Summary
Ten-eleven translocation (Tet) enzymes are involved in DNA demethylation, important in regulating embryo development, stem cell pluripotency and tumorigenesis. Alterations of DNA methylation with age have been shown in various somatic cell types. We investigated whether Tet1 and Tet2 regulate aging. We showed that Tet1-deficient mice undergo a progressive reduction of spermatogonia stem cells and spermatogenesis and thus accelerated infertility with age. Tet1 deficiency decreases 5hmC levels in spermatogonia and downregulates a subset of genes important for cell cycle, germ cell differentiation, meiosis and reproduction, such as Ccna1 and Spo11, resulting in premature reproductive aging. Moreover, Tet1 and 5hmC both regulate signaling pathways key for stem cell development, including Wnt and PI3K-Akt, autophagy and stress response genes. In contrast, effect of Tet2 deficiency on male reproductive aging is minor. Hence, Tet1 maintains spermatogonia stem cells with age, revealing an important role of Tet1 in regulating stem cell aging.
Subject Areas: Age, Male Reproductive Endocrinology, Cell Biology, Stem Cells Research
Graphical Abstract
Highlights
-
•
Tet1 regulates stem cell aging and differentiation
-
•
Tet1 plays an important role in maintaining spermatogonial stem cells
-
•
Loss of Tet1 results in exhaustion of spermatogonia and premature reproductive aging
-
•
Effect of Tet2 deficiency on reproductive aging in males is minor
Age; Male Reproductive Endocrinology; Cell Biology; Stem Cells Research
Introduction
Mammalian aging involves many aspects of cellular and molecular changes, such as genomic instability, telomere attrition, epigenetic alterations, stem cell exhaustion etc (Lopez-Otin et al., 2013). Deeper understanding of epigenetic alterations in aging is of particular interest as epigenetic modifications can be reversed, allowing manipulation to potentially reverse aging and thus therapeutic potential (Chen and Kerr, 2019), unlike genomic mutations which are not readily fixable.
As the best studied epigenetic modifications, DNA methylation at the 5-position of cytosine (5-methylcytosine, 5mC) plays essential roles in development, aging and disease (Greenberg and Bourc'his, 2019, Tan and Shi, 2012). DNA methylation changes have become a hallmark of aging (Lopez-Otin et al., 2013). The DNA methylation signature as 'epigenetic clocks' can predict the stage of body aging for any tissue across the entire life course (Horvath, 2013). Most methylation changes occur in a programmed manner in a subpopulation of tissue cells during natural aging, probably predisposing them toward tumorigenesis (Klutstein et al., 2016). Additionally, age-associated methylation perturbations represent a plausible mechanism by which the increased incidence of disease in the offspring of older fathers may be transmitted (Jenkins et al., 2014, Jenkins et al., 2015).
Cytosine methylation is introduced by DNA methyltransferases such as Dnmt1, Dnmt3a and Dnmt3b. Active DNA demethylation is mainly mediated by Ten-eleven translocation (Tet) enzymes, including Tet1, Tet2, and Tet3, that oxidize 5-methylcytosine (5mC) sequentially to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) (He et al., 2011, Ito et al., 2010, Ito et al., 2011, Tahiliani et al., 2009). The roles of Tet enzymes were well documented in in vivo and in vitro study. Tet1 plays important roles in pluripotency and differentiation of embryonic stem cells (ESCs) (Ito et al., 2010, Koh et al., 2011, Yang et al., 2016), development, female meiosis (Yamaguchi et al., 2012) and tumorigenesis (Wu and Zhang, 2017). Both Tet1 and Tet2 are involved in lineage differentiation and pluripotency of ESCs (Khoueiry et al., 2017, Koh et al., 2011, Pastor et al., 2013, Williams et al., 2011, Wu et al., 2018), while Tet3 is indispensable for embryo epigenetic reprogramming (Gu et al., 2011, Wossidlo et al., 2011). Tet2 is also widely expressed in a variety of somatic organs and cell types, especially in hematopoietic cells (Moran-Crusio et al., 2011). Tet1/2/3 triple knockout mice are embryonic lethal with severe gastrulation defects (Dai et al., 2016, Li et al., 2016).
However, Tet1 or Tet2 knockout mice are viable (Ko et al., 2011), suggesting that they are redundant for embryo development. Loss of both Tet1 and Tet2 enzymes is compatible with development but promotes hypermethylation and compromises imprinting, and most double mutant mice die perinatally, suggesting that these enzymes have overlapping roles in development (Dawlaty et al., 2013). Tet1 regulates meiotic gene expression and loss of Tet1 causes female meiosis defect in fetal ovaries (Yamaguchi et al., 2012). Nevertheless, Tet1 knockout mice are fertile albeit displaying reduced body mass and smaller litter size suggesting a subtle role for Tet1 in animal physiology (Dawlaty et al., 2011). Like Tet1, Tet2 is dispensable for embryonic development and adult mutants also are fertile.
Although genome-wide DNA methylation studies have shown that in most tissue types in humans, methylation associated with promoters increases with age, and genome-wide DNA methylation decreases, 5hmC can be acquired or lost in different regions (Torano et al., 2016). The decline in age-dependent overall 5mC levels is consistent with previously known age-related genome-wide hypomethylation (Fuke et al., 2004, Heyn et al., 2012, Wilson et al., 1987). The level of 5hmC in human blood cells decreases with age, and some of them were associated with TET2 mutations. The reduction of 5hmC is much greater than the reduction of 5mC (Buscarlet et al., 2016). Multiple tissue DNA methylation epigenetic clocks are age-related. However, it remains elusive whether TET enzymes play a regulatory role in aging in the adult.
Moreover, hormonal differences in mice affect "biological age", as shown by accelerated epigenetic aging in ovariectomized mice (Stubbs et al., 2017). During spermatogenesis, 5hmC level is changed dynamically and correlated with gene expression, and RNAseq data shows that Tet1 gene is expressed in spermatogonia (Gan et al., 2013, Hammoud et al., 2014, Nettersheim et al., 2013). The role of Dnmt1, 5mC, and 5hmC in the mammalian germline is to facilitate DNA demethylation and meiosis at the appropriate time (Hargan-Calvopina et al., 2016). Moreover, Tet1 expression levels during human spermatogenesis also decease with age and are associated with reduced fertility (Ni et al., 2016). However, loss of Tet1 in male mice minimally affects testis morphology and function or fertility, but leads to dysregulation of imprinted genes and a mild developmental delay shown as smaller body size (Dawlaty et al., 2011). Thus far, the function of Tet in male spermatogenesis and during aging remains to be explored. Given the importance of stem cell aging in the degeneration and dysfunction of aging tissues and the reversible nature of epigenetic regulation, a comprehensive understanding of the epigenetics of stem cell aging is central to the basic biology of aging (Chen and Kerr, 2019). Using Tet-deficient mice, we show that Tet1 deficiency accelerates spermatogonial stem cell aging and leads to premature reproductive aging in males. Male mice deficient in Tet1 exhibit progressive loss of germ cells, meiosis defect, increased apoptosis and subfertility with age. Furthermore, the mechanisms of Tet1 in regulating stem cell aging were investigated.
Results
Tet1 Deficiency Reduces Male Fertility with Age
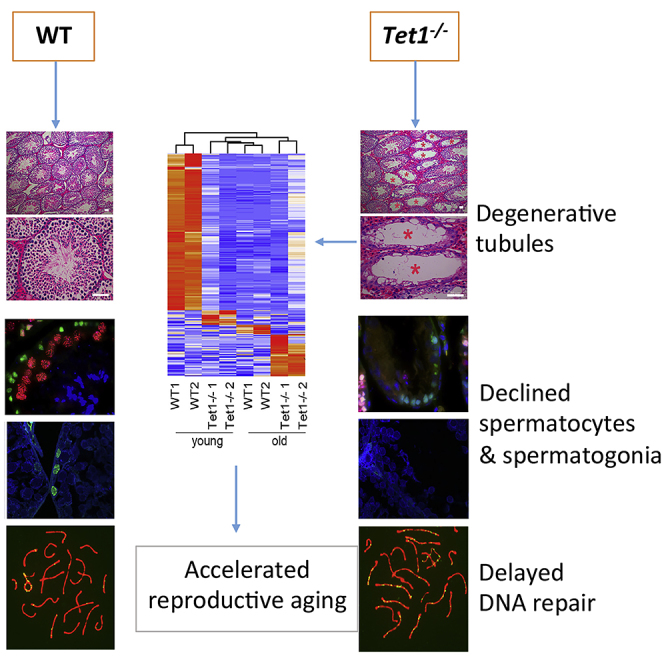
Heterozygous Tet1 or Tet2 mice (Zhang et al., 2013) were intercrossed to obtain Tet1 knockout (Tet1−/−) or Tet2 knockout (Tet2−/−) and control wild-type (WT) mice. Genotyping by PCR (Figure S1A) indicated that Tet1−/− mice were derived at the unexpected Mendelian frequency, with approximately a half of the number as expected, and this was consistent with a previous report (Yamaguchi et al., 2012). Quantitative real-time PCR showed that Tet1 mRNA was expressed but at low level relative to GAPDH in WT testis and Tet1 mRNA could not be detected in Tet1−/− testis (Figure S1B). We further determined the genotypes by immunofluorescence of Tet1 (green) and Oct4 (Red) in the developed embryos (Figure S1C). Consistent with loss of Tet1, dot-blot analyses showed a reduction of 5-hydroxymethylcytosine (5hmC) levels in Tet1−/− testis (Figure S1D). Co-immunofluorescence staining of 5hmC and 5mC followed by standard exposure time demonstrated that 5hmC strongly stained cells consistent with Sertoli cells and peritubular myoid cells, smooth muscle cells-like, by their locations (Figure S1E). Absolute quantification of 5hmC of mouse spermatogenic cells isolated from neonatal mice demonstrated that the Sertoli cells indeed had higher 5hmC content than male germ cells while mESC had a 5hmC content similar to spermatogonia cells (Gan et al., 2013). In contrast, 5mC moderately stained spermatogonia and spermatocytes. Moreover, 5mC fluorescence intensity was increased in Tet1−/− young (3 month-old) and old (11month-old) mouse tubules, compared with young (3 month-old) WT tubules. By overexposure of 5hmC staining, 5hmC expression could be revealed in the spermatogonia and spermatocytes of young WT tubules, and was reduced in Tet1−/− young and old mouse tubules (Figure S1F). Reduction of 5hmC if any in Sertoli cells and myoid cells after Tet1 deficiency is not obvious likely due to high expression of 5hmC and partly to the overexposure-caused exaggeration. Collectively, these data indicate that Tet1 is effectively abolished and the low 5hmC expression level in spermatogonia and spermatocytes is further reduced in Tet1−/− testis.
Immunofluorescence of 5mC or 5hmC showed that 5mC staining can be found in different cell types including spermatogonia, spermatocytes and spermatid and that Tet1 deficiency generally increased 5mC fluorescence. Yet, 5hmC immunostaining pattern was intriguing. The question was whether the low fluorescence signal of 5hmC in spermatogonia and spermatocytes in WT tubules, and overall reduced 5hmC staining in Tet1−/− tubules and old mouse tubules represents real 5hmC expression level. Immunohistochemistry of cytosine modifications in human testes sections showed that levels of 5hmC are decreasing as spermatogenesis proceeds, while 5mC levels remain constant, indicate that during spermatogenesis active DNA demethylation becomes downregulated leading to a conservation of the methylation marks in mature sperm (Nettersheim et al., 2013). To estimate the 5mC and 5hmC levels in spermatogonia cells, we performed co-immunostaining using promyelocytic leukemia zinc-finger (PLZF) as a marker of undifferentiated spermatogonia (Buaas et al., 2004, Costoya et al., 2004, Hobbs et al., 2012), a less abundant spermatogonia population containing spermatogonial stem cells (SSCs) (Fayomi and Orwig, 2018). Spermatogonia cells are enriched in neonatal mouse testis (Gan et al., 2013), but very few cells in tubules were stained positive for PLZF in adult testis, as shown in a recent study (Grive et al., 2019). We observed that 5hmC levels in spermatogonia decrease in 11 month-old mice, compared with 3 month-old mice (Figures S2A and S2B). Furthermore, 5mC levels are increased but 5hmC levels reduced in Tet1−/− spermatogonia of young and old mice, compared with those of age-matched WT mice (Figures S2A–S2D), consistent with the well-established role of Tet1 in DNA demethylation, now shown also in spermatogonia.
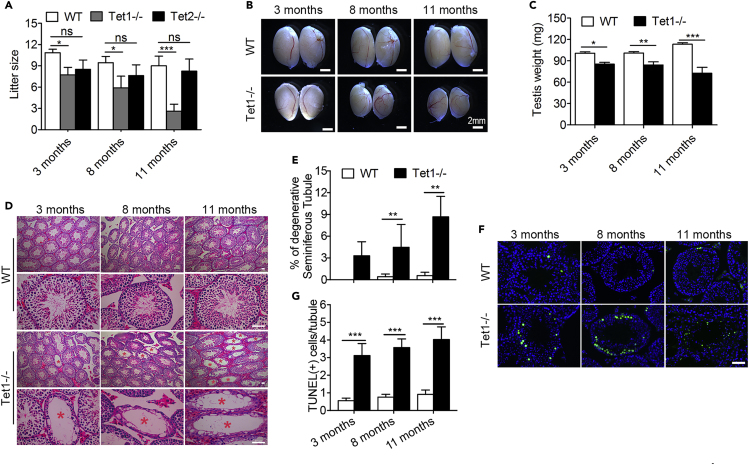
To investigate the effects of Tet deficiency on male reproductive aging, we set up mating with young WT females of Tet1−/−, or Tet2−/− males compared with WT males at three age groups (3 month-, 8 month- and 11 month-old) and counted the produced viable progeny after observing successful mating by plug next morning. The average litter size of WT males was slightly reduced with age, and did not reach significant difference by the age of 11 months, consistent with robust reproductive performance of hybrid male mouse strains. Compared to WT mice, loss of Tet1 in males reduced (p < 0.05) number of pups at young age (3 month) and at the age of 8 months. But by 11 month-old, Tet1−/− males produced markedly (p < 0.001) reduced number of pups compared to age-matched WT males (Figure 1A). While WT mice produced litter size of an average of 8 pups at the 11 month-old, Tet1−/− males had only about 3 pups. This shows that the effect of loss of Tet1 on male reproduction is age-related. Testis weight is an important reproductive trait in males. Consistent with age-related decline in fertility, the testis of Tet1−/− males was getting smaller with age, in contrast to WT mouse testes that became slightly larger with age. Tet1−/− males had significantly lighter testis than did age-matched WT males (Figures 1B and 1C).
Figure 1.
Tet1-Deficient Males Show Progressive Reduction in Fertility with Age and Spermatogenesis Defects
(A) Reduction in the fertility in Tet1−/− male mice with age, but not in Tet2−/− males, compared with WT males. Experiments were repeated at least three times for each age group males. n ≥ 8 males that had successful mating by the presence of plug with young female mice (2-3 months) were counted for number of pups produced (litter size) and evaluation of fertility. Data are represented as mean ± SEM.
(B) Representative images showing the testis collected from WT and Tet1−/− mice.
(C) Average testis weight. n = 8 mice for each group. Data are represented as mean ± SD.
(D and E) H&E-staining of the cross sections of seminiferous tubules from 3-, 8- and 11-month-old WT and Tet1−/− mice (D). Red asterisks represent tubules with loss of germ cells. (E) The ratios of degenerated tubules to the total number of tubules are presented. Four mice for each group were used. Scale bar, 50 μm. Data are represented as mean ± SD.
(F and G) Representative images (F) and quantification (G) of TUNEL positive cells in WT and Tet1−/− testes at 3, 8 and 11 months of age. Scale bar, 50 μm. Three mice for each group were used. Approximately 70 tubules each group were randomly counted. Green, apoptotic cells by TUNEL assay; Blue, nuclei stained with DAPI. Data are represented as mean ± SEM.*p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant (p > 0.05). Student's t-test for A, C, E and G.
See also Figures S1–S4.
Tet2 Deficiency Has Only Minor Effect on Male Fertility with Age
To know whether Tet2 also may regulate male germ cell function with age, we examined fertility of Tet2−/− males with age, compared with age-matched WT controls. Genotyping by PCR indicated Tet2 deficiency in Tet2−/− mice and normal Tet2 in WT (Figure S3A). By qPCR, Tet2 mRNA was expressed at low level relative to GAPDH in WT testis and Tet2 mRNA level was minimal in Tet2−/− testis (Figure S3B). Moreover, immunofluorescence of Tet2 and synaptonemal complex protein 3 (Sycp3) in testis sections showed that Tet2 was not detectable in the testis of Tet2−/− mice and that both Tet2−/− and WT mice exhibited similar staining of Sycp3 positive cells, indicative appropriate homologous pairing. Tet2 was expressed mainly in primary spermatocytes, secondary spermatocytes and round spermatids (Figure S3C). 5mC and 5hmC immunofluorescence intensity was slightly changed following Tet2 deficiency (Figure S3E).
Loss of Tet2 in males slightly reduced number of pups at young age (3 month-old) but did not reach significant difference, compared to WT mice (Figure 1A). By the age of 8 month or 11 month-old, Tet2−/− males produced number of pups comparable to that of age-matched WT males (Figure 1A). The weight of Tet2−/− testis did not differ from that of WT mouse testes at the age of 3 or 8 month-old, but became lighter compared to WT when the mice reached the age of 11 months (Figure S3D). H&E staining of cross-sections of testis revealed that normal spermatogenesis were observed in most of seminiferous tubules of Tet2−/− males (Figure S3F). Only few seminiferous tubules of Tet2−/− mice showed reduced germ cells. These results suggest that the effect of loss of Tet2 on male reproduction and aging is insignificant.
Tet1 Deficiency Causes Progressive Loss of Germ Cells with Age
To understand the causes of subfertility with age in Tet1−/− mice, we examined histology of testes by H&E staining of cross-sections and immunofluorescence microscopy. Seminiferous tubules with disrupted spermatogenesis were observed in testes of Tet1−/− males. Some seminiferous tubules of Tet1−/− mice lacked a number of germ cell types such as round spermatid, and others were even depleted of germ cells, but only contained Sertoli cells. The percentage of degenerated tubules increased with age in Tet1−/− mice (Figures 1D and 1E). At the age of 3 month-old, about 3.3% tubules with disrupted spermatogenesis were observed, with increasing to 4.5% and 8.6% at the age of 8 months and 11 months (n = 4, four testes from four different mice examined at each age, respectively). Degenerated seminiferous tubules were not observed in testes of control WT males at 3 months and very few at the age of 8 months and 11months. Concurrent with degenerated seminiferous tubules in Tet1−/− males, a significant increase in germ cell apoptosis was observed. The TUNEL assay showed that the number of germ cell underwent apoptosis per tubule increased slightly in WT males with age, but increased significantly (p < 0.001) in Tet1−/− males when compared to age-matched WT males (Figures 1F and 1G). This result suggests that increased apoptosis is probably a contributing factor for progressive germ cell loss in Tet1−/− males, and this phenotype is age-related.
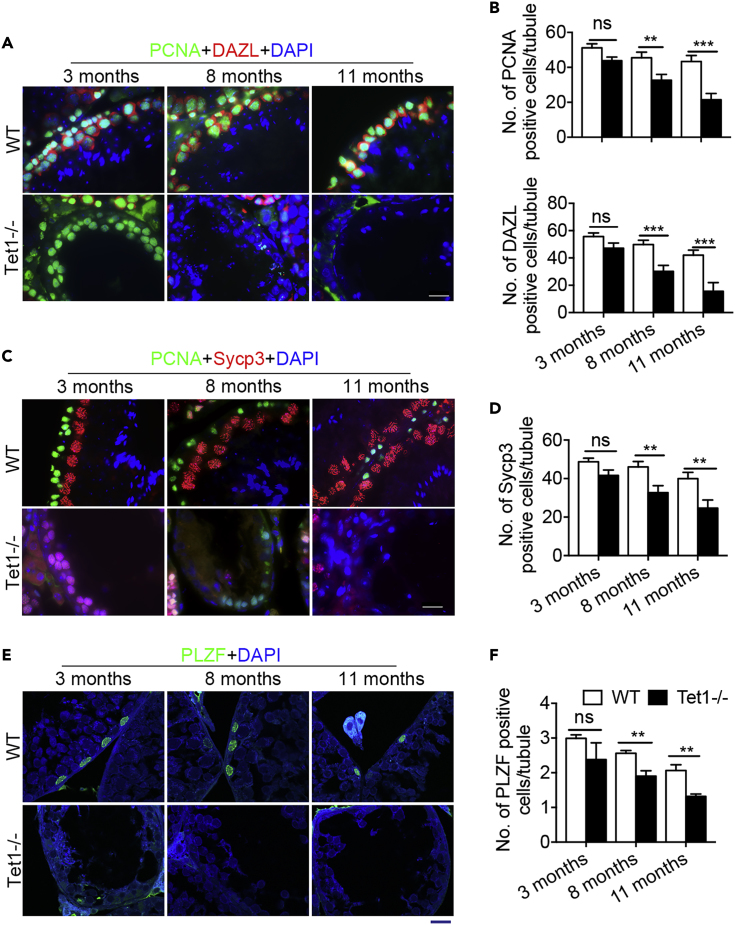
To further confirm the loss of germ cells in the degenerative seminiferous tubules, we examined the expression of proliferating cell nuclear antigen (PCNA), a marker for proliferative cells (Wrobel et al., 1996), and used Dazl to reveal germ cells or Sycp3 to detect prophase I spermatocytes (Yuan et al., 2000). Both the numbers of PCNA and Dazl positive cells were significantly reduced in Tet1−/− mouse testis and further declined with age, compared to WT testis (Figures 2A and 2B). Normal seminiferous tubules of testes in Tet1−/− mice still had PCNA and Sycp3 positive cells, but the degenerative tubules showed very few PCNA and Sycp3 positive cells and other types of germ cell such as round sperm (Figures 2A and 2B). Furthermore, the number of PCNA positive spermatogonia and Sycp3 positive prophase I spermatocytes per seminiferous tubule both were significantly (p < 0.001) decreased in Tet1−/− mice compared to age-matched WT mice (Figures 2C and 2D). Hence, these results indicate that lack of Tet1 leads to a progressive germ cell loss and spermatogenesis defect with age.
Figure 2.
Tet1 Deficiency Leads to Loss of Male Germ Cells with Age
(A) Sections of WT and Tet1−/− testis were co-stained with antibodies specific for PCNA (green) and DAZL (red).
(B) Number of PCNA and DAZL positive cells per seminiferous tubule. n = 10 tubules, three repeats.
(C) Sections of WT and Tet1−/− testis were co-stained with antibodies specific for PCNA (green) and Sycp3 (red).
(D) Number of Sycp3 positive cells per seminiferous tubule. n = 10 tubules, three repeats.
(E) Immunofluorescence of PLZF (green) in the sections of WT and Tet1−/− testis. DAPI (in blue) stains nucleus.
(F) Number of PLZF positive cells per seminiferous tubule. n = 20 tubules, three repeats. Data are represented as mean ± SEM. **p < 0.01, ***p < 0.001, ns, not significant (p > 0.05). Student's t-test for B, D, and F. Scale bar, 20 μm.
See also Figures S2 and S4.
Adult stem cells play essential roles in maintenance of tissue homeostasis and regeneration. In mouse testis, SSCs are able to self-renew and differentiate, maintaining the continuous spermatogenesis to produce spermatozoa (Fayomi and Orwig, 2018, Kubota and Brinster, 2018). Because some complete degeneration of the seminiferous epithelium with a Sertoli cell-only phenotype was observed in Tet1−/− testis and Tet1 was expressed in spermatogonia (containing SSCs) (Gan et al., 2013), this inspired us to hypothesize that loss of Tet1 might impact self-renewal of SSCs, causing a progressive loss of germ cells with age. To substantiate this hypothesis, we carefully examined and compared PLZF positive cells between Tet1−/− and WT seminiferous tubules. Interestingly, the number of PLZF positive cells per seminiferous tubule also was decreased in WT mice with age, and significantly decreased in Tet1−/− mice compared to age-matched WT mice (Figures 2E and 2F). Previous findings also showed reduced number and proliferation of SSCs with age (Schmidt et al., 2011). Overall, these results indicate that loss of Tet1 results in exhaustion of undifferentiated spermatogonia and degenerative tubules, suggesting that Tet1 plays an important role in maintaining SSCs.
Tet1 Deficiency Leads to Meiosis Defect with Age
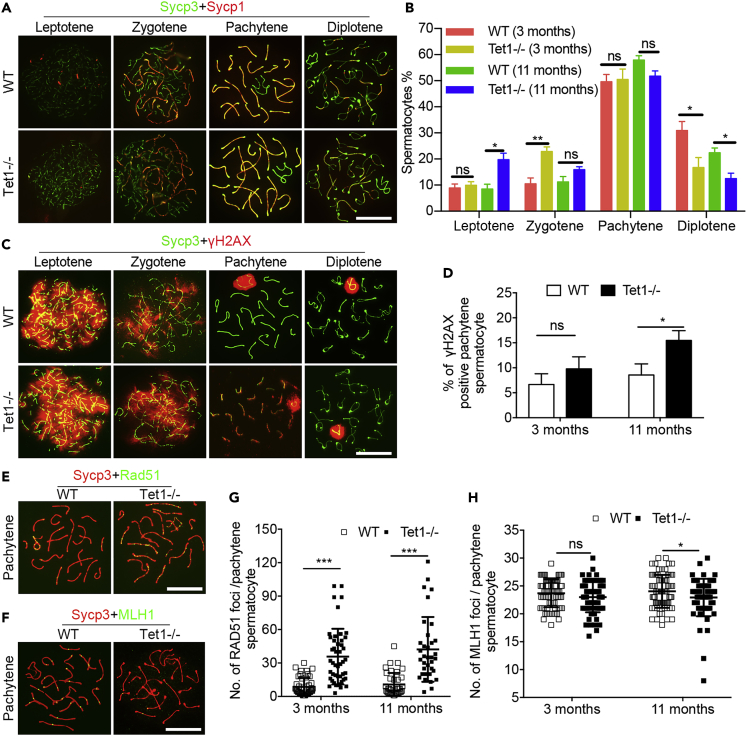
As shown above, Tet1−/− testis has morphologically normal tubules, so we asked whether they undergo meiosis correctly in these tubules. We tested whether loss of Tet1 leads to meiosis defect with age. Co-immunostaining of spermatocytes spreads with the meiotic synapsis proteins Sycp3 and Sycp1 revealed that progression of meiotic prophase I was impaired in Tet1−/− males. At the age of 3 months, the percentage of leptotene and pachytene did not differ between Tet1−/− and WT males, but the percentage of zygotene in Tet1−/− males was increased, and the percentage of diplotene was decreased, compared with WT mice. At the age of 11 month-old, the percentage of leptotene and zygotene both were higher, and the percentage of diplotene was lower in Tet1−/− mice than in WT mice (Figures 3A and 3B).
Figure 3.
Tet1 Deficiency Leads to Meiosis Defect with Age
(A) Co-immunofluorescence of Sycp3 (green) and Sycp1 (red) in spermatocytes. Scale bar, 20 μm.
(B) Frequencies of meiotic stages in WT and Tet1−/− testis suspensions. Approximately 200 meiocytes from 3-6 mice were counted for each stage.
(C) Co-immunofluorescence of Sycp3 (green) and γH2AX (red) in spermatocytes. Scale bar, 20 μm.
(D) Percentage of cells retaining γH2AX outside of the sex chromosomes in pachytene and diplotene spermatocytes. Approximately 50 pachytene meiocytes from 3 mice were counted.
(E and F) Co-immunofluorescence of Sycp3 (red) and Rad51(green) (E) or MLH1 (green, F) in spermatocytes. Scale bar, 20 μm.
(G) Quantification of the Rad51 foci per pachytene spermatocyte.
(H) Quantification of the MLH1 foci per pachytene spermatocyte. Approximately 30 pachytene meiocytes from three mice were counted. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant (p > 0.05). Student's t-test for B, D, G, and H.
See also Figure S4.
Although changes in the percentage of four stages of prophase I spermatocytes indicated that the progression of meiosis prophase I was impaired in Tet1−/− males, no defect was observed in synapsis formation at pachytene stage. We asked whether Tet1−/− deficiency influences DNA repair and recombination. At the meiosis prophase I, meiotic recombination is essential for accurate segregation of homologous chromosomes (Hunter, 2015). The initiating event of meiotic recombination is a programmed DNA double-strand breaks (DSBs) introduced to the genome by Spo11 (Keeney et al., 1997). The DSBs which can be detected by the presence of γH2AX, must be subsequently repaired to form crossovers or noncrossovers (Gray and Cohen, 2016). As expected, many DSBs were present from leptotene to zygotene stages both in WT and Tet1−/− spermatocytes, and staining of γH2AX nearly disappeared at autosome except for XY body in pachytene and diplotene stage spermatocytes. However, staining of γH2AX still remained at autosome in some pachytene stage spermatocytes (termed as γH2AX positive cells), and the percentage of γH2AX positive pachytene cells was increased in Tet1−/− males at the age of 11 months compared to WT males (Figures 3C and 3D). These data suggest that Tet1 deficiency caused DSB repair defect. The increased apoptotic cell death might be partially caused by meiosis defects.
In line with a DSBs repair defect, Rad51, the DSBs repair associated recombinase (Baier et al., 2014, Bannister and Schimenti, 2004), exhibited more foci in pachytene spermatocytes of Tet1−/− than in age-matched WT males (Figures 3E and 3G). The presence of γH2AX and delayed removal of RAD51 in the chromosomes indicate that homologous recombination and repair were still occurring, but impaired in Tet1−/− spermatocytes. MLH1 is an important marker for meiotic recombination and represents crossover (Baker et al., 1996, Guillon et al., 2005, Hassold et al., 2009). Co-immunostaining of spermatocytes surface spreads with Sycp3 and MLH1 showed that MLH1 foci decreased dramatically in pachytene spermatocytes of 11 month-old Tet1−/− males, but showed no difference at the age of 3 months (Figures 3F and 3H). Taken together, Tet1 deficiency results in meiotic recombination defect with age.
Tet1 Deficiency Shortens Telomeres
Telomeres were shortened in mouse embryonic stem cells (ESCs) and in epiblast differentiation depleted of Tet1 (Khoueiry et al., 2017, Yang et al., 2016), but it is unclear whether Tet1 loss leads to telomere shortening in male germ cell. We preformed telomere quantitative fluorescence in situ hybridization (QFISH) and immunofluorescence of PLZF and Sycp3 on testis section and spermatocytes. Telomeres slightly shortened with age, and shortened further in Tet1−/− SSCs and pachytene spermatocytes, compared with those of WT mice (Figures S4A and 4B), which was further corroborated by Southern blot measurement of telomere TRF (Figure S4C). Although telomere length did not show dramatic reduction in the definite spermatogonia and pachytene spermatocytes in Tet1−/− mice, these were survived cells, when used for preparation for telomere QFISH analysis, so they expectedly had better telomere maintenance. Others with shorter or dysfunctional telomeres might have undergone apoptosis that could not be detected due to the limitation of the assay.
Transcriptome Feature of Spermatogonia Cells with Age and Associated with Tet1 Deficiency
During spermatogenesis, Tet1 and Tet2 are expressed at highest levels in type A & B spermatogonia, at reduced levels in primary spermatocytes and at minimal levels in spermatid, but Tet3 is expressed at much higher levels than do Tet1 and Tet2 in all these cells (Gan et al., 2013). These data on spermatogonia and spermatocytes were based on the materials obtained from neonatal mice. We attempted to localize Tet1 and Tet2 protein expression by immunofluorescence in these different cell types in the adult and aging males, and found that Tet2 protein is mainly expressed in primary and secondary spermatocytes and spermatid (Figure S2C). Unfortunately we could not reveal Tet1 localization by immunofluorescence staining, although we tested at least four Tet1 antibodies obtained commercially or custom-made. Next we explored the mechanism of Tet1 in regulating male fertility by further assessing spermatogonia cells. We purified spermatogonia from testis by FACS and profiled their transcriptome by RNA-sequencing to investigate the molecular changes and mechanism after loss of Tet1. Based on cell sorting by Hoechst-blue and Hoechst red, P3 cell populations contained mostly spermatogonia cells, which took about 0.1-0.3% of whole cell populations (Figures S5A–S5D). Primary spermatocytes in P4 took about 1-5% of cell populations. P3 and P4 populations also were confirmed by co-immunofluorescence of Dnmt3b and absence of Sycp3, and Dnmt3b-negative and Sycp3-positive spermatocytes (Figure S5E). By immunofluorescence of PLZF, P3 cell populations after cell sorting contained few spermatocytes which were PLZF positive and had relatively large decondensed nuclei (Figure S5F). The purity of spermatogonia varied from 90%–98% each time.
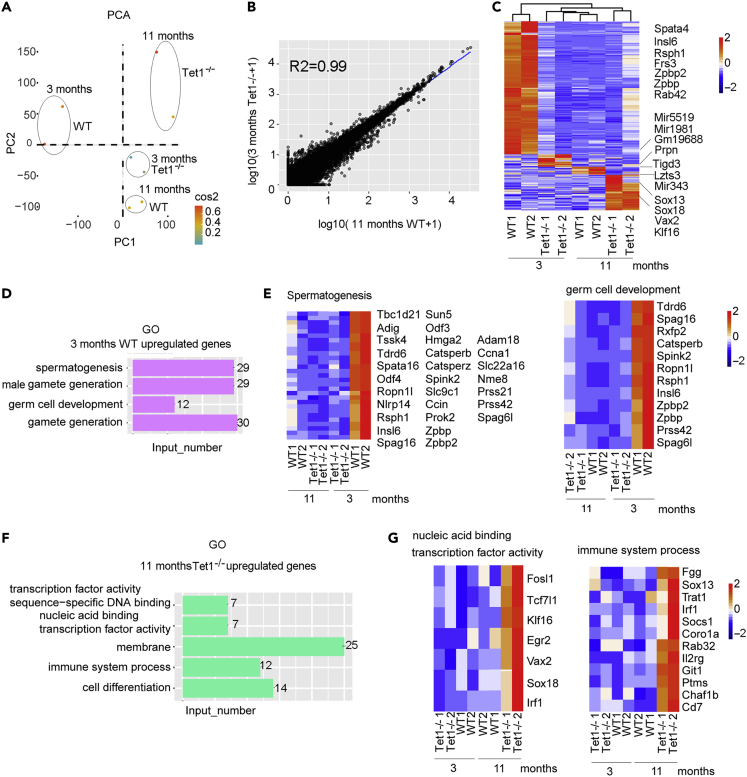
We performed global principal component analysis (PCA) using transcripts per kilobase of exon model per million mapped reads (TPM) of all four groups: 3 month-old WT and Tet1−/−, and 11 month-old WT and Tet1−/− mice. Transcriptome of spermatogonia from four groups could be mostly separated by clustering of PC1 and PC2 (Figure 4A), in which 11 month-old WT and 3 month-old Tet1−/− clustered close together, in consistency with correlation analysis, and the correlation coefficient of the two groups was up to 0.99 (Figure 4B). This is also consistent with Pearson's correlation coefficient diagram (Figure S5G). Specific genes were highly expressed in each group and also on chromosome Y shown by pheatmap in young male mice at the age of 3 month-old and were down-regulated in 3 month- Tet1−/− and 11 month- WT and 11 month-old Tet1−/− mice (Figures 4C and S5H). These data indicated that genes expressed in spermatogonia cells mostly were down-regulated after Tet1 deficiency, and suggested that Tet1 loss could accelerate the reproductive aging by impairing spermatogonia cells.
Figure 4.
Transcriptome Feature of Spermatogonia Cells following Tet1 Deficiency or Aging
(A) PCA analysis of TPM showing differential gene expression pattern among 3 month-old WT and Tet1−/−, and 11 month-old WT and Tet1−/− spermatogonia and few spermatocytes.
(B) Correlation analysis. X axis represented the log10(TPM+1) of 11 month-old WT group, and Y axis represented the 1og10(TPM+1) of 3 month-old Tet1−/− spermatogonia cells. Their correlation coefficient was 0.99.
(C) Differential gene expression profile shown by pheatmap.
(D) GO enrichment analysis of specific genes with high expression levels in 3 month-old WT mice.
(E) Pheatmap showing the genes enriched in spermatogenesis and germ cell development.
(F) GO enrichment analysis of specific genes with high expression levels in 11 month-old Tet1−/− males.
(G) Pheatmap illustrating the genes enriched in nucleic acid binding transcription factor activity and immune system process.
See also Figures S5 and S6.
We enriched the genes by KOBAS (Xie et al., 2011), which were downregulated in 3 month-old Tet1−/− and 11 month-old WT and Tet1−/− mice. The GO results showed that these genes were enriched in spermatogenesis (29 genes), male gamete generation (29 genes), germ cell development (12 genes) and gamete generation (30 genes) (Figure 4D), based on the database of GO (http://geneontology.org/). Genes enriched in spermatogenesis and germ cell development were shown by pheatmap, and both exhibited higher expression in 3 months WT group, compared with other three groups (Figure 4E). We also looked at the enrichment of genes highly expressed in the 11 months old Tet1−/− mice. These genes were enriched in the categories of transcription factor activity, sequence-specific DNA binding, nucleic acid binding transcription factor activity, membrane, immune system process and cell differentiation (Figures 4F and 4G). These data show that Tet1 deficiency accelerates spermatogonia cell aging.
Differential Gene Expression in Spermatogonia in Young and Old Males
To explore transcriptome changes during natural aging of spermatogonia cells, we further selected and performed RNA-seq analysis of 3 month and 11 month-old male mice. By analysis of differentially expressed genes (up: log2Foldchange >1, p value<0.05; down: log2Foldchange < -1, p value<0.05), 3,449 genes were highly expressed in 3 month-old WT males, in contrast to only 125 highly expressed genes in 11 month-old WT males, indicating that the expression of most genes decreased with age (Figure S6A and S6B), including Dppa3, Cyp26b1, Kitl and Aurkc. Dppa3/Stella as germline pluripotency marker is expressed at highest levels in one cluster of spermatogonia stem cells (Law et al., 2019), and activates spermatogenesis-associated genes (Dong et al., 2017). Elimination of Cyp26b1 activity within both germ and Sertoli cells resulted in severe male subfertility, with a loss of advanced germ cells from the seminiferous epithelium, indicating that Cyp26 activity within either Sertoli or germ cells is essential for the normal progression of spermatogenesis (Hogarth et al., 2015, Saba et al., 2014). Reduction of Cyp26b1 could alter meiosis differentiation.
Genes downregulated in 11-month-old WT males were enriched in PI3K-Akt signaling pathway, retinol metabolism, cAMP signaling and AMPK signaling pathway (Figures S6C and S6D). PI3K-Akt signaling pathway regulates the proliferation and differentiation of SSCs (Zhou et al., 2015). AMPK and Wnt signaling pathway were all highly expressed in 3 month-old WT mice. GO enrichment results showed that genes downregulated in 11 months WT group were focused on reproductive structure development, meiotic cell cycle, chromatin organization, cellular response to DNA damage stimulus, and actin cytoskeleton (Dunleavy et al., 2019) (Figure S6E), which play important roles in spermatogonia differentiation. Genes enriched in chromatin organization and meiotic cell cycle are shown by pheatmap (Figure S6F). Notably, Spo11, Ccna1, Piwil1, Syce1l, Aurka, and Haspin etc. were consistently downregulated in spermatogonia cells in old mice. SSC subtypes contain six clusters, and cluster 1 is highly enriched for categories of sexual reproduction, meiosis, and gametogenesis and includes Piwil1, Sohlh2, Mael, Stra8, Rad51, Sycp, and Syce (Hammoud et al., 2015). Meiotic genes are DNA methylated in PGCs but fully DNA-hypomethylated in adult SSCs (and “poised” by low/moderate H3K4me3) (Hammoud et al., 2014). Single-cell RNA-seq identified four spermatogonial subtypes expressing many different markers for undifferentiated (e.g., Gfra1, Lin28, Id4, Pax7, Etv5, Zbtb16, and Tert) and differentiating spermatogonia (e.g., Kit, Stra8, Sycp1, Sycp3, Dmrts, and Sohlhs) (Green et al., 2018). Together, natural aging negatively affects transcriptome of spermatogenesis in mice.
Roles of Tet1 on Spermatogonia
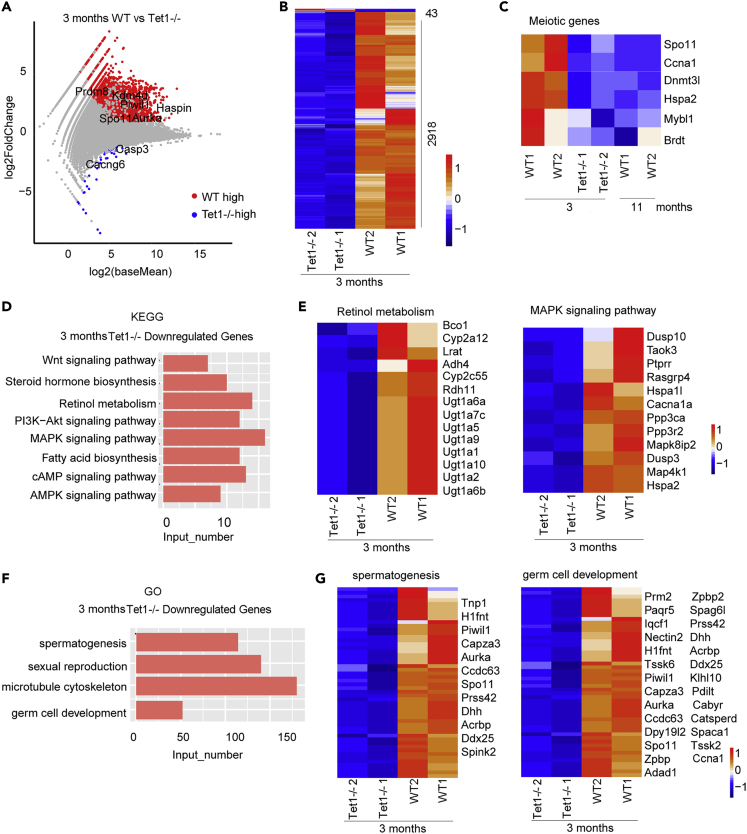
Next question was how Tet1 regulates the aging of spermatogonia cells. We analyzed RNA-seq data from spermatogonia of 3 month-old Tet1−/− males and compared with the age-matched WT males. Scatter diagram showed that more genes were highly expressed in the 3-month-old WT males than in Tet1−/− males, indicating that the expression of most genes decreased after Tet1 deficiency, including Prdm8, Spo11, Aurka, Piwil1, Haspin (Figure 5A). Other genes including Casp3, which plays important roles in apoptosis, and Cacng6 were highly expressed in Tet1−/− males. There were 2,918 genes downregulated and only 43 upregulated in Tet1−/− males, compared to WT males, shown by pheatmap (Figure 5B). Spermatogonial cells express germ cell marker genes and meiotic marker genes (Sharma et al., 2019). Interestingly, genes important for meiosis progression, Spo11, Piwil1, Ccna1, Hspa2, and Dnmt3l were downregulated in Tet1−/− deficient germ cells, like those of natural aging (Figure 5C). As the third Piwi family member, Piwil1, also known as Miwi, is specifically highly expressed in adult testis and essential for spermatogenesis (Deng and Lin, 2002, Yue et al., 2014). Piwil1/Miwi is required for the continued maintenance of repeat-derived piRNAs long after transposon silencing is established in germline stem cells and is essential for male fertility (Reuter et al., 2011). Genes downregulated in Tet1−/− males were enriched in PI3K-Akt signaling pathway, cAMP signaling, AMPK signaling, MAPK signaling and retinol metabolism (Figures 5D and 5E), like those of aging mice shown above.
Figure 5.
Tet1 Deficiency Alters Transcriptome of Germ Cells
(A) Scatterplot showing differential gene expression between 3 months WT and Tet1−/− spermatogonia and spermatocytes. Red represents genes with higher expression in 3 months WT, and blue represents genes with higher expression in Tet1−/−, gray represented no significance between 3 month and Tet1−/− spermatogonia and spermatocytes.
(B) Differential gene expression shown by pheatmap. Differential genes were selected by the standard of log2Foldchange>1 or log2Foldchange < -1, and p value < 0.05.
(C) Pheatmap showing meiotic related genes including Spo11, Ccna1 and Dnmt3l that are downregulated.
(D) KEGG results enriched by the 2,918 genes downregulated in 3 months Tet1−/− spermatogonia and spermatocytes by KOBAS websites.
(E) Pheatmap showing the genes enriched in retinal metabolism and MAPK signaling pathway.
(F) GO results enriched by the 2,918 genes downregulated in 3 months Tet1−/− spermatogonia and spermatocytes.
(G) Pheatmap showing the genes enriched in spermatogenesis and germ cell development (http://zfin.org/action/ontology/term-detail/GO:0007281).
See also Figure S6.
GO enrichment results showed that genes downregulated in 3 month-old Tet1−/− males were focused on spermatogenesis, sexual reproduction, microtubule cytoskeleton, meiosis I cell cycle phase, and germ cell development (Figures 5F and 5G), indicating that Tet1 deficiency leads to abnormal spermatogenesis. Similar effects of age and Tet1 deficiency on specific pathways together with PCA data suggest that Tet1 deficiency mimics aging-associated dysfunction in spermatogenesis.
Methylation Aging Clock of Germ Cells
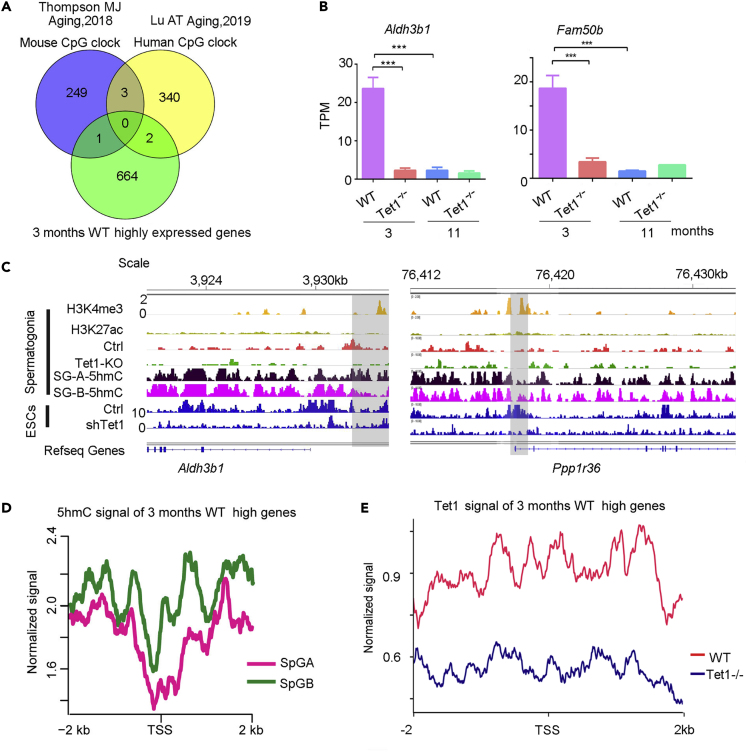
Methylation aging clock has been proposed and validated in somatic cell aging (Horvath, 2013, Horvath and Raj, 2018). Mouse aging clock appeared to differ from human aging clock by comparison analysis (Figure 6A). Previous studies have shown that male germ cells in adult mice have highly different methylation state (Cui et al., 2016, Gan et al., 2013), and different patterns of epigenetic dysregulation in DNA methylation occur in tissue-specific over time (Thompson et al., 2010), but how methylation plays a role in spermatogonia and germ cells aging remains to be determined. DNA methylation of transcription start site (TSS) usually suppresses gene transcription (review (Xiao et al., 2019)). We overlapped the genes with high expression in 3 month-old mouse WT spermatogonia but low expression presumably associated with methylation in 11-month old WT males and 3 month-old Tet1−/− and 11 month-old Tet1−/− spermatogonia, with mouse (Thompson et al., 2018) and human aging clock genes (including 253 genes in mice and 345 genes in humans) (Lu et al., 2019) (Figure 6A). Interestingly, only two genes Aldh3b1 and Fam50b that are highly expressed in the 3 month-old mice but lower expressed in 11 month-old WT males and 3 month- Tet1−/− and 11 month-old Tet1−/− males (Figure 6B), overlapped with aging clock genes in humans, and only Ppp1r36 overlapped with the mouse aging clock genes. Tet1 deficiency or age can lead to a significant decline in the expression of both genes.
Figure 6.
Transcriptome of Spermatogonia Cells and Methylation Aging Clock
(A) Venn diagram in comparison of highly expressed genes in 3 month-old WT mice but downregulated in other three groups shown in Figure 4C, with methylation aging clock of mouse (Thompson et al., 2018) and human genes (Lu et al., 2019).
(B) Bar plot showing Aldh3b1 and Fam50b expressed at significantly higher levels in 3 months WT group. Data are represented as mean ± SEM. (n = 3). ***p < 0.001, Student's t-test.
(C) Integrative Genomics Viewer (IGV) demonstrating binding site of Aldh3b1and Ppp1r36. H3K4me3 was used to show the promoter location of Aldh3b1. Tet1 biding to the Control and Tet1 knockout spermatogonia cells (our data) is compared with 5hmC enrichment in both type A and type B spermatogonia cells. Tet1 abundance in ESCs and Tet1 knocked down ESCs served another control. H3K4me3 and H3K27me3 data were obtained from (Hammoud et al., 2014), SG-A-5hmC and SG-B-5hmC from (Gan et al., 2013), and Tet1-Ctrl and shTet1 from (Williams et al., 2011).
(D) Average signal level of 5hmC binding to 667 genes in 3 months WT near TSS region of the spermatogonia cells. Pink represents Type A spermatogonia cells and green represents Type B spermatogonia cells.
(E) Average signal level of Tet1 binding to 667 genes in 3 months WT near TSS region. The signal was decreased in Tet1 knockout spermatogonia cells.
See also Figure S7.
To understand whether these two genes are regulated by methylation, we used the Integrative Genomics Viewer (IGV) to see if 5hmC regulates the promoter of Aldh3b1. H3K4me3 is used to show the promoter region of the gene (i.e. the gray region in Figure 6C), from which it can be seen that 5hmC is bound to the Aldh3b1 promoter region regardless of type A or B-type spermatogonia cells, and the signal binding to the coding exon of Aldh3b1 was stronger. This suggests that the expression of Aldh3b1 could be regulated by 5hmC. It has been shown that 5hmC is enriched in gene bodies and proximal promoters but depleted in intergenic regions and TSS in spermatogonia and spermatocytes and the study further provided evidence for a positive regulatory role of 5hmC in gene activation during spermatogenesis (Gan et al., 2013). Additionally, we compared the abundance of Tet1, 5hmC and 5mC on Aldh3b1. Tet1 is bound to the promoter of Aldh3b1 in WT spermatogonia, but the binding reduced in Tet1−/− spermatogonia (Figure 6C). Binding of 5hmC on Aldh3b1 in ES cells is similar to that of spermatogonia cells, on both the promoter and exon of Aldh3b1. Signal of 5hmC on the intergenic and coding of Ppp1r36 in spermatogonia cells also is strong. These data suggest that Aldh3b1 and Ppp1r36 could be regulated by Tet1 and its mediated-5hmC.
To determine whether reproductive cell aging have its own unique clock or pattern, we analyzed 5hmC signals at 667 genes highly expressed in the 3-month WT group in spermatogonia cells but with lower expression in other three groups. 5hmC strongly bound to intergenic and intragenic region of the genes but reduced at the promoter region (near TSS) of spermatogonia cells (Figure 6D). However, Tet1 could bind to both TSS as well as intergenic and intragenic region of these genes, and the binding to these regions was noticeably reduced in Tet1−/− spermatogonia (Figure 6E). These data suggest that both Tet1 and 5hmC are involved in transcriptional regulation of these genes in spermatogonia cells. It remains to be validated whether these genes can mark germ cell aging clock. Nevertheless, from our initial analysis, aging clock of somatic cells may differ from that of germ cells.
Mechanism of Transcriptional Regulation by Tet1 and Age on Spermatogonia Cells
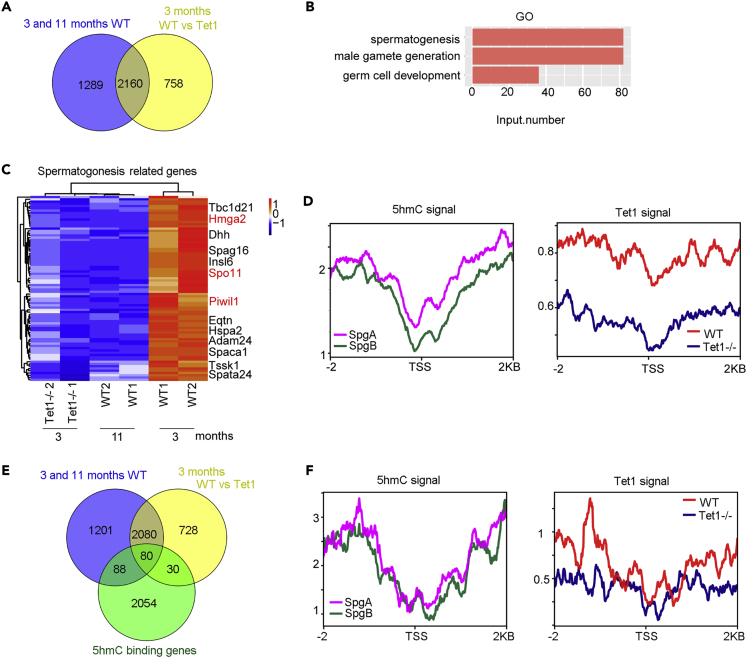
Based on the data analysis shown above, there were more down-regulated genes than up-regulated genes in 11 month-old WT and 3 month-old Tet1−/− males compared with 3 month-old WT males. We tested the hypothesis that Tet1 deficiency may lead to premature senility of mouse spermatogonia cells. We draw a Venn diagram of these down-regulated genes, showing that 2,160 genes were both downregulated in 3 month Tet1−/− and 11 month-old WT spermatogonia cells (Figure 7A). GO results showed that these 2,160 genes were enriched in spermatogenesis, gamete generation and germ cell development (Figure 7B). Genes associated with spermatogenesis were downregulated in 3 month-old Tet1−/− and 11 month-old WT spermatogonia cells (Figure 7C). Normalized 5hmC signal of type A and type B spermatogonia was decreased in TSS region. Tet1 signal also decreased in TSS region and the signal of Tet1 on the genes in WT spermatogonia cells were noticeably reduced in Tet1−/− spermatogonia cells (Figure 7D), implying similarly regulatory mechanisms of these genes by 5hmC and Tet1. We also analyzed the genes downregulated in 3 month-old Tet1−/− and 11 month-old WT spermatogonia cells and the genes with 5hmC binding, which revealed 80 genes overlapped among them (Figure 7E). Again, 5hmC or Tet1 signal on the 80 genes shared was decreased in TSS region. Signal of Tet1 on these genes in WT spermatogonia cells also were reduced in Tet1−/− spermatogonia cells but to less extent (Figure 7F). These data suggested that reduction of 5hmC and Tet1 can contribute to the declined expression of the genes associated with spermatogenesis with age or Tet1 deficiency.
Figure 7.
ChIP-seq Analysis of the Genes Downregulated with Age and Tet1 Deficiency
(A) Venn diagram showing the genes downregulated in 3 month-old WT vs 11 month-old WT, or 3 month-old WT vs Tet1−/− spermatogonia cells.
(B) GO enrichments of the downregulated genes in spermatogenesis, gamete generation and germ cell development.
(C) Pheatmap illustrating the genes related to spermatogenesis that are highly expressed in 3 month-old WT mice.
(D) Average signal of 5hmC or Tet1 by ChIP-seq on the genes downregulated in both Tet1−/− and 11 month-old spermatogonia cells.
(E) Venn diagram showing the genes downregulated in 3 months WT vs 11 months WT, or 3 months WT vs Tet1−/− spermatogonia cells, and 80 of them are shared by 5hmC binding.
(F) Average signal of 5hmC or Tet1 on the 80 shared genes in spermatogonia cells.
See also Figure S7.
Further comparison of 5hmC binding genes in ESCs (Williams et al., 2011), type A and type B spermatogonia (Gan et al., 2013), revealed that there were 1,920 genes common in ESCs and spermatogonia cells, and 2,375 genes in the spermatogonia A and B cell types (Figure S7A). These genes are involved in various signaling transduction pathways and biological functions (Figures S7B–S7D). Again, 99 genes downregulated in 3 month-old Tet1−/− and 11 month-old WT mice were co-occupied by 5hmC in both ESCs and spermatogonia cells (Figures S7E and S7F). While 5hmC signal in the TSS region of these genes was decreased, the signal of Tet1 in the TSS region of the genes was elevated in spermatogonia cells, but reduced in Tet1−/− spermatogonia cells (Figure S7G). These data support the notion that Tet1 has a role in transcriptional regulation of the bound genes. Tet1 deficiency can result in declined expression of its bound genes.
Discussion
We provide a direct evidence to demonstrate the link of Tet enzyme to aging. In this study, we present an important role of Tet enzymes in reproductive aging using knockout mouse model, which has advantages in study of aging in general. We have shown that Tet enzyme and its intermediate product 5hmC regulate germline and stem cell aging. Although Tet1 or Tet2 deficiency leads to reduced 5hmC and increased 5mC to similar levels, compared with wild-type mice, they result in differential impact on reproductive aging and perhaps aging in general. It is likely that transcriptional regulation by Tet1 in combination with methylation/demethylation together regulate reproductive aging.
Increasing evidence shows reduced levels of 5hmC and Tet enzymes with age and these suggest their potential participation in aging. Aging is associated with decreased TET1 and TET2 expression (Jessop and Toledo-Rodriguez, 2018). Tet1 expression levels also decease with age and are associated with reduced fertility (Ni et al., 2016), as shown here in mice. Similarly, a decrease in Tet2 expression and 5hmC levels was detected in the aged hippocampus associated with adult neurogenesis (Gontier et al., 2018). An age-related decline of TET1, TET3 and TDG gene expression along with a decrease of 5hmC and an accumulation of 5caC, and the observed impairment of 5hmC-mediated DNA demethylation pathway in blood cells may lead to aberrant transcriptional programs in the elderly (Valentini et al., 2016). Also, it has been demonstrated that 5hmC content decreased with age in the peripheral blood T cells, and that the mRNA expression levels of TET1 and TET3 decreased with age, while those of TET2 were not influenced by age (Truong et al., 2015). In contrast, evidence also is reported that global 5hmC content was increased during aging in the absence of 5mC decrease, suggesting that 5hmC could act as an epigenetic marker and not only as an intermediary in DNA demethylation (Chen et al., 2012). Similar to cerebrum, the human cerebellum shows an age-dependent increase in 5hmC content, although at lower absolute levels (Wagner et al., 2015), consistent with others suggesting that increased 5hmC may play a role in age-related neurodegeneration (Song et al., 2011, Szulwach et al., 2011). However, age-related changes in DNA hydroxymethylation could be more complicated, and cell type specific and gene specific (Kochmanski et al., 2018). These age-correlated observations also could not determine whether levels of 5hmC or Tet enzymes contribute to aging, or are the result of aging.
We intended to address these questions by using Tet knockout mice as prove of principles. We sought firstly to investigate whether Tet deficiency can actually cause premature aging using Tet knockout mice as a model, and then to understand how Tet deficiency and/or 5hmC levels accelerates aging. To shorten the observation period, here we focused on reproductive aging in males.
Indeed, our data reveals that Tet1 plays a major role in regulating reproductive aging unlike Tet2. Tet1 deficiency results in reduced expression of genes, like those of natural aging. The genes downregulated in aging males or in Tet1-deficient males are enriched in in spermatogonia cell cycle progression, spermatogenesis, meiosis, germ cell differentiation and DNA repair etc. For instance, Ccna1 expression is greatly reduced in Tet1-deficient males, like that of natural reproductive aging males, compared with young mice. Ccna1 (Cyclin A1) is expressed in mice exclusively in the germ cell lineage and is required for meiosis in the male mouse. Ccna1−/− males were sterile due to a block of spermatogenesis before the first meiotic division, whereas females were normal. Meiosis arrest in Ccna1−/− males was associated with increased germ cell apoptosis and desynapsis abnormalities (Liu et al., 1998). Ccna1+/− male mice also show reduced sperm production and fertility (van der Meer et al., 2004). Reduced expression of Ccna1 following Tet1 deficiency could negatively affect self-renewal of spermatogonia cells and delays meiotic cell cycle, consistent with the observation that PLZF-positive spermatogonia cells are greatly reduced in Tet1-deficient males and in natural aging. Also, Spo11, critical for meiosis homologous pairing and recombination initiation (Boateng et al., 2013, Keeney et al., 1997), is down-regulated in Tet1-deficient spermatogonia and spermatocytes, like that of natural reproductive aging males. We observed significantly delayed meiosis progression and homologous pairing and recombination in Tet1-deficient meiocytes. DNA methyltransferase 3-like (Dnmt3L) is expressed in SSCs and their precursors. Loss of Dnmt3L from early germ cells also caused meiotic failure in spermatocytes and infertility (Bourc'his and Bestor, 2004). In Dnmt3L−/- meiotic spermatocytes, homologous chromosomes fail to align and form synaptonemal complexes, spermatogenesis arrests, and spermatocytes are lost by apoptosis and sloughing (Webster et al., 2005).
Moreover, aldehyde dehydrogenase (ALDH) enzymes are critical in the detoxification of aldehydes. ALDH3B1 belongs to the ALDH3 family and protects cells from the damaging effects of oxidative stress (Marchitti et al., 2010). Ppp1r36, a regulatory subunit of protein phosphatase 1, is highly enriched in human (Fagerberg et al., 2014) and mouse testis, and enhances autophagy during spermatogenesis (Zhang et al., 2016). These genes showed significant reduction in the expression in both Tet1 deficient mice and old mice. It is also interesting to note that both Tet1 deficient mice and natural aging mice showed similarly declined signal pathways, including Wnt, Retinol metabolism, Ras/MAPK, PI3K-Akt, and AMPK. These are involved in aging, or spermatogonia stem cell proliferation (Griswold, 2016, Grive et al., 2019, Takase and Nusse, 2016). Tet1−/− spermatogonia cells had abnormal retinol metabolism, reducing meiosis entry (Bowles et al., 2006, Feng et al., 2014). MAPK signaling pathway plays a key role in maintaining self-renewal capacity of mouse male germline stem cells (Gao et al., 2018, Hasegawa et al., 2013). Actually, mutations dysregulating RAS-MAPK signaling are often found in aged human testes (Maher et al., 2018). It is likely that reduced expression of these important genes and defective signaling pathways together contribute to accelerated reproductive aging resulting from Tet1 deficiency, similar to natural aging.
Telomere shortening also leads to aging or accelerated aging (Armanios et al., 2009, Armanios and Blackburn, 2012, Blackburn et al., 2015, Blasco, 2005). High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells (Pech et al., 2015). Telomerase activity is expressed at high levels in the type A spermatogonial stem cells, is down-regulated during spermatogenesis, and is absent in the differentiated spermatozoa (Ravindranath et al., 1997). Tet enzyme also is involved in the regulation of telomere length in mouse ESCs and epiblasts (Khoueiry et al., 2017, Lu et al., 2014, Yang et al., 2016). In epiblast cells, Tet1 demethylates gene promoters via hydroxylation of 5mC and maintains telomere stability (Khoueiry et al., 2017). We show that telomeres are shortened in spermatogonia cells as well as in spermatocytes of Tet1 deficient mice.
Our study highlights an interplay between the catalytic and non-catalytic activities of Tet1 that plays important role in regulating transcription of spermatogonia and spermatogenesis, contributing to both normal development and aging. Generally, DNA methylation in regions near the TSS is closely associated with the suppression of gene expression (Xiao et al., 2019). Tet1 and Tet2 function to regulate locus-specific methylation during PGC development. Tet depletion induces promoter and gene body hypermethylation that is consistent with 5hmC having a locus-specific role in DNA demethylation in PGCs (Hackett et al., 2013, Vincent et al., 2013). There is a possibility that reduced expression of genes related to germ cells and spermatogenesis might result from hypermethylation when Tet1 is deficient. There has been very little information on how the age-related changes in DNA methylation affect the transcriptome or function of a tissue or cell. Methylation changes, in association with reduced RNA expression levels, in germ cells with age or following Tet1 deficiency, may not be the same as those in blood which strongly predicts lifespan and healthspan (Lu et al., 2019). Tissues have heterogeneous cell populations, and it is well established that different cell types have different epigenetic profiles. The effect of aging on DNA methylation could be cell-type specific. TET1 stimulates transcription of germline reprogramming-responsive genes via a DNA demethylation-independent mechanism, thus enabling progression toward gametogenesis (Hill et al., 2018). Despite reduced gene expression, methylation levels of spermatogenesis-related promoters remain relatively stable, and no substantial difference in the global DNA methylation patterns was observed between 18 week and 17 month samples (Kobayashi et al., 2016). Our data shows that 5hmC also does not enrich at the TSS of those genes downregulated with age or due to Tet1 deficiency in male germline cells. Whether spermatogonia cells may have their unique methylome aging marks requires further investigation.
Limitations of the Study
Tet1 knockout decreases 5hmC levels in spermatogonia cells and downregulates a clear set of genes important for cell cycle progression, germ cell differentiation, meiosis and reproduction, such as Ccna1 and Spo11. Furthermore, Tet1 and 5hmC both regulate signaling pathways important for stem cell development, retinol metabolism, MAPK, PI3K-Akt, and AMPK, etc. These gene expression changes were obtained by bioinformatics analysis of RNA-seq data and in partial combination with ChIP-seq data. Further experiments will be needed to look at whether the gene expression can actually lead to changes at the protein levels by proteomics analysis and by western blot.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31571546, 91749129).
Author Contributions
G.H. designed and performed major experiments and prepared manuscript. L.L.L. analyzed the RNA-seq and CHIP-seq data. H.W. conducted major experiments. M.G. J.Y. and W.D. helped genotyping and other tests. P.G. assisted IF-FISH experiment. C.T. helped TRF and CHIP assay. T.Z provided reagents and materials. G.X. provided the Tet1 and Tet2 knockout mice, advised the study, and revised the manuscript. L.L. conceived the study, designed experiments and wrote the manuscript.
Declaration of Interests
The authors declare they have no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100908.
Contributor Information
Guo-Liang Xu, Email: glxu@sibcb.ac.cn.
Lin Liu, Email: liulin@nankai.edu.cn.
Data and Code Availability
Data and code related to this paper may be requested from the authors. Raw RNA-seq sequences are available online (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE140014) and raw ChIP-seq sequences are available online (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE140999). The accession number for the dataset reported in this paper is NCBI: [GSE140014 and GSE140999].
Supplemental Information
References
- Armanios M., Blackburn E.H. The telomere syndromes. Nat. Rev. Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M., Alder J.K., Parry E.M., Karim B., Strong M.A., Greider C.W. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B., Hunt P., Broman K.W., Hassold T. Variation in genome-wide levels of meiotic recombination is established at the onset of prophase in mammalian males. PLoS Genet. 2014;10:e1004125. doi: 10.1371/journal.pgen.1004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.M., Plug A.W., Prolla T.A., Bronner C.E., Harris A.C., Yao X., Christie D.M., Monell C., Arnheim N., Bradley A. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- Bannister L.A., Schimenti J.C. Homologous recombinational repair proteins in mouse meiosis. Cytogenet. Genome Res. 2004;107:191–200. doi: 10.1159/000080597. [DOI] [PubMed] [Google Scholar]
- Blackburn E.H., Epel E.S., Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- Blasco M.A. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Boateng K.A., Bellani M.A., Gregoretti I.V., Pratto F., Camerini-Otero R.D. Homologous pairing preceding SPO11-mediated double-strand breaks in mice. Dev. Cell. 2013;24:196–205. doi: 10.1016/j.devcel.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourc'his D., Bestor T.H. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–99. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- Bowles J., Knight D., Smith C., Wilhelm D., Richman J., Mamiya S., Yashiro K., Chawengsaksophak K., Wilson M.J., Rossant J. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Buaas F.W., Kirsh A.L., Sharma M., McLean D.J., Morris J.L., Griswold M.D., de Rooij D.G., Braun R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Buscarlet M., Tessier A., Provost S., Mollica L., Busque L. Human blood cell levels of 5-hydroxymethylcytosine (5hmC) decline with age, partly related to acquired mutations in TET2. Exp. Hematol. 2016;44:1072–1084. doi: 10.1016/j.exphem.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Chen D., Kerr C. The epigenetics of stem cell aging comes of age. Trends Cell Biol. 2019;29:563–568. doi: 10.1016/j.tcb.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Dzitoyeva S., Manev H. Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus. Restor. Neurol. Neurosci. 2012;30:237–245. doi: 10.3233/RNN-2012-110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoya J.A., Hobbs R.M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K.E., Wolgemuth D.J., Pandolfi P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Cui X., Jing X., Wu X., Yan M., Li Q., Shen Y., Wang Z. DNA methylation in spermatogenesis and male infertility. Exp. Ther. Med. 2016;12:1973–1979. doi: 10.3892/etm.2016.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H.Q., Wang B.A., Yang L., Chen J.J., Zhu G.C., Sun M.L., Ge H., Wang R., Chapman D.L., Tang F. TET-mediated DNA demethylation controls gastrulation by regulating Lefty-Nodal signalling. Nature. 2016;538:528–532. doi: 10.1038/nature20095. [DOI] [PubMed] [Google Scholar]
- Dawlaty M.M., Ganz K., Powell B.E., Hu Y.C., Markoulaki S., Cheng A.W., Gao Q., Kim J., Choi S.W., Page D.C. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9:166–175. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawlaty M.M., Breiling A., Le T., Raddatz G., Barrasa M.I., Cheng A.W., Gao Q., Powell B.E., Li Z., Xu M. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell. 2013;24:310–323. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- Dong G., Shang Z., Liu L., Liu C., Ge Y., Wang Q., Wu L., Chen F., Li B., Liu X. Retinoic acid combined with spermatogonial stem cell conditions facilitate the generation of mouse germ-like cells. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy J.E.M., O'Bryan M., Stanton P.G., O'Donnell L. The cytoskeleton in spermatogenesis. Reproduction. 2019;157:R53–R72. doi: 10.1530/REP-18-0457. [DOI] [PubMed] [Google Scholar]
- Fagerberg L., Hallstrom B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayomi A.P., Orwig K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018;29:207–214. doi: 10.1016/j.scr.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C.W., Bowles J., Koopman P. Control of mammalian germ cell entry into meiosis. Mol. Cell. Endocrinol. 2014;382:488–497. doi: 10.1016/j.mce.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Fuke C., Shimabukuro M., Petronis A., Sugimoto J., Oda T., Miura K., Miyazaki T., Ogura C., Okazaki Y., Jinno Y. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann. Hum. Genet. 2004;68:196–204. doi: 10.1046/j.1529-8817.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- Gan H., Wen L., Liao S., Lin X., Ma T., Liu J., Song C.X., Wang M., He C., Han C. Dynamics of 5-hydroxymethylcytosine during mouse spermatogenesis. Nat. Commun. 2013;4:1995. doi: 10.1038/ncomms2995. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao X., Liu C., Shao B., Zhang X., Li K., Cai J., Wang S., Huang X. Somatic angiotensin I-converting enzyme regulates self-renewal of mouse spermatogonial stem cells through the mitogen-activated protein kinase signaling pathway. Stem Cells Dev. 2018;27:1021–1032. doi: 10.1089/scd.2017.0287. [DOI] [PubMed] [Google Scholar]
- Gontier G., Iyer M., Shea J.M., Bieri G., Wheatley E.G., Ramalho-Santos M., Villeda S.A. Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 2018;22:1974–1981. doi: 10.1016/j.celrep.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S., Cohen P.E. Control of meiotic crossovers: from double-strand break formation to designation. Annu. Rev. Genet. 2016;50:175–210. doi: 10.1146/annurev-genet-120215-035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.D., Ma Q., Manske G.L., Shami A.N., Zheng X., Marini S., Moritz L., Sultan C., Gurczynski S.J., Moore B.B. A comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-seq. Dev. Cell. 2018;46:651–667.e10. doi: 10.1016/j.devcel.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.V.C., Bourc'his D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019;20:590–607. doi: 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- Griswold M.D. Spermatogenesis: the commitment to meiosis. Physiol. Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grive K.J., Hu Y., Shu E., Grimson A., Elemento O., Grenier J.K., Cohen P.E. Dynamic transcriptome profiles within spermatogonial and spermatocyte populations during postnatal testis maturation revealed by single-cell sequencing. PLoS Genet. 2019;15:e1007810. doi: 10.1371/journal.pgen.1007810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T.P., Guo F., Yang H., Wu H.P., Xu G.F., Liu W., Xie Z.G., Shi L., He X., Jin S.G. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–610. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- Guillon H., Baudat F., Grey C., Liskay R.M., de Massy B. Crossover and noncrossover pathways in mouse meiosis. Mol. Cell. 2005;20:563–573. doi: 10.1016/j.molcel.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Hackett J.A., Sengupta R., Zylicz J.J., Murakami K., Lee C., Down T.A., Surani M.A. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339:448–452. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud S.S., Low D.H., Yi C., Carrell D.T., Guccione E., Cairns B.R. Chromatin and transcription transitions of mammalian adult germline stem cells and spermatogenesis. Cell Stem Cell. 2014;15:239–253. doi: 10.1016/j.stem.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Hammoud S.S., Low D.H., Yi C., Lee C.L., Oatley J.M., Payne C.J., Carrell D.T., Guccione E., Cairns B.R. Transcription and imprinting dynamics in developing postnatal male germline stem cells. Genes Dev. 2015;29:2312–2324. doi: 10.1101/gad.261925.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargan-Calvopina J., Taylor S., Cook H., Hu Z., Lee S.A., Yen M.R., Chiang Y.S., Chen P.Y., Clark A.T. Stage-specific demethylation in primordial germ cells safeguards against precocious differentiation. Dev. Cell. 2016;39:75–86. doi: 10.1016/j.devcel.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Namekawa S.H., Saga Y. MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem Cells. 2013;31:2517–2527. doi: 10.1002/stem.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Hansen T., Hunt P., VandeVoort C. Cytological studies of recombination in rhesus males. Cytogenet. Genome Res. 2009;124:132–138. doi: 10.1159/000207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.F., Li B.Z., Li Z., Liu P., Wang Y., Tang Q., Ding J., Jia Y., Chen Z., Li L. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn H., Li N., Ferreira H.J., Moran S., Pisano D.G., Gomez A., Diez J., Sanchez-Mut J.V., Setien F., Carmona F.J. Distinct DNA methylomes of newborns and centenarians. Proc. Natl. Acad. Sci. U S A. 2012;109:10522–10527. doi: 10.1073/pnas.1120658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill P.W.S., Leitch H.G., Requena C.E., Sun Z., Amouroux R., Roman-Trufero M., Borkowska M., Terragni J., Vaisvila R., Linnett S. Epigenetic reprogramming enables the transition from primordial germ cell to gonocyte. Nature. 2018;555:392–396. doi: 10.1038/nature25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs R.M., Fagoonee S., Papa A., Webster K., Altruda F., Nishinakamura R., Chai L., Pandolfi P.P. Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell. 2012;10:284–298. doi: 10.1016/j.stem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C.A., Evans E., Onken J., Kent T., Mitchell D., Petkovich M., Griswold M.D. CYP26 enzymes are necessary within the postnatal seminiferous epithelium for normal murine spermatogenesis. Biol. Reprod. 2015;93:19. doi: 10.1095/biolreprod.115.129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- Hunter N. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 2015;7:a016618. doi: 10.1101/cshperspect.a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., D'Alessio A.C., Taranova O.V., Hong K., Sowers L.C., Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Shen L., Dai Q., Wu S.C., Collins L.B., Swenberg J.A., He C., Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T.G., Aston K.I., Pflueger C., Cairns B.R., Carrell D.T. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014;10:e1004458. doi: 10.1371/journal.pgen.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T.G., Aston K.I., Meyer T., Carrell D.T. The sperm epigenome, male aging, and potential effects on the embryo. Adv. Exp. Med. Biol. 2015;868:81–93. doi: 10.1007/978-3-319-18881-2_4. [DOI] [PubMed] [Google Scholar]
- Jessop P., Toledo-Rodriguez M. Hippocampal TET1 and TET2 expression and DNA hydroxymethylation are affected by physical exercise in aged mice. Front. Cell Dev. Biol. 2018;6:45. doi: 10.3389/fcell.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S., Giroux C.N., Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- Khoueiry R., Sohni A., Thienpont B., Luo X., Velde J.V., Bartoccetti M., Boeckx B., Zwijsen A., Rao A., Lambrechts D. Lineage-specific functions of TET1 in the postimplantation mouse embryo. Nat. Genet. 2017;49:1061–1072. doi: 10.1038/ng.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klutstein M., Nejman D., Greenfield R., Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76:3446–3450. doi: 10.1158/0008-5472.CAN-15-3278. [DOI] [PubMed] [Google Scholar]
- Ko M., Bandukwala H.S., An J., Lamperti E.D., Thompson E.C., Hastie R., Tsangaratou A., Rajewsky K., Koralov S.B., Rao A. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc. Natl. Acad. Sci. U S A. 2011;108:14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Okae H., Hiura H., Chiba H., Shirakata Y., Hara K., Tanemura K., Arima T. Genome-scale assessment of age-related DNA methylation changes in mouse spermatozoa. PLoS One. 2016;11:e0167127. doi: 10.1371/journal.pone.0167127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochmanski J., Marchlewicz E.H., Cavalcante R.G., Sartor M.A., Dolinoy D.C. Age-related epigenome-wide DNA methylation and hydroxymethylation in longitudinal mouse blood. Epigenetics. 2018;13:779–792. doi: 10.1080/15592294.2018.1507198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K.P., Yabuuchi A., Rao S., Huang Y., Cunniff K., Nardone J., Laiho A., Tahiliani M., Sommer C.A., Mostoslavsky G. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H., Brinster R.L. Spermatogonial stem cells. Biol. Reprod. 2018;99:52–74. doi: 10.1093/biolre/ioy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law N.C., Oatley M.J., Oatley J.M. Developmental kinetics and transcriptome dynamics of stem cell specification in the spermatogenic lineage. Nat. Commun. 2019;10:2787. doi: 10.1038/s41467-019-10596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yue X., Pastor W.A., Lin L., Georges R., Chavez L., Evans S.M., Rao A. Tet proteins influence the balance between neuroectodermal and mesodermal fate choice by inhibiting Wnt signaling. Proc. Natl. Acad. Sci. U S A. 2016;113:E8267–E8276. doi: 10.1073/pnas.1617802113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Matzuk M.M., Sung W.K., Guo Q., Wang P., Wolgemuth D.J. Cyclin A1 is required for meiosis in the male mouse. Nat. Genet. 1998;20:377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Liu Y., Jiang L., Yamaguchi S., Zhang Y. Role of Tet proteins in enhancer activity and telomere elongation. Genes Dev. 2014;28:2103–2119. doi: 10.1101/gad.248005.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A.T., Quach A., Wilson J.G., Reiner A.P., Aviv A., Raj K., Hou L., Baccarelli A.A., Li Y., Stewart J.D. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11:303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher G.J., Ralph H.K., Ding Z., Koelling N., Mlcochova H., Giannoulatou E., Dhami P., Paul D.S., Stricker S.H., Beck S. Selfish mutations dysregulating RAS-MAPK signaling are pervasive in aged human testes. Genome Res. 2018;28:1779–1790. doi: 10.1101/gr.239186.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitti S.A., Brocker C., Orlicky D.J., Vasiliou V. Molecular characterization, expression analysis, and role of ALDH3B1 in the cellular protection against oxidative stress. Free Radic. Biol. Med. 2010;49:1432–1443. doi: 10.1016/j.freeradbiomed.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K., Reavie L., Shih A., Abdel-Wahab O., Ndiaye-Lobry D., Lobry C., Figueroa M.E., Vasanthakumar A., Patel J., Zhao X. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettersheim D., Heukamp L.C., Fronhoffs F., Grewe M.J., Haas N., Waha A., Honecker F., Waha A., Kristiansen G., Schorle H. Analysis of TET expression/activity and 5mC oxidation during normal and malignant germ cell development. PLoS One. 2013;8:e82881. doi: 10.1371/journal.pone.0082881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K., Dansranjavin T., Rogenhofer N., Oeztuerk N., Deuker J., Bergmann M., Schuppe H.C., Wagenlehner F., Weidner W., Steger K. TET enzymes are successively expressed during human spermatogenesis and their expression level is pivotal for male fertility. Hum. Reprod. 2016;31:1411–1424. doi: 10.1093/humrep/dew096. [DOI] [PubMed] [Google Scholar]
- Pastor W.A., Aravind L., Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013;14:341–356. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech M.F., Garbuzov A., Hasegawa K., Sukhwani M., Zhang R.J., Benayoun B.A., Brockman S.A., Lin S., Brunet A., Orwig K.E. High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 2015;29:2420–2434. doi: 10.1101/gad.271783.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath N., Dalal R., Solomon B., Djakiew D., Dym M. Loss of telomerase activity during male germ cell differentiation. Endocrinology. 1997;138:4026–4029. doi: 10.1210/endo.138.9.5488. [DOI] [PubMed] [Google Scholar]
- Reuter M., Berninger P., Chuma S., Shah H., Hosokawa M., Funaya C., Antony C., Sachidanandam R., Pillai R.S. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature. 2011;480:264–267. doi: 10.1038/nature10672. [DOI] [PubMed] [Google Scholar]
- Saba R., Wu Q., Saga Y. CYP26B1 promotes male germ cell differentiation by suppressing STRA8-dependent meiotic and STRA8-independent mitotic pathways. Dev. Biol. 2014;389:173–181. doi: 10.1016/j.ydbio.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Schmidt J.A., Abramowitz L.K., Kubota H., Wu X., Niu Z., Avarbock M.R., Tobias J.W., Bartolomei M.S., Brinster R.L. In vivo and in vitro aging is detrimental to mouse spermatogonial stem cell function. Biol. Reprod. 2011;84:698–706. doi: 10.1095/biolreprod.110.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Wistuba J., Pock T., Schlatt S., Neuhaus N. Spermatogonial stem cells: updates from specification to clinical relevance. Hum. Reprod. Update. 2019;25:275–297. doi: 10.1093/humupd/dmz006. [DOI] [PubMed] [Google Scholar]
- Song C.X., Szulwach K.E., Fu Y., Dai Q., Yi C., Li X., Li Y., Chen C.H., Zhang W., Jian X. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat. Biotechnol. 2011;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs T.M., Bonder M.J., Stark A.K., Krueger F., Team B.I.A.C., von Meyenn F., Stegle O., Reik W. Multi-tissue DNA methylation age predictor in mouse. Genome Biol. 2017;18:68. doi: 10.1186/s13059-017-1203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach K.E., Li X., Li Y., Song C.X., Wu H., Dai Q., Irier H., Upadhyay A.K., Gearing M., Levey A.I. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase H.M., Nusse R. Paracrine Wnt/beta-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc. Natl. Acad. Sci. U S A. 2016;113:E1489–E1497. doi: 10.1073/pnas.1601461113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Shi Y.G. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139:1895–1902. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.F., Atzmon G., Gheorghe C., Liang H.Q., Lowes C., Greally J.M., Barzilai N. Tissue-specific dysregulation of DNA methylation in aging. Aging Cell. 2010;9:506–518. doi: 10.1111/j.1474-9726.2010.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.J., Chwialkowska K., Rubbi L., Lusis A.J., Davis R.C., Srivastava A., Korstanje R., Churchill G.A., Horvath S., Pellegrini M. A multi-tissue full lifespan epigenetic clock for mice. Aging (Albany NY) 2018;10:2832–2854. doi: 10.18632/aging.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torano E.G., Bayon G.F., Del Real A., Sierra M.I., Garcia M.G., Carella A., Belmonte T., Urdinguio R.G., Cubillo I., Garcia-Castro J. Age-associated hydroxymethylation in human bone-marrow mesenchymal stem cells. J. Transl. Med. 2016;14:207. doi: 10.1186/s12967-016-0966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong T.P., Sakata-Yanagimoto M., Yamada M., Nagae G., Enami T., Nakamoto-Matsubara R., Aburatani H., Chiba S. Age-dependent decrease of DNA hydroxymethylation in human T cells. J. Clin. Exp. Hematop. 2015;55:1–6. doi: 10.3960/jslrt.55.1. [DOI] [PubMed] [Google Scholar]
- Valentini E., Zampieri M., Malavolta M., Bacalini M.G., Calabrese R., Guastafierro T., Reale A., Franceschi C., Hervonen A., Koller B. Analysis of the machinery and intermediates of the 5hmC-mediated DNA demethylation pathway in aging on samples from the MARK-AGE Study. Aging (Albany NY) 2016;8:1896–1922. doi: 10.18632/aging.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer T., Chan W.Y., Palazon L.S., Nieduszynski C., Murphy M., Sobczak-Thepot J., Carrington M., Colledge W.H. Cyclin A1 protein shows haplo-insufficiency for normal fertility in male mice. Reproduction. 2004;127:503–511. doi: 10.1530/rep.1.00131. [DOI] [PubMed] [Google Scholar]
- Vincent J.J., Huang Y., Chen P.Y., Feng S., Calvopina J.H., Nee K., Lee S.A., Le T., Yoon A.J., Faull K. Stage-specific roles for tet1 and tet2 in DNA demethylation in primordial germ cells. Cell Stem Cell. 2013;12:470–478. doi: 10.1016/j.stem.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Steinbacher J., Kraus T.F., Michalakis S., Hackner B., Pfaffeneder T., Perera A., Muller M., Giese A., Kretzschmar H.A. Age-dependent levels of 5-methyl-, 5-hydroxymethyl-, and 5-formylcytosine in human and mouse brain tissues. Angew. Chem. Int. Ed. 2015;54:12511–12514. doi: 10.1002/anie.201502722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K.E., O'Bryan M.K., Fletcher S., Crewther P.E., Aapola U., Craig J., Harrison D.K., Aung H., Phutikanit N., Lyle R. Meiotic and epigenetic defects in Dnmt3L-knockout mouse spermatogenesis. Proc. Natl. Acad. Sci. U S A. 2005;102:4068–4073. doi: 10.1073/pnas.0500702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Christensen J., Pedersen M.T., Johansen J.V., Cloos P.A., Rappsilber J., Helin K. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V.L., Smith R.A., Ma S., Cutler R.G. Genomic 5-methyldeoxycytidine decreases with age. J. Biol. Chem. 1987;262:9948–9951. [PubMed] [Google Scholar]
- Wossidlo M., Nakamura T., Lepikhov K., Marques C.J., Zakhartchenko V., Boiani M., Arand J., Nakano T., Reik W., Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat. Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- Wrobel K.H., Bickel D., Kujat R. Immunohistochemical study of seminiferous epithelium in adult bovine testis using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen (PCNA) Cell Tissue Res. 1996;283:191–201. doi: 10.1007/s004410050529. [DOI] [PubMed] [Google Scholar]
- Wu X., Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat. Rev. Genet. 2017;18:517–534. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- Wu X., Li G., Xie R. Decoding the role of TET family dioxygenases in lineage specification. Epigenetics Chromatin. 2018;11:58. doi: 10.1186/s13072-018-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F.H., Wang H.T., Kong Q.P. Dynamic DNA methylation during aging: a "prophet" of age-related outcomes. Front. Genet. 2019;10:107. doi: 10.3389/fgene.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Mao X., Huang J., Ding Y., Wu J., Dong S., Kong L., Gao G., Li C.Y., Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Hong K., Liu R., Shen L., Inoue A., Diep D., Zhang K., Zhang Y. Tet1 controls meiosis by regulating meiotic gene expression. Nature. 2012;492:443–447. doi: 10.1038/nature11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Guo R.P., Wang H., Ye X.Y., Zhou Z.C., Dan J.M., Wang H.Y., Gong P., Deng W., Yin Y. Tet enzymes regulate telomere maintenance and chromosomal stability of mouse ESCs. Cell Rep. 2016;15:1809–1821. doi: 10.1016/j.celrep.2016.04.058. [DOI] [PubMed] [Google Scholar]
- Yuan L., Liu J.G., Zhao J., Brundell E., Daneholt B., Hoog C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol. Cell. 2000;5:73–83. doi: 10.1016/s1097-2765(00)80404-9. [DOI] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.R., Cui Q.Y., Murai K., Lim Y.C., Smith Z.D., Jin S., Ye P., Rosa L., Lee Y.K., Wu H.P. Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell. 2013;13:237–245. doi: 10.1016/j.stem.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Gao M., Zhang Y., Song Y., Cheng H., Zhou R. The germline-enriched Ppp1r36 promotes autophagy. Sci. Rep. 2016;6:24609. doi: 10.1038/srep24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Shao H., Zhang D., Dong J., Cheng W., Wang L., Teng Y., Yu Z. PTEN signaling is required for the maintenance of spermatogonial stem cells in mouse, by regulating the expressions of PLZF and UTF1. Cell Biosci. 2015;5:42. doi: 10.1186/s13578-015-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code related to this paper may be requested from the authors. Raw RNA-seq sequences are available online (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE140014) and raw ChIP-seq sequences are available online (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE140999). The accession number for the dataset reported in this paper is NCBI: [GSE140014 and GSE140999].