Abstract
ProBiotic-4 is a probiotic preparation composed of Bifidobacterium lactis, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus acidophilus. This study aims to investigate the effects of ProBiotic-4 on the microbiota–gut–brain axis and cognitive deficits, and to explore the underlying molecular mechanism using senescence-accelerated mouse prone 8 (SAMP8) mice. ProBiotic-4 was orally administered to 9-month-old SAMP8 mice for 12 weeks. We observed that ProBiotic-4 significantly improved the memory deficits, cerebral neuronal and synaptic injuries, glial activation, and microbiota composition in the feces and brains of aged SAMP8 mice. ProBiotic-4 substantially attenuated aging-related disruption of the intestinal barrier and blood–brain barrier, decreased interleukin-6 and tumor necrosis factor-α at both mRNA and protein levels, reduced plasma and cerebral lipopolysaccharide (LPS) concentration, toll-like receptor 4 (TLR4) expression, and nuclear factor-κB (NF-κB) nuclear translocation in the brain. In addition, not only did ProBiotic-4 significantly decreased the levels of γ-H2AX, 8-hydroxydesoxyguanosine, and retinoic-acid-inducible gene-I (RIG-I), it also abrogated RIG-I multimerization in the brain. These findings suggest that targeting gut microbiota with probiotics may have a therapeutic potential for the deficits of the microbiota–gut–brain axis and cognitive function in aging, and that its mechanism is associated with inhibition of both TLR4-and RIG-I-mediated NF-κB signaling pathway and inflammatory responses.
Key words: Microbiota–gut–brain axis, Cognitive decline, TLR4, RIG-I, NF-κB, Probiotics, SAMP8 mice
Abbreviations: 8-OHdG, 8-hydroxydesoxyguanosine; AD, Alzheimer's disease; AAMI, age-associated memory impairment; BBB, blood–brain barrier; CFU, colony-forming units; ELISA, enzyme-linked immunosorbent assay; F/B, Firmicutes/Bacteroidetes; GFAP, glial fibrillary acidic protein; HE, hematoxylin and eosin; Iba-1, ionized calcium binding adaptor molecule-1; IHC, immunohistochemistry; IL-6, interleukin-6; LPS, lipopolysaccharide; MCI, mild cognitive impairment; NF-κB, nuclear factor-κB; NMDS, non-metric multidimensional scaling; OTU, operational taxonomic unit; PAMP, pathogen-associated molecular pattern; RIG-I, retinoic-acid-inducible gene-I; SAMP8, senescence-accelerated mouse prone 8; SYN, synaptophysin; TEM, transmission electron microscopy; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; VE-cadherin, vascular endothelial-cadherin; ZO-1, zona occluden-1
Graphical abstract
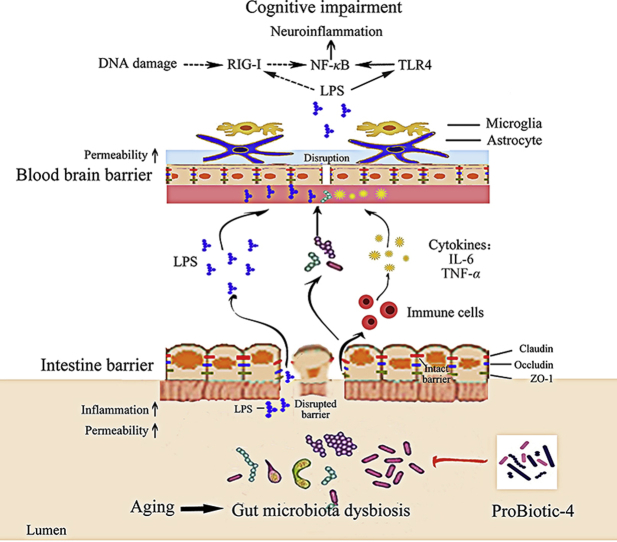
Long-term oral administration with ProBiotic-4, a complex probiotic preparation, could significantly improve the deficits of the microbiota–gut–brain axis and cognitive function in aged SAMP8 mice. The underlying mechanism is associated with inhibition of both TLR4-and RIG-I-mediated NF-κB signaling pathway and inflammatory responses.
1. Introduction
Aging-related cognitive impairments have become the common health threats to the elderly population. The aging-related cognitive decline includes age-associated memory impairment (AAMI), mild cognitive impairment (MCI) and senile dementia (known as Alzheimer's disease, AD). MCI, a cognitive state intermediate between normal cognitive aging and dementia, is associated with increased risk for AD1. Clinical studies suggest that individuals with MCI may progress to AD at a rate of 10%–15% per year2. Although accumulated evidence shows that early interventions may prevent the progression of MCI to AD in aging, there are no effective therapeutic approaches for the cognitive impairments so far.
Gut microbiota, comprising more than trillions of bacteria, contribute to basic physiological processes and alter the host's susceptibility to diseases3, 4. Recent studies show that AD histological and behavioral manifestations are correlated with gut microbiome dysbiosis, and the potential prevention of probiotics against AD progression is one of the recent innovations in the field of neurodegenerative diseases5, 6. Multiple probiotics (Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum) and selenium combination or probiotic mixture (L. acidophilus, Lactobacillus casei, B. bifidum, and Lactobacillus fermentum) improved mini-mental state examination (MMSE) score and some metabolic profiles in AD patients7, 8. A mixture of probiotics (L. acidophilus, L. fermentum, Bifidobacterium lactis, and B. longum) has shown modulating gut microbiota and improving memory deficits and oxidative stress in β-amyloid (1–42) injected rats9. Although these data display the potential disease-modifying effects of probiotics on AD, the mechanism remains unclear.
Based on the bidirectional communication between the gut microbiota and the brain, the concept of the microbiota–gut–brain axis was proposed10. There are three recognized mechanisms of microbiota–gut–brain axis: modification of autonomic/sensorimotor connections, immune activation (such as inflammatory response), and neuroendocrine pathway regulation10, 11, 12. Various lines of evidence suggest that aging-induced changes in gut microbiota composition might lead to inflammaging (a chronic low-grade inflammation), a risk factor linked to cognitive decline in the elderly13, 14, 15, 16, 17. Studies have shown that gut microbiota dysbiosis is often accompanied with gut-leak, increased plasma lipopolysaccharide (LPS) content and blood–brain barrier (BBB) breakdown18, 19. LPS is a well-known ligand for toll-like receptor 4 (TLR4), and can induce inflammatory responses through activation of TLR4/nuclear factor-κB (NF-κB) signaling pathway20, 21. These studies imply that the influence of aging-related gut microbiota dysbiosis on cognitive function is possibly associated with LPS-activated TLR4/NF-κB pathway and neuroinflammatory responses in the brain. In addition, it was reported that Listeria monocytogenes increased the level of retinoic-acid-inducible gene-I (RIG-I)22, an important viral RNA recognition molecule, and induced NF-κB pathway activation and subsequent inflammatory responses. The data suggests that RIG-I-induced NF-κB pathway activation is also possibly in correlation with microbiota community. Taken together, accumulating evidence suggests that both TLR4-and RIG-I-mediated NF-κB pathway activation is involved in the gut microbiota dysbiosis associated inflammaging and cognitive impairments in aging. However, to our best knowledge, the potential role of TLR4-and RIG-I-mediated NF-κB pathway activation in the pathogenesis of aging-related cognitive decline and the neuroprotective mechanism of probiotics have not been reported yet.
The present study investigated the effects of probiotics on the deficits of the microbiota–gut–brain axis and cognitive function in MCI and explored the underlying mechanism. Senescence-accelerated mouse prone 8 (SAMP8) is an excellent model of memory deficits and a suitable animal model for MCI associated research23. Notably, studies have shown that multiplexes probiotics (i.e., combining different strains of specific genera) can be more effective than a single-strain probiotic in the treatment of certain clinical conditions, such as irritable bowel syndrome, ulcerative colitis, and atopic dermatitis24, 25. In addition, multiple probiotics are normally applied in AD research and clinical practice7, 8, 9. Based on the clinical effect of probiotics on cognitive dysfunction and the doses of probiotics administrated to mice in literature review26, 27, we considered ProBiotic-4 (a mixture of B. lactis, L. casei, B. bifidum, and L. acidophilus) at a dose of 2 × 109 colony-forming units (CFU) per day as the intervention of choice for SAMP8 mice. In the present study, ProBiotic-4 was orally administered to 9-month-old SAMP8 mice for 12 weeks. Our results unearthed that ProBiotic-4 was able to significantly attenuate deficits of microbiota–gut–brain axis and cognitive function in aged SAMP8 mice, and its mechanism was associated with inhibition of both TLR4-and RIG-I-mediated NF-κB signaling pathway and inflammatory responses.
2. Materials and methods
2.1. Materials
ProBiotic-4, a probiotic preparation composed of B. lactis (50%), L. casei (25%), B. bifidum (12.5%), and L. acidophilus (12.5%), were purchased from Swanson (Fargo, ND, USA). The other reagents were of the highest quality available and obtained from commercial sources.
2.2. Animal treatment
All of the animal studies were conducted in accordance with the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People's Republic of China. The procedures were approved by the Animal Research Committee of West China School of Pharmacy (Chengdu, China). Male 9-month-old SAMP8 and SAMR1 mice were purchased from the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (Tianjin, China). The animals were housed at 22 ± 2 °C under a 12 h/12 h light/dark cycle with free access to food and water. SAMP8 mice were divided into two groups (n = 12/group) using the grading score system and Y-maze test before the experiment (Supporting Information Fig. S1)28, and received vehicle (water) or ProBiotic-4 (2 × 109 CFU) once daily for 12 weeks. The age-matched SAMR1 mice (n = 12) were used as normal controls and were similarly given vehicle.
2.3. Y-maze and passive avoidance tests
At 4, 8 and 12 weeks of ProBiotic-4 treatment, the Y-maze alternation test was performed to assess short-term working memory in mice. Twenty-four hours after the last Y-maze alternation test, the passive avoidance test was performed to assess long-term working memory. Detailed procedures for these tests were described previously29.
2.4. Tissue and blood collection
After neurobehavioral evaluation, mouse plasma samples were collected for further analysis. The mice were perfused with cold normal saline via the ascending aorta, and the brains were removed. The left hemisphere was frozen in liquid nitrogen and used for transmission electron microscopy (TEM), microbiota analysis, Western blotting, and biochemical analyses. The right hemisphere was placed in 4% paraformaldehyde, dehydrated in 15% and 30% sucrose solutions and then frozen for 20-μm-thick sections. The intestine was collected, frozen in liquid nitrogen, and stored at −80 °C for further analyses.
2.5. Nissl staining
Nissl staining was used to detect neuronal injury as reported previously30. The number of neurons in per field of cerebral cortex and hippocampal CA1 was digitized (400 × magnification) using Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
2.6. TEM
Fresh brain tissues were taken from the left cortex, cut into about 1-mm3 cubes, and fixed in 2.5% glutaraldehyde for 24 h. The samples were fixed in 1% osmium tetroxide for 2 h, dehydrated in graded acetone, and embedded in araldite. Sections were cut at 50 nm and stained with uranyl acetate and lead citrate. The ultrastructures of the BBB were observed under an H-600IV transmission electron microscope (Hitachi, Tokyo, Japan).
2.7. Microbiota analysis
Fresh feces samples were collected after the behavioral tests. Brain tissues and feces were used for 16S rRNA gene analysis of microbiota profiling with barcoded amplicons from the V3–V4 region. Illumina MiSeq sequencing was conducted at Novogene Co., Ltd. (Beijing, China). 16S rRNA data analysis was performed using the QIIME data analysis package (the Caporaso and Knight Labs, at Northern Arizona University and University of Colorado, respectively).
2.8. Immunofluorescence analysis
The expression of ionized calcium binding adapter molecule 1 (Iba-1) and glial fibrillary acidic protein (GFAP) was detected as described previously31. The selected brain sections were incubated with respective primary antibodies (Supporting Information Table S1) at 4 °C overnight, followed by detection with a secondary antibody conjugated with fluorescein isothiocyanate (Boster, Wuhan, Hubei, China). The area or the number of immunoreactivity in per field of cerebral cortex or hippocampal CA1 was digitized (400 × magnification) using Image Pro Plus 6.0 software.
2.9. Histology
Sections of the ileum were collected and fixed in 10% neutral buffered formalin. Tissues were embedded in paraffin, and 5 μm sections were cut and placed on glass slides. The slides were stained with hematoxylin and eosin (HE). Digital images were acquired at 100 × magnification by using Eclipse NI-U upright microscope (Nikon, Tokyo, Japan).
2.10. Immunohistochemistry
Immunohistochemistry (IHC) was performed to analyze ileum tight junction protein expression and distribution in the mice. Primary antibodies against claudin-1, occludin, and zona occluden-1 (ZO-1) were probed with target proteins, respectively. Quantification of the positive area was performed (200 × magnification) using Image Pro Plus 6.0 software.
2.11. Quantitative real-time polymerase chain reaction
Tissue RNA was isolated from the left hemisphere of the brain and intestine using TRIzol reagent (Thermo Fisher Scientific, Shanghai, China) and processed for cDNA. Quantitative real-time polymerase chain reaction (qPCR) was performed according to our previously published methods30. The sequences of the primers and probes for each gene are listed in Supporting Information Table S2.
2.12. Enzyme-linked immunosorbent assay
Brain 8-hydroxydesoxyguanosine (8-OHdG) levels were measured using the 8-OHdG Assay Kit (Jiancheng Bioengineering, Nanjing, China). Plasma levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined using the mouse enzyme-linked immunosorbent assay (ELISA) kits (Dakewe Bioengineering, Shenzhen, China). LPS levels in brain and plasma were measured using the High Sensitive ELISA Kit (Cloud-Clone Corp, Wuhan, China).
2.13. Western blotting analysis
Protein samples were isolated from left brain tissue homogenates using RIPA buffer (Beyotime, Shanghai, China) according to the manufacturer's instructions. The detailed procedure of Western blotting analysis was described previously31. Primary antibodies against synaptophysin (SYN), γ-H2AX, β-actin, TLR4, RIG-I, NF-κB P65, lamin B, claudin-5, occludin, ZO-1 and vascular endothelial-cadherin (VE-cadherin) were probed with target proteins, respectively. All antibodies and dilutions are listed in Table S1.
2.14. Statistical analysis
The data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The data are expressed as mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA) followed by the Tukey post hoc test was used for the statistical analysis. Correlations were performed by one-tailed Spearman's analysis with 95% confidence intervals. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. ProBiotic-4 improved memory deficits and neuronal injury in aged SAMP8 mice
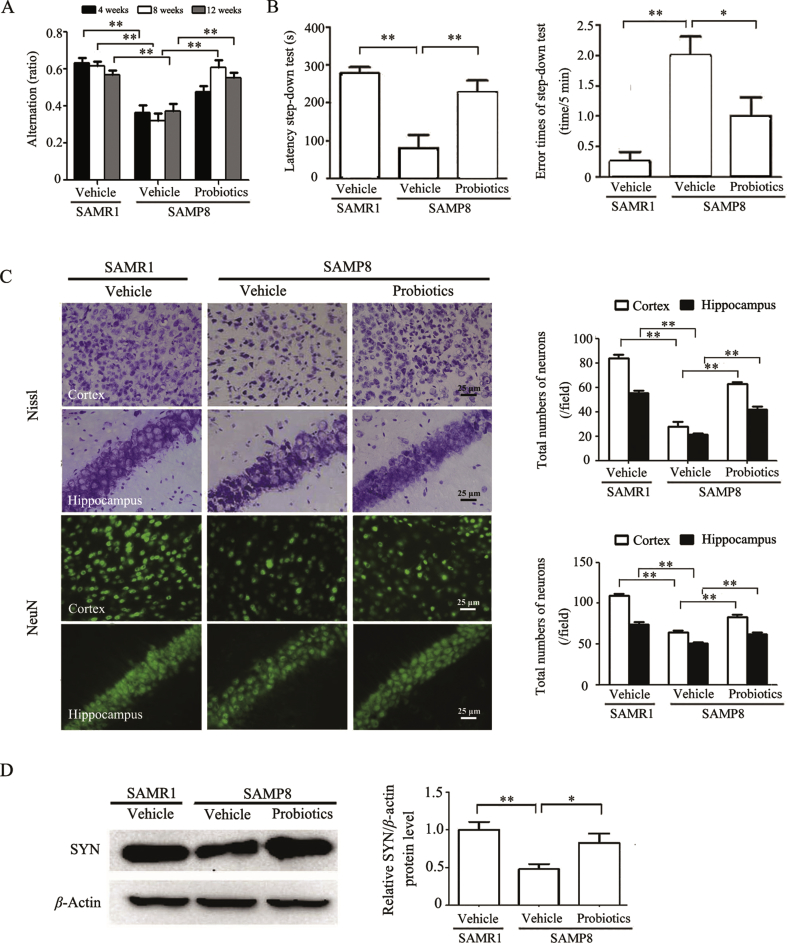
The Y-maze test was applied to evaluate the effects of ProBiotic-4 on memory function in aged SAMP8 mice. As shown in Fig. 1A, alternation significantly decreased in vehicle-treated SAMP8 group compared with vehicle-treated SAMR1 group throughout the entire process (P < 0.01). At 4 weeks of ProBiotic-4 treatment, no significant differences were found in alternation between the two SAMP8 groups. In contrast, alternation significantly increased in probiotic-treated SAMP8 group compared with vehicle-treated SAMP8 group at 8 weeks (P < 0.01), suggesting that ProBiotic-4 significantly improved short-term working memory in SAMP8 mice starting from 8 weeks. Also, alternation significantly increased in probiotic-treated SAMP8 group compared with vehicle-treated SAMP8 group at 12 weeks (P < 0.01), suggesting that ProBiotic-4 could effectively improve short-term working memory. Therefore, the passive avoidance test was performed afterwards. As shown in Fig. 1B, step-down latency significantly decreased, and error times of step-down test significantly increased in vehicle-treated SAMP8 group compared with vehicle-treated SAMR1 group (P < 0.01). Treatment with ProBiotic-4 significantly increased alternation and step-down latency and reduced the error times in aged SAMP8 mice compared with vehicle-treated SAMP8 group (P < 0.05 or P < 0.01). Meanwhile, the aging degree score, which is positively correlated with the degree of senescence, was lower in probiotic-treated SAMP8 mice than those in vehicle-treated SAMP8 mice (P < 0.05; Fig. S1A).
Figure 1.
ProBiotic-4 treatment improved memory deficits and neuronal injury in aged SAMP8 mice. (A) Alternation in the Y-maze test at 4, 8 and 12 weeks after ProBiotic-4 treatment. (B) Step-down latency and error times in the step-down passive avoidance test. (C) Representative photomicrographs and quantitative analysis of the number of neurons per field in the cerebral cortex and hippocampal CA1 areas, analyzed by Nissl staining and NeuN immunostaining. (D) Representative immunoblots and quantitative analysis of SYN expression in brain tissues. The level of SYN was normalized to β-actin. The results are expressed as the normalized optical density value relative to vehicle-treated SAMR1 group. The data are expressed as mean ± SEM (n = 10–12/group in A and B; n = 5–6/group in C and D). *P < 0.05, **P < 0.01 versus SAMP8 group treated with vehicle (one-way ANOVA followed by the Tukey post hoc test).
Nissl staining and NeuN immunostaining showed that the number of neurons in the cerebral cortex and hippocampal CA1 areas significantly decreased in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice (P < 0.01). ProBiotic-4 significantly preserved neuronal survival in the cortex and hippocampal CA1 areas in aged SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.01; Fig. 1C). In addition, synaptic plasticity plays an important role in memory processes in the brain. Therefore, we evaluated the expression of SYN, a major synaptic vesicle protein, by Western blotting in brain tissues. As shown in Fig. 1D, SYN expression significantly reduced in vehicle-treated SAMP8 mice compared with age-matched SAMR1 mice (P < 0.01), which was significantly rescued by ProBiotic-4 (P < 0.05). Altogether, these findings suggested that ProBiotic-4 could improve memory deficits and neuronal injury in aged SAMP8 mice.
3.2. ProBiotic-4 modified microbiota composition in aged SAMP8 mice
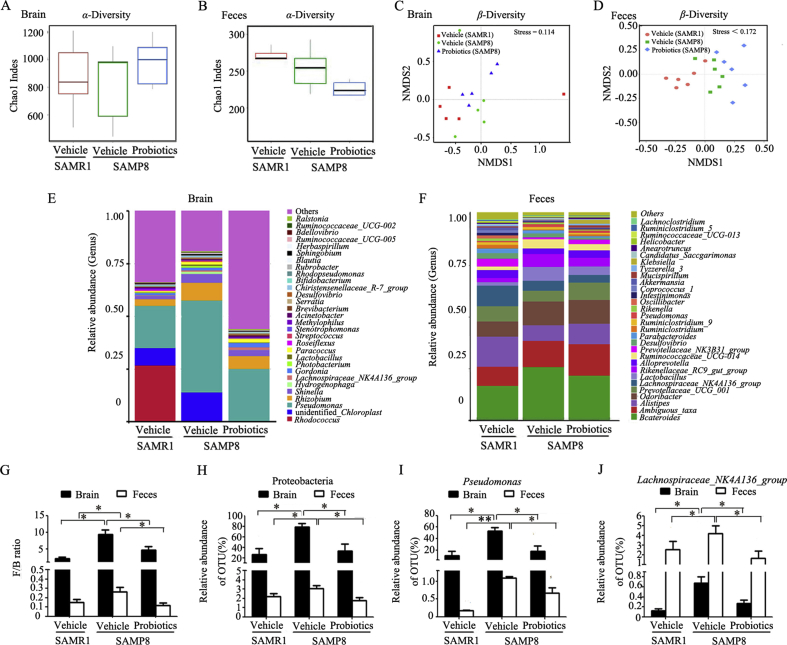
Probiotics are known to balance bacterial populations and have beneficial metabolic actions. We detected alterations of microbiota composition in both brain tissues and feces. The comparison of α-diversity indices (chao1 indes) revealed no significant differences in bacterial species between groups (Fig. 2A and B). We also assessed the dissimilarity of individual microbial communities (β-diversity). Non-metric multidimensional scaling (NMDS) showed a distinct clustering effect in probiotic-treated SAMP8 mice compared with vehicle-treated SAMP8 mice. NMDS also revealed a similar distinct clustering effect in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice (Fig. 2C and D). To identify microbial community membership of the brain and feces in probiotic- and vehicle-treated mice, we performed taxonomic classification analysis of operational taxonomic units (OTUs) that were derived from the primary sequencing data, revealing alterations of microbial composition in the three groups (Fig. 2E and F). Dramatic alterations of the microbiota composition were observed. Both in the brain and feces, the phylum Firmicutes/Bacteroidetes (F/B) ratio significantly increased in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice (P < 0.05). Probiotic-treated SAMP8 mice had a significantly lower F/B ratio than vehicle-treated SAMP8 mice (P < 0.05; Fig. 2G). Furthermore, Proteobacteria (phylum), Pseudomonas (genus) and Lachnospiraceae_NK4A136_group (genus) markedly decreased after ProBiotic-4 treatment in aged SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.05; Fig. 2H–J). Altogether, these data indicated that ProBiotic-4 might modify the microbiota composition both in the brain and intestine of aged SAMP8 mice.
Figure 2.
ProBiotic-4 treatment modified brain and gut microbiota composition in aged SAMP8 mice. (A) and (B) α-Diversity of flora in the brain and feces. (C) and (D) β-Diversity of flora in the brain and feces. (E) and (F) Diversity of microbial composition based on quality-controlled OTU reads in the brain and feces. (G) F/B ratio. (H)–(J) Effects of ProBiotic-4 on Proteobacteria, Pseudomonas and Lachnospiraceae_NK4A136_group. The data are expressed as mean ± SEM (n = 5–6/group). *P < 0.05, **P < 0.01 versus SAMP8 group treated with vehicle (one-way ANOVA followed by the Tukey post hoc test).
3.3. ProBiotic-4 significantly decreased intestinal barrier injury and inflammation in aged SAMP8 mice
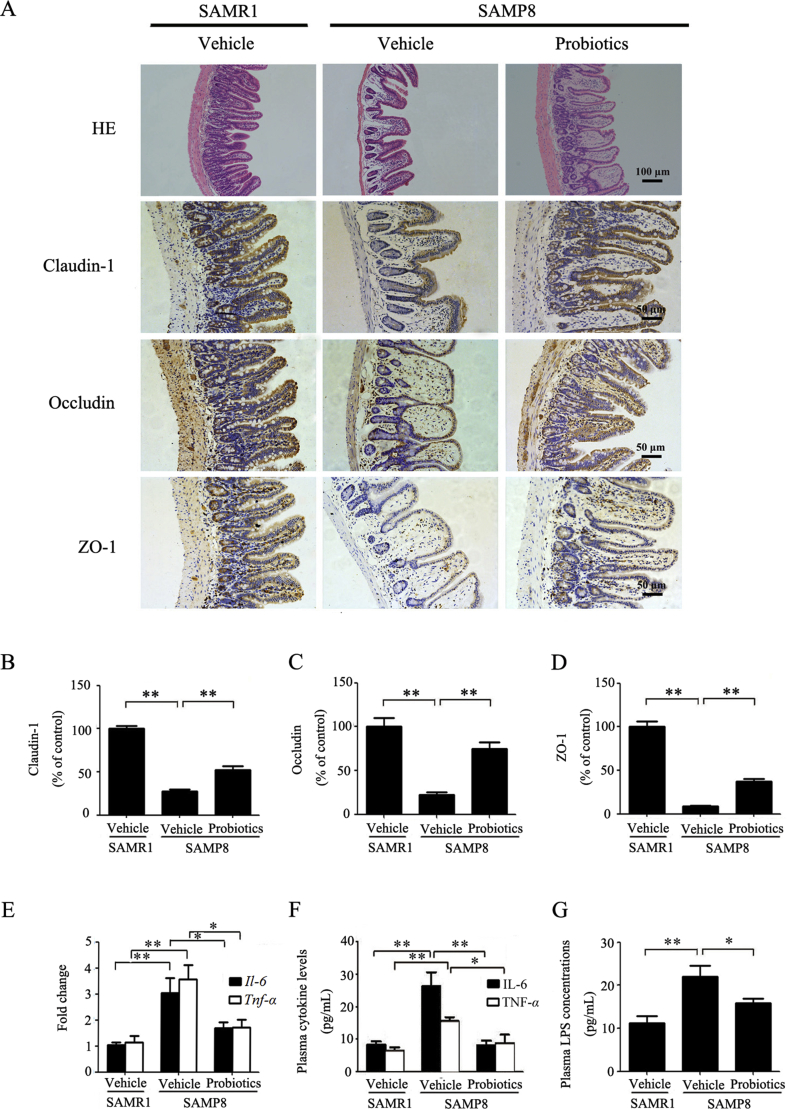
The intestinal barrier dysfunction and subsequent inflammatory responses are implicated in the occurrence and development of cognitive impairment32, 33, 34. We performed histological staining in the ileum. HE staining showed mucous layer atrophy, crypt loss, and villus fracture in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice (Fig. 3A), indicating that aged SAMP8 mice had significant deficits in intestinal barrier integrity. ProBiotic-4 treatment mitigated the magnitude of histological injuries in the ileum. We further performed IHC to assess the expression of intestine tight junction proteins, a multi-protein complex crucial for maintaining the intestinal barrier. Significant decreases in protein expression levels of claudin-1, occludin, and ZO-1 were observed in vehicle-treated SAMP8 mice compared with age-matched SAMR1 mice (P < 0.01). ProBiotic-4 significantly increased the expression of these proteins in the intestine in aged SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.01; Fig. 3A–D).
Figure 3.
Effects of ProBiotic-4 treatment on intestinal tight junction proteins and inflammation in aged SAMP8 mice. (A) Representative photomicrographs of HE staining and immunostaining of intestine tight junction markers claudin-1, occludin, and ZO-1 in the intestine. (B)–(D) Quantitative image analysis of claudin-1, occludin, and ZO-1 protein expressions based on the integrated optical density (IOD). (E) PCR analysis of Il-6 and Tnf-α mRNA levels in the intestine. The relative mRNA levels of Il-6 and Tnf-α were normalized to Gapdh. The results are expressed as the normalized fold change relative to vehicle-treated SAMR1 group. (F) and (G) IL-6, TNF-α and LPS levels in Plasma were measured by ELISA. The data are expressed as mean ± SEM (n = 6–7/group). *P < 0.05, **P < 0.01 versus SAMP8 group treated with vehicle (one-way ANOVA followed by the Tukey post hoc test).
It has been reported that age-related gut microbiota dysbiosis can trigger the inflammatory responses35. Therefore, we examined the effects of ProBiotic-4 on inflammatory cytokine expression in the intestine and plasma in aged SAMP8 mice. We observed that both the intestinal mRNA levels and the plasma protein contents of proinflammatory cytokines (IL-6 and TNF-α) significantly decreased in probiotic-treated SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.05 or P < 0.01; Fig. 3E and F). Meanwhile, plasma LPS levels significantly decreased in probiotic-treated SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.05; Fig. 3G). Collectively, these results indicated that oral ProBiotic-4 administration improved intestinal barrier injury and reduced inflammation in aged SAMP8 mice.
3.4. ProBiotic-4 significantly attenuated BBB injury, and inhibited neuroinflammation and TLR4/NF-κB signaling pathway in aged SAMP8 mice
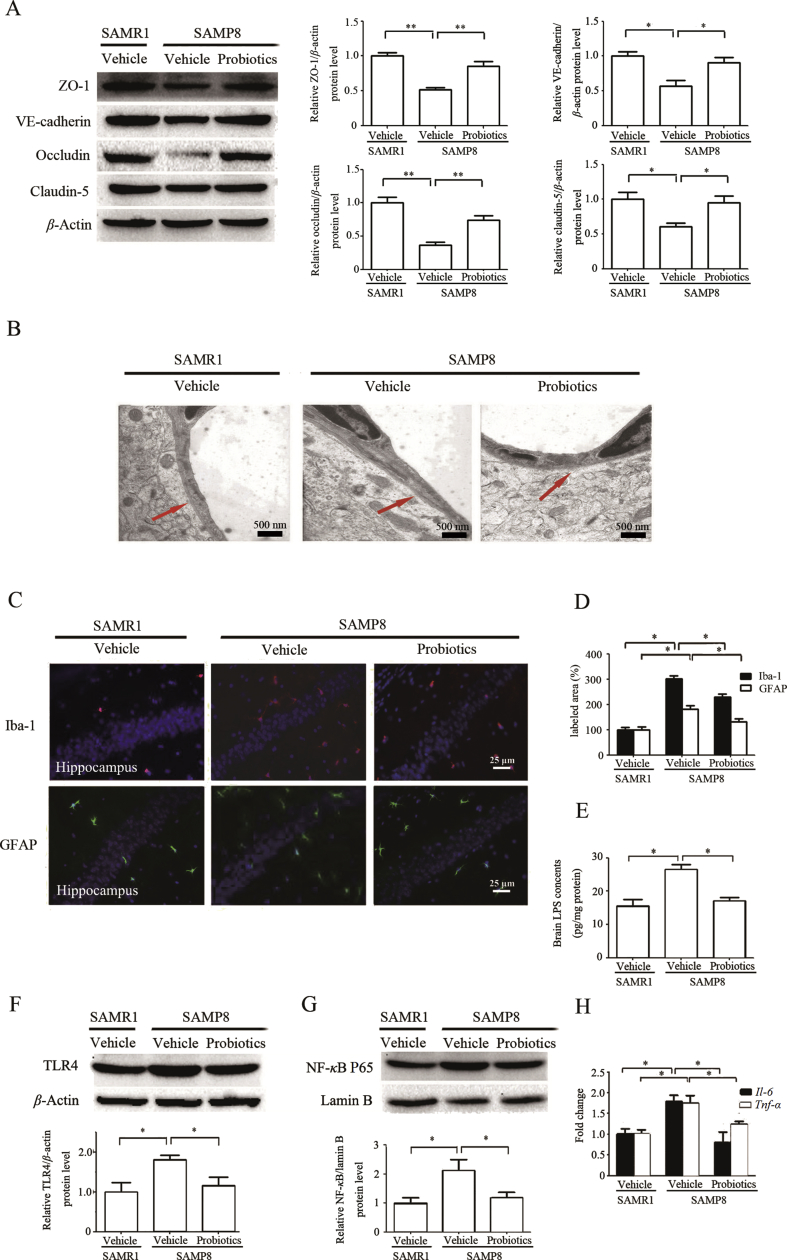
To evaluate the effects of ProBiotic-4 on BBB function, tight junction proteins and adherens junction protein was performed by Western blotting. As shown in Fig. 4A, the protein levels of occludin, claudin-5, ZO-1, and VE-cadherin significantly decreased in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice (P < 0.05 or P < 0.01). Significant increase in the protein levels of occludin, claudin-5, ZO-1, and VE-cadherin was observed in probiotic-treated SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.05 or P < 0.01). Moreover, the ultrastructures of BBB were confirmed by TEM. As shown in Fig. 4B, the endothelial cells were swollen and the basement membrane was incomplete in vehicle-treated SAMP8 mice. In contrast, the vascular endothelial cells and the basement membrane exhibited smooth and intact surfaces with clear layers in vehicle-treated SAMR1 mice and probiotic-treated SAMP8 mice.
Figure 4.
ProBiotic-4 alleviated the BBB injury and inflammationin aged SAMP8 mice. (A) Representative immunoblots and quantitative analysis of ZO-1, VE-cadherin, occludin, and claudin-5 expressions in the brain tissues. The levels of ZO-1, VE-cadherin, occludin, and claudin-5 were normalized to β-actin. The results are expressed as the normalized optical density value relative to the vehicle-treated SAMR1 group. (B) TEM observation of ultrastructure of the BBB. (C) Representative photomicrographs of Iba-1-positive microglia and GFAP-positive astrocytes in the hippocampal CA1 area. (D) Quantitative photomicrograph analysis of the Iba-1- and GFAP-positive area in the hippocampal CA1 area. (E) LPS level in the brain was measured by ELISA. (F) Representative immunoblots and quantitative analysis of TLR4 expression in the brain tissues. The levels of TLR4 were normalized to β-actin. The results are expressed as the normalized optical density value relative to the vehicle-treated SAMR1 group. (G) Representative immunoblots and quantitative analysis of the NF-κB nuclear translocation in the brain tissues. The levels of NF-κB were normalized to lamin B. The results are expressed as the normalized optical density value relative to the vehicle-treated SAMR1 group. (H) PCR analysis of Il-6 and Tnf-α mRNA levels in the brain. The relative mRNA levels of Il-6 and Tnf-α were normalized to Gapdh. The results are expressed as the normalized fold change relative to vehicle-treated SAMR1 group. The data are expressed as mean ± SEM (n = 5–6/group). *P < 0.05, **P < 0.01 versus SAMP8 group treated with vehicle (one-way ANOVA followed by the Tukey post hoc test).
To assess the effect of ProBiotic-4 on the activation of immune cells, GFAP and Iba-1, specific markers of astrocyte and microglia/macrophage activation, were detected. Vehicle-treated SAMP8 mice had markedly enhanced GFAP and Iba-1 immunoreactivity in the hippocampal CA1 area compared with vehicle-treated SAMR1 mice. Such immunoreactivity was decreased by ProBiotic-4 treatment (Fig. 4C and D). Importantly, brain LPS levels also significantly increased in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice (P < 0.05), which were effectively decreased by ProBiotic-4 treatment (P < 0.05; Fig. 4E). This indicates that the gut-derived LPS may reach the brain through the BBB. Dysbiosis in the gut microbiota increases the production of LPS, which can activate the TLR4 signaling pathway36. We next investigated the effect of ProBiotic-4 on the TLR4 pathway and production of downstream proinflammatory cytokines. ProBiotic-4 significantly decreased TLR4 protein levels and Il-6 and Tnf-α mRNA levels compared with vehicle-treated SAMP8 mice (P < 0.05), and significantly inhibited the nuclear translocation of NF-κB P65 compared with vehicle-treated SAMP8 mice (P < 0.05; Fig. 4F–H). These findings indicated that ProBiotic-4 treatment significantly improved BBB function and reduced neuroinflammation, and the mechanism might be associated with the TLR4/NF-κB signaling pathway.
3.5. ProBiotic-4 reduced oxidative DNA damage and inhibited RIG-I activation in the brain in aged SAMP8 mice
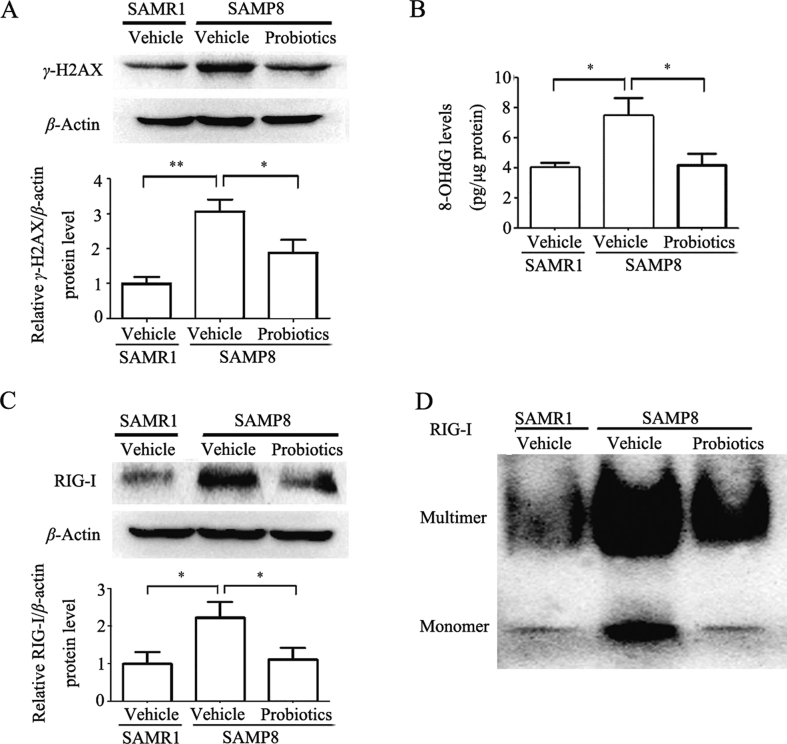
RIG-I is known as one of RIG-I-like receptors that plays a crucial role in the innate immune response to virus infection37. Recent studies have demonstrated that bacterial LPS and age-related increase in oxidative DNA damage may act as potent positive regulators of RIG-I expression and multimerization, leading to NF-κB signaling activation thereby enhancing inflammatory responses37, 38, 39. Therefore, we examined the effects of ProBiotic-4 on oxidative DNA damage markers (γ-H2AX and 8-OHdG) and RIG-I activation. As shown in Fig. 5A, γ-H2AX expression significantly decreased in probiotic-treated SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.05). The levels of 8-OHdG also significantly decreased in probiotic-treated SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.05, Fig. 5B). Meanwhile, the levels of RIG-I expression significantly increased in the brain in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice (P < 0.05), and decreased in probiotic-treated SAMP8 mice compared with vehicle-treated SAMP8 mice (P < 0.05; Fig. 5C). RIG multimerization obviously increased in the brain in vehicle-treated SAMP8 mice compared with vehicle-treated SAMR1 mice, and ProBiotics-4 improved it (Fig. 5D). The current results indicated that ProBiotic-4 might exert inhibitory effects on RIG-I-mediated neuroinflammation in aged SAMP8 mice.
Figure 5.
ProBiotic-4 reduced oxidative DNA damage and inhibited RIG-I activation in the brain in aged SAMP8 mice. (A) Representative immunoblots and quantitative analysis of γ-H2AX expression in the brain tissues. The levels of γ-H2AX was normalized to β-actin. The results are expressed as the normalized optical density value relative to the vehicle-treated SAMR1 group. (B) The DNA oxidation product 8-OHdG level in the brain was measured by ELISA. (C) Representative immunoblots and quantitative analysis of RIG-I expression in the brain tissues. The levels of RIG-I was normalized to β-actin. The results are expressed as the normalized optical density value relative to vehicle-treated SAMR1 group. (D) Representative immunoblots of RIG-I multimerization in the brain tissues. The data are expressed as mean ± SEM (n = 5–6/group). *P < 0.05, **P < 0.01 versus SAMP8 group treated with vehicle (one-way ANOVA followed by the Tukey post hoc test).
3.6. Correlation between the effects of ProBiotic-4 on TLR4-and RIG-I-mediated NF-κB pathway and microbiota–gut–brain axis in aged SAMP8 mice
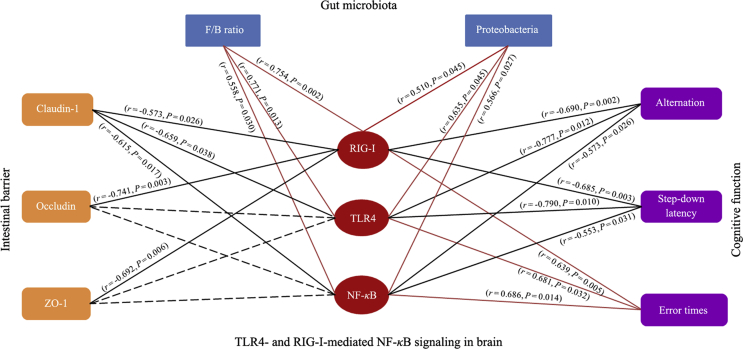
To investigate whether the effect of ProBiotic-4 on the microbiota–gut–brain axis imbalance in aging was implicated in the TLR4-and RIG-I-mediated NF-κB signaling pathway, the correlation analysis was performed based on the experimental data of vehicle- and probiotic-treated SAMP8 mice. As shown in Fig. 6, the F/B ratio and community abundance of Proteobacteria in gut microbiota, and the error times in the passive avoidance test were positively correlated with the levels of RIG-I, TLR4, and NF-κB p65 nuclear translocation in the brain, while the levels of claudin-1 in the intestine, the alternation in the Y-maze test, and the step-down latency in the passive avoidance test were negatively correlated with the levels of RIG-I, TLR4 and NF-κB P65 nuclear translocation in the brain. Meanwhile, the levels of occludin and ZO-1 in the intestine were negatively correlated with the RIG-I levels in the brain. The correlation analysis indicates that the microbiota–gut–brain axis deficits may be linked to activation of TLR4-and RIG-I-mediated NF-κB signaling pathway in the brain of aged SAMP8 mice and that ProBiotic-4 improved the deficits of the microbiota–gut–brain axis and cognitive function at least partly by inhibiting the TLR4-and RIG-I-mediated NF-κB signaling pathway.
Figure 6.
Correlation between TLR4-and RIG-I-mediated NF-κB signaling pathway in the brain and microbiota–gut–brain axis imbalance. The red oval node represents the TLR4-and RIG-I-mediated NF-κB signaling pathway in the brain, and the blue rectangular node represents gut microbiota. The orange rectangular node represents the intestinal barrier, and the purple rectangular node represents the cognitive function. The red edge represents the positive correlation, the black edge represents the negative correlation, and the gray dotted edge represents no correlation. The r represents Spearman's correlation coefficient. Data were collected from vehicle-treated and probiotic-treated SAMP8 mice (n = 5–6/group). One-tailed Spearman's analysis, confidence interval 95%.
4. Discussion
A large number of researches have demonstrated that neuroinflammation plays an essential role in the progression of aging-related cognitive deficits. So far, however, no effective agents are available for the prevention and treatment of the cognitive impairments in the elderly. Recent studies have drawn attention to the beneficial potentials of various probiotics on the microbiota–gut–brain axis imbalance and cognitive decline in aging7, 8, 9. Results from different research groups have indicated a neuroprotective effect of some probiotics, such as B. lactis, L. casei, B. bifidum, and L. acidophilus, on aging-related cognitive dysfunction in clinic and preclinic studies7, 8, 9. In the present study, therefore, we investigated the effects and underlying mechanism of ProBiotic-4, a probiotic preparation composed of B. lactis, L. casei, B. bifidum, and L. acidophilus, on the deficits of the microbiota–gut–brain axis and cognitive function in aged SMAP8 mice. We observed that oral administration of ProBiotic-4 for 12 weeks significantly improved the memory deficits, cerebral neuronal and synaptic injuries, glial activation, and modified the microbiota composition in the gut and brain in aged SAMP8 mice. Meanwhile, ProBiotic-4 treatment attenuated aging-related disruptions of the intestinal barrier and BBB, decreased Il-6 and Tnf-α in the intestine and brain at mRNA levels, lowered LPS concentrations in the plasma and brain, reduced the levels of γ-H2AX, 8-OHdG, TLR4, RIG-I, and NF-κB nuclear translocation in the brain. Furthermore, correlation analysis showed that the microbiota–gut–brain axis imbalance was linked to the TLR4-and RIG-I-mediated NF-κB signaling pathway activation in aging. Interestingly, ProBiotic-4 treatment was able to modulate aging-related gut microbiota dysbiosis and improve the deficits of the microbiota–gut–brain axis and cognitive function. To our best knowledge, the current findings suggest for the first time that the inhibition of TLR4-and RIG-I-mediated NF-κB signaling pathway contributes to the neuroprotective effect of probiotics (ProBiotic-4) against aging associated cognitive impairments.
SAMP8 is a widely used mouse model of aging and dementia40. It has been reported that 9-month-old SAMP8 mice begin to develop early cognitive dysfunction and neuronal injury compared with age-matched SAMR1 mice41. Moreover, our previous studies observed the increased oxidative stress and neuroinflammation in the brain of 10-month-old SAMP8 mice30, 42. Therefore, the 9-month-old SAMP8 and SMAR1 mice were orally administrated with ProBiotic-4 or vehicle for 12 weeks in this study, respectively. Consistent with previous reports29, 42, the impaired memory ability was observed in vehicle-treated SAMP8 mice compared with age-matched SAMR1 mice. In addition, ProBiotic-4 treatment is able to significantly improve the cognitive performance of aged SAMP8 mice in the Y-maze and passive avoidance tests. Meanwhile, ProBiotic-4 obviously alleviated the neuropathological alterations, such as neuronal loss, synaptic injury and glial activation, and improved neuroinflammation and oxidative DNA damage in the brain of aged SAMP8 compared with vehicle-treated SAMP8 controls. These findings indicate that ProBiotic-4 may ameliorate aging-related memory impairment at least partly through inhibition of oxidative stress and neuroinflammation in the aging brain.
Emerging evidence suggests that the microbiota–gut–brain axis deficits are involved in the pathogenesis of aging-related cognitive decline43, 44. For example, bacteria have been recently identified in the brain of AD patients, suggesting that microbes are a possible contributing factor in AD-associated neuroinflammation45. It has been shown that gut microbiota imbalance can induce intestinal inflammatory response and intestinal barrier damage, which facilitates the entrance of gut bacteria-derived pathogens and toxins (e.g., LPS) into the circulatory system, resulting in BBB destruction and neuroinflammation46, 47, 48. Previous studies showed that gut dysbiosis, such as increased F/B ratio and distinctive expansion of potential pathogens (e.g., Proteobacteria or Lachnospiraceae), was highly associated with various inflammation-related diseases49, 50, 51. To decipher the mechanism by which ProBiotic-4 improves MCI in aged SAMP8 mice, we first examined the effects of probiotic intervention on the gut and brain microbiota profiles by 16S rRNA gene analysis. We observed the disturbance of the gut and brain microbiota in vehicle-treated SAMP8 mice compared with SAMR1 controls. Consistent with previous reports49, 52, we found elevated F/B ratio, increased relative abundance of Proteobacteria in aged SAMP8 mice. In addition, changes in other bacterial genera were also identified in our study. For instance, the relative abundance of Pseudomonas and Lachnospiraceae_NK4A136_group was increased in these mice. Intestingly, ProBiotic-4 treatment was able to significantly modulate gut microbiota dysbiosis in aged SAMP8 mice. Specifically, ProBiotic-4 dramatically reduced F/B ratio, lowered the relative abundance of Proteobacteria, Pseudomonas and Lachnospiraceae_NK4A136_group in the brains of aged SAMP8 mice. These findings suggest that ProBiotic-4 may reduce neuroinflammation by influencing microbes in the brain. Further studies are needed to investigate the potential impact of bacteria in the brain on cognitive performance. Taken together, these results suggest that the identified microbes may be involved in aging-related MCI pathogenesis.
Moreover, immunostaining and Western blotting analyses were applied to examine the effects of ProBiotic-4 on the intestinal barrier and BBB integrity in aged mice, respectively. Our results showed that the expression of tight junction proteins and adherens junction protein in the brain and that of tight junction proteins in the intestine were significantly decreased in vehicle-treated SAMP8 mice compared with SAMR1 controls. Importantly, ProBiotic-4 treatment significantly upregulated the expression of these tight junctions and adherens junction proteins in SAMP8 mice. Notably, transmission electron microscopy study showed a protective effect of ProBiotic-4 on the BBB ultrastructure injury such as basement membrane impairment. Furthermore, qPCR and ELISA assays were used to detect the inflammatory responses in the intestine and the systemic circulation in mice. Compared with SAMR1 group, both the cerebral mRNA levels and plasma protein contents of proinflammatory cytokines (IL-6 and TNF-α) were significantly increased in vehicle-treated SAMP8 mice, which could be effectively reduced by ProBiotic-4 intervention. These data suggest that the neuroprotective effect of ProBiotic-4 is possibly associated with its modulation on the microbiota–gut–brain axis deficits and inhibition of inflammaging in aged SAMP8 mice.
It is well known that bacterial pathogen-associated molecular patterns (PAMPs) activated pattern recognition receptor (PRR) signaling pathways play vital roles in innate host immunity against pathogenic microorganisms. LPS is a classical PAMP derived from Gram-negative bacteria, and may induce the activation of TLR4/NF-κB signaling pathway and subsequent inflammatory response. To further explore the underlying molecular mechanism by which ProBiotic-4 protects cognitive deficits in aged SAMP8 mice, we investigated the effect of ProBiotic-4 on the TLR4/NF-κB signaling pathway in SAMP8 mice. Compared to vehicle-treated SAMR1 mice, LPS contents in the plasma and brain and TLR4/NF-κB pathway activation in the brain were significantly upregulated in vehicle-treated SAMP8 mice. Notably, ProBiotic-4 significantly inhibited TLR4/NF-κB signaling pathway in SAMP8 mice. In addition, RIG-I is regarded as an important PRR, which activates NF-κB activation and exerts antiviral and antibacterial innate immune responses by recognizing viral and bacterial RNAs and LPS36, 39. Given that oxidative stress is identified as a positive regulator of RIG-I activation38, we examined the effects of ProBiotic-4 on oxidative DNA damage and RIG-I activation. We found, for the first time, that ProBiotic-4 reduced the oxidative DNA damage and inhibited RIG-I expression and multimerization in aged SAMP8 mice. Collectively, these results suggest that the observed activation of TLR4-and RIG-I-mediated NF-κB signaling pathway in the brain could be secondary to changes in the gut microbiota, and the neuroprotective effect of ProBiotic-4 is possibly associated with the inhibitory effect on TLR4-and RIG-I-mediated NF-κB signaling pathway.
5. Conclusions
In summary, the present study demonstrates for the first time that probiotics (ProBiotic-4) may modulate the gut microbiota dysbiosis and the microbiota–gut–brain axis deficits, resulting in improvement of the cognitive dysfunction in senile mice. Moreover, inhibition of TLR4-and RIG-I-mediated NF-κB signaling pathway contributes to the neuroprotective effect of ProBiotic-4. These findings provide a novel gut microbiota-targeted therapeutic strategy for aging-related cognitive decline. Furthermore, the comparative studies of the neuroprotective efficacies of ProBiotic-4 multiplexes and the corresponding four single-strain probiotics against MCI should be performed in the future. This study will be helpful for the clarification of the relationship between specific probiotics and aging-related MCI. In addition, further experiments using loss-of-function studies (e.g., TLR4−/−) and/or pharmacological modulators (e.g., LPS) will provide insight into the mechanism of probiotics-based therapies.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81473219 and 81973307) and partly by 111 Project of the National Ministry of Education (B18035, China).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Author contributions
Junrong Du conceptualized this study. Junrong Du and Xueqin Yang designed the experiment. Xueqin Yang and Li Xue conducted the experiments and data analysis. Hui Li assisted in some acquisition of data. Xueqin Yang and Dongke Yu prepared the draft and Junrong Du rewrote the manuscript.
Conflicts of interest
All authors state that there are no conflicts of interests.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.07.001.
Appendix A. Supporting information
The following is the Supplementary data to this article:
References
- 1.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choiniere J., Wang L. Exposure to inorganic arsenic can lead to gut microbe perturbations and hepatocellular carcinoma. Acta Pharm Sin B. 2016;6:426–429. doi: 10.1016/j.apsb.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancuso C., Santangelo R. Alzheimer's disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res. 2018;129:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Shen L., Liu L., Ji H.F. Alzheimer's disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimer's Dis. 2017;56:385–390. doi: 10.3233/JAD-160884. [DOI] [PubMed] [Google Scholar]
- 7.Tamtaji O.R., Heidari-Soureshjani R., Mirhosseini N., Kouchaki E., Bahmani F., Aghadavod E. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38:1594–1598. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O.R. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azm S.A.N., Djazayeri A., Safa M., Azami K., Ahmadvand B., Sabbaghziarani F. Lactobacilli and Bifidobacteria ameliorate memory and learning deficits and oxidative stress in beta-amyloid1–42 injected rats. Appl Physiol Nutr Metabol. 2018;43:718–726. doi: 10.1139/apnm-2017-0648. [DOI] [PubMed] [Google Scholar]
- 10.Powell N., Walker M.M., Talley N.J. The mucosal immune system: master regulator of bidirectional gut–brain communications. Nat Rev Gastroenterol Hepatol. 2017;14:143–159. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- 11.Forsythe P., Bienenstock J., Kunze W.A. Vagal pathways for microbiome–brain–gut axis communication. Adv Exp Med Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 12.Cani P.D., Knauf C. How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab. 2016;5:743–752. doi: 10.1016/j.molmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makivuokko H., Tiihonen K., Tynkkynen S., Paulin L., Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103:227–234. doi: 10.1017/S0007114509991553. [DOI] [PubMed] [Google Scholar]
- 14.Claesson M.J., Cusack S., O'Sullivan O., Greene-Diniz R., de Weerd H., Flannery E. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108:4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Pena C., Alvarez-Cisneros T., Quiroz-Baez R., Friedland R.P. Microbiota and aging. A review and commentary. Arch Med Res. 2017;48:681–689. doi: 10.1016/j.arcmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Fransen F., van Beek A.A., Borghuis T., Aidy S.E., Hugenholtz F., van der Gaast-de C. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. doi: 10.3389/fimmu.2017.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atienza M., Ziontz J., Cantero J.L. Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging. Sleep Med Rev. 2018;42:171–183. doi: 10.1016/j.smrv.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y.H., Gao C.L., Guo H.L., Zhang W.Q., Huang W., Tang S.S. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J Endocrinol. 2018;238:231–244. doi: 10.1530/JOE-18-0137. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar S.R., Banerjee S. Gut microbiota in neurodegenerative disorders. J Neuroimmunol. 2019;328:98–104. doi: 10.1016/j.jneuroim.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Jiao F.Z., Wang Y., Zhang H.Y., Zhang W.B., Wang L.W., Gong Z.J. Histone deacetylase 2 inhibitor CAY10683 alleviates lipopolysaccharide induced neuroinflammation through attenuating TLR4/NF-κB signaling pathway. Neurochem Res. 2018;43:1161–1170. doi: 10.1007/s11064-018-2532-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhao C.Y., Hou W.Z., Lei H., Huang L.J., Wang S., Cui D.D. Potassium 2-(l-hydroxypentyl)-benzoate attenuates neuroinflammatory responses and upregulates heme oxygenase-1 in systemic lipopolysaccharide-induced inflammation in mice. Acta Pharm Sin B. 2017;7:470–478. doi: 10.1016/j.apsb.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imaizumi T., Sashinami H., Mori F., Matsumiya T., Yoshida H., Nakane A. Listeria monocytogenes induces the expression of retinoic acid-inducible gene-I. Microbiol Immunol. 2006;50:811–815. doi: 10.1111/j.1348-0421.2006.tb03857.x. [DOI] [PubMed] [Google Scholar]
- 23.Kang L., Li S., Xing Z., Li J., Su Y., Fan P. Dihydrotestosterone treatment delays the conversion from mild cognitive impairment to Alzheimer's disease in SAMP8 mice. Horm Behav. 2014;65:505–515. doi: 10.1016/j.yhbeh.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman H.M., Koning C.J.M., Mulder L., Rombouts F.M., Beynen A.C. Monostrain, multistrain and multispecies probiotics—a comparison offunctionality and efficacy. Int J Food Microbiol. 2004;96:219–233. doi: 10.1016/j.ijfoodmicro.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Chapman C.M.C., Gibson G.R., Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50:1–17. doi: 10.1007/s00394-010-0166-z. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi Y., Sugahara H., Shimada K., Mitsuyama E., Kuhara T., Yasuoka A. Therapeutic potential of Bifdobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease. Sci Rep. 2017;7:13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H.J., Hwang Y.H., Kim D.H. Lactobacillus plantarum C29-fermented soybean (DW2009) alleviates memory impairment in 5XFAD transgenic mice by regulating microglia activation and gut microbiota composition. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201800359. [DOI] [PubMed] [Google Scholar]
- 28.Takeda T., Hosokawa M., Takeshita S., Irino M., Higuchi K., Matsushita T. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 29.Kuang X., Chen Y.S., Wang L.F., Li Y.J., Liu K., Zhang M.X. Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer's disease mouse model. Neurobiol Aging. 2014;35:169–178. doi: 10.1016/j.neurobiolaging.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H.J., Zeng C.Y., Yang T.T., Long F.Y., Kuang X., Du J.R. Lentivirus-mediated klotho up-regulation improves aging-related memory deficits and oxidative stress in senescence-accelerated mouse prone-8 mice. Life Sci. 2018;200:56–62. doi: 10.1016/j.lfs.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H.J., Li H., Shi M.Q., Mao X.N., Liu D.L., Chang Y.R. Protective effect of Klotho against ischemic brain injury is associated with inhibition of RIG-I/NF-κB signaling. Front Pharmacol. 2017;8:950. doi: 10.3389/fphar.2017.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oriá R.B., Murray-Kolb L.E., Scharf R.J., Pendergast L.L., Lang D.R., Kolling G.L. Early-life enteric infections: relation between chronic systemic inflammation and poor cognition in children. Nutr Rev. 2016;74:374–386. doi: 10.1093/nutrit/nuw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellegrini C., Antonioli L., Colucci R., Blandizzi C., Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol. 2018;136:345–361. doi: 10.1007/s00401-018-1856-5. [DOI] [PubMed] [Google Scholar]
- 34.Bettcher B.M., Kramer J.H. Inflammation and clinical presentation in neurodegenerative disease: a volatile relationship. Neurocase. 2013;19:182–200. doi: 10.1080/13554794.2011.654227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L., Li J., Zhou Y., Huang X., Qin X., Du G. Effects of baicalein on cortical proinflammatory cytokines and the intestinal microbiome in senescence accelerated mouse prone 8. ACS Chem Neurosci. 2018;9:1714–1724. doi: 10.1021/acschemneuro.8b00074. [DOI] [PubMed] [Google Scholar]
- 36.Mobarak E., Haversen L., Manna M., Rutberg M., Levin M., Perkins R. Glucosylceramide modifies the LPS-induced inflammatory response in macrophages and the orientation of the LPS/TLR4 complex in silico. Sci Rep. 2018;8:13600. doi: 10.1038/s41598-018-31926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoneyama M., Onomoto K., Jogi M., Akaboshi T., Fujita T. Viral RNA detection by RIG-I-like receptors. Curr Opin Immunol. 2015;32:48–53. doi: 10.1016/j.coi.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Liu F., Wu S., Ren H., Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13:254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 39.Kong L., Sun L., Zhang H., Liu Q., Liu Y., Qin L. An essential role for RIG-I in toll-like receptor-stimulated phagocytosis. Cell Host Microbe. 2009;6:150–161. doi: 10.1016/j.chom.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi R. Anti-aging studies on the senescence accelerated mouse (SAM) strains. Yakugaku Zasshi. 2010;130:11–18. doi: 10.1248/yakushi.130.11. [DOI] [PubMed] [Google Scholar]
- 41.del Valle J., Bayod S., Camins A., Beas-Zarate C., Velazquez-Zamora D.A., Gonzalez-Burgos I. Dendritic spine abnormalities in hippocampal CA1 pyramidal neurons underlying memory deficits in the SAMP8 mouse model of Alzheimer's disease. J Alzheimer's Dis. 2012;32:233–240. doi: 10.3233/JAD-2012-120718. [DOI] [PubMed] [Google Scholar]
- 42.Li X., Yang X.Q., Shi M.Q., Du J.R. Anti-oxidative protective effect and mechanism of klotho on ischemic brain injury. Acta Pharm Sin. 2018;53:1332–1337. [Google Scholar]
- 43.Köhler C.A., Maes M., Slyepchenko A., Berk M., Solmi M., Lanctôt K.L. The gut–brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer's disease. Curr Pharmaceut Des. 2016;22:6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 44.Hu X., Wang T., Jin F. Alzheimer's disease and gut microbiota. Sci China Life Sci. 2016;59:1006–1023. doi: 10.1007/s11427-016-5083-9. [DOI] [PubMed] [Google Scholar]
- 45.Emery D.C., Shoemark D.K., Batstone T.E., Waterfall C.M., Coghill J.A., Cerajewska T.L. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer's post-mortem brain. Front Aging Neurosci. 2017;9:195. doi: 10.3389/fnagi.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso F.L., Kittel A., Veszelka S., Palmela I., Toth A., Brites D. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular e ndothelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin L., Nation R.L., Li J., Nicolazzo J.A. Species-dependent blood–brain barrier disruption of lipopolysaccharide: amelioration by Colistin in vitro and in vivo. Antimicrob Agents Chemother. 2013;57:4336–4342. doi: 10.1128/AAC.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman J.D., Parikh I., Green S.J., Chlipala G., Mohney R.P., Keaton M. Age drives distortion of brain betabolic, vascular and cognitive functions, and the gut microbiome. Front Aging Neurosci. 2017;9:298. doi: 10.3389/fnagi.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng H., Ishaq S.L., Zhao F.Q., Wright A.G. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J Nutr Biochem. 2016;35:30–36. doi: 10.1016/j.jnutbio.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Shin N.R., Whon T.W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.