Abstract
Ulcerative colitis (UC) manifests as an etiologically complicated and relapsing gastrointestinal disease. The enteric nervous system (ENS) plays a pivotal role in rectifying and orchestrating the inflammatory responses in gut tract. Berberine, an isoquinoline alkaloid, is known as its anti-inflammatory and therapeutic effects in experimental colitis. However, little research focused on its regulatory function on ENS. Therefore, we set out to explore the pathological role of neurogenic inflammation in UC and the modulating effects of berberine on neuro–immune interactions. Functional defects of enteric glial cells (EGCs), with decreased glial fibrillary acidic protein (GFAP) and increased substance P expression, were observed in DSS-induced murine UC. Administration of berberine can obviously ameliorate the disease severity and restore the mucosal barrier homeostasis of UC, closely accompanying by maintaining the residence of EGCs and attenuating inflammatory infiltrations and immune cells overactivation. In vitro, berberine showed direct protective effects on monoculture of EGCs, bone marrow-derived dendritic cells (BMDCs), T cells, and intestinal epithelial cells (IECs) in the simulated inflammatory conditions. Furthermore, berberine could modulate gut EGCs–IECs–immune cell interactions in the co-culture systems. In summary, our study indicated the EGCs–IECs–immune cell interactions might function as a crucial paradigm in mucosal inflammation and provided an infusive mechanism of berberine in regulating enteric neurogenic inflammation.
Keywords: Ulcerative colitis, Berberine, Mucosal inflammation, Enteric glial cells, Enteric nervous system
Abbreviations: APCs, antigen-presenting cells; BDNF, brain-derived neurotrophic factor; BMDCs, bone marrow-derived dendritic cells; CGRP, calcitonin gene-related peptide; DSS, dextran sulfate sodium; EGCs, enteric glial cells; ENS, enteric nervous system; GDNF, glial cell derived neurotrophic factor; GFAP, glial fibrillary acidic protein; IBD, inflammatory bowel diseases; IECs, intestinal epithelial cells; LMPC, lamina propria mononuclear cells; MAPK, mitogen-activated protein kinases; MLNs, mesenteric lymph nodes; MPO, myeloperoxidase; UC, ulcerative colitis; VIP, vasoactive intestinal polypeptide
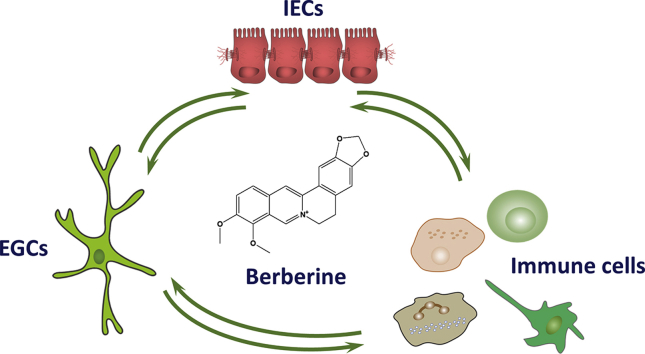
Graphical abstract
This study demonstrates the pathological role of functional defects of enteric glial cells (EGCs) and an infusive mechanism of therapeutic effects of berberine in ulcerative colitis. Berberine could maintain the intestinal residence of EGCs and modulate the interactions between EGCs, epithelial cells and immune cells.
1. Introduction
Ulcerative colitis (UC) is a relapsing and etiologically idiopathic inflammatory disease, characterized by intestinal tract bleeding and mucosal erosions, which commonly afflicts patients aged 30–40 years and leads to disability and severe body weight loss1. Evidence from immunological and genetic studies reveals that the inflammation in UC patients is closely associated with burst of cytokines or/and chemokines production from immune cells and epithelial cells, which are linked to the dysfunction of intestinal barrier homeostasis2. The intestinal epithelium, functioning as a physical barrier against external environment, is formed by a tight and continuous monolayer of intestinal epithelial cells (IECs)3. Disruption of IECs results in further inflammatory responses, referring to microbe-derived responses and overexpression of chemokines and adhesive molecules3. Under such inflammatory circumstances, the dysregulation of immune responses is initialed by abnormal activation and infiltration of innate lymphoid cells (ILCs) and antigen-presenting cells (APCs). Pathogenic factors derived from active immune cells further intend to destroy the gut barrier integrity, which contributes to the exacerbation of UC4. Additionally, overproduction of inflammatory cytokines plays a critical role in exaggerating adaptive immune responses, including T cells differentiation, activation and reactivity5.
Over the past decade, increasing studies have identified the close interaction and communication between the immunology and nervous system in inflammatory diseases6. Immune cells activation could modulate multiple neuronal circuits and the activity of sensory neurons in the local gut tract through cytokines receptors, G-protein-coupled receptors and other receptors, which in turn result in generation of secondary messengers, cyclic adenosine monophosphate (cAMP) and Ca2+, and activation of protein kinases A (PKA) and mitogen-activated protein kinase (MAPK) signaling. As a consequence, plenty of neuropeptides, such as vasoactive intestinal peptide (VIP), substance P, and calcitonin gene-related peptide (CGRP), are synthesized and result in the concept of generation of neurogenic inflammation6. As located in gut tract, enteric glial cells (EGCs), the major constituent of the enteric nervous system, have been demonstrated to closely associated with regulation and maintenance of intestinal barrier function and reduction or/and functional defects of EGCs contribute to the barrier dysfunction in gut inflammatory diseases7, 8. Mucosal EGCs produce several physiological and pathological mediators, including glial-derived neurotrophic factor (GDNF), substance P, neurotrophins, and transforming growth factor-β1 (TGF-β1), which are more or less implicated in modulating mucosal barrier function9, 10. Therein, increased expression of inflammatory substance P and its receptors NK-1R and NK-2R has been identified in the colon biopsy of UC and Crohn's disease (CD) patients11, 12. Collectively, the role of EGCs on barrier function has drawn interests in inflammatory diseases, which may provide an alternative therapeutic strategy in UC treatment13, 14. Furthermore, role of EGCs in mucosal inflammation has also been explored. Similar to astrocytes in central nervous system (CNS), EGCs have been recognized as APCs and could also produce TNF-α, IL-1β, and IL-6 and other inflammatory mediators, which then lead to activation of macrophages, mast cell and T cells10.
In the gut microenvironment, delicate modulation and communication among EGCs, IECs and immune cells contribute to the mucosal barrier homeostasis and balance of immune system8. Accumulating studies have revealed immune cell–IECs interaction are also critical for UC initiation and aggravation3, 15. Nevertheless, the pathological role of interactions among EGCs–IECs–immune cells in the development of UC remains elusive and little research has been published to investigate whether overactivation of immune cells showed regulatory effects on EGCs and hyperreaction of EGCs influenced the inflammatory responses and intestinal barrier homeostasis.
Berberine, an isoquinoline alkaloid, is present in Hydrastis canadensis, Berberis aquifolium, and Berberis vulgaris. Previous researches16, 17, 18 have demonstrated that berberine could alleviate acute or chronic experimental colitis by modulating the responses of Th17 cells, improving epithelial barrier function19, 20, inhibiting lipid peroxidation21, and balancing the gut microbiota18. Recently, a phase 4 clinical trial from Xijing Hospital of Digestive Diseases is ongoing to uncover the therapeutic effects of berberine on the annual recurrence rate of UC in remission (NCT02962245, ClinicalTrials.gov). In the present study, we aimed to investigate the pathological role of enteric neuro-inflammation in UC and explore whether berberine exert modulating effects on EGCs–IECs–immune cells interactions.
2. Materials and methods
2.1. Reagents and chemicals
Dextran sulfate sodium (DSS, 36–50 kDa) was obtained from MP Biomedicals (Irvine, CA, USA). Berberine chloride, FITC-dextran and substance P were obtained from Sigma–Aldrich (St. Louis, MO, USA). The fecal occult blood test kits and myeloperoxidase (MPO) activity assay kits were obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). L-012 sodium was obtained from Tocris (Bristol, UK). Cell Counting Kit-8 (CCK-8) was obtained from Dojindo (Kumamoto, Japan). Superoxide dismutase (SOD), and malondialdehyde (MDA) assay kits were obtained from Beyotime (Haimen, China). Mouse TNF-α, IFN-γ, IL-1β, IL-2, IL-6, IL-10, IL-12p40, and IL-17A ELISA kits were obtained from BD Pharmingen (San Diego, CA, USA). GDNF, brain-derived neurotrophic factor (BDNF), and substance P ELISA kits were obtained from Abcam (Cambridge, MA, USA), R&D Systems (Minneapolis, MN, USA), and Raybiotech (Norcross, GA, USA), respectively. FITC-conjugated CD62L, FITC-conjugated CD25, FITC-conjugated γδTCR, FITC-conjugated Gr-1, PE-conjugated CD25, PE-conjugated F4/80, PE-conjugated IL-17A, FITC Annexin V Apoptosis Detection Kit, Percp-Cy5.5-conjugated CD11b, APC-conjugated CD11c, APC-conjugated IFN-γ, BV421-conjugated CD3, and BUV395-conjugated CD45 were obtained from BD Biosciences (San Jose, CA, USA). Percp-Cy5.5-conjugated CD44, and Percp-Cy5.5-conjugated Foxp3 were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Recombinant murine IL-4, GM-CSF, GDNF and recombinant human TNF-α, GDNF, BDNF were obtained from PeproTech (Rocky Hill, NJ, USA).
2.2. Cell culture
Human adherent colon cancer cell lines HT-29, Caco-2 cells, human monocytic leukemia cell line THP-1 cells, and rat enteroglial cell (EGC) line CRL-2690 were purchased form American Type Culture Collection (ATCC, Manassas, VA, USA). HT-29 cells were cultured in McCoy's 5a medium containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Caco-2 cells and CRL-2690 cells were cultured in DMEM containing 10% FBS, 2 mmol/L l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. THP-1 cells were culture in RMPI 1640 medium containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained at 37 °C in a humidified incubator of 5% CO2.
2.3. Animals and DSS-induced murine experimental colitis
Male C57BL/6 mice (8-week old, 23–25 g, IACUC: 2018-03-TW-06) were obtained from Shanghai Lingchang Biotechnology Co., Ltd. (Certificate No.2013-0018, China). Male Sprague–Dawley rats (160–200 g, IACUC: 2018-05-ZJP-77) were obtained from Shanghai Laboratory Animal Center of Chinese Academy of Sciences. All animals were maintained under specific pathogen-free (SPF) animal facilities with 12 h of light/12 h of dark cycle, 22 ± 1 °C and 55 ± 5% of relative humidity, and free access to food and water. All experiments were carried out performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, revised 2011) and were approved by the Bioethics Committee of the Shanghai Institute of Materia Medica (SIMM, Shanghai, China). Upon arrival, the mice were acclimatized for 1 week before induction of colitis and the mice wellbeing was monitored at least daily throughout the experiments.
Mice were randomly divided into three groups, including normal, vehicle and berberine-treated vehicle group with 8 mice per group. To induce experimental colitis, 3% DSS (w/v) was added to the drinking water over a 7-day period and then was replaced with normal drinking water for an additional 4 days (Fig. 1A). Berberine chloride (100 mg/kg) was dissolved in sterile water and administrated daily through gavage. Mice were killed on Day 11 for the following analysis.
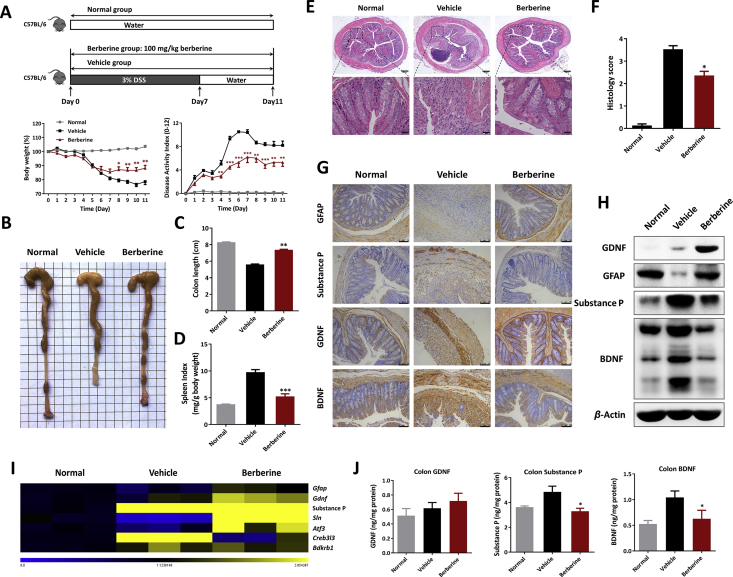
Figure 1.
Berberine exerted protective effects and enhanced residence of EGCs in DSS-induced ulcerative colitis. (A) Mice were treated with 3% DSS (from Day 0–7) and sterile water for additional 4 days. Berberine (100 mg/kg) was administrated daily and body weight (shown as the percentage of initial body weight) and DAI were monitored. (B) Typical pictures of colon. (C) Colon length. (D) Spleen index expressed as spleen weight (mg)/body weight (g). (E) Typical histological sections stained with H&E (50 × and 400 × magnification). (F) Statistics of histological score. (G) Immunohistochemistry staining of GFAP, substance P, GDNF, and BDNF of colonic tissue (scale = 75 μm). (H) Western blot assay of the protein level of GDNF, substance P, and BDNF. (I) The mRNA expression of Gfap, Gdnf, substance P, and their related genes in colon. (J) GDNF, substance P, and BDNF in colonic homogenates, determined by ELISA. All data were presented as mean ± SEM (n = 8 mice per group). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the vehicle group. Normal, mice with no treatment; Vehicle, mice treated only with DSS; Berberine, mice treated with DSS and berberine.
2.4. Morphological and histopathological examination
During the process of colitis, body weight loss, stool consistency, and fecal blood were monitored daily by 3 independent observer and accumulated as disease activity index (DAI, shown in Table 1) as previously reported22. On Day 11, colon length from the anus to appendix and spleen index calculated by spleen weight (mg)/body weight (g) were recorded.
Table 1.
Scoring system for calculating a disease activity index (DAI).
| Score | Weight loss (%) | Stool consistency | Blood |
|---|---|---|---|
| 0 | None | Normal | Negative hemoccult |
| 1 | 1–5 | Soft but still formed | Weakly positive hemoccult |
| 2 | 6–10 | Soft | Positive hemoccult |
| 3 | 11–20 | Very soft; wet | Blood traces in stool visible |
| 4 | >20 | Watery diarrhea | Gross rectal bleeding |
Colon sections from each mouse in each group were fixed in 4% phosphate-buffered saline (PBS)-buffered formaldehyde, embedded in paraffin, and then cut into 5-μm fractions. The sections were stained with hematoxylin and eosin (H&E). Images were acquired using Leica laser microdissection systems (DM6B, Heidelberg, Germany). Histopathological scores were obtained from three investigators who were blinded to the experimental conditions in the Center for Drug Safety Evaluation and Research, SIMM, Chinese Academy of Sciences (CAS). The criteria were shown as: 0, no evidence of inflammation; 1, low level inflammation with scattered mononuclear cells; 2, moderate inflammation with multiple foci of mononuclear cells; 3, high level inflammation with increased vascular density and marked wall thickening; and 4, maximal inflammation with transmural leukocyte infiltration and loss of goblet cells.
2.5. Biochemical and oxidative stress measurement
Mice were anesthetized, and the serum samples were collected for biochemical indexes analysis, including albumin (ALB), alkaline phosphatase (ALP), total cholesterol (TC), and triglyceride (TG), using a HITACHI-7080 automatic biochemical analyzer (Tokyo, Japan) according to the instructions.
Freshly excised colon sections were homogenized in tissue lysis buffer and centrifuged at 10,000 rpm (5424R, Eppendorf, Hamburg, Germany) for the supernatant. Individual activities of MPO, SOD and MDA in serum and colon homogenates were determined using an MPO, SOD, and MDA assay kit according to the manufacturer's instructions.
2.6. Full-thickness colon tissue culture
The 1-cm colon sections were isolated at the same region, washed with cold PBS for three times, and subsequently cut into three or four defined biopsies. The freshly isolated colon tissues were cultured for 24 h containing 1 mL of RMPI 1640 medium at 37 °C incubator as previous description22. The supernatants were centrifuged at 12,000 rpm (5424R, Eppendorf) and stored at −20 °C for following measurement of cytokines.
2.7. In vivo living imaging and intestinal permeability assay
Intestinal inflammation was determined using a luminal-based chemiluminescent probe L-012 sodium. Mice were anesthetized with 1.5%–2.0% isoflurane, injected intraperitoneally with 25 mg/kg L-012 solution as previously described23. Mice were then placed into the IVIS Spectrum CT bioluminescence imaging system (Perkin–Elmer, Waltham, MA, USA) and the images were acquired using the autoexposure option to optimize signal intensity.
Intestinal permeability measurement was performed upon oral administration of FITC-dextran solution (3–5 kDa, 600 mg/kg)24, 25. After 4 h absorption and excretion, mice were exposed to the IVIS Spectrum CT system and the fluorescent retentions were determined at 480 nm excitation and 520 nm emission. Whereafter, mice were anesthetized and serum samples were prepared and the fluorescence intensity of FITC-dextran were measured at 480 nm excitation and 520 nm emission using a microplate reader (Spectramax M5, Molecular Devices Corporation, Sunnyvale, CA, USA).
2.8. Single cell preparation and flow cytometry assay
Single cell suspensions from spleen and mesenteric lymph nodes (MLNs) were prepared through a 70 μm nylon mesh strainer. Colons were cut into small pieces and incubated in RPMI 1640 containing 10% FBS and 5 mmol/L EDTA for 15 min in a shaking incubator at 37 °C for three times to remove epithelial cells. The remaining tissue was digested using RPMI 1640 containing 10% FBS, 0.5 mg/mL Type IV Collagenase, 3 mg/mL Dispase Ⅱ (Sigma–Aldrich) and 0.1 mg/mL DNase Ⅰ (Sigma–Aldrich) for 30 min in a 37 °C shaking incubator. Lamina propria (LP) cells were collected and layered on a Percoll gradient (GE Healthcare, Buckinghamshire, UK) and centrifuged at 600×g for 20 min. The lymphocyte enriched population was recovered from the 40%–75% interface.
Single-cell suspensions were washed with PBS and stained with fixable viability dye for 30 min at 4 °C to identify the viable cells. Then, cells were blocked with 2.4G2 and stained with BUV395, FITC, PE, Percp-Cy5.5, APC and BV421-labeled antibodies. For intracellular staining, cells were first stained with surface markers, followed by fixation and permeabilization using Foxp3 Staining Buffer set. Subsequently, cells were labeled intracellularly with PE-conjugated anti-IL-17A and APC-conjugated anti-IFN-γ. Flow cytometry was performed on BD LSRFortessa (BD biosciences, Franklin Lakes, NJ, USA) and data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
2.9. Ex vivo stimulation and CD4+ T cells purification
CD4+ T cells were isolated from spleen and MLNs cells of each group using EasySep™ mouse CD4+ T Cell isolation kit (Stemcell, Vancouver, BC, Canada) according to the instructions. The purity of CD4+ T cells was identified with >98%, determined by flow cytometry. Purified CD4+ T cells were primed with anti-CD3 (5 μg/mL) and anti-CD28 antibodies (2 μg/mL, Thermo Fisher Scientific). After incubation, cells were pulsed with 0.5 μCi/well [3H-TdR] thymidine to detect cell proliferation and the supernatants were used to quantify the cytokines production.
2.10. Development and in vitro induction of bone marrow-derived dendritic cells (BMDCs)
BMDCs were prepared as previously described26, 27. Briefly, bone marrow cells (1 × 106/mL) from the tibia fibula and femur bones of normal C57BL/6 mice were cultured in RMPI 1640 medium containing 10% FBS in the presence of 15 ng/mL GM-CSF and 10 ng/mL IL-4. On Day 7, loosely adherent cells were collected, and assayed for purity measurement and further mature. The purity of BMDCs was determined through flow cytometry and was consistently >95%.
Immature BMDCs were plated in 24-well plates with medium alone, or in the presence of berberine, substance P, GDNF, and followed by LPS stimulation. After 24 h culture, the supernatants were collected to measure TNF-α production.
2.11. Enzyme-linked immunosorbent assay (ELISA)
Cytokines concentrations in colon homogenates and tissue culture were detected using mouse TNF-α, IFN-γ, IL-1β, IL-2, IL-6, IL-10, IL-12p40, GDNF, BDNF, substance P, and IL-17A ELISA kits according to the manufacturer's instructions. The concentration of total protein in homogenates was determined by BCA protein assay kit (Thermo Fisher Scientific) to normalize the cytokines concentration.
Cytokines in serum were quantified using the Luminex x-MAP technology (Luminex Corp, Austen TX, USA). Serum samples were analyzed using a Milliplex multi-analyte magnetic bead panel obtained from Thermo Fisher Scientific and all data were collected on a Lumine ×200 instrument as previously reported28.
2.12. In vitro leukocyte adhesion and chemotaxis assay
Human HT-29 cells were treated with berberine in the absence or presence of TNF-α (100 ng/mL) for 24 h. THP-1 cells labeled with 5 μmol/L calcein AM (BD Biosciences) were added onto HT-29 monolayer cells and incubated for additional 30 min. Non-adherent THP-1 cells were washed away with PBS and then the fluorescent images were acquired using a microscope (Olympus IX73, Tokyo, Japan).
For in vitro chemotaxis, the HT-29 cell supernatants from above treatment were added in the lower chamber of Trans-well and then Calcein AM-labeled THP-1 cells were added into the upper chamber for additional 2 h. The number of THP-1 cells were counted and detected under a microscope (Olympus IX73).
2.13. In vitro assay of EGC functions
Rat EGC cell line, CRL-2690 cell, were seeded in 6-well or 24-well plates and exposed to berberine or/and recombinant human BDNF (100 ng/mL, Peprotech) in the presence of brefeldin A (used for inhibiting protein release, Thermo Fisher Scientific). Cells were harvested for gene and protein expression as following measurement. For EGCs–immune cell co-culture, splenocytes from SD rats were stimulated with Concanavalin A (ConA, Sigma–Aldrich) for 24 h incubation in the absence or presence of berberine. Subsequently, the supernatants were added into the EGC-adherent plates and cultured for 4 h in the presence of Brefeldin A for total RNA extraction and real-time PCR. After 24 h incubation, EGCs were collected for annexin V and PI staining using FITC Annexin V Apoptosis Detection Kit. EGCs apoptosis were analyzed by flow cytometry using a FACSCalibur (BD biosciences).
2.14. Immunohistochemistry and immunofluorescence
Paraffin-embedded colon sections were deparaffinized in xylene and rehydrated through graded alcohol to water. After unmasking antigens by 0.01 mol/L citrate buffer solution, the colon sections were blocked with 5% BSA and stained with anti-ZO-1 (Proteintech, Rosemont, USA), anti-E-cadherin (Cell Signaling Technology, Danvers, MA, USA), anti-GFAP (Cell Signaling Technology), anti-substance P (Abcam, Cambridge, MA, USA), anti-GDNF (Novus Biologicals, Littleton, CO, USA), anti-BDNF (Abcam), anti-CD11b (Abcam), anti-F4/80 (Abcam), anti-Ly6G (BioLegend, San Diego, CA, USA), and anti-CCR6 (Abcam) primary antibodies overnight at 4 °C. Immunohistochemistry was analyzed by biotinylated horse anti-rabbit IgG secondary antibody (Bio-Rad, Hercules, CA, USA) with streptavidin-horseradish peroxidase and then signals were detected using diaminobenzidine. For immunofluorescence, signals were determined using FITC-conjugated secondary antibodies (Proteintech) and then counterstained with DAPI (Abcam) to stain the nuclei. Images were collected on Leica TCS SPS microscope (Wetzlar, Germany).
2.15. RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from colonic biopsies and cells using RNAsimple total RNA kit (Tiangen, Beijing, China) and then reverse transcribed by Hifair™ Ⅱ 1st Strand cDNA Synthesis SuperMix for qPCR (Yeasen, Shanghai, China). Real-time PCR was performed with SYBR® Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan) on an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster city, CA, USA). The primers used for PCR amplification are listed in Supporting Information Table S1. The fold change in mRNA expression of gene was normalized to β-actin or Gapdh using the ΔΔ Ct method.
2.16. Western blot analysis
Colon tissues and cell samples were lysed with sodium dodecyl sulfate (SDS) sample buffer containing proteinase and phosphatase inhibitor. The protein concentrations were measured by the BCA protein assay kit. Equal amounts of total protein (5–20 μg) were subjected and separated to 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Bio-Rad). After blocking with 5% BSA, the membranes were incubated with rabbit or mouse primary antibodies (Supporting Information Table S2) overnight at 4 °C. Signals were acquired with HRP-conjugated anti-rabbit IgG (1:20,000, Bio-Rad) or HRP-conjugated anti-mouse IgG (1:10,000, Kangcheng, Shanghai, China) using SuperSigna West Femto Maximum Sensitivity Substrate under visualization in a ChemiDoc™ MP Imaging System (Bio-Rad).
2.17. Statistical analysis
All experiments were repeated at least three times and data were presented as mean ± SEM. Statistical differences were analyzed by GraphPad Prism 6.0 software (La Jolla, CA, USA) using Student's t-test or one-way ANOVA with Dunnet's multiple comparisons test with no significant variance inhomogeneity (F achieved P < 0.05). P-values of less than 0.05 were considered statistically significant.
3. Results
3.1. Berberine ameliorated the experimental features in DSS-induced murine colitis
UC manifestations developed in all mice following DSS uptake, as evidenced by continuous body weight loss, severe DAI score, and colon shortening (Fig. 1A–C). Additionally, DSS-treated mice also exhibited increased spleen indexes (Fig. 1D), which were generally related to the extent of inflammation and anemia29. In line with previous publications, berberine treatment showed therapeutic effects on DSS-induced murine UC, referred to the facts of reversed body weight loss (Fig. 1A) and colon shortening (Fig. 1B and C), along with a lower DAI score (Fig. 1A) and reduced splenomegaly (Fig. 1D). The histological examination demonstrated that colitis mice treated with berberine received a significant improvement in colon damage and inflammation, including mucosal ulcers, loss of crypt and goblet cells, epithelial barrier disorders, and infiltration of multiple inflammatory cells (Fig. 1E and F). Consistently, berberine could reduce the serum level of several inflammatory cytokines, including TNF-α, IFN-γ, IL-1β, IL-6, and IL-17A, detected by Luminex assay (Supporting Information Fig. S1A). Owing to excessive bleeding and diarrhea, DSS-treated mice pathologically displayed metabolic disturbance and oxidative stress damage. As indicated in Fig. S1B, levels of serum ALB and ALP in vehicle group were much lower, while serum TC and TG higher than normal controls, and berberine strikingly restored these biochemical indices. Moreover, upon DSS exposure, the body immune system immediately initiated antioxidant defenses and subsequently led to imbalance between reactive oxygen species and tissue repair30. Compared to control mice, the levels of MPO and MDA were much higher in colitis, which were significantly decreased in serum and colon after berberine treatment (Supporting Information Fig. S2), whereas, berberine upregulated the SOD activities that were decreased both in serum and colon tissue of DSS-treated mice (Fig. S2).
3.2. Berberine modulated neuropeptides production and enhanced the residence of EGCs in DSS-colitis mice
Linked with numerous pathogenic triggers in UC, multiple highlights of cross-talk and communication between the nervous and immune system have been identified in active researches6. To uncover the underlying mechanism of berberine on the points of neuropeptides expression and EGCs functions in gut local inflamed microenvironment, in situ immunohistochemistry staining were performed. We first identified substantial decreased the population of EGCs (identified as GFAP+ positive staining), obviously increased expression of substance P and BDNF, and slightly increased expression of GDNF in DSS-treated mice and berberine showed modulatory effects on the residence of EGCs and neuropeptides production (Fig. 1G). Consistently, berberine treatment could increase the protein level of GFAP, GDNF and decrease the level of BDNF, substance P, as determined by Western blot and ELISA assay (Fig. 1H and J). Furthermore, gene expression in inflamed colon tissue indicated that berberine regulate the neuropeptides expression in a similar tendency as exhibited in Fig. 1H and J, and their related genes expression as well (Fig. 1I).
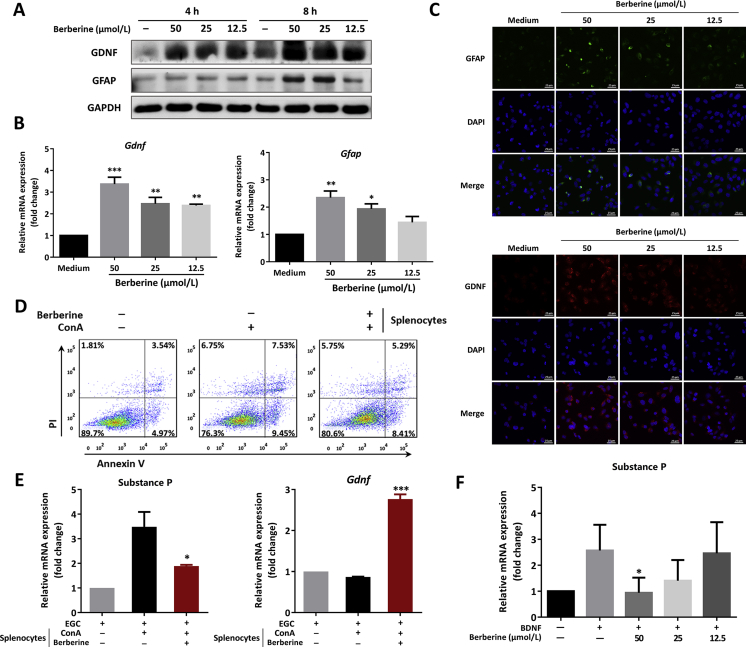
3.3. Berberine maintained the function of EGCs in monoculture and modulated the cross-talk between EGCs and immune cells
In light of the above results, rat EGC cell line, CRL-2690, was included in the present research. As demonstrated in Fig. 2A, berberine could increase the protein level of GFAP and GDNF, especially after 4 and 8 h incubation. Notably, during the monoculture with berberine, there was no change on inflammatory substance P expression (data not shown). Moreover, the results in Fig. 2B showed that berberine dose-dependently upregulated the mRNA expression of Gfap and Gdnf and the immunofluorescent staining further verified that berberine could maintain the function of EGCs (Fig. 2C).
Figure 2.
Berberine modulated the function of EGCs and decreased the expression of substance P in vitro. (A)–(C), Rat EGC cell line, CRL-2690, were treated with indicated concentrations of berberine. (A) Western blot assay of GFAP and GDNF of EGCs at various timepoints. (B) The mRNA expression level of Gfap and Gdnf of EGCs after 4 h incubation was determined, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with medium control. (C) Immunofluorescence staining with GFAP and GDNF in EGC (Scale = 25 μm). (D) and (E) Rat splenocytes were activated with ConA stimulation for 24 h, and subsequently the supernatants were added into EGC cells culture. (D) Apoptosis assay of EGCs following annexin V and PI staining after 24 h incubation. (E) The mRNA expression of substance P and Gdnf of EGCs. (F) CRL-2690 cells were culture with recombinant BDNF (100 μg/mL) in the presence or absence of indicated concentrations and real-time PCR was performed to determine the expression of substance P. (E) and (F) *P < 0.05, **P < 0.01, and ***P < 0.001 compared with splenocytes supernatants or BDNF-primed cells. All data were presented as mean ± SEM of three independent experiments.
To gain insights to the communication and interaction between EGCs and immune cells, rat splenocytes were activated with ConA and then the supernatants were added into the EGCs cultures. It was worth mentioning that the supernatants from activated splenocytes resulted in apoptosis of EGCs (Fig. 2D), increased expression of substance P, and slightly decreased expression of Gdnf (Fig. 2E). Berberine showed protective effects on the function of EGCs in the co-culture system, as evidenced by suppressing EGCs apoptosis (Fig. 2D), inhibiting substance P expression and increasing Gdnf expression (Fig. 2E), which were in line with the in vivo findings in Fig. 1G–J. Furthermore, it was previously demonstrated recombinant BDNF could directly activate EGCs through the TrκB–PLCγ1 signaling, which contributed to colonic hypersensitivity31. In the present study, we found that berberine could inhibit substance P expression in BDNF-primed EGCs in a dose-dependent manner (Fig. 2F).
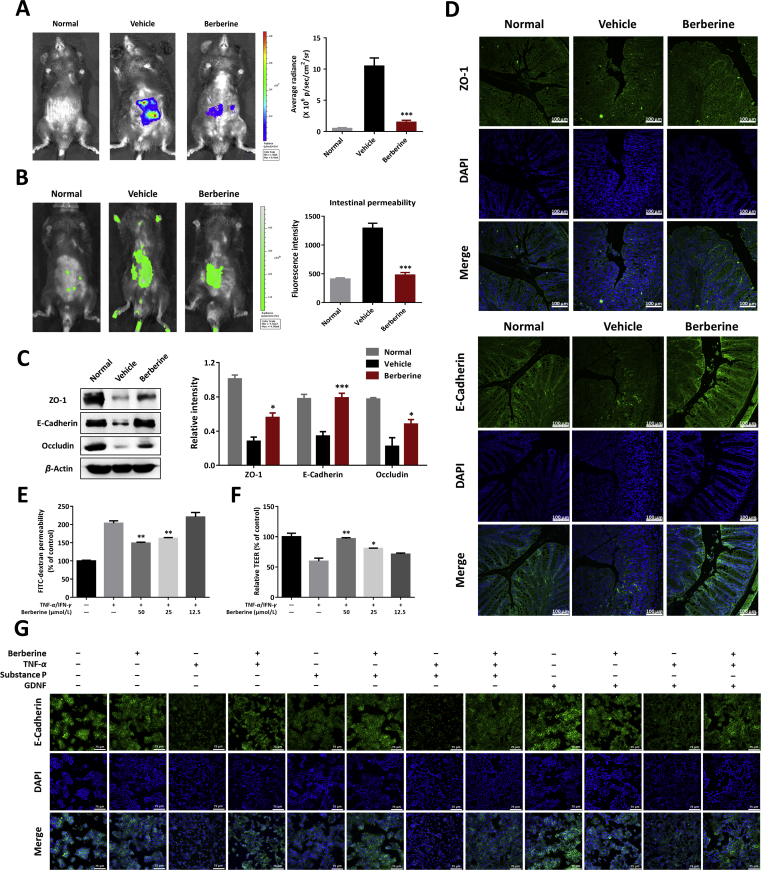
3.4. Berberine rebuilt intestinal barrier homeostasis and maintained a normal intestinal permeability
The colonic epithelium functions as the physiological construction for maintaining mucosal homeostasis, depending on intestinal epithelial cells and tight junction proteins5. EGCs have been demonstrated to possess an available and supporting relationship with IECs; and EGCs lesions or/and functional defects might be involved in barrier dysfunction7. Given the interactions between EGCs and IECs, mucosal barrier function in colitic mice was measured in Fig. 3. In vivo living imaging with L-012 probe was conducted to explore the inflammatory conditions in gut. The results indicated the evident inflammation signals appeared in the abdominal regions of colitis mice and berberine treatment significantly reduced the gut local inflammation (Fig. 3A). As described previously, FITC-dextran was used to evaluate intestinal permeability in vivo32. We found that berberine-treated mice exhibited much lower retention of fluorescent signals than vehicles and in turn, the serum fluorescence intensity results further confirmed this finding (Fig. 3B). Consistently, by contrast to control mice, significant decreases of colonic tight junction proteins (ZO-1, E-cadherin, and occludin) were observed in DSS-colitis mice and berberine upregulated the expression level of tight junction proteins (Fig. 3C), which were further identified using in situ fluorescent staining with ZO-1 and E-cadherin (Fig. 3D).
Figure 3.
Berberine maintained the intestinal barrier homeostasis in DSS-colitis mice and TNF-α and/or neuropeptides-cultured epithelial cells. (A) Left, typical in vivo imaging pictures with bioluminescent imaging by injecting L-012 sodium (25 mg/kg). Right, quantification of bioluminescent images by ROI determination. (B) Intestinal permeability measurement by oral administration of FITC-dextran (600 mg/kg). Fluorescent images (left) and serum fluorescence intensity (right) of FITC-dextran were determined. (C) Left, colonic homogenates were analyzed by Western blotting to detect the expression of tight junction protein (ZO-1, E-cadherin, and occludin). Right, quantitative analysis of tight junction protein expression. (D) Immunofluorescence staining with ZO-1 and E-cadherin of colonic sections (Scale = 100 μm). (E) and (F) Barrier function in vitro was assayed in TNF-α and IFN-γ-primed Caco-2 cells. FITC-dextran permeability (E) and trans-epithelial electrical resistance (TEER, F) were determined. (G) HT-29 cells were cultured with berberine, substance P, or GDNF in the absence or presence of TNF-α induction. E-cadherin expression by immunofluorescence staining was performed (Scale = 75 μm). (A)–(D), all data were presented as mean ± SEM; n = 8 mice per group. (E)–(G), all data were presented as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001: (A)–(C), compared with the vehicle group; (E) and (F), compared with TNF-α/IFN-γ-stimulated cells.
In vitro, cytokines-primed human epithelial cell lines, HT-29 and Caco-2 cells, were introduced to validate the effects of berberine on intestinal permeability and formation of tight junctions. As presented in Supporting Information Fig. S3, normal HT-29 cells were grown with a gathering manner and formation of tight junction, however, TNF-α addition intended to destroy the epithelial barrier structure. Expectedly, berberine could dose-dependently reverse TNF-α-triggered destruction of E-cadherin (Fig. S3). Additionally, berberine also showed inhibitory effects on the permeability in TNF-α-primed Caco-2 cells monolayers, including the increased FITC-dextran flux (Fig. 3E) and decreased trans epithelial electric resistance (TEER, Fig. 3F).
3.5. Berberine ameliorated TNF-α/substance P-induced tight junction damage in human HT-29 cells
Previously, it has been demonstrated that GDNF possessed strong antiapoptotic properties in the colonic epithelial cells, SW48013. However, the role of GDNF and substance P in epithelial barrier function remains unclear. We therefore investigated whether the neuropeptides demonstrated effects on the formation of tight junction protein in TNF-α stimulated HT-29 cells. Strikingly, GDNF could maintain the barrier function, in the presence or absence of berberine, with increasing formation of E-cadherin, however, substance P showed a tendency to decreases the expression and localization of E-cadherin and berberine treatment could alleviate TNF-α and substance P-induced tight junction injury (Fig. 3G).
3.6. Berberine suppressed inflammatory cells infiltration to the inflamed colon with decreased expression of adhesive molecules, chemokines and chemokine receptors
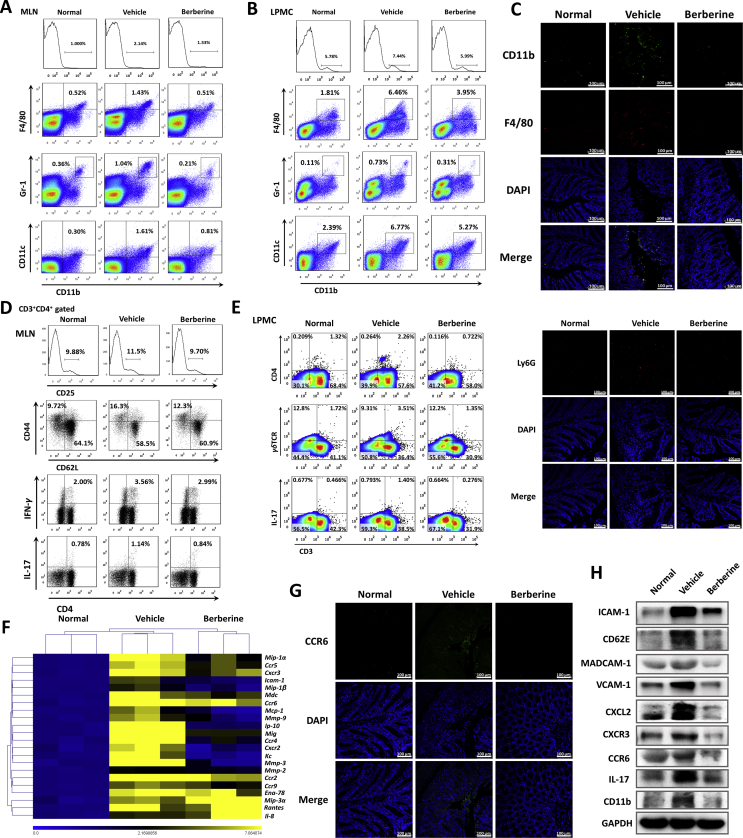
The intestine barrier and mucosal immune system have evoked to maintain the tolerance to diverse pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs)3, 33. To elucidate the communication between epithelial turbulence and immune cells infiltration, mesenteric lymph nodes (MLN) and gut lamina propria mononuclear cells (LPMC) were assayed by flow cytometry. Following epithelial barrier disruption, the population of monocytes (CD11b+) macrophage (CD11b+F4/80+), neutrophils (CD11b+Gr-1+), and dendritic cells (CD11b+CD11c+) were increased in DSS-treated mice, which were obviously decreased after berberine treatment (Fig. 4A and B). The immunofluorescent staining with CD11b, F4/80, and Ly6G in Fig. 4C further confirmed the inflammatory infiltrations in the colon sections.
Figure 4.
Berberine suppressed the colonic leukocytes infiltration by regulating chemotaxis and adhesion. The percentage of macrophage (CD11b+F4/80+), neutrophils (CD11b+Gr-1+), and dendritic cells (CD11b+CD11c+) were assayed in MLNs (A) and colonic lamina propria (B). (C) Colonic sections were immunofluorescence stained with Ly6G, CD11b, and F4/80 and the nuclei were stained with DAPI (Scale = 100 μm). (D) The percentage of CD25-positive cells, naïve T cells (CD44‒CD62L+) and effector T cells (CD44+CD62L‒) gated on CD3+CD4+ subpopulation, Th1 (CD4+IFN-γ+) and Th17 cells (CD4+IL-17+) in MLNs. (E) The percentage of CD3+CD4+ cells, CD3+γδTCR+ cells, and CD3+IL-17+ cells in colonic lamina propria. (F) Heatmap of the mRNA expression of chemokines, chemokine receptors, and adhesion molecules. (G) Immunofluorescence staining with CCR6 and DAPI of colonic tissue (Scale = 100 μm). (H) Western blot assay of certain chemokines, chemokine receptors, and adhesion molecules expression. All data were presented as mean ± SEM; n = 8 mice per group.
It has been elucidated that EGCs manifested with immunosuppressive effects referring to inhibiting T cells expansion and proliferation34. The crosstalk and interaction between T cells and EGCs also attracted our interests25, 35, 36, 37. In addition to innate immune cells infiltrations, T cells activation and subpopulations were analyzed in the present study. Berberine obviously restrained T cells activation from MLNs, as evidenced by decreased percentage of CD25-positive cells (gated on CD3+CD4+), effector T cells (CD44+CD62L–, gated on CD3+CD4+), and increased percentage of naïve T cells (CD44‒CD62L+, gated on CD3+CD4+, Fig. 4D). Furthermore, Th1 (CD4+IFN-γ+) and Th17 (CD4+IL-17+) cells were also reduced in berberine-treated mice (Fig. 4D). In colonic LP, berberine consistently suppressed the infiltration of CD4+ T cells, γδT cells, and IL-17-producing cells (Fig. 4E). Moreover, the proliferation abilities of CD4+ T cells, purified from splenocytes and MLN cells in berberine-treated mice, were weaker than that in DSS-colitis mice (Supporting Information Figs. S4A and C). In accordance with proliferation, cytokines production, including IL-2, IFN-γ, and IL-17A, from purified T cells were also decreased upon berberine treatment (Figs. S4B and D).
Inspired from the inflammatory cell infiltrations, gene expression profile of colon tissue was performed, which indicated berberine inhibited the mRNA expression level of multiple chemokines, chemokine receptors, and adhesive molecules (Fig. 4F), which were further confirmed in protein level by Western blot assay (Fig. 4H). CCR6, highly expressed on a variety of immune cells including dendritic cells, activated B cells, memory T cells, and Th17 cells, are implicated in many acute and chronic inflammatory diseases38. In the present study, increased expression of CCR6 was identified in the inflamed colon tissue and a dramatic reduction of CCR6 expression exhibited in berberine-treated mice (Fig. 4F–H).
3.7. Berberine suppressed the adhesion and chemotaxis of immune cells toward IECs in multi-chamber culture of human HT-29 cells
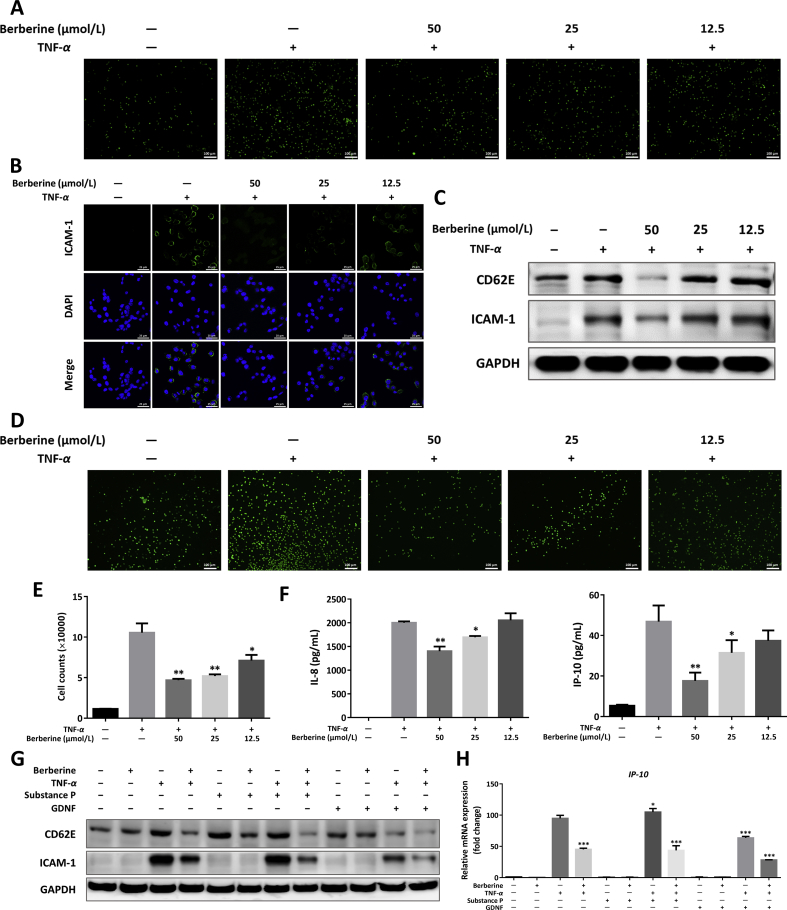
Next, in vitro analysis of IECs–immune cells interaction between HT-29 cells and THP-1 cells were designed to verify the above conclusion. As demonstrated in Fig. 5A, D, and E, berberine dose-dependently suppressed THP-1 cells adhering and migrating to TNF-α-primed human HT-29 cells. Functionally, TNF-α addition showed obvious increased expression of ICAM-1 and slightly increased expression of CD62E, accordingly, berberine possessed the capacity to downregulate expression of the two adhesive molecules (Fig. 5B and C), which were in line with the results in Fig. 4F and H. Moreover, berberine could inhibit chemokines, IP-10 and IL-8, production from HT-29 cells in a dose-dependent manner (Fig. 5F). To further understand whether the neuropeptides from EGCs were involved in the cross-talk between IEC and immune cells, GDNF and substance P were added into the culture system of TNF-α-stimulated HT-29 cells. Interestingly, GDNF significantly inhibited the expression of ICAM-1 and CD62E. However, substance P showed a mild-to-moderate upregulation on adhesive molecules expression and berberine could reverse the expression of ICAM-1 and CD62E in the presence of substance P (Fig. 5G). Continuously, a similar effect was observed in the mRNA expression of IP-10 (Fig. 5H), which suggested the neuropeptides not only regulated the barrier function of epithelial cells, but also modulated the interaction between gut IECs and immune cells.
Figure 5.
Berberine inhibited chemotaxis and adhesion in TNF-α and/or neuropeptides-cultured human HT-29 cells. (A) Calcein AM-labeled THP-1 cells were incubated onto HT-29 monolayer cells for 30 min. THP-1 cells adhered to the TNF-α-activated HT-29 cells were observed after washing (Scale = 100 μm). (B) Immunofluorescence staining with ICAM-1 of TNF-α-activated HT-29 cells (Scale = 25 μm). (C) Western blot assay of CD62E and ICAM-1 expression in TNF-α-activated HT-29 cells. (D) Representative images of THP-1 cells migrated to the HT-29 cell supernatants as described in Methods (Scale = 100 μm). (E) The counting number of THP-1 cells chemotactic to the lower chamber containing HT-29 cell supernatants. (F) Secretion of IP-10 and IL-8 in the supernatants of TNF-α-activated HT-29 cells. (G) and (H) HT-29 cells were cultured with berberine, substance P, or GDNF, in the absence or presence of TNF-α induction. Western blot assay of CD62E and ICAM-1 (G), and the mRNA expression of IP-10 (H) were performed. All data were presented as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001, compared with TNF-α-stimulated cells.
3.8. Effects of berberine, substance P, and GDNF on LPS-activated BMDCs
Lastly, to gain insights toward the effects of neuropeptides on immune cells, BMDCs were introduced in our study. The results showed that berberine and GDNF could inhibit TNF-α release by LPS stimulation, respectively. Meanwhile, GDNF showed a superimposed effect in the presence of berberine (Supporting Information Fig. S5). Oppositely, substance P functioned as a pro-inflammatory mediator in LPS-activated BMDCs, nevertheless, berberine possess the capacity to inhibit the release of TNF-α (Fig. S5).
3.9. Berberine inhibited the inflammatory responses and related signaling pathways in DSS-induced colitis
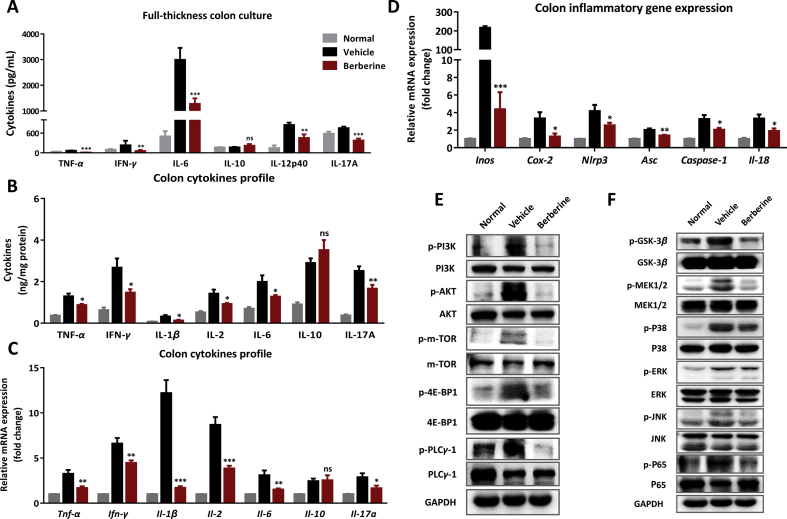
As described above in the DSS-induced murine UC, defects of EGCs function and residence, destruction of barrier integrity and immune cell infiltrations were observed, which collectively contributed to the severe inflammatory responses in the colon tissue. In our previous research, increased inflammatory cytokines were observed in full-thickness colon culture from colitis mice, which directly referred to gut inflammatory stages25. As shown in Fig. 6A, berberine-treated colon synthesized less cytokines than vehicle mice. Correspondingly, in the inflamed lesions of colon, various cytokines were increased as illustrated in both protein and mRNA levels, to some extent, berberine suppressed the inflammatory cytokines expression of TNF-α, IFN-γ, IL-1β, IL-2, IL-6, and IL-17A (Fig. 6B and C). Meanwhile, berberine treatment lead to a significant reduction of Inos, Cox-2, and inflammasome-related genes, namely Nlrp3, Asc, caspase-1, and Il-18 (Fig. 6D). Furthermore, PI3K-AKT-mTOR (Fig. 6E), MAPK and P65 NF-κB signaling (Fig. 6F), were assayed in our research to deeply understand the mechanisms of abnormal immune activation and neuropeptides expression, which indicated that berberine could suppress the overactivation of signaling transduction.
Figure 6.
Berberine inhibited the overexpression of inflammatory mediators and related signaling pathway in DSS-induced colitis. (A) Cytokines secretion in the full-thickness colon tissue culture by ELISA. (B) Cytokines protein levels in the colonic homogenates. (C) mRNA was isolated from the colon for real-time PCR analysis of indicated gene expression. The mRNA expression level in normal group was set as 100%. (D) The mRNA expression of Inos, Cox-2, and inflammasome-related genes. Western blot assay of neuropeptides-related signaling pathways, including PI3K-AKT-mTOR (E), MAPK, and P65 NF-κB signaling (F) were performed. All data were presented as mean ± SEM; n = 8 per group. *P < 0.05, **P < 0.01, and ***P < 0.001, compared with the vehicle group.
4. Discussion
Berberine hydrochloride tablets have been applied to treating intestinal bacterial and parasitic infection, diarrhea, and dysentery. In the past decades, there has been a great and increasing interest in exploring the therapeutic effects and mechanisms of berberine in treating inflammatory bowel diseases, including UC and CD. Recently, in addition to the clinical trial monitoring annual recurrence rate of UC in remission (NCT02962245, ClinicalTials.gov), a pilot phase 1 trial was designed to uncover the side effects of berberine in treating UC patients and those who are in remission to reduce the risk of colorectal cancer (NCT02365480, ClinicalTials.gov). In experimental colitis, berberine showed dramatic therapy effects with the manifestation of ameliorating colonic macromorphological and histopathological inflammation20, 39. Up to now, the underlying mechanism of berberine in colitis is continuously a topic for current research. The present study was designed to deeply elucidate the pathological role of neuro-immune turbulence in UC and find out whether the effects of berberine were associated with modulating neuro-immune interactions.
The enteric nervous system (ENS), also known as “little brain of the gut”, is similar to CNS and has been implicated in the pathophysiology of colitis and other gut inflammatory conditions. The ENS neuro-immune axis is a complicated and extensive network of sensory neurons, interneurons, and glial cells within the gastrointestinal tract11. EGCs, dominantly existing in ENS and identified by expression of unique marker GFAP and the calcium-binding protein S-100β, play a critical role in maintaining gut epithelial integrity8, 40, 41. Previous study showed that addition of EGCs prevented barrier failure and ameliorated expression and localization of tight junction proteins7. In DSS-induced acute murine UC, a significant decrease of GFAP was found in our study and berberine significantly recovered the residence of EGCs in the inflamed tissue (Fig. 1G–J).
Neuropeptides, secreted by mucosal EGCs, such as GNDF, substance P, neurotrophins, and CGRP, play an important role in regulating intestinal inflammation and barrier homeostasis42, 43. Several neuropeptides could directly or indirectly function immune responses, including chemotaxis, lymphocyte mitogenesis, phagocytosis, and homing patterns of immune cells11. Moreover, it has been suggested that human EGCs inhibited the proliferation and division of activated T cells and possessed the immunosuppressive capacity34. Collectively, the role of EGCs and its neurotransmitters, GDNF and substance P, on UC drew our interests. GDNF serves as a novel number in the family set of protective mucosal factors in gut inflammation. It has been indicated that GDNF is slightly upregulated in IBD patients and is likely involved in the initiation stage of inflammation, which may refer to an early feedback mechanism13. Moreover, GDNF and its receptor GFP-α1 have been investigated in epithelial cell line SW480 cells, which showed that GDNF could prevent apoptosis in SW480 cells depending on MAPK and PI3K-AKT pathways13. Liu et al.14 demonstrated that recombinant adenoviral vectors encoding GDNF dramatically reduced colonic inflammation, prevented partially the loss of enteric neurons, and improved delayed colonic transit in DSS-induced rat UC. Consistently, in the present research, GDNF was firstly found an increased expression in DSS-induced colitis, while, berberine strongly further enhanced GDNF expression (Fig. 1G–J). Substance P, another vital mediator, is widely distributed in the ENS and CNS. Previous study showed substance P could increase adhesion of neutrophils to human umbilical vein endothelial cells (HUVECs) via NK1 and NK2 receptors, which is critical for recruitment of leukocytes into the inflamed tissues during the early and late inflammation stages12. Administration of NK1 receptor antagonist, CP-96345, alleviated the colonic inflammation, oxidative stress, and colon structural damage44. Strikingly, an increased expression of substance P was observed in the colon tissue of DSS-induced UC mice, which was reversed following berberine treatment.
Intestinal barrier disturbances, including a defective production of various antimicrobial peptides, and decreased thickness or defects of mucus layer are thought to contribute to the pathogenesis of UC4. Colitis in DSS-treated mice possesses pathologic changes with destruction of natural intestinal epithelial barrier function, crypt injury and formation of ulceration, disturbance of detrimental pathogenic microbes in the intestinal lumen29. In the present research, extensive inflammation and increased intestinal permeability were appeared in DSS-induced mice as shown in the results of in vivo living imaging with L-012 probe and FITC-dextran absorption (Fig. 3A and B). Correspondingly, in situ immunofluorescent staining of inflamed colonic sections revealed chaos localization of tight junction proteins, ZO-1 and E-cadherin (Fig. 3D). Upon epithelial barrier disturbance, various inflammatory cells were recruited into the mucosal layers accompanying with increased expression of adhesive molecules and chemokines (Fig. 4). We found berberine could remodel the interaction between epithelial barrier and immune system, referring to restore a normal intestinal permeability and decrease inflammatory infiltrations (Fig. S3 and Fig. 4).
Collectively, it was indicated that berberine could maintain the residence and function of EGCs, rebuild epithelial barrier homeostasis, and balance mucosal immunity, respectively. To clarify the gut EGCs–IEC–immune cells interactions in depth, co-culture and/or multi-chamber culture system were conducted in our research. We found that berberine could modulate the cross-talk between EGCs and immune cells, as evidenced by preventing EGCs apoptosis, inhibiting substance P expression and increasing Gdnf expression (Fig. 2D and E, and Fig. 7). Expectedly, GDNF, derived from EGCs, also showed inhibitory effects on TNF-α release, whereas substance P increased TNF-α production from LPS-activated BMDCs (Fig. S5 and Fig. 7). Moreover, GDNF and substance P exhibited regulatory effects on the formation of tight junctions in epithelial cells (Fig. 3G), and also demonstrated effects on the communication between IEC and immune cells, characterized by adhesion and chemotaxis (Fig. S5 and Fig. 7).
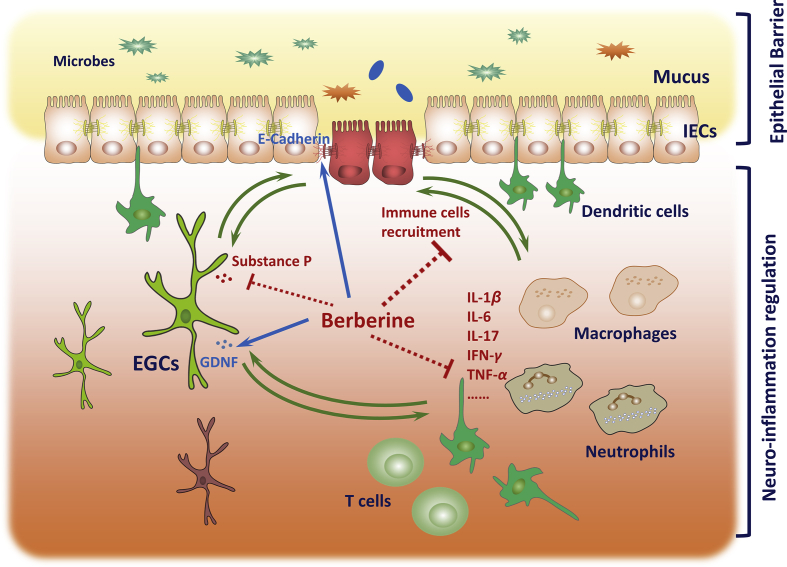
Figure 7.
Diagram of gut EGCs–IECs–immune cells interaction affected by berberine to ameliorate UC. Upon DSS exposure, epithelial barrier disturbance and functional defects of EGCs and inflammatory cell infiltrations were observed. Berberine ameliorated the disease severity and intestinal inflammation, accompanying by directly restoring the mucosal barrier homeostasis, maintaining the residence of EGCs and attenuating inflammatory infiltrations and immune cells overactivation, respectively. In depth, berberine could modulate gut EGCs–IECs–immune cell interactions, providing an infusive mechanism of berberine in regulating enteric neurogenic inflammation. Briefly, berberine could prevent EGCs apoptosis, inhibiting substance P expression and increasing GDNF expression in the presence of activated immune cells. By contrast, berberine showed anti-inflammatory effects involving in GDNF and substance P. Moreover, berberine, along with GDNF and substance P could interfere with the communication between immune cell and epithelial cells, including the barrier function, adhesion and chemotaxis. Abbreviations: EGCs, enteric glial cells; IEC, intestinal epithelial cells.
In addition, Cui et al.18 have indicated that berberine could modulate gut microbiota in alleviating murine UC. A decreased abundance of destructive bacteria, Desulfovibrio, and an increased population of probiotics, Eubacterium limosum, were observed upon berberine treatment. It has been demonstrated that metabolites (such as short chain fatty acids, SCFAs, including pyruvic, lactic, acetic, propionic, and butyric acids), are derived from fermentation of nondigestible carbohydrates (fibers) by gut anaerobic bacteria45. Fecal level of SCFAs is closely related to the development of UC and differs between UC patients and healthy individuals, which might due to the distribution of intestinal microbiota changes and mucosal disorders46, 47. Over the past decades, it has been identified that SCFAs exerted modulatory effects via predominately binding to FFAR2 (GPR43) and FFAR3 (GRP41), involving in the immune cells activation, intestinal barrier integrity, and the functions of neurons48, 49. In addition to the direct effects of berberine on EGCs, IECs, and immune cells, it can be speculated that berberine might modulate gut EGCs–IECs–immune cells interaction by gut microbiota in an indirect way.
Herein, the current research suggested that berberine treatment exerted protective effects in DSS-induced experimental UC by inhibiting colonic inflammation, maintaining epithelial barrier function, suppressing inflammatory recruitments, and restrained T cells reactivity. Deeply, we found the therapeutic capacity is mainly mediated by enhancing the population and residence of EGCs and regulating the enteric glial–immune–epithelial cells interactions (Fig. 7).
5. Conclusions
The present research indicated that such neuro–immune–epithelial cell crosstalk might be considered as an alternative paradigm in mucosal physiology and inflammatory regulation and modulating the neuro-inflammation might be available for the therapy strategy in UC patients.
Acknowledgments
This work was supported by Science and Technology Commission of Shanghai Municipality, China (No. 18431907100). We are kindly grateful for technical assistance from the Platform of Molecular Imaging and Research, SIMM, CAS, Beijing, China.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Author contributions
H Li, W Tang and JP Zuo conceived and designed the study. H Li, C Fan, CL Feng, HM Lu, CG Xiang, PL He, XQ Yang performed the experiments. H Li analyzed all the data and written the manuscript. W Tang and JP Zuo revised the manuscript, obtained the funding and supervised the whole study. All authors approved the final version of the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.08.006.
Contributor Information
Jianping Zuo, Email: jpzuo@simm.ac.cn.
Wei Tang, Email: tangwei@simm.ac.cn.
Appendix A. Supporting information
The following is the Supplementary data to this article:
References
- 1.Ungaro R., Mehandru S., Allen P.B., Peyrin-Biroulet L., Colombel J.-F. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H., Zuo J.P., Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1–21. doi: 10.3389/fphar.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 4.Antoni L., Nuding S., Wehkamp J., Stange E.F. Intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2014;20:1165–1179. doi: 10.3748/wjg.v20.i5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh K., Antanaviciute A., Fawkner-Corbett D., Jagielowicz M., Aulicino A., Lagerholm C. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49–55. doi: 10.1038/s41586-019-0992-y. [DOI] [PubMed] [Google Scholar]
- 6.Chavan S.S., Pavlov V.A., Tracey K.J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46:927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheadle G.A., Costantini T.W., Lopez N., Bansal V., Eliceiri B.P., Coimbra R. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0069042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y.B., Li Y.Q. Enteric glial cells and their role in the intestinal epithelial barrier. World J Gastroenterol. 2014;20:11273–11280. doi: 10.3748/wjg.v20.i32.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabarrocas J., Savidge T.C., Liblau R.S. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81–93. doi: 10.1002/glia.10169. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor T.M., O'Connell J., O'Brien D.I., Goode T., Bredin C.P., Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 11.Gross K.J., Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–932. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 12.Dianzani C., Collino M., Lombardi G., Garbarino G., Fantozzi R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br J Pharmacol. 2003;139:1103–1110. doi: 10.1038/sj.bjp.0705344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinkamp M., Geerling I., Seufferlein T., von Boyen G., Egger B., Grossmann J. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology. 2003;124:1748–1757. doi: 10.1016/s0016-5085(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu G.X., Yang Y.X., Yan J., Zhang T., Zou Y.P., Huang X.L. Glial-derived neurotrophic factor reduces inflammation and improves delayed colonic transit in rat models of dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2014;19:145–152. doi: 10.1016/j.intimp.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Wittkopf N., Neurath M.F., Becker C. Immune–epithelial crosstalk at the intestinal surface. J Gastroenterol. 2014;49:375–387. doi: 10.1007/s00535-013-0929-4. [DOI] [PubMed] [Google Scholar]
- 16.Li Y.H., Xiao H.T., Hu D.D., Fatima S., Lin C.Y., Mu H.X. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016;110:227–239. doi: 10.1016/j.phrs.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Xi Y., Li S., Zhao Q., Cheng W., Wang Z. Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Mol Immunol. 2015;67:444–454. doi: 10.1016/j.molimm.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Cui H., Cai Y., Wang L., Jia B., Li J., Zhao S. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front Pharmacol. 2018;9:1–17. doi: 10.3389/fphar.2018.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L.C., Wang Y., Tong L.C., Sun S., Liu W.Y., Zhang S. Berberine alleviates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Exp Ther Med. 2017;13:3374–3382. doi: 10.3892/etm.2017.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan F., Wang L., Shi Y., Cao H., Liu L., Washington M.K. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G504–G514. doi: 10.1152/ajpgi.00312.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee I.A., Hyun Y.J., Kim D.H. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-kappaB activation. Eur J Pharmacol. 2010;648:162–170. doi: 10.1016/j.ejphar.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 22.Wirtz S., Popp V., Kindermann M., Gerlach K., Weigmann B., Fichtner-Feigl S. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 23.Kielland A., Blom T., Nandakumar K.S., Holmdahl R., Blomhoff R., Carlsen H. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic Biol Med. 2009;47:760–766. doi: 10.1016/j.freeradbiomed.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Llorente C., Hartmann P., Yang A.M., Chen P., Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. 2015;421:44–53. doi: 10.1016/j.jim.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H., Fan C., Feng C., Wu Y., Lu H., He P. Inhibition of phosphodiesterase-4 attenuates murine ulcerative colitis through interference with mucosal immunity. Br J Pharmacol. 2019;176:2209–2226. doi: 10.1111/bph.14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei H.J., Letterio J.J., Pareek T.K. Development and functional characterization of murine tolerogenic dendritic cells. J Vis Exp. 2018;135:1–12. doi: 10.3791/57637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He S.J., Lin Z.M., Wu Y.W., Bai B.X., Yang X.Q., He P.L. Therapeutic effects of DZ2002, a reversible SAHH inhibitor, on lupus-prone NZBxNZW F1 mice via interference with TLR-mediated APC response. Acta Pharmacol Sin. 2014;35:219–229. doi: 10.1038/aps.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reslova N., Michna V., Kasny M., Mikel P., Kralik P. Xmap technology: applications in detection of pathogens. Front Microbiol. 2017;8:1–17. doi: 10.3389/fmicb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:1–14. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton G.J., Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P., Du C., Chen F.X., Li C.Q., Yu Y.B., Han T. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2016;6:1–15. doi: 10.1038/srep20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta J., Nebreda A.R. Analysis of intestinal permeability in mice. Bio-protocol. 2014;4:1–4. [Google Scholar]
- 33.Cader M.Z., Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653–1664. doi: 10.1136/gutjnl-2012-303955. [DOI] [PubMed] [Google Scholar]
- 34.Kermarrec L., Durand T., Neunlist M., Naveilhan P., Neveu I. Enteric glial cells have specific immunosuppressive properties. J Neuroimmunol. 2016;295–296:79–83. doi: 10.1016/j.jneuroim.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 35.von Boyen G.B., Degenkolb N., Hartmann C., Adler G., Steinkamp M. The endothelin axis influences enteric glia cell functions. Med Sci Monit. 2010;16:Br161–Br167. [PubMed] [Google Scholar]
- 36.Ruhl A., Franzke S., Collins S.M., Stremmel W. Interleukin-6 expression and regulation in rat enteric glial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1163–G1171. doi: 10.1152/ajpgi.2001.280.6.G1163. [DOI] [PubMed] [Google Scholar]
- 37.Morgan M.E., Zheng B., Koelink P.J., van de Kant H.J., Haazen L.C., van Roest M. New perspective on dextran sodium sulfate colitis: antigen-specific T cell development during intestinal inflammation. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0069936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruth J.H., Shahrara S., Park C.C., Morel J.C.M., Kumar P., Qin S. Role of macrophage inflammatory protein-3α and its ligand CCR6 in rheumatoid arthritis. Lab Investig. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Liu X., Hua W., Wei Q., Fang X., Zhao Z. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-kappaB signaling pathway to protect against DSS-induced colitis. Int Immunopharmacol. 2018;57:121–131. doi: 10.1016/j.intimp.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 40.Veiga-Fernandes H., Mucida D. Neuro–immune interactions at barrier surfaces. Cell. 2016;165:801–811. doi: 10.1016/j.cell.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coelho-Aguiar Jde M., Bon-Frauches A.C., Gomes A.L., Verissimo C.P., Aguiar D.P., Matias D. The enteric glia: identity and functions. Glia. 2015;63:921–935. doi: 10.1002/glia.22795. [DOI] [PubMed] [Google Scholar]
- 42.Di Giovangiulio M., Verheijden S., Bosmans G., Stakenborg N., Boeckxstaens G.E., Matteoli G. The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front Immunol. 2015;6:590. doi: 10.3389/fimmu.2015.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boesmans W., Lasrado R., Vanden Berghe P., Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229–241. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- 44.Stucchi A.F., Shofer S., Leeman S., Materne O., Beer E., McClung J. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1298–G1306. doi: 10.1152/ajpgi.2000.279.6.G1298. [DOI] [PubMed] [Google Scholar]
- 45.Goncalves P., Araujo J.R., Di Santo J.P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:558–572. doi: 10.1093/ibd/izx029. [DOI] [PubMed] [Google Scholar]
- 46.Huda-Faujan N., Abdulamir A.S., Fatimah A.B., Anas O.M., Shuhaimi M., Yazid A.M. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J. 2010;4:53–58. doi: 10.2174/1874091X01004010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaworska K., Konop M., Bielinska K., Hutsch T., Dziekiewicz M., Banaszkiewicz A. Inflammatory bowel disease associates with increased gut-to-blood penetration of SCFA: a new, noninvasive marker of a functional intestinal lesion. Exp Physiol. 2019;104:1226–1236. doi: 10.1113/EP087773. [DOI] [PubMed] [Google Scholar]
- 48.Bolognini D., Tobin A.B., Milligan G., Moss C.E. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. 2015;89:388–398. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- 49.Nohr M.K., Pedersen M.H., Gille A., Egerod K.L., Engelstoft M.S., Husted A.S. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.