Abstract
Radiotherapy (RT) has always been a mainstay for malignant tumors therapy, but it is also used for benign pathology. The application of low or intermediate doses of RT has been widely studied. This topic was presented and discussed in the last XX GOCO (Grup Oncològic Català-Occità) meeting. The aim of this article is to review the indications of low dose irradiation (LD-RT), total dose and different fractionations, the public to whom it can be directed, and to offer an analysis about secondary effects. We believe it can be useful not only for radiation oncologists, but for other physicians to consider this option for future patients.
Keywords: Benign, Low dose radiation, Anti-Inflammatory, Non-Malignant, Radio-Induced cancer risk, Anti-Proliferation
1. Introduction
Radiotherapy has always been a mainstay for malignant tumors therapy, but its use for benign conditions should not be ignored. The application of low or intermediate doses of radiotherapy (RT) has been widely studied and in some countries, like Germany, the use of this technique is even more frequent among benign cases than for malignant pathologies.
In 1898, a juvenile arthritis case was the first benign pathology ever reported to be treated with radiotherapy.1,2 Further investigations place us in a new technology era with a considerable range of alternatives for a single non-neoplasic pathology, giving patients an effective opportunity away from polipharmacy and surgery.
This topic was presented and discussed in the last XX GOCO (Grup Oncològic Català-Occità) meeting, which took place at Hospital del Mar of Barcelona. GOCO is an association of Radiation oncology professionals of Catalonia and Occitania.
Dra Arenas and Dr Biete presented their last publications emphasizing the use of External Beam RT (EBRT) for a widely benign presentations and its mechanisms of effectiveness.
The aim of this article is to sum up the utility of low dose irradiation (LD-RT) starting with a brief about the molecular and cellular interactions that take place, different scenarios where it can be indicated, the most frequent dose fractionations, and some case examples.
Although there is poor evidence about secondary malignancy effects due to this LD-RT, this consideration has been contemplated and discussed as it might be a reason for some physicians to avoid this treatment.
1.1. Radiobiology principles
Radiobiology effects of LD-RT differ from the high doses used at malignancies.
Many researchers have studied how this radiation modulates the function of inflammatory pathways. Although the understanding of this process is still under investigation, many advances, in vivo and in vitro, have enlightened important mechanisms which explain the reason for its effectiveness.3, 4, 5
Inflammatory and degenerative diseases are the result of patho-physiological complexes based on immunological networks and LD-RT can modulate some of the pathways involved.
There are two basic hypothetical mechanisms: anti-inflammatory and anti-proliferative:
1.1.1. Anti-inflammatory effect (2−6 Gy)
With LD-RT (<0.5 Gy/fraction) different interactions take place in the inflammatory process.
Under normal conditions, inflammatory responses are regulated by a tightly sequential process that depends on the production of inflammatory mediators, cell adhesion molecules expression and leukocyte-endothelial cell interaction: rolling, adhesion and migration to the interstitial space.
During the rolling phenomenon, leukocytes are activated through local inflammatory mediators, slowing the blood flow and stimulating immunocompetent cells to migrate to the surrounding tissue, regulated by membrane-bound receptors (Selectines), producing cytokines responsible for vasodilatation and vascular permeability (Interleukine-1 (IL-1), Tumor Necrosis Factor-α (TNF-α), Nitric Oxide (NO)). This process carries on edema, erythema and pain.6,7
When LD-RT is applied (<1 Gy), it inhibits the expression of Inducible Nitric Oxide Synthase (iNOS) and that reduces endothelial cell-leukocyte interactions as well as vasodilatation. Other anti-inflammatory cytokines are produced (IL-10, Transforming Growth Factor-β1 (TGF-β1), increasing apoptosis and diminishing NO production. TGF-β1 is known to be an endogenous important factor in inflammatory regulation.8
There is also a decreased expression of adhesion molecules (P-, L-, E- Selectins, Intercellular Adhesion Molecule (ICAM), Vascular Cell Adhesion Molecule (VCAM)), and lower secretion of CCL20 (C-C motif chemokine ligand 20) of neutrophiles, which correlates to a lower adhesion to endothelial cells.9
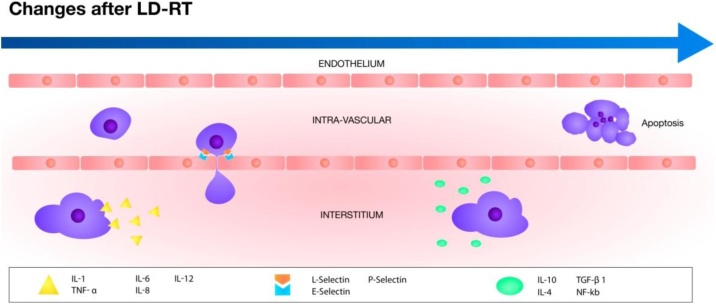
A resume of this complex process is offered in Fig. 1 and Table 1.
Fig. 1.
This figure shows schematically which molecules take prominenceduring the inflammatory process and after irradiation at low doses.
Table 1.
Differences between the effects of irradiation at low and high doses.
| Low irradiation doses (<1 Gy) | High irradiation doses (>2 Gy) |
|---|---|
| Anti-inflamatory properties | Pro-inflamatory cytokines production |
| IL-10, TGF-β1, IL-4, NF-κB, HO-1, HSP70 | IL-1, TNF-α, IL-6, IL-8, IL-12 |
| L-Selectin, E-Selectin, AP-1, ROS, iNOS | ROS, iNOS |
| Apoptosis |
AP-1: Activator Protein- 1; HO-1: Heme oxygenase-1; HSP70: Inductible Heat Shock protein 70; IL: Interleukine;
NF-κB: Nuclear factor-κB; ROS: Reactive Oxygen Species; TGF-β1: Transforming Growth Factor-β1; TNF- α: Tumor Necrosis Factor- α; iNOS: Inductible Nitric Oxide Synthase.
Studies in vitro find anti-inflammatory effects at doses <0.7 Gy. Furthermore, in vivo studies have identified an overexpression of TGF-β1 at 24 h of LD-RT and decreased levels of pro-inflammatory cytokines (TNF-α, IL-1β, iNOs) and concomitant increase of Heme oxygenase-1(HO-1) and inducible Heat Shock protein 70 (HSP70).10 Moreover, through mice studies, a decreased adhesion of leucocytes has been showed with 0.3 Gy as lowest effective dose, and it is suggested that anti-inflammatory action of LD-RT does not depend on ICAM expression.11
Therefore, doses ranged between 0.1-0.3 Gy could offer anti-inflammatory effects minimizing toxicity.
1.1.2. Antiproliferative effect (8−10 Gy)
Irradiation produces a delay in cell mitotic cycle that prevents cellular growth in the irradiated tissue during a dose-dependent period of time. In some phenomena (like heterotrophic ossification after prosthetic replacement), when there is a critical period of time where cellular repopulation is essential for the expression of damage, the LD-RT could provoke an initial blockage and prevent the appearance of damage.
1.1.3. Immunomodulatory (>10 Gy)
A regulation of lymphocytes antigenic stimulus takes place over 10 Gy, suppressing the local autoimmune process.
1.2. LD-RT Indications
Non-malignant disorders can be safely treated through LD-RT, especially in elderly patients, who are less exposed to possible secondary effects.12,13 This therapeutic method can reduce the long-term use of pain drugs. However, its use must be balanced against possible side effects or contraindications.
Despite the well-known efficacy of LD-RT, there are numerous fractionation regimens. Therefore, some protocols have been proposed to unify prescriptions in every benign pathology. Updated guidelines defining RT indications clinically evident are available since the year 2000, and have been reevaluated and improved following the last levels of evidence published14,15 (Table 2). Even though, properly designed studies are needed to determine the most efficacious treatment dose and schedules.
Table 2.
Most common used fractionations and Clinical Target Volume for non-malignant pathologies.
| Benign disorder | Fractionation | Total dose | CTV* |
|---|---|---|---|
| Acute/chronic inflammatory disorders | |||
| Hidradenitis suppurativa | 0.2−1 Gy 5fx*/week until response | 0.6−5 Gy | Gland affection |
| Sialorrhea | 4 Gy 2-3fx/week | 10-20 Gy | Submandibular gland and 2/3 parotids |
| Acute/chronic painful degenerative diseases | |||
| Insertion tendinitis | 0.5−1 Gy 2-3fx/week |
5−12 Gy | Tendon insertion |
| Synovitis | Synovial Bag | ||
| Osteoarthritic disease | Osteoarthritic affected articulation | ||
| Hypertrophic disorders of soft tissues | |||
| Dupuytren/ Ledderhose/ Peyronie | 2−4 Gy 5fx/week | 20-40 Gy | Regional involvement with margin |
| Keloids | 2−5 Gy 1–2 fx/week | 12−21 Gy | Surgical bed +0.5 cm |
| Pterygium | 10 Gy 1fx/week | 30-60 Gy | Conjunctive injury |
| Gynecomastia | 2−5 Gy 5fx/week | 20-30 Gy | Breast |
| Functional diseases | |||
| Dysthytoid ophthalmopathy | 1.5−2 Gy 5fx/week | 10-20 Gy | Retrobulbar orbital volume |
| Orbital pseudotumor | 2 Gy 5fx/week | 20 Gy | |
| Arteriovenous malformations | 16−25 Gy Single fraction | 16−25 Gy | Vascular alteration with margin |
| Age-related macular degeneration | 1.8 Gy 4fx/week | 14.4 Gy | Posterior chamber |
| Lymphatic fistula | 0.5−1 Gy 4-5fx/week | 3−10 Gy | Fistulous path |
| Dermatologic diseases | |||
| Eczemas | 0.5−1 Gy 1–2fx/week | 4−5 Gy | Affected area with margins |
| Psoriasic nail bed lesions | 1−2 Gy 1–2/week | 6−8 Gy | Nail bed |
| Heterotrophic ossification | 7 Gy Single fraction | 7 Gy | Articulation and peri-articular soft tissues |
| Calcaneal spur | 0.5 Gy 2fx/week | 5 Gy | Calcaneus and achilles tenson insertion |
| Vertebral hemangioma | 2 Gy 5fx/week | 30-40 Gy | Spinal body affected |
CTV: Clinical Target Volume; fx: fractions.
RT of non-malignant disorders is performed with the same techniques as malignant diseases,16 it is the fractionation that makes the difference.
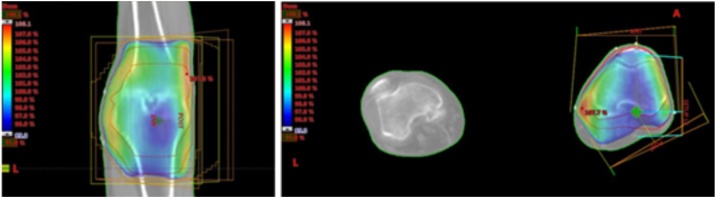
Trying to delimitate properly the location with LD-RT and avoiding surrounding tissues can help in diminishing secondary effects and radio-induced cancer (RIC) risk (Fig. 2). In case that the patient reaches partially positive clinical outcome, a second course of LD-RT can be added some weeks later in order to complete the pain relief effectiveness.
Fig. 2.
This figure shows the planification (with its dosimetry) of a knee osteoarthrosis from a 87 years old man with sever cardiovascular disease. It was offered a LD-RT (6 Gy, 0.6 Gy fractionation) with good long-term results.
Two clinical trials about LD-RT symptomatic effectiveness have been published so far, and it is important to highlight that overall conclusions show a negative impact on this issue.17, 18, 19 Outlining the well-designed studies, randomized (irradiation group vs. sham treatment group) and double-blinded, some considerations have been already discussed.20,21
First of all, these studies contemplate patients with hand and knee osteoarthritic disorders, when it is known that LD-RT has a greater response in other non-malignant diseases, like enthesopathies.22 Degenerative Osteoarthritis (DOA) is a chronic disorder with joint destruction where irradiation takes action on antiinflamatory process, but the articulation does not experience regeneration and pathophysiological mechanisms carry on despite the pain relief that a patient might notice at the beginning (Table 3).
Table 3.
This table resumes schematically the most important aspects to consider before LD-RT planning.
| To sum up |
|---|
| Radiobiological effects at LD-RT* - anti-proliferation - anti-inflammation |
| Important Key factors: Age and RFS* |
| Patient individualization: radiosensitivity syndromes, pacemaker |
| Organs at risk at LD-RT: eyes, spinal cord, Kidneys, heart, breast |
| Adjusted CTV* |
| LD-RT (Low dose radiotherapy); RFS(Radiation Field Size); CTV (Clinical Target Volume) |
Secondly, no patient in the randomized trials received a second irradiation, where a complete benefit has been experienced before in several clinical studies and is a well-known practice used frequently by expert physicians.
Third, different clinical studies with big data, considering DOA in hand and knee as well, describe significant symptomatic relief.23
In addition, the number of patients is considered too low to conclude that LD-RT in DOA has to be excluded in the clinical practice. Moreover, a higher body mass index was reported in the irradiated group, which might have had a false negative impact in the results.
Finally, among the randomized studies we are discussing, the early outcome at 3 months was evaluated, and nowadays the long-term results at 6–12 months after irradiation24 is recognized.
To sum it all up, there is a need to resolve the doubts about fractionation and the efficacy for each benign disorder. Nevertheless, there are remarkable published reviews that highlight the indications for benign diseases treatment with LD-RT. Focusing specifically on DOA, vascular and soft tissues diseases; they have shown the reliability of this treatment, as well as the careful evaluation that has to be done on every patient.25 On top of all that, the DEGRO guidelines for non-malignant disorders written by a German group resume in 4 different reviews: the physical principles, radiobiological mechanisms and radiogenic risk16; and the treatment indications for degenerative skeletal disorders,26 hyperproliferative disorders,27 and symptomatic functional disorders28 with LD-RT.
1.3. Organs at risk
Since the total dose used for benign conditions is below the recognized thresholds for secondary effects, surrounding tissues would not be a limiting factor. The lower the dose, the less is the risk, especially if no important organs are exposed in the radiation field. However, specific tissue damage at low dose should be known and avoided.29
Some tissues are known to be more sensitive to radiation, e.g. eyes, the spinal cord or kidneys, and need to be controlled specifically, maintaining the dose to them as low as possible and following the dose constraints used for malignant treatments.
1.4. Secondary malignancies
It is believed that the fear of radiation-induced tumors can explain the lack of LD-RT use.
We cannot deny the lack of controlled studies involving the manner, but several retrospective series have reported the insufficient evidence to affirm this condition.
Most of the irradiation side effects have been extrapolated from radioactive accidents that took place in the past when people were exposed to whole body low dose irradiation (survivors of the atomic bomb in Hiroshima and Nagasaki, nuclear plant accident in Chernobyl, irradiation for ankylosing spondylitis, fluoroscopic repeated examinations in tuberculous patients, and so on). Recent analyses confirm that the risk of RIC increases almost linearly with dose.
Large follow-ups in the literature describe high risks of RIC, however, it was analyzed on pathologies that are no longer treated with RT and using old RT techniques, which makes it very discordant with the indications and techniques used in modern medicine; for this reason, we should be cautious with historic interpretations. On the other hand, current studies are based on case reports and small retrospective series, with short term follow-up due to the life expectancy of patients treated. Therefore, long-term benefits and risks of treatments are difficult to ascertain.
Radiation Field Size (RFS) is an important variable to contemplate, since the smaller irradiation fields would reduce the chance to initiation or promotion that might ultimately lead to a tumor.
Different mathematical models have tried to resolve the doubts of dose effect in RIC, and after a variety of hypotheses,30,31 there is a higher belief in linear relationship between dose administered and RIC. This pattern was confirmed by Hodgkin lymphoma irradiated patients, who developed secondary lung and breast cancer with a reported dose-dependent increase risk,32 and bone and soft-tissue sarcomas after high-dose fractionated radiation exposure.33
The hormesis or cellular adaptive model, first described in 1980, defends that cell response to very LD-RT can later resist exposure to higher doses.31,34 This hypothetical statement became controversial with the linear no threshold theory.
The studies have confirmed the importance of age, as younger the patient was at the time of RT, the higher the risk of developing RIC. Furthermore, in utero exposure has lower risk than being irradiated during infancy.35
Skin cancer: The incidence of non-melanoma skin cancer (NMSC) increases after occupational or therapeutic radiation exposure. It is thought that carcinogenic effects of radiation and Ultra-Violet exposure are synergic and can increase RIC.
RIC has a latency of 10 years. Exposure to RT is associated with an increased risk of Basal Cell Carcinoma (BCC), showing an increase with younger age exposure.36,37
Soft-tissue sarcoma and bone sarcoma: There is no dose-response relationship described for soft tissue sarcomas after RT. A genetic link has been identified between sarcoma and retinoblastoma, increasing the risk of sarcomas in the irradiated field of this population.38
Leukemia: In contrast to the linear dose relation contemplated since now, haematological malignancies have a non-linear dose response. Therefore, it is important to mention that risk with LD-RT, as the risk to develop leukemia remains for at least 25 years and its maximum risk depends on the age at exposure. Moreover, for patients receiving a mean bone marrow dose of 1 Gy (like in ankylosing spondylitis), the leukemia risk is about 0.2%.
Brain tumors: LD-RT and High-dose EBRT encompassing the brain field may increase the incidence of benign brain tumors (meningioma, neurilemmomas) and also, but less frequently, malignant tumors.39,40
Thyroid cancer: Thyroid gland is an important radiosensitive organ during childhood, papillary thyroid cancer being the most common RIC.
Breast Cancer: For breast cancer age is again a key factor. It might occur in women who received RT for postpartum mastitis, thoracic hemangioma, fibroadenomatosis, etc.41
Lung cancer: An increased incidence of lung cancer has been reported in patients treated previously for ankylosing spondylitis and peptic ulcers.
There are a few well known radiosensitive syndromes, such as retinoblastoma, neurofibromatosis type 1, Li Fraumeni Syndrome, Nijmegen Breakage Syndrome, Nevoid basal cell carcinoma Syndrome, Ataxia telangiectasia. These patients are likely to show more severe reaction to RT and we all should be aware of them.
2. Conclusions
Irradiation to benign pathology is effective due to the antiinflammatory effect, and it has already been demonstrated in clinical trials.
LD-RT offers a low-risk, genuinely conservative, noninvasive therapeutic alternative for elderly patients, with low toxicity. Nowadays it is reserved as a rescue treatment when standard management has failed.
Radiogenic risks should be taken into account in each and every indication for irradiation.
References
- 1.Schmid-Monnard. Über Heilung des Gelenkrheumatismus durch Röntgenstrahlen bei Kindern. s.l. : Fortschritte auf dem Gebiet der Röntgenstrahlen, 1898. 1:209.
- 2.Stenbek. Om behandling of kronsik ledgangs-rheumatism med Röntgenstralar. s.l. : Sv Läk Förh, 1898. 117.
- 3.Frey B., Gaipl U.S., Sarter K. Whole body low dose irradiation improves the course of beginning polyarthritis in human TNF-transgenic mice. Autoimmunity. 2009;42(4):346–348. doi: 10.1080/08916930902831738. PMID: 19811297. [DOI] [PubMed] [Google Scholar]
- 4.Arenas M., Gil F., Gironella M. Anti-inflammatory effects of low-dose radiotherapy in an experimental model of systemic inflammation in mice. Int J Radiat Oncol Biol Phys. 2006;66(2):560–567. doi: 10.1016/j.ijrobp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Arenas M., Gil F., Gironella M. Time course of anti-inflammatory effect of low-dose radiotherapy: Correlation with TGF-beta(1) expression. Radiother Oncol. 2008;86(3):399–406. doi: 10.1016/j.radonc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Panés J., Granger D.N. Leukocyte-endothelial cell interactions: Molecular mechanisms and implicationsin gastrointestinal disease. Gastroenterology. 1998;114(5):1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 7.Rödel F., Frey B., Manda K. 2012. Immunomodulatory properties and molecular effects in inflammatory diseases of low-dose X-irradiation. München, Germany : Frontiers in Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith W.B., Noack L., Khew-Goodall Y., Isenmann S., Vadas M.A., Gamble J.R. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol. 1996 PMID 8683138. [PubMed] [Google Scholar]
- 9.Rödel F., Hofmann D., Auer J. The anti-inflammatory effect of low-dose radiation therapy involves a diminished CCL20 chemokine expression and granulocyte/endothelial cell adhesion. Strahlenther Onkol. 2008 doi: 10.1007/s00066-008-1776-8. [DOI] [PubMed] [Google Scholar]
- 10.Schaue D., Jahns J., Hildebrandt G., Trott K.R. Radiation treatment of acute inflammation in mice. Radiat Biol. 2005 doi: 10.1080/09553000500385556. [DOI] [PubMed] [Google Scholar]
- 11.Arenas M., Sabater S., Hernández V. Springer-Verlag; Spain: 2012. Anti-inflammatory effects of low-dose radiotherapy. [DOI] [PubMed] [Google Scholar]
- 12.Micke O., Seegenschmiedt M.H., Adamietz I.A. 2018. Low-dose radiation therapy for benign painful skeletal disorders: The typical treatment for the elderly patient? Germany. DOI 0.1016/j.ijrobp.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Park Shin-Hyung, Lee Jeong Eun. 2017. Radiotherapy, a new treatment option for non-malignant disorders: Radiobiological mechanisms, clinical applications, and radiation risk. Daegu, Korea. [Google Scholar]
- 14.Seegenschmiedt M.H., Micke O., Muecke R. Radiotherapy for non-malignant disorders: State of the art and update of the evidence-based. Br J Radiol. 2015 doi: 10.1259/bjr.20150080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2015. Radiologists, the Royal College of. A review of the use of radiotherapy in the UK for the treatment of benign clinical conditions and benign tumours. London. [Google Scholar]
- 16.Reichl B., Block A., Schäfer U. Springer Berlin Heidelberg; Weiden, Germany: 2015. DEGRO practical guidelines for radiotherapy of non-malignant. [DOI] [PubMed] [Google Scholar]
- 17.Minten M.J., Mahler E.A., Leseman-Hoogenboom M.M. Low-dose radiation therapy as treatment for hand and knee osteoarthritis: Two double-blinded RCTs. Radiother Oncol. 2018 [Google Scholar]
- 18.Mahler E.A.M., Minten M.J., Leseman-Hoogenboom M.M. Effectiveness of low-dose radiation therapy on symptoms in patients with knee osteoarthritis: A randomised, double-blinded, sham-controlled trial. Ann Rheum Dis. 2018 doi: 10.1136/annrheumdis-2018-214104. [DOI] [PubMed] [Google Scholar]
- 19.Minten M.J.M., Leseman-Hoogenboom M.M., Kloppenburg M. Lack of beneficial effects of low-dose radiation therapy on hand osteoarthritis symptoms and inflammation: A randomised, blinded, sham-controlled trial. Osteoarthr Cartil. 2018 doi: 10.1016/j.joca.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Montero A., Sabater S., Rödel F. Is it time to redefine the role of low-dose radiotherapy for benign disease? Ann Rheumatic Diseases. 2018 doi: 10.1136/annrheumdis-2018-214873. [DOI] [PubMed] [Google Scholar]
- 21.Ott O.J., Micke O., Mücke R. Low-dose radiotherapy: Mayday, mayday. We’ve been hit! Strahlenther Onkol. 2018 doi: 10.1007/s00066-018-1412-1. [DOI] [PubMed] [Google Scholar]
- 22.Micke O., Seegenschmiedt M.H., Adamietz I.A. Low-dose radiation therapy for benign painful skeletal disorders: The typical treatment for the elderly patient? Int J Radiat Oncol Biol Phys. 2017 doi: 10.1016/j.ijrobp.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Mücke R., Seegenschmiedt M.H., Heyd R. 2010. Radiotherapy in painful gonarthrosis. Results of a national patterns-of-care study]. Germany : Strahlenther Onkol. [DOI] [PubMed] [Google Scholar]
- 24.Keller S., Müller K., Kortmann R.D. Efficacy of low-dose radiotherapy in painful gonarthritis: Experiences from a retrospective East German bicenter study. Radiat Oncol. 2013 doi: 10.1186/1748-717X-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montero Luis A., Hernanz de Lucas R., Hervás Morón A. Radiation therapy for the treatment of benign vascular, skeletal and soft tissue diseases. Clin Transl Oncol. 2008 doi: 10.1007/s12094-008-0209-6. [DOI] [PubMed] [Google Scholar]
- 26.Oliver J.Ott, Niewald Marcus, Weitmann Hajo-Dirk. DEGRO guidelines for the radiotherapy of non-malignant disorders. Strahlenther Onkol. 2014 doi: 10.1007/s00066-014-0757-3. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich Seegenschmiedt M., Micke Oliver. DEGRO guidelines for the radiotherapy of non-malignant disorders. Strahlenther Onkol. 2015 doi: 10.1007/s00066-015-0818-2. [DOI] [PubMed] [Google Scholar]
- 28.Reinartz Gabriele, Eich Hans Theodor, Pohl Fabian. 2014. DEGRO practical guidelines for the radiotherapy of non-malignant disorders – Part IV. s.l. : Strahlenther Onkol. [DOI] [PubMed] [Google Scholar]
- 29.Stewart F.A., Akleyev A.V., Hauer-Jensen M. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs--Threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 2012 doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Brenner D.J., Sachs R.K. Estimating radiationinduced cancer risks at very low doses: Rationale for using a linear no-threshold approach. Radiat Environ Biophys. 2006 doi: 10.1007/s00411-006-0029-4. [DOI] [PubMed] [Google Scholar]
- 31.Sagan L.A. What is hormesis and why haven't we heard about it before? Health Phys. 1987 PMID 3570794. [PubMed] [Google Scholar]
- 32.Sachs R.K., Brenner D.J. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0506648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berrington de Gonzalez A., Kutsenko A., Rajaraman P. Sarcoma risk after radiation exposure. Clin Sarcoma Res. 2012 doi: 10.1186/2045-3329-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luckey T.D. CRC Press; Boca Raton: 1980. Hormesis with ionizing radiation. [Google Scholar]
- 35.Preston D.L., Cullings H., Suyama A. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst. 2008 doi: 10.1093/jnci/djn045. [DOI] [PubMed] [Google Scholar]
- 36.Shore R.E., Albert R.E., Reed M., Harley N., Pasternack B.S. 1984. Skin cancer incidence among children irradiated for ringworm of the scalp. PMID 6494429. [PubMed] [Google Scholar]
- 37.Karagas M.R., McDonald J.A., Greenberg E.R. Risk of basal cell and squamous cell skin cancers after ionizing radiation therapy. For the Skin Cancer prevention Study Group. J Natl Cancer Inst. 1996 doi: 10.1093/jnci/88.24.1848. [DOI] [PubMed] [Google Scholar]
- 38.Draper G.J., Sanders B.M., Kingston J.E. Second primary neoplasms in patients with retinoblastoma. Br J Cancer. 1986 doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ron E., Modan B., Boice J.D., Jr Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988 doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 40.Neglia J.P., Robison L.L., Stovall M. New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the Childhood Cancer survivor Study. J Natl Cancer Inst. 2006 doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 41.Mattsson A., Rudén B.I., Palmgren J., Rutqvist L.E. Dose- and time-response for breast cancer risk after radiation therapy for benign breast disease. Br J Cancer. 1995 doi: 10.1038/bjc.1995.461. [DOI] [PMC free article] [PubMed] [Google Scholar]