Abstract
Acute viral infection or vaccination generates highly functional memory CD8 T cells following the Ag resolution. In contrast, persistent antigenic stimulation in chronic viral infection and cancer leads to a state of T-cell dysfunction termed T-cell exhaustion. We and other have recently identified a novel subset of exhausted CD8 T cells that act as stem cells for maintaining virus-specific CD8 T cells in a mouse model of chronic lymphocytic choriomeningitis virus infection. This stem cell-like CD8 T-cell subset has been also observed in both mouse and human tumor models. Most importantly, in both chronic viral infection and tumor models, the proliferative burst of Ag-specific CD8 T cells driven by PD-1-directed immunotherapy comes exclusively from this stem cell-like CD8 T-cell subset. Therefore, a better understanding of the mechanisms how CD8 T-cell subsets are regulated during chronic viral infection and cancer is required to improve the current immunotherapies that restore the function of exhausted CD8 T cells. In this review, we discuss the differentiation of virus-specific CD8 T cells during chronic viral infection, the characteristics and function of CD8 T-cell subsets, and the therapeutic intervention of PD-1-directed immunotherapy in cancer.
Keywords: T-cell exhaustion, PD-1, Immunotherapy, Stem cell-like CD8 T-cell subset
INTRODUCTION
In contrast to highly functional memory CD8 T cells that are generated following the resolution of pathogen infection, continuous antigenic stimulation results in T-cell dysfunction, which is called T-cell exhaustion (1,2,3). This functional exhaustion of antigen (Ag)-specific CD8 T cells was first documented in the mouse model of persistent lymphocytic choriomeningitis virus (LCMV) infection in which they persist but lose effector function (4,5) and these findings were then extended to chronic viral infections in non-human primates (6) and humans (7,8,9,10,11,12). T-cell exhaustion has also been described in mouse tumor models (13,14) and in human cancers (15,16,17,18,19). A characteristic feature of exhausted CD8 T cells is the expression of various inhibitory receptors (20,21,22,23). Notably, PD-1 is a central regulator of this process. The discovery that PD-1 blockade could restore anti-viral immunity and reduce viral titers in the mouse model of persistent LCMV infection (23) rapidly expanded to other chronic viral infections (24,25,26) and cancers (27,28,29). PD-1 blockade has had a revolutionary impact on cancer treatment by high efficacy against various cancer models with limited side effects. Recent clinical studies have shown that PD-1 blockade significantly improved anti-tumor immunity and resulted in objective responses in several types of cancer including melanoma, non-small-cell lung cancer (NSCLC), kidney cancer, non-Hodgkin lymphoma and head and neck cancer, then eventually has been approved by the US Food and Drug Administration (30,31,32). While successful, some patients do not respond to anti-PD-1 therapy and not all patients undergo a complete response. For this reason, ongoing research into the mechanisms that control T-cell exhaustion, and more specifically, how PD-1 blockade reverses this condition, is needed.
We and others recently discovered a novel subset of exhausted CD8 T cells which acted as stem cells to maintain the pool of virus-specific T-cell population in a chronic Ag setting (33,34,35,36). These stem cell-like cells lack many common effector function, however they can generate large numbers of terminally differentiated cells while also undergoing self-renewal to maintain their numbers. In addition, the proliferation of exhausted CD8 T cells that occurs after PD-1 blockade is exclusively derived from the stem cell-like CD8 T cells. These stem cell-like CD8 T cells have been also referred to as “stem cell-like progenitor”, “memory-like”, “precursor exhausted” or “progenitor exhausted” cells (37,38). Here, we will use a nomenclature of the “stem cell-like” CD8 T cells because they could differentiate into new CD8 T-cell subsets having distinct features from terminally differentiated CD8 T cells after cytokine treatment or exposure to the environment without the Ag (in our unpublished data) and it has not been validated yet whether they could survive in the absence of Ag as canonical memory CD8 T cells do. Here, we review recent studies focused on the characteristics of the stem cell-like and terminally differentiated CD8 T cells, and the mechanism by which PD-1 blockade regulates CD8 T-cell immunity in a mouse model of chronic LCMV infection. Then, we discuss whether the same differentiation program of CD8 T cells is applicable to other disease models such as cancer and autoimmune diseases in which CD8 T cells are continuously exposed to antigenic stimulation. Finally, we discuss the therapeutic implications of this knowledge for therapy in cancer patients.
DEFINING STEM CELL-LIKE CD8 T CELLS DURING CHRONIC VIRAL INFECTION
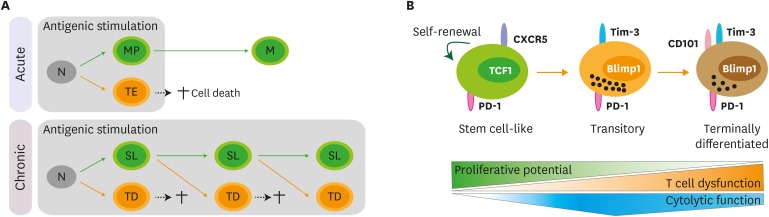
It has been thought that canonical memory CD8 T cells are not generated during chronic viral infections (39). Instead of homeostatic cytokines such as IL-7 and IL-15, viral Ags drove the proliferation of exhausted CD8 T cells for maintaining the pool of CD8 T cells during chronic LCMV infection (39,40). Therefore, when the exhausted CD8 T cells were exposed to Ag-free environment, their population was dramatically contracted. However, Utzschneider et al. (41) proposed memory-like exhausted CD8 T cells, which could survive in the absence of Ags and robustly proliferate after Ag restimulation similar to classic memory CD8 T cells, during chronic LCMV infection (42) (Fig. 1A). We and others recently identified a novel population of TCF1+CXCR5+ virus-specific CD8 T cells during chronic viral infection that act as stem cells and maintain the pool of virus-specific CD8 T cells (33,34,35,36). These stem cell-like CD8 T cells maintained the population sustaining their native phenotypes by self-renewal and differentiated into the TCF1−Tim-3+ terminally differentiated cells accompanied with active proliferation. More robust proliferation and differentiation of the stem cell-like CD8 T cells were observed when the cells were exposed to a setting much more favorable for activation of CD8 T cells by transferring them into naive mice, followed by Cl13 or Arm infection (33,35). This event occurred when we used the stem cell-like CD8 T cells isolated from both early and late phase of chronic LCMV infection, reflecting that the capability of reconstitution potential is maintained during the course of chronic viral infection. We recently further divided the TCF1−Tim-3+ CD8 T cells into 2 subsets of CD101−Tim-3+ and CD101+Tim-3+ CD8 T cells (43) (Fig. 1B). CD101−Tim-3+ CD8 T cells are recently-generated cells from stem cell-like CD8 T cells and are able to proliferate and differentiate into further differentiated CD101+Tim-3+ CD8 T-cell subset, suggesting that this CD101−Tim-3+ subset is a transitory population during the differentiation between stem cell-like and CD101+Tim-3+ terminally differentiated cells. The stem cell-like CD8 T cells possess memory-like features (33,34,35,36). For example, the stem cell-like CD8 T cells showed higher expression of genes such as Il7r, Sell (CD62L), Ccr7, Id3, Bcl6, Eomes, and Tcf7 (TCF1). As the differentiation progressed, genes related to the differentiation of effector T cells such as Prdm1 (Blimp1), Tbx21 (T-bet) and Id2, the cytolytic functions such as Gzma, Gzmb and Prf1, and the apoptosis such as Fasl and Tnfsf10 (TRAIL), were significantly increased. Of note, although the stem cell-like CD8 T cells showed the absence of Granzyme B expression, there was a hierarchy in the production of an effector cytokine, IFNγ, and a degranulation marker, CD107, after ex vivo stimulation among different CD8 T-cell subsets; the highest in the stem cell-like CD8 T cells, middle in newly generated cells and the lowest in old terminally differentiated cells (34). We confirmed that the CD101−Tim-3+ transitory subset had a role in viral control with the highest expression of Granzyme B (43). Taken together, these results strongly support the differentiation pathway for maintaining CD8 T-cell immunity during chronic viral infection as follows: TCF1+Tim-3− stem cell-like cells → CD101−Tim-3+ transitory cells → CD101+Tim-3+ terminally differentiated cells (Fig. 1B).
Figure 1. Differentiation pathway of Ag-specific CD8 T cells during chronic viral infection. (A) Upon acute viral infection, naïve CD8 T cells activate and differentiate into memory precursors (MP) and terminal effectors (TE). Terminal effectors die by AICD and memory precursors survive and become memory CD8 T cells (M) after the clearance of viral infection. Similarly, naïve CD8 T cells (N) are activated and differentiate into a stem cell-like subset (SL) and terminally differentiated cells (TD) upon chronic viral infection. Analogous to terminal effectors, terminally differentiated cells also die by AICD. Different from the acute infection, sustained antigenic stimulation during chronic viral infection resulted in the continual differentiation of stem cell-like CD8 T cells into terminally differentiated CD8 T cells. (B) TCF1+CXCR5+ stem cell-like CD8 T cells maintain their population by slow self-renewal. Upon antigenic stimulation, these stem cell-like CD8 T cells differentiate into CD101−Tim-3+ transitory population. This CD101−Tim-3+ subset possesses in vivo proliferative potential after antigenic stimulation, can differentiate further into terminally differentiated CD101+Tim-3+ CD8 T cells, and contributes to viral control with the highest cytolytic activity. With upregulation of CD101, terminally differentiated CD101+Tim-3+ CD8 T cells lost in vivo proliferative potential and possessed impaired cytolytic function.
LOCALIZATION AND MIGRATION OF CD8 T-CELL SUBSETS DURING CHRONIC VIRAL INFECTION
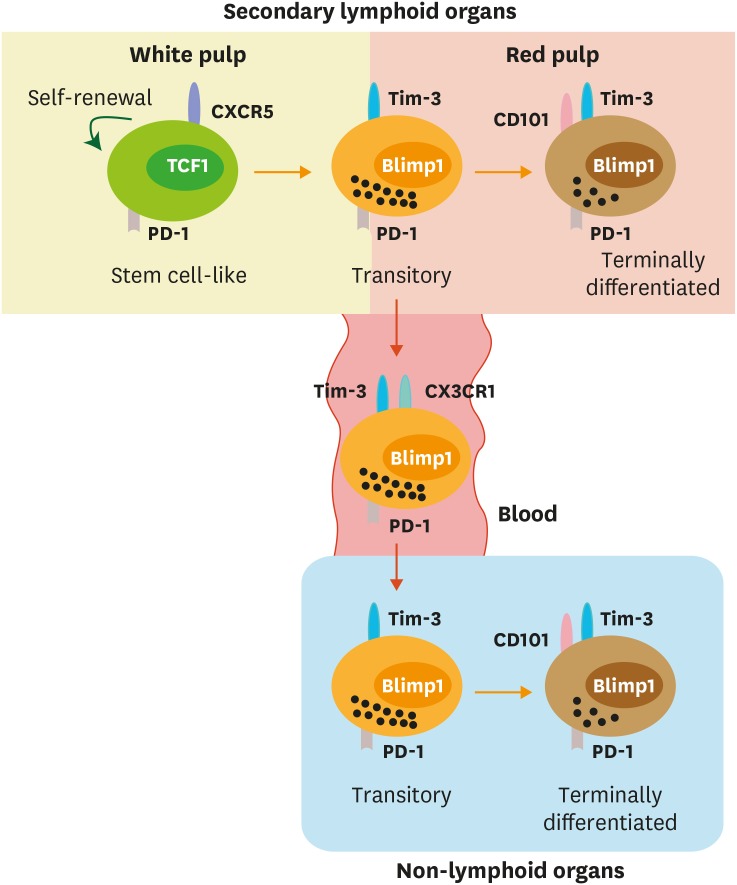
The stem cell-like CD8 T cells were mainly present in the lymphoid tissues but were rarely shown in the non-lymphoid tissues whereas the terminally differentiated cells localized in both lymphoid and non-lymphoid tissues (33,34). Although the location of the stem cell-like CD8 T cells in the spleen is arguable, we observed that stem cell-like CD8 T cells are preferentially localized in the T cells zone (33). The T-cell zone is where T cells interact with dendritic cells (DCs) to induce activation (44,45,46). One plausible hypothesis is that the stem cell-like CD8 T cells continuously interact with a subset of Ag presenting cells (APCs) in the T-cell zones and these APCs act as niches for the maintenance of the stemness of the stem cell-like CD8 T cells. Consistent of this postulation, the stem cell-like CD8 T cells highly expressed Xcl1 (33). XCL1 recruit XCR1-expressing CD8α+ lymphoid DCs (47), which are specialized APCs for the cross-presentation (48,49,50,51,52). The result that the stem cell-like CD8 T cells highly expressed co-stimulatory molecules such as ICOS and CD28, but did not have cytolytic molecules such as granzymes and perforin, could support this notion as well (33). In contrast, the terminally differentiated cells were mainly resided in the red pulp. LCMV Cl13 strain inducing chronic infection was detected mainly in the red pulp macrophages and stromal cells and minimal numbers of DCs (33,53,54), suggesting that the terminally differentiated cells are interacting with infected cells to kill them in the red pulp of the spleen, while the stem cell-like CD8 T cells residing in the T-cell zone are protected from excessive exposure to Ag stimulation (Fig. 2). We previously determined that PD-L1 on bone-marrow-derived cells such as APCs negatively regulates cell expansion and cytokine production, whereas PD-L1 on infected non-hematopoietic cells suppresses viral clearance and immunopathology during chronic LCMV infection (55). Considering that only the stem cell-like CD8 T cells possess a proliferative potential while the further differentiated exhausted cells highly produce cytolytic molecules, this result supports the idea that PD-L1 on APCs in the T cell zone inhibits the proliferation and differentiation of stem cell-like CD8 T cells into the terminally differentiated cells and PD-L1 on non-hematopoietic cells in the red pulp and non-lymphoid tissues dampers the cytolytic activity of the terminally differentiated cells.
Figure 2. Migration of CD8 T-cell subsets during chronic viral infection. Both stem cell-like and terminally differentiated CD8 T cells are circulating after the onset of viral infection. After the establishment of T-cell exhaustion, stem cell-like cells become resident and mainly reside in the lymphoid organs, especially in the white pulp of the spleen. A fraction of the transitory CD101−Tim-3+ CD8 T cells circulate via the blood and migrate other inflamed tissues corresponding to the expression of a fractalkine receptor CX3CR1. In the red pulp of the spleen and non-lymphoid tissues, CD101−Tim-3+ CD8 T-cell subset differentiates into terminally differentiated CD101+Tim-3+ CD8 T cells and become resident.
We recently discovered that virus-specific CD8 T cells during chronic viral infection exhibited minimal migration using a parabiosis experiment (56). The stem cell-like CD8 T-cell subset was much more stationary compared to terminally differentiated cells. Of interest, circulating virus-specific CD8 cells during chronic viral infection were recently-generated cells having the CD101−Tim-3+ phenotype (56), the transitory subset that was previously descried (43), and expressed CX3CR1, which involves in the migration to the inflamed tissues (57,58). Overall, these results suggest that the early step differentiation from stem cell-like CD8 T cells to terminally differentiated CD8 T cells usually occurs in the lymphoid organs such as spleen, and then a fraction of recently-generated transitory cells go into the circulation, move to non-lymphoid organs, and further differentiate into CD101+Tim-3+ terminally differentiated cells as illustrated in Fig. 2.
TRANSCRIPTIONAL PROGRAMMING FOR CD8 T-CELL SUBSETS DURING CHRONIC VIRAL INFECTION
TCF1-Bcl-6-Id2-E2A
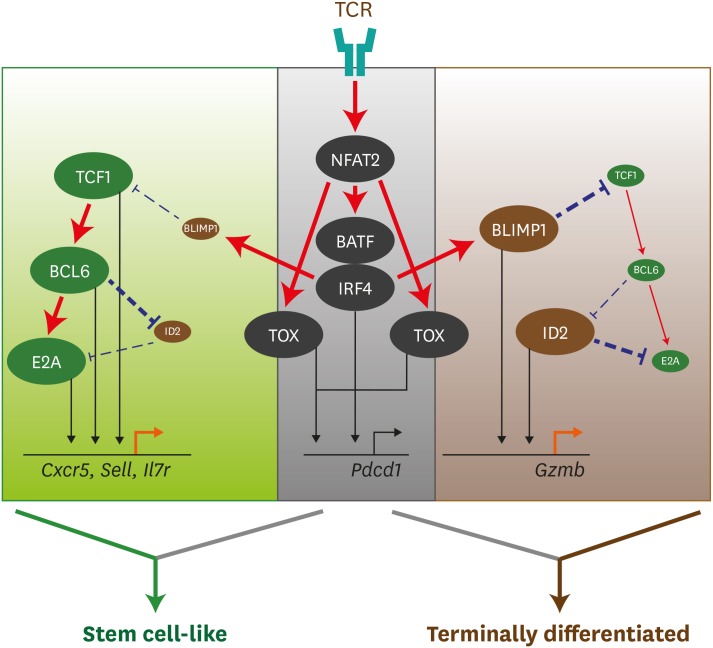
The transcription factors that regulate the differentiation of the stem cell-like CD8 T cells were shared with memory CD8 T cells, CD4 follicular helper T-cell (TFH) T cells, and hematopoietic stem cells (HSCs) (33,34,35,36). For example, TCF1 plays a role in the generation of memory CD8 T cells (59,60,61,62) and CD4 TFH cells (63,64), and also in the maintenance of an undifferentiated state of HSCs (65). Consistently, we and others observed that the loss of TCF1 resulted in a striking defect in the generation of the stem cell-like CD8 T cells during chronic LCMV infection (33,35,36) (Fig. 3). However, the initial expansion of the terminally differentiated cells was normal in the absence of TCF1, suggesting that the differentiation programs are different between the stem cell-like CD8 T cells and the terminally differentiated cells during chronic viral infection. In contrast, overexpression of TCF1 contributed to the increased frequency of the stem cell-like CD8 T cells and the improved LCMV-specific CD8 T-cell responses at a later time point, accompanied with enhanced Bcl-6 expression (35,36). By chromatin immunoprecipitation (ChIP) sequencing, it was revealed that TCF1 preferentially bound to a promoter region of Bcl-6 and Cish, an intron of Prdm1 (Blimp1), and an upstream region of Havcr2 (Tim-3) (66). These results suggest that TCF1 is indispensable for the differentiation into the stem cell-like CD8 T cells by directly regulating genes related to CD8 T-cell exhaustion during chronic viral infection. Bcl-6 has an intrinsic role in the generation of central memory CD8 T cells (67,68). In addition, it is well established that Bcl-6 is a central regulator for CD4 TFH differentiation (69). Similarly, when virus-specific CD8 T cells overexpressed Bcl-6, more stem cell-like CD8 T cells were generated after chronic infection (36), suggesting that Bcl-6 imposes the differentiation into the stem cell-like CD8 T cells. Meanwhile, Id2 plays a role in the differentiation of terminal effector CD8 T cells during acute viral infection (70). Id2-E2A axis has been also known to regulate the differentiation of Ag-specific CD4 T cells. Id2 represses CD4 TFH differentiation by inhibiting the expression of E2A (71,72). Consistently, the loss of Id2 led to an aberrant differentiation skewed into the stem cell-like CD8 T cells during chronic LCMV infection (34). Different from Id2, E2A promoted CXCR5 expression in exhausted CD8 T cells by binding to its intron region as assessed by ChIP assay. Overexpression of E2A in LCMV-specific CD8 T cells increased their differentiation into the stem cell-like CD8 T cells, but this effect was compromised when Id2 was co-overexpressed. Combining with the result that Bcl-6 bound to Id2 locus (71), it is likely that the sequential event via “TCF1 - Bcl-6 - Id2 - E2A” transcription factors axis regulates the differentiation of the stem cell-like CD8 T cells during chronic viral infection.
Figure 3. Transcriptional network to regulate differentiation of CD8 T cells during chronic viral infection. TCR stimulation by persistent Ags initiates a set of transcription factors, including NFAT2 followed by BATF and IRF4. TOX is also activated by NFAT2 via separate route. TCR-driven factors generally contribute to PD-1 upregulation in both stem cell-like and terminally differentiated CD8 T cells undergoing exhaustion process. Although IRF4 together with BATF directly represses TCF1 expression and upregulates BLIMP1, highly upregulated TCF1 in stem cell-like subset increases BCL6 expression, which also accelerates E2A expression. This TCF1-BCL6-E2A axis altogether or independently induces the genes related to stem cell-like CD8 T cell signature, such as Cxcr5, Sell, and Il7r. In the other hand, terminally differentiated CD8 T cells shows a typical feature with high level of BLIMP1 and low level of TCF1. Repressed level of TCF1 are not enough to upregulate BCL6, which allows the induction of ID2 and subsequent suppression of E2A. Both BLIMP1 and ID2 contribute to the enhancement of Gzmb expression, leading to the differentiation of terminally differentiated CD8 T cells. Straight red lines with arrow symbol indicate stimulation or activation. Dotted blue lines with block symbol indicate inhibition or suppression. Thickness of lines defines a strength of signal. Straight black lines with arrow symbol and orange lines with arrow symbol exhibit a binding to upstream of the corresponding genes and their transcription, respectively.
TOX
Despite the shared transcriptional programming between stem cell-like and memory CD8 T cells, their epigenetic profiles are significantly distinct (73,74,75,76), suggesting the unique transcriptional regulation during persistent antigenic stimulation. It has been recently highlighted that TOX, the thymocyte selection-associated high mobility group box, is a transcription factor that plays a major role in the generation of stem cell-like CD8 T-cell subset, the maintenance of the pool of virus-specific CD8 T cells, and the T-cell dysfunction during chronic viral infection (Fig. 3) (77,78,79,80). TOX is marginally increased at the peak response after acute infection and is dispensable for the differentiation of effector and memory CD8 T cells. In contrast, TOX is highly expressed in exhausted CD8 T cells compared to effector and memory CD8 T cells and, of note, stem cell-like CD8 T-cell subset exhibited higher TOX expression than terminally differentiated CD8 T cells. TOX expression is regulated by Ag-induced Ca2+ signaling and the transcription factor NFAT2 because the calcineurin inhibitors FK506 or cyclosporin A inhibited TOX expression. Absence of TOX expression resulted in the defect of the generation of stem cell-like CD8 T-cell subset. In addition, TOX deficiency led to the production of KLRG1+ functional virus-specific CD8 T cells corresponding to the polyfunctional cytokine production and the decreased expression of inhibitory receptors. However, the generation of highly functional Ag-specific CD8 T cells might be detrimental in terms of immunopathology as Tox-deficient mice notably experienced the weight loss and tissue damage (77). Therefore, TOX might make a balance in the maintenance of Ag-specific CD8 T cells and the protection from excessive immunopathology. However, deletion of Tox around 3 weeks post chronic viral infection has minimal effect on the regulation of exhaustion-related genes (77), suggesting that TOX downstream effects might be restricted in early time course of chronic viral infection.
IRF4
IRF4 is another transcription factor instantly induced by TCR signaling (81,82). Similar to TOX, IRF4 also contributed to the exhausted features of CD8 T cells such as upregulation of inhibitory receptors, impaired cytokine production, and the repression of anabolic metabolism (Fig. 3) (83). However, in contrast to TOX, IRF4 inhibited the development of stem cell-like CD8 T-cell subset during chronic viral infection. Together with BATF and NFAT, IRF4 suppress TCF1 expression by directly binding to Tcf7 loci. These results suggest that IRF4 and TOX differently regulate the development of stem cell-like CD8 T cells despite they are under downstream of NFAT and displayed similar role in T-cell dysfunction. Therefore, further investigations are needed to verify the circuit of transcription factors for the differentiation of CD8 T-cell subsets during persistent antigenic stimulation.
ACTION MECHANISM OF PD-1 BLOCKADE DURING CHRONIC VIRAL INFECTION
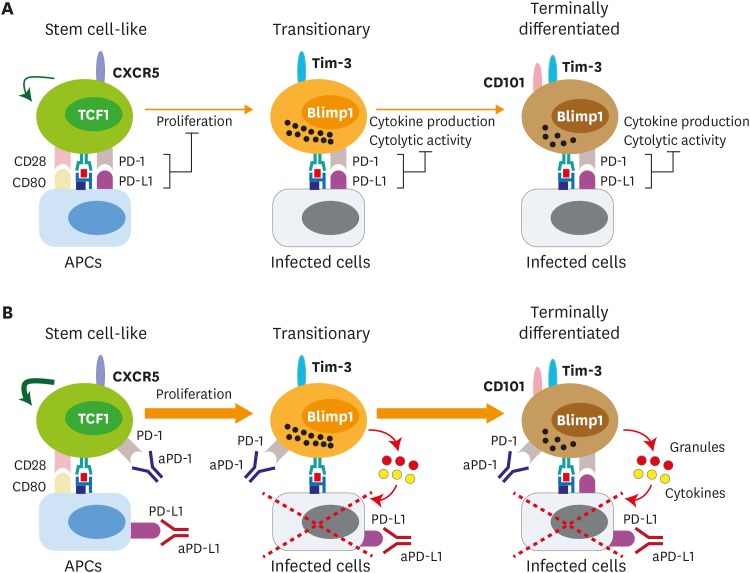
Heterogeneity of exhausted CD8 T cells raises a possibility that different subsets of exhausted CD8 T cells would respond differently to PD-1 blockade. Blackburn et al. demonstrated that PD-1intCD44hi exhausted CD8 T-cell subset responded better to PD-1 blockade than PD-1hiCD44int exhausted CD8 T-cell subset during chronic LCMV infection (84). Three independent studies confirmed that only the stem cell-like CD8 T cells possessing PD-1intCD44hi phenotype proliferated and differentiated into terminally differentiated CD8 T cells after PD-1 blockade, whereas the terminally differentiated cells having PD-1hiCD44int phenotype did not (Fig. 4) (33,34,35). Therefore, PD-1 blockade led to increased proportion of terminally differentiated CD8 T cells among Ag-specific CD8 T cells and this is consistent with the stable transcriptional and epigenetic profiles of Ag-specific CD8 T cells after PD-1-directed immunotherapy when they were analyzed as a bulk population (74). Despite differentiation of the stem cell-like CD8 T cells into the more differentiated cells, the absolute number of the stem cell-like CD8 T cells was also marginally increased after PD-1 blockade, indicating that PD-1 blockade increased the self-renewal of the stem cell-like CD8 T cells as well.
Figure 4. Proposed mechanism of PD-1-directed immunotherapy during chronic viral infection and cancer. (A) After the establishment of T-cell exhaustion, PD-1/PD-L1 axis plays a role in 2 different ways. First, PD-1/PD-L1 interaction inhibits the proliferation and differentiation of stem cell-like CD8 T cells into the more differentiated CD8 T-cell subsets. CD28 is a major target for PD-1-mediated suppression. Next, PD-L1 on infected cells or tumor cells impairs the cytolytic function and cytokine production of PD-1+ Ag-specific differentiated CD8 T-cell subsets. (B) In the absence of PD-1-mediagted signals, the proliferation and differentiation of stem cell-like CD8 T cells into transitory and terminally differentiated CD8 T cells are accelerated and this happens in a CD28/B7-dependent manner. The increased population of transitory subset showing the highest anti-viral/tumor activity is critical for the viral clearance and tumor regression. In terms of the quality, the cytolytic function and cytokine production of transitory and terminally differentiated CD8 T-cell subsets might be increased after PD-1 blockade.
Two recent observations revealed the requirement of CD28 for the proliferation of exhausted CD8 T cells after PD-1 blockade. Hui et al. (85) showed that CD28 is the primary target for PD-1-mediated suppression of CD8 T-cell function using a cell-free membrane reconstitution system. During PD-1/PD-L1 ligation, the PD-1/SHP-2 complex favorably mediates dephosphorylation of CD28 over the T-cell receptor, leading to the inhibition of CD8 T-cell activity. Using in vivo mouse models, Kamphorst et al. (86) demonstrated that genetic loss of CD28 or blockade of B7 molecules abrogated the proliferation of CD8 T cells during chronic viral infection and tumor suppression driven by PD-1 blockade. In addition, the CD8 T cells that underwent proliferation in patients with advanced NSCLC after PD-1 blockade were largely PD-1+CD28+. Considering that B7 molecules are mainly expressed on professional APCs, not on infected cells or tumor cells, and that only stem cell-like CD8 T cells could proliferate after PD-1 blockade, these results supported the idea again that the stem cell-like CD8 T cells are interacting with PD-L1- and B7 molecules-expressing APCs in the T-cell zone (Fig. 3).
As discussed, the terminally differentiated CD8 T cells did not proliferate in response to PD-1 blockade. However, when PD-L1 is expressed on infected non-hematopoietic cells, viral clearance and immunopathology during chronic LCMV infection is suppressed (55). This suggests that the PD-1/PD-L1 interaction still plays a role inhibiting CTL activity of the terminally differentiated cells in the infected tissues (Fig. 4). Moreover, it has been demonstrated that the PD-1/PD-L1 interaction inhibits CD8 T-cell motility by forming a stable and mature immunological synapse in chronically infected mice (87). Taken together, PD-1 blockade manipulates the T-cell response in 2 different ways: 1) PD-1 blockade induces the proliferation and differentiation of the stem cell-like CD8 T cells into the terminally differentiated cells in the CD28-dependent manner. 2) Newly generated terminally differentiated cells actively migrate into the infected tissues and kill the infected cells with improved CTL activity in the absence of PD-1/PD-L1 ligation (Fig. 3).
PRESENCE OF STEM CELL-LIKE T CELLS IN CANCER AND AUTOIMMUNE DISEASE MODELS HAVING PERSISTENT ANTIGENIC STIMULATION
One of the interesting question after the discovery of stem cell-like T cells in the model of chronic viral infection was whether the stem cell-like T-cell subset would exist in other models having continuous antigenic stimulation such as cancer and autoimmunity. First of all, the presence of TCF1+PD-1+ stem cell-like CD8 tumor-infiltrating lymphocytes (TILs) were also revealed in various mouse and human cancers (36,88,89,90,91,92). stem cell-like and terminally differentiated CD8 TILs exhibited similar transcriptional and epigenetic profiles to the corresponding subsets in the spleen of chronically infected mice. Similar to mouse chronic LCMV model, stem cell-like CD8 TILs reproduce themselves by self-renewal and produce terminally differentiated CD8 TILs within tumors. In addition, only stem cell-like CD8 TILs exhibited the proliferative burst upon PD-1 blockade. Parallel with these observations, the frequency of stem cell-like TILs was associated with a better clinical outcome (88,89,90,91,92). It is worth noting that stem cell-like TIL subset was localized in the area having dense APCs as niches in kidney, prostate, and bladder tumors (92). However, the tumor-specificity of TILs were uncertain in human tumors. In an independent study, the stem cell-like TILs were observed in both tertiary lymphoid structures and their outside in melanomas and lung cancers (89). Therefore, in-depth analysis regarding the specificity of stem cell-like TIL subset and its location in tumors is required.
Yost et al. (93) also recently presented that clones showing high TCF7+ signature expanded more significantly than those having low TCF7+ signature in advanced basal cell carcinoma. Of interest, however, among clones with exhausted features, around two-thirds of clones expanded following PD-1-directed immunotherapy were derived from novel clonotypes not from pre-existing TILs. This result suggests several possibilities as follows. First, these novel clones might derive from secondary lymphoid organs as stem cell-like CD8 T cells mainly reside in the spleen of chronically LCMV-infected mice. Second, PD-1 blockade lessen the threshold of the activation of minor clones, leading to new activation of naïve T cells in tumor sites. Finally, these novel clones might be not tumor-specific but autoantigen-specific since some patients have exhibited autoimmune disease as a side effect of immune checkpoint blockades. Although this observation is very interesting in terms of the clonality after the immunotherapy, further investigations are required about the source of these novel clones and their clinical relevance in addition to their Ag specificity.
In addition to the importance of TCF1 in the generation of stem cell-like CD8 TILs, TOX is also essential for the maintenance of tumor-specific CD8 T cells and T-cell dysfunction in cancer (80,94,95,96). Recent report showed that partially Tox-deficient tumor-specific CD8 T cells delayed tumor growth compared to wild type cells in mouse tumor model (78). In addition, knockdown of Tox in human CD8 TILs led to an increased production of effector cytokines and an enhanced cytotoxicity in vitro (97). In the model of adoptive transfer of T cells expressing chimeric Ag receptors (CAR T cells), CAR T cells deficient in both Tox and Tox2 were highly effective in tumor control and led to prolonged survival of tumor-bearing mice, corresponding to increased cytokine production and decreased expression of inhibitory receptors (94). Furthermore, NR4A was recently reported to cooperate with TOX and play a regulatory role in the efficacy of CAR T cells similar to TOX (96,98). It would be worthwhile to note that initially Tox-deleted CD8 T cells fail to persist in tumors, suggesting the role of TOX in preventing T cell overstimulation and activation-induced cell death (AICD) (94).
During autoimmune diseases, CD4 T-cell subset with progenitor potential was also observed. Shin et al. (99) found that IFNγ-TCF1+ CD4 T cells exhibited a similar gene signature to counterpart subsets in the spleen of chronically infected mice such as high expression of Tcf7, Id3, Cxcr5, and Kit and the absence of Prdm1, Id2, Prf1, and Gzmb expression. This stem cell-like subset had the potential to elicit pathogenic CD4 T cells and confer colitis in mice. Of interest, the glycosyltransferase ST6Gal-I was required for the generation of stem cell-like T-cell subset in this setting. Stem cell-like T helper17 CD4 T cells (Th17) was also determined in mice with experimental autoimmune encephalomyelitis (EAE) (100). The stem cell-like Th17 cells highly expressed CD27 and could differentiate into CD27-Tbethi subpopulation, inferring stemness features. Taken together, these results suggest that common core programs exist in the T-cell differentiation program in the environment with persistent antigenic stimulation.
REMAINING QUESTIONS AND FUTURE DIRECTIONS
One of important remaining questions is how the stem cell-like CD8 T cells are maintained during chronic viral infection. He et al. (34) demonstrated that the stem cell-like CD8 T cells were derived from the thymus. However, considering their slow self-renewal in the spleen, other mechanisms could still contribute to their maintenance. We confirmed in our unpublished data that the population of stem cell-like CD8 T-cell subset and the pool of virus-specific CD8 T cells were sustained during the course of chronic LCMV infection in thymectomized mice, supporting this postulation. We have considered several potential mechanisms for the maintenance of the stem cell-like CD8 T cells during chronic viral infection: 1) Ag-independent survival and homeostatic cytokines-driven proliferation. A decade ago, a few reports have previously argued that exhausted CD8 T cells could not survive in an Ag-free condition and did not respond to homeostatic cytokines such as IL-7 and IL-15 (39,40). However, when exhausted CD8 T cells isolated from chronically infected mice were transferred into naive mice, a quarter of the transferred cells was still detected in the blood and tissues around a month after the transfer (39). In addition, 10% to 15% of the exhausted CD8 T cells exhibited IL-7- or IL-15-driven proliferation both in vitro and in vivo. Furthermore, Utzschneider et al. (41) also demonstrated that a fraction of exhausted CD8 T cells could respond to the rechallenge with LCMV virus after long-term Ag withdrawal. Considering memory-like features of the stem cell-like CD8 T cells, these results suggest that the stem cell-like CD8 T cells could be maintained by an Ag-independent, but homeostatic cytokine-dependent manner during chronic viral infection. 2) IL-21-dependent maintenance. Although an exact mechanism is not revealed yet, several reports have verified that IL-21 is crucial for the maintenance of virus-specific CD8 T cells and the control of virus infection during chronic LCMV infection (101,102,103). It is of interest to investigate whether IL-21 contributes to the maintenance of the stem cell-like CD8 T cells or to their conversion to the terminally differentiated cells during chronic viral infection. 3) Interaction with professional APCs which act as niches. As previously discussed, it is expected that the stem cell-like CD8 T cells might continuously interact with professional APCs in the T-cell area of lymphoid tissues, that is, the professional APCs might act as niches for the maintenance of stemness of the stem cell-like CD8 T cells during chronic viral infection. It has been revealed that the stem cell-like CD8 T-cell usually resided in APC dense regions which might act as niches in human tumors (92). Therefore, it is of interest which subset of DCs act as niches and which cellular ligands and soluble factors are involved in this interaction between APCs and the stem cell-like CD8 T cells. These examinations could promote a significant improvement in cancer therapies. Although PD-1 blockade increased the number of the stem cell-like CD8 T cells, this therapy is specialized in the expansion of the more differentiated cells derived from the stem cell-like CD8 T cells. If the new regimen is developed for increasing the self-renewal of the stem cell-like CD8 T cells, there might result in remarkable synergistic effect when combined with PD-1 therapy. Moreover, these investigations could advance adoptive cell therapy. In adoptive cell therapy, more differentiated effector CD8 T cells exhibited less in vivo therapeutic efficacy despite enhanced in vitro anti-tumor activity compared to naive T cells (104,105,106,107,108). This result might be due to the short-lived fate of the terminally differentiated cells and a defect in the generation of the stem cell-like CD8 T cells. If the stem cell-like CD8 T cells isolated from cancer patients could be numerically expanded ex vivo in a quiescent status and transferred back into the patients, long-term CD8 T-cell immunity and improved therapeutic efficacy would be expected.
Combining with the current PD-1 blockade, various regimens have been being examined such as immune checkpoint blockades, cytokines, the depletion of Tregs, therapeutic vaccination, and adoptive cell therapy against chronic viral infection and cancer (20,109,110,111,112,113,114,115,116,117,118). It is of interest to examine whether only the stem cell-like CD8 T cells could still respond to these combined therapies or the terminally differentiated cells could expand by novel therapeutics. For example, we previously presented that Tim-3 blockade synergistically enhanced virus-specific CD8 T-cell immunity and the control of viral infection combined with PD-1 blockade during chronic LCMV infection despite that Tim-3 blockade alone was not effective (109). Considering that the stem cell-like CD8 T cells do not express Tim-3, it is intriguing whether the CD101+Tim-3+ terminally differentiated cells proliferate in response to the co-blockade or CD101−Tim-3+ transitory CD8 T cells newly generated from the stem cell-like CD8 T cells expand when Tim-3 is up-regulated, but simultaneously blocked.
Finally, in-depth analysis is required regarding the function of exhausted CD8 T cells during disease progress. Recent reports have discovered that the stem cell-like CD8 T cells could robustly proliferate in vivo after antigenic restimulation regardless of the time points when they were isolated (33,35). However, Angelosanto et al. (119) observed that exhausted CD8 T cells isolated early (day 8) from chronically infected mice could differentiate into classic memory CD8 T cells after transfer into Ag-free mice whereas exhausted CD8 T cells isolated later (day 30) could not. In this study, it is more likely that the stem cell-like CD8 T-cell subset could differentiate into memory CD8 T cells in Ag-free environment considering the memory-like features of the stem cell-like CD8 T cells. In HIV model, there is a negative correlation between the severity of disease and frequency of circulating CD8 memory stem T cells cells (120). Taken together, the stem cell-like CD8 T cells might maintain their proliferative potential but lose multipotent potential as the disease is progressed. Most PD-1-directed therapies have been being used as a second-line setting when the first-line therapy such as chemotherapy is failed. If time-dependent exhaustion occurs in the stem cell-like CD8 T cells during chronic viral infection and cancers, earlier treatment of PD-1 blockade should be considered for the improved clinical outcomes.
CONCLUDING REMARK
Although PD-1 blockade has provided a breakthrough in cancer treatment, there is still room for improvement. To achieve this, a better understanding of T-cell exhaustion and a further investigation for the working mechanism of PD-1 blockade are required. Recent studies have revealed the stem cell-like CD8 T-cell subset which maintains CD8 T-cell immunity during chronic LCMV infection and cancer and preferentially proliferates after PD-1 blockade. Because many features of T-cell exhaustion are shared between chronic viral infections and cancer, these discoveries possess a noteworthy clinical relevance for cancers as well. The discovery of this new stem cell-like CD8 T-cell subset will provide new insights for the development of new improved therapeutics in the combined therapies with PD-1 blockade and the adoptive cell therapy for cancers.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2017R1A5A1014560, 2018R1A2A1A05076997, 2018M3A9H3024850, 2019M3A9B6065221).
Abbreviations
- AICD
activation-induced cell death
- APC
Ag presenting cell
- CAR
chimeric Ag receptor
- ChIP
chromatin immunoprecipitation
- DC
dendritic cell
- HSC
hematopoietic stem cell
- LCMV
lymphocytic choriomeningitis virus
- NSCLC
non-small-cell lung cancer
- TFH
follicular helper T-cell
- TIL
tumor-infiltrating lymphocyte
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Im SJ, Ha SJ.
- Funding acquisition: Ha SJ.
- Supervision: Ha SJ.
- Writing - original draft: Im SJ.
- Writing - review & editing: Im SJ, Ha SJ.
References
- 1.Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, Araki K, Ahmed R. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. 2018;69:301–318. doi: 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 3.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 4.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goepfert PA, Bansal A, Edwards BH, Ritter GD, Jr, Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- 9.Kostense S, Ogg GS, Manting EH, Gillespie G, Joling J, Vandenberghe K, Veenhof EZ, van Baarle D, Jurriaans S, Klein MR, et al. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31:677–686. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumprecht S, Schürch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 14.Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hämmerling GJ, et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389–395. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, Elliott S, Gill D, Marlton P, Seymour J, Khanna R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108:2280–2289. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 22.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 24.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 25.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 32.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. Follicular CXCR5- expressing CD8+ T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 35.Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol. 2016;1:eaai8593. doi: 10.1126/sciimmunol.aai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kallies A, Zehn D, Utzschneider DT. Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol. 2020;20:128–136. doi: 10.1038/s41577-019-0223-7. [DOI] [PubMed] [Google Scholar]
- 39.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utzschneider DT, Legat A, Fuertes Marraco SA, Carrié L, Luescher I, Speiser DE, Zehn D. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol. 2013;14:603–610. doi: 10.1038/ni.2606. [DOI] [PubMed] [Google Scholar]
- 42.Speiser DE, Utzschneider DT, Oberle SG, Münz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol. 2014;14:768–774. doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- 43.Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, Lin JX, Konieczny BT, Im SJ, Freeman GJ, et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity. 2019;51:1043–1058.e4. doi: 10.1016/j.immuni.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calabro S, Liu D, Gallman A, Nascimento MS, Yu Z, Zhang TT, Chen P, Zhang B, Xu L, Gowthaman U, et al. Differential intrasplenic migration of dendritic cell subsets tailors adaptive immunity. Cell Reports. 2016;16:2472–2485. doi: 10.1016/j.celrep.2016.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, Fukuda Y, Yano T, Nobuoka M, Hirashima T, et al. Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J Immunol. 2013;190:6071–6082. doi: 10.4049/jimmunol.1202798. [DOI] [PubMed] [Google Scholar]
- 48.Kretzer NM, Theisen DJ, Tussiwand R, Briseño CG, Grajales-Reyes GE, Wu X, Durai V, Albring J, Bagadia P, Murphy TL, et al. RAB43 facilitates cross-presentation of cell-associated antigens by CD8α+ dendritic cells. J Exp Med. 2016;213:2871–2883. doi: 10.1084/jem.20160597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greyer M, Whitney PG, Stock AT, Davey GM, Tebartz C, Bachem A, Mintern JD, Strugnell RA, Turner SJ, Gebhardt T, et al. T cell help amplifies innate signals in CD8+ DCs for optimal CD8+ T cell priming. Cell Reports. 2016;14:586–597. doi: 10.1016/j.celrep.2015.12.058. [DOI] [PubMed] [Google Scholar]
- 50.Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Güttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 51.Kroczek RA, Henn V. The role of XCR1 and its ligand XCL1 in antigen cross-presentation by murine and human dendritic cells. Front Immunol. 2012;3:14. doi: 10.3389/fimmu.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, Brown BD, Schmolke M, Miller JC, Leboeuf M, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Im SJ, Konieczny BT, Hudson WH, Masopust D, Ahmed R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc Natl Acad Sci U S A. 2020:201917298. doi: 10.1073/pnas.1917298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamon P, Loyher PL, Baudesson de Chanville C, Licata F, Combadière C, Boissonnas A. CX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in mice. Blood. 2017;129:1296–1307. doi: 10.1182/blood-2016-08-732164. [DOI] [PubMed] [Google Scholar]
- 58.Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, et al. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med. 2010;16:1305–1312. doi: 10.1038/nm.2253. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Xue HH. Cutting edge: generation of memory precursors and functional memory CD8+ T cells depends on T cell factor-1 and lymphoid enhancer-binding factor-1. J Immunol. 2012;189:2722–2726. doi: 10.4049/jimmunol.1201150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gullicksrud JA, Li F, Xing S, Zeng Z, Peng W, Badovinac VP, Harty JT, Xue HH. Differential requirements for Tcf1 long isoforms in CD8+ and CD4+ T cell responses to acute viral infection. J Immunol. 2017;199:911–919. doi: 10.4049/jimmunol.1700595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeannet G, Boudousquié C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue HH, Crotty S. LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol. 2015;16:980–990. doi: 10.1038/ni.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, He R, Hao Y, Wang H, Zhao T, et al. The transcription factor TCF-1 initiates the differentiation of TFH cells during acute viral infection. Nat Immunol. 2015;16:991–999. doi: 10.1038/ni.3229. [DOI] [PubMed] [Google Scholar]
- 65.Wu JQ, Seay M, Schulz VP, Hariharan M, Tuck D, Lian J, Du J, Shi M, Ye Z, Gerstein M, et al. Tcf7 is an important regulator of the switch of self-renewal and differentiation in a multipotential hematopoietic cell line. PLoS Genet. 2012;8:e1002565. doi: 10.1371/journal.pgen.1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 67.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 68.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 69.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D'Cruz LM, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaw LA, Bélanger S, Omilusik KD, Cho S, Scott-Browne JP, Nance JP, Goulding J, Lasorella A, Lu LF, Crotty S, et al. Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nat Immunol. 2016;17:834–843. doi: 10.1038/ni.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyazaki M, Rivera RR, Miyazaki K, Lin YC, Agata Y, Murre C. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat Immunol. 2011;12:992–1001. doi: 10.1038/ni.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jadhav RR, Im SJ, Hu B, Hashimoto M, Li P, Lin JX, Leonard WJ, Greenleaf WJ, Ahmed R, Goronzy JJ. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc Natl Acad Sci U S A. 2019;116:14113–14118. doi: 10.1073/pnas.1903520116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell. 2017;170:142–157.e19. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–269. doi: 10.1038/s41586-019-1326-9. [DOI] [PubMed] [Google Scholar]
- 78.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao C, Sun HW, Lacey NE, Ji Y, Moseman EA, Shih HY, Heuston EF, Kirby M, Anderson S, Cheng J, et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat Immunol. 2019;20:890–901. doi: 10.1038/s41590-019-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mann TH, Kaech SM. Tick-TOX, it's time for T cell exhaustion. Nat Immunol. 2019;20:1092–1094. doi: 10.1038/s41590-019-0478-y. [DOI] [PubMed] [Google Scholar]
- 81.Nayar R, Enos M, Prince A, Shin H, Hemmers S, Jiang JK, Klein U, Thomas CJ, Berg LJ. TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation. Proc Natl Acad Sci U S A. 2012;109:E2794–E2802. doi: 10.1073/pnas.1205742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nayar R, Schutten E, Bautista B, Daniels K, Prince AL, Enos M, Brehm MA, Swain SL, Welsh RM, Berg LJ. Graded levels of IRF4 regulate CD8+ T cell differentiation and expansion, but not attrition, in response to acute virus infection. J Immunol. 2014;192:5881–5893. doi: 10.4049/jimmunol.1303187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, Pellegrini M, Zehn D, Berberich-Siebelt F, Febbraio MA, et al. Transcription factor IRF4 promotes CD8+ T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity. 2017;47:1129–1141.e5. doi: 10.1016/j.immuni.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 84.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zinselmeyer BH, Heydari S, Sacristán C, Nayak D, Cammer M, Herz J, Cheng X, Davis SJ, Dustin ML, McGavern DB. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–774. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, et al. Intratumoral Tcf1+ PD-1+ CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50:195–211.e10. doi: 10.1016/j.immuni.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 90.Brummelman J, Mazza EM, Alvisi G, Colombo FS, Grilli A, Mikulak J, Mavilio D, Alloisio M, Ferrari F, Lopci E, et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J Exp Med. 2018;215:2520–2535. doi: 10.1084/jem.20180684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175:998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, Cardenas M, Wilkinson S, Lake R, Sowalsky AG, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576:465–470. doi: 10.1038/s41586-019-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. 2019;25:1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, He Q, Shen H, Xia A, Tian W, Yu W, Sun B. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol. 2019;71:731–741. doi: 10.1016/j.jhep.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 96.Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, López-Moyado IF, Georges RO, Zhang W, Onodera A, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc Natl Acad Sci U S A. 2019;116:12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee H, Kim TS, Choi SJ, Kim HD, Han JW, et al. VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol. 2019;4:eaay0555. doi: 10.1126/sciimmunol.aay0555. [DOI] [PubMed] [Google Scholar]
- 98.Chen J, López-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567:530–534. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin B, Kress RL, Kramer PA, Darley-Usmar VM, Bellis SL, Harrington LE. Effector CD4 T cells with progenitor potential mediate chronic intestinal inflammation. J Exp Med. 2018;215:1803–1812. doi: 10.1084/jem.20172335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karmaus PW, Chen X, Lim SA, Herrada AA, Nguyen TM, Xu B, Dhungana Y, Rankin S, Chen W, Rosencrance C, et al. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature. 2019;565:101–105. doi: 10.1038/s41586-018-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fröhlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 104.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O'Connor RS, Hwang WT, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klebanoff CA, Crompton JG, Leonardi AJ, Yamamoto TN, Chandran SS, Eil RL, Sukumar M, Vodnala SK, Hu J, Ji Y, et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2:e95103. doi: 10.1172/jci.insight.95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klebanoff CA, Scott CD, Leonardi AJ, Yamamoto TN, Cruz AC, Ouyang C, Ramaswamy M, Roychoudhuri R, Ji Y, Eil RL, et al. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest. 2016;126:318–334. doi: 10.1172/JCI81217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol Rev. 2014;257:264–276. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O'Mara L, Yang S, Konieczny BT, Sharpe AH, et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng W, Lizée G, Hwu P. Blockade of the PD-1 pathway enhances the efficacy of adoptive cell therapy against cancer. OncoImmunology. 2013;2:e22691. doi: 10.4161/onci.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 116.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ribeiro SP, Milush JM, Cunha-Neto E, Kallas EG, Kalil J, Somsouk M, Hunt PW, Deeks SG, Nixon DF, SenGupta D. The CD8+ memory stem T cell (TSCM) subset is associated with improved prognosis in chronic HIV-1 infection. J Virol. 2014;88:13836–13844. doi: 10.1128/JVI.01948-14. [DOI] [PMC free article] [PubMed] [Google Scholar]