic targeting of the IL-22 signaling pathway in atopic dermatitis. Immune Netw. 2018;18:e42. doi: 10.4110/in.2018.18.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nardinocchi L, Sonego G, Passarelli F, Avitabile S, Scarponi C, Failla CM, Simoni S, Albanesi C, Cavani A. Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. Eur J Immunol. 2015;45:922–931. doi: 10.1002/eji.201445052. [DOI] [PubMed] [Google Scholar]
- 97.Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: driving protection and pathology. Eur J Immunol. 2017;47:607–614. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- 98.Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, Gao Y, Yao A, Wang X, Yu L, et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 2013;13:59. doi: 10.1186/1471-2407-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lim C, Savan R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014;25:257–271. doi: 10.1016/j.cytogfr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 100.Wen Z, Liao Q, Zhao J, Hu Y, You L, Lu Z, Jia C, Wei Y, Zhao Y. High expression of interleukin-22 and its receptor predicts poor prognosis in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21:125–132. doi: 10.1245/s10434-013-3322-x. [DOI] [PubMed] [Google Scholar]
- 101.Zhang W, Chen Y, Wei H, Zheng C, Sun R, Zhang J, Tian Z. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–6439. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 102.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 105.Chen Y, Zhao Z, Chen Y, Lv Z, Ding X, Wang R, Xiao H, Hou C, Shen B, Feng J, et al. An epithelial-to-mesenchymal transition-inducing potential of granulocyte macrophage colony-stimulating factor in colon cancer. Sci Rep. 2017;7:8265. doi: 10.1038/s41598-017-08047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated CD8+ T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother. 2011;60:1473–1484. doi: 10.1007/s00262-011-1054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qian X, Gu L, Ning H, Zhang Y, Hsueh EC, Fu M, Hu X, Wei L, Hoft DF, Liu J. Increased Th17 cells in the tumor microenvironment is mediated by IL-23 via tumor-secreted prostaglandin E2. J Immunol. 2013;190:5894–5902. doi: 10.4049/jimmunol.1203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 110.Lee JW, Wang P, Kattah MG, Youssef S, Steinman L, DeFea K, Straus DS. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J Immunol. 2008;181:6536–6545. doi: 10.4049/jimmunol.181.9.6536. [DOI] [PubMed] [Google Scholar]
- 111.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chan IH, Jain R, Tessmer MS, Gorman D, Mangadu R, Sathe M, Vives F, Moon C, Penaflor E, Turner S, et al. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 2014;7:842–856. doi: 10.1038/mi.2013.101. [DOI] [PubMed] [Google Scholar]
- 115.Bagheri N, Azadegan-Dehkordi F, Shirzad H, Rafieian-Kopaei M, Rahimian G, Razavi A. The biological functions of IL-17 in different clinical expressions of Helicobacter pylori-infection. Microb Pathog. 2015;81:33–38. doi: 10.1016/j.micpath.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 116.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Q, Anderson CD, Egilmez NK. Inhaled IL-10 suppresses lung tumorigenesis via abrogation of inflammatory macrophage-Th17 cell axis. J Immunol. 2018;201:2842–2850. doi: 10.4049/jimmunol.1800141. [DOI] [PubMed] [Google Scholar]
- 118.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen X, Cai G, Liu C, Zhao J, Gu C, Wu L, Hamilton TA, Zhang CJ, Ko J, Zhu L, et al. IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1+ stem cells. J Exp Med. 2019;216:195–214. doi: 10.1084/jem.20171849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fanok MH, Sun A, Fogli LK, Narendran V, Eckstein M, Kannan K, Dolgalev I, Lazaris C, Heguy A, Laird ME, et al. Role of dysregulated cytokine signaling and bacterial triggers in the pathogenesis of cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:1116–1125. doi: 10.1016/j.jid.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haudenschild D, Moseley T, Rose L, Reddi AH. Soluble and transmembrane isoforms of novel interleukin-17 receptor-like protein by RNA splicing and expression in prostate cancer. J Biol Chem. 2002;277:4309–4316. doi: 10.1074/jbc.M109372200. [DOI] [PubMed] [Google Scholar]
- 122.Steiner GE, Newman ME, Paikl D, Stix U, Memaran-Dagda N, Lee C, Marberger MJ. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171–182. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- 123.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 124.Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6:e21816. doi: 10.1371/journal.pone.0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang B, Kang H, Fung A, Zhao H, Wang T, Ma D. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediators Inflamm. 2014;2014:623759. doi: 10.1155/2014/623759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang T, Long H, He L, Han X, Lin K, Liang Z, Zhuo W, Xie R, Zhu B. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene. 2015;34:165–176. doi: 10.1038/onc.2013.537. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Q, Liu S, Parajuli KR, Zhang W, Zhang K, Mo Z, Liu J, Chen Z, Yang S, Wang AR, et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene. 2017;36:687–699. doi: 10.1038/onc.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, Bardeesy N. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ho PL, Lay EJ, Jian W, Parra D, Chan KS. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Punt S, Langenhoff JM, Putter H, Fleuren GJ, Gorter A, Jordanova ES. The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review. OncoImmunology. 2015;4:e984547. doi: 10.4161/2162402X.2014.984547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med. 2018;50:109. doi: 10.1038/s12276-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, Curé H, Mascaux C, Alberici G, Bonnefoy N, et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci Rep. 2013;3:3456. doi: 10.1038/srep03456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sui G, Qiu Y, Yu H, Kong Q, Zhen B. Interleukin-17 promotes the development of cisplatin resistance in colorectal cancer. Oncol Lett. 2019;17:944–950. doi: 10.3892/ol.2018.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 135.Nuñez S, Saez JJ, Fernandez D, Flores-Santibañez F, Alvarez K, Tejon G, Ruiz P, Maldonado P, Hidalgo Y, Manriquez V, et al. T helper type 17 cells contribute to anti-tumour immunity and promote the recruitment of T helper type 1 cells to the tumour. Immunology. 2013;139:61–71. doi: 10.1111/imm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Majchrzak K, Nelson MH, Bailey SR, Bowers JS, Yu XZ, Rubinstein MP, Himes RA, Paulos CM. Exploiting IL-17-producing CD4+ and CD8+ T cells to improve cancer immunotherapy in the clinic. Cancer Immunol Immunother. 2016;65:247–259. doi: 10.1007/s00262-016-1797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu J, Zhou G, Zhang L, Zhao Q. Building Potent Chimeric Antigen Receptor T Cells With CRISPR Genome Editing. Front Immunol. 2019;10:456. doi: 10.3389/fimmu.2019.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng J, Zhao L, Zhang Y, Qin Y, Guan Y, Zhang T, Liu C, Zhou J. Understanding the mechanisms of resistance to CAR T-cell therapy in malignancies. Front Oncol. 2019;9:1237. doi: 10.3389/fonc.2019.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood. 2007;110:2013–2019. doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Min B, Choi H, Her JH, Jung MY, Kim HJ, Jung MY, Lee EK, Cho SY, Hwang YK, Shin EC. Optimization of Large-Scale Expansion and Cryopreservation of Human Natural Killer Cells for Anti-Tumor Therapy. Immune Netw. 2018;18:e31. doi: 10.4110/in.2018.18.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahalingam D, Wang JS, Hamilton EP, Sarantopoulos J, Nemunaitis J, Weems G, Carter L, Hu X, Schreeder M, Wilkins HJ. Phase 1 open-label, multicenter study of first-in-class RORγ agonist LYC-55716 (cintirorgon): safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res. 2019;25:3508–3516. doi: 10.1158/1078-0432.CCR-18-3185. [DOI] [PubMed] [Google Scholar]
- 142.Bie Q, Sun C, Gong A, Li C, Su Z, Zheng D, Ji X, Wu Y, Guo Q, Wang S, et al. Non-tumor tissue derived interleukin-17B activates IL-17RB/AKT/β-catenin pathway to enhance the stemness of gastric cancer. Sci Rep. 2016;6:25447. doi: 10.1038/srep25447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Majchrzak K, Nelson MH, Bowers JS, Bailey SR, Wyatt MM, Wrangle JM, Rubinstein MP, Varela JC, Li Z, Himes RA, et al. β-catenin and PI3Kδ inhibition expands precursor Th17 cells with heightened stemness and antitumor activity. JCI Insight. 2017;2:90547. doi: 10.1172/jci.insight.90547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, Frigault MJ, Lee J, Posey AD, Jr, Scholler J, Scholler N, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee M, Rhee I. Cytokine Signaling in Tumor Progression. Immune Netw. 2017;17:214–227. doi: 10.4110/in.2017.17.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]