Abstract
Most patients with hepatocellular carcinoma (HCC) are diagnosed at an advanced stage of disease. Until recently, systemic treatment options that showed survival benefits in HCC have been limited to tyrosine kinase inhibitors, antibodies targeting oncogenic signaling pathways or VEGF receptors. The HCC tumor microenvironment is characterized by a dysfunction of the immune system through multiple mechanisms, including accumulation of various immunosuppressive factors, recruitment of regulatory T cells and myeloid-derived suppressor cells, and induction of T cell exhaustion accompanied with the interaction between immune checkpoint ligands and receptors. Immune checkpoint inhibitors (ICIs) have been interfered this interaction and have altered therapeutic landscape of multiple cancer types including HCC. In this review, we discuss the use of anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies in the treatment of advanced HCC. However, ICIs as a single agent do not benefit a significant portion of patients. Therefore, various clinical trials are exploring possible synergistic effects of combinations of different ICIs (anti-PD-1/PD-L1 and anti-CTLA-4 antibodies) or ICIs and target agents. Combinations of ICIs with locoregional therapies may also improve therapeutic responses.
Keywords: Carcinoma, hepatocellular; Immune checkpoint inhibitor; Therapeutics
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most frequently diagnosed cancers and is a leading cause of cancer-related death. Cirrhosis induced by infection, such as by hepatitis B or C virus, is the principal cause of HCC. Other factors, e.g., alcohol, drugs, autoimmune hepatitis, and non-alcoholic fatty liver disease are also associated with HCC development. The incidence of HCC is gradually increasing worldwide despite the development of potent antiviral agents (1,2,3). Chronic inflammation and subsequent fibrosis can induce the development of HCC; inflammation also results in increased tumor immunogenicity.
In the early stages of HCC, curative treatment is possible. However, 70%–80% of patients are diagnosed with advanced-stage HCC (4). Sorafenib is the first-line systemic therapy for patients with Child-Pugh A cirrhosis and Barcelona clinic liver cancer-stage C (5). Sorafenib is an oral multi-tyrosine kinase inhibitor that targets a number of signaling pathways, such as the pathway centered on VEGF (6). Lenvatinib is an alternative first-line therapy and is non-inferior to sorafenib (5,7). Until 2017, there was no second-line treatment for patients in whom sorafenib treatment failed. Regorafenib and cabozantinib are systemic therapies that have recently been used as second-line treatments (8,9). Ramucirumab after sorafenib in patients with advanced HCC and increased α-fetoprotein showed improved overall survival compared with placebo group (10). However, improvements in the overall survival rate have been unsatisfactory. Clearly, new approaches for HCC remain necessary.
Recent advances in molecular and tumor biology have dramatically changed the paradigm of cancer treatment. The development of immune checkpoint inhibitors (ICIs) was clinical breakthrough. Two major targets of immunotherapy are CTLA-4 (also known as CD152) and PD-1 with PD-L1. These molecules inhibit T cell activation and promote a state of T cell dysfunction known as T cell exhaustion (11). ICIs, such as anti-CTLA-4 (e.g., ipilimumab, tremelimumab), anti-PD-1 (e.g., nivolumab, pembrolizumab), and anti-PD-L1 (e.g., durvalumab, atezolizumab) antibodies, are currently approved for several types of hematologic and solid malignancies. HCC occurs in the context of inflammatory environments. Numerous studies have demonstrated the role of immune tolerance in the development of this cancer, suggesting that suppression of ICIs may be an effective treatment strategy (12). In this review, we discuss the current status and future directions of ICIs for HCC (Table 1).
Table 1. Clinical trials associated with ICIs in hepatocellular carcinoma.
| Drug name | Trial name | Phase | Line of therapy | Design | NCT number | Status | |
|---|---|---|---|---|---|---|---|
| Anti-PD-1 | |||||||
| Nivolumab | CheckMate 040 | I/II | 1L/2L | Nivolumab vs. sorafenib | NCT01658878 | Completed | |
| CheckMate459 | III | 1L | Nivolumab vs. sorafenib | NCT02576509 | Completed | ||
| - | Ib/II | 2L | Nivolumab+galunisertib (TGF-β receptor I kinase inhibitor) | NCT02423343 | Recruiting | ||
| Pembrolizumab | KEYNOTE-224 | II | 2L | Pembrolizumab | NCT02702414 | Completed | |
| KEYNOTE-240 | III | 2L | Pembrolizumab vs. placebo | NCT02702401 | Recruiting | ||
| - | I | 2L | Pemrolizumab+lenvatinib | NCT03006926 | Recruiting | ||
| Tislelizumab | RATIONALE-301 | III | 1L | Tislelizumab vs. sorafenib | NCT03412773 | Recruiting | |
| Camrelizumab | - | II/III | 2L | Camrelizumab | NCT02989922 | Recruiting | |
| - | II | 1L/2L | Camrelizumab+apatinib vs. Camrelizumab+FOLFOX4 | NCT03092895 | Recruiting | ||
| Sintilimab | ORIENT-32 | III | 1L | Sintilimab+bevacizumab (VEGF Ab) vs. sorafenib | NCT03794440 | Recruiting | |
| Anti-PD-L1 | |||||||
| Durvalumab | HIMALAYA | III | 1L | Durvalumab+tremelimumab (CTLA-4 Ab) vs. durvalumab | NCT03298451 | Recruiting | |
| - | II | 1L/2L | Durvalumab; tremelimumab; durvalumab+tremelimumab | NCT02519348 | Recruiting | ||
| - | I | 2L | Durvalumab+ramucirumab (VEGFR2 inhibitor) | NCT02572687 | Recruiting | ||
| Atezolizumab | - | I | 1L | Atezolizumab+bevacizumab | NCT02715531 | Recruiting | |
| - | III | 1L | Atezolizumab+bevacizumab vs. sorafenib | NCT03434379 | Recruiting | ||
| Avelumab | - | I | 1L | Avelumab+axitinib (tyrosine kinase inhibitor) | NCT03289533 | Recruiting | |
| Anti-CTLA-4 | |||||||
| Tremelimumab | - | II | 2L | Tremelimumab (HCV) | NCT01008358 | Completed | |
| Ipilimumab | - | II | Neoadjuvant | Ipilimumab+nivolumab vs. nivolumab | NCT03222076 | Recruiting | |
1L, first line; 2L, second line; HCV, hepatitis C virus.
IMMUNOLOGY IN HCC
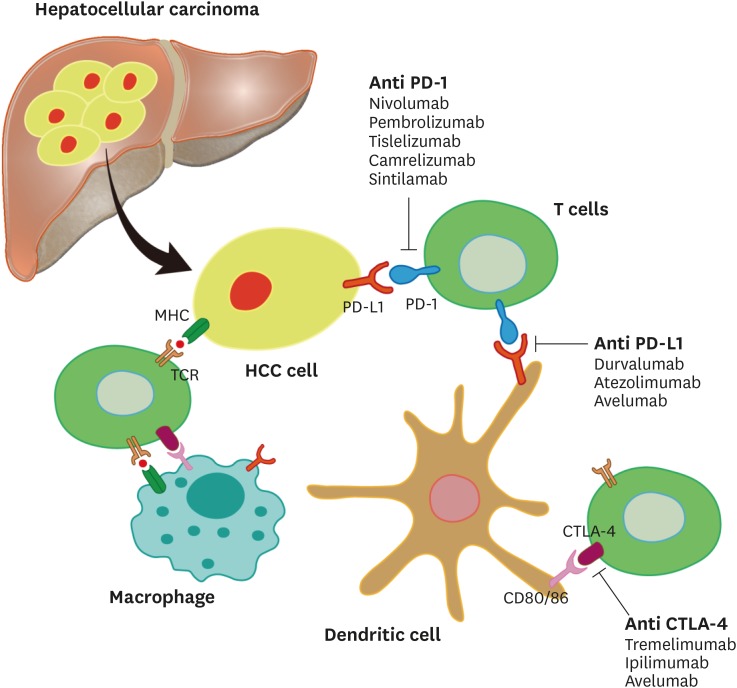
The liver receives blood from hepatic artery and portal vein, enabling it to detect and initiate immunological responses against viruses, tumors, and parasites (13). However, the inflammatory response causes hepatocellular DNA damage, promotes immune tolerance, and confers transformed hepatocytes to evade host immune surveillance, which cooperatively contribute to initiation and progression of HCC (14,15). Furthermore, the immunosuppressive tumor microenvironment mediates HCC immune tolerance and evasion (16,17). HCC development and progression involves the dysfunction of various human immune components, including immune cells and cytokines involved in HCC proliferation, invasion, and drug resistance (Fig. 1) (18). The infiltrating myeloid-derived suppressor cells and lower numbers of tumor-infiltrating lymphocytes in fibrotic HCC tissue cause damage to effector T cells, reduction of NK cell cytotoxicity, and activation of phenotypes associated with aggressive tumorigenicity (19,20). The recruitment of myeloid-derived suppressor cells by tumor-derived TGF-β selectively suppresses the effector function of T cells, diminishes metabolic fitness for T cells, and eventually leads to T cell apoptosis (21). Moreover, tumor-associated macrophages and neutrophils activated by TGF-β facilitate tumor growth, metastasis, and resistance to sorafenib. In addition, they induce immune tolerance through nuclear factor kappa-light-chain-enhancer of activated B cells signaling (22,23). A positive feedback loop triggering immune evasion occurs when secretion of HCC-derived cytokine (e.g., chemokine C-C motif ligand2, interleukin-4, interleukin-13, and C-X-C motif chemokine 12) induces differentiation of tumor-associated macrophages and activation of tumor-associated neutrophils. These effects result in further recruitment of tumor-associated macrophages and tumor-infiltrating regulatory T cells, as well as apoptosis of cytotoxic T lymphocytes and fatigue of anti-tumor immunity via interleukin-10 (24,25). In addition, pro-angiogenic cytokine VEGF is up-regulated by hypoxia inducible factor-depending pathway in the hypoxic tumor environment, which affects immune suppression in the tumor microenvironment through expressing higher levels of pro-inflammatory cytokines and immunosuppressive mediators (26). NK cells, as modulators of the balance between immune defense and tolerance in the liver, are directly and indirectly affected by the tumor microenvironment. Hypoxic stress and expression of α-fetoprotein in HCC tissue result in suppression of interleukin-12 secretion from dendritic cells, activating receptors on NK cells and causing NK cell dysfunction (27,28). Recent studies also indicate that HCC-associated fibroblast-derived indoleamine-2,3-dioxygenase and prostaglandin E2 inhibit secretion of tumor necrosis factor-alpha and interferon-gamma by NK cells, resulting in persistent fibrosis in HCC and tumor cell immune evasion (29,30). Thus, there are multiple mechanisms by which the intratumoral accumulation of immunosuppressive cells and activation of an inhibitory immune network in the tumor microenvironment induce cancer stem cell-like characteristics and sustain HCC carcinogenesis (31,32).
Figure 1. Schematic diagram of T cell Interaction with hepatocellular tumor cells and dendritic cells.
CURRENT STATUS OF ICIs IN HCC
Anti-PD-1
Nivolumab
Nivolumab (Opdivo®), a fully humanized IgG4 anti-PD-1 monoclonal Ab, was approved by the Food and Drug Administration (FDA) on September 23, 2017, for patients with HCC who experienced sorafenib treatment failure. The CheckMate 040 trial was a phase I/II, open label, noncomparative, dose escalation and expansion trial for patients with advanced HCC and variety of underlying chronic liver diseases (33). The efficacy of nivolumab as a first-line treatment was evaluated in patients with advanced HCC who were treatment-naive or as a second-line treatment in patients on sorafenib with disease progression. Patients were treated with nivolumab at 0.1–10 mg/kg once every 2 wk (dose-escalating cohort) or at a dose of 3 mg/kg once every 2 wk (expansion cohort). In this trial, 46 (96%) of 48 patients discontinued treatment in the dose-escalation phase, 42 (88%) due to disease progression. The objective response rate was 20% (95% confidence interval [CI], 15%–26%) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI, 6%–28%) in the dose-escalation phase. The most common treatment-related adverse events were fatigue, rash, pruritus, and an increase in liver enzyme levels. Grade 3/4 adverse events (e.g., adrenal insufficiency, diarrhea, hepatitis, and acute kidney injury) occurred in 12 of 48 patients. The baseline tumor cell expression of PD-L1 did not have an obvious effect on the response rate. That study revealed the therapeutic potential of nivolumab, showing favorable efficacy and good safety in patients with HCC who had few treatment options. Regrettably, the CheckMate 459 trial, a randomized phase III study evaluating nivolumab versus sorafenib as a first-line treatment in patients with unresectable HCC, did not achieve significance for its primary endpoint of overall survival as defined in the pre-specified analysis plan (hazard ratio [HR]=0.85; 95% CI=0.72–1.02; p=0.075) (34). Although the results failed to meet the predefined threshold of statistical significance because p-value for overall survival of this trial is borderline, there was a clear trend suggestive of improved overall survival for patients treated with nivolumab compared to sorafenib. A phase I/II trial of nivolumab, ipilimumab, and their combination at different doses and intervals is ongoing (35).
Pembrolizumab
Pembrolizumab (Keytruda®) is an IgG4/κ isotype humanized monoclonal Ab targeting the PD-1 receptor in immune cells. Pembrolizumab was first approved for the treatment of metastatic melanoma, metastatic non-small-cell lung cancer, recurrent or metastatic squamous cell carcinoma of the head and neck, recurrent locally advanced or metastatic gastric cancer, locally advanced or metastatic urothelial cancer and classical Hodgkin lymphoma (36). The results of the KEYNOTE-224 trial led the FDA to approve pembrolizumab as s a second-line agent for treatment of HCC after sorafenib therapy on November 10, 2018 (37). The KEYNOTE-224 study was a non-randomized, open-label, multicenter phase II trial in which 104 patients were treated with intravenous pembrolizumab (200 mg) at 3-wk intervals for 2 years or until disease progression, or any other reason to stop treatment. The trial enrolled sorafenib-refractory or -intolerant patients (cohort 1) and patients with no history of systemic treatment (cohort 2) (37). The objective response rate was 17% (complete, 1%; partial, 16%). Forty-six (44%) patients had stable disease, while 34 (33%) had progressive disease. However, serious adverse events occurred in 15% of patients. One died due to treatment-related ulcerative esophagitis.
The phase III KEYNOTE-240 trial is a confirmatory trial for pembrolizumab. Pembrolizumab was granted an accelerated approval in November 2018 for use in patients with HCC who were previously treated with sorafenib, based on data from the phase II KEYNOTE-224 trial. A total of 413 patients with advanced HCC who had previously received systemic therapy were randomized to receive pembrolizumab plus best supportive care or placebo plus best supportive care (38). Although results from the final analysis showed that improved overall survival when compared with placebo, the differences did not reach statistical significance per predefined criteria (HR=0.781; 95% CI=0.611–0.998; p=0.0238). In addition, patients treated with pembrolizumab exhibited improvement in progression-free survival, but this difference also failed to meet predefined threshold for significance. A phase III trial involving 5 Asian countries is underway (KEYNOTE-394, NCT03062358) (39).
Tislelizumab
Tislelizumab (BGB-A317) is an anti-PD-1 Ab undergoing development by BeiGene (Beijing, China). Its safety was confirmed in a phase I trial involving 61 patients with cancers, including HCC. The RATIONALE-301 randomized phase III trial of tislelizumab versus sorafenib as the first-line regimen is ongoing (NCT03412773) (40). Patients were treated with tislelizumab 200 mg intravenously every 3 wk. The primary endpoint is overall survival and the secondary endpoint is non-inferiority of tislelizumab compared to sorafenib.
Camrelizumab
Camrelizumab (SHR-1210) is a fully humanized anti-PD1 IgG4 monoclonal Ab undergoing development by Incyte (Wilmington, DE, USA) and Jiangsu HengRui (Lianyungang, China). A phase I trial confirmed its safety in 58 patients with solid cancers (41). A phase II/III trial is underway, and involves patients who failed to respond or were intolerant to prior systemic treatment (NCT02989922) (42). Camrelizumab was administered intravenously at 3 mg/kg on day 1 every 2 wk (cohort 1) with the same dose every 3 wk (cohort 2). According to the interim results of the phase II trial, the objective response rate was 13.8% (95% CI, 9.5%–19.1%) and 6-month overall survival rate was 74.7% (95% CI, 68.3%–79.9%).
Sintilimab
The anti-PD1 Ab, sintilimab, is undergoing a phase III trial. The ORIENT-32 study (NCT03794440) is a randomized, open-label, multicenter trial in China of sintilimab and bevacizumab (anti-VEGF Ab, IBI-305) versus sorafenib as a first-line treatment. Patients are treated with sintilimab (200 mg) and bevacizumab (15 mg/kg) intravenously on day 1 every 3 wk.
Anti-PD-L1
Durvalumab
Durvalumab (MEDI4736) is an anti-PD-L1 Ab undergoing development by MedImmune/AstraZeneca (Cambridge, UK). Durvalumab has been approved for locally advanced or metastatic urothelial carcinoma. A phase I/II trial of durvalumab monotherapy for solid cancers, including HCC, showed a 10% response rate and a median survival duration of 13.2 months in patients with HCC (43). A phase I/II study evaluating a combination of durvalumab, and tremelimumab (an anti-CTLA-4 Ab) confirmed its safety (44). No unexpected side effects of durvalumab and tremelimumab were observed in patients with unresectable HCC. A phase III trial of durvalumab plus tremelimumab combination therapy as a first-line regimen is ongoing (HIMALAYA study, NCT03298451) (45). The trial is a 4-arm comparing patients receiving durvalumab monotherapy, durvalumab plus tremelimumab combination therapy (regimens 1 and 2), and sorafenib monotherapy. The primary endpoint of the study is overall survival; study completion is anticipated in 2020.
Atezolizumab
Atezolizumab (MPDL3280A) is an anti-PD-L1 Ab undergoing development by Roche (Basel, Switzerland). Atezolizumab has been proven effective for locally advanced or metastatic urothelial carcinoma and metastatic non-small-cell lung cancer. A phase I trial of atezolizumab plus bevacizumab (anti-VEGF Ab) therapy is ongoing (46). According to the interim results, the response rate at presentation was 32%, based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Recently, the phase III IMbrave150 study found that combined treatment with atezolizumab and bevacizumab was associated with statistically significant improvements in both overall survival and progression-free survival, compared with sorafenib, in patients with unresectable HCC who had not received previous systemic therapy. Bevacizumab presumably enhances the ability of the PD-L1 inhibitor to restore antitumor immunity. An open-label, randomized phase III trial focusing on survival in patients receiving atezolizumab plus bevacizumab therapy and sorafenib monotherapy as first-line regimens is underway (NCT03434379) (47). Patients receive 1,200 mg atezolizumab plus 15 mg/kg bevacizumab, both administered intravenously, on day 1 of each 21-day cycle. FDA grants breakthrough therapy designation for atezolizumab/bevacizumab combination as the first-line treatment for advanced or metastatic HCC last year.
Avelumab
Avelumab (MSB0010718C) is an anti-PD-L1 Ab undergoing development by Merck KGaA (Darmstadt, Germany), Pfizer (New York, NY, USA) and Eli Lilly (Indianapolis, IN, USA). Avelumab plus axitinib (AG-013736) is undergoing a phase I trial of safety and tolerability (NCT03289533). Patients will receive avelumab 10 mg/kg every 2 wk in combination with axitinib 5 mg twice a day.
Anti-CLTA-4
Tremelimumab
Tremelimumab (CP 675206) is a CTLA-4 blocking monoclonal Ab undergoing development by MedImmune/AstraZeneca. The first small phase II clinical trial of tremelimumab monotherapy for patients with HCC and chronic hepatitis C virus infection has been conducted (48). Tremelimumab at 15 mg/kg intravenously every 90 days was administered until tumor progression or severe toxicity; partial response rate was 17.6%, disease control rate was 76.4%, and the time to progression was 6.48 months (95% CI, 3.95–9.14 months). Although a significant proportion (42.9%) of patients in Child-Pugh stage B were included in the study, the safety profile of treatment was also acceptable. As mentioned above, a phase III study of efficacy and safety of durvalumab (anti-PD-L1) plus tremelimumab combination therapy and durvalumab monotherapy versus sorafenib is ongoing.
Tremelimumab combined with tumor ablation was evaluated in a second small pilot study (49). Locoregional therapy was expected to have a synergistic effect by inducing immunogenic tumor cell death. A confirmed partial response was achieved in 26.3% of patients. This proof-of-concept study demonstrated that immunotherapy in combination with tumor ablation could be used as treatment for patients with advanced HCC.
Ipilimumab
Ipilimumab (YERVOY®) is an anti-CTLA-4 Ab undergoing development by Bristol-Myers Squibb (New York, NY, USA)/Ono (Osaka, Japan). The CheckMate 040 study includes evaluation of nivolumab plus ipilimumab in a subcohort of sorafenib-treated patients (NCT01658878). Preliminary results showed an objective response rate of 31%, with a median duration of response of 17 months. Two other clinical studies of nivolumab plus ipilimumab as a neoadjuvant therapy are ongoing. One is a randomized phase II trial in the US comparing nivolumab monotherapy with nivolumab plus ipilimumab combination therapy (NCT03222076); the other is a planned phase II trial in Taiwan and will evaluate the combination therapy alone (NCT03510871).
OTHER IMMUNOTHERAPIES
T cell immunoglobulin and mucin-domain containing-3 is a transmembrane protein expressed by CT4+ Th1 cells and CD8+ Tc1 (cytotoxic) cells (50). A phase II trial of an anti-T cell immunoglobulin and mucin-domain containing-3 Ab (TSR-022) and anti-PD-1 (TSR-042) Ab for HCC is planned (NCT03680508). TGF-β is involved in induction of maintenance of regulatory T cells. A phase I trial of anti-TGF-β monoclonal Ab NIS793 and PD-1 inhibitor spartalizumab for advanced malignancies, including HCC, is underway (NCT02947165). Anti-TGF-β Ab is administered every 2 or 3 wk and anti-PD-1 Ab is administered every 3 or 4 wk.
Cellular immunotherapies, such as chimeric antigen receptor T cells, reportedly benefit patients with hematologic malignancies (51). Few studies have evaluated the efficacies of cellular immunotherapies against solid cancers, such as HCC. In the recent phase 3 trial, adjuvant immunotherapy with activated cytokine-induced killer cells prolonged recurrence-free and overall survival of patients who underwent curative treatment for HCC (52). clinical trial of autologous T cell receptor-engineered T cells that recognize alpha-fetoprotein, involving patients with HCC and lung cancer, is underway (NCT03441100). Finally, a trial involving T cells that recognize glypican-3 (glypican-3-specific chimeric antigen receptor expressing T cells) is now recruiting patients (NCT02905188). Glypican-3 is a membrane factor expressed by most HCC cells.
ICIs-BASED COMBINATION THERAPY
Because HCC has various causes and uses numerous mechanisms to evade the immune system, an attractive therapeutic approach involves combining different treatment mechanisms. Potential synergistic combinations include two ICIs and ICIs with conventional therapies (e.g., transarterial chemoembolization, transarterial radioembolization, radiation therapy, and targeted therapies). Table 2 summarizes the results of studies associated with ICI-based combination therapies.
Table 2. Summary of clinical trials of ICIs-based combination treatment in hepatocellular carcinoma.
| Characteristic | Phase | Line of therapy | Treatment | No. of patients | Results | |
|---|---|---|---|---|---|---|
| Combination of 2 ICIs | ||||||
| Nivolumab+ipilimumab (62) | II | 2L | (A) Nivolumab 1 mg/kg+ipilimumab 3 mg/kg Q3W (4 doses) or (B) nivolumab 3 mg/kg+ipilimumab 1 mg/kg Q3W (4 doses), each followed by nivolumab 240 mg Q2W, or (C) nivolumab 3 mg/kg Q2W+ipilimumab 1 mg/kg Q6W | 148 | (A) ORR: 32%, CR: 8%, PR: 8%, DCR: 54%, mOS: 23 months or (B) ORR: 32%, CR: 6%, PR: 24%, DCR: 43%, mOS: 12 months or (C) ORR: 31%, CR: 0%, PR: 31%, DCR: 49%, mOS: 13 months | |
| Durvalumab+tremelimumab (44) | I/II | 1L/2L | 11 HBV positive, 9 HCV positive, 20 uninfected | 40 | ORR: 15%, CR+PR: 20%, DCR16: 57.5% | |
| ICIs+angiogenesis inhibitors | ||||||
| Atezolizumab+bevaxizumab (63) | III | 1L | (A) Atezolizumab 1,200 mg IV Q3W+bevaxizumab 15 mg/kg IV Q3W or (B) sorafenib 400 mg BID | 501 | (A) mOS: NA, mPFS: 6.8 months | |
| (B) mOS: 13.2 months, mPFS: 4.3 months | ||||||
| Pembrolizumab+lenvatinib (64) | Ib | 1L | Lenvatinib (BW ≥60 kg: 12 mg/day; <60 kg: 8 mg/day QD) and pembrolizumab (200 mg IV Q3W) | 67 | ORR: 44.8%, CR: 6.0%, PR: 26%, SD: 37.3%, PD: 9.0% | |
| Avelumab+axitinib (56) | I | 1L | Avelumab 10 mg/kg IV Q2W+axitinib 5 mg orally BID | 22 | ORR: 31.8%, mPFS: 3.8 months, 6-month PFS: 30.9% (mRECIST) | |
| Camrelizumab+apatinib (57) | Ib | 2L | Camrelizumab 200 mg every 2 wk and apatinib 125–500 mg once daily | 16 | PR: 44.4%, ORR: 50%, DCR: 93.8%, mPFS: 5.8 months | |
| ICIs+locoregional therapy | ||||||
| Tremelimumab+tumor ablation (49) | III | 1L/2L | Tremelimumab (3.5 and 10 mg/kg IV) every 4 wk for 6 doses, followed by 3-monthly infusions. On day 36, patients underwent ablation | 32 | 6-month PFS: 57.1%, 12-month PFS: 33.1%, mTTP: 7.4 months, mOS: 12.3 months | |
| ICIs+yttrium-90 radioembolization (65) | NA | 1L/2L | Nivolumab alone or ipilimumab and nivolumab or ipilimumab and nivolumab following nivolumab | 26 | TTP: 5.7 months, PFS: 5.7 months | |
1L, first line; 2L, second line; Q3W, every 3 weeks; Q2W, every 2 weeks; Q6W, every 6 weeks; ORR, objective response rate; CR, complete response; PR, partial response; DCR, disease control rate; mOS, median overall survival; HBV, hepatitis B virus; HCV, hepatitis C virus; IV, intravenously; PFS, progression free survival; mPFS, median progression free survival; TTP, time to tumor progression; mTTP, median time to tumor progression; NA, not available; mRECIST, median Response Evaluation Criteria in Solid Tumors; BID, twice a day; SD, standard deviation; BW, body weight; QD, once daily.
Combinations of two ICIs
Combinations of two ICIs are considered promising because they can target multiple mechanisms. The high efficacy of combination therapy has been proven in other solid tumors (53). Targeting the PD-1/PD-L1 pathway alone might not inhibit development of the immunosuppressive microenvironment if the required CD8+ T cells are not adequately represented in the tumor microenvironment. However, simultaneous inhibition of the CTLA-4 pathway might increase the number of activated CD8+ T cells in lymph nodes; this would be followed by an increase in the number of activated CD8+ T cells infiltrating tumor tissue and an enhancement of their antitumor effects. As stated above, combinations of durvalumab plus tremelimumab (NCT03298451) and nivolumab plus ipilimumab (NCT01658878, NCT03222076, NCT03510871) are examples of possible therapies.
ICIs and angiogenesis inhibitors
HCC is a highly vascularized tumor with predominantly arterial blood flow. Thus, angiogenesis inhibitors are good options for combination treatment of HCC. Proangiogenic growth factors, which are mainly produced by tumor cells, tumor-associated macrophages, and tumor-associated fibroblasts, include VEGF-A, platelet-derived growth factor, IGF-1, and TGF-β (54). Atezolizumab plus bevacizumab (NCT03434379), pembrolizumab plus lenvatinib, camrelizumab plus apatinib, and avelumab plus axitinib are representative combinations of ICIs and angiogenesis inhibitors. Atezolizumab plus bevacizumab was discussed earlier in this review. A phase I trial for pembrolizumab plus lenvatinib is underway. Preliminary results showed that 46% of patients with HCC exhibited a radiological response (55). Consequently, a phase 3 study was initiated to compare lenvatinib to pembrolizumab plus lenvatinib in treatment-naive patients with advanced HCC (NCT03713593). A phase Ib trial of avelumab plus axitinib in 22 naive patients with HCC showed 13.6% and 31.8% objective response rates according to RECIST and mRECIST criteria, respectively (56). Camrelizumab plus apatinib showed that 50% of patients with HCC achieved a partial response (57). Trials of nivolumab plus bevacizumab (NCT03382886), nivolumab plus lenvatinib (NCT03418922), and pembrolizumab plus lenvatinib (NCT03713593) are underway.
Combinations of ICIs and locoregional therapy
Several trials are evaluating ICIs as (neo)adjuvant setting following by curative resection of ablation, such as nivolumab versus placebo following resection or ablation (NCT03343458) and the MK-3475-937/KEYNOTE-937 trial with pembrolizumab (NCT03867084). Tremelimumab combined with tumor ablation is a potential treatment option for patients with advanced HCC (49).
Transarterial chemoembolization is associated with enhanced spread of tumor-associated antigens and an increase in VEGF. A study of transarterial chemoembolization plus nivolumab is underway (NCT03143270). More complex approaches have recently been proposed, including those used in the LEAP-01 study (chemoembolization combined with pembrolizumab and lenvatinib, NCT03713593) and the EMERALD-1 study (chemoembolization combined with durvalumab and bevacizumab, NCT03778957). Transarterial radioembolization also promotes radiation-induced tumor damage similar to that induced by stereotactic radiation therapy (58). Several phase I and II studies combining locoregional therapy with ICIs are expected to begin soon (NCT02837029, NCT03033446, NCT03099564, and NCT03380130).
CONCLUSIONS AND FUTURE PERSPECTIVES
Sorafenib and lenvatinib are currently the first-line agents for advanced HCC. However, the prognosis for advanced HCC remains unsatisfactory. ICIs might improve the prognosis of patients with advanced HCC. Recent data have shown that immunotherapies enhance survival and are safe, but their effects are limited. Combination therapies using ICIs with other agents are expected to overcome tumor-induced immunosuppression. Various combinations of immunotherapies are undergoing trials; the results are eagerly anticipated.
In addition, it is not yet possible to determine which patients can be treated effectively with immunotherapy. High expression of PD-L1 is reportedly associated with poor outcome in patients with HCC (59). However, the predictive role of PD-L1 expression in HCC patients treated with ICIs is unclear. In addition, investigating noninvasive biomarkers predicting response to ICIs is warranted (60,61).
In conclusion, advances in immunotherapy have opened a new chapter in the treatment of HCC. However, further investigation of the immune biology of HCC is needed to facilitate development of more effective therapies for patients with HCC. In addition, overcoming issues such as the lack of biomarkers and combination therapies will improve the prognosis of patients with advanced HCC.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2018R1A2B2005901).
Abbreviations
- CI
confidence interval
- FDA
Food and Drug Administration
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- ICI
immune checkpoint inhibitor
- RECIST
Response Evaluation Criteria in Solid Tumors
Footnotes
Conflict of Interests: The authors declare no potential conflicts of interest.
- Conceptualization: Park JY.
- Resources: Cho KJ.
- Supervision: Park JY.
- Writing - original draft: Lee HW.
- Writing - review & editing: Park JY.
References
- 1.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol. 2018;24:1–9. doi: 10.3350/cmh.2017.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JS, Cho IR, Lee HW, Jeon MY, Lim TS, Baatarkhuu O, Kim DY, Han KH, Park JY. Conditional survival estimates improve over time for patients with hepatocellular carcinoma: an analysis for Nationwide Korea Cancer Registry Database. Cancer Res Treat. 2019;51:1347–1356. doi: 10.4143/crt.2018.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 12.Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of hepatocellular carcinoma: facts and hopes. Clin Cancer Res. 2018;24:1518–1524. doi: 10.1158/1078-0432.CCR-17-0289. [DOI] [PubMed] [Google Scholar]
- 13.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 14.Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31:1409–1420. doi: 10.1038/aps.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:396. doi: 10.1186/s13046-019-1396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariani E, Missale G. Immune landscape of hepatocellular carcinoma microenvironment: implications for prognosis and therapeutic applications. Liver Int. 2019;39:1608–1621. doi: 10.1111/liv.14192. [DOI] [PubMed] [Google Scholar]
- 17.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 18.Chon YE, Park H, Hyun HK, Ha Y, Kim MN, Kim BK, Lee JH, Kim SU, Kim DY, Ahn SH, et al. Development of a new nomogram including neutrophil-to-lymphocyte ratio to predict survival in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancers (Basel) 2019;11:E509. doi: 10.3390/cancers11040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo Y, Shimosegawa T. Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis B virus persistent infection and hepatitis B virus-related HCCs. Int J Mol Sci. 2015;16:3307–3322. doi: 10.3390/ijms16023307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Zhou J, Liu X, Feng Y, Yang W, Wu F, Cheung OK, Sun H, Zeng X, Tang W, et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut. 2020;69:365–379. doi: 10.1136/gutjnl-2018-317257. [DOI] [PubMed] [Google Scholar]
- 21.Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185:1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015;64:1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D, Wu K, Xia L. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015;149:1053–1067.e14. doi: 10.1053/j.gastro.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 25.Cai H, Zhu XD, Ao JY, Ye BG, Zhang YY, Chai ZT, Wang CH, Shi WK, Cao MQ, Li XL, et al. Colony-stimulating factor-1-induced AIF1 expression in tumor-associated macrophages enhances the progression of hepatocellular carcinoma. OncoImmunology. 2017;6:e1333213. doi: 10.1080/2162402X.2017.1333213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasmim M, Messai Y, Ziani L, Thiery J, Bouhris JH, Noman MZ, Chouaib S. Critical role of tumor microenvironment in shaping NK cell functions: implication of hypoxic stress. Front Immunol. 2015;6:482. doi: 10.3389/fimmu.2015.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto M, Tatsumi T, Miyagi T, Tsunematsu H, Aketa H, Hosui A, Kanto T, Hiramatsu N, Hayashi N, Takehara T. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. Clin Exp Immunol. 2011;165:211–219. doi: 10.1111/j.1365-2249.2011.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–161. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Muhanna N, Abu Tair L, Doron S, Amer J, Azzeh M, Mahamid M, Friedman S, Safadi R. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60:90–98. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 31.Zhou SL, Yin D, Hu ZQ, Luo CB, Zhou ZJ, Xin HY, Yang XR, Shi YH, Wang Z, Huang XW, et al. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology. 2019;70:1214–1230. doi: 10.1002/hep.30630. [DOI] [PubMed] [Google Scholar]
- 32.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH, 3rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P, et al. LBA38_PR - CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30:v874–v875. [Google Scholar]
- 35.Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24:S3–S10. doi: 10.1634/theoncologist.2019-IO-S1-s01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudo M. Pembrolizumab for the treatment of hepatocellular carcinoma. Liver Cancer. 2019;8:143–154. doi: 10.1159/000500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 38.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 39.Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open. 2018;3:e000455. doi: 10.1136/esmoopen-2018-000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, Mercade TM, Tomasello G, Boisserie F, Hou J, et al. A phase 3, randomized, open-label, multicenter study to compare the efficacy and safety of tislelizumab, an anti-PD-1 antibody, versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2018;36:TPS3110 [Google Scholar]
- 41.Huang J, Mo H, Wu D, Chen X, Ma L, Lan B, Qu D, Yang Q, Xu B. Phase I study of the anti-PD-1 antibody SHR-1210 in patients with advanced solid tumors. J Clin Oncol. 2017;35:e15572 [Google Scholar]
- 42.Qin SK, Ren ZG, Meng ZQ, Chen ZD, Chai XL, Xiong JP, Bai YX, Yang L, Zhu H, Fang WJ, et al. LBA27 - A randomized multicentered phase II study to evaluate SHR-1210 (PD-1 antibody) in subjects with advanced hepatocellular carcinoma (HCC) who failed or intolerable to prior systemic treatment. Ann Oncol. 2018;29:mdy424.029 [Google Scholar]
- 43.Wainberg ZA, Segal NH, Jaeger D, Lee KH, Marshall J, Antonia SJ, Butler M, Sanborn RE, Nemunaitis JJ, Carlson CA, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC) J Clin Oncol. 2017;35:4071. [Google Scholar]
- 44.Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, Morse M, Kang YK, Rebelatto M, Makowsky M, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J Clin Oncol. 2017;35:4073. [Google Scholar]
- 45.Abou-Alfa GK, Chan SL, Furuse J, Galle PR, Kelley RK, Qin S, Armstrong J, Darilay A, Vlahovic G, Negro A, et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J Clin Oncol. 2018;36:TPS4144 [Google Scholar]
- 46.Pishvaian MJ, Lee MS, Ryoo BY, Stein S, Lee KH, Verret W, Spahn J, Shao H, Liu B, Iizuka K, et al. LBA26 - Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC) Ann Oncol. 2018;29:mdy424.028 [Google Scholar]
- 47.Finn RS, Ducreux M, Qin S, Galle PR, Zhu AX, Ikeda M, Kim TY, Xu DZ, Verret W, Liu J, et al. IMbrave150: A randomized phase III study of 1L atezolizumab plus bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma. J Clin Oncol. 2018;36:TPS4141 [Google Scholar]
- 48.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 51.Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: a new era for cancer treatment (Review) Oncol Rep. 2019;42:2183–2195. doi: 10.3892/or.2019.7335. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 53.Giannini EG, Aglitti A, Borzio M, Gambato M, Guarino M, Iavarone M, Lai Q, Levi Sandri GB, Melandro F, Morisco F, et al. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA.LI. Cancers (Basel) 2019;11:E1689. doi: 10.3390/cancers11111689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, Kaneko S, Zhu AX, Kubota T, Kraljevic S, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC) J Clin Oncol. 2018;36:4076. [Google Scholar]
- 56.Kudo M, Motomura K, Wada Y, Inaba Y, Sakamoto Y, Kurosaki M, Umeyama Y, Kamei Y, Yoshimitsu J, Fujii Y. doi: 10.1159/000514420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25:515–523. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 58.Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol. 2019;25:2416–2429. doi: 10.3748/wjg.v25.i20.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung HI, Jeong D, Ji S, Ahn TS, Bae SH, Chin S, Chung JC, Kim HC, Lee MS, Baek MJ. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat. 2017;49:246–254. doi: 10.4143/crt.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HW, Cho KJ, Shin SY, Kim HY, Lee EJ, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, et al. Serum PD-1 levels change with immunotherapy response but do not predict prognosis in patients with hepatocellular carcinoma. J Liver Cancer. 2019;19:108–116. [Google Scholar]
- 61.Liu CQ, Xu J, Zhou ZG, Jin LL, Yu XJ, Xiao G, Lin J, Zhuang SM, Zhang YJ, Zheng L. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br J Cancer. 2018;119:80–88. doi: 10.1038/s41416-018-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al. Nivolumab (NIVO)+ ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37:4012. [Google Scholar]
- 63.Cheng AL, Qin S, Ikeda M, Galle P, Ducreux M, Zhu A, Kim TY, Kudo M, Breder V, Merle P, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo)+ bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC) Ann Oncol. 2019;30:ix186–ix187. [Google Scholar]
- 64.Llovet J, Shepard K, Finn R, Ikeda M, Sung M, Baron A, Kudo M, Okusaka T, Kobayashi M, Kumada H, et al. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Ann Oncol. 2019;30:v286–v287. [Google Scholar]
- 65.Zhan C, Ruohoniemi D, Shanbhogue KP, Wei J, Welling TH, Gu P, Park JS, Dagher NN, Taslakian B, Hickey RM. Safety of combined yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31:25–34. doi: 10.1016/j.jvir.2019.05.023. [DOI] [PubMed] [Google Scholar]