Abstract
Cancer immunotherapy, in the form of vaccination, adoptive cellular transfer, or immune checkpoint inhibitors, has emerged as a promising practice within the field of oncology. However, despite the developing field's potential to revolutionize cancer treatment, the presence of immunotherapeutic-resistant tumor cells in many patients present a challenge and limitation to these immunotherapies. These cells not only indicate immunotherapeutic resistance, but also show multi-modal resistance to conventional therapies, abnormal metabolism, stemness, and metastasis. How can immunotherapeutic-resistant tumor cells render multi-malignant phenotypes? We reasoned that the immune-refractory phenotype could be associated with multi-malignant phenotypes and that these phenotypes are linked together by a factor that acts as the master regulator. In this review, we discussed the role of the embryonic transcription factor NANOG as a crucial master regulator we named “common factor” in multi-malignant phenotypes and presented strategies to overcome multi-malignancy in immunotherapeutic-resistant cancer by restraining the NANOG-mediated multi-malignant signaling axis. Strategies that blunt the NANOG axis could improve the clinical management of therapy-refractory cancer.
Keywords: Immunotherapy, Therapy-refractory cancer, NANOG, Common factor, Multi-malignant phenotypes
INTRODUCTION
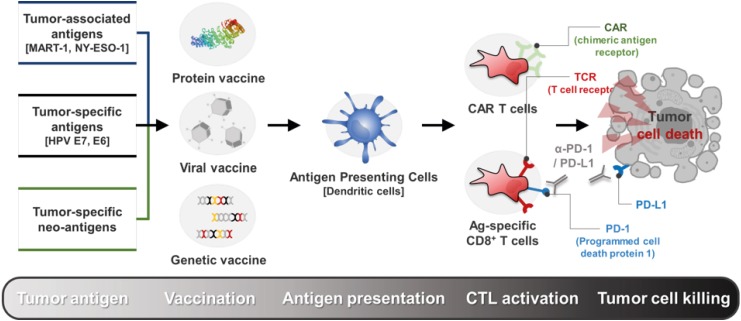
Over the past few decades, tremendous progress has been made in the field of cancer immunotherapy and its clinical efficacy. Key developments in understanding the immune system and its anti-cancer capacity have allowed for the rapid development and clinical implementation of cancer immunotherapy (1,2,3). Harnessing the immune system to recognize and control tumor growth, cancer immunotherapy induces the patient's immune system's ability to recognize and control tumor cells through tumor-specific Ags, tumor-associated Ags, and killer T cells (4,5,6,7). Immunotherapy can be materialized by vaccination or adoptive cell transfer (ACT), involving the transfer of CTLs that have been expanded to target tumor Ags. Recently, immune checkpoint blockade (ICB) therapy has taken the center stage in cancer treatment to address the ability of tumors to evade the immune system. After several conceptual breakthroughs in understanding the limitations of anti-tumor immune responses, ICB therapy normalizes defective anti-tumor immune responses by the use of monoclonal anti-bodies and aims to combat against cancer with immune-suppressive functions (8) (Fig. 1).
Figure 1. Emerging trends in the cancer immunotherapy. Cancer immunotherapy induces the patient's immune system's ability to recognize and kill tumor cells through tumor-specific Ags, tumor-associated Ags, and killer T cells. Immunotherapy can be materialized by vaccination or ACT, involving the transfer of CTLs that have been expanded to target tumor Ags. Recently, ICB therapy has taken the center stage in cancer treatment to address the ability of tumors to evade the immune system.
MART1, melanoma Ag recognized by T cells 1; NY-ESO-1, cancer/testis Ag 1; HPV, human papillomavirus; CAR, chimeric Ag receptor.
Cancer immunotherapy elicits a marked clinical response in patients with various tumor types and changes the paradigm for cancer treatment. Although the developing field's potential to revolutionize cancer treatment, it also has critical limitations. For example, even though anti-PD-1 therapy has demonstrated remarkable results in tumor progression and long-term survival rates, a large of proportion of patients are unresponsive to the therapy, and of those who do respond, a fraction of them experienced relapse after treatment. Previous studies have reported a considerable population of patients that exhibit both innate and acquired resistance, presenting a big hurdle to overcome in order to achieve effective treatment (9,10,11).
The dynamics of these immunotherapeutic-resistant tumor cells have been the focal point of various studies aimed at gaining a novel insight to advance the field of immunotherapy. Interestingly, these cells not only exhibit immunotherapeutic-resistance (12,13,14,15,16), but also express multi-malignant phenotypes, which includes resistance to conventional treatments, such as chemo- and radiation-therapy (17,18,19,20), stemness (12,13), metastasis (21,22), and abnormal metabolism (15,18). So how can these immunotherapeutic-resistant tumor cells also show multi-malignant phenotypes? We reasoned on the possibility that the immunotherapeutic resistance could also be associated with the multi-malignant phenotypes as well, and that the various refractory phenotypes are linked together by a factor that acts as a master regulator. We believe that finding the factor, what we termed “a common factor”, could provide a novel perspective on immunotherapeutic-resistant cells and help derive strategies to overcome the multi-malignant phenotypes these tumor cells express.
THE CAUSE OF MULTI-MALIGNANCY PRESENTED IN IMMUNOTHERAPEUTIC-RESISTANT TUMOR CELLS: CANCER IMMUNOEDITING
To understand the multi-malignant phenotypes presented in immunotherapeutic-resistant tumor cells, we must first understand how they are propagated in the first place. For gaining better insights into the limitations of immunotherapy against the occurrence of multiple malignancies, conceptual developments have been made to describe how the immune system can paradoxically constrain and promote tumor progression and development. This complex process, often referred to as cancer immunoediting, proceeds through 3 phases of elimination, equilibrium, and escape (23). During the elimination phase, components of the immune systems cooperate to recognize and kill transformed cells that have escaped cell-intrinsic tumor suppression mechanisms. Rare tumor subclones capable of surviving elimination can progress to the equilibrium phase, in which the net tumor growth is limited and, over time, can be stalled (24). However, the constant or immune selective pressure from the host immune system can select for tumor subclones with enhanced immunotherapeutic resistance, as well as multi-malignant phenotypes. Although this process is known to occur during the natural tumor progression in the host, the results of various studies have indicated that immunoediting also occurs in response to immunotherapies as well (12,13,17,24). This means that the application of immunotherapy acts as an immune selective pressure to promote rare tumor subclones with multi-malignant phenotypes, which notably include immunotherapeutic resistance.
MULTI-MALIGNANT PHENOTYPES: THE COMMON FEATURES BETWEEN IMMUNOTHERAPEUTIC-RESISTANT AND STEM-LIKE TUMOR CELLS
Because it is not yet known how these immunotherapeutic-resistant tumor cells subclones also have the ability to demonstrate other multi-malignant phenotypes, we searched for working models that would allow us to investigate the mechanisms of the multi-malignant phenotypes of immunotherapeutic-resistant cells. This led us to the studies of a specific subclone in cancer, termed stem-like tumor cells (13,25,26,27).
With a high capacity to survive, self-renew, and advance to malignancy, these cells represent a huge obstacle to overcome in the fight against cancer. The persistence of stem-like tumor cells is thought to be primarily responsible for tumor progression and relapse after therapy (28). Interestingly, the emergence of a stem-like properties, as well as multi-modal resistance, metastasis, and abnormal metabolism, has been seen in stem-like tumor cells. Although the dynamics of stem-like tumor cell maintenance and propagation remain unknown, both stem-like tumor cells and immunotherapeutic-resistant tumor cells show similar phenotypes, particularly regarding multiple malignancy, as described in this review (Fig. 2).
Figure 2. Concept of common factor which regulates multi-malignant phenotypes. Phenotypes common to stem-like tumor cells and immunotherapeutic-resistant tumor cells provide a first clue to identify a common factor that confers the multi-malignant phenotypes.
Attempting to better understand the mechanisms behind these phenotypes, we have developed a system to demonstrate how immune selection can act as the perpetuating force that drives tumor cells towards multi-malignant phenotypes. By using a vaccination or ACT regime, which induces CTLs-mediated immune selective pressure, we were able to simulate the evolution of cancer cells in live hosts. We applied in vivo immune selection for 3 rounds, leaving us with a line of immunotherapeutic-resistant tumor cells (termed as P3) (13). Interestingly, these P3 cells exhibited the multi-modal therapeutic resistance, metastasis, and abnormal metabolism. In addition, these cells had stem-like properties enabling them to form spheres in vitro and tumors in vivo when transplanted into NOD/SCID mice unlike parental tumor cells (P0). Notably, the P3 population was enriched in cells expressing a panel of ‘stemness’ markers, such as epithelial cell adhesion molecule, CD166, and CD44 (13). Phenotypes common to stem-like tumor cells and immunotherapeutic-resistant tumor cells provide a first clue to identify a common factor that confers the multi-malignant phenotypes.
IDENTIFICATION OF THE MULTI-MALIGNANT “COMMON FACTOR”: NANOG
Numerous studies have indicated that stem-like tumor cells express embryonic transcription factors, such as c-MYC, Kruppel-like factor 4, NANOG, octamer-binding transcription factor 4, or SRY (sex determining region Y)-box 2, that exist only in embryonic stem cells (29). Interestingly, these transcription factors have been reported to be associated with multiple malignancies, including multi-modal resistance, stem-like properties, metastasis and abnormal metabolism (13,17). Therefore, we hypothesized that certain embryonic transcription factors may confer a survival advantage to tumor cells against immunotherapy and promote multi-malignant phenotypes in immunotherapeutic-resistant tumor cells. By examining the molecular basis of the ‘stemness’ of immunotherapeutic-resistant tumor (P3) cells, we assessed the expression of a panel of proteins known to be important for the pluripotency of stem cells. Among the factors, we found that the NANOG expression was increased with sequential rounds of immune selection (13,16). The total level of NANOG protein was about 10 times more abundant in P3 cells compared to P0 cells. Notably, the overall increase in NANOG expression in the P3 cells was likely due to the enrichment of NANOG+ cells, as opposed to the up-regulation of NANOG, since the frequency of NANOG+ cells rose from around 5% in the P0 cells to around 90% in the P3 cells. Thus, immune selection depletes cells lacking NANOG and spares those containing NANOG (13). This suggests that NANOG may promote the formation of immunotherapeutic-resistant tumor cells that resemble stem-like tumor cells by conferring a valuable survival advantage.
The elevated expression of NANOG has been reported by several groups to be an indicator of poor prognosis for patients with breast, cervix, oral, kidney, prostate, lung, gastric, brain and ovarian cancer (29,30,31,32,33,34,35,36,37,38). Notably, a higher expression of NANOG was associated with advanced cancer stage and shorter patient survival rates (13,39,40). To test the possibility that NANOG could play a crucial role in multi-malignant phenotypes as a common factor, we examined multi-malignant phenotypes with varying NANOG expressions. We first silenced NANOG expression in P3 cells using siRNA. Compared to siGFP-transfected control cells, siNANOG transfection in P3 cells reduced multi-modal resistance to immuno-, chemo-, and radiotherapy and decreased the stem-like properties and metastatic capacity. Conversely, the overexpression of NANOG alone in P0 cells was sufficient for the induction of the multi-malignant properties (13,17,21). The finding that NANOG as a common factor could play a crucial role in multi-malignancy makes it a potentially ideal target for therapy-refractory cancer (Fig. 2).
UNDERSTANDING OF THE NANOG-MEDIATED SIGNALING PATHWAY IN MULTI-MALIGNANT PHENOTYPES
The analysis of downstream signaling pathways directly or indirectly regulated by NANOG suggests that NANOG could regulate various aspects of therapeutic-refractory tumor development and progression such as multi-modal resistance to cancer therapies, stemness, metastasis, and abnormal metabolism. Therefore, the elucidation of NANOG signaling offers a basic understanding of how therapy-refractory tumor cells acquire multi-malignancy, and key factors in the NANOG signaling pathway could be potentially promising therapeutic targets in clinical applications to control therapy-refractory cancer.
NANOG-driven stem-like proliferative potential
NANOG is involved in the regulation of self-renewal in embryonic stem cells. In cancer cells, the overexpression of NANOG has been associated with increased proliferation rates and tumor growth. Several reports have indicated that the knockdown of NANOG in several cancer types suppressed proliferation and clonogenic growth. Indeed, the ectopic expression of NANOG alone induced increased proliferation, spheroid body formation in vitro and tumor growth in vivo (13,41,42). A global gene expression profile using NANOG siRNA-transfected embryonic carcinoma cells suggested that NANOG is involved in the cell cycle-signaling pathway (43). This group showed that NANOG up-regulated several cell cycle-related genes (such as cyclins D1, D2, D3, and E1) and p53-related signaling pathway components (such as Bcl6 and Atf3), suggesting a role of NANOG in cell cycle and survival (29). Notably, Han et al. found that NANOG transcriptionally up-regulated the cyclin D1 expression by directly binding to its promoter region, driving the cell cycle forward and accelerating cell proliferation (42). Interestingly, we previously reported that the overexpression of cyclin D1 promoted uncontrolled cyclin D1-CDK4/6 activation, which in turn up-regulated NANOG expression (16), suggesting that the NANOG axis promotes stem-like proliferative potentials through a formation of positive feedback loop (Fig. 3). These results indicated that the NANOG axis could be potentially promising therapeutic targets in clinical applications to control abnormal tumor growth.
Figure 3. Key targets for overcoming NANOG-mediated multi-malignancy. Diagrammatic representation of the NANOG signaling axis, which trigger the multi-malignant properties of tumor cells, and different therapeutic strategies to target the NANOG axis.
TWIST1, twist-related protein 1; MMP, matrix metallopeptidase; HIF-1, hypoxia-inducible factor-1; MCL1, myeloid cell leukemia 1; BMI1, B lymphoma Mo-MLV insertion region 1 homolog.
NANOG-mediated multi-modal resistance to various cancer therapies
Cancer immunotherapy, such as cancer vaccination or ACT, was once thought to be a specific method targeting cancer cells. However, promising outcomes in clinical trials of this approach are still insufficient. This may partially, if not entirely, be based on the capability of cancer cells to adapt themselves to the host immune system and escape killing mediated by CTLs. We found that the application of cancer vaccination or ACT resulted in increased NANOG expression and stemness properties in tumor cells and the elevated NANOG drove tumor cells toward a therapeutic-resistant state, rendering cells tolerant to the killing by CTLs, chemotherapeutic-reagents or radiation (13,17). We further demonstrated that the NANOG-dependent multi-modal therapeutic resistance in tumor cells was mediated through a T cell leukemia/lymphoma protein 1A (TCL1A)-AKT pathway. The hyperactive NANOG-TCL1A-AKT pathway has been shown to promote multi-modal resistance to cancer therapies (13,17). Furthermore, we identified histone deacetylase 1 (HDAC1) as a key mediator of the NANOG-associated phenotype. NANOG upregulated HDAC1 through promoter occupancy or AKT-mediated protein stabilization. HDAC1-driven epigenetic silencing of E3 ubiquitin-protein ligase TRIM17 and NOXA promoted multi-modal resistance in tumor cells by increasing anti-apoptotic myeloid cell leukemia 1 (17) (Fig. 3). Similar phenomena were also found in breast, ovarian, and oral squamous cell carcinomas, where elevated NANOG expression was correlated with resistance to chemotherapeutic drugs (41). A report by Bourguignon et al. (44) demonstrated that NANOG up-regulated the drug-resistance-related genes (such as ABCB1 and ABCG2) through transcriptional activation of STAT3, suggesting a direct regulatory link between NANOG and the drug-resistance mechanism in various cancer cells. These results suggest that targeting of the NANOG axis is a potential strategy for overcoming therapy-refractory cancer.
NANOG-induced epithelial-mesenchymal transition (EMT)-like metastatic phenotypes
Elevated NANOG expression is associated with tumor metastasis in various types of cancer (34,40,45,46). In cancer patient samples, the overexpression of NANOG in the nucleus was significantly associated with high-grade, serious histological subtypes, and poor disease-free survival (13,47). The correlation between elevated NANOG expression and advanced stages of cancer or metastatic incidence showed a critical role of NANOG in tumor progression. We found that hyper-activating the NANOG-TCL1A-AKT signaling axis conferred tumor cells with enhanced migration, infiltration, and invasiveness functions through the up-regulation of EMT-associated molecule B lymphoma Mo-MLV insertion region 1 homolog and twist-related protein 1 expression, whereas NANOG knockdown decreased them (21) (Fig. 3). Indeed, Wu et al. reported that NANOG-mediated cell motility and tumor metastasis involved the transcriptional up-regulation of Slug (41). Their results indicated that NANOG was one of the important regulators of EMT and tumor invasion and that blockade of the NANOG axis is a promising strategy to control the metastatic capability of therapy-refractory cancer.
NANOG-mediated reprogramming of mitochondrial metabolism
Mitochondria are complex organelles that influence cancer initiation, growth, survival, and metastasis, and many facets of mitochondrial biology beyond energy production actively contribute to tumorigenesis (48,49,50,51,52). Recently, we found that multi-malignant phenotypes arise through mitochondrial dysfunction initiated by HDAC1-mediated epigenetic silencing of the ATP synthase subunit ATP5H, leading to ROS accumulation and hypoxia-inducible factor-1α stabilization under normoxic conditions (18) (Fig. 3). In the Warburg effect phenomenon, that tumor cells rely mainly on glycolysis, instead of mitochondrial oxidative phosphorylation, for ATP synthesis (53,54). Our results demonstrated that NANOG-HDAC1-driven ATP5H loss in tumor cells leads to mitochondrial metabolic reprogramming, such as increased glucose uptake and lactate production, suggesting that NANOG axis-mediated mitochondrial metabolic reprogramming contributes to the Warburg effect. Thus, we postulate that the disruption of glycolysis in tumor cells may also restore susceptibility to therapy in NANOGhigh refractory tumor cells.
THERAPEUTIC OPPORTUNITIES THROUGH ACTIONABLE DRUGS TARGETING THE NANOG SIGNALING AXIS
Theoretically, there are several advantages to choosing NANOG as a therapeutic target. First of all, NANOG plays a key role in activating several well-known oncogenic pathways, as shown in Fig. 3, which makes it a highly desirable target candidate. Second, NANOG is commonly not expressed in ordinary differentiated somatic cells. Therefore, the off-target effects of NANOG-targeting should be limited. Finally, according to the cellular functions mediated by NANOG downstream effectors, the specific suppression of NANOG enhances the immune surveillance of the host and apoptosis of tumor cells, prevents resistance to chemo- or radiotherapy, reduces tumor growth and metastasis, and inhibits stemness functions (29). Unfortunately, however, pharmacologic inhibitors of NANOG have yet to be developed. Therefore, an in-depth understanding of the underlying molecular mechanisms of NANOG-mediated multi-malignancy is essential for developing strategies to overcome therapy-refractory cancer.
Accumulating evidence has demonstrated the therapeutic potential of targeting the NANOG signaling axis in several cancer cells. For example, small-molecule inhibitors of CDK4/6, a main intermediate of the NANOG signaling cascade, such as palbociclib (PD-0332991), could reverse the NANOG-mediated multi-malignant phenotypes in various cancer cells. Notably, a combination of palbociclib with the adoptive transfer of CTLs resulted in robust anti-tumor effects and survival benefits against NANOGhigh therapy-refractory tumor cells (16) (Fig. 3). Another example is the inhibition of HDAC1, a crucial mediator of the NANOG-associated phenotypes, using romidepsin (FK228). We found that HDAC1 promotes multi-malignant phenotypes mediated by NANOG. Despite its important role in enhancing NANOG-mediated multi-malignancy, HDAC1 also serves as a vulnerability factor by acting as the Achilles' heel, potentially leading to the decline of tumors if it is selectively targeted (17). Indeed, epigenetic therapies have increasingly been recognized as new approaches to treat various relapsed cancers after therapy (55) (Fig. 3). These results demonstrate that inhibition of the NANOG axis using CDK4/6 or HDAC1 inhibitor represents an attractive, widely applicable strategy for the control of NANOGhigh refractory cancer, either as a sole modality or synergistically, as part of an immune-based therapy.
CONCLUSION
Tumor cells undergo molecular evolution under various therapeutic pressures such as immunoediting caused by intrinsic or extrinsic anti-tumor immunity (23,24). As a result, current data from clinical trials indicate that immunotherapy rarely yields significant benefits for cancer patients in terms of tumor progression and long-term survival. The amplifying propensity is that the poor clinical outcomes of immunotherapy are associated with multi-malignant phenotypes including the resistance to immunotherapeutic agents (24). We report that anti-immunity drives the evolution of tumor cells toward a stem-like phenotype that promotes both tumor growth and EMT-like metastatic phenotypes and nullifies CTLs, as well as other types of cancer therapeutic agents, such as chemotherapeutic-reagents and gamma-irradiation. The emergence of these phenotypes requires the transcription factor NANOG, which is a common factor regulating the multi-malignant-related genes of therapy-refractory tumor cells. Thus, therapeutic strategies aiming to blunt NANOG expression or target the NANOG signaling pathway may represent promising ways to manage cancer that is refractory to various immune-therapeutic agents, including immune-checkpoint blockade agents.
ACKNOWLEDGMENTS
This work was supported by funding from the National Research Foundation of Korea (NRF-2019R1A6A3A01096338 to S.J. Oh and NRF-2017R1A2A1A17069818, NRF-2019R1A4A1029000, and NRF-2019M3A9A8066884 to T.W. Kim).
Abbreviations
- ACT
adoptive cell transfer
- CDK
cyclin-dependent kinase
- EMT
epithelial-mesenchymal transition
- HDAC1
histone deacetylase 1
- ICB
immune checkpoint blockade
- TCL1A
T cell leukemia/lymphoma protein 1A
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kim TW, Oh SJ.
- Investigation: Kim M, Jung H.
- Writing - original draft: Oh SJ, Lee J, Kim Y.
- Writing - review & editing: Oh SJ, Song KH, Cho E.
References
- 1.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park YM, Lee SJ, Kim YS, Lee MH, Cha GS, Jung ID, Kang TH, Han HD. Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw. 2013;13:177–183. doi: 10.4110/in.2013.13.5.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JS, Kim YG, Park EJ, Kim B, Lee HK, Hong JT, Kim Y, Han SB. Cell-based immunotherapy for colorectal cancer with cytokine-induced killer cells. Immune Netw. 2016;16:99–108. doi: 10.4110/in.2016.16.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son KJ, Choi KR, Lee SJ, Lee H. Immunogenic cell death induced by ginsenoside rg3: significance in dendritic cell-based anti-tumor immunotherapy. Immune Netw. 2016;16:75–84. doi: 10.4110/in.2016.16.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho JH. Immunotherapy for non-small-cell lung cancer: current status and future obstacles. Immune Netw. 2017;17:378–391. doi: 10.4110/in.2017.17.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noh KH, Lee YH, Jeon JH, Kang TH, Mao CP, Wu TC, Kim TW. Cancer vaccination drives Nanog-dependent evolution of tumor cells toward an immune-resistant and stem-like phenotype. Cancer Res. 2012;72:1717–1727. doi: 10.1158/0008-5472.CAN-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noh KH, Kim BW, Song KH, Cho H, Lee YH, Kim JH, Chung JY, Kim JH, Hewitt SM, Seong SY, et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J Clin Invest. 2012;122:4077–4093. doi: 10.1172/JCI64057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song KH, Cho H, Kim S, Lee HJ, Oh SJ, Woo SR, Hong SO, Jang HS, Noh KH, Choi CH, et al. API5 confers cancer stem cell-like properties through the FGF2-NANOG axis. Oncogenesis. 2017;6:e285. doi: 10.1038/oncsis.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YH, Bae HC, Noh KH, Song KH, Ye SK, Mao CP, Lee KM, Wu TC, Kim TW. Gain of HIF-1α under normoxia in cancer mediates immune adaptation through the AKT/ERK and VEGFA axes. Clin Cancer Res. 2015;21:1438–1446. doi: 10.1158/1078-0432.CCR-14-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SJ, Cho H, Kim S, Noh KH, Song KH, Lee HJ, Woo SR, Kim S, Choi CH, Chung JY, et al. Targeting cyclin d-cdk4/6 sensitizes immune-refractory cancer by blocking the scp3-nanog axis. Cancer Res. 2018;78:2638–2653. doi: 10.1158/0008-5472.CAN-17-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song KH, Choi CH, Lee HJ, Oh SJ, Woo SR, Hong SO, Noh KH, Cho H, Chung EJ, Kim JH, et al. Hdac1 upregulation by nanog promotes multidrug resistance and a stem-like phenotype in immune edited tumor cells. Cancer Res. 2017;77:5039–5053. doi: 10.1158/0008-5472.CAN-17-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song KH, Kim JH, Lee YH, Bae HC, Lee HJ, Woo SR, Oh SJ, Lee KM, Yee C, Kim BW, et al. Mitochondrial reprogramming via ATP5H loss promotes multimodal cancer therapy resistance. J Clin Invest. 2018;128:4098–4114. doi: 10.1172/JCI96804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang HS, Woo SR, Song KH, Cho H, Chay DB, Hong SO, Lee HJ, Oh SJ, Chung JY, Kim JH, et al. API5 induces cisplatin resistance through FGFR signaling in human cancer cells. Exp Mol Med. 2017;49:e374. doi: 10.1038/emm.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo SR, Lee HJ, Oh SJ, Kim S, Park SH, Lee J, Song KH, Kim TW. Stabilization of HDAC1 via TCL1-pAKT-CHFR axis is a key element for NANOG-mediated multi-resistance and stem-like phenotype in immune-edited tumor cells. Biochem Biophys Res Commun. 2018;503:1812–1818. doi: 10.1016/j.bbrc.2018.07.118. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Noh KH, Lee YH, Song KH, Oh SJ, Kim SY, Kim TW. NANOG signaling promotes metastatic capability of immunoedited tumor cells. Clin Exp Metastasis. 2015;32:429–439. doi: 10.1007/s10585-015-9717-2. [DOI] [PubMed] [Google Scholar]
- 22.Song KH, Kim SH, Noh KH, Bae HC, Kim JH, Lee HJ, Song J, Kang TH, Kim DW, Oh SJ, et al. Apoptosis inhibitor 5 increases metastasis via erk-mediated mmp expression. BMB Rep. 2015;48:330–335. doi: 10.5483/BMBRep.2015.48.6.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 24.O'Donnell JS, Teng MW, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 25.Schatton T, Frank MH. Antitumor immunity and cancer stem cells. Ann N Y Acad Sci. 2009;1176:154–169. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 28.Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18:669–680. doi: 10.1038/s41568-018-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ML, Chiou SH, Wu CW. Targeting cancer stem cells: emerging role of Nanog transcription factor. Onco Targets Ther. 2013;6:1207–1220. doi: 10.2147/OTT.S38114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696–3705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- 31.Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255–2265. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- 32.Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104:2092–2098. doi: 10.1002/cncr.21435. [DOI] [PubMed] [Google Scholar]
- 33.Hoei-Hansen CE, Almstrup K, Nielsen JE, Brask Sonne S, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005;47:48–56. doi: 10.1111/j.1365-2559.2005.02182.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin T, Ding YQ, Li JM. Overexpression of Nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med Oncol. 2012;29:878–885. doi: 10.1007/s12032-011-9860-9. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, Zhang Y, Ling EA, Gao J, Hao A. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763–775. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- 36.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622–626. doi: 10.1097/MPA.0b013e3181c75f5e. [DOI] [PubMed] [Google Scholar]
- 37.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X, Zhou YP, Huang GR, Gong BL, Yang B, Zhang DX, Hu P, Xu SR. Expression of the stem cell marker, Nanog, in human endometrial adenocarcinoma. Int J Gynecol Pathol. 2011;30:262–270. doi: 10.1097/PGP.0b013e3182055a1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagata T, Shimada Y, Sekine S, Hori R, Matsui K, Okumura T, Sawada S, Fukuoka J, Tsukada K. Prognostic significance of NANOG and KLF4 for breast cancer. Breast Cancer. 2014;21:96–101. doi: 10.1007/s12282-012-0357-y. [DOI] [PubMed] [Google Scholar]
- 40.Lee M, Nam EJ, Kim SW, Kim S, Kim JH, Kim YT. Prognostic impact of the cancer stem cell-related marker NANOG in ovarian serous carcinoma. Int J Gynecol Cancer. 2012;22:1489–1496. doi: 10.1097/IGJ.0b013e3182738307. [DOI] [PubMed] [Google Scholar]
- 41.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Zhang F, Yu M, Zhao P, Ji W, Zhang H, Wu B, Wang Y, Niu R. RNA interference-mediated silencing of NANOG reduces cell proliferation and induces G0/G1 cell cycle arrest in breast cancer cells. Cancer Lett. 2012;321:80–88. doi: 10.1016/j.canlet.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Choi SC, Choi JH, Park CY, Ahn CM, Hong SJ, Lim DS. Nanog regulates molecules involved in stemness and cell cycle-signaling pathway for maintenance of pluripotency of P19 embryonal carcinoma stem cells. J Cell Physiol. 2012;227:3678–3692. doi: 10.1002/jcp.24076. [DOI] [PubMed] [Google Scholar]
- 44.Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-cd44 interaction activates stem cell marker nanog, stat-3-mediated mdr1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu F, Dai C, Zhang R, Zhao Y, Peng S, Jia C. Nanog: a potential biomarker for liver metastasis of colorectal cancer. Dig Dis Sci. 2012;57:2340–2346. doi: 10.1007/s10620-012-2182-8. [DOI] [PubMed] [Google Scholar]
- 46.Tsai LL, Yu CC, Chang YC, Yu CH, Chou MY. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:621–628. doi: 10.1111/j.1600-0714.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 47.Siu MK, Wong ES, Kong DS, Chan HY, Jiang L, Wong OG, Lam EW, Chan KK, Ngan HY, Le XF, et al. Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene. 2013;32:3500–3509. doi: 10.1038/onc.2012.363. [DOI] [PubMed] [Google Scholar]
- 48.Biswas G, Anandatheerthavarada HK, Avadhani NG. Mechanism of mitochondrial stress-induced resistance to apoptosis in mitochondrial DNA-depleted C2C12 myocytes. Cell Death Differ. 2005;12:266–278. doi: 10.1038/sj.cdd.4401553. [DOI] [PubMed] [Google Scholar]
- 49.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 50.Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaría G, Kim H, Zapata JM, Marusawa H, Chamorro M, Reed JC. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62:6674–6681. [PubMed] [Google Scholar]
- 51.Isidoro A, Martínez M, Fernández PL, Ortega AD, Santamaría G, Chamorro M, Reed JC, Cuezva JM. Alteration of the bioenergetic phenotype of mitochondria is a hallmark of breast, gastric, lung and oesophageal cancer. Biochem J. 2004;378:17–20. doi: 10.1042/BJ20031541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin YK, Yoo BC, Chang HJ, Jeon E, Hong SH, Jung MS, Lim SJ, Park JG. Down-regulation of mitochondrial F1F0-ATP synthase in human colon cancer cells with induced 5-fluorouracil resistance. Cancer Res. 2005;65:3162–3170. doi: 10.1158/0008-5472.CAN-04-3300. [DOI] [PubMed] [Google Scholar]
- 53.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell. 2014;54:716–727. doi: 10.1016/j.molcel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]