Abstract
Background
Central post-stroke pain (CPSP) is refractory to pharmacotherapy (eg, NSAIDs, opioids, antidepressants, and anticonvulsants), and may require transcranial or deep brain stimulation.
Case Presentation
A 67-year-old woman presented with severe paroxysmal cramp-like pain on the right side, including the head and both upper and lower extremities. The pain started 5 years earlier, was initially mild and occasional, but gradually intensified to an unbearable degree with an average of 10–15 daily episodes, each lasting for 5–10 mins. The patient disclosed “hemorrhagic stroke” 10 years ago that resulted in hemiplegia on the right side. CT examination verified the lesion. The patient received daily injection of 2-mL 2% lidocaine under ultrasound guidance to block the stellate ganglion. Pain subsided rapidly in both intensity and frequency. On the seventh day, the patient no longer had pain episodes. At the last follow-up, 9 months later, the patient was free from pain.
Conclusion
Ultrasound-guided stellate ganglion block is a viable alternative for CPSP that is refractory to pharmacotherapy.
Keywords: central post-stroke pain, cramp-like pain, ultrasound, stroke, stellate ganglion block
Introduction
Central post-stroke pain (CPSP) is the central equivalent of peripheral neuropathic pain.1 The incidence of CPSP after stroke is unknown, but has been estimated at 1–12%.2–5 CPSP typically develops in the sub-acute or chronic stages of ischemic or hemorrhagic stroke. CPSP was originally referred to as Dejerine-Roussy syndrome, and later as thalamic pain syndrome.6 Since brain sites other than the thalamus (for example, medulla oblongata), the current formal term is CPSP.4,7 CPSP is typically refractory to pharmacotherapy (NSAIDs, opioids, antidepressants, and anticonvulsants). More recent treatment options included stereotaxic irradiation of the pituitary with γ-irradiation, trans-cranial cortex stimulation and deep brain stimulation.8–10 Stellate ganglion block is a method to treat reflex sympathetic dystrophy,11 and was reported to be effective for thalamic pain in a case report in 2007.12 We now report a case of successful treatment of cramp-like pain after thalamic hemorrhage using ultrasound-guided stellate ganglion block.
Written informed consent has been provided by the patient to have the case details and accompanying images published.
Case Presentation
A 67-year-old woman presented with severe paroxysmal cramp-like pain on the right side, including the head and both upper and lower extremities. The pain started 5 years ago, and there was no obvious cues that preceded or elicited the episodes. The pain was initially mild and occasional, but gradually intensified to an unbearable degree with an average of 10–15 daily episodes, each lasting for 5–10 mins. The pain had profound impact on daily life: the patient was practically confined to a wheelchair due to muscle stiffness. She was profoundly anxious and depressive, and attempted a few unsuccessful suicides.
The patient had a “hemorrhagic stroke” 10 years ago that resulted in hemiplegia on the right side. Both the right arm and legs were completely paralyzed, but voluntary movements gradually recovered. She was able to walk alone slowly using a cane and manage an independent life. Five years after the stroke, cramp-like pain emerged. Despite of treatments with NSAIDs, tramadol, duloxetine and gabapentin, the pain gradually increased in intensity and episode frequency. Motor cortical stimulation and deep brain stimulation were offered but refused.
Upon assessment using a 10-point visual analogue scale, the pain intensity was 7–10. The Barthel activity of daily living score was 40 (the 100 maximum indicates full functioning13) (Table 1). Hospital anxiety and depression scale (HADS) was 12 for anxiety and 14 for depression.14 The patient reported stiffness throughout the right side of the body, and heavy sweating and on the right side of the face. Thermosensation in the right arm and leg, particularly to cold stimuli, was impaired. Muscle tone was significantly increased, with intact but diminished deep tendon reflex. Cranial CT and MRI were consistent with lesions to the left dorsal thalamus and medial temporal lobe (Figure 1A). Extensive hemosiderosis was apparent, indicating hemorrhagic stroke. She had hypertension for 10 years, but blood pressure was well controlled.
Table 1.
Preoperative and 9 Months Postoperative VAS, ADL and HADS Scores
| Preoperative | Postoperative | ||
|---|---|---|---|
| VAS | 7–9 | 0–1 | |
| ADL | 40 | 80 | |
| HADS | Anxiety | 12 | 6 |
| Depression | 14 | 8 | |
Abbreviations: VAS, visual analog scale; ADL, barthel activity of daily living; HADS, hospital anxiety and depression scale.
Figure 1.
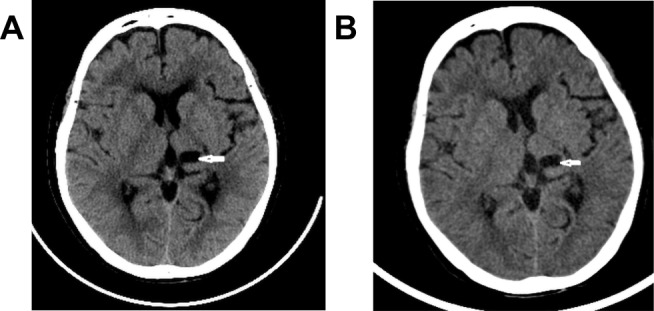
Cranial CT (A) upon presentation and (B) 9 months after stellate ganglion block. White arrows: lesions.
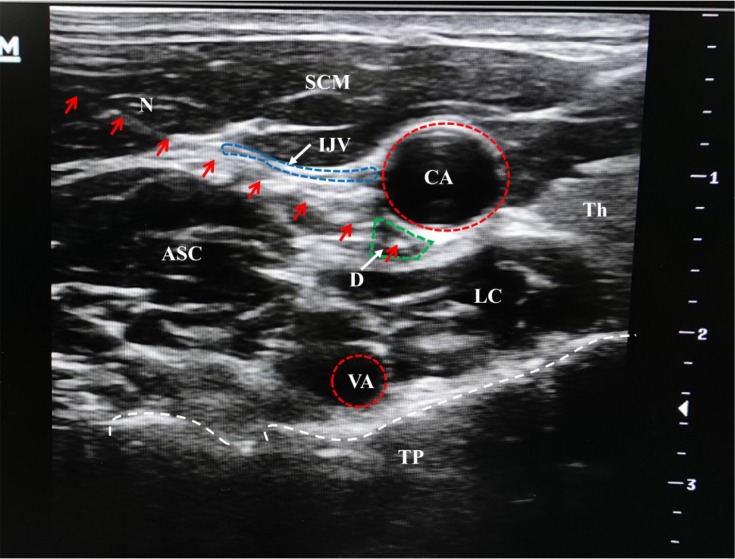
Stellate ganglion block was performed, as described previously. Briefly, 2-mL 2% lidocaine was injected between the internal carotid artery and the longus colli at the C7 level under ultrasound guidance (Figure 2).15,16 The block was repeated once every day, and the pain subsided rapidly in both intensity and frequency along with the advancement of daily treatment. On the seventh day after treatment initiation, the patient no longer had pain episode. Thermosensation normalized, and she was able to walk for 200 m on her own. A physical workup showed reduced muscle tension and normalized deep tension reflex. Stiffness persisted, but at a reduced degree that could be readily managed with gabapentin at small doses. At the last follow-up 9 months later, the patient was free from pain and only took gabapentin on-demand occasionally. A repeat cranial CT (Figure 1B) was unremarkable. ADL score increased to 80. HADS score was 6 for anxiety and 8 for depression.
Figure 2.
A schematic illustration of ultrasound-guided lidocaine injection.
Notes: Red arrows: needle trajectory; white arrows: arrows for indications; red-dotted circles: the shapes of the arteries; blue-dotted circle: the wall of internal jugular vein; green-dotted circle: diffusion of local anesthetics; white-dotted line: the shape of transverse process.
Abbreviations: ASC, Anterior scalene muscle; CA, Carotid artery; D, Drug; IJV, Internal jugular vein; LC, Longus colli muscle; N, puncture needle; SCM, Sternocleidomastoid muscle; TH; Thyroid gland; TP, Transverse process; VA, Vertebral artery.
Discussion
In the index case, the patient had 5-years of increasing severe and frequent episodes of cramp-like CPSP apparently due to previous hemorrhagic stroke. The pain did not responded to attempted treatments with a variety of pharmacotherapy that included NSAIDs, tramadol, duloxetine and gabapentin. Treatment with ultrasound-guided stellate ganglion block, in contrast, abrogated the pain. Stiffness was also alleviated. It is noteworthy that the pain tin the leg was also abrogated since stellate ganglion block has been typically used to treat reflex sympathetic dystrophy of the arms.11 This is a successful application of stellate ganglion block in central cramp-like pain.17
The neuroanatomy and neurophysiology of thalamus have been extensively studied. Thalamus is a key relay station for the transmission of nociceptive information to the cerebral cortex. However, the mechanism CPSP is unknown. Proposed factors include excessive excitability of the central nervous system, and more specifically, the release of top-down inhibition of the spinal cord due to the loss of sensory afferent neurons.18–21 In addition to the cardinal feature of severe pain, patients often have other less clear symptoms, such as sensory abnormalities and abnormal reflexes.20
CPSP is typically refractory to pharmacotherapy. More recently, cortex stimulation and deep brain stimulation have been used to treat CPSP.8 A case report published 3 years earlier claimed that stellate ganglion block is effective for CPSP.12 Ultrasound guidance could improve the accuracy of local anesthetic injection into target site.15,16 The use of ultrasound-guided in the index case it apparently helpful in achieving therapeutic action.
The cause-effect relationship between stellate ganglion block and pain relief in our patient is apparent based on the temporal event sequence. Reduction in pain intensity and attack frequency is obvious on the second day after the treatment; on the seventh day, the pain completely disappeared. The diagnosis of CPSP, however, is completely based on the history of stroke and the development of increasingly severe cramp-like pain 5 years later and not by a thorough neurological and electrophysiological investigation. Also, whether repeated injection of lidocaine is necessary is unknown. Future studies, ideally randomized controlled trials, are needed to verify the efficacy and to examine the potential harm.
Conclusion
Ultrasound-guided stellate ganglion block is a viable treatment option for CPSP that is refractory to pharmacotherapy.
Acknowledgment
The authors thank the patient who participated in this study.
Funding Statement
Supported by grant 2019HXFH069 from 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of the First People’s Hospital, Zigong, Sichuan, China. The patient provided written informed consent to have the case details and any accompanying images published. The study was undertaken in strict accordance with the Declaration of Helsinki.
Data Sharing Statement
Original dataset (including images) is available from the corresponding author on reasonable request.
Author Contributions
Qian Liu, Guoqiang Tang, and Qing Zhong contributed equally to the work and should be regarded as co-first authors. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Merskey HM, Bogduk N. Classification of Chronic Pain. 2nd ed. Seattle: IASP Press; 1994. [Google Scholar]
- 2.Widar M, Samuelsson L, Karlsson-Tivenius S, Ahlstrom G. Long term pain conditions after a stroke. J Rehabil Med. 2002;34:165–170. doi: 10.1080/16501970213237 [DOI] [PubMed] [Google Scholar]
- 3.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8:857–868. doi: 10.1016/S1474-4422(09)70176-0 [DOI] [PubMed] [Google Scholar]
- 4.Andersen G, Vestergaard K, Ingeman-Nielsen M, et al. Incidence of central post-stroke pain. Pain. 1995;61(2):187–193. doi: 10.1016/0304-3959(94)00144-4 [DOI] [PubMed] [Google Scholar]
- 5.Delpont B, Blanc C, Osseby GV, Hervieu-Bègue M, Giroud M, Béjot Y. Pain after stroke: a review. Rev Neurol (Paris). 2018;174:671–674. doi: 10.1016/j.neurol.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 6.Dejerine J, Roussy G. Le syndrome thalamique. Rev Neurol (Paris). 1906;14:521–532. [Google Scholar]
- 7.De Castro-costa CM, Do Vale OC, Siqueira Neto JI. An association of central post-stroke pain and thalamic hand. Arq Neuropsiquiatr. 1992;50:120–122. doi: 10.1590/S0004-282X1992000100021 [DOI] [PubMed] [Google Scholar]
- 8.Holland MT, Zanaty M, Li L, et al. Successful deep brain stimulation for central post-stroke pain and dystonia in a single operation. J Clin Neurosci. 2018;50:190–193. doi: 10.1016/j.jocn.2018.01.036 [DOI] [PubMed] [Google Scholar]
- 9.Hesami O, Gharagozli K, Beladimoghadam N, Assarzadegan F, Mansouri B, Sistanizad M. The efficacy of gabapentin in patients with central post-stroke pain. Iran J Pharm Res. 2015;14:95–101. [PMC free article] [PubMed] [Google Scholar]
- 10.Bordet R, Ihl R, Korczyn AD, et al. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: a consensus report. BMC Med. 2017;15(1):107. doi: 10.1186/s12916-017-0869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen-Falkenberg A, Olsen KS. Continuous stellate ganglion blockade for reflex sympathetic dystrophy. Anesth Analg. 1992;75(6):1041–1042. doi: 10.1213/00000539-199212000-00032 [DOI] [PubMed] [Google Scholar]
- 12.Liao C, Yang M, Liu P, Zhong W, Zhang W. Thalamic pain alleviated by stellate ganglion block: a case report. Medicine (Baltimore). 2017;96(5):e6058. doi: 10.1097/MD.0000000000006058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wade DT, Skilbec CE, Hewer RL. Predicting Barthel ADL score at 6 months after an acute stroke. Arch Phys Med Rehabil. 1983;64(1):24–28. [PubMed] [Google Scholar]
- 14.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–3670. doi: 10.1111/acp.1983.67.issue-6 [DOI] [PubMed] [Google Scholar]
- 15.Lee MH, Kim KY, Song JH, et al. Minimal volume of local anesthetic required for an ultrasound-guided SGB. Pain Med. 2012;13(11):1381–1388. doi: 10.1111/j.1526-4637.2012.01495.x [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Song SO, Jung G. A lateral paracarotid approach for ultrasound-guided stellate ganglion block with a linear probe. J Anesth. 2017;31:458–462. doi: 10.1007/s00540-017-2354-y [DOI] [PubMed] [Google Scholar]
- 17.Sposato LA, Sharma HA, Khan AR, Bartha R, Hachinski V. Thalamic cramplike pain. J Neurol Sci. 2014;336:269–272. doi: 10.1016/j.jns.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Kumar G, Soni CR. Central post-stroke pain: current evidence. J Neurol Sci. 2009;284:10–17. doi: 10.1016/j.jns.2009.04.030 [DOI] [PubMed] [Google Scholar]
- 19.Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol. 1994;72(4):1570–1587. doi: 10.1152/jn.1994.72.4.1570 [DOI] [PubMed] [Google Scholar]
- 20.Bowsher D. Central post-stroke (‘thalamic syndrome’) and other central pains. Am J Hosp Palliat Care. 1999;16(4):593–597. doi: 10.1177/104990919901600408 [DOI] [PubMed] [Google Scholar]
- 21.Boivie J. Chapter 48 Central post-stroke pain. Handb Clin Neurol. 2006;81:715–730. [DOI] [PubMed] [Google Scholar]