Abstract
Purpose
The purpose of this study was to review the microsurgical anatomy and clipping of ruptured anterior communicating artery (AComA) aneurysms and to plan and avoid complications before operation.
Methods
A total of 523 cases of cerebral aneurysms admitted to the neurosurgery department of the Third Affiliated Hospital of Sun Yat-Sen University from September 2010 to October 2018 were analyzed retrospectively. Among them, 85 patients had ruptured AComA aneurysms. This study was limited to 85 of these cases, whose satisfactory preoperative angiographic diagnostic films can be retrieved from the hospital database system because of the need for detailed review.
Results
We performed supraorbital eyebrow keyhole approach (SOEK) craniotomy in 85 patients to clip 85 AComA aneurysms, in the setting of subarachnoid hemorrhage (SAH). Patients’ mean age was (52.69 ± 9.94) years (range, 28–78 years). The proportions of small, medium and large aneurysms were 83.5%, 15.3%, and 1.2%, respectively. The average size of the aneurysms was (5.07 ± 2.36) mm. There were 77.8% of patients with inferior aneurysms and 81.3% of patients with superior aneurysms achieved good results. There was a significant correlation between A1 dominance and operation method (p < 0.001). There was no significant relationship between surgical approach and aneurysm projection or A2 plane (p = 0.157 & p = 0.318).
Conclusion
Regardless of whether the A2 plane is open or closed, the A1 dominant side is still a better choice for accessing AComA aneurysms to avoid dangerous premature bleeding.
Keywords: Anterior communicating artery, Aneurysm projection, Clipping, Ruptured aneurysm, Surgical approach
Introduction
In order to achieve accurate clipping of the aneurysm, A1 dominance, the anatomy of the aneurysm neck with A1 and A2 segment, presence of perforators and other vascular abnormalities are very important.1 Magnetic resonance angiography (MRA), three dimensions (3D) computed tomography angiography (CTA), and 3D digital subtraction angiography (DSA) can easily observe the detailed anatomy around the anterior communicating artery (AComA) complex.2
The decision of the surgical approach is based on the relationship between A1 dominance with projection, and relation of the projection with the plane of both A2 vessels. According to Yasargil et al,3 projection is the main anatomical factor.
In this study, we analyzed the microanatomical relationship between AComA complex and its adjacent vessels, and individualized the selection strategy of the surgical side to avoid or reduce the complications associated with the dissection and clipping of AComA aneurysms, and further clarified the importance of using the dominant A1 segment during the microsurgical clipping of AComA aneurysm via supraorbital eyebrow keyhole (SOEK) approach.
Methods
General data collection
From September 2010 to October 2018, 523 cases of cerebral aneurysms were treated in the Department of Neurosurgery of the Third Affiliated Hospital of Sun Yat-Sen University. Among them, 85 cases of AComA aneurysm with subarachnoid hemorrhage were included in our study. All 85 cases had complete cerebral vascular imaging data and clinical data before and after the operation. We collected the general data, clinical manifestations, imaging data, intraoperative findings and postoperative recovery of 85 patients.
Research and analysis
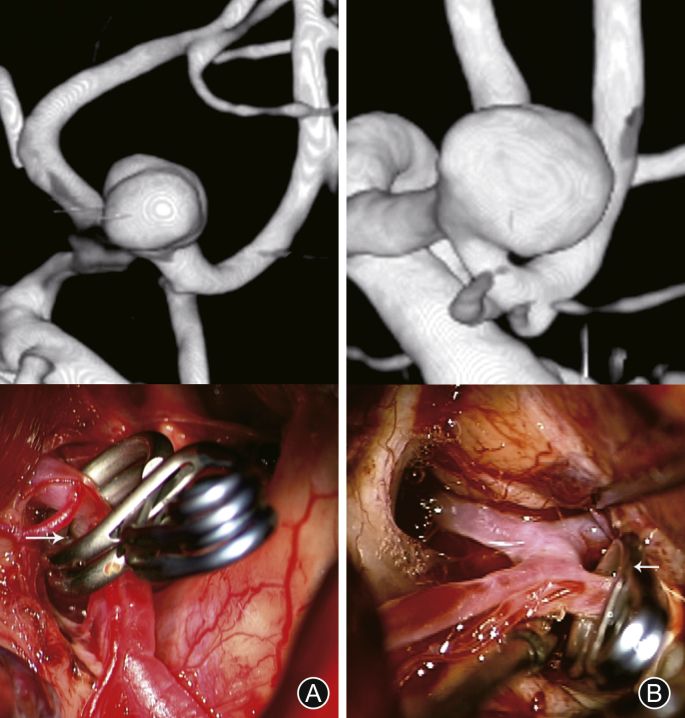
The general data and angiographic features of 85 patients, such as size, projection, multiplicity, lobulations, dominant blood supply, relationship between the aneurysm and A2 plane, and surgical complications were analyzed in detail. The open A2 plane is defined as the presence of A2 of the SOEK approach more posteriorly than the contralateral A2. The closed A2 plane is defined as the position where the ipsilateral A2 is located more anteriorly, which results in the A1-A2 junction and the A2 to hide the neck of the aneurysm (Fig. 1). At the same time, world federation of neurological surgeon (WFNS) score and Fisher grade of patients at admission were analyzed. All patients were assessed by the modified ranking scale (MRS) when they were discharged from the hospital.
Fig. 1.
Computed tomography angiography and intraoperative photographs of the procedure. Supraorbital eyebrow keyhole approach. (A) Open A2 plane; (B) Closed A2 planes4.
Statistical analysis
Quantitative variables are expressed as mean ± SD and categorical variables are expressed as frequency distribution. Quantitative variables of the two groups were compared using an independent sample t-test. The correlation between categorical variables was analyzed by Chi-square test. p < 0.05 was considered significant. SPSS software (version 21.0; IBM, SPSS, Chicago, IL, USA) was used for statistical analysis.
Results
The average age of 85 patients in this study was (52.69 ± 9.94) years (range, 28–78 years). There were 50 male patients (58.8%). All the patients presented with ruptured AComA aneurysm. Among ruptured aneurysms, 28 (32.9%) were WFNS grade I, 20 (23.5%) were WFNS grade II, 12 (14.1%) were WFNS grade III, 23 (27.1%) were WFNS grade IV, and 2 (2.4%) were WFNS grade V (Table 1). A number of 49 (57.6%) patients were operated within 48 h and 36 (42.4%) were operated after 48 h of initial symptoms.
Table 1.
Demographics of the 85 patients with cerebral aneurysms.
| Patients | n (%) |
|---|---|
| Age (years) | |
| <40 | 4 (4.7) |
| 40–49 | 6 (7.1) |
| 50–59 | 10 (11.7) |
| >60 | 65 (76.5) |
| Gender | |
| Male | 50 (58.8) |
| Female | 35 (41.2) |
| WFNS score | |
| I | 28 (32.9) |
| II | 20 (23.5) |
| III | 12 (14.1) |
| IV | 23 (27.1) |
| V | 2 (2.4) |
| Total | 85 (100) |
WFNS: World Federation of Neurological Surgeon.
For all ruptured aneurysms, the severity of bleeding was determined by Fisher classification. Of 85 ruptured aneurysms, 42 were Fisher II (49.4%), 13 were Fisher III (15.3%) and 30 were IV (35.3%). Preoperative infarction occurred in 5 patients (5.3%).
Preoperative CTA/DSA of 85 cases was analyzed to study the morphology of aneurysms. The average size was (5.07 ± 2.36) mm (range, 2–12 mm). There were 52 patients had dominant circulation, 36 of them had dominant left A1 and 16 had dominant right A1 circulation. A total of 33 cases (38.8%) were co-dominant. With 48 patients had superior projection (56.5%), 18 patients had inferior projection (21.2%), 16 patients (18.8%) and 3 patients (3.5%) had anterior and posterior projection respectively. We found that 24 cases had multiple projections due to multiple lobules, of which dominant projections were considered the main projections. The A2 plane was open in 37 cases (43.5%) and closed in 48 cases (56.5%).
Out of 85 patients, 84 AComA aneurysms were clipped and 1 was wrapped. Three patients (3.5%) had ruptured aneurysms during operation. Three patients (3.5%) had a perforator injury. Rectus gyrus aspiration was performed in 5 patients (5.9%). Twenty-five patients (29.4%) received temporary clipping of dominant A1. Temporary clipping duration of dominant A1 was 1–20 min (average 6.9 min).
Of the 85 patients, 2 (2.4%) had post-operative contusion and 17 (20.0%) had infarction. Of these 10 (11.8%) patients suffered from severe infarction. Postoperative hematoma and intraventricular hemorrhage occurred in 5 cases (5.9%) and which resolved in subsequent computed tomography (CT) scan. Nine cases (10.6%) underwent cerebrospinal fluid (CSF) diversion due to hydrocephalus.
We evaluated the MRS for 85 patients at discharge. Two patients who had preoperative Hunt & Hess grade V died. Sixty-nine patients (81.2%) had a good prognosis (Table 2).
Table 2.
Outcome assessment of the 85 patients with cerebral aneurysms.
| Modified rankin scale | n (%) |
|---|---|
| 0 | 11 (12.9) |
| 1 | 24 (28.3) |
| 2 | 34 (40) |
| 3 | 3 (3.5) |
| 4 | 3 (3.5) |
| 5 | 8 (9.4) |
| 6 | 2 (2.4) |
Among 85 patients, 52 patients had A1 dominance and 33 patients had co-dominance. Thirty-six patients had left side A1 dominance and 16 patients had right A1 dominance. Of the 36 patients with left A1 dominance, 33 underwent left-sided surgery and 3 underwent right-sided surgery. All 16 patients with dominant right A1 underwent right-sided surgery. The 3 cases which underwent surgery from the non-dominant circulation, all were anterio-inferiorly directing aneurysms (Table 3).
Table 3.
A1 dominance, projection, A2 plane and side of the approach, n (%).
| Variables | Side of approach |
Total (n = 85) | p value | |
|---|---|---|---|---|
| Left (n = 43) | Right (n = 42) | |||
| A1 dominance | <0.001 | |||
| Left | 33 (76.7) | 3 (7.1) | 36 (42.4) | |
| Right | 0 (0) | 16 (38.1) | 16 (18.8) | |
| No dominance | 10 (23.3) | 23 (54.8) | 33 (38.8) | |
| Projections | 0.157 | |||
| Superior | 26 (60.5) | 22 (52.4) | 48 (56.5) | |
| Inferior | 5 (11.6) | 13 (30.9) | 18 (21.2) | |
| Anterior | 10 (23.3) | 6 (14.3) | 16 (18.8) | |
| Posterior | 2 (4.6) | 1 (2.4) | 3 (3.5) | |
| A2 Plane | 0.318 | |||
| Open | 21 (48.8) | 16 (38.1) | 37 (43.5) | |
| Closed | 22 (51.2) | 26 (61.9) | 48 (56.5) | |
A total of 48 cases of superiorly projecting aneurysms, 3 (3.5%) had an intraoperative rupture (IOR), gyrus rectus aspiration was done in the 3 patients (6.3%) and 1 had perforator injury. There were 13 cases of temporary clipping, 8 cases (16.7%) of infarction and 6 cases (12.5%) of postoperative hematoma. One case (2.1%) had hydrocephalus and 6 patients underwent CSF diversion due to hydrocephalus. Out of 18 cases of inferiorly projecting aneurysms, IOR occurred in 1 case (2.1%), gyrus rectus aspiration was done in 2 cases (11.1%) and 1 patient had perforator injury. Temporary clipping applied in 4 cases. Infarction occurred in 5 cases (27.8%) and hematoma occurred in 1 case (5.6%) (Table 4).
Table 4.
Projection of aneurysm, operative complications and outcomes evaluated by the modified ranking scale, n (%).
| Complications | Projections |
Total (n = 85) | p value | |||
|---|---|---|---|---|---|---|
| Superior (n = 48) | Inferior (n = 18) | Anterior (n = 16) | Posterior (n = 3) | |||
| Intraoperative complications | ||||||
| Intraoperative rupture | 1 (2.08) | 1 (5.6) | 1 (6.3) | 0 (0) | 3 (3.5) | 0.793 |
| Gyrus rectus resection | 3 (6.3) | 2 (11.1) | 0 (0) | 0 (0) | 5 (5.9) | 0.554 |
| Perforator injury | 1 (2.1) | 1 (5.6) | 0 (0) | 1 (33.3) | 3 (3.5) | 0.002 |
| Temporary clipping | 13 (27.1) | 4 (22.2) | 7 (43.8) | 1 (33.3) | 25 (29.4) | 0.536 |
| Postoperative complication | ||||||
| Contusions | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 1 (1.2) | 0.671 |
| Infarction | 8 (16.7) | 5 (27.8) | 3 (18.8) | 1 (33.3) | 17 (20.0) | 0.680 |
| Hematoma | 6 (12.5) | 1 (5.6) | 0 (0) | 0 (0) | 7 (8.2) | 0.857 |
| VP shunt | 6 (12.5) | 2 (11.1) | 1 (6.3) | 0 (0) | 9 (10.6) | 0.834 |
| Modified ranking scale | ||||||
| 0 | 6 (12.5) | 0 (0) | 4 (25.0) | 1 (33.4) | 11 (13.0) | |
| 1 | 13 (27.1) | 5 (27.7) | 5 (31.2) | 1 (33.3) | 24 (28.2) | |
| 2 | 20 (41.6) | 9 (50.0) | 4 (25.0) | 1 (33.3) | 34 (40.0) | |
| 3 | 2 (4.2) | 1 (5.6) | 0 (0) | 0 (0) | 3 (3.5) | |
| 4 | 1 (2.1) | 2 (11.1) | 0 (0) | 0 (0) | 3 (3.5) | |
| 5 | 5 (10.4) | 1 (5.6) | 2 (12.5) | 0 (0) | 8 (9.4) | |
| 6 | 1 (2.1) | 0 (0) | 1 (6.3) | 0 (0) | 2 (2.4) | |
VP shunt: ventriculoperitoneal shunt.
One patient with superior projecting aneurysms died, 39 cases (81.3%) had a good prognosis. There was no mortality in the inferior projecting aneurysms, and 14 patients (77.8%) had a good prognosis. One patient with anterior projection died, however, there was no mortality in posteriorly projecting aneurysm. There was no statistical difference in outcome according to different aneurysm projections (Table 4).
Among 48 cases of superior projecting aneurysms, 19 cases had open A2 plane and 29 cases had closed A2 plane on the operative side. Of 26 cases were dominant circulation and 22 cases were co-dominant. In this dominant circulation, 14 cases appeared in closed A2 plane and 12 cases in open A2 plane (Table 5).
Table 5.
A2 plane, side of approach, A1 dominance, intraoperative and postoperative complications and outcome in superiorly projecting aneurysms.
| Variables | A2 plane |
Total (n = 48) | p value | |
|---|---|---|---|---|
| Open (n = 19) | Closed (n = 29) | |||
| Side of approach | 0.312 | |||
| Left | 12 (63.1) | 14 (48.3) | 26 (54.1) | |
| Right | 7 (36.8) | 15 (51.7) | 22 (45.8) | |
| A1 dominance | 0.50 | |||
| Left | 9 (47.4) | 9 (31.1) | 18 (37.5) | |
| Right | 3 (15.8) | 5 (17.2) | 8 (16.7) | |
| No dominance | 7 (36.8) | 15 (51.7) | 22 (45.8) | |
| Complications | ||||
| IOR | 0 (0) | 1 (3.4) | 1 (2.1) | 0.475 |
| Gyrus rectus resection | 2 (10.5) | 3 (10.3) | 3 (6.2) | 0.322 |
| Perforator injury | 0 (0) | 1 (3.4) | 1 (2.1) | 0.531 |
| Temporary clipping | 5 (26.3) | 8 (27.6) | 13 (27.1) | 0.923 |
| Contusion | 0 (0) | 1 (3.4) | 1 (2.1) | 0.475 |
| Infarction | 2 (10.5) | 6 (20.7) | 8 (16.7) | 0.455 |
| Hematoma | 2 (10.5) | 4 (13.8) | 6 (12.5) | 0.69 |
| VP shunt | 2 (10.5) | 4 (13.8) | 6 (12.5) | 0.147 |
| MRS | 0.503 | |||
| 0 | 4 (21.1) | 2 (6.9) | 6 (12.5) | |
| 1 | 5 (26.4) | 8 (27.6) | 13 (27.1) | |
| 2 | 6 (31.6) | 14 (48.3) | 20 (41.6) | |
| 3 | 1 (5.2) | 1 (3.4) | 2 (4.2) | |
| 4 | 1 (5.2) | 0 (0) | 1 (2.1) | |
| 5 | 2 (10.5) | 4 (13.8) | 6 (12.5) | |
| 6 | 0 (0) | 0 (0) | 0 (0) | |
IOR: intraoperative rupture, VP shunt: ventriculoperitoneal shunt, MRS: modified rankin scale.
In these closed A2 plane (n = 29), IOR was found in 1 case (3.4%) and 1 case had perforator injury. Postoperative complications like infarction were found in 4 cases (13.8%), hematoma in 4 cases (13.8%), contusion in 1 case and CSF diversion was done in 4 cases. In the open A2 plane (n = 19), no patients had IOR and no patients had perforator injury. Postoperative infarction occurred in 2 cases (10.5%), hematoma in 2 cases and 2 cases underwent CSF diversion (Table 5).
Therefore, there were more surgical and postoperative complications in close A2 plane cases. But in all parameters, the p value was not significant. The average duration of temporary clipping in an open plane was (6.13 ± 4.19) min, while that in the closed plane was (8.32 ± 5.39) min (p = 0.32).
MRS showed no mortality at discharge for superior projecting aneurysms. Thus, in our study, there is no significant statistical difference in outcome between these two group (Table 5).
Discussion
The most important factor of microvascular surgery for AComA aneurysms is the conceptualization and clarification of 3D spatial structure, which is more common in superior aneurysms and anterior superior protrusion aneurysms. Among these protrusions, the dome of aneurysm is closely related to segment A2, masking the AComA complex and adjacent to the critical perforating artery.
According to Hernesniemi et al,5 the factors affecting the surgical exposure of AComA aneurysms include rupture status, size, projection of aneurysm dome, dominance of A1, plane of A2, neurovascular variation, and the existence of related intraparenchymal and intraventricular hematomas. AComA aneurysms are more likely to bleed in the frontal lobes and adjacent ventricular systems. Intracerebral hematoma affects clinical outcomes and therefore requires early surgical resection.
In our study, 85 aneurysms were ruptured. Twenty-eight cases (32.9%) were WFNS grade I, 20 (23.5%) were WFNS grade II, 12 (14.1%) were WFNS grade III, 23 (27.1%) were WFNS grade IV, and 2 (2.4%) were WFNS V. Fisher grade III was found in 13 cases (15.3%), Fisher grade IV in 30 (35.3%) and rectus gyrus hematoma in 5 (5.9%).
Dashti et al6 reported that 30% of patients had intraventricular hemorrhage (IVH), which is the second most common cause of aneurysmal intracranial hemorrhage and requires urgent evacuation. Pasqualin et al7 reported that 32% of intracranial hemorrhage in 402 patients was caused by rupture of aneurysm.
The surgical strategy for AComA aneurysms requires a detailed review of AComA complexes to protect perforators and avoid dangerous premature rupture. Although DSA is the gold standard in many centers, we perform both DSA and multi-slice spiral CTA simultaneously in our center.
A1 dominance, A2 plane and aneurysm projection are the main factors determining the approach of SOEK according to the literature review.
A1 dominance
Unilateral dominance can lead to the occurrence of AComA aneurysms. Cohen and Samson8 reported that 57% of AComA patients had A1 dominance. Yasargil et al3 found that 80% of patients with AComA aneurysms had A1 dominance, and explained that these aneurysms originated from the dominant A1 side. It also pointed out that AComA aneurysms may originate in the middle of the AComA complex when A1 supply is equal, indicating that hemodynamic disturbance is a predisposing factor. Lawton9 reported 80% frequency of A1 dominance and right-sided approach was taken for symmetric A1 and in asymmetric A1, dominant A1 was the criteria to approach the aneurysm.
In our study, 52 (61.2%) patients had A1 dominance and 33 patients had co-dominance among 85 patients. Among them, 36 patients had left side A1 dominance and 16 patients had right A1 dominance. Of the 36 patients with left A1 dominance, 33 underwent left-sided surgery and 3 underwent right-sided surgery. All 16 patients with right A1 dominance underwent right-sided surgery. The 3 cases which underwent surgery from the non-dominant circulation, all were anterio-inferiorly directing aneurysms.
According to Sano,10 the A1 dominance should be the most important factor for small to large sized aneurysms because it is sometimes difficult to ensure the opposite side of A1. However, there was no significant difference in the difficulty of left and right approaches.
Hyun et al4 found that 78.9% of patients had left A1 dominance in the superior projecting aneurysms, compared with 51.5% previously reported. They chose the right side to approach 36.8% and the left side to approach 63.2%. Suzuki et al11 said that inferior projecting aneurysms were treated with A1 dominance and superior projected aneurysms were treated with A2 plane. They found that A1 dominance was 35.6% on the right, 51.1% on the left, and had no dominance on 13.3%. Approach side was selected according to A1 dominance.
Hernesniemi et al5 reviewed 921 AComA aneurysms; side selection for pterional approach was A1 dominance. However, our study reported no statistical differences between the right and left methods. In addition, in order to avoid dangerous rupture during surgery, when selecting an approach, care should be taken to identify which A1 side is dominant. Therefore, the dominant A1 side is the better side.
Aneurysm projection
In our study, the most common projection was the superior projection aneurysm (56.5%), followed by the inferior projection aneurysm (21.2%). Different authors found different predictions of AComA. Norlen and Barnum12 considered that the most common type of aneurysm was inferior process aneurysm. Yasargil et al3 considered that the most common was posterior projecting aneurysm (34%), followed by superior projecting aneurysm (22.7%).
Superior projecting aneurysms are usually associated with dominant ipsilateral A1 vessels, which usually do not cover the contralateral optic nerve. This projection enters the junction of hemispherical fissure and the contralateral A1/A2, and is covered by the fundus of the aneurysm. This is the most common projection direction of the aneurysmal fundus and may be partially embedded in the contralateral gyrus rectus. These aneurysms are usually easier to control than the aneurysms projected elsewhere. Rectus gyrus resection contributes to mobilize the fundus.14
According to Suzuki et al,11 AComA with inferior projection is easily clipped without extensive dissection of hemispheric fissures and preparation of bilateral A2 segments, but aneurysms with inferior projection are more likely to rupture prematurely due to adhesion to the optic chiasm or frontal base. So that the inferiorly projecting aneurysm was treated by A1 dominance and conversely in the superior projecting aneurysm, preparation of bilateral A1 segment is feasible, but the key step of neck dissection requiring detailed visualization is difficult, if the same side A1-A2 complex or A2 hides the neck. Thus, superiorly projecting aneurysm was approached by the open A2 plane.
Sano10 pointed out that A1 was bilaterally secured before approaching the aneurysm when the aneurysm was located superiorly. Therefore, entry into the open part of the A2 fork (i.e. the side of A2 facing posteriorly) facilitates clipping. If the aneurysm is located posteroinferiorly and back of the neck, entry into the side of the A2 located more anteriorly is recommended.
In our study, there were no statistical differences in terms of operative strategy between different projections. There was no statistical difference in complications and outcomes.
Superiorly projecting aneurysm with closed A2 plane
Higher requirement for gyrus rectus aspiration and higher incidence of residual neck remnant in closed A2 plane was observed by Suzuki et al,11 but there was no significant difference in the related vascular injury. A significant difference in contusion was observed in patients with closed A2 plane (p < 0.0092). They believe that the open A2 plane has obvious advantages in approaching the superiorly projecting AComA aneurysms.
According to Hyun et al4 the plane of the both A2 blood vessels was more important in selecting the approach side than the dominant side of A1. It has been suggested that the spatial distribution around AComA should be determined prior to the determining the approach side for superior projecting AComA aneurysms. Ten patients (52.6%) were approached from the A2 anterior displacement side (closed A2 plane). In these 10 cases, 2 (20%) had A1 dominancy on the right side and 8 (80%) had A1 dominancy on the left side. The right approach was selected in 3 patients (30%), while the remaining 7 were treated from the left side (70%). For patients with closed A2 plane (9 out of 10 patients, p = 0.041), the requirement of gyrus aspiration was higher. However, according to the Glasgow outcome scale score, there was no significant correlation between the surgical approach side and the outcome.
In our study, there was no significant difference between open and closed A2 plane in terms of operative, post-operative and outcomes at the time of discharge. In addition, the development of clipping technology and the use of fenestrated clips help surgeons approach from either side.
Our study focused only on ruptured aneurysms. These aneurysms need proximal control, so the AComA aneurysms were approached from the dominant side. In literature review, the A2 posterior displacement side (open A2 plane) approach in patients with superior projecting aneurysms enables neurosurgeons to secure the neck of the aneurysm and prevent postoperative complications. On the contrary, our study, where cases were operated based on dominant circulation showed no significant correlation between open and closed A2 plane in terms of operative, post-operative complications, and outcomes.
Therefore, regardless of whether the A2 plane is open or closed, the A1 dominant side is still a better choice for accessing AComA aneurysms to avoid dangerous premature bleeding.
The limitations of our study are mainly related to small sample size, retrospective design, lack of randomization, and outcome assessment by surgical surgeons. Finally, our results are related to the single-center experience of specific surgical techniques and schemes, which limits the generalization. Only large-scale prospective studies can overcome these weaknesses.
Funding
Nil.
Ethical Statement
This study has been approved by the local ethical committee.
Declaration of Competing Interest
No benefits in any form have been received or will be received from a commercial party-related directly or indirectly with regard to this article and there are no competing interests related to this article.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.van Lindert E., Perneczky A., Fries G. The supraorbital keyhole approach to supratentorial aneurysms: concept and technique. Surg Neurol. 1998;49:481–489. doi: 10.1016/s0090-3019(96)00539-3. discussion 489-490. [DOI] [PubMed] [Google Scholar]
- 2.Figueiredo E.G., Deshmukh P., Nakaji P. The minipterional craniotomy: technical description and anatomic assessment. Neurosurgery. 2007;61(5 Suppl 2):256–264. doi: 10.1227/01.neu.0000303978.11752.45. discussion 264-265. [DOI] [PubMed] [Google Scholar]
- 3.Yasargil M.G., For J.L., Ray W. The operative approaches to aneurysms of the anterior communicating artery. In: Krayenbuhl H., editor. Advances and Technical Standards in Neurosurgery. Georg Thieme Verlag; New York, NY: 1975. pp. 114–170. [Google Scholar]
- 4.Hyun S.J., Hong S.C., Kim J.S. Side selection of the pterional approach for superiorly projecting anterior communicating artery aneurysms. J Clin Neurosci. 2010;17:592–596. doi: 10.1016/j.jocn.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Hernesniemi J., Dashti R., Lehecka M. Microneurosurgical management of anterior communicating artery aneurysms. Surg Neurol. 2008;70:8–28. doi: 10.1016/j.surneu.2008.01.056. discussion 29. [DOI] [PubMed] [Google Scholar]
- 6.Dashti R., Hernesniemi J., Niemelä M. Microneurosurgical management of middle cerebral artery bifurcation aneurysms. Surg Neurol. 2007;67:441–456. doi: 10.1016/j.surneu.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 7.Pasqualin A., Bazzan A., Cavazzani P. Intracranial hematomas following aneurysmal rupture: experience with 309 cases. Surg Neurol. 1986;25:6–17. doi: 10.1016/0090-3019(86)90107-2. [DOI] [PubMed] [Google Scholar]
- 8.Anterior communicating artery aneurysm, Samson D interviewed by Cohen A. https://www.neurosurgicalatlas.com/grand-rounds/microsurgical-clip-ligation-of-anterior-communicating-artery-aneurysms Neurosurgical Atlas January 30 2011.
- 9.Lawton T.M. Anterior communicating artery aneurysms. In: Conerly K., editor. Seven Aneurysms Tenets and Techniques for Clipping. Thieme; New York, NY: 2011. pp. 94–120. [Google Scholar]
- 10.Sano H. Anterior communicating artery aneurysms surgical approaches review. In: Laligam S., Fessler R., editors. Atlas of Neurosurgical Techniques. New York, NY; Thieme. 2006. pp. 142–152. [Google Scholar]
- 11.Suzuki M., Fujisawa H., Ishihara H. Side selection of pterional approach for anterior communicating artery aneurysms – surgical anatomy and strategy. Acta Neurochir. 2007;150:31–39. doi: 10.1007/s00701-007-1466-9. [DOI] [PubMed] [Google Scholar]
- 12.Norlén G., Barnum A.S. Surgical treatment of aneurysms of the anterior communicating artery. J Neurosurg. 1953;10:634–650. doi: 10.3171/jns.1953.10.6.0634. [DOI] [PubMed] [Google Scholar]