Abstract
Background & Aims
Surgical resection is the only potentially curative therapy for patients with biliary tract cancer (BTC), but 5-year survival rates after tumor resection have remained below 30%, corroborating the need for better stratification tools to identify the ideal surgical candidates. The soluble urokinase plasminogen activator receptor (suPAR) represents a mediator of inflammation and has been associated with distinct types of cancer. In this study, we evaluated a potential role of suPAR as a novel biomarker in patients undergoing BTC resection.
Methods
Tumor expression of uPAR was analyzed by immunohistochemistry in 108 BTC samples. Serum levels of suPAR were analyzed by ELISA in a training and validation cohort comprising a total of 117 patients with BTC and 76 healthy controls.
Results
High tumoral uPAR expression was associated with an adverse outcome after BTC resection. Accordingly, circulating levels of suPAR were significantly elevated in patients with BTC compared to healthy controls, as well as in patients with primary sclerosing cholangitis. Using a small training set, we established an optimal prognostic suPAR cut-off value of 3.72 ng/ml for patients with BTC. Importantly, preoperative suPAR serum levels above this cut-off value were associated with significantly impaired overall survival in both the training and validation cohort. Multivariate Cox-regression analysis including various clinicopathological parameters such as tumor stage, markers of inflammation and organ dysfunction, as well as tumor markers, revealed circulating suPAR levels as an independent prognostic marker following BTC resection. Finally, high preoperative suPAR levels were indicative of acute kidney injury after tumor resection.
Conclusion
Circulating suPAR represents a previously unrecognized biomarker in patients with resectable BTC, which might help to preoperatively identify the ideal candidates for liver surgery.
Lay summary
Surgical resection represents the only curative treatment option for patients with biliary tract cancer, but not all patients benefit to the same extent in terms of overall survival. Here, we provide evidence that serum levels of an inflammatory mediator (suPAR) are indicative of a patient's postoperative outcome and might thus help to identify the ideal surgical candidates.
Keywords: suPAR, biomarker, BTC, cholangiocarcinoma, CCA, acute kidney injury, CEA, CA19-9
Abbreviations: AKI, acute kidney injury; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BTC, biliary tract cancer; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRP, C-reactive protein; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IRS, immunoreactive score; OR, odds ratio; OS, overall survival; PSC, primary sclerosing cholangitis; suPAR, soluble uPAR; uPAR, urokinase plasminogen activator receptor
Graphical abstract
Highlights
-
•
Biliary tract cancer is associated with poor outcomes and increasing incidence.
-
•
Surgical resection is the only potentially curative treatment option for patients with biliary tract cancer.
-
•
The identification of ideal surgical candidates has remained challenging.
-
•
Circulating suPAR represents a novel diagnostic and prognostic biomarker in resectable patients.
-
•
SuPAR might be useful to identify patients with biliary tract cancer who will benefit most from tumor resection.
Introduction
Biliary tract cancer (BTC) comprises a group of epithelial tumors with cholangiocellular differentiation that has a very poor prognosis compared to other GI cancers.1 It represents the second most common primary liver malignancy and incidence rates, especially for intrahepatic cholangiocarcinoma (CCA), have constantly risen over the last years.2,3 Surgical resection has remained the cornerstone of a curative therapeutic approach, although liver transplantation can be considered for selected patients.1,3 If surgical tumor resection is not feasible, chemotherapy with gemcitabine and cisplatin represents the standard of care, resulting in a median overall survival (OS) of 10–12 months.[4], [5], [6] Importantly, the postoperative outcome following BTC resection is very heterogeneous and the majority of successfully resected patients (R0 resection) face disease recurrence,7 resulting in an overall 5-year survival rate of less than 30%.[8], [9], [10] Moreover, while current data suggest a benefit of more aggressive adjuvant treatment regimens in pancreatic cancer, which shares many clinicopathological similarities with BTC,11 data from lager clinical trials evaluating this concept in patients with BTC are still missing (NCT02170090). Thus, there is a vital need for novel preoperative stratification strategies to enable the identification of the ideal surgical candidates who will particularly benefit from extensive tumor resection.
The soluble urokinase plasminogen activator receptor (suPAR) represents the circulating form of the cell surface receptor uPAR (CD87), which is expressed by a variety of cells including epithelial or immune cells and has key functions in inflammation.12 SuPAR might also be an interesting circulating biomarker as recently shown for patients with sepsis13 and cancer.[14], [15], [16] In BTC, preliminary data described a correlation between high tumoral uPAR expression and tumor invasion and metastasis.17,18 However, there is insufficient data on a potential role of circulating suPAR as a biomarker in patients with BTC.
In this study, we therefore evaluated a potential role of circulating suPAR as a biomarker in 2 cohorts of patients undergoing BTC resection at our institution between 2011 and 2017.
Patients and methods
Study design and patient characteristics
This observational cohort study was performed to analyze circulating levels of suPAR and their potential diagnostic and/or prognostic role in patients with BTC undergoing surgical tumor resection. Patients with BTC who were admitted to the Department of Visceral and Transplantation Surgery at University Hospital RWTH Aachen for tumor resection were prospectively recruited in 2 cohorts between 2011 and 2017 and enrolled into this study (training cohort: n = 23 patients, validation cohort: n = 95 patients, see Table 1 and Table S1 for detailed patient characteristics). Blood samples were collected prior to surgery and 6–7 days after BTC resection, centrifuged for 10 min at 2,000 g, and serum samples were then stored at −80°C until use. The postoperative timepoint of serum collection was determined for the following reason: Several patients with BTC who were enrolled in this study were referred to our tertiary referral center from peripheral hospitals for surgery only. Because these patients are transferred back to the initial healthcare provider shortly after surgery on a regular basis, we had to collect postoperative serum samples at a rather early postoperative timepoint. Diagnosis of BTC was confirmed histologically in the resected tumor sample. As a control population we analyzed a total of 76 (training cohort: n = 10, validation cohort: n = 66) healthy, cancer-free blood donors with normal values for blood counts, C-reactive protein, kidney and liver function as well as a cohort of 11 patients with primary sclerosing cholangitis (PSC) without evidence of malignancy. Postoperative acute kidney injury (AKI) I was defined according to the KDIGO criteria.19 The study protocol was approved by the ethics committee of the University Hospital RWTH Aachen, Germany (EK 206/09) and conducted in accordance with the ethical standards laid down in the Declaration of Helsinki. Written informed consent was obtained from the patients.
Table 1.
Patient characteristics.
| Training cohort | Validation cohort | |
|---|---|---|
| BTC patients, n | 23 | 95 |
| Gender [%]: | ||
| Male-female | 73.9–26.1 | 52.0–48.0 |
| Age [years, median and range] | 65 [39–80] | 68 [37–84] |
| BMI [kg/m2, median and range] | 23.94 [20.05–36.73] | 26.17 [18.83–46.36] |
| Anatomic location of BTC [%] | ||
| Intrahepatic | 56.5 | 38.6 |
| Klatskin | 43.5 | 37.6 |
| Distal | – | 14.9 |
| Gallbladder | – | 8.9 |
| Staging [%] | ||
| T1-T2-T3-T4 | 30.0-25.0-30.0-15.0 | 6.0-39.8-36.1-18.1 |
| N0-N1 | 57.9-42.1 | 44.2-55.8 |
| M0-M1 | 94.1-5.9 | 78.6-21.4 |
| G2-G3 | 75.0-25.0 | 57.1-42.9 |
| R0-R1 | 90.0-10.0 | 60.3-39.7 |
| ECOG PS [%] | ||
| ECOG 0 | 40.0 | 52.6 |
| ECOG 1 | 40.0 | 29.2 |
| ECOG 2 | 20.0 | 8.2 |
| Healthy controls, n | 10 | 66 |
| Gender [%]: | ||
| Male-female | 70-30 | 78.6-21.4 |
| Age [years, median and range] | 46 [20-74] | 33 [19-65] |
BMI, body mass index; BTC, biliary tract cancer; ECOG PS, Eastern Cooperative Oncology Group performance status.
Immunohistochemistry of BTC tissue microarray
A tissue microarray (TMA) containing 108 cholangiocarcinoma samples was constructed as described previously and then stained for uPAR20 (see Table S2 for detailed characteristics). In more detail, 3 μm sections of formalin-fixed, paraffin-embedded tissue were obtained and the PT-Link module pH9, (DAKO, Glostrup, Denmark) was used for antigen retrieval. The primary anti-uPA Receptor antibody (1:1000, ab218106, Cambridge, UK) was incubated for 60 min at RT. Visualization was performed using Envision Flex kit (DAKO) according to the manufacturer's instructions. After counterstain with haematoxylin, sections were dehydrated and cover slipped. Staining was assessed using the immunoreactive score as described previously.21
Evaluation of suPAR serum levels
Serum levels of suPAR were analyzed by a commercial ELISA according to the manufacturer's instructions (Nr. A001, suPARnostic, ViroGates, Birkerød, Denmark). Standard laboratory parameters were measured in the laboratory center at University Hospital RWTH Aachen.
Evaluation of cytokine serum levels
Serum levels of IL-1β, IL-8, TNF-α, CCL3, CXCL5 and CX3CL1 were measured by multiplex immunoassay according to the manufacture's instruction using a Bio-Plex 200 system and Bio-Plex Manager 6.0 software (Bio-Plex Pro Human Chemokine Panel, #171AK99MR2, Bio Rad, Hercules, CA, USA).
Statistical analysis
Serum data are displayed as median and range. Shapiro-Wilk-Test was used to test for normal distribution. Non-parametric data were compared using the Mann-Whitney U test or the Kruskal-Wallis-Test for multiple group comparisons. Related samples were compared using the Wilcoxon signed-rank test. Correlation analyses were performed using the Spearman's correlation coefficient. Box plot graphics display the median, quartiles and ranges. We generated ROC curves by plotting the sensitivity against 1-specificity. Optimal cut-off values for ROC curves were calculated with the Youden-index method (YI = sensitivity + specificity - 1). The predictive value of circulating suPAR for the prediction of AKI was analyzed with a binary logistic regression model. The odds ratio (OR) and the 95% CI are shown. Kaplan-Meier curves display the impact of a specific parameter on the OS. The Log-rank test was used to test for statistical differences between subgroups. The ideal cut-off value for the identification of patients with an impaired OS was calculated using biometric software, which fits Cox proportional hazard models to the dichotomized survival status as well as survival time and defines the optimal cut-off as the point with the most significant split in the log-rank test.22 The prognostic value of variables was further tested by uni- and multivariate Cox-regression analyses. Parameters with a p value of <0.250 in univariate testing were included into multivariate testing. The hazard ratio (HR) and the 95% CI are displayed. All statistical analyses were performed with SPSS 23 (SPSS, Chicago, IL, USA).13 A p value of <0.05 was considered statistically significant (*p <0.05; **p <0.01; ***p <0.001).
Results
High tumoral uPAR expression is associated with poor prognosis in resectable BTC
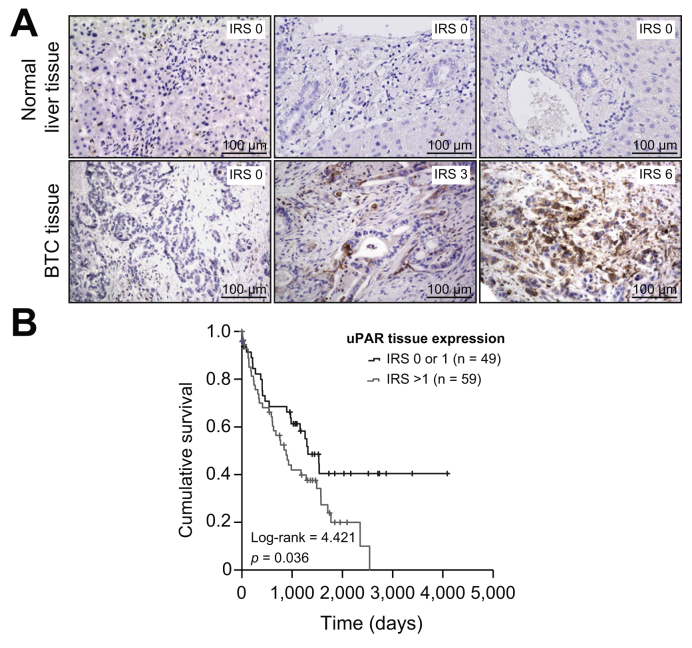
The cell surface receptor uPAR (CD87) represents a key mediator of inflammation and tumorigenesis and is associated with distinct pro-malignant characteristics including tumor cell migration, invasiveness and angiogenesis.23,24 We first evaluated tumoral expression levels of uPAR in 108 BTC tissue samples by immunohistochemistry (IHC, see Table S2 for detailed characteristics). While normal liver tissue samples (n = 108) showed no relevant uPAR expression in hepatocytes or bile duct cells (Fig. 1A, upper panel), we observed a positive uPAR immunoreactive score (IRS) of ≥1 in 79.7% of BTC samples with uPAR being expressed in tumor, immune and stromal cells (Fig. 1A, lower panel). We divided our cohort of patients into 2 groups based on their uPAR expression level (no relevant uPAR expression [IRS 0 or 1, n = 49 patients, Fig. 1A, lower panel, left] vs. relevant uPAR expression [IRS >1, n = 59 patients, Fig. 1A, lower panel, middle and right]) to evaluate a potential impact of tumoral uPAR expression on patient outcome. Interestingly, Kaplan-Meier curve analysis showed significantly impaired long-term survival for patients with relevant uPAR expression (IRS >1) compared to patients without relevant uPAR expression (IRS 0 or 1, Fig. 1B). The median OS was 890 days for patients with an IRS >1 vs. 1,321 days for patients with an IRS of 0 or 1, respectively. Of note, none of the patients with significant uPAR expression (IRS >1) reached long-term survival beyond 7 years (Fig. 1B). We finally evaluated if tumoral uPAR expression was associated with different tumor (TNM stage, tumor grading, resection status, perineural, vascular or lymphatic invasion and tumor localization) or patient characteristics (age, sex, BMI, and ECOG performance status). However, uPAR IRS levels were not significantly altered between these subgroups, suggesting that tumoral uPAR was expressed independently of these clinicopathological parameters in our cohort of patients with BTC (Fig. S1A-K).
Fig. 1.
Tumor expression of uPAR in biliary tract cancer.
(A) Representative images of uPAR expression in BTC tissue samples and normal liver tissue as detected by immunohistochemistry (200× magnification). While normal liver tissue shows no uPAR expression in hepatocytes or bile duct cells (upper panel), we subdivided patients with BTC into a group with no relevant uPAR expression (IRS: 0 or 1, lower panel, left) and a group with relevant uPAR expression (IRS >1, lower panel, middle and right). (B) Patients with BTC and a tumoral uPAR expression >1 had significantly impaired long-term survival. BTC, biliary tract cancer; IRS, immunoreactive score; uPAR, urokinase plasminogen activator receptor.
Circulating levels of suPAR are elevated in patients with BTC and predict outcome
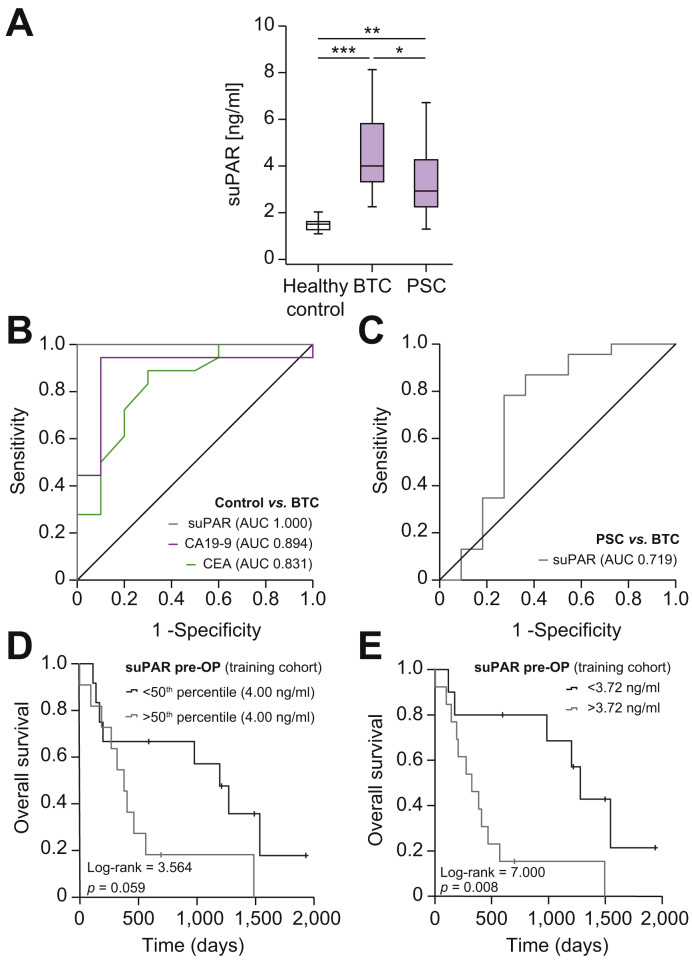
As tissue-based biomarkers like tumoral uPAR expression level have certain clinical limitations, we analyzed whether suPAR levels in the serum might also reflect patient prognosis. We first compared circulating levels of suPAR between a training cohort of patients with BTC (n = 23), healthy control samples (n = 10) and patients with PSC (n = 11) who showed no evidence of cancer. In this analysis, patients with BTC had significantly elevated serum levels of suPAR compared to both control populations (Fig. 2A). In a ROC curve analysis, circulating suPAR showed an AUC value of 1.0 which was higher than the standard diagnostic markers carcinoembryonic antigen (CEA, AUCCEA: 0.831) and carbohydrate antigen 19-9 (CA19-9, AUCCA19-9: 0.894, Fig. 2B). At the optimal diagnostic cut-off value of 2.14 ng/ml that we established using the Youden-index, circulating suPAR showed a sensitivity and specificity of 100% each to discriminate between patients with BTC and healthy controls. Interestingly, suPAR levels were also significantly elevated in patients with PSC compared to healthy controls, although this analysis was only performed in a small cohort of 11 patients with PSC (Fig. 2A). Nevertheless, circulating suPAR still had diagnostic potential to discriminate between patients with BTC and PSC, with an AUC of 0.719 (Fig. 2C). The diagnostic sensitivity and specificity in this setting was 78.3% and 72.7% using an optimal cut-off value of 3.25 ng/ml.
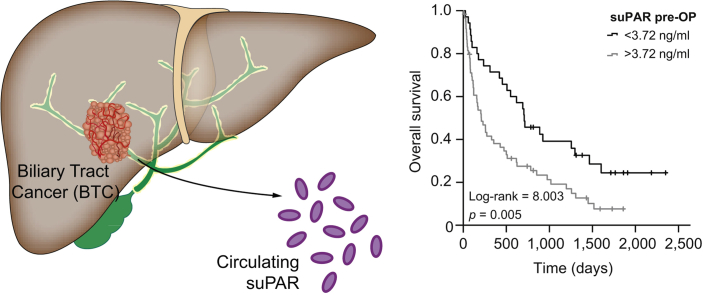
Fig. 2.
Circulating suPAR in patients with biliary tract cancer – results from the training cohort.
(A) Patients with BTC from the training cohort have significantly elevated serum suPAR levels compared to healthy controls and patients with PSC. (B) Circulating suPAR has an AUC value of 1.0 for the discrimination between patients with BTC and healthy controls. (C) suPAR has a diagnostic potential to discriminate between patients with BTC and PSC (AUC: 0.719). (D) Patients with BTC and initial suPAR levels above the 50th percentile show a trend towards an impaired postoperative outcome. (E) At the optimal cut-off value of 3.72 ng/ml, preoperative suPAR serum levels identify patients with BTC who have significantly impaired postoperative overall survival. *p <0.05; **p <0.01; *** p <0.001. BTC, biliary tract cancer; PSC, primary sclerosing cholangitis; suPAR, soluble urokinase plasminogen activator receptor.
Based on this finding, we next hypothesized that preoperative suPAR levels might be indicative of postoperative outcomes, so we compared the OS of patients with high or low suPAR levels using the median suPAR concentration of the training cohort (4.0 ng/ml) as a cut-off value. Interestingly, patients with initial suPAR levels above the 50th percentile showed a trend towards an impaired postoperative outcome (p = 0.059, Fig. 2D). To further increase the prognostic relevance of circulating suPAR, we established an ideal prognostic cut-off value by fitting Cox proportional hazard models to the survival status and time as recently described,22 which revealed a suPAR concentration of 3.72 ng/ml as an ideal discriminative cut-off value. Patients with BTC and preoperative suPAR levels below 3.72 ng/ml had a significantly better outcome, with a median OS of 1,280 days compared to patients with suPAR levels above the cut-off value who had a median OS of only 326 days (Fig. 2E). Importantly, no patients with a preoperative suPAR level above the ideal cut-off value of 3.72 ng/ml reached a 5-year OS (Fig. 2E). In line with this finding, univariate Cox-regression analysis revealed preoperative suPAR serum levels as a prognostic parameter for OS after tumor resection, with an HR of 1.392 (95% CI 1.004–1.929, p = 0.047). The patient number in this training cohort was too small to perform multivariate analysis.
Validation of the prognostic relevance of circulating suPAR
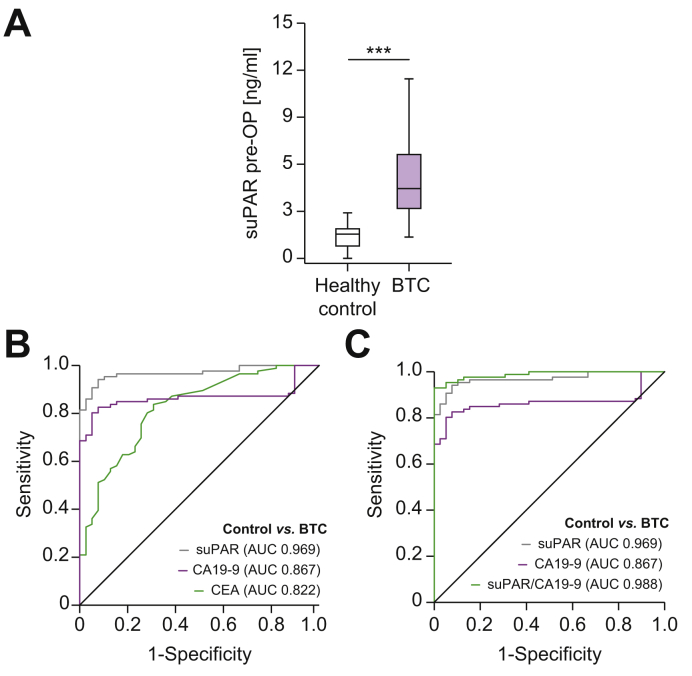
Based on these promising results of a potential role of circulating suPAR in patients with resectable BTC from our training cohort, we next established a validation cohort of 95 patients with BTC (not including patients from the training cohort) who underwent surgical tumor resection. Like the training cohort, patients with BTC in the validation cohort showed significantly higher initial serum levels of suPAR compared to healthy controls (Fig. 3A). The median preoperative suPAR level was 4.45 ng/ml in patients with BTC and 1.55 ng/ml in the control group. In the validation cohort, circulating suPAR levels showed an AUC value of 0.969 for the discrimination between patients with BTC and healthy controls (Fig. 3B), which again was higher than the most commonly used biomarkers CEA (AUCCEA: 0.822) and CA19-9 (AUCCA19-9: 0.867). Using the optimal diagnostic cut-off value that we established in the training cohort (2.14 ng/ml), circulating suPAR showed a diagnostic sensitivity and specificity of 95.3% and 89.7%, respectively. Importantly, the diagnostic power was highest when we combined serum suPAR and CA19-9 levels, showing and AUC value of 0.988 in ROC curve analysis (Fig. 3C). The combination of these 2 biomarkers increased the diagnostic sensitivity to 95.3% and the specificity to 94.9%.
Fig. 3.
Serum levels of suPAR are elevated in patients with biliary tract cancer.
(A) Patients with BTC (validation cohort) show significantly elevated serum suPAR levels compared to healthy control samples. (B) Circulating suPAR reveals an AUC value of 0.969 regarding the discrimination between patients with BTC and healthy controls. (C) The diagnostic power is highest when combining suPAR and CA19–9 levels. ***p <0.001. BTC, biliary tract cancer; CA19-9, carbohydrate antigen 19-9; suPAR, soluble urokinase plasminogen activator receptor.
We then compared circulating levels of suPAR between patients with different tumor and patient characteristics to evaluate whether suPAR might reflect individual tumor properties or disease stage and to gain further insight into functional regulation mechanisms that are involved in the upregulation of circulating suPAR in patients with BTC. SuPAR serum levels were unaltered between different subtypes of BTC (intrahepatic CCA, Klatskin tumors, distal CCA, and gallbladder carcinoma, Fig. S2A) patients with T1- to T4-tumor stage (Fig. S2B), with or without lymph node involvement (Fig. S2C) as well as non-metastasized or metastasized patients who were still resectable (Fig. S2D). Moreover, initial (pre-surgery) suPAR levels did not differ between patients achieving R0 or R1 tumor resection status (Fig. S2E) or male and female patients (Fig. S2F). We did observe significantly elevated levels of circulating suPAR in patients with poorly differentiated BTC (G3) compared to moderately differentiated tumors (G2, Fig. S2G). Moreover, we found no significant differences in circulating suPAR between patients with histologically relevant signs of liver inflammation or liver fibrosis (Desmet classification ≥ grade25) in the resected non-tumorous liver samples and patients without any signs of chronic liver disease, arguing that preexisting liver damage did not influence circulating suPAR levels (Fig. S2H). Although the cohorts for uPAR IHC analysis and suPAR serum analysis were not identical, there was an overlap of 23 individuals between the IHC cohort and the suPAR validation cohort. In this exploratory setting, we compared circulating suPAR levels in patients with relevant (IRS >1) or irrelevant (IRS 0 or 1) tumoral uPAR expression. Interestingly, we observed a strong trend towards higher suPAR levels in patients with a relevant uPAR expression, though statistical significance was not reached (p = 0.082, Fig. S1L). Next, we performed correlation analyses between initial suPAR serum levels and standard laboratory parameters of liver function, cholestasis and inflammation to identify further drivers of elevated suPAR levels in our cohort of patients with BTC. Interestingly, we observed a positive correlation between suPAR serum levels and bilirubin, alkaline phosphatase (ALP) and C-reactive protein (CRP) as indicators of cholestasis and inflammation, respectively. Gamma glutamyltransferase (GGT) showed a strong trend towards a positive correlation, but serum alanine aminotransferase (ALT) levels did not, suggesting that cholestasis and cholestasis-driven inflammation but not acute hepatocyte damage may represent a driver of elevated serum suPAR levels before tumor resection (Table S3).
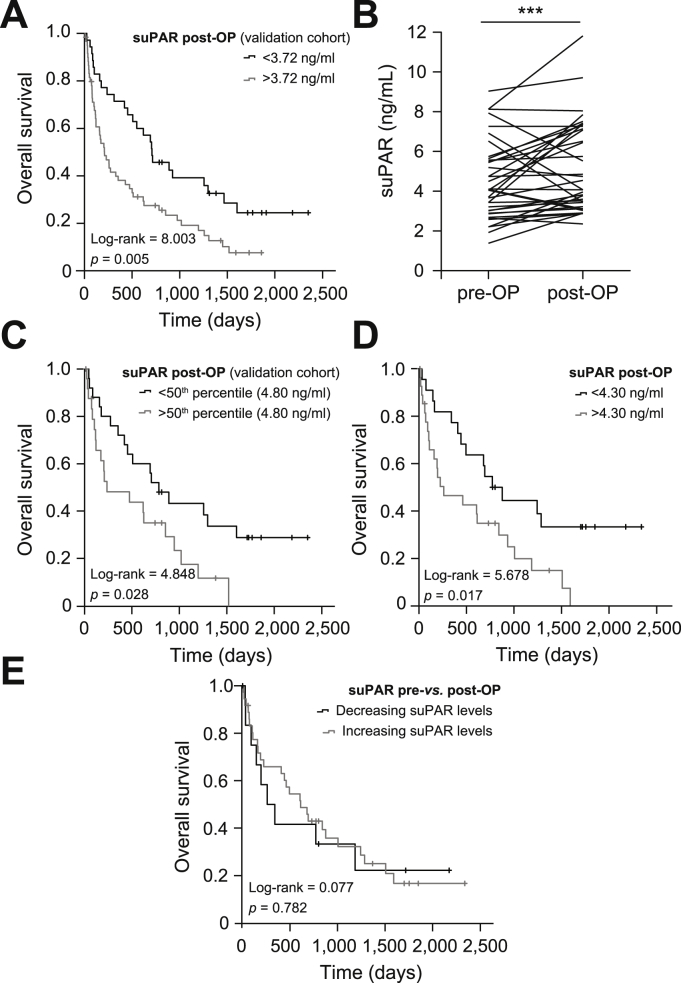
We next aimed to validate the prognostic relevance of circulating suPAR that we had established in the training cohort (3.72 ng/ml) in the present validation cohort. In this analysis, the subgroup of patients with serum suPAR concentrations above the ideal prognostic cut-off value showed a significantly impaired OS (Fig. 4A). The median OS in the high suPAR group was 204 days compared to 703 days for patients with suPAR serum levels below 3.72 ng/ml. To further elaborate the prognostic impact of preoperative suPAR levels on patients' postoperative outcomes and to exclude potential confounders, we performed uni- and multivariate Cox-regression testing. We included a variety of potential prognostic factors including established tumor markers (CA19-9), markers of systemic inflammation (CRP, leucocyte count) and impaired kidney or liver function (creatinine, aspartate aminotransferase, ALT, GGT, ALP) as well as tumor- and patient-specific characteristics (T-stage, ECOG PS, age, BMI, sex) into univariate analyses (Table 2). Subsequently, we included parameters with a p value <0.250 in univariate analyses into multivariate Cox-regression analysis which revealed initial suPAR concentrations to be an independent prognostic factor in the context of BTC resection (Table 2). Finally, we measured serum levels of CXCL5, CX3CL1 and IL-8, representing cytokines with a known pro-malignant function in BTC cancer and other malignancies[26], [27], [28], [29] to gain further information on the association between elevated suPAR levels and impaired outcomes. Importantly, we observed a positive correlation between all 3 cytokines and circulating suPAR levels, suggesting that suPAR might also reflect a pro-malignant tumor biology in patients with BTC (Table S3).
Fig. 4.
Elevated levels of circulating suPAR are associated with an impaired overall survival after BTC resection.
(A) Patients with BTC (validation cohort) and a preoperative suPAR level above 3.72 ng/ml have significantly impaired overall survival. (B) Postoperative suPAR levels are significantly higher compared to preoperative levels. (C) Postoperative suPAR serum concentrations above the 50th percentile (4.80 ng/ml) are associated with impaired long–term survival. (D) At the ideal cut-off value (4.30 ng/ml), postoperative suPAR levels significantly discriminate between long–term survivors and patients who died early. (E) The individual course of suPAR serum levels before and after surgery does not allow a prediction of overall survival. ***p <0.001. BTC, biliary tract cancer; suPAR, soluble urokinase plasminogen activator receptor.
Table 2.
Uni- and multivariate Cox-regression analyses for the prediction of overall survival.
| Parameter | Univariate Cox regression |
Multivariate Cox regression |
||
|---|---|---|---|---|
| p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | |
| suPAR pre–OP | <0.001 | 1.098 (1.047–1.150) | 0.041 | 1.050 (1.002–1.100) |
| CA19–9 | <0.001 | 1.000 (1.000–1.000) | 0.022 | 1.000 (1.000–1.000) |
| Leukocyte count | 0.245 | 1.037 (0.975–1.104) | 0.770 | 0.986 (0.900–1.081) |
| CRP | <0.001 | 1.011 (1.005–1.016) | 0.125 | 1.007 (0.998–1.017) |
| Platelets | 0.731 | 1.000 (0.999–1.002) | ||
| Potassium | 0.482 | 1.190 (0.773–1.932) | ||
| AST | 0.467 | 0.999 (0.998–1.001) | ||
| ALT | 0.355 | 0.999 (0.997–1.001) | ||
| Bilirubin | 0.601 | 0.983 (0.921–1.049) | ||
| ALP | 0.707 | 1.000 (0.999–1.001) | ||
| GGT | 0.471 | 1.000 (0.999–1.000) | ||
| Creatinine | 0.411 | 1.350 (0.660–2.761) | ||
| BMI | 0.336 | 1.021 (0.978–1.067) | ||
| ECOG PS | 0.132 | 1.325 (0.919–1.910) | 0.649 | 1.124 (0.680–1.859) |
| Age | 0.021 | 1.025 (1.004–1.048) | 0.231 | 1.018 (0.988–1.049) |
| Sex | 0.908 | 1.027 (0.658–1.603) | ||
| T–stage | 0.040 | 1.429 (1.016–2.010) | 0.030 | 1.560 (1.045–2.328) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CA19–9, carbohydrate antigen 19–9, CRP, C–reactive protein; ECOG PS, Eastern Cooperative Oncology Group performance status; GGT, gamma–glutamyltransferase; suPAR, soluble urokinase plasminogen activator receptor.
Postoperative suPAR concentrations predict patient outcomes after tumor resection
For 50 patients from the validation cohort, postoperative suPAR levels were available at 1 week after tumor resection. When postoperative suPAR concentrations were compared with the respective preoperative values, we observed a significant increase after tumor resection (mean value: 4.05 ng/ml vs. 4.74 ng/ml, p = 0.001, Fig. 4B), possibly related to surgery (trauma)-related inflammation.30 Again, post-surgery suPAR concentrations did not significantly differ among patients with different TNM stages (Fig. S3A-C), male and female patients (Fig. S3D) and patients with poorly or moderately differentiated tumors (Fig. S3E). However, we observed significantly higher postoperative suPAR levels in patients with residual tumor cells on microscopy (R1 status, Fig. S3F).
Hypothesizing that suPAR levels after tumor resection might also be indicative of patients' postoperative outcome, we compared the OS of patients with high or low postoperative suPAR concentrations (above or below the 50th percentile). Interestingly, Kaplan-Meier curve analysis revealed a significantly impaired long-term survival for patients with high postoperative suPAR serum levels (Fig. 4C). We again defined an optimal prognostic cut-off value for postoperative suPAR levels of 4.3 ng/ml,22 that further increased the prognostic power of postoperative suPAR concentrations (Fig. 4D). These results were corroborated by Cox-regression analysis which identified postoperative suPAR levels above 4.30 ng/ml as a negative prognostic factor, with a HR of 2.230 (95% CI 1.134–4.386, p = 0.020). Next, we investigated whether the individual course of circulating suPAR before and after surgery was associated with the patients' OS. However, we observed no significant difference in OS between patients with increasing or decreasing suPAR levels 1 week after tumor resection (Fig. 4E).
Finally, we aimed at further investigating potential functional drivers of elevated postoperative suPAR levels. Hypothesizing that systemic inflammation might be of relevance in this setting, we measured circulating levels of pro-inflammatory cytokines (IL-1β, TNF-α, CCL3 and IL-8) after tumor resection. Importantly, we observed a strong positive correlation between postoperative suPAR levels and lL-1β, TNF-α, CCL3 and IL-8 serum levels (Fig. S4A-D), arguing that systemic inflammation is one of the key drivers of elevated suPAR levels 1 week after BTC resection.
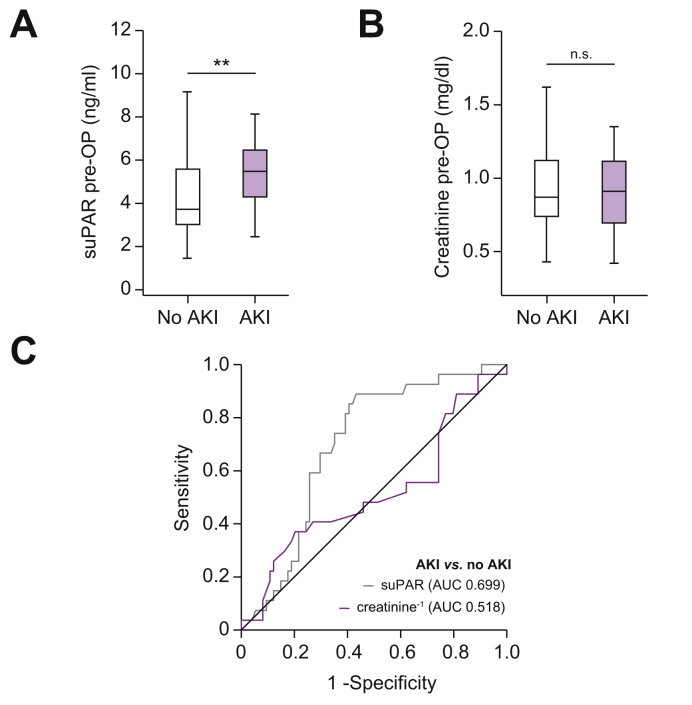
High baseline suPAR serum levels indicate AKI after BTC resection
Circulating suPAR has recently been associated with chronic kidney disease31 and elevated suPAR serum levels were suggested to predict impaired renal function following open cardiac surgery.32 Hence, we evaluated if circulating levels of suPAR might be indicative of the occurrence of AKI following tumor resection. According to the current KDIGO guidelines,19 a total of 28 patients fulfilled the criteria of AKI stage I after tumor resection. Interestingly, these patients displayed significantly elevated baseline levels of circulating suPAR compared to patients with an unimpaired postoperative renal function (Fig. 5A). In contrast, circulating creatinine levels did not significantly differ between patients with or without postoperative AKI (Fig. 5B). Preoperative suPAR levels outperformed creatinine (AUC1/creatinine: 0.518) for the discrimination between AKI and non-AKI patients in ROC curve analysis, showing an AUC value of 0.699 (Fig. 5C). At a cut-off value of 4.03 ng/ml, circulating suPAR revealed a diagnostic sensitivity and specificity of 88.9% and 56.8% for the occurrence of postoperative AKI. This finding was corroborated by binary logistic regression analysis that identified initial suPAR (OR 1.340; 1.071–1.676; p = 0.010) but not creatinine (OR 1.025; 0.249-4.226; p = 0.972) levels as a predictor of postoperative AKI.
Fig. 5.
Initial suPAR serum levels can predict AKI after BTC resection.
(A) Initial suPAR serum levels are significantly higher in patients with BTC and postoperative AKI I° when compared to non-AKI patients. (B) Initial serum creatinine levels are unaltered between non-AKI and AKI patients. (C) Initial suPAR levels show a superior AUC value of 0.669 compared to creatinine levels (AUC 0.518) for the discrimination between AKI and non-AKI patients. **p <0.01. AKI, acute kidney injury; BTC, biliary tract cancer; suPAR, soluble urokinase plasminogen activator receptor.
Hypothesizing that postoperative AKI might also be a reason for elevated suPAR levels after tumor resection, we finally compared postoperative suPAR levels between patients with and without AKI. Interestingly, this analysis revealed significantly higher postoperative suPAR levels in patients who presented with an impaired renal function after surgery (Fig. S4E), suggesting that AKI was also a driver of elevated suPAR levels at day 6 or 7 after tumor resection.
Discussion
BTC represents a rare but highly aggressive type of cancer.1 Surgical tumor resection is the only available curative treatment option but the long-term outcome of successfully resected patients is often limited due to the high risk of tumor recurrence.7 Nevertheless, the clinically available tumor markers CEA and CA19-9 are mostly used to monitor tumor response to chemotherapy, and the existing preoperative stratification tools often fail to identify the ideal candidates for a surgical treatment approach.33,34 Herein, we showed that serum levels of suPAR are elevated in 2 independent cohorts of patients with BTC who underwent surgical tumor resection compared to patients with PSC and healthy controls. Moreover, our data provided evidence that suPAR serum levels before and after surgery might yield valuable information on the patients' postoperative outcome. As such, elevated initial suPAR serum levels above a calculated optimal cut-off value (3.72 ng/ml) identified a high-risk subgroup of patients with BTC who had a substantially impaired long-term prognosis, with a median OS of only 204 days (see Fig. 4). The prognostic relevance of circulating suPAR was further corroborated by uni- and multivariate Cox-regression analyses, including various clinical and pathophysiological confounders (see Table 2). Moreover, we established a predictive role of preoperative suPAR regarding the occurrence of postoperative AKI following extensive tumor resection. Finally, we could show that a high tumoral expression of uPAR, the membrane bound source of circulating suPAR, was also associated with an impaired postoperative outcome.
While the membrane bound plasminogen activator receptor (uPAR, CD87) is expressed on various cell types such as immune and epithelial cells,35 up to now there was only limited data on circulating suPAR in BTC. A high expression of uPA in human CCA tissue samples correlated with lymphatic invasion and metastasis and a high uPA/uPAR expression in CCA cell lines (HuCCA-1 and KKU-M213) was associated with increased invasiveness in vitro.18 Moreover, stromal expression of uPAR was linked to an invasive growth of cancer cells into the surrounding tissue in resected CCA tumor samples.36 In line with these findings arguing for a pro-malignant role of uPAR in BTC, we observed significantly impaired postoperative long-term survival in patients with relevant tumoral uPAR expression (IRS >1) compared to patients with no relevant uPAR expression (IRS 0 or 1, see Fig. 1). In terms of a potential association between increased tumoral uPAR expression and elevated levels of circulating suPAR, experimental data suggest a positive correlation between the total tumor volume and plasma suPAR levels.37 We observed a strong trend towards higher suPAR serum levels in patients with a relevant tumoral uPAR expression (IRS >1) in a smaller subset of patients with available uPAR and suPAR data (see Fig. S1L). We also identified cholestasis and cholestasis-driven inflammation as drivers of elevated suPAR levels before tumor resection. Moreover, an upregulated shedding of uPAR was described for several tumor entities such as breast cancer,38 and was suggested to reflect increased inflammation39 which is commonly observed in BTC.40 We also observed higher suPAR serum levels in patients with poorly differentiated tumors (G3) compared to moderately differentiated tumors (G2), which might reflect the more inflammatory phenotype of poorly differentiated tumors and their microenvironment.41 Importantly, suPAR serum concentrations were significantly higher after tumor resection, suggesting that tumor-related uPAR expression did not represent the only source of circulating suPAR in patients with BTC. While patients with complete tumor resection (R0) had significantly lower postoperative suPAR levels compared to R1-resected patients, it is likely that elevated suPAR levels after tumor resection were also driven by postoperative inflammation, as well as impaired renal function after tumor resection. As such, postoperative suPAR levels correlated with pro-inflammatory cytokines such as IL-1β, TNF-α, CCL3 and IL-8 and patients who developed postoperative AKI had higher serum suPAR levels after surgery. Serum measurements at later timepoints are warranted to fully elucidate the longitudinal course of circulating suPAR after tumor resection. The molecular link between a high tumoral uPAR expression and/or elevated levels of circulating suPAR and a poor postoperative outcome remains unknown. It was demonstrated that uPAR influences the phosphorylation state and signaling activity of the epidermal growth factor receptor, thereby providing cancer cells with a proliferative advantage.42 Other data provided evidence that both uPAR and suPAR are capable of downregulating the tumor suppressor PTEN in endothelial cells to support angiogenesis.43 In our study, we found a positive correlation between initial suPAR levels and cytokines (CXCL5, CX3CL1 and IL-8) with a known pro-malignant function in BTC and other malignancies,[26], [27], [28], [29] suggesting that circulating suPAR might also reflect a more aggressive individual tumor biology. However, functional data in the context of BTC are still limited and further molecular studies are warranted to elucidate the underlying pathophysiology of increased uPAR/suPAR expression and impaired survival in patients with BTC.
Our data further support the predictive relevance of initial suPAR levels with respect to the occurrence of AKI after tumor resection. AKI represents a common postoperative complication after abdominal surgery and liver resection in particular and is associated with higher postoperative morbidity and mortality.44 The prediction of postoperative AKI, however, has remained challenging, and creatinine, the most established laboratory marker to monitor renal function, only has a limited potential to predict AKI.45 In our study, patients with preoperative suPAR levels above 4.03 ng/ml had a significantly higher likelihood of developing postoperative AKI. This finding is in line with previous studies arguing for a potential predictive function of suPAR for AKI following major abdominal or thoracic surgery,32 as well as for a chronic deterioration of renal function in patients with cardiovascular events.31 On a pathophysiological level, it was recently shown that uPAR/suPAR activates integrin αvβ3 on glomerular podocytes leading to proteinuria and focal segmental glomerulosclerosis.46 Together, circulating suPAR could thus help to preoperatively identify certain patients with BTC who are susceptible to AKI after tumor resection. This could in turn trigger specific clinical measures such as perioperative liquid substitution to improve patient outcomes.
The clinical implementation of circulating biomarkers into patient selection algorithms has remained difficult and it is unlikely that a patient who was considered a surgical candidate based on imaging and his/her performance status is denied surgery because of an elevated preoperative suPAR level. We suggest that preoperative suPAR measurements might prove useful to identify a subgroup of high-risk patients with BTC that should be given particular attention, e.g. in terms of clinical measurement against postoperative AKI. One could discuss a more aggressive perioperative treatment for patients with high-risk BTC. While more aggressive adjuvant chemotherapy regimens are currently being investigated in clinical trials (e.g. ACTICCA trial, NCT02170090), biomarkers such as suPAR might help to identify patients who would particularly benefit from perioperative treatment. Regarding a potential implementation of suPAR as a diagnostic marker, it should be stated that suPAR does not represent a specific marker for BTC but was also shown to be elevated in patients with other tumor entities14,15 as well as non-malignant conditions.31,47 We found elevated levels of suPAR in patients with PSC and no signs of malignant transformation. It is thus likely that suPAR might be implemented into diagnostic algorithms in combination with other markers rather than being used as a stand-alone screening parameter. As an example, we could show that the combination of suPAR and CA19-9 had a higher diagnostic sensitivity and specificity compared to either marker alone. However, before suPAR might become a diagnostic parameter in clinical routine, a standardized diagnostic cut-off value needs to be established and validated, as individual studies, including ours, have reported various cut-offs which are mainly due to differing suPAR serum levels in control populations.14,15
Our study has some limitations. Although the single center design of our study implicates a comparability of the 2 cohorts with respect to eligibility criteria and surgical procedures, this design warrants a confirmation in a multicenter approach. Moreover, our study did not include alternative treatment approaches such as chemotherapy or loco-regional therapies, but only investigated patients undergoing surgical resection of BTC. Thus, we cannot answer the decisive question regarding whether a patient with BTC and an initial suPAR serum level above our ideal prognostic cut-off value might have had a similar or even better outcome if treated differently. On a methodological level, our study lacks full-scale correlation analysis between tumoral uPAR expression and circulating suPAR levels, and the presented IHC data do not allow a reliable discrimination between uPAR expression in tumor, immune or stromal cells, which could be achieved using multiparameter staining in future analysis. Moreover, the control cohorts (healthy controls and patients with PSC) were not matched for age, sex or BMI. Thus, further multicenter clinical trials including different treatment modalities and larger patient numbers are needed to gain full insight into the pathophysiological and clinical importance of uPAR/suPAR in the context of BTC.
Financial support
Work in the lab of T.L. was funded from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program through the ERC Consolidator Grant PhaseControl (Grant Agreement n° 771083). The lab of T.L. was further supported by the German Cancer Aid (Deutsche Krebshilfe 110043 and a Mildred-Scheel-Professorship), the German-Research-Foundation (SFB-TRR57/P06 and LU 1360/3-1), the Ernst-Jung-Foundation Hamburg, the IZKF (interdisciplinary centre of clinical research) Aachen and a grant from the medical faculty of the RWTH Aachen. The suPAR ELISA kits were kindly provided by Virogates (Denmark).
Authors’ contributions
TL, SHL, CR and UPN designed the study; UPN, TFU, GL, JB, AAR, SS and PHA recruited and operated on patients; SHL and AB performed experiments; SHL, JNK and AB performed statistical analysis and generated figures and tables; TLo and TR performed IHC analysis; CT, FT, JNK, JG, PP, JFB and AK provided intellectual input; SHL and TL drafted the manuscript; all authors approved the paper.
Conflict of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100080.
Contributor Information
Ulf P. Neumann, Email: uneumann@ukaachen.de.
Tom Luedde, Email: tluedde@ukaachen.de.
Supplementary data
References
- 1.Razumilava N., Gores G.J. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergquist A., von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2017;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle J., Wasan H., Palmer D.H., Cunningham D., Anthoney A., Maraveyas A. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 5.Valle J.W., Furuse J., Jitlal M., Beare S., Mizuno N., Wasan H. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R., Sendilnathan A., Siddiqi N.I., Gulati S., Ghose A., Xie C. Advanced biliary tract cancer: clinical outcomes with ABC-02 regimen and analysis of prognostic factors in a tertiary care center in the United States. J Gastrointest Oncol. 2016;7:996–1003. doi: 10.21037/jgo.2016.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komaya K., Ebata T., Yokoyama Y., Igami T., Sugawara G., Mizuno T. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163:732–738. doi: 10.1016/j.surg.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Kang M.J., Jang J.-Y., Chang J., Shin Y.C., Lee D., Kim H.B. Actual long-term survival outcome of 403 consecutive patients with hilar cholangiocarcinoma. World J Surg. 2016;40:2451–2459. doi: 10.1007/s00268-016-3551-9. [DOI] [PubMed] [Google Scholar]
- 9.Bi C., Wang L.M., An S.L., Huang J., Feng R.M., Wu F. Analysis of the survival of 123 patients with intrahepatic cholangiocarcinoma after surgical resectionZhonghua Zhong Liu Za Zhi. 2016;38:466–471. doi: 10.3760/cma.j.issn.0253-3766.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Buettner S., Margonis G.A., Kim Y., Gani F., Ethun C.G., Poultsides G. Conditional probability of long-term survival after resection of hilar cholangiocarcinoma. HPB (Oxford) 2016;18:510–517. doi: 10.1016/j.hpb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 12.Thunø M., Macho B., Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. doi: 10.3233/DMA-2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch A., Voigt S., Kruschinski C., Sanson E., Dückers H., Horn A. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. doi: 10.1186/cc10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidan E., Mentese A., Ozdemir F., Deger O., Kavgaci H., Caner Karahan S. Diagnostic and prognostic significance of CA IX and suPAR in gastric cancer. Med Oncol. 2013;30:540. doi: 10.1007/s12032-013-0540-9. [DOI] [PubMed] [Google Scholar]
- 15.Chounta A., Ellinas C., Tzanetakou V., Pliarhopoulou F., Mplani V., Oikonomou A. Serum soluble urokinase plasminogen activator receptor as a screening test for the early diagnosis of hepatocellular carcinoma. Liver Int. 2015;35:601–607. doi: 10.1111/liv.12705. [DOI] [PubMed] [Google Scholar]
- 16.Loosen S.H., Tacke F., Binneboesel M., Leyh C., Vucur M., Heitkamp F. Serum levels of soluble urokinase plasminogen activator receptor (suPAR) predict outcome after resection of colorectal liver metastases. Oncotarget. 2018;9(43):27027–27038. doi: 10.18632/oncotarget.25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue S.-Q., Yang Y.-L., Zhou J.-S., Li K.-Z., Dou K.-F. Relationship between urokinase-type plasminogen activator receptor and vascular endothelial growth factor expression and metastasis of gallbladder cancer. World J Gastroenterol. 2004;10:2750–2752. doi: 10.3748/wjg.v10.i18.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thummarati P. High level of urokinase plasminogen activator contributes to cholangiocarcinoma invasion and metastasis. World J Gastroenterol. 2012;18:244. doi: 10.3748/wjg.v18.i3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 20.Longerich T., Breuhahn K., Odenthal M., Petmecky K., Schirmacher P. Factors of transforming growth factor beta signalling are co-regulated in human hepatocellular carcinoma. Virchows Arch. 2004;445:589–596. doi: 10.1007/s00428-004-1118-x. [DOI] [PubMed] [Google Scholar]
- 21.Schlaeger C., Longerich T., Schiller C., Bewerunge P., Mehrabi A., Toedt G. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology. 2008;47:511–520. doi: 10.1002/hep.22033. [DOI] [PubMed] [Google Scholar]
- 22.Budczies J., Klauschen F., Sinn B.V., Győrffy B., Schmitt W.D., Darb-Esfahani S. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugdahl E., Bachmann I.M., Schuster C., Ladstein R.G., Akslen L.A. Prognostic value of uPAR expression and angiogenesis in primary and metastatic melanoma. PLoS One. 2019;14:e0210399. doi: 10.1371/journal.pone.0210399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poettler M., Unseld M., Mihaly-Bison J., Uhrin P., Koban F., Binder B.R. The urokinase receptor (CD87) represents a central mediator of growth factor-induced endothelial cell migration. Thromb Haemost. 2012;108:357–366. doi: 10.1160/TH11-12-0868. [DOI] [PubMed] [Google Scholar]
- 25.Desmet V.J., Gerber M., Hoofnagle J.H., Manns M., Scheuer P.J. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 26.Liu W., Liang Y., Chan Q., Jiang L., Dong J. CX3CL1 promotes lung cancer cell migration and invasion via the Src/focal adhesion kinase signaling pathway. Oncol Rep. 2019;41:1911–1917. doi: 10.3892/or.2019.6957. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S.L., Dai Z., Zhou Z.J., Chen Q., Wang Z., Xiao Y.S. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597–605. doi: 10.1093/carcin/bgt397. [DOI] [PubMed] [Google Scholar]
- 28.Isse K., Harada K., Zen Y., Kamihira T., Shimoda S., Harada M. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506–516. doi: 10.1002/hep.20582. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q., Li F., Sun F., Niu J. Interleukin-8 is a prognostic indicator in human hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2015;8:8376–8384. [PMC free article] [PubMed] [Google Scholar]
- 30.Gussen H., Hohlstein P., Bartneck M., Warzecha K.T., Buendgens L., Luedde T. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J Intensive Care. 2019;7:26. doi: 10.1186/s40560-019-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayek S.S., Sever S., Ko Y.A., Trachtman H., Awad M., Wadhwani S. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossanen J., Pracht J., Jansen T., Buendgens L., Stoppe C., Goetzenich A. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci. 2017;18:1662. doi: 10.3390/ijms18081662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B., Chen L., Chang H.-T. Potential diagnostic and prognostic biomarkers for cholangiocarcinoma in serum and bile. Biomark Med. 2016;10(6):613–619. doi: 10.2217/bmm-2015-0062. [DOI] [PubMed] [Google Scholar]
- 34.Loosen S.H., Vucur M., Trautwein C., Roderburg C., Luedde T. Circulating biomarkers for cholangiocarcinoma. Dig Dis. 2018;36(4):281–288. doi: 10.1159/000488342. [DOI] [PubMed] [Google Scholar]
- 35.Pyke C., Ralfkiaer E., Rønne E., Høyer-Hansen G., Kirkeby L., Danø K. Immunohistochemical detection of the receptor for urokinase plasminogen activator in human colon cancer. Histopathology. 1994;24:131–138. doi: 10.1111/j.1365-2559.1994.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 36.Akahane T., Ishii M., Ohtani H., Nagura H., Toyota T. Stromal expression of urokinase-type plasminogen activator receptor (uPAR) is associated with invasive growth in primary liver cancer. Liver. 1998;18:414–419. doi: 10.1111/j.1600-0676.1998.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 37.Holst-Hansen C., Hamers M.J.A.G., Johannessen B.E., Brünner N., Stephens R.W. Soluble urokinase receptor released from human carcinoma cells: a plasma parameter for xenograft tumour studies. Br J Cancer. 1999;81:203–211. doi: 10.1038/sj.bjc.6690678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henic E., Sixt M., Hansson S., Høyer-Hansen G., Casslén B. EGF-stimulated migration in ovarian cancer cells is associated with decreased internalization, increased surface expression, and increased shedding of the urokinase plasminogen activator receptor. Gynecol Oncol. 2006;101:28–39. doi: 10.1016/j.ygyno.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 39.Koch A., Zimmermann H.W., Gassler N., Jochum C., Weiskirchen R., Bruensing J. Clinical relevance and cellular source of elevated soluble urokinase plasminogen activator receptor (suPAR) in acute liver failure. Liver Int. 2014;34:1330–1339. doi: 10.1111/liv.12512. [DOI] [PubMed] [Google Scholar]
- 40.Liu B., Yan S., Jia Y., Ma J., Wu S., Xu Y. TLR2 promotes human intrahepatic cholangiocarcinoma cell migration and invasion by modulating NF-κB pathway-mediated inflammatory responses. FEBS J. 2016;283:3839–3850. doi: 10.1111/febs.13894. [DOI] [PubMed] [Google Scholar]
- 41.Milione M., Miceli R., Barretta F., Pellegrinelli A., Spaggiari P., Tagliabue G. Microenvironment and tumor inflammatory features improve prognostic prediction in gastro-entero-pancreatic neuroendocrine neoplasms. J Pathol Clin Res. 2019;5(4):217–226. doi: 10.1002/cjp2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D., Aguirre Ghiso J., Estrada Y., Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 43.Unseld M., Chilla A., Pausz C., Mawas R., Breuss J., Zielinski C. PTEN expression in endothelial cells is down-regulated by uPAR to promote angiogenesis. Thromb Haemost. 2015;114:379–389. doi: 10.1160/TH15-01-0016. [DOI] [PubMed] [Google Scholar]
- 44.Lim C., Audureau E., Salloum C., Levesque E., Lahat E., Merle J.C. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB. 2016;18:540–548. doi: 10.1016/j.hpb.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Geus H.R.H., Betjes M.G., Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5:102–108. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dande R.R., Peev V., Altintas M.M., Reiser J. Soluble urokinase receptor and the kidney response in diabetes mellitus. J Diabetes Res. 2017;2017:3232848. doi: 10.1155/2017/3232848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koch A., Voigt S., Kruschinski C., Sanson E., Dückers H., Horn A. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. doi: 10.1186/cc10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.