Abstract
Aim
The goal of this study was to determine whether a delay in starting treatment via surgery or neoadjuvant chemotherapy is related to a decrease in cancer-specific survival (CSS) in women with operable breast cancer (BrCr).
Background
Limited medical infrastructure and a lack of cancer prevention awareness in low- and middle-income countries have caused high BrCr incidence and mortality rates.
Methods
We analyzed a retrospective cohort of 720 women treated at a single center from 2005 to 2012. CSS estimates were obtained by the Kaplan-Meier method. A Cox model of proportional risks was performed to obtain the risk of dying from BrCr. We also obtained the risk according to the category of treatment initiation.
Results
Women with locally advanced stages and without hormone receptor expression were more likely to initiate treatment after 45 days. Patients in Stage IIIA had a 78.1% survival if treatment was initiated before 45 days (95% CI, 0.70–0.84) and 63.6% survival if treatment was started after 45 days (95% CI, 0.44–0.78; p < 0.001). Patients in Stage IIIB had a 62.9% survival if treatment was initiated before 45 days (95% CI, 0.53–0.72) and 57.4% survival if treatment started after 45 days (95% CI, 0.31-0.89; p < 0.001). Prognostic factors in which lower survival was recognized were Stage IIIA, Stage IIIB, treatment initiation after 45 days, and triple-negative tumors.
Conclusions
The initiation of treatment within the first 45 days of diagnosis of BrCr in women portends better survival compared with those who began treatment longer than 45 days from diagnosis.
Abbreviations: BrCa, breast cancer; CSS, cancer-specific survival; ER, estrogen receptor; HR, hazard ratio; IQR, interquartile range; OR, odds ratio
Keywords: Timing of cancer treatment, Treatment delay, Breast cancer chemotherapy, Breast cancer surgery, Breast cancer radiotherapy
1. Background
In recent decades, breast cancer (BrCr) has become a leading cause of cancer death among women worldwide, second to lung cancer.1 Up to 50% of diagnoses of BrCr and 60% of deaths due to this tumor occur in women living in middle-income countries. BrCr is also the leading cause of cancer death and disability-adjusted life-years for women.2 The BrCr incidence and mortality rates in these countries are high due to limited medical infrastructure and lack of promotion of cancer prevention and breast self-examination practices.3,4 Among high-income countries, the average interval for a patient to start cancer treatment is 30–48 days, and up to 60% of patients begin treatment in the first three months after symptoms were discovered. However, in low- to middle-income countries, the interval can range from 165 days in Malaysia to 240 days in Brazil, where < 30% of patients start treatment soon after diagnosis. With respect to the time between the first medical consultation and the start of cancer treatment, Germany’s average interval is 15 days—a stark contrast to Brazil, Colombia, Mexico, and Turkey, where this time ranges from 78 days to 240 days.5 Some studies report an increased range longer than 90 days for starting treatment with decreased survival.6 The literature uses “total delay” in BrCr care, which is defined as delays greater than three months (approximately 90 days) and is measured from symptoms demonstrated to the date treatment started.7 Delays are classified into two types of delays: those associated with the patient, and those associated with health services.8, 9, 10 Another indicator of health services delay is the interval from diagnosis to initiation of treatment, defined as the time between the histological diagnosis of cancer and the onset of cancer treatment. The latter indicator is the subject of this study.11 This retrospective study aimed to determine whether a delay in starting treatment via surgery or neoadjuvant chemotherapy is related to a decrease in cancer-specific survival (CSS) of women with operable BrCr treated at our institute.
2. Materials and Methods
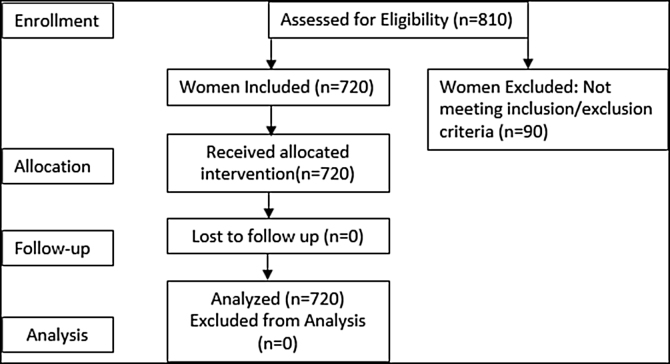
A random sample of 810 women was obtained from a large database created to assess treatment delays from 2005 to 2012. A total of 720 women met the inclusion criteria (Fig. 1).
Fig. 1.
Flow chart of breast cancer patient enrollment.
2.1. Data collection and variable definition
The data were extracted from medical records. Our study sample included women older than 18 years with a histopathological diagnosis of ductal and lobular infiltrating cancer. A pathological review was performed for women included in the study. Immunohistochemical assays were used to characterize the BrCr subtypes according to the expression levels of estrogen receptors (ER), progesterone receptors, the transmembrane tyrosine kinase receptor HER2, and the KI67 index value. For triple-negative BrCr determinations of the epidermal growth factor receptor, Actin, cytokeratins 7 and 14, and androgen receptor analyses were performed. Accurate dates of diagnosis and treatment initiation in women with an initial clinical Stage I–IIIB, according to the TNM (tumor, node, metastasis) staging system, were recorded. We excluded men with BrCr, women with cancer in situ, patients operated outside the hospital, women with tumors showing features of inoperability (e.g., extensive breast edema, satellite nodules in the skin, inflammatory cancer, parasternal tumor nodules, confirmed supraclavicular metastases, arm edema, and distant metastases), women with synchronous or metachronous BrCr, women with a history of any other type of cancer except nonmelanoma skin cancer, women in whom the standard treatment had not been granted due to comorbidities that put lives at risk (e.g., morbid obesity, heart disease, and renal failure), and women with metastatic disease at diagnosis. Women with institutional records who had not completed treatment at the hospital or women whose initial treatment took more than seven months for any reason were eliminated. The study was approved by the ethics committee as required by our institution.
We calculated the time between BrCr diagnosis and the onset of treatment for all patients. For CSS survival analysis, the initial event was the date of treatment initiation for BrCr, and the final event was death due to BrCr. Death for other causes was not considered for the analysis. Beside the descriptive analysis of demographic and clinical characteristics, survival estimates were obtained by the Kaplan-Meier method. To evaluate the effect of prognostic factors on the population, survival curves comparisons were performed by a log-rank test. A Cox proportional-hazards model was performed to obtain the risk of dying from BrCr, adjusted by clinical stage, lymphovascular invasion, molecular classification, and categories of delay in treatment. Furthermore, the risk according to category at the initial treatment was obtained. Statistical analysis was performed using STATA Statistical Software Version 14 (StataCorp LP, College Station, TX).
3. Results
The study population consisted of 720 women with BrCr, whose median age was 50 years (range, 27–89), meaning 67.6% of the study participants were older than 45 years. The median time of follow-up was 5.8 years (range, 6 months to 11.5 years). The median time for a patient to start treatment at our institute was 26 days (interquartile range, 1–158 days). Demographic and clinical characteristics are displayed in Table 1.
Table 1.
Demographic and clinical characteristics of women with operable breast cancer.
| Variable | n = 720 (%) |
|---|---|
| Age at diagnosis | |
| <45 years | 233 (32.4) |
| ≥45 years | 487 (67.6) |
| Age in years, median (SD) | 50 (11.8) |
| Body Mass Index | |
| Normal | 186 (25.8) |
| Overweight | 293 (40.7) |
| Obesity | 241 (33.5) |
| Hormonal status | |
| Premenopausal | 235 (32.6) |
| Postmenopausal | 485 (67.4) |
| Clinical stage | |
| I | 90 (12.5) |
| IIA | 157 (21.8) |
| IIB | 190 (26.4) |
| IIIA | 172 (23.9) |
| IIIB | 111 (15.4) |
| Histology | |
| Infiltrating ductal | 656 (91.1) |
| Infiltrating lobular | 64 (8.9) |
| Tumor size | |
| <2.0cm | 194 (26.9) |
| 2.1-5.0 cm | 278 (38.6) |
| >5.0 cm | 248 (34.5) |
| Nuclear grade | |
| Well differentiated | 165 (23.0) |
| Moderately differentiated | 261 (36.4) |
| Poorly differentiated | 292 (40.6) |
| Lymphovascular invasion | |
| Absent | 621 (86.2) |
| Present | 99 (13.8) |
| Estrogen receptors | |
| Negative | 251 (34.9) |
| Positive | 469 (65.1) |
| Progesterone receptors | |
| Negative | 213 (29.6) |
| Positive | 507 (70.4) |
| Molecular classification | |
| Luminal A | 468 (65.0) |
| Luminal B | 39 (5.4) |
| Her2Neu | 67 (9.3) |
| Triple negative | 146 (20.3) |
| Lymph nodes invasion | |
| No | 232 (32.2) |
| Yes | 488 (67.8) |
Abbreviation: SD, standard deviation.
Of our study sample, 74.2% began treatment 15 days after their admission to the hospital. Most women started treatment between 15 and 30 days from diagnosis confirmation (32.5%). The preferred modality of treatment was neoadjuvant chemotherapy (53.5%), which is not surprising given the number of women presenting with locally advanced disease (Table 2).
Table 2.
Treatment features of women with breast cancer.
| Variables | n = 720 (%) |
|---|---|
| Delay time for starting | |
| <15 days | 186 (25.8) |
| 15–29 days | 234 (32.5) |
| 30–44 days | 170 (23.6) |
| >45 days | 130 (18.1) |
| First treatment performed | |
| Surgery | 318 (44.2) |
| Neoadjuvant chemotherapy | 385 (53.5) |
| Neoadjuvant hormone therapy | 17 (2.3) |
| Surgery type | |
| Breast-conserving | 118 (16.4) |
| Mastectomy | 602 (83.6) |
| Adjuvant treatment | |
| Radiotherapy | 222 (30.8) |
| Chemotherapy | 306 (42.5) |
| Hormone therapy | 130 (18.1) |
| Surveillance | 62 (8.6) |
| Timing of chemotherapy | |
| Neoadjuvant | 385 (54.0) |
| Adjuvant | 306 (31.0) |
| None | 29 (15.0) |
| Adjuvant radiotherapy | |
| No | 243 (33.8) |
| Yes | 477 (66.2) |
The 5-year CSS was 83.9%, (95% CI, 0.81–0.86). Women in Stage I had a 5-year CSS of 95.4% (95% CI 0.88–0.98), women in Stage IIA had a CSS of 91.3% (95% CI, 0.85-0.95), those in stage IIB had 89.4% survival (95% CI, 0.84-0.93), patients in Stage IIIA had a 76.1% CSS (95% CI, 0.69–0.82), and 64.3% (95% CI, 0.55–0.73) for Stage IIIB (p < .001). The CSS of patients stratified by tumor size was 90.9% (95% CI, 0.8–0.94) when the tumor size was <2 cm, 89.2% (95% CI, 0.85–0.92) in tumors sized 2 cm–4.9 cm, and 71.7% (95% CI, 0.66–0.77) in patients with tumors >5 cm (p < .001). Patients categorized as Luminal A had a CSS rate of 88.4% (95% CI, 0.85–0.91); Luminal B patients had a CSS rate of 86.1% (95% CI, 0.69–0.94). Her2Neu and triple-negative patients had 85.1% (95% CI, 0.74–0.92) and 67.5% CSS (95% CI, 0.59–0.75), respectively (p < .001). Age at diagnosis, body mass index, hormonal status, histology, nuclear grade, lymphovascular invasion, and expression of ER did not show significant differences between groups. Survival started to decrease at treatment delays of longer than 45 days (Table 3).
Table 3.
CSS of women with breast cancer according to treatment initiation before and after 45 Days.
| Variable | Calculated survival at 5 years from initial treatment (%) |
p-value* | |
|---|---|---|---|
| <45 days | >45 days | ||
| 590 (%)a | 130 (%)a | ||
| Age at diagnosis (years) | |||
| <45 | 83.9 | 81.6 | 0.434 |
| >45 | 83.5 | 84.5 | |
| Body Mass Index | |||
| Normal | 84.6 | 81.3 | |
| Overweight | 82.1 | 79.9 | 0.058 |
| Obesity | 84.5 | 84.8 | |
| Hormonal status | |||
| Premenopausal | 82.3 | 78.9 | 0.732 |
| Postmenopausal | 84.4 | 84.3 | |
| Clinical stage | |||
| I | 95.2 | 90.6 | |
| IIA | 92.0 | 87.8 | |
| IIB | 89.5 | 89.2 | <0.001 |
| IIIA | 78.1 | 63.6 | |
| IIIB | 62.9 | 57.4 | |
| Histology | |||
| Infiltrating ductal | 83.6 | 83.5 | 0.303 |
| Infiltrating lobular | 86.4 | 75.0 | |
| Tumor size | |||
| <2 cm | 92.7 | 84.3 | |
| 2–5 cm | 88.2 | 86.4 | <0.001 |
| >5 cm | 71.6 | 72.0 | |
| Nuclear grade | |||
| Well differentiated | 92.7 | 90.6 | |
| Moderately differentiated | 87.6 | 80.7 | 0.162 |
| Poorly differentiated | 77.4 | 78.0 | |
| Lymphovascular invasion | |||
| Absent | 84.2 | 84.9 | 0.361 |
| Present | 81.3 | 81.4 | |
| Estrogen receptor expression | |||
| Negative | 77.1 | 74.8 | 0.219 |
| Positive | 87.6 | 87.2 | |
| Progesterone receptor expression | |||
| Negative | 79.1 | 80.1 | <0.001 |
| Positive | 87.4 | 86.7 | |
| Molecular classification | |||
| Luminal A | 88.5 | 88.0 | |
| Luminal B | 86.9 | 53.3 | <0.001 |
| Her2 Neu | 84.7 | 62.5 | |
| Triple negative | 67.7 | 66.0 | |
| Lymph node invasion | .001 | ||
| No | 94.5 | 93.7 | <0.001 |
| Yes | 79.0 | 75.9 | |
Kaplan Meier Method.
Log-Rank Test.
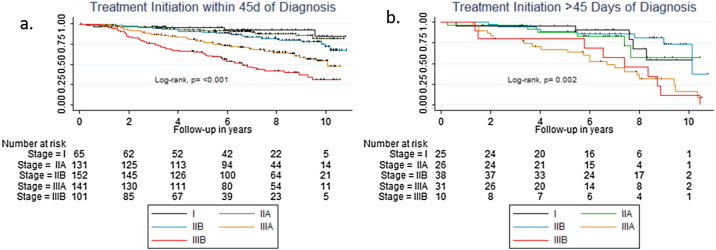
We performed a stratified analysis to determine which patients may benefit from treatment within 45 days of diagnosis. Patients in Stage I had a 95.2% survival if treatment was initiated before 45 days (95% CI, 0.86–0.98) and 90.6% if treatment started after 45 days (95% CI, 0.67–0.98). Patients in Stage IIA had a 92.0% survival if treatment was initiated before 45 days (95% CI, 0.86–0.96) and 87.8% if treatment started after 45 days (95% CI, 0.67–0.96). Patients in Stage IIB had an 89.5% survival if treatment was initiated before 45 days (95% CI, 0.83–0.94) and 89.2% if treatment started after 45 days (95% CI, 0.74–0.96). Patients in Stage IIIA had a 78.1% survival if treatment was initiated before 45 days (95% CI, 0.70–0.84) and 63.6% if treatment started after 45 days (95% CI, 0.44–0.78). Patients in Stage IIIB had a 62.9% survival if treatment was initiated before 45 days (95% CI, 0.53–0.72) and 57.4% if treatment started after 45 days (95% CI, 0.31–0.89; Fig. 2).
Fig. 2.
Kaplan–Meier estimates of cancer-specific survival (CSS) by clinical stage according to treatment initiation within 45 days of diagnosis (a), or after 45 days of cancer diagnosis (b).
Prognostic factors in which lower survival was recognized were Stage IIIA (95% CI, 1.9–7.6; hazard ratio [HR], 3.9), stage IIIB (95% CI, 3.4–13.2; HR, 6.7), treatment initiation after 45 days (95% CI, 1.4–2.6; HR, 1.8), and triple-negative tumor status (95% CI, 1.3–2.4; HR, 1.7; Table 4).
Table 4.
Prognostic factors in women with breast cancer.
| Variable | HRa | 95% CI | p-value | HRc | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Clinical stage | ||||||
| I | 1.0b | 1.0b | ||||
| IIA | 1.1 | 0.5-2.4 | 0.728 | 1.1 | 0.5-2.5 | 0.718 |
| IIB | 1.5 | 0.8-3.1 | 0.243 | 1.4 | 0.7-2.9 | 0.311 |
| IIIA | 3.9 | 2.0-7.5 | <0.001 | 3.9 | 1.9-7.6 | <0.001 |
| IIIB | 6.5 | 3.3-12.6 | <0.001 | 6.7 | 3.4-13.2 | <0.001 |
| Delayed of initial treatment | ||||||
| <45 days | 1.0‡ | 1.0b | ||||
| ≥45 days | 1.6 | 1.1-2.2 | 0.006 | 1.8 | 1.4-2.6 | <0.001 |
| Lymphovascular invasion | ||||||
| Absent | 1.0b | 1.0b | ||||
| Present | 1.2 | 0.8-1.7 | 0.361 | 1.4 | 0.90-2.0 | 0.090 |
| Molecular classification | ||||||
| Luminal A | 1.0b | 1.0b | ||||
| Luminal B | 1.4 | 0.8-2.7 | 0.247 | 1.7 | 0.9-3.1 | 0.106 |
| Her2Neu | 1.3 | 0.8-2.1 | 0.319 | 1.3 | 0.8-2.1 | 0.361 |
| Triple Negative | 1.9 | 1.4-2.3 | <0.001 | 1.7 | 1.3-2.4 | <0.001 |
Raw hazard ratio.
Reference Category.
Adjusted hazard ratio for clinical stage, lymphovascular invasion, categories of delay in treatment and molecular classification.
4. Discussion
In recent years, BrCr has been the subject of prevention and early detection campaigns that reinforce the concept of early diagnosis given that cancer diagnosis at advanced stages carries a poor prognosis for survival.12 Although cancer treatment should be immediate in all cases, no guidelines suggest the acceptable interval between diagnosis and treatment initiation. For over 70 years, research regarding delayed treatment as a prognostic factor for survival in women with BrCr has been contradictory. A meta-analysis in 1999 reported patients treated between the first three to six months of diagnosis had a reduction in five-year survival of 12% (odds ratio [OR], 1.47; 95% CI, 1.42–1.53) compared with women who started treatment within three months of diagnosis and in whom survival decreased 7% (OR, 1.24; 95% CI, 1.17–1.30). Sixty-two percent of the studies included in the meta-analysis were published before 1970, the most recent of which was published in the 1990s.13 Our findings suggest that patients with BrCr should begin treatment preferably within the first 45 days of diagnosis, because survival decreases with delay in treatment initiation, especially in women with tumors in more advanced clinical stages. Recently, Unger-Saldaña et al. conducted a qualitative multicentric study whose objective was the evaluation of the delays in cancer care in women with BrCr.14 Ninety percent of patients in the study (N = 886) experienced delays in cancer care, while 57% had a delay of longer than six months. For each month of delay in seeking medical care, Unger-Saldaña reported a 1.8% increase in the probability of having a more advanced clinical stage. For each month of delay in care from health services, the likelihood of starting the treatment at a more advanced clinical stage was 1%. Also, for each year of age of the patient, the likelihood of starting treatment at an advanced clinical stage decreased to 0.4%.14
Researchers have used various intervals to explain the impact of treatment delay in cancer survival. Smith et al. and Redondo et al. found no significant differences in survival for a treatment delay of 30 days.15,16 Because of their findings, we chose 45 days as the threshold for treatment delays. We stratified patients by clinical stage at diagnosis to analyze the effect of treatment delay on survival. The lack of stratification in other studies may explain why those studies failed to show a significant decrease in survival. Although the delay in treatment initiation decreased survival in women with early disease, this decrease was not significant. However, survival decreased significantly in patients with locally advanced disease. Our study suggests a delayed start of treatment longer than 45 days increases the risk of dying from cancer. Two studies from Korea reported similar results; both studies used national records and insurance data (which could present errors in data collection).17,18 Shin et al. suggest the risk of dying from BrCr doubles in women for whom surgery is delayed more than 12 weeks.17 Yun et al. suggested that in hospitals with a high volume of patients, a delay longer than four weeks from diagnosis increases the risk of death 1.6 times.18 Our study’s use of electronic records derived from a single institution offered more detailed clinicopathologic information and excluded patients with major delays of six months who may have ignored their diagnosis, rejected standard treatment, or chosen alternative treatment outside the institution. McLaughlin et al. reported the extended time between histologic confirmation and initiation of treatment decreased the survival of patients in more advanced clinical stages but not in women with tumors in clinical Stage I.19 McLaughlin used a Cox proportional hazards model to adjust for the clinical stage to assess overall survival and specific survival for BrCr. They found that while the delay in treatment did not affect overall survival (p = 0.37) or specific survival (p = 0.49) among patients with early BrCr, a delay to start treatment >60 days for patients with advanced clinical disease was associated with a lower overall survival (HR, 1.66; 95% CI, 1.00–2.77; p = 0.05) and a decrease in specific survival for BrCr (HR, 1.85; 95% CI 1.04–3.27, p = 0.04).19 Delays between surgery and adjuvant radiotherapy may be due to a lack of linear accelerators and trained personnel. However, existing knowledge regarding adjuvant radiotherapy delay after surgery is poor, and some authors suggest the implementation of satellite units for decentralizing radiotherapy services, reducing delays and radiotherapy treatment interruptions.20,21 A recent Mexican study found a statistical decrease in disease-specific survival in women with locally advanced BrCr receiving radiotherapy after a delay >60 days.22
Given our study’s retrospective nature, it is not without limitations and biases. Our findings cannot be generalized to all populations; it is geared towards a specific group of women in whom treatment should start in the first 45 days of diagnosis confirmation (i.e., women with Stage IIIA disease and more advanced stages, triple-negative molecular category, and lymphovascular invasion). Identifying specific factors that contributed to the delayed initiation of treatment would have been helpful (e.g., multiple biopsies, breakdown of mammography or computed tomography equipment, and a long waiting list in operating rooms) in addition to cultural and socioeconomic factors related to the patient. Another limitation was the size of the sample—a larger sample should have been selected for the analysis of each clinical stage.
5. Conclusions
The initiation of treatment within the first 45 days of diagnosis of BrCr in women is associated with better survival compared with those who began treatment longer than 45 days from diagnosis. Any study that aims to analyze the effect of a delay in starting treatment faces the ethical dilemma of not being able to randomize subjects by delay categories in a prospective trial; so, when retrospective studies are conducted, they open an area of opportunity for further research to dictate a policy for patients with cancer to be treated in a timely manner. While our study design may prohibit generalizations for other populations, our findings should encourage other institutions to create their own data given the growing waiting lists for treatment initiation in cancer centers worldwide.
Financial disclosure
None declared.
Conflict of interest
None declared.
Acknowledgements
None declared.
Contributor Information
Christian H. Flores-Balcázar, Email: christian.floresb@incmnsz.mx.
Ma. L. Flores-Luna, Email: mflor@insp.mx.
Cynthia M. Villarreal-Garza, Email: cynvg@gmail.com.
Juan E. Bargalló-Rocha, Email: ebargallo@yahoo.com.
References
- 1.DeSantis C.E., Ma J., Gaudet M.M. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.Fitzmaurice C., Allen C., Barber R.M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villarreal-Garza C., Aguila C., Magallanes-Hoyos M. Breast cancer in young women in Latin America: an unmet, growing burden. Oncologist. 2013;18(12):1298–1306. doi: 10.1634/theoncologist.2013-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villarreal-Garza C., Lopez-Martinez E.A., Muñoz-Lozano J.P., Unger-Saldaña K. Locally advanced breast cancer in young women in Latin America. Ecancermedicalscience. 2019;13:894. doi: 10.3332/ecancer.2019.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unger-Saldaña K. Breast cancer early diagnosis in developing countries. World J Clin Oncol. 2014;5(3):465–477. doi: 10.5306/wjco.v5.i3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivotto I.A., Bancej C., Goel V. Waiting times from abnormal breast screen to diagnosis in 7 Canadian provinces. CMAJ. 2001;165:277–283. [PMC free article] [PubMed] [Google Scholar]
- 7.Unger-Saldaña K., Infante-Castañeda C. Delay of medical care for symptomatic breast cancer: a literature review. Salud Publica Mex. 2009;51(Suppl 2):S270–85. doi: 10.1590/s0036-36342009000800018. [DOI] [PubMed] [Google Scholar]
- 8.Bright K., Barghash M., Donach M., de la Barrera M.G., Schneider R.J., Formenti S.C. The role of health system factors in delaying final diagnosis and treatment of breast cancer in Mexico city, Mexico. Breast. 2011;20(Suppl 2):S54–9. doi: 10.1016/j.breast.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Knaul F., Bustreo F., Ha E., Langer A. Breast cancer: why link early detection to reproductive health interventions in developing countries? Salud Publica Mex. 2009;51(Suppl 2):s220–7. doi: 10.1590/s0036-36342009000800012. [DOI] [PubMed] [Google Scholar]
- 10.Barber M.D., Jack W., Dixon J.M. Diagnostic delay in breast cancer. Br J Surg. 2004;91(1):49–53. doi: 10.1002/bjs.4436. [DOI] [PubMed] [Google Scholar]
- 11.Facione N.C. Delay versus help seeking for breast cancer symptoms: a critical review of the literature on patient and provider delay. Soc Sci Med. 1993;36:1521–1534. doi: 10.1016/0277-9536(93)90340-a. [DOI] [PubMed] [Google Scholar]
- 12.Dianatinasab M., Fararouei M., Mohammadianpanah M., Zare-Bandamiri M. Impact of social and clinical factors on diagnostic delay of breast cancer: a cross-sectional study. Medicine (Baltimore) 2016;95(38):e4704. doi: 10.1097/MD.0000000000004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards M.A., Westcombe A.M., Love S.B., Littlejohns P., Ramirez A.J. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 14.Unger-Saldaña K., Miranda A., Zarco-Espinosa G., Mainero-Ratchelous F., Bargalló-Rocha E., Miguel Lázaro-León J. Health system delay and its effect on clinical stage of breast cancer: multicenter study. Cancer. 2015;121(13):2198–2206. doi: 10.1002/cncr.29331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith E.R., Adams S.A., Das I.P., Bottai M., Fulton J., Hebert J.R. Breast cancer survival among economically disadvantaged women: the influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev. 2008;17:2882–2890. doi: 10.1158/1055-9965.EPI-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redondo M., Rodrigo I., Pereda T. Prognostic implications of emergency admission and delays in patients with breast cancer. Support Care Cancer. 2009;17(5):595–599. doi: 10.1007/s00520-008-0513-2. [DOI] [PubMed] [Google Scholar]
- 17.Shin D.W., Cho J., Kim S.Y. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20:2468–2476. doi: 10.1245/s10434-013-2957-y. [DOI] [PubMed] [Google Scholar]
- 18.Yun Y.H., Kim Y.A., Min Y.H. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012;23:2731–2737. doi: 10.1093/annonc/mds101. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin J., Anderson R., Ferketich A., Seiber E., Balkrishnan R., Paskett E. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30:4493–4500. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arenas M., Gomez D., Sabater S., Rovirosa A., Biete A., Colomer J. Decentralisation of radiation therapy. Is it possible and beneficial to patients? Experience of the first 5 years of a satellite radiotherapy unit in the province of Tarragona, Spain. Rep Pract Oncol Radiother. 2014;20(2):141–144. doi: 10.1016/j.rpor.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arenas M., Sabater S., Gascón M. Quality assurance in radiotherapy: analysis of the causes of not starting or early radiotherapy withdrawal. Radiat Oncol. 2014;9:260. doi: 10.1186/s13014-014-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores-Balcázar C.H., Flores-Luna L., Villarreal-Garza C., Mota-García A., Bargalló-Rocha E. Impact of delayed adjuvant radiotherapy in the survival of women with breast cancer. Cureus. 2018;10(7):e3071. doi: 10.7759/cureus.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]