Abstract
Immune checkpoint inhibitors (ICIs), including anti-PD-1 and anti-CTLA-4 therapeutic agents, are now approved by the Food and Drug Administration for treatment of various types of cancer. However, the therapeutic efficacy of ICIs varies among patients and cancer types. Moreover, most patients do not develop durable antitumor responses after ICI therapy due to an ephemeral reversal of T-cell dysfunction. As co-stimulatory receptors play key roles in regulating the effector functions of T cells, activating co-stimulatory pathways may improve checkpoint inhibition efficacy, and lead to durable antitumor responses. Here, we review recent advances in our understating of co-stimulatory receptors in cancers, providing the necessary groundwork for the rational design of cancer immunotherapy.
Keywords: Cancer, Immunotherapy; Costimulatory T-cell receptors; T-Lymphocytes
INTRODUCTION
Immunotherapy has long been considered an important cancer treatment option, and immune checkpoint inhibitors (ICIs) targeting PD-1, PD-L1, and CTLA-4 have revolutionized cancer treatment (1). Many studies have examined the features of tumor-infiltrated lymphocytes (TILs) in the tumor microenvironment and the mechanisms by which cancers evade immune response, elucidating the concept of CD8 T-cell exhaustion, which is critical for improving cancer immunotherapy. T-cell exhaustion was first described in chronic viral infection, and is defined as an impaired capacity of T cells to secrete cytokines and proliferate, caused by prolonged antigenic stimulation-induced overexpression of immune checkpoint receptors, such as PD-1, CTLA-4, T-cell Ig and mucin-domain containing (TIM)-3, and lymphocyte-activation gene 3 (2). Since response to ICI treatment substantially varies among patients and cancer types, it is important to develop novel immuno-therapeutic strategies with improved therapeutic efficacy.
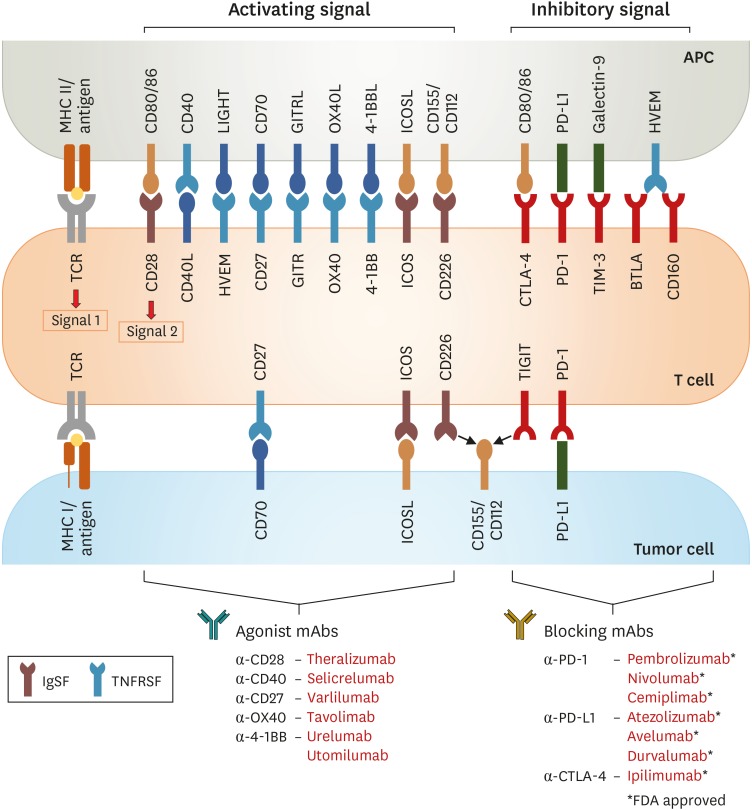
Various therapeutic strategies that have been considered to improve the anti-tumor response to checkpoint blockades, include combinations of multiple checkpoint blockers, agonistic mAb targeting co-stimulatory receptors, soluble mediators (cytokines), and chimeric Ag receptor (CAR) T cells (2). In addition to inhibitory receptors, activated T cells and exhausted T cells in tumor microenvironment also exhibit upregulation of numerous co-stimulatory receptors (3) (Fig. 1). Since immune-stimulating co-stimulatory receptors are an essential element of anti-cancer immunity, effective activation of these receptors may contribute to the therapeutic repertoires against cancers. Moreover, it has been suggested that T-cell exhaustion and T-cell activation are closely interconnected and share features related to the cell cycle pathway, T-cell migration, and cytotoxic effector function (4,5). Therefore, one promising therapeutic approach is to target co-stimulatory receptors using agonistic mAb for re-invigorating T-cell responses in cancers.
Figure 1. Schematic overview of co-stimulatory/inhibitory receptors expressed by T cells interacting with their counterpart on APCs or tumor cells. Inhibitory and stimulatory receptors expressed on T cells in the tumor microenvironment may be targeted for therapeutic intervention by development of agonist targeting co-stimulatory receptors and/or blocking Abs targeting immune inhibitory receptors.
CD40L, CD40 ligand; LIGHT, lymphocyte activation gene 3 protein; HVEM, herpes virus-entry mediator; BTLA, B-lymphocyte and T-lymphocyte attenuator.
Here, we review recent advances in our understating of various co-stimulatory receptors belonging to the Ig receptor superfamily (IgSF) and the TNF receptor superfamily (TNFRSF) in cancers. We focus on the immunological characteristics of these co-stimulatory receptors and also highlight the current status of anti-cancer immunotherapeutic strategies targeting each co-stimulatory receptor.
CO-STIMULATORY RECEPTOR EXPRESSIONS ON TILs
Co-stimulatory receptors on T cells are cell surface molecules that can positively induce signaling to fully activate T cells with TCR signaling and cytokine stimulation (6). This co-signaling plays critical roles in T-cell priming and activation, and in modulating T-cell differentiation, effector function, and survival. Co-stimulatory receptors are commonly categorized into 2 groups: the IgSF and TNFRSF (Fig. 1). Most co-stimulatory receptors are upregulated by TCR engagement and downregulated with reduced TCR signaling. However, some co-stimulatory receptors (e.g., CD27 and CD28) are constitutively expressed on all T cells, and show distinct expression patterns depending on ligation with their counterparts or tissue environment (6). Therefore, to target co-stimulatory receptors for promoting anti-tumor immune responses, it is important to understand the repertoires of co-stimulatory receptors and their impacts on other immune cells in the tumor environment. We summarize the molecular/immunological features and clinical implications of various co-stimulatory receptors, highlighting the therapeutic promise and development challenges.
IgSF
CD28
Upon finding that TCR engagement alone is insufficient for full T-cell activation (7,8), the discovery of CD28 revealed that co-stimulatory receptors may be important in secondary signaling along with TCR ligation (9). CD28 and the B7 family are among the most well-known co-stimulatory molecules. Part of the IgSF, CD28 binds to multiple ligands, including B7-1 (CD80) and B7-2 (CD86) (10). CD28 engagement with ligands initiates the induction of signaling by specific proteins that bind to phosphorylated or unphosphorylated motifs in the cytoplasmic tail (11). This signal transduction leads to activation of NF-AT, AP-1, and NF-κB transcriptional factors that are important for IL-2 induction (12,13).
CD28 is expressed in almost 80% of human CD4 T cells and 50% of CD8 T cells, and its expression decreases with aging (14). Additionally, CD28 expression on CD8 T cells is reduced by repeated Ag stimulation in tumors and chronic infection (15). Persistent Ag exposure strongly upregulates PD-1 expression on exhausted CD8 T cells, and PD-1 can strongly repress CD28 signaling by direct dephosphorylation of CD28 (16). Additionally, the ligands of CD28 can also bind CTLA-4, which induces CD28 downregulation via endocytosis (17). Therefore, repeated TCR signaling and high expression of PD-1 and CTLA-4 in certain environments, such as cancers and chronic infections, lead to downregulated CD28 expression and block CD28 signaling. This reduction of CD28 is accelerated with tumor malignancy, and greater numbers of CD28−CD8 T cells are found hematogenous solid tumors and in the peripheral blood of patients with solid tumors (18,19,20).
Conditional CD28 deletion in CD8 T cells reduces the effects of anti-PD-1 treatment during chronic lymphocytic choriomeningitis virus (LCMV) infection and in mouse tumor models, indicating that CD28 on CD8 T cells plays critical roles in both fundamental co-stimulatory signaling and in effective PD-1 blockade therapy (21). The loss of CD28 expression on exhausted tumor-infiltrating T cells is one obstacle to harnessing the anti-tumor effect of PD-1 blocking. On the other hand, CD28−CD8 T cells themselves exert some anti-tumor effects in melanoma through the expression of perforin (22). In another study, CD28−CD8 T cells showed cytotoxic function in addition to a regulatory role in human cancers (19). Notably, in lung cancer patients, these cells exhibit upregulated FOXP3 expression and play an immune-regulatory role by suppressing T-cell proliferation (20).
A CD28 agonist was developed to reinvigorate exhausted CD8 T cells, and has been tested in many cancer types and inflammatory diseases (23). However, in a phase I trial of the anti-CD28 mAb TGN1412, non-specific CD28 signaling led to systemic inflammatory response (cytokine storm) (24). To overcome this adverse effect of CD28 agonist treatment, modified CD28 agonist mAb strategies have been developed and are currently in clinical trials in solid neoplasms and rheumatoid arthritis to determine the optimal dose, and examine specific targeting using CAR-T cells (25,26,27).
Inducible T-cell co-stimulator (ICOS)
ICOS is a member of the IgSF, which exhibits homology to CD28 and CTLA-4 (28). The expression of ICOS on CD4 and CD8 T cells is rapidly increased by TCR engagement and/or CD28 co-stimulatory signaling (29). Despite the substantial homology, ICOS does not share ligands with CD28 and CTLA-4 due to the lack of a specific MYPPPY motif required for ligation with CD80 and CD86 (30). Thus, ICOS-ICOS ligand (ICOSL) ligation can be differently regulated based on a distinct expression pattern of ICOSL. Additionally, downstream signaling differs between ICOS and CD28. Although ICOS and CD28 ligation similarly induce downstream PI3K and MAPK signaling, ICOS has slightly different signaling pathways, with stronger PI3K signaling, weaker MAPK signaling, and ICOS-specific recruitment of TBK1 (31).
ICOS is constitutively expressed by FOXP3+CD25+CD4+ Treg (32). It is also highly expressed on tonsillar T cells, which are important for germinal center formation and B-lymphocyte maturation (33). In the tumor environment, CD4 T cells are a major population of ICOS-positive T cells. As expected, ICOS is also highly expressed by follicular helper T (Tfh) cells among the TILs in lymphoma patients. Interestingly, CD8 T cells also show upregulated ICOS expression in some lymphoma samples (34). Likewise, highly increased ICOS expression has been observed on Tregs and CD4 and CD8 effector T cells among TILs in mouse syngeneic tumors (35).
Similar to the function of CD28 in efficient anti-PD-1 therapy, ICOS also plays a role in the anti-tumor immune response. Stem-like CXCR5+PD-1int CD8 T cells, which are an important target of anti-PD-1 treatment, express ICOS in a mouse infection model (36). This ICOS expression pattern has also been observed in a mouse tumor model and human cancers, and higher ICOS expression is associated with better overall survival in colorectal cancer patients (37,38,39). This suggests that co-targeting PD-1 and ICOS may be an improved means of further reinvigorating TILs, leading to improved anti-tumor response. ICOS co-stimulation reportedly also enhances the effect of CTLA-4 blockade on tumor immunity (40,41).
The expression of ICOS on Tregs means that ICOS agonism may also have adverse effects on anti-tumor response. ICOS agonist treatment could lead to Treg population expansion, potentially improving the suppressive Treg function in the tumor environment (42,43). However, despite the pro-tumor influence of ICOS stimulation on Tregs, ICOS agonistic treatment with CTLA-4 still has an overall anti-tumor effect. Considering the dual effects of ICOS in tumors, combination treatment with Treg depletion plus inhibitory receptor blockades may be a promising strategy for eliminating side effects and increasing the anti-tumor response to ICOS stimulation. In a mouse model, anti-OX40-mediated Treg depletion plus ICOS signaling promoted an anti-tumor response (35).
CD226
CD226 (also known as DNAM1) is a co-stimulatory receptor of the IgSF, which was first discovered as the human T lineage-specific molecule TLiSA1 (44). CD226 is predominantly expressed on CD8 T cells and NK cells, and is also expressed on activated CD4 T cells and monocytes in humans and mice (45). Upon interaction with its ligands via an ITAM, homo-dimeric CD226 plays important roles in NK-cell and T-cell activation and function (46). Ligands for CD226 include CD155 (necl-5 or PVR) and CD112 (nectin-2 or PVRL2), which are normally expressed on epithelial, endothelial, and Ag-presenting cells (APCs) and are highly upregulated on tumor cells.
The CD226 ligands CD155 and CD112 are also ligands for another IgSF member, T cell Ig and ITIM domain (TIGIT) (47). Compared to TIGIT, CD226 shows much lower binding affinity to its ligands, but still strongly contributes to various immune responses, including T-cell and NK-cell migration, activation, differentiation, and function. The levels of CD226 and TIGIT expression during infection or at tumor sites remain unknown. CD226 mediates the cytotoxicity of NK cells towards tumor cells expressing its ligands, and NK-cell activation is modulated by CD155-expressing dendritic cells (DCs) (45). CD226 also affects CD4 and CD8 T cells by interacting with LFA-1 and lipid rafts, leading to IFN-γ production and increased anti-tumor activity of T cells (48,49). These studies demonstrate that CD226 has dual roles in terms of activation and adhesion receptors.
CD226 signaling can be inhibited by TIGIT due to competition for the same ligands, as well as by TIGIT's ability to directly prevent CD226 homo-dimerization (50). This interfering effect results in a distinct CD226 expression pattern in tumor-infiltrating T cells. In peripheral blood, CD226 expression is highly upregulated on over 80% of tumor-associated Ag (NY-ESO-1)-specific CD8 T cells and total CD8 T cells, in healthy donors and in advanced melanoma patients (51). However, metastatic melanoma exhibits an opposite expression pattern, highly upregulated TIGIT expression in TILs, and a decreased frequency of CD226+ CD8 T cells (51). Low CD226 expression on effector memory CD8 T cells has also been observed in follicular lymphoma and multiple myeloma (52,53). Interestingly, TIGIT+ CD4 T cells exhibit higher CD226 expression than CD8 T cells, and high CD226 expression has been reported on Tfh cells in follicular lymphoma. However, CD25hiFoxp3+Tregs in PBMCs from melanoma patients and healthy donors displayed lower CD226 expression compared to CD25–Foxp3– effector CD4 T cells (52,54).
Considering the competition between CD226 and TIGIT for the same ligands, utilizing the CD226 and TIGIT axis in tumor immunotherapy requires an understanding of T-cell exhaustion and the implications of immune checkpoint blockades, especially TIGIT mAbs. Combined immunotherapy involving TIGIT and PD-1 or PD-L1 increases the cellular proliferation and functionality of tumor-associated Ag-specific CD8 T cells in vitro and induces anti-tumor responses in mouse tumor models (50,51). With the combined blockade of TIGIT and PD-L1, additionally blocking CD226 abrogated the effects on tumor size, survival rate, and increased frequency of IFN-γ-producing CD8 T cells among TILs; however, the effect of upregulated IFN-γ production among CD8 T cells in tumor-draining lymph nodes remained (50). These findings revealed that CD226 plays a critical role in the reinvigoration of CD8 T cells, which induces anti-tumor responses after blocking TIGIT. Additionally, investigations in a mouse model of spontaneous multiple myeloma (Vk*MYC transgenic mice) crossed with CD226 KO mice have demonstrated that CD226 controlled multiple myeloma development, and that this anti-tumor effect of CD226 was modulated by CD8 T cells and NK cells using perforin and IFN-γ (55). Moreover, in melanoma, CD226 signaling upon ligation with PVR abrogates the suppressive function and stability of Tregs, while TIGIT signaling increases Treg-mediated suppression (54). All available data suggest that the interplay between CD226 and TIGIT has a critical role in anti-tumor immunotherapy.
TIM-1, CD2, and signaling lymphocytic activation molecule family member 6 (SLAMF6)
TIM domain family is part of the IgSF, which includes both co-stimulatory and co-inhibitory receptors (56). The TIM family includes 8 molecules in mice (TIMs 1-8) and three molecules in humans (TIM-1, TIM-3, and TIM-4) (57). TIM-1 is a typical co-stimulatory molecule, and its main ligands are TIM-4 and phophatidylserine (58,59). TIM-1 is not expressed in naïve T cells, but its expression is upregulated after activation. Other immune cell types can also express TIM-1, including NK cells, B cells, macrophages, DCs, and mast cells (56,57). Agonistic TIM-1 mAb directly enhances effector T-cell expansion and stability, and inhibits Treg generation and suppressive functions (60). Additionally, DCs that constitutively express TIM-1, TIM-1 signaling induces co-stimulatory molecules and pro-inflammatory cytokine production, indirectly promoting enhanced effector T-cell response (61). Few reports describe the anti-tumor effect of TIM-1; however, agonistic TIM-1 signaling could be a promising new target for anti-tumor treatment based on its potential to stimulate effector T cells.
The IgSF also includes CD2 and members of the signaling lymphocytic activation molecule (SLAM) family, for which the IgV and IgC domains are co-stimulatory receptors (6). Like CD226, CD2 has plays dual roles as co-stimulatory receptor and adhesion molecule for T-cell activation, cytotoxicity of NK and T cells, cytokine production, and formation of the immunologic synapse between T cells and APCs (62). CD2 is expressed on T, NK, and B cells and its ligands are CD48 in mice, and CD58 (LFA-3) in humans. Since CD2 exhibits co-stimulatory function and strong expression in all T and NK cells, irrespective of differentiation and activation status, an agonistic CD2 bispecific Ab has been used to therapeutically target EGFR-expressing tumors (63). Additionally, CD2 shows ligation as an endogenous natural receptor on first-generation CAR T cells, which is important for the IL-2 production of CAR T cells in B-cell lymphoma (64).
SLAMF6 (also known as NTB-A) is a SLAM family member that is expressed on T, NK, and B cells. It upregulates Th1 responses, and through homophilic interaction activates NK cells in terms of proliferation, cytotoxicity, and IFN-γ production (65,66). Interestingly, SLAMF6 expression is highly correlated expression of T-cell factor 1 (TCF-1), which is used as a marker of exhaustion. Both TCF-1 and SLAMF6 are highly upregulated in progenitor exhausted CD8 T cells, but not in terminally exhausted CD8 T cells during chronic infection (67). This study highlighted SLAMF6 as a useful cell surface marker for isolating progenitor exhausted CD8 T cells, as an alternative to TCF-1. In addition to its role as a marker, treatment with the soluble ectodomain of SLAMF6 reportedly improved the CD8 T-cell response in melanoma (68). This homotypic binding of SLAMF6 reduced activation-induced cell death and protected tumor-infiltrating CD8 T cells from apoptosis, to a greater degree than IL-2 (68). Additionally, SLAMF6 directly affects the functions of melanoma-specific CD8 T cells, increasing IFN-γ production and cytotoxicity (68). In vivo studies in a mouse melanoma model revealed that systemic treatment with the soluble ectodomain of SLAMF6 played a role in the maintenance of tumor-specific CD8 T cells and delayed tumor growth (68).
TNFRSF
4-1BB (CD137)
The TNFRSF includes the inducible co-stimulatory receptor 4-1BB, also known as CD137 and TNF receptor (TNFR) 9. Its major ligand is 4-1BB ligand (4-1BBL), which is expressed on activated DCs, macrophages, and B cells (69). Ligation of 4-1BB on T cells induces co-stimulatory signaling, which recruits the key adapter molecules TNFR-associated factor (TRAF) 1 and TRAF2, and leads to activation of NF-κB and MAPKs (70). Additionally, the 4-1BB signaling pathway affects metabolic reprogramming within T cells in terms of promoting mitochondrial function and biogenesis (71). After ligation with 4-1BB agonist Abs, 4-1BB surface expression rapidly decreases due to internalization to the endocellular compartment, where it blocks continuous co-stimulatory signaling (72).
Although 4-1BB is highly upregulated in activated T cells upon TCR signaling and used as a marker of T-cell activation, its expression is also widely observed in immune cells (e.g., DCs, activated monocytes, NK, neutrophils, eosinophils, and mast cells) and in non-hematopoietic cells, such as endothelial cells (72). NK cells upregulate 4-1BB surface expression upon ligation of FcRγIII Fc receptor, and the 4-1BB signaling induces the NK cells' cytotoxic function (73). On endothelial cells, 4-1BB expression can be increased by exposure to TNF-α, liposaccharide, and IL-1β, as well as to hypoxia-inducible factor 1-α in the tumor microenvironment. This suggests that 4-1BB expression, especially on blood vessel walls, may mediate immune responses against inflammation or tumor microvasculature by enhancing migration of monocytes and DCs (74,75,76). Tregs also express 4-1BB; however, its role on Tregs is controversial as 4-1BB can reportedly inhibit Treg suppressive function or induce Treg proliferation following ligation or 4-1BB agonism (72). With regards to T cells, engagement of 4-1BB with TCR signaling induces IL-2 production, regardless of CD28 expression (77). Additionally, ligation of 4-1BB enhances CD8 T-cell proliferation, survival, and effector function (72).
In TILs, 4-1BB expression can represent the tumor reactivity of T cells, meaning that 4-1BB-expressing TILs have specific TCRs that recognize tumor Ags, and ongoing TCR signaling irrespective of T-cell differentiation status or cytokine production (78). With regards to activation-induced 4-1BB expression, TILs show different 4-1BB expression patterns depending on the tumor type, degree of tumor Ag exposure, and stage of T-cell exhaustion. CD8 TILs exhibit greater 4-1BB expression in hepatocellular carcinoma (HCC) and ovarian cancer (OC) than in other cancer types, such as non-small-cell lung cancer (NSCLC), intrahepatic cholangiocellular carcinoma, colorectal cancer, and glioblastoma (79,80). Moreover, 4-1BB is predominantly expressed in PD-1high CD8 TILs, which show tumor reactivity, and is expressed at a significantly higher level in CD8 TILs than in PBMCs from the same HCC patients and normal intrahepatic lymphocytes (79,81). Notably, 4-1BB+ CD8 TILs show fewer exhausted features in terms of proliferation potential and transcriptional level (e.g., TCF-1, T-bet, and Eomes) compared to 4-1BB− CD8 TILs among PD-1high CD8 TILs (79). These findings suggest that distinct 4-1BB expression on CD8 TILs indicates tumor-reactive CD8 T cells, as well as potentially functional CD8 T cells, which may be reinvigorated by immunotherapies targeting exhausted PD-1+ CD8 T cells.
The meaning of 4-1BB expression on CD8 TILs has made 4-1BB signaling a prominent target for anti-tumor immunity. In a mouse melanoma model, mAb-induced 4-1BB signaling reversed a loss of IL-2 production in CD8 TILs. Notably, this co-stimulatory effect of 4-1BB was not due to an increase of newly primed T cells but rather to reinvigoration of dysfunctional TILs (82). Moreover, anti-4-1BB mAb treatment prolonged the intra-tumor persistence of adoptively transferred OT-1 effector T cells, which are specific to the B16-OVA tumor model, and even promoted an effector phenotype and cytotoxicity (83). In an A20 mouse lymphoma model, anti-4-1BB agonistic mAb exerted anti-tumor effects, reducing tumor size and increasing survival rate more than treatment with anti-OX40, anti-CTLA-4, or anti-glucocorticoid-induced TNFR-related protein (GITR) agents (84). This anti-tumor immunity was dependent on CD8 T cells and NK cells, and persisted beyond 100 days after tumor clearance in a rechallenge experiment (84). However, this critical effect of 4-1BB therapy requires 4-1BB expression on CD8 TILs or NK cells, as well as specific tumor environmental factors, such as IFN-γ expression, to allow CD8 T cells to infiltrate the tumor site (85). In human HCC, the combination of 4-1BB agonist plus anti-PD-1 treatment improved CD8 TIL proliferation and cytokine production. This synergistic effect of 4-1BB with PD-1 blockade could be due to the high 4-1BB expression of HCC, and the further upregulation of 4-1BB expression caused by PD-1 blockade of CD8 TILs (79). The significant effect of 4-1BB agonistic mAb in anti-tumor immunity suggests that targeting 4-1BB may be a potent strategy for improving immunotherapy, and a breakthrough for tumors that do not respond to immune checkpoint blockades.
Two 4-1BB mAb evaluated in recent clinical trials are urelumab (BMS-663513) and utomilumab (PF-05082566). Urelumab was the first anti-4-1BB mAb developed with human IgG4 for therapeutic purposes, and does not inhibit binding of endogenous 4-1BBL (86). On the other hand, utomilumab includes hIgG2 and has a blocking effect against endogenous 4-1BBL. Clinical trials of both anti-4-1BB mAbs were unsuccessful due to grade 3–4 treatment-related adverse events, liver toxicity of urelumab, and low efficacy of utomilumab (72). Various strategies have been applied to modify anti-4-1BB mAbs to overcome their liver toxicity and improve their specific agonism. Modifications of the Fc portion of anti-4-1BB have been tested to optimize activation and inhibit FcγR binding that causes hepatotoxicity and low efficacy. Additionally, bi-specific Abs have been developed that have specificity for 4-1BB and tumor Ags, such as HER2 and fibroblast activation protein or PD-L1 (87,88,89,90).
OX40
Another co-stimulatory receptor of the TNFRSF is OX40 (also called CD134 and TNFRSF4), which is a well-known T-cell activation marker, like 4-1BB (91). The ligand of OX40 (OX40L or CD252) is expressed on all activated APCs, including DCs, B cells, and macrophages, as well as on non-hematopoietic cells, including endothelial cells and smooth muscle cells (92). Ligation between OX40 and OX40L plays roles in T-cell survival, maintenance of memory CD8 T cells, differentiation of CD4 T cells, and Treg inhibition (93). OX40 signaling is critical for survival of effector and memory T cells, but not for priming naïve T cells, since OX40-deficient T cells exhibit normal differentiation to effector T cells via TCR signaling but are unable to survive (94). This OX40 signaling required for T-cell survival is dependent on induction of the anti-apoptotic proteins Bcl2 and Bcl-xL, as well as survivin, regulated by NF-κB and AKT signaling (95,96). Additionally, the OX40-OX40L axis significantly inhibits the generation, differentiation, and suppressive function of IL-10-producing CD4 type 1 regulatory T cells (97).
Although T cells consistently express some co-stimulatory receptors, such as CD28, OX40 is only transiently expressed on activated CD4 and CD8 T cells after TCR engagement with inflammatory cytokines, and not on naïve T cells (98). OX40 is constitutively expressed on Tfh cells in mice and humans, and on Tregs only in mice (91). However, OX40 shows a distinct expression pattern in the tumor environment. OX40 is consistently expressed on CD4 TILs in mouse models of glioma and melanoma, and on CD8 TILs of mouse carcinomas (99,100). OX40 expression is also observed on CD4 TILs in human malignant melanoma and colorectal cancer, suggesting persistent TCR engagement against tumor Ags. Moreover, the degree of OX40 expression may be associated with anti-tumor response and patient outcomes (101,102). In an exhausted model with chronic viral infection, OX40 expression on virus-specific CD4 T cells significantly correlated with PD-1 expression, indicating that OX40 expression is dependent on TCR stimulation regardless of T-cell exhaustion status (103).
Many trials have examined OX40 agonistic signaling as anti-tumor therapy through TIL regulation, and the anti-tumor effects of OX40 agonist Abs has been observed in numerous mouse tumor models and preclinical human studies (91). The main mechanisms underlying the anti-tumor effects of OX40 agonism might be explained by modulation of CD8 and CD4 TILs. Anti-OX40 Ab treatment increases the anti-tumor ability of CD8 T cells in the MC38 tumor model and in human melanoma samples, and also promotes the generation of tumor-specific T-cell memory in a murine cancer vaccine model (104). Additionally, in a human clinical trial, OX40 agonist expanded non-Treg CD4 and CD8 T cells with upregulation of the activation markers CD38 and HLA-DR (105). Considering the constitutive OX40 expression on Tregs, OX40 signaling dominantly affects Tregs in tumors. However, the functions of anti-OX40 mAbs on Tregs are controversial, with some reports showing that OX40 blocks the suppressive function of Tregs, while another group demonstrated enhanced Treg proliferation (106,107). These conflicting results indicate that OX40 signaling may regulate Tregs in multiple ways, meaning that the effect of OX40 agonist on Tregs may differ depending on various factors, such as cytokines and other stimulation.
Although OX40 agonists play roles in anti-tumor immunity, such treatment is not sufficient in all tumors. On possible means of overcoming this limitation is by combining OX40 agonistic therapy with immune checkpoint blockades. Anti-OX40 mAb treatment plus CTLA-4 blockade increased the survival rate in a mouse sarcoma model by increasing CD8 and CD4 T-cell function (108). Additionally, combination of anti-OX40 mAb treatment plus the HER2 cancer vaccine promoted anti-tumor functions of CD8 T cells and led to tumor regression (100). PD-1 blockade also exerted a synergistic anti-tumor effect with OX40 agonist treatment in a murine OC model that which showed poor responses to both monotherapies (109). However, in a cancer vaccine model, combination therapy with PD-1 blockade inhibited the anti-tumor effect of anti-OX40 mAb by reducing T-cell infiltration to the tumor and inducing apoptosis of tumor-reactive CD8 T cells (110). Therefore, depending on the context, combination therapy may or may not be conducive to anti-tumor effects. Other efforts to improve anti-tumor immunity with OX40 agonist include co-treatment with IL-2 and CTLA-4 plus OX40 bispecific Ab (111,112).
GITR
GITR protein—also known as TNFRSF18, CD357, or activation-inducible TNFR family receptor—is another co-stimulatory receptor of the TNFRSF (113). GITR signaling is commonly mediated through binding with its ligand GITRL, which is also a member of the TNFRSF and is mainly expressed on activated APCs (114). Similar to OX40L, GITR:GITRL expression is observed on various cell types, and is not restricted to hematopoietic cells—for example, showing intermediate expression on keratinocytes and osteoclast precursors, and high expression on endothelial cells stimulated with type I IFN (114). Due to the absence of intrinsic enzymatic activity, GITR signaling is mediated by recruitment of TRAFs. For example, upon GITR ligation, TRAF2 and TRAF5 induce NF-κB, leading to upregulation of the anti-apoptotic protein Bcl-Xl on activated CD8 T cells—meaning that GITR signaling functions in T-cell survival (115). GITR and GIRTL engagement also induces IL-9-producing Th cells in a manner dependent on TRAF6 and NF-κB activation, yielding improved responses of tumor-specific cytotoxic T cells (116). Thus, GITR signaling mediates several functions via differential TRAF recruitment, depending on context and cell type.
Tregs exhibit high GITR expression induced by Foxp3, while naïve and memory T cells typically do not express GITR until activation. Activated T cells rapidly upregulate GITR expression, which is modulated by NF-κB signaling, and ligation with GITR promotes IL-2 and IFN-γ production via further upregulation of CD25 (117). Importantly, during chronic LCMV infection, mice with genetic deletion of GITR exhibit increased T-cell exhaustion and uncontrolled viral load, which depend on the effects of GITR on CD4 T cells. This suggests that GITR signaling is directly and indirectly critical for the maintenance of T-cell effector function and exhaustion status (118). In the context of tumors, GITR expression has been reported in TILs from HCC, NSCLC, renal cell carcinoma, melanoma, and OC—with high GITR expression observed in Tregs, compared to lower expression in CD4 and CD8 T cells (119). In mouse tumor models, after treatment with anti-mouse GITR agonistic Ab (DTA-1), GITR expression was still highest in Tregs among TILs. However, non-Treg CD4 and CD8 TILs also exhibited high GITR expression and the GITR expression on CD8 and CD4 TILs was highly correlated with the anti-tumor effects of GITR treatment (119).
It appears that tumor regression by GITR signaling to TILs is regulated by the co-stimulatory function of GITR on CD4 and CD8 TILs, as well as effects on Tregs. DTA-1 treatment induces IL-9 production and Th9 differentiation, which enhances the responses of tumor-specific cytotoxic T cells via DC activation in mouse tumors (116). Additionally, GITR agonism increases CD8 T-cell infiltration into the tumor, as well as CD8-TIL metabolism, leading to improved proliferation and production of cytokines, such as IFN-γ (120,121). The effect of GITR signaling on Treg destabilization also promoted anti-tumor immunity against melanoma in mice, by reducing Foxp3 expression in intratumor Tregs—not in circulating Tregs (122). However, when defining the effects of co-stimulatory receptor agonistic Abs, we must also consider the Fc effector function induced by Ab-dependent cellular cytotoxicity (ADCC). In particular, DTA-1 (which has rat IgG2b as its backbone) has controversial effects on Tregs. For example, while DTA-1 induces MC38 tumor regression, this effect is abrogated when using the pentamerized form of the GITRL extracellular domain (pGITRL), which lacks the Fc portion, instead of DTA-1. Additionally, pGITRL treatment increased the number of activated Tregs in tumor tissue and draining lymph nodes (123). These findings indicate that the effects of GITR on Tregs must be considered to effectively raise the anti-tumor response with anti-GITR mAb, and that the effects of DTA-1 may be based on FcγR-mediated Treg depletion.
Numerous studies have demonstrated the anti-tumor effects of GITR, and have suggested various strategies to improve its therapeutic efficacy. The most promising so far is combined treatment with GITR agonist plus immune checkpoint blockade, such as PD-1, which has induced restoration of dysfunctional CD8 TILs by rescuing CD226 and reducing TIGIT expression (124). Another option is local administration of GITR agonistic mAb into tumor-draining lymph nodes (125).
CD27 and death receptor 3 (DR3)
In contrast to other activation-inducible TNFRSF members, CD27 is constitutively expressed on both naïve and effector T cells. The CD27 ligand CD70 is transiently induced by several stimuli on DCs, B cells, and T cells. In particular, the ligation of CD27 and CD70 generates an important key molecule in the helping mechanism of CD4 T cells, leading to priming and effector differentiation of cytotoxic T cells, and optimal anti-tumor response (126). However, continuous CD27 signaling is considered an important factor in inducing T-cell exhaustion and affecting Treg survival, suggesting that CD27 agonism is also involved in tumor progression (127,128). Nevertheless, agonistic Abs targeting CD27 have exhibited anti-tumor effects in various mouse tumor models, and a newly engineered anti-CD27 mAb that induces ADCC by replacing the Fc portion (mouse IgG1 to mIgG2a) promoted better anti-tumor response via limited T-cell activation and FcγR-mediated depletion of CD27hi Tregs (129,130).
DR3 is a co-stimulatory receptor in the TNFRSF, which is encoded by TNFRSF25. DR3 binds to a cognate ligand, called TL1A or TNFSF15, and is expressed on the surface of activated T cells, APCs, and phagocytes (131). Unlike other co-stimulatory receptors, DR3 uses TNFR-associated death domain adaptor protein to recruit TRAF2 and RIPK1, subsequently inducing NF-κB activation and MAPK pathways in T cells (131). Ligation of DR3 with TL1A induces T-cell proliferation and enhanced IFN-γ production in an infection model (132). DR3 agonistic Ab (4C12) or TL1A-lg fusion protein causes expansion of the Treg population; however, at the cellular level, DR3 engagement reduces the suppressive function of Tregs (133). Like GITR, co-stimulatory DR3 signaling induces Th1 and Th9 differentiation of CD4 T cells, and diminishes the suppressive function of Tregs, suggesting that agonistic mAb targeting DR3 is a potential candidate for anti-tumor immunotherapy (134).
Overall, the available data indicate that various co-stimulatory molecules can modulate TILs and target specific co-stimulatory receptors. Treatments targeting these costimulatory molecules could be a potential way to improve standard anti-tumor therapies, or even a breakthrough treatment for tumors that are non-reactive to recent immunotherapies.
CONCLUSION
In this review, we have discussed recent studies about the characteristics of co-stimulatory receptors and the current status of developing agonist Abs targeting co-stimulatory receptors in cancers. In addition to inhibitory receptors, activated T cells and exhausted T cells also exhibit upregulation of numerous co-stimulatory receptors (3). As immune-stimulating co-stimulatory receptors are an essential element of anti-cancer immunity, it is clear that effective activation of these receptors can contribute to the therapeutic repertoires against cancers. Previous preclinical experiments and clinical data clearly support that agonizing a co-stimulatory pathway can be a potently effective strategy for re-invigorating T-cell responses in cancers, particularly when used in combination with other immune-activating strategies. Moreover, newly-developed agonist Abs targeting novel co-stimulatory receptors such as anti-TNFR2 are being investigated for therapy of cancer (135).
However, more cross-disciplinary studies need to be carried out to maximize patient benefit despite overwhelming evidences demonstrating the potent therapeutic effectiveness of agonist Abs targeting co-stimulatory receptors. It has been suggested that some co-stimulatory agonist Abs function by a dual mechanism: 1) by agonist activation of tumor-specific T cell populations, and 2) by immune cell depletion (such as Tregs) via ADCC. In addition, the interaction between Fc domain of Abs and FcγRs has been suggested as a requisite for optimal function of agonist Abs targeting co-stimulatory receptors (86). Therefore, IgG isotype selection and full characterization of Fc domain property is critical for the design of co-stimulatory agonist Abs, particularly with those having human IgG1, with a consideration of the relative effect of immune cell activation versus immune cell depletion via ADCC in cancer patients. Moreover, the expression profiles and distinct roles of various co-stimulatory receptors on Tregs need to be studied. Finally, as the immune status context may vary widely in patients even with the same type of cancer, patient selection based on immune profiles may become critical for the effective use of agonist Abs.
In this regard, recent advances in our understating of co-stimulatory receptor pathways and next-generation approaches under development will provide rationale and evidence for the logical design of immunotherapies targeting costimulatory receptors.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A2C2005176).
Abbreviations
- 4-1BBL
4-1BB ligand
- ADCC
Ab-dependent cellular cytotoxicity
- APC
Ag-presenting cell
- CAR
chimeric Ag receptor
- DC
dendritic cell
- DR-3
death receptor 3
- GITR
glucocorticoid-induced TNF receptor-related protein
- GITRL
GITR ligand
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibitor
- ICOS
inducible T-cell co-stimulator
- ICOSL
ICOS ligand
- IgSF
Ig receptor superfamily
- LCMV
lymphocytic choriomeningitis virus
- NSCLC
non-small-cell lung cancer
- OC
ovarian cancer
- OX40L
OX40 ligand
- pGITRL
pentamerized form of the GITRL extracellular domain
- SLAM
signaling lymphocytic activation molecule
- SLAMF6
signaling lymphocytic activation molecule family member 6
- TCF-1
T-cell factor 1
- Tfh
follicular helper T
- TIGIT
T cell Ig and ITIM domain
- TIM
T-cell Ig and mucin-domain containing
- TIL
tumor-infiltrated lymphocyte
- TNFR
TNF receptor
- TNFRSF
TNF receptor superfamily
- TRAF
TNF receptor-associated factor
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Jeong S, Park SH.
- Writing - original draft: Jeong S, Park SH.
- Writing - review & editing: Jeong S, Park SH.
References
- 1.Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11:8. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 3.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D, et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell. 2016;166:1500–1511.e9. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. [PubMed] [Google Scholar]
- 8.Mueller DL, Jenkins MK, Schwartz RH. An accessory cell-derived costimulatory signal acts independently of protein kinase C activation to allow T cell proliferation and prevent the induction of unresponsiveness. J Immunol. 1989;142:2617–2628. [PubMed] [Google Scholar]
- 9.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 10.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian R, Wang H, Gish GD, Petsalaki E, Pasculescu A, Shi Y, Mollenauer M, Bagshaw RD, Yosef N, Hunter T, et al. Combinatorial proteomic analysis of intercellular signaling applied to the CD28 T-cell costimulatory receptor. Proc Natl Acad Sci U S A. 2015;112:E1594–E1603. doi: 10.1073/pnas.1503286112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 13.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8+CD28− T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, et al. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 20.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, Ferrari M, Cosentino M, Marino F, Pozzi E, et al. Foxp3 expressing CD4+ CD25+ and CD8+ CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casado JG, Soto R, DelaRosa O, Peralbo E, del Carmen Muñoz-Villanueva M, Rioja L, Peña J, Solana R, Tarazona R. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–1171. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huff WX, Kwon JH, Henriquez M, Fetcko K, Dey M. The evolving role of CD8+CD28− immunosenescent T cells in cancer immunology. Int J Mol Sci. 2019;20:2810. doi: 10.3390/ijms20112810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 25.Tyrsin D, Chuvpilo S, Matskevich A, Nemenov D, Römer PS, Tabares P, Hünig T. From TGN1412 to TAB08: the return of CD28 superagonist therapy to clinical development for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:45–48. [PubMed] [Google Scholar]
- 26.Tang XY, Sun Y, Zhang A, Hu GL, Cao W, Wang DH, Zhang B, Chen H. Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open. 2016;6:e013904. doi: 10.1136/bmjopen-2016-013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabo M, Offringa R, Zitvogel L, Kroemer G, Muntasell A, Galluzzi L. Trial Watch: immunostimulatory monoclonal antibodies for oncological indications. OncoImmunology. 2017;6:e1371896. doi: 10.1080/2162402X.2017.1371896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 29.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 30.Peach RJ, Bajorath J, Brady W, Leytze G, Greene J, Naemura J, Linsley PS. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180:2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wikenheiser DJ, Stumhofer JS. ICOS co-stimulation: friend or foe? Front Immunol. 2016;7:304. doi: 10.3389/fimmu.2016.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 33.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 34.Le KS, Amé-Thomas P, Tarte K, Gondois-Rey F, Granjeaud S, Orlanducci F, Foucher ED, Broussais F, Bouabdallah R, Fest T, et al. CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas. Blood Adv. 2018;2:1889–1900. doi: 10.1182/bloodadvances.2018017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzger TC, Long H, Potluri S, Pertel T, Bailey-Bucktrout SL, Lin JC, Fu T, Sharma P, Allison JP, Feldman RM. ICOS promotes the function of CD4+ effector T cells during anti-OX40-mediated tumor rejection. Cancer Res. 2016;76:3684–3689. doi: 10.1158/0008-5472.CAN-15-3412. [DOI] [PubMed] [Google Scholar]
- 36.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyrend G, van der Gracht E, Yilmaz A, van Duikeren S, Camps M, Höllt T, Vilanova A, van Unen V, Koning F, de Miranda NF, et al. PD-L1 blockade engages tumor-infiltrating lymphocytes to co-express targetable activating and inhibitory receptors. J Immunother Cancer. 2019;7:217. doi: 10.1186/s40425-019-0700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Luo Y, Qin SL, Mu YF, Qi Y, Yu MH, Zhong M. The clinical impact of ICOS signal in colorectal cancer patients. OncoImmunology. 2016;5:e1141857. doi: 10.1080/2162402X.2016.1141857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soldevilla MM, Villanueva H, Meraviglia-Crivelli D, Menon AP, Ruiz M, Cebollero J, Villalba M, Moreno B, Lozano T, Llopiz D, et al. ICOS costimulation at the tumor site in combination with ctla-4 blockade therapy elicits strong tumor immunity. Mol Ther. 2019;27:1878–1891. doi: 10.1016/j.ymthe.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211:715–725. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q, Mo L, Cai X, Wei L, Xie Z, Li H, Li J, Hu Z. ICOS signal facilitates Foxp3 transcription to favor suppressive function of regulatory T cells. Int J Med Sci. 2018;15:666–673. doi: 10.7150/ijms.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amatore F, Gorvel L, Olive D. Inducible co-stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin Ther Targets. 2018;22:343–351. doi: 10.1080/14728222.2018.1444753. [DOI] [PubMed] [Google Scholar]
- 44.Burns GF, Triglia T, Werkmeister JA, Begley CG, Boyd AW. TLiSA1, a human T lineage-specific activation antigen involved in the differentiation of cytotoxic T lymphocytes and anomalous killer cells from their precursors. J Exp Med. 1985;161:1063–1078. doi: 10.1084/jem.161.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou S, Ge K, Zheng X, Wei H, Sun R, Tian Z. CD226 protein is involved in immune synapse formation and triggers natural killer (NK) cell activation via its first extracellular domain. J Biol Chem. 2014;289:6969–6977. doi: 10.1074/jbc.M113.498253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan CJ, Andrews DM, Smyth MJ. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr Opin Immunol. 2012;24:246–251. doi: 10.1016/j.coi.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188:3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsbottom KM, Hawkins ED, Shimoni R, McGrath M, Chan CJ, Russell SM, Smyth MJ, Oliaro J. Cutting edge: DNAX accessory molecule 1-deficient CD8+ T cells display immunological synapse defects that impair antitumor immunity. J Immunol. 2014;192:553–557. doi: 10.4049/jimmunol.1302197. [DOI] [PubMed] [Google Scholar]
- 50.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Josefsson SE, Huse K, Kolstad A, Beiske K, Pende D, Steen CB, Inderberg EM, Lingjærde OC, Østenstad B, Smeland EB, et al. T cells expressing checkpoint receptor TIGIT are enriched in follicular lymphoma tumors and characterized by reversible suppression of T-cell receptor signaling. Clin Cancer Res. 2018;24:870–881. doi: 10.1158/1078-0432.CCR-17-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guillerey C, Harjunpää H, Carrié N, Kassem S, Teo T, Miles K, Krumeich S, Weulersse M, Cuisinier M, Stannard K, et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood. 2018;132:1689–1694. doi: 10.1182/blood-2018-01-825265. [DOI] [PubMed] [Google Scholar]
- 54.Fourcade J, Sun Z, Chauvin JM, Ka M, Davar D, Pagliano O, Wang H, Saada S, Menna C, Amin R, et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight. 2018;3:e121157. doi: 10.1172/jci.insight.121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guillerey C, Ferrari de Andrade L, Vuckovic S, Miles K, Ngiow SF, Yong MC, Teng MW, Colonna M, Ritchie DS, Chesi M, et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J Clin Invest. 2015;125:2077–2089. doi: 10.1172/JCI77181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kane LP. T cell Ig and mucin domain proteins and immunity. J Immunol. 2010;184:2743–2749. doi: 10.4049/jimmunol.0902937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du P, Xiong R, Li X, Jiang J. Immune regulation and antitumor effect of TIM-1. J Immunol Res. 2016;2016:8605134. doi: 10.1155/2016/8605134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi N, Karisola P, Peña-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, Alexopoulos S, Oukka M, Umetsu DT, DeKruyff RH, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao S, Zhu B, Jin H, Zhu C, Umetsu DT, DeKruyff RH, Kuchroo VK. Tim-1 stimulation of dendritic cells regulates the balance between effector and regulatory T cells. Eur J Immunol. 2011;41:1539–1549. doi: 10.1002/eji.201040993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musgrave BL, Watson CL, Hoskin DW. CD2-CD48 interactions promote cytotoxic T lymphocyte induction and function: anti-CD2 and anti-CD48 antibodies impair cytokine synthesis, proliferation, target recognition/adhesion, and cytotoxicity. J Interferon Cytokine Res. 2003;23:67–81. doi: 10.1089/107999003321455462. [DOI] [PubMed] [Google Scholar]
- 63.Wild MK, Strittmatter W, Matzku S, Schraven B, Meuer SC. Tumor therapy with bispecific antibody: the targeting and triggering steps can be separated employing a CD2-based strategy. J Immunol. 1999;163:2064–2072. [PubMed] [Google Scholar]
- 64.Cheadle EJ, Rothwell DG, Bridgeman JS, Sheard VE, Hawkins RE, Gilham DE. Ligation of the CD2 co-stimulatory receptor enhances IL-2 production from first-generation chimeric antigen receptor T cells. Gene Ther. 2012;19:1114–1120. doi: 10.1038/gt.2011.192. [DOI] [PubMed] [Google Scholar]
- 65.Valdez PA, Wang H, Seshasayee D, van Lookeren Campagne M, Gurney A, Lee WP, Grewal IS. NTB-A, a new activating receptor in T cells that regulates autoimmune disease. J Biol Chem. 2004;279:18662–18669. doi: 10.1074/jbc.M312313200. [DOI] [PubMed] [Google Scholar]
- 66.Flaig RM, Stark S, Watzl C. Cutting edge: NTB-A activates NK cells via homophilic interaction. J Immunol. 2004;172:6524–6527. doi: 10.4049/jimmunol.172.11.6524. [DOI] [PubMed] [Google Scholar]
- 67.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eisenberg G, Engelstein R, Geiger-Maor A, Hajaj E, Merims S, Frankenburg S, Uzana R, Rutenberg A, Machlenkin A, Frei G, et al. Soluble SLAMF6 receptor induces strong CD8+ T-cell effector function and improves anti-melanoma activity in vivo. Cancer Immunol Res. 2018;6:127–138. doi: 10.1158/2326-6066.CIR-17-0383. [DOI] [PubMed] [Google Scholar]
- 69.Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Jenkins NA, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 70.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 71.Menk AV, Scharping NE, Rivadeneira DB, Calderon MJ, Watson MJ, Dunstane D, Watkins SC, Delgoffe GM. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J Exp Med. 2018;215:1091–1100. doi: 10.1084/jem.20171068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 73.Chester C, Ambulkar S, Kohrt HE. 4-1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunother. 2016;65:1243–1248. doi: 10.1007/s00262-016-1829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE, Lim YC, Schwarz H. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB J. 2007;21:456–463. doi: 10.1096/fj.05-4739com. [DOI] [PubMed] [Google Scholar]
- 75.Teijeira Á, Palazón A, Garasa S, Marré D, Aubá C, Rogel A, Murillo O, Martínez-Forero I, Lang F, Melero I, et al. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J. 2012;26:3380–3392. doi: 10.1096/fj.11-201061. [DOI] [PubMed] [Google Scholar]
- 76.Palazón A, Teijeira A, Martínez-Forero I, Hervás-Stubbs S, Roncal C, Peñuelas I, Dubrot J, Morales-Kastresana A, Pérez-Gracia JL, Ochoa MC, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71:801–811. doi: 10.1158/0008-5472.CAN-10-1733. [DOI] [PubMed] [Google Scholar]
- 77.Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HD, Park S, Jeong S, Lee YJ, Lee H, Kim CG, Kim KH, Hong SM, Lee JY, Kim S, et al. 4-1BB delineates distinct activation status of exhausted tumor-infiltrating CD8+ T cells in hepatocellular carcinoma. Hepatology. 2019 doi: 10.1002/hep.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ., Jr CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014;20:44–55. doi: 10.1158/1078-0432.CCR-13-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24:994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams JB, Horton BL, Zheng Y, Duan Y, Powell JD, Gajewski TF. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J Exp Med. 2017;214:381–400. doi: 10.1084/jem.20160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weigelin B, Bolaños E, Teijeira A, Martinez-Forero I, Labiano S, Azpilikueta A, Morales-Kastresana A, Quetglas JI, Wagena E, Sánchez-Paulete AR, et al. Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb. Proc Natl Acad Sci U S A. 2015;112:7551–7556. doi: 10.1073/pnas.1506357112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, Torchia JA, Kolstad A, Chen L, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayes PA, Hance KW, Hoos A. The promise and challenges of immune agonist antibody development in cancer. Nat Rev Drug Discov. 2018;17:509–527. doi: 10.1038/nrd.2018.75. [DOI] [PubMed] [Google Scholar]
- 87.Qi X, Li F, Wu Y, Cheng C, Han P, Wang J, Yang X. Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat Commun. 2019;10:2141. doi: 10.1038/s41467-019-10088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hinner MJ, Aiba RS, Jaquin TJ, Berger S, Dürr MC, Schlosser C, Allersdorfer A, Wiedenmann A, Matschiner G, Schüler J, et al. Tumor-localized costimulatory t-cell engagement by the 4-1bb/her2 bispecific antibody-anticalin fusion prs-343. Clin Cancer Res. 2019;25:5878–5889. doi: 10.1158/1078-0432.CCR-18-3654. [DOI] [PubMed] [Google Scholar]
- 89.Claus C, Ferrara C, Xu W, Sam J, Lang S, Uhlenbrock F, Albrecht R, Herter S, Schlenker R, Hüsser T, et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019;11:eaav5989. doi: 10.1126/scitranslmed.aav5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Labrijn AF, Janmaat ML, Reichert JM, Parren PW. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 91.Linch SN, McNamara MJ, Redmond WL. Ox40 agonists and combination immunotherapy: Putting the pedal to the metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishii N, Takahashi T, Soroosh P, Sugamura K. OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol. 2010;105:63–98. doi: 10.1016/S0065-2776(10)05003-0. [DOI] [PubMed] [Google Scholar]
- 93.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 94.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 95.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 96.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willoughby J, Griffiths J, Tews I, Cragg MS. OX40: structure and function - what questions remain? Mol Immunol. 2017;83:13–22. doi: 10.1016/j.molimm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 99.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 100.Linch SN, Kasiewicz MJ, McNamara MJ, Hilgart-Martiszus IF, Farhad M, Redmond WL. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc Natl Acad Sci U S A. 2016;113:E319–E327. doi: 10.1073/pnas.1510518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ladányi A, Somlai B, Gilde K, Fejös Z, Gaudi I, Tímár J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10:521–530. doi: 10.1158/1078-0432.ccr-1161-03. [DOI] [PubMed] [Google Scholar]
- 102.Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134) Am J Surg. 2002;183:512–518. doi: 10.1016/s0002-9610(02)00831-0. [DOI] [PubMed] [Google Scholar]
- 103.Boettler T, Moeckel F, Cheng Y, Heeg M, Salek-Ardakani S, Crotty S, Croft M, von Herrath MG. OX40 facilitates control of a persistent virus infection. PLoS Pathog. 2012;8:e1002913. doi: 10.1371/journal.ppat.1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng W, Williams LJ, Xu C, Melendez B, McKenzie JA, Chen Y, Jackson HL, Voo KS, Mbofung RM, Leahey SE, et al. Anti-OX40 antibody directly enhances the function of tumor-reactive CD8+ T cells and synergizes with PI3Kβ inhibition in PTEN loss melanoma. Clin Cancer Res. 2019;25:6406–6416. doi: 10.1158/1078-0432.CCR-19-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73:7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Polesso F, Sarker M, Weinberg AD, Murray SE, Moran AE. OX40 agonist tumor immunotherapy does not impact regulatory T cell suppressive function. J Immunol. 2019;203:2011–2019. doi: 10.4049/jimmunol.1900696. [DOI] [PubMed] [Google Scholar]
- 108.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2:142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo Z, Wang X, Cheng D, Xia Z, Luan M, Zhang S. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One. 2014;9:e89350. doi: 10.1371/journal.pone.0089350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shrimali RK, Ahmad S, Verma V, Zeng P, Ananth S, Gaur P, Gittelman RM, Yusko E, Sanders C, Robins H, et al. Concurrent pd-1 blockade negates the effects of OX40 agonist antibody in combination immunotherapy through inducing T-cell apoptosis. Cancer Immunol Res. 2017;5:755–766. doi: 10.1158/2326-6066.CIR-17-0292. [DOI] [PubMed] [Google Scholar]
- 111.Redmond WL, Triplett T, Floyd K, Weinberg AD. Dual anti-OX40/IL-2 therapy augments tumor immunotherapy via IL-2R-mediated regulation of OX40 expression. PLoS One. 2012;7:e34467. doi: 10.1371/journal.pone.0034467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kvarnhammar AM, Veitonmäki N, Hägerbrand K, Dahlman A, Smith KE, Fritzell S, von Schantz L, Thagesson M, Werchau D, Smedenfors K, et al. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J Immunother Cancer. 2019;7:103. doi: 10.1186/s40425-019-0570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10. doi: 10.1016/j.ejca.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 114.Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv Exp Med Biol. 2009;647:156–173. doi: 10.1007/978-0-387-89520-8_11. [DOI] [PubMed] [Google Scholar]
- 115.Snell LM, McPherson AJ, Lin GH, Sakaguchi S, Pandolfi PP, Riccardi C, Watts TH. CD8 T cell-intrinsic GITR is required for T cell clonal expansion and mouse survival following severe influenza infection. J Immunol. 2010;185:7223–7234. doi: 10.4049/jimmunol.1001912. [DOI] [PubMed] [Google Scholar]
- 116.Kim IK, Kim BS, Koh CH, Seok JW, Park JS, Shin KS, Bae EA, Lee GE, Jeon H, Cho J, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat Med. 2015;21:1010–1017. doi: 10.1038/nm.3922. [DOI] [PubMed] [Google Scholar]
- 117.Zhan Y, Gerondakis S, Coghill E, Bourges D, Xu Y, Brady JL, Lew AM. Glucocorticoid-induced TNF receptor expression by T cells is reciprocally regulated by NF-kappaB and NFAT. J Immunol. 2008;181:5405–5413. doi: 10.4049/jimmunol.181.8.5405. [DOI] [PubMed] [Google Scholar]
- 118.Clouthier DL, Zhou AC, Wortzman ME, Luft O, Levy GA, Watts TH. GITR intrinsically sustains early type 1 and late follicular helper CD4 T cell accumulation to control a chronic viral infection. PLoS Pathog. 2015;11:e1004517. doi: 10.1371/journal.ppat.1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vence L, Bucktrout SL, Fernandez Curbelo I, Blando J, Smith BM, Mahne AE, Lin JC, Park T, Pascua E, Sai T, et al. Characterization and comparison of GITR expression in solid tumors. Clin Cancer Res. 2019;25:6501–6510. doi: 10.1158/1078-0432.CCR-19-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sabharwal SS, Rosen DB, Grein J, Tedesco D, Joyce-Shaikh B, Ueda R, Semana M, Bauer M, Bang K, Stevenson C, et al. GITR agonism enhances cellular metabolism to support CD8+ T-cell proliferation and effector cytokine production in a mouse tumor model. Cancer Immunol Res. 2018;6:1199–1211. doi: 10.1158/2326-6066.CIR-17-0632. [DOI] [PubMed] [Google Scholar]
- 121.Cho JS, Hsu JV, Morrison SL. Localized expression of GITR-L in the tumor microenvironment promotes CD8+ T cell dependent anti-tumor immunity. Cancer Immunol Immunother. 2009;58:1057–1069. doi: 10.1007/s00262-008-0622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim YH, Shin SM, Choi BK, Oh HS, Kim CH, Lee SJ, Kim KH, Lee DG, Park SH, Kwon BS. Authentic GITR signaling fails to induce tumor regression unless Foxp3+ regulatory T cells are depleted. J Immunol. 2015;195:4721–4729. doi: 10.4049/jimmunol.1403076. [DOI] [PubMed] [Google Scholar]
- 124.Wang B, Zhang W, Jankovic V, Golubov J, Poon P, Oswald EM, Gurer C, Wei J, Ramos I, Wu Q, et al. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8+ T cell dysfunction and maintain memory phenotype. Sci Immunol. 2018;3:eaat7061. doi: 10.1126/sciimmunol.aat7061. [DOI] [PubMed] [Google Scholar]
- 125.Narumi K, Miyakawa R, Shibasaki C, Henmi M, Mizoguchi Y, Ueda R, Hashimoto H, Hiraoka N, Yoshida T, Aoki K. Local administration of GITR agonistic antibody induces a stronger antitumor immunity than systemic delivery. Sci Rep. 2019;9:5562. doi: 10.1038/s41598-019-41724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahrends T, Spanjaard A, Pilzecker B, Babala N, Bovens A, Xiao Y, Jacobs H, Borst J. CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity. 2017;47:848–861.e5. doi: 10.1016/j.immuni.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 127.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 128.Claus C, Riether C, Schürch C, Matter MS, Hilmenyuk T, Ochsenbein AF. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer Res. 2012;72:3664–3676. doi: 10.1158/0008-5472.CAN-11-2791. [DOI] [PubMed] [Google Scholar]
- 129.Wasiuk A, Testa J, Weidlick J, Sisson C, Vitale L, Widger J, Crocker A, Thomas LJ, Goldstein J, Marsh HC, et al. CD27-mediated regulatory T cell depletion and effector T cell costimulation both contribute to antitumor efficacy. J Immunol. 2017;199:4110–4123. doi: 10.4049/jimmunol.1700606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He LZ, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, Pilsmaker CD, Round SM, Tutt A, Glennie MJ, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191:4174–4183. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]
- 131.Ward-Kavanagh LK, Lin WW, Šedý JR, Ware CF. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44:1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Twohig JP, Marsden M, Cuff SM, Ferdinand JR, Gallimore AM, Perks WV, Al-Shamkhani A, Humphreys IR, Wang EC. The death receptor 3/TL1A pathway is essential for efficient development of antiviral CD4+ and CD8+ T-cell immunity. FASEB J. 2012;26:3575–3586. doi: 10.1096/fj.11-200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meylan F, Richard AC, Siegel RM. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244:188–196. doi: 10.1111/j.1600-065X.2011.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Richard AC, Tan C, Hawley ET, Gomez-Rodriguez J, Goswami R, Yang XP, Cruz AC, Penumetcha P, Hayes ET, Pelletier M, et al. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J Immunol. 2015;194:3567–3582. doi: 10.4049/jimmunol.1401220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tam EM, Fulton RB, Sampson JF, Muda M, Camblin A, Richards J, Koshkaryev A, Tang J, Kurella V, Jiao Y, et al. Antibody-mediated targeting of TNFR2 activates CD8+ T cells in mice and promotes antitumor immunity. Sci Transl Med. 2019;11:eaax0720. doi: 10.1126/scitranslmed.aax0720. [DOI] [PubMed] [Google Scholar]