Abstract
Background
Although the clinical importance of the immunological benefits of breastfeeding has been emphasized for decades, their direct relationship with acute pyelonephritis (APN) is still not clear. Our goal was to determine whether breastfeeding truly provides protection against APNs, while investigating the effects of other factors such as sex, age, mode of delivery, and birth weight on APN.
Methods
A total of 62 infants under 6 months of age who had both microbiologically and radiologically-confirmed APN were enrolled in the case group. Healthy infants (n = 178) who visited the hospital for scheduled vaccinations were enrolled in the control group. The following participant characteristics were compared between the case and control groups: age, sex, birth order among siblings, feeding methods, weight percentile by month, birth weight percentile by gestational age, gestational age at birth, and mode of delivery.
Results
Babies exclusively fed with manufactured infant formulae before 6 months of age had significantly higher risk for APN than breastfed or mixed-fed infants (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.687–7.031; P = 0.001). Firstborn babies had lower risk for APN than 2nd- or 3rd-born babies (OR, 0.43; 95% CI, 0.210–0.919). Other factors that increased the risk for APN were low birth weight percentiles (OR, 8.33; 95% CI, 2.300–30.166) and birth via caesarean section (OR, 2.32; 95% CI, 1.097–4.887). There were more preterm births in the case group (10.9% vs. 1.7%; P = 0.002), but this did not increase the risk for APN (OR, 4.47; P = 0.063).
Conclusion
Feeding exclusively with formula before 6 months of age was related to higher risk for APN, which demonstrates that breastfeeding has a protective effect against APN. The other risk factors for APN were birth order (≥ 2nd-born), low birth weight, and birth via caesarean section.
Keywords: Breastfeeding, Urinary Tract Infections, Pyelonephritis, Low Birth Weight, Caesarean Section, Infants
Graphical Abstract
INTRODUCTION
Breast milk is the ideal nutritional food for babies, and the World Health Organization and the American Academy of Paediatrics both recommend maintaining breastfeeding exclusively for at least 6 months after birth and supplementing breast milk with baby food during late infancy.1 Although the components of manufactured formula milk are quite similar to those of breast milk, immunoglobulins and other trace amounts of beneficial components naturally contained in breast milk have been proven to be protective against infections during infants' immunologically immature period.2,3 There are also other broad spectrum benefits of breastfeeding, such as the prevention of sudden infant death syndrome, improved intellectual development, and even long-term effects on adulthood.4,5 Among the various categories of infectious diseases, there have been many studies demonstrating the protective effects of breastfeeding for respiratory and gastrointestinal infections or otitis media,6,7 but only a few regarding urinary tract infections (UTIs). Only a “probable” level8 of evidence regarding the protective relationship between breastfeeding and UTIs has been identified. Currently known risk factors for UTIs in children include young age, sex, circumcision status if applicable, diaper use, toilet training, and colonization of virulent types of bacteria.9 The aims of the present study were to investigate the relationship between feeding method and risk for acute pyelonephritis (APN) in infants under 6 months of age and to determine whether the benefits of breastfeeding truly provide protection against APNs. Furthermore, the effects of additional factors such as sex, age, mode of delivery, and birth weight on APN were also investigated.
METHODS
This was a case-control study conducted using retrospective chart reviews from a single tertiary medical centre in Korea. Case groups were selected from among 114 infants under 6 months of age who were admitted to the hospital from January 2012 to July 2017 with diagnoses of UTI or APN. Among these patients, we included 67 patients who had: 1) bacterial growth of more than 105 colony-forming units in catheterized urine cultures; 2) abnormal findings on ultrasonography (USG) or Tc-99 m dimercaptosuccinic acid (DMSA) renal scintigraphy suggesting renal involvement; and 3) no previous medical history of APN. Five patients were excluded due to anatomic anomalies of the genitourinary system on image work ups, including USGs, DMSA scans, or voiding cystouretherographies (VCUG), or recurrent UTIs or APNs (≥ 2 times) without positive VCUG results. Ultimately, 62 patients with APN were enrolled in the case group.
The control group was selected from among 573 infants who were born at our hospital from October 2014 to June 2016 with no specific perinatal history. Healthy infants under 6 months of age (n = 180) who visited the hospital for scheduled vaccinations were enrolled in the control group. Two were excluded due to late-appearing APN; therefore, 178 infants were ultimately enrolled in the control group.
The following participant characteristics were investigated: age, sex, birth order among siblings, feeding methods, weight percentile by month, birth weight percentile by gestational age, gestational age at birth, and mode of delivery. Participant age in the case group was based on age at first day of admission for APN treatment. None of the subjects had histories of severe systemic disease, congenital/acquired immune deficiency, or any contraindications for breastfeeding. A physician asked about feeding methods at the time of admission for the case group, or at the time of out-patient clinic visits for the control group. According to the feeding methods, subjects were grouped into either an exclusive breastfeeding, mixed-feeding, or exclusive formula feeding group. The “exclusive breastfeeding/formula feeding” group in this study included babies being fed entirely with full breast milk or formulae milk in recent days, respectively, regardless of the feeding methods in the distant past. Participants were also divided into the following 4 groups based on weight percentile charts according to months of age and gestational age: 1) under 10th percentile, 2) from 10th percentile to 50th percentile, 3) from 50th percentile to 90th percentile, and 4) over 90th percentile (Tables 1 and 2).10,11 “Preterm baby” indicates a baby born before 37 weeks of pregnancy.
Table 1. Weight percentiles by month (based on Moon et al.10).
| Age, mon | Percentiles, kg | |||||
|---|---|---|---|---|---|---|
| 10th | 50th | 90th | ||||
| Male | Female | Male | Female | Male | Female | |
| 1 | 4.4 | 4.2 | 5.3 | 5.0 | 6.0 | 5.8 |
| 2 | 5.2 | 4.9 | 6.1 | 5.7 | 7.0 | 6.6 |
| 3 | 5.7 | 5.4 | 6.8 | 6.4 | 7.8 | 7.4 |
| 4 | 6.3 | 5.9 | 7.4 | 7.0 | 8.4 | 8.0 |
| 5 | 6.6 | 6.3 | 7.8 | 7.4 | 8.9 | 8.4 |
| 6 | 7.1 | 6.6 | 8.2 | 7.8 | 9.4 | 8.8 |
Table 2. Birth weight percentiles by gestational age (based on Lee et al.11).
| Gestational age, wk | Percentiles, kg | ||
|---|---|---|---|
| 10th | 50th | 90th | |
| 34 | 1.85 | 2.31 | 2.78 |
| 35 | 2.04 | 2.53 | 3.03 |
| 36 | 2.24 | 2.74 | 3.26 |
| 37 | 2.43 | 2.93 | 3.46 |
| 38 | 2.60 | 3.11 | 3.63 |
| 39 | 2.75 | 3.25 | 3.78 |
| 40 | 2.88 | 3.37 | 3.89 |
| 41 | 2.98 | 3.46 | 3.99 |
Statistical analysis
SPSS version 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Categorical variables were analysed using the chi-squared test to compare case and control group proportions, and Student's t-test was used for continuous variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were derived from binary logistic regression analyses. Statistical significance was defined as a P value < 0.05.
Ethics statement
Ethical approval for this study was obtained from the Kangbuk Samsung Hospital Institutional Review Board (approval No. 2019-07-046), and the need for informed consent was waived.
RESULTS
The general characteristics of the 240 participants ultimately included in the study are shown in Table 3. Variables including sex, age in months, birth order among siblings, feeding method, weight and birth weight, gestational age, and delivery method were investigated.
Table 3. Characteristics of the study population.
| Variables | Total (n = 240) | |
|---|---|---|
| Age, mon | 3.85 ± 1.668 (median age, 4) | |
| 1–2 | 69 (28.8) | |
| 3–4 | 87 (36.3) | |
| 5–6 | 84 (35.0) | |
| Sex, male | 141 (58.8) | |
| Firstborn baby | 159 (67.7) | |
| Feeding | ||
| Formula | 70 (29.2) | |
| Mixed | 102 (42.5) | |
| Breast milk | 68 (28.3) | |
| Weight, p | ||
| < 10 | 33 (13.8) | |
| 10–50 | 88 (36.7) | |
| 50–90 | 83 (34.6) | |
| > 90 | 36 (15.0) | |
| Birth weight, p | ||
| < 10 | 15 (6.5) | |
| 10–50 | 101 (43.5) | |
| 50–90 | 97 (41.8) | |
| > 90 | 19 (8.2) | |
| Preterm birth, < 37 wk | 9 (3.9) | |
| Cesarean section | 68 (28.3) | |
Data were shown as mean ± standard deviation or number (%).
In Table 4, each variable was compared between patients with or without APN. The mean age of the two groups was similar, with the same median age (4 months), and there were no statistically significant differences in age distribution. The percentage of males (71%) was higher in the case group than in the control group (54.5%). There were more 2nd- or 3rd-born babies in the case group than in the control group.
Table 4. Comparisons between the case and control groups.
| Variables | Control (n = 178) | Case (n = 62) | P value | |
|---|---|---|---|---|
| Age, mon | 3.86 ± 1.736 | 3.81 ± 1.496 | 0.609 | |
| 1–2 | 54 (30.3) | 15 (24.2) | ||
| 3–4 | 62 (34.8) | 25 (40.3) | ||
| 5–6 | 62 (34.8) | 22 (26.2) | ||
| Sex, male | 97 (54.5) | 44 (71.0) | 0.023 | |
| Firstborn baby | 126 (71.2) | 33 (56.9) | 0.043 | |
| Feeding | < 0.001 | |||
| Formula | 39 (21.9) | 31 (50.0) | ||
| Mixed | 85 (47.8) | 17 (27.4) | ||
| Breast milk | 54 (30.3) | 14 (22.6) | ||
| Weight, p | 0.182 | |||
| < 10 | 21 (11.8) | 12 (19.4) | ||
| 10–50 | 66 (37.1) | 22 (35.5) | ||
| 50–90 | 67 (37.6) | 16 (25.8) | ||
| > 90 | 24 (13.5) | 12 (19.4) | ||
| Birth weight, p | 0.014 | |||
| < 10 | 7 (4.0) | 8 (14.5) | ||
| 10–50 | 84 (47.5) | 17 (30.9) | ||
| 50–90 | 73 (41.2) | 24 (43.6) | ||
| > 90 | 13 (7.3) | 6 (10.9) | ||
| Preterm birth, < 37 wk | 3 (1.7) | 6 (10.9) | 0.002 | |
| Cesarean section | 44 (25.0) | 24 (38.7) | 0.040 | |
Data were shown as mean ± standard deviation or number (%).
The proportion of participants in the exclusive formula feeding category was significantly higher in the case group than in the control group (50.0% vs. 21.9%, P < 0.001).
The proportion of low birth weights under the 10th percentile by gestational age was higher in the case group than in the control group (14.5% vs. 4.0%, P = 0.014). In contrast, there was no statistically significant difference among the percentiles of current body weight between the case and control groups. Premature infants and births by caesarean section were also greater in the case group. Fisher's exact test revealed that the percentage of caesarean sections according to the number of preterm births was not statistically different (P = 0.126) (data not shown).
Table 5 shows the relationship between breastfeeding and the other variables, to identify whether any of them affect breastfeeding. The case group was divided into a breastfeeding group (both exclusive and mixed) and an exclusive formula feeding group. Among the variables for each group, only the age of the case group was statistically significant, while sex, birth order among siblings, weight percentile, birth weight percentile, prematurity, and mode of delivery were not related to breastfeeding. The same analysis was performed in the control group, but there was no significant relationship at all (data not shown). We investigated whether the presence of a pathogen in the urine cultures of the APN group has any relationship with the feeding methods and mode of delivery, but neither was statistically significant (the latter, P = 0.669, is not shown in the tables).
Table 5. Comparisons between breastfeeding (both exclusive and mixed) and exclusive formula feeding in the case group.
| Variables | Formula (n = 31) | Breast milk (n = 31) | P value | ||
|---|---|---|---|---|---|
| Age, mon | 0.035 | ||||
| 1–2 | 4 (12.9) | 11 (35.5) | |||
| 3–4 | 17 (54.8) | 8 (25.8) | |||
| 5–6 | 10 (32.3) | 12 (38.7) | |||
| Sex, male | 22 (71.0) | 22 (71.0) | 1.000 | ||
| Firstborn baby | 21 (67.7) | 12 (44.4) | 0.074 | ||
| Weight, p | 0.450 | ||||
| < 10 | 4 (12.9) | 8 (25.8) | |||
| 10–50 | 13 (41.9) | 9 (29.0) | |||
| 50–90 | 7 (22.6) | 9 (29.0) | |||
| > 90 | 7 (22.6) | 5 (16.1) | |||
| Birth weight, p | 0.530 | ||||
| < 10 | 6 (21.4) | 2 (7.4) | |||
| 10–50 | 8 (28.6) | 9 (33.3) | |||
| 50–90 | 11 (39.3) | 13 (48.1) | |||
| > 90 | 3 (10.7) | 3 (11.1) | |||
| Preterm birth, < 37 wk | 4 (14.3) | 2 (7.4) | 0.669 | ||
| Cesarean section | 12 (38.7) | 12 (38.7) | 1.000 | ||
| Urine culture | 0.671 | ||||
| Escherichia coli | 29 (93.5) | 27 (87.1) | |||
| Othersa | 2 (6.5) | 4 (12.9) | |||
Data were shown as mean ± standard deviation or number (%).
aOther pathogens: Klebsiella pneumonia 3 (2 of them were in the formula-feeding group), Enterobacter cloacae 1, Enterococcus faecalis 1, Serratia fonticola 1.
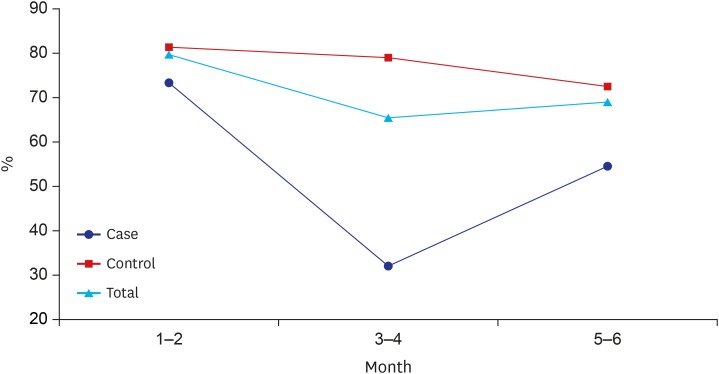
Fig. 1 shows the percentage of breastfeeding by age with the breastfeeding group including both exclusive and mixed-fed infants. The percentage of breastfeeding in the control group was significantly higher than that of the case group and decreased gradually with increasing age.
Fig. 1. Percentages of infants who received exclusive or mixed breastfeeding. The percentage in the control group decreased with increasing age, and was greater than in the case group.
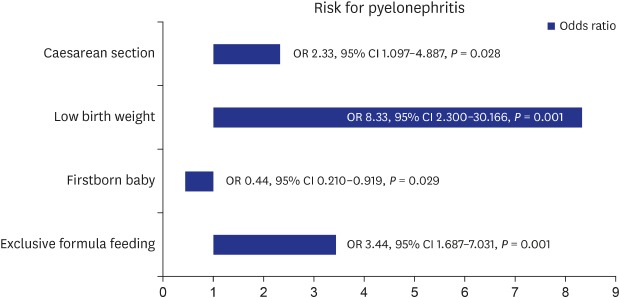
Table 6 presents the results of the logistic regression analysis of the variables which showed statistical significance in Table 4. OR for males was 2.23 with age not being significant in this analysis. In addition, the risk for APN was low (OR, 0.43; 95% CI, 0.210–0.919) for firstborn babies and 3.44 times higher for the exclusive formula feeding category compared with the exclusively breast-fed or mixed-fed categories (OR, 3.4; 95% CI, 1.687–7.031; P = 0.001). OR for preterm infants was high (4.47), but not statistically significant (P = 0.063). High ORs were also seen in infants with low birth weight and birth by caesarean section (OR, 8.33; 95% CI, 2.300–30.166 and OR, 2.32; 95% CI, 1.097–4.887, respectively).
Table 6. The risk for acute pyelonephritis by logistic regression analysis.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Sex, male | 2.229 | 1.029–4.831 | 0.042 |
| Age | 0.931 | 0.747–1.161 | 0.526 |
| Firstborn baby | 0.439 | 0.210–0.919 | 0.029 |
| Formula feeding only | 3.444 | 1.687–7.031 | 0.001 |
| Preterm birth | 4.470 | 0.923–21.640 | 0.063 |
| Low birth weight | 8.329 | 2.300–30.166 | 0.001 |
| Cesarean section | 2.316 | 1.097–4.887 | 0.028 |
OR = odds ratio, CI = confidence interval.
DISCUSSION
This study was conducted to determine whether breastfeeding truly has a protective effect against APN. As a result of the statistical comparisons between participants with and without APN, infants whose feeding methods switched from breast milk to formula milk before 6 months of age had a significantly higher risk for APN than breast-fed or mixed-fed infants. There have been several proposed theories explaining the differences in immunity between breastfed and formula-fed infants.12 First of all, secretory immunoglobulin A (SIgA), which is abundant in human breast milk, prevents mobilization and adherence of pathogens to intestinal and urinary epithelial cells3,13 with neutralizing toxins.14 The amount of SIgA in human breast milk is approximately 0.5–1.5 g/day, while an average adult produces 2.5 g/day of SIgA.15 SIgA cannot be transferred via the placenta directly and the serum immunoglobulin A (IgA) level of newborns is too low to be effective against infections; therefore, breastfeeding becomes the only pathway of transfer of maternal SIgA to newborns. It was found that IgA level was relatively higher in breastfed neonates' faeces, compared to that of formula-fed babies.16 Secondly, lactoferrin, another milk protein with microbicidal and immune-stimulatory effects, has been proven to prevent Escherichia coli UTI in mice.17 Likewise, “non-E.coli” bacteria were the most commonly found pathogens in the breastfeeding group in our study (Table 5), even though the number was too small to represent any statistical significance. There are also other potentially defensive components such as lysozyme, lactadherin, anti-secretory factor, immune receptors, and oligosaccharides in human breast milk.15 Another study proved that the size of the thymus was even larger in breastfed babies compared to that of non-breastfed babies at age 4 months, and decreased in size after discontinuing breastfeeding.18 This result implies that some of the immune-stimulatory components are present in human breast milk. Thirdly, the most beneficial long-term effect of breastfeeding is the increase in diversity of the intestinal microbiome. Breastfed infants had greater diversity of gut microbiome than non-breastfed infants,19 and immune development and reinforcement by gut microbes in the perinatal period are accelerated by various means, such as regulation of gene expression controlling immune reactions, promotion of maturation of intestinal mucosa and lymphoid tissue, stimulus of the intestinal epithelium to secrete antimicrobial peptides,20,21,22,23 and enhancement of colonization by beneficial lactobacilli and other probiotic microbes.24,25,26 The interesting point is that this initial colonization may persist throughout adulthood.27 This “gut microbiota theory” is also linked to the increased APN incidence of infants born by caesarean section.
The relationship between caesarean section and mother's APN diagnosis is well-known; however, the relationship between caesarean section and babies' potential APN diagnosis is not. There have been numerous studies suggesting that birth mode itself is related to babies' health, and even with morbidity in adulthood, such as that caused by allergic and/or autoimmune diseases.28,29 Intrauterine immune response during labour directly affects immune activation in the neonate by increasing cytokines and hormones.23 Subsequently, through exposure to the mother’s vaginal and faecal microbes, early acquisition of microbial diversity in the gastrointestinal environment promotes early immune development, as previously mentioned. These processes occur only in spontaneous vaginal delivery, not in elective caesarean section, therefore making a difference in the immune development of babies born by the two different birth modes.
There have been a few studies regarding the relationship between the benefits of breastfeeding and UTIs,30,31 but the diagnosis of a UTI is ambiguous, especially in infants, and may not be easily confirmed simply by the presence of fever and positive urine culture. Additionally, since anomalies of the genitourinary system, a very important risk factor, were hardly considered, we added another inclusion criterion (any abnormal nuclear medicine-radiologic finding consistent with APN) to clarify the diagnosis differentiating it from non-invasive infections in the urinary system or non-specific bacteriuria combined with fever. We also excluded any cases with recurrence of APN to minimize any compounding factors. We only evaluated feeding methods present at the time of the visits to minimize memory bias and to conduct a more objective study.
Considering that the average age of participants was 3.8 months, the proportions of each feeding method were consistent with the results of national surveys and other epidemiologic studies which showed that breastfeeding represented 29%–38% of each of the 3 groups at 4 months of age.32,33 As seen in Fig. 1, the decrease in percentages of breastfed infants by age showed a similar pattern in the general population, although it was remarkable that the percentage was approximately 10% higher than those in the national surveys. Presumably, parents who visited a tertiary medical centre for a scheduled vaccination with a healthy infant might be very meticulous regarding their babies' health, which indicates the possibility of selection bias in our control group.
Sex was one of the statistically significant variables with an OR of 2.23, and the male to female ratio was 2.4:1. This result was also consistent with previous findings, which adds reliability to our results.9,34
We analysed the factors thought to affect breastfeeding, such as age, prematurity, mode of delivery, and birth order (Table 5), but most of them did not show a direct relationship with breastfeeding itself. There was also an interesting national survey in the United States which indicated that feeding methods were independent of the number of siblings, which was consistent with our result.35 However, breastfed firstborn babies had lower risk factors (for APNs) than 2nd- or 3rd-born babies in our study, and we speculated as to why. In fact, whether the firstborn babies were only children or not could not be determined from this study. However, we could not find any scientific evidence for this matter and it might be one of the limitations of this study.
Small for gestational age status was another strong risk factor for APN, yet prematurity alone was not a statistically significant risk factor. There were several studies regarding UTI and very low birth weight, which were mostly combined with preterm birth. Our study differed from these studies in that it compared the two groups by weight percentile at the same age or at the same gestational age, and participants were not treated in intensive care units that typically utilize indwelling urine catheters. Even though our control group included babies born at > 35 weeks of pregnancy, there were only two babies who were born at < 34 weeks in the case group; thus, any differences would have been very minimal.
There were several limitations of this study, which were mostly of a retrospective nature. The first one was that while obesity has been occasionally mentioned as a risk factor for UTI,36 we could not evaluate it with weight-height percentile charts or body mass index because there were too few data regarding height values for the control group. Accurately measuring the heights of infants under 6 months of age is difficult in out-patient clinics and should be addressed in future studies. Secondly, “exclusive breastfeeding” was generally defined in many other studies as “a baby having never been fed with formulae milk in his/her entire life”. However, we defined “exclusive breastfeeding” as a baby being fed entirely with breast milk “during recent days or weeks”. The reasons for the different definitions were, first, that we were concerned about recall error and, second, the unique Korean culture called “Sanhujori”. It is “non-professional postpartum care after delivery”37 and usually caregivers working at the Sanhujori centres nurse the babies (with formulae milk, of course) instead of their mothers. More than 50 percent of babies were admitted to a Sanhujori centre.33 Therefore, if we use the term “exclusive breastfeeding” strictly, there would be very few “exclusively breastfed” babies in Korea. Thirdly, VCUG was not performed in every patient with APN, so vesicoureteral reflux and other urinary tract anomalies might not be thoroughly excluded. To compensate for this limitation, we excluded all participants who had recurrent APN even without any positive imaging study showing a genitourinary anomaly. The last limitation is that circumcision is known to have a relationship with the incidence of UTIs, but this retrospective study did not include these data.
As mentioned earlier, although many official committees recommend breastfeeding exclusively until 6 months of age, it can be quite challenging due to a number of social factors, such as the increase in number of women returning to work and the lack of education or facilities that provide comfortable breastfeeding environments.38 However, the benefits of breastfeeding have been proven in numerous studies, and individuals and society in general should promote this activity for the long-term health of all children.
In conclusion, exclusive formula feeding before 6 months of age was related to a higher risk for APN, which demonstrates that breastfeeding has a protective effect against APN. Firstborns also had a lower risk for developing APN. Other risk factors for APN included low birth weight and birth via caesarean section.
ACKNOWLEDGMENTS
The authors thank Mrs. Mi Yeon Lee of Kangbuk Samsung Hospital, for her statistical assistance for this study.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee YJ, Kim KM, Shim JW.
- Data curation: Lee YJ, Shim JW, Shim JY, Jung HL, Kim DS.
- Formal analysis: Lee YJ.
- Investigation: Lee YJ, Shim JW, Shim JY, Jung HL, Kim DS.
- Methodology: Lee YJ, Kim KM, Shim JW.
- Software: Lee YJ.
- Validation: Lee YJ, Kim KM.
- Writing - original draft: Lee YJ, Kim KM.
- Writing - review & editing: Lee YJ, Shim JW.
References
- 1.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;(8):CD003517. doi: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RA. Breastfeeding: benefits, risks and alternatives. Curr Opin Obstet Gynecol. 2000;12(6):519–524. doi: 10.1097/00001703-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Hanson LA. Protective effects of breastfeeding against urinary tract infection. Acta Paediatr. 2004;93(2):154–156. [PubMed] [Google Scholar]
- 4.Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 5.Horta BL, Victora CG. Geneva: World Health Organization; 2013. [Google Scholar]
- 6.Bachrach VR, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med. 2003;157(3):237–243. doi: 10.1001/archpedi.157.3.237. [DOI] [PubMed] [Google Scholar]
- 7.Long SS. Breastfeeding—protection against hospitalization in a developed country. J Pediatr. 2015;166(3):507–510. [Google Scholar]
- 8.Allen J, Hector D. Benefits of breastfeeding. N S W Public Health Bull. 2005;16(3-4):42–46. doi: 10.1071/nb05011. [DOI] [PubMed] [Google Scholar]
- 9.Chang SL, Shortliffe LD. Pediatric urinary tract infections. Pediatr Clin North Am. 2006;53(3):379–400. vi. doi: 10.1016/j.pcl.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean national growth charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51(1):1–25. [Google Scholar]
- 11.Lee JJ, Kim MH, Ko KO, Kim KA, Kim SM, Kim ER, et al. The study of growth measurements at different gestatioal ages of Korean newborn the survey and statistics. J Korean Soc Neonatol. 2006;13(1):47–57. [Google Scholar]
- 12.Winberg J, Wessner G. Does breast milk protect against septicaemia in the newborn? Lancet. 1971;1(7709):1091–1094. doi: 10.1016/s0140-6736(71)91836-8. [DOI] [PubMed] [Google Scholar]
- 13.Coppa GV, Gabrielli O, Giorgi P, Catassi C, Montanari MP, Varaldo PE, et al. Preliminary study of breastfeeding and bacterial adhesion to uroepithelial cells. Lancet. 1990;335(8689):569–571. doi: 10.1016/0140-6736(90)90350-e. [DOI] [PubMed] [Google Scholar]
- 14.Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: a review on its composition and bioactivity. Early Hum Dev. 2015;91(11):629–635. doi: 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Hanson LÅ, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol. 2002;7(4):275–281. doi: 10.1016/s1084-2756(02)90124-7. [DOI] [PubMed] [Google Scholar]
- 16.Jatsyk GV, Kuvaeva IB, Gribakin SG. Immunological protection of the neonatal gastrointestinal tract: the importance of breast feeding. Acta Paediatr Scand. 1985;74(2):246–249. doi: 10.1111/j.1651-2227.1985.tb10958.x. [DOI] [PubMed] [Google Scholar]
- 17.Håversen LA, Engberg I, Baltzer L, Dolphin G, Hanson LA, Mattsby-Baltzer I. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect Immun. 2000;68(10):5816–5823. doi: 10.1128/iai.68.10.5816-5823.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasselbalch H, Jeppesen DL, Engelmann MD, Michaelsen KF, Nielsen MB. Decreased thymus size in formula-fed infants compared with breastfed infants. Acta Paediatr. 1996;85(9):1029–1032. doi: 10.1111/j.1651-2227.1996.tb14211.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, Dahl DB, et al. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13(4):r32. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabeerdoss J, Ferdous S, Balamurugan R, Mechenro J, Vidya R, Santhanam S, et al. Development of the gut microbiota in southern Indian infants from birth to 6 months: a molecular analysis. J Nutr Sci. 2013;2:e18. doi: 10.1017/jns.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson-Chagoyán OC, Maldonado J, Gil A. Colonization and impact of disease and other factors on intestinal microbiota. Dig Dis Sci. 2007;52(9):2069–2077. doi: 10.1007/s10620-006-9285-z. [DOI] [PubMed] [Google Scholar]
- 22.Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. 2016;16(1):86. doi: 10.1186/s12876-016-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francino MP. Birth mode-related differences in gut microbiota colonization and immune system development. Ann Nutr Metab. 2018;73(Suppl 3):12–16. doi: 10.1159/000490842. [DOI] [PubMed] [Google Scholar]
- 24.Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Björkstén B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J Pediatr Gastroenterol Nutr. 2009;49(3):349–354. doi: 10.1097/MPG.0b013e31818f091b. [DOI] [PubMed] [Google Scholar]
- 25.Soto A, Martín V, Jiménez E, Mader I, Rodríguez JM, Fernández L. Lactobacilli and bifidobacteria in human breast milk: influence of antibiotherapy and other host and clinical factors. J Pediatr Gastroenterol Nutr. 2014;59(1):78–88. doi: 10.1097/MPG.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amdekar S, Singh V, Singh DD. Probiotic therapy: immunomodulating approach toward urinary tract infection. Curr Microbiol. 2011;63(5):484–490. doi: 10.1007/s00284-011-0006-2. [DOI] [PubMed] [Google Scholar]
- 27.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38(2):321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houghteling PD, Walker WA. Why is initial bacterial colonization of the intestine important to infants' and children's health? J Pediatr Gastroenterol Nutr. 2015;60(3):294–307. doi: 10.1097/MPG.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falakaflaki B, Ahmadiafshar A. Protective effect of breast milk against urinary tract infection. Hong Kong J Paediatr. 2008;13:235–238. [Google Scholar]
- 31.Mårild S, Hansson S, Jodal U, Odén A, Svedberg K. Protective effect of breastfeeding against urinary tract infection. Acta Paediatr. 2004;93(2):164–168. doi: 10.1080/08035250310007402. [DOI] [PubMed] [Google Scholar]
- 32.Lee F, Edmunds LS, Cong X, Sekhobo JP. Trends in breastfeeding among infants enrolled in the special supplemental nutrition program for women, infants and children - New York, 2002–2015. MMWR Morb Mortal Wkly Rep. 2017;66(23):610–614. doi: 10.15585/mmwr.mm6623a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HR. Breastfeeding trends, affecting factors and policy options for breastfeeding promotion in Korea. Health Welf Policy Forum. 2013;201:49–60. [Google Scholar]
- 34.Wu JH, Chiou YH, Chang JT, Wang HP, Chen YY, Hsieh KS. Urinary tract infection in infants: a single-center clinical analysis in southern Taiwan. Pediatr Neonatol. 2012;53(5):283–288. doi: 10.1016/j.pedneo.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Taylor JS, Geller L, Risica PM, Kirtania U, Cabral HJ. Birth order and breastfeeding initiation: results of a national survey. Breastfeed Med. 2008;3(1):20–27. doi: 10.1089/bfm.2007.0006. [DOI] [PubMed] [Google Scholar]
- 36.Mahyar A, Ayazi P, Gholmohammadi P, Moshiri SA, Oveisi S, Esmaeily S. The role of overweight and obesity in urinary tract infection in children. Infez Med. 2016;24(1):38–42. [PubMed] [Google Scholar]
- 37.Kim J. Survey on the programs of Sanhujori centers in Korea as the traditional postpartum care facilities. Women Health. 2003;38(2):107–117. doi: 10.1300/J013v38n02_08. [DOI] [PubMed] [Google Scholar]
- 38.Lauer EA, Armenti K, Henning M, Sirois L. Identifying barriers and supports to breastfeeding in the workplace experienced by mothers in the new hampshire special supplemental nutrition program for women, infants, and children utilizing the total worker health framework. Int J Environ Res Public Health. 2019;16(4):529. doi: 10.3390/ijerph16040529. [DOI] [PMC free article] [PubMed] [Google Scholar]