Abstract
Immune checkpoint inhibitors (ICIs) have shown remarkable benefit in the treatment of patients with non-small-cell lung cancer (NSCLC) and have emerged as an effective treatment option even in the first-line setting. ICIs can block inhibitory pathways that restrain the immune response against cancer, restoring and sustaining antitumor immunity. Currently, there are 4 PD-1/PD-L1 blocking agents available in clinics, and immunotherapy-based regimen alone or in combination with chemotherapy is now preferred option. Combination trials assessing combination of ICIs with chemotherapy, targeted therapy and other immunotherapy are ongoing. Controversies remain regarding the use of ICIs in targetable oncogene-addicted subpopulations, but their initial treatment recommendations remained unchanged, with specific tyrosine kinase inhibitors as the choice. For the majority of patients without targetable driver oncogenes, deciding between therapeutic options can be difficult due to lack of direct cross-comparison studies. There are continuous efforts to find predictive biomarkers to find those who respond better to ICIs. PD-L1 protein expressions by immunohistochemistry and tumor mutational burden have emerged as most well-validated biomarkers in multiple clinical trials. However, there still is a need to improve patient selection, and to establish the most effective concurrent or sequential combination therapies in different NSCLC clinical settings. In this review, we will introduce currently used ICIs in NSCLC and analyze most recent trials, and finally discuss how, when and for whom ICIs can be used to provide promising avenues for lung cancer treatment.
Keywords: Non-small cell lung cancer, Immunotherapy, Programmed cell death protein 1
INTRODUCTION
Immune checkpoint proteins, such as PD-1 or CTLA-4 emerged as promising targets of immunotherapy, and have improved clinical outcomes of non-small-cell lung cancer (NSCLC) patients tremendously. Currently, the anti-PD-1 agent pembrolizumab is approved for use as first- and second-line therapy in patients with advanced NSCLC whose tumors express PD-L1 in immunohistochemistry analysis (1,2). Nivolumab (anti-PD-1) and atezolizumab (anti-PD-L1) are both indicated for use as second-line therapies regardless of PD-L1 expression (1,3). Durvalumab (anti-PD-L1) is approved as a maintenance therapy in patients with unresectable, stage 3 NSCLC whose disease has not progressed following concurrent platinum-based chemoradiotherapy (4). However, many issues are still not resolved regarding the biomarker status, choice in the first-line setting, immunotherapy in oncogene-addicted tumors, and how to combine immunotherapy with other agents.
NSCLC is a heterogeneous disease that is categorized into 2 broad histologic subtypes, adenocarcinoma and squamous cell carcinoma. Recent investigation of tumor immune microenvironment suggested that lung adenocarcinoma and squamous cell carcinoma show significant differences in immune landscape (5). Understanding the differences in immune microenvironment may suggest heterogeneous response to immunotherapy. Several microenvironmental factors differentially induce lung adenocarcinoma and squamous cell carcinoma immune subtypes, as well as immune checkpoint expression (6). For example, tumor-associated macrophages are key immune cells in lung squamous cell carcinoma, whereas regulatory B cells play immunosuppressive role in lung adenocarcinoma. In addition, the complexity of immune landscape of NSCLC arises from molecular subtype, oncogenic drivers, nonsynonymous mutational load, tumor aneuploidy, clonal heterogeneity and tumor evolution (7).
Tumor expression of PD-L1 has been most widely investigated as a predictive marker of response, but the sensitivity and specificity of this approach is modest (8,9). PD-L1 testing shows variable results because of the different Abs and cutoff values used (10), thus PD-L1 alone cannot accurately reflect the complexity of the tumor microenvironment involved in the response to immunotherapy. At the genomic level, tumor mutational burden (TMB) has been correlated with the clinical response to anti-PD-1 therapy and associated with favorable responses in smokers (11). The role of TMB as a marker predictive of response has been also evaluated in several clinical trials (CheckMate026, CheckMate568, CheckMate227) (12,13,14), which showed that patients with high TMB showed enhanced response to immunotherapy, regardless of PD-L1 expression. However, overall survival (OS) was not affected by TMB alone, and further understanding of the role of TMB as a biomarker is warranted before the integration into clinical practice.
Recent pivotal studies have assessed the role of immunotherapy in previously untreated metastatic NSCLCs in both squamous and nonsquamous histology, and 4 studies have shown an OS benefit from adding PD-1 or PD-L1 inhibitor to standard chemotherapy (KEYNOTE-189, IMpower150, IMpower130, KEYNOTE-407). Chemotherapy-sparing regimens such as PD-1 or PD-L1 inhibitor alone or in combination with a CTLA-4 inhibitor have also demonstrated a survival benefit in biomarker-selected, treatment-naïve NSCLC patients (KEYNOTE-024, KEYNOTE-042, and CheckMate227). Therefore, it is recommended that treatment-naïve, metastatic NSCLC patients receive 1st line treatment with immunotherapy, alone or in combination with chemotherapy. The only subsets of patients that should not receive first-line immunotherapy regimens are those with genomic-driven lung cancer, such as EGFR-mutant or ALK-positive NSCLC. First-line treatment with a tyrosine kinase inhibitor (TKI) is recommended, and guidelines for genomic testing in newly diagnosed metastatic NSCLC remain unchanged.
In this review, we focus on pivotal clinical trials (Table 1) which changed the treatment landscape in advanced, stage 4, NSCLC and discuss open issues on how to choose the best therapeutic strategy and to select patients for the different treatment options.
Table 1. Pivotal studies of ICIs in advanced NSCLC.
| Study name | Phase | Histology, PD-L1 | Line of treatment | Study design | Control arm outcome | Experimental arm outcome | Hazard ratio (95% Confidence interval, p value) | |
|---|---|---|---|---|---|---|---|---|
| First-line ICI only | ||||||||
| KEYNOTE-024 | III | NSCLC, PD-L1 TPS≥50% | Treatment-naïve | Pembrolizumab vs. chemotherapy | mOS 14.2 months | mOS 30.0 months | 0.63 (0.47–0.86), p=0.002 | |
| KEYNOTE-042 | III | NSCLC, PD-L1 TPS≥1% | Treatment-naïve | Pembrolizumab vs. chemotherapy | mOS 12.1 months | mOS 16.7 months | 0.85 (0.71–0.93), p=0.0018 | |
| CheckMate026 | III | NSCLC, PD-L1 TPS≥1% | Treatment-naïve | Nivolumab vs. chemotherapy | mOS 13.2 months | mOS 14.4 months | 1.02 (0.80–1.30), p=NS | |
| MYSTIC | III | NSCLC | Treatment-naïve | D vs. D+Tr vs. chemotherapy | mOS 12.9 months | mOS 16.3 months (D) | D vs. Chemotherapy: 0.76 (0.56–1.02). p=NS | |
| mOS 11.9 months (D+Tr) | D+Tr vs. Chemotherapy: 0.85 (0.61–1.17), p=NS | |||||||
| First-line ICI+Chemotherapy combination | ||||||||
| KEYNOTE-189 | III | Nonsquamous | Treatment-naïve | Pem/C±pembrolizumab vs. placebo | 12-month OS 49.4% | 12-month OS 69.2% | 0.49 (0.38–0.64), p<0.001 | |
| IMpower150 | III | Nonsquamous, including EGFR/ALK+ | Treatment-naïve | B/Pac/C±atezolizumab | mOS 14.7 months | mOS 19.2 months | 0.78 (0.64–0.96), p=0.02 | |
| IMpower132 | III | Nonsquamous | Treatment-naïve | Pem/P±atezolizumab | mPFS 5.2 months | mPFS 7.6 months | 0.60 (0.49–0.73), p<0.0001 | |
| KEYNOTE-407 | III | Squamous | Treatment-naïve | T/C±pembrolizumab | mOS 11.3 months | mOS 15.9 months | 0.64 (0.49–0.85), p<0.001 | |
| IMpower131 | III | Squamous | Treatment-naïve | Nab/C±atezolizumab | mPFS 5.6 months | mPFS 6.3 months | 0.715 (0.603–0.848), p=0.0001 | |
| Later-line ICI | ||||||||
| CheckMate017 | III | Squamous | Second or later | Nivolumab vs. docetaxel | mOS 6.0 months | mOS 9.2 months | 0.62 (0.47–0.80) | |
| CheckMate057 | III | Nonsquamous | Second or later | Nivolumab vs. docetaxel | mOS 12.2 months | mOS 9.5 months | 0.75 (0.63–0.91) | |
| KEYNOTE-010 | II/III | NSCLC, PD-L1 TPS≥1% | Second or later | Pembrolizumab 2 mg/kg or 10 mg/kg vs. docetaxel | mOS 8.5 months | 2 mg/kg: mOS 10.4 months | 2 mg/kg: 0.71, p=0.0008 | |
| 10 mg/kg: mOS 12.7 months | 10 mg/kg: 0.61, p<0.0001 | |||||||
| OAK | III | NSCLC | Second or later | Atezolizumab vs. docetaxel | mOS 9.6 months | mOS 13.8 months | 0.73 (0.62–0.87), p=0.0003 | |
ICI, immune checkpoint inhibitor; NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression free survival.
EFFICACY OF KEY TRIALS
PD-1/PD-L1 inhibitor monotherapy in previously treated NSCLC
Three agents (nivolumab, pembrolizumab, atezolizumab) have been investigated for efficacy in phase III trials involving previously treated NSCLC patients.
In the open-label, randomized, phase III CheckMate017 trial, nivolumab was compared with docetaxel in the second-line setting in NSCLC patients with squamous histology (1). Patients were 1:1 randomized to receive either nivolumab 3 mg/kg every 2 weeks or docetaxel 75 mg/m2 every 3 weeks until disease progression or unacceptable toxicity. Nivolumab showed a significant OS benefit (9.2 months vs. 6.0 months; hazard ratio [HR], 0.59; 95% confidence interval [CI], 0.44–0.79; p<0.001), and the objective response rate (ORR) was 20% in the nivolumab arm, as compared to 9% in the docetaxel arm. The expression of PD-L1 stratified at 1%, 5%, or 10% was not found to be predictive of benefit. Treatment-related adverse events (AEs) occurred less frequently in nivolumab arm (58% any grade and 7% grade 3 or 4 AEs vs. 86% any-grade and 55% grade 3 or 4 AEs) compared with docetaxel arm.
CheckMate057 trial, which assessed efficacy of nivolumab compared to docetaxel in the second-line setting in NSCLC patients with nonsquamous histology also showed survival benefit for nivolumab (1). The same dose and schedule of nivolumab and docetaxel were used as in CheckMate017 trial, and the median OS was superior in the nivolumab arm (12.2 months vs. 9.4 months; HR, 0.73; 95% CI, 0.59–0.89; p<0.002). The ORR was 19% for nivolumab and 12% for docetaxel, and efficacy was greater with nivolumab at pre-specified PD-L1 expressions of 1%, 5% or 10%. Treatment-related AEs occurred less frequently in nivolumab arm (69% any-grade and 10% grade 3 or 4 AEs vs. 88% any-grade and 54% grade 3 or 4 AEs).
The 3-year OS data were recently presented, showing ongoing progression free survival (PFS) and OS benefits for nivolumab for both the squamous and nonsquamous histologies (15). The 3-year OS rates for CheckMate017 and CheckMate057 were 16% and 18% respectively, and among patients who showed response to nivolumab, 26% and 23% showed ongoing responses, respectively.
KEYNOTE010 trial was an open-label, phase II/III trial which randomized NSCLC patients 1:1:1 to receive pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks (2). Both squamous and nonsquamous histologies were included in this trial, and patients were required to have tumors expressing PD-L1. The OS was superior for both doses of pembrolizumab compared to docetaxel (10.4 months for pembrolizumab 2 mg/kg, 12.7 months for pembrolizumab 10 mg/kg, and 8.5 months for docetaxel). Patients who expressed PD-L1 expression of ≥50% showed greater benefit with pembrolizumab, and the median PFS was also statistically improved in these group of patients. Safety was improved for patients in the pembrolizumab arm, grade 3 or greater toxicities occurring at 13% in pembrolizumab 2 mg/kg arm, 16% in pembrolizumab 10 mg/kg arm, and 35% in docetaxel arm.
OAK trial evaluated the efficacy and safety of atezolizumab compared with docetaxel in NSCLC patients of both squamous and nonsquamous cell histologies (3). PD-L1 expression was not required for eligibility and patients were randomized to receive either atezolizumab 1,200 mg or docetaxel 75 mg/m2 every 3 weeks. The median OS was prolonged in the atezolizumab arm compared to docetaxel arm (13.8 months vs. 9.6 months; HR, 0.73; 95% CI, 0.62–0.87; p=0.0003), and benefit was consistent regardless of PD-L1 expression. The greatest OS benefit was observed in patients having highest PD-L1 expression (20.5 months vs. 8.9 months; HR, 0.41; 95% CI, 0.27–0.64; p<0.0001). Intriguingly, patients with brain metastases seemed to benefit from atezolizumab treatment in a subgroup analysis, which was not observed in other studies with PD-1 inhibitors (1,16). Atezolizumab had a better safety profile, showing fewer treatment-related AEs compared to docetaxel (15% vs. 43%).
Overall, the above trials showed consistent improvement in OS and ORR with PD-L1 or PD-L1 inhibitor monotherapy compared with standard chemotherapy, with less toxicity. However, the trials showed heterogeneous cut-offs and diagnostic methods for PD-L1 testing, and whether PD-L1 expression should be required in selecting patients for second-line immunotherapy remains unclear. Several immunohistochemistry assays are available for evaluating PD-L1 expression levels (17). The 22C3 pharmDx assay (Agilent) was used in the pivotal pembrolizumab studies and it is approved as a companion diagnostic assay to categorize PD-L1 expression on tumor cells according to the tumor proportion score. In addition, 28-8 PharmDx (Agilent) was recognized as a complementary diagnostic assay of nivolumab based on evidence that patients with positive PD-L1 expression in tumor cells have a higher clinical benefit of nivolumab. The Ventana platform was used to develop the SP142 Abs in conjunction with atezolizumab, but recent study sponsored by the National Comprehensive Cancer Network and the Blueprint Project showed that SP142 had lower sensitivity because pathologists do not concordantly read PD-L1 expression on immune cells (18).
PD-1/PD-L1 inhibitor monotherapy in previously untreated NSCLC
The KEYNOTE-024 and -042 studies compared the efficacy of pembrolizumab monotherapy to standard platinum-based chemotherapy in previously untreated NSCLC patients. The phase III KEYNOTE-024 trial enrolled the patients with squamous and nonsquamous NSCLC with PD-L1 expression on at least 50% of the tumor cells (8). The results showed that pembrolizumab had superior PFS compared to chemotherapy (10.3 months vs. 6.0 months; HR, 0.50; 95% CI, 0.37–0.68; p<0.001). The frequency of treatment-related AEs of any grade and grade ≥3 were significantly lower in the pembrolizumab arm than in the chemotherapy arm (73.4% vs. 90% and 26.6% vs. 53.3%, respectively). According to KEYNOTE-024 trial, pembrolizumab monotherapy is now regarded as a standard of care therapy for NSCLC patients (squamous or nonsquamous histology) with PD-L1 expression of at least 50%.
The KEYNOTE-042 trial assessed the efficacy of pembrolizumab monotherapy in patients with PD-L1 expression on at least 1% of tumor cells (19). NSCLC patients with squamous or nonsquamous histologies were randomized 1:1 to receive either pembrolizumab 200 mg or platinum-based chemotherapy, without crossover to pembrolizumab. Pembrolizumab monotherapy significantly improved OS in all pre-specified PD-L1 expression subgroups (TPS≥1%, ≥20%, ≥50%). The magnitude of OS benefit was greatest in the patients with TPS≥50% (20 months vs. 12.2 months; HR, 0.69; 95% CI, 0.56–0.85; p=0.0003), but the OS benefit was not seen patients with TPS ≥1%–49%. These results suggest that patients with PD-L1 TPS≥50% were driving the OS benefit, which is consistent with the results from KEYNOTE-024 trial.
On the contrary, CheckMate026 trial, a phase III study of nivolumab versus platinum-based chemotherapy in patients with treatment-naïve advanced NSCLC with a PD-L1 TPS of ≥1%, did not show any clinical benefit (20). In the primary efficacy analysis of patients with a PD-L1 TPS of ≥5%, nivolumab did not show significant improvement in either PFS or OS. Additional exploratory subgroup analyses also did not show any significant difference in PFS or OS in patients with a PD-L1 TPS≥50%. Of note, an exploratory analysis was conducted to see the role of TMB, and patients with high TMB (as defined as ≥243 missense mutations) showed higher response rate (47% vs. 28%) and prolonged PFS (9.7 months vs. 5.8 months; HR, 0.62; 95% CI, 0.38–1.00). However, no significant difference was observed in OS regardless of TMB.
MYSTIC trial compared both durvalumab monotherapy and durvalumab in combination with tremelimumab with the platinum-doublet chemotherapy in the first-line setting in patients 25% or greater PD-L1 expression (21). This trial, however, did not meet its primary endpoint of improved PFS compared with the chemotherapy in either the durvalumab monotherapy or durvalumab plus tremelimumab. Therefore, pembrolizumab remains the only FDA-approved single-agent, immune checkpoint inhibitor (ICI) in the first-line setting in advanced NSCLC patients.
PD-1/PD-L1 inhibitor in combination with chemotherapy
The addition of PD-1/PD-L1 inhibitor to standard chemotherapy in treatment-naïve NSCLC patients was investigated, regardless of PD-L1 expression. The rationales behind combining immunotherapy to chemotherapy are that cytotoxic chemotherapeutic agents may 1) induce immunological activity (22); 2) cytotoxic agents may increase presentation of tumor Ags (23); 3) reduce regulatory T cells (24) and myeloid derived suppressor cells (25); 4) induce PD-L1 expression on tumor cells (23).
KEYNOTE-189 was a phase III, placebo-controlled double-blinded trial which assessed first-line platinum-based chemotherapy with or without pembrolizumab in EGFR/ALK-wild type, nonsquamous NSCLC patients (26). The ORR was 47.6% in the pembrolizumab-chemotherapy arm vs. 18.9% in the placebo-chemotherapy arm (p<0.001). The median OS was not reached at the time of analysis for the pembrolizumab-chemotherapy arm vs. 11.3 months for placebo-chemotherapy arm (HR, 0.49; 95% CI, 0.38–0.64; p<0.001), and the OS advantage was achieved in all PD-L1 subgroups. The median PFS was 8.8 months vs. 4.9 months (HR, 0.52; 95% CI, 0.43–0.64; p<0.001), but no PFS benefit was evident adding pembrolizumab in patients with PD-L1 TPS<1%. In terms of safety, neither an increase in AEs nor an increase in immune-mediated AEs were reported in pembrolizumab-chemotherapy arm. On the basis of KEYNOTE-189 results, pembrolizumab in combination with pemetrexed and carboplatin as first-line treatment in metastatic nonsquamous NSCLC became a new standard, regardless of PD-L1 expression (27).
Impower150 evaluated the role of atezolizumab combined with chemotherapy for the first-line treatment of metastatic nonsquamous NSCLC. Patients were randomized to 3 groups: atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP); ACP; and BCP. While the data comparing ABCP and BCP are available, both the median PFS and OS were improved in the atezolizumab-containing arm (PFS, 8.3 months vs. 6.8 months; OS, 19.2 months vs. 14.7 months) compared with the patients treated with BCP (28). Of note, patients with EGFR and ALK alterations were included in this trial, and they also had benefit from atezolizumab-containing arm (HR, 0.59; 95% CI, 0.37–0.94). A higher incidence of grade ≥3 AEs was observed in the atezolizumab-containing arm (55.7% vs. 47.7%), mainly anorexia, nausea, diarrhea, neutropenia, thrombocytopenia and febrile neutropenia. A total of 77.% of the immune-related AEs in ABCP group were grade 1 or 2 and manageable, and none were grade 5.
IMpower132 also assessed the role of atezolizumab in first-line chemotherapy combinations for advanced nonsquamous NSCLC. There was an improvement in PFS in the atezolizumab-containing arm (7.6 months vs. 5.2 months) and benefit was seen in both PD-L1 positive and negative group (29).
KEYNOTE-407 and IMpower131 trial investigated the efficacy of PD-1/PD-L1 inhibitor in metastatic squamous NSCLC in combination with chemotherapy. In KEYNOTE-407 trial, patients were randomized to receive 4 cycles of carboplatin and a taxane with or without pembrolizumab (30). As expected, patients in the pembrolizumab-containing group showed a significantly improved OS compared with those in the chemotherapy group (15.9 months vs. 11.3 months; HR, 0.64; 95% CI, 0.49–0.85; p<0.001). Benefit was seen in all PD-L1 TPS groups, and pembrolizumab did not significantly increase treatment-related toxicity.
IMpower131 trial examined atezolizumab with chemotherapy consisting of carboplatin with either paclitaxel (ACP) or nab-paclitaxel (ACnP) against carboplatin plus nab-paclitaxel (CnP) control (31). While the results were positive in terms of its primary endpoint of median PFS for ACnP versus CnP (6.3 months vs. 5.6 months; HR, 0.715; 95% CI, 0.603–0.848; p=0.0001), the median OS were not different between 2 groups.
Immunotherapy combinations
In CheckMate227, treatment-naïve patients with advanced NSCLC were randomized to nivolumab plus ipilimumab, nivolumab, and histology-based chemotherapy arms (14). According to PD-L1 expression, patients were divided into PD-L1≥1% and <1%, and further randomized 1:1:1 to nivolumab plus ipilimumab, platinum-based chemotherapy, or nivolumab monotherapy (PD-L1≥1% group) or nivolumab plus platinum-based chemotherapy (PD-L1<1% group). The study protocol was later modified to include a co-primary endpoint of PFS in patients with high TMB, as defined by ≥10 mutations per megabase. This was due to the previous finding that high TMB was associated with enhanced response rate and PFS, independent of tumor PD-L1 expression (11). Among patients with high TMB, nivolumab plus ipilimumab arm showed a significantly prolonged PFS than chemotherapy arm (7.2 months vs. 5.5 months; HR, 0.58; 95% CI, 0.41–0.81; p<0.001). However, in patients with lower TMB, median PFS was shorter in patients in nivolumab plus ipilimumab arm compared with those in chemotherapy arm (3.2 months vs. 5.5 months; HR, 1.07, 95% CI, 0.56–1.10). However, the median OS was not significantly different among high TMB (32). Incidence of grade 3 or higher AEs were similar (31.2% in nivolumab plus ipilimumab vs. 36.1% in chemotherapy arm).
In CheckMate568, the association of efficacy with PD-L1 expression and TMB was assessed in patients who received first-line nivolumab plus ipilimumab (33). Higher response rates and improved PFS were observed in patients with TMB of 10 or more mut/Mb versus TMB of fewer than 10 mut/Mb, irrespective of PD-L1 expression. This analysis supported TMB of 10 or more mut/Mb as a clinically meaningful cutoff for response and PFS in patients receiving first-line nivolumab plus ipilimumab.
Currently, novel targets and combinations are underway to enhance antitumor immune response. Supplementary Table 1 shows a selected list of novel immunotherapy trials in clinical development in solid tumors and lymphomas. Novel combination strategies can be classified according to mechanism of action: 1) Co-inhibitory blockade; 2) Costimulation; 3) Bispecific T cell engager Ab constructs; and 4) Priming. First of all, agents that block co-inhibitory receptors apart from CTLA-4 and PD-1/PD-L1 include V-domain immunoglobulin suppressor of T cell activation, lymphocyte-activation gene-3 (LAG-3), T cell immunoglobulin- and mucin-domain-containing module 3 (TIM-3), T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain, and B7-H3. Since a considerable proportion of patients remain unresponsive to immunotherapeutics targeting the inhibitory receptors CTLA-4, PD-1 or PD-L1, targeting novel inhibitory pathways in combination with current immunotherapies may improve clinical outcomes. Therefore, clinical trials involving novel checkpoint targets such as V-domain immunoglobulin suppressor of T cell activation /PD-1H (NCT02671955), B7-H3 (NCT01391143, NCT02475213, NCT00844064), LAG-3 (NCT01968109, NCT02460224, NCT00732082, NCT00349934) are ongoing, often in combination with PD-1 inhibitors. A first-in-human study of human LAG-3 monoclonal Ab (REGN3767) in combination with PD-1 inhibitor (cemiplimab) was recently reported, and early efficacy signals were detected with acceptable safety profiles (34). Secondly, costimulatory agents are OX40, GITR, ICOS, 4-1BB, and CD40. Besides, costimulatory molecules such as OX40, GITR, ICOS, 4-1BB (CD137), CD40 can augment immunological responses against malignant cells. Bispecific Abs, vaccines, oncolytic viruses and cytokines are also passive and active immunotherapies. Bispecific T cell engager Ab constructs are a type of fusion protein that is designed by linking the targeting regions of 2 Abs. One arm of the molecule binds to the surface of cytotoxic T cells, and the other arm binds to a specific protein found primarily on tumor cells (35). Moreover, bispecific Abs to TIM-3 have also been developed. RO7121661 (bispecific Ab to TIM-3 and PD-1) and LY3415244 (bispecific Ab to TIM-3 and PD-L1) are currently under clinical investigation. Oncolytic viruses selectively kill cancer cells and stimulate the immune system (e.g., T-Vec), while dendritic cell vaccines (e.g., sipuleucel-T) involve the extraction of dendritic cells from the patient, exposure to cancer cells or Ags, and reintroduction of now active immune cells to the patient (36). STimulator of INterferon Genes agonist, a phase 1b study of MIW815 in combination with PD-1 inhibitor (PDR001), was recently reported in patients with advanced solid tumors. The preliminary response was higher in patients with moderate to high baseline PD-L1 expression (ORR 25.0%) as compared to patients with low PD-L1 expression (ORR 4.5%), and increase in CD8+ T cells in tumor after drug injection was suggested as a pharmacodynamics marker, reflecting therapeutic benefit (37). Further understanding of the basic biology of these novel targets is imperative to the development of effective cancer immunotherapy.
PD-1/PD-L1 inhibitor in EGFR and ALK altered patients
The role of PD-1/PD-L1 inhibitor in oncogene-addicted NSCLC is still unclear, and currently TKIs are the standard treatment options in patients with EGFR- or ALK-altered tumors. Targeted therapy in combination with PD-1/PD-L1 inhibitors have been tried, trials have been prematurely stopped due to toxicity issues. A phase 1b TATTON trial testing osimertinib (EGFR TKI) plus nivolumab was closed early due to high incidence of interstitial lung disease (38%) (38). In addition, the CAURAL phase III trial evaluating the combination of osimertinib and durvalumab in EGFR T790M positive patients was also prematurely stopped due to safety concerns. However, in ALK-rearranged NSCLC patients, combination of alectinib (ALK TKI) and atezolizumab showed acceptable toxicity, but ORR was not improved compared to alectinib alone (39). Due to high incidence of high-grade toxicities with combination of TKI and immunotherapy, further development should be cautiously considered.
A single agent pembrolizumab was investigated in a phase II trial of patients with EGFR-mutant, PD-L1-positive (≥1%) advanced NSCLC. All patients were treatment-naïve, but after enrolling 11 out of 25 patients, the trial was terminated early due to lack of efficacy (40). To date, ICIs are not considered effective in oncogene-addicted patients. Other interventional strategies are underway to evaluate the combination of ICIs plus chemotherapy in the resistant setting (NCT02864251, NCT03256136, NCT03515837), as well as combination with anti-CD73 therapies (NCT03454451) based on preclinical rationale (41).
PATIENT SELECTION AND BIOMARKERS
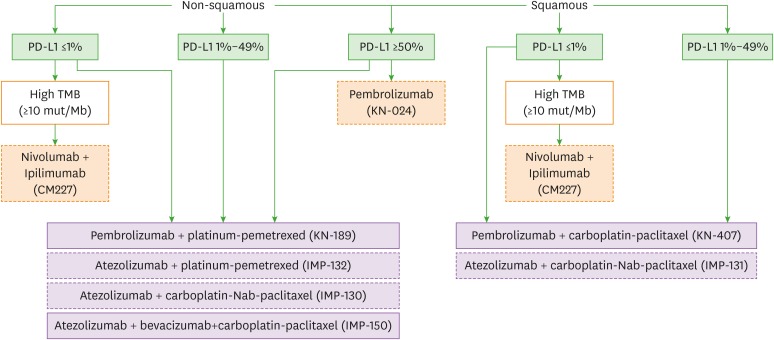
Figure 1 shows the first-line treatment algorithm. So far, PD-L1 expression by immunohistochemistry is the only approved biomarker to select patients for immunotherapy. As mentioned earlier, there are different methods of cutoffs and interpretation because companies use different PD-L1 assays (Dako 28-8, Dako 22C3, Ventana SP142, Ventana SP263 for nivolumab, pembrolizumab, atezolizumab and durvalumab, respectively). Moreover, even PD-L1 negative patients may respond to anti-PD-1/PD-L1 inhibitor, while some PD-L1 highly positive patients do not show response. Therefore, PD-L1 expression is still an incomplete marker.
Figure 1. First-line treatment algorithm. Dashed boxes indicate treatments which did not receive approval from regulatory agencies yet.
TMB, tumor mutational burden.
According to results of CheckMate227 trial, TMB was considered as a potential and new biomarker, independently from PD-L1 expression. Recently, blood TMB was also evaluated in pretreated NSCLC patients who were enrolled in OAK and POPLAR studies (42), in treatment-naïve NSCLC patients in a phase II, B-F1RST trial receiving atezolizumab (43), and in patients in MYSTIC trial (21). These studies suggested that TMB was feasible in the majority of patients, and the rate of high TMB (≥10 mut/megabase) was between 23% and 30%. In addition, blood TMB and tissue TMB correlated significantly in the pooled analysis, suggesting that TMB may be more easily tested on blood rather than on tumor tissues. However, like PD-L1, TMB results may vary according to different platforms of sequencing, and testing costs are high for routine clinical practice. Although TMB can be complementary to PD-L1 immunohistochemistry, future prospective randomized studies are required to assess the clinical value of TMB as a predictive biomarker for anti-PD-1/PD-L1 inhibitors.
TOXICITY PROFILES
Immune-related adverse events (irAEs) are ICI-mediated inflammatory side effects (44). The pathophysiology underlying irAEs is largely unknown but is believed to be related to the disruption of immunologic homeostasis (45). Recent studies suggest that the occurrence of irAEs predicts the treatment efficacy of ICIs in NSCLC (46,47), but at the same time, is more likely to be associated with treatment discontinuation. Adverse events from ICIs can affect one or several different systemic organ systems. Toxicities can occur as various symptoms and signs: skin (rash, pruritus), gastrointestinal (colitis), liver (transaminitis), pancreas (pancreatitis), endocrine (thyroiditis, adrenal insufficiency, hypophysitis, type 1 diabetes mellitus), lung (pneumonitis), kidney (proteinuria), eye (uveitis, episcleritis), nervous system (myasthenia gravis, peripheral neuropathy, encephalitis, transverse myelitis), cardiovascular (myocarditis), and musculoskeletal (arthritis). The incidence of grade 3 or higher toxicities is 7% to 13% in NSCLC patients treated with PD-1 axis inhibitors (48). As ICIs increase the activity of the immune system, T cells can attack healthy cells in the body, causing inflammatory conditions that mimic a range of autoimmune conditions, some of which can be serious (49). These immune-related AEs can occur at any time during treatment or even after treatment is discontinued. The severity of AEs can range from asymptomatic to severe or life-threatening and they may cumulate over the course of therapy. Combination treatment may increase the severity of adverse events, so more caution is required. Regular monitoring including laboratory tests and physical exams needs to be conducted to detect any potential immune-related AEs, because most AEs can be managed effectively if detected and treated early (50).
TREATMENT DURATION
While current dosing and duration guidelines are based primarily on initial clinical trials conducted for approval of the ICIs, the optimal duration of treatment still needs to be explored. Similar to chemotherapeutic agents, the duration of treatment of all 5 currently approved PD-1/PD-L1 inhibitors are until disease progression or unacceptable toxicity. However, since immunotherapy work with a completely different mechanism compared to chemotherapy, using the same therapy duration may not be optimal. For example, it remains undecided whether we can discontinue the therapy in patients with complete response. In a retrospective study, they suggest stopping treatment is a viable option in patients with complete response as the durability of response is maintained in about 80%–90% of patients (51). Treatment holidays and possibly stopping immune based therapy early is a concept that needs further research using novel trial designs.
HYPERPROGRESSION
There is an emerging evidence that PD-1/PD-L1 inhibitors can lead to hyperprogressive disease (HPD), similar to a flare-up of tumor growth leading to dismal outcome. A recent meta-analysis to identify baseline patient factors associated with risks of developing HPD was reported. Although there was no standard definition of HPD, the incidence of HPD ranged from 1% to 30% (52). In this report, they identified serum LDH above the upper normal limit, more than 2 metastatic sites, liver metastases, Royal Marsden Hospital prognostic score of 2 or above as positively associated with HPD, and positive PD-L1 expression status that was inversely correlated with HPD. In another analysis, genomic alterations in genes such as EGFR, MDM2/4 and DNMT3A were associated with HPD (53). In a recent exploratory biomarker analysis of patients with HPD, a lack of pre-existing antitumor immunity correlated with HPD, represented by a lower frequency of effector/memory subsets and a higher frequency of exhausted T cells populations in patients with HPD (54). Molecular mechanisms of hyperprogression are yet to be elucidated.
CONCLUSIONS
Although ICIs have been adopted for a limited amount of time so far, they ushered in a new era in the management of advanced NSCLC. Clinicians are now faced with numerous options of treatment such as PD-1 inhibitor monotherapy or PD-1/PD-L1 inhibitor plus chemotherapy, and a growing number of patients are achieving durable responses. Better predictive biomarkers are required to optimize the benefit of immunotherapy, and further studies are needed to determine the mechanism of resistance to ICIs and how to overcome it. In summary, ICIs have changed the treatment landscape in advanced NSCLC and ongoing translational and clinical studies are highly awaited to further improve outcomes for these patients. Expanding clinical benefit to the majority of patients and preventing drug resistance requires a better understanding of the mechanisms that lead to an effective anti-tumor response. The development of new combination strategies will shed the light to the next advances of cancer immunotherapy.
ACKNOWLEDGEMENTS
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2017M3A9E9072669 (CTC), NRF-2019M3A9B6065231, 2017M3A9E8029717 to HRK, and Bio & Medical Technology Development Program of the NRF funded by the Korean government (No. 2018M3A9E8066245, NRF-2019R1A2C4069993) to SML.
Abbreviations
- ABCP
atezolizumab, bevacizumab, carboplatin, and paclitaxel
- AE
adverse event
- CI
confidence interval
- HPD
hyperprogressive disease
- HR
hazard ratio
- ICI
immune checkpoint inhibitor
- irAE
immune-related adverse event
- LAG-3
lymphocyte-activation gene-3
- NSCLC
non-small-cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PFS
progression free survival
- TIM-3
T cell immunoglobulin- and mucin-domain-containing module 3
- TKI
tyrosine kinase inhibitor
- TMB
tumor mutational burden
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lim SM, Hong MH, Kim HR.
- Data curation: Lim SM, Hong MH, Kim HR.
- Formal analysis: Lim SM, Kim HR.
- Funding acquisition: Kim HR.
- Investigation: Lim SM, Kim HR.
- Methodology: Lim SM, Kim HR.
- Project administration: Lim SM.
- Supervision: Lim SM.
- Validation: Lim SM.
- Visualization: Lim SM.
- Writing - original draft: Lim SM.
- Writing - review & editing: Lim SM, Kim HR.
SUPPLEMENTARY MATERIAL
Selected trials of novel immunotherapy combination
References
- 1.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 3.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 5.Faruki H, Mayhew GM, Serody JS, Hayes DN, Perou CM, Lai-Goldman M. Lung adenocarcinoma and squamous cell carcinoma gene expression subtypes demonstrate significant differences in tumor immune landscape. J Thorac Oncol. 2017;12:943–953. doi: 10.1016/j.jtho.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo JS, Kim A, Shin JY, Kim YT. Comprehensive analysis of the tumor immune micro-environment in non-small cell lung cancer for efficacy of checkpoint inhibitor. Sci Rep. 2018;8:14576. doi: 10.1038/s41598-018-32855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anichini A, Tassi E, Grazia G, Mortarini R. The non-small cell lung cancer immune landscape: emerging complexity, prognostic relevance and prospective significance in the context of immunotherapy. Cancer Immunol Immunother. 2018;67:1011–1022. doi: 10.1007/s00262-018-2147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehnert JM, Monjazeb AM, Beerthuijzen JM, Collyar D, Rubinstein L, Harris LN. The challenge for development of valuable immuno-oncology biomarkers. Clin Cancer Res. 2017;23:4970–4979. doi: 10.1158/1078-0432.CCR-16-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters S, Creelan B, Hellmann MD, Socinski MA, Reck M, Bhagavatheeswaran P, Chang H, Geese WJ, Paz-Ares L, Carbone DP. Abstract ct082: Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage IV or recurrent non-small cell lung cancer: an exploratory analysis of checkmate 026. Cancer Res. 2017;77:CT082. [Google Scholar]
- 13.Ramalingam SS, Hellmann MD, Awad MM, Borghaei H, Gainor J, Brahmer J, Spigel DR, Reck M, O'Byrne KJ, Paz-Ares L, et al. Abstract ct078: Tumor mutational burden (tmb) as a biomarker for clinical benefit from dual immune checkpoint blockade with nivolumab (nivo) + ipilimumab (ipi) in first-line (1l) non-small cell lung cancer (nsclc): Identification of tmb cutoff from checkmate 568. Cancer Res. 2018;78:CT078. [Google Scholar]
- 14.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felip Font E, Gettinger SN, Burgio MA, Antonia SJ, Holgado E, Spigel DR, Arrieta O, Domine Gomez M, Aren Frontera O, Brahmer J, et al. Three-year follow-up from checkmate 017/057: Nivolumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC) Ann Oncol. 2017;28(Suppl 5):mdx380.004 [Google Scholar]
- 16.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Büttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F, Penault-Llorca F, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35:3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- 18.Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou TY, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes GW, Kudaba I, Kowalski D, Cho BC, Castro G, et al. Pembrolizumab versus platinum-based chemotherapy as first-line therapy for advanced/metastatic nsclc with a PD-L1 tumor proportion score >=1%: open-label, phase 3 keynote-042 study. J Clin Oncol. 2018;36:36. [Google Scholar]
- 20.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Chul Cho B, Reinmuth N, Lee KH, Ahn MJ, Luft A, van den Heuvel M, Cobo M, Smolin A, Vicente D, et al. Lba6durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: mystic. Ann Oncol. 2018;29(Suppl 10):mdy511.005 [Google Scholar]
- 22.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, Farsaci B, Donahue R, Gulley JL, Schlom J, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. OncoImmunology. 2013;2:e27025. doi: 10.4161/onci.27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. OncoImmunology. 2017;6:e1331807. doi: 10.1080/2162402X.2017.1331807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi L, Garassino MC. Pembrolizumab plus chemotherapy in lung cancer. N Engl J Med. 2018;379:e18. doi: 10.1056/NEJMc1808567. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Non-small cell lung cancer [Internet] [accessed on 2019]. Available at https://www.nccn.org/professionals/physician_gls/default.aspx.
- 28.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 29.Papadimitrakopoulou VA, Cobo M, Bordoni R, Longeras PD, Szalai Z, Ursol G, Novello S, Orlandi F, Ball S, Goldschmidt J, et al. Impower132: PFS and safety results with 1l atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC; Proceedings of the IASLC 19th World Conference on Lung Cancer 2018; 2018 September 23-26; Toronto, Canada. Aurora, CO: International Association for the Study of Lung Cancer; 2018. [Google Scholar]
- 30.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 31.Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein MA, Soo RA, Conter HJ, Kozuki T, Silva C, et al. Impower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36:LBA9000 [Google Scholar]
- 32.Bristol-Meyers Squibb. Bristol-Myers Squibb to announce results for fourth quarter 2018 on January 24 [Internet] Available at https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announce-results-fourth-quarter-2018-janu.
- 33.Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (checkmate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37:992–1000. doi: 10.1200/JCO.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadopoulos KP, Lakhani NJ, Johnson ML, Park H, Wang D, Yap TA, Dowlati A, Maki RG, Lynce F, Ulahannan SV, et al. First-in-human study of REGN3767 (R3767), a human LAG-3 monoclonal antibody (mAb), ± cemiplimab in patients (pts) with advanced malignancies. J Clin Oncol. 2019;37:2508. [Google Scholar]
- 35.Mølhøj M, Crommer S, Brischwein K, Rau D, Sriskandarajah M, Hoffmann P, Kufer P, Hofmeister R, Baeuerle PA. CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol Immunol. 2007;44:1935–1943. doi: 10.1016/j.molimm.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meric-Bernstam F, Sandhu SK, Hamid O, Spreafico A, Kasper S, Dummer R, Shimizu T, Steeghs N, Lewis N, Talluto CC, et al. Phase ib study of miw815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J Clin Oncol. 2019;37:2507. [Google Scholar]
- 38.Ahn MJ, Yang J, Yu H, Saka H, Ramalingam S, Goto K, Kim SW, Yang L, Walding A, Oxnard GR. 136o: osimertinib combined with durvalumab in egfr-mutant non-small cell lung cancer: results from the tatton phase ib trial. J Thorac Oncol. 2016;11:S115. [Google Scholar]
- 39.Kim DW, Gadgeel SM, Gettinger SN, Riely GJ, Oxnard GR, Mekhail T, Schmid P, Dowlati A, Heist RS, Wozniakm AJ, et al. Safety and clinical activity results from a phase ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC) J Clin Oncol. 2018;36:9009. doi: 10.1016/j.jtocrr.2022.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M, Hunt J, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13:1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 42.Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 43.Kim ES, Velcheti V, Mekhail T, Leal TA, Dowell JE, Tsai ML, Dakhil CS, Stella P, Shen V, Hu S, et al. Primary efficacy results from b-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC) Ann Oncol. 2018;29(Suppl 8):29. [Google Scholar]
- 44.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 45.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12:1798–1805. doi: 10.1016/j.jtho.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 51.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, Hwu WJ, Weber JS, Gangadhar TC, Joseph RW, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 52.Kim JY, Lee KH, Kang J, Borcoman E, Saada-Bouzid E, Kronbichler A, Hong SH, de Rezende LF, Ogino S, Keum N, et al. Hyperprogressive disease during anti-PD-1 (PDCD1) / PD-L1 (CD274) therapy: a systematic review and meta-analysis. Cancers (Basel) 2019;11:11. doi: 10.3390/cancers11111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30:1104–1113. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selected trials of novel immunotherapy combination