Abstract
Purpose
Cervical cancer is one of the most common causes of death among women globally. Combinations of cisplatin, paclitaxel, bevacizumab, carboplatin, topotecan, and gemcitabine are recommended as first-line therapies.
Methods
This study focuses on the development of folate-decorated, pH-sensitive lipid-polymer hybrid nanoparticles (LPNs). Loading carboplatin (CBP) and paclitaxel (PTX), LPNs were expected to combine the therapeutic effects of CBP and PTX, thus show synergistic ability on cervical cancer.
Results
FA-CBP/PTX-LPNs showed the sizes of 169.9 ± 5.6 nm, with a narrow size distribution of 0.151 ± 0.023. FA-CBP/PTX-LPNs exhibited pH-responsive drug release, high cellular uptake efficiency (66.7 ± 3.1%), and prominent cell inhibition capacity (23 ± 1.1%). In vivo tumor distribution and tumor inhibition efficiency of FA-CBP/PTX-LPNs was the highest, with no obvious body weight lost.
Conclusion
High tumor distribution and remarkable antitumor efficiency obtained using in vitro as well as in vivo models further proved the FA-CBP/PTX-LPNs is a promising tool for cervical cancer therapy.
Keywords: cervical cancer, folate, pH-sensitive, carboplatin, paclitaxel, lipid-polymer hybrid nanoparticles
Introduction
Cervical cancer is a malignant epithelial tumor that forms in the uterine cervix.1 It is one of the most common causes of death among women globally.2 Cervical cancer treatment approaches include surgery, radiation therapy, chemotherapy, and targeted therapy.3 Chemotherapy is a powerful therapeutic approach for the cancer therapy. However, using a single therapeutic agent is not effective in eradicating cancer cells, and hence the use of combinatorial therapy is necessary and inevitable.4 Various combinations of cisplatin, paclitaxel, bevacizumab, carboplatin, topotecan, and gemcitabine are recommended as first-line therapies.5 However, conventional chemotherapy is lack of cell specificity thus may cause serious side effects: both normal cells and cancer cells may be killed together by anticancer drugs.6
Nanoparticles have been widely investigated in the treatment of cancer because nanoparticles have special characters that can load small molecules for biomedical applications.7 Therefore, various nanoparticles including polymeric nanoparticles, lipid nanoparticles, dendrimers, and micelles have been designed to encapsulate anticancer drugs.8 Lipid-polymer hybrid nanoparticles (LPNs) usually contained a polymer inner core and a phospholipid surface layer.9 LPNs combine advantages of both liposomes and polymers into a single platform, which is an ideal system for combinatorial delivery based on the dual-component structure.10 pH-sensitive nanoparticles have been widely used to deliver drugs in cancer therapy due to the lower pH in tumors than in normal tissues.11–13 In this study, pH-responsive LPNs were applied for the carboplatin and paclitaxel delivery.
Surface decorated nanoparticles (by conjugating specific ligands) could potentially be delivered to specific organs, tissues, cells, or even cellular organelles.14 Nanoparticles surface modified with different ligands may elicit specific cellular interactions, so the in vivo fate and efficacy of these nanoparticles can be dramatically affected.15 Folate (FA), a nonimmunogenic receptor-specific ligand, has emerged as an attractive specific ligand for targeted anticancer drug delivery.16 FA showed immense potential to target cancer cells owing to its high affinity for folate receptors, which are normally over-expressed in various human carcinomas, including cervical cancer.17 So FA decorated nanoparticle formulations were developed for targeted cancer therapy.18–20
The present research focuses on the development of folate-decorated, pH-sensitive LPNs. Loading carboplatin (CBP) and paclitaxel (PTX), LPNs were expected to combine the therapeutic effects of CBP and PTX, thus show synergistic ability on cervical cancer.
Materials and Methods
Materials
Docetaxel was obtained from Sanwei Pharmaceutical Co. Ltd (Shanghai, China). Poly (D,L-lactic-co-glycolic) (PLGA, 50:50, MW 20,000) was purchased from Shandong Institute of Medical Instrument (Shandong, China). poly(vinyl alcohol) (PVA), dimethyl sulfoxide (DMSO), coumarin-6 (Cou-6), and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma–Aldrich Co. (St. Louis, MO, USA). Fetal Bovine Serum (FBS) was purchased from Invitrogen Corporation (Carlsbad, CA). Injectable soy lecithin (ISL), Dimethyl sulfoxide (DMSO), 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (EDCI), and 1-Hydroxybenzotriazole (HOBt) were obtained from Aladdin Reagent Co. Ltd (Shanghai, China). Glyceride oleate (GO) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Folate-polyethylene glycol-COOH (FA-PEG-COOH) was provided by Xi’an Ruixi Biological Technology Co., Ltd (Xi’an, China). Eagle’s Minimum Essential Medium (EMEM) and human cervix adenocarcinoma cell line (HeLa cells) were obtained from the American type culture collection (ATCC® CCL-2™, Manassas, VA, USA). Female BALB/c nude mice (6–8weeks) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). The animal experiments were carried out in accordance with the UK Animals Act, 1986, and associated guidelines, EU Directive 2010/63/EU for animal experiments and were approved by the Laboratory Animal Ethics Committee of Zhejiang Cancer Hospital (No. 2019-09-001).
Synthesis of Folate-Contained, pH-Sensitive Ligands
Folate-contained, pH-sensitive ligands were synthesized by conjugating FA-PEG-COOH with GO through a hydrazone bond (adipohydrazide) (Figure 1). Firstly, DMSO was used to dissolve FA-PEG-COOH (1 mmol) and reacted with the amine groups of adipohydrazide (HZ, 1 mmol) for 12 h by adding DCC (1 mmol) and NHS (4 mmol) under nitrogen atmosphere in dark to get FA-PEG-HZ.21 Then, GO (1 mmol) was dissolved in DMSO and added to the FA-PEG-HZ solution, in the mean time, EDCI (0.25 mmol) and HOBt (0.25 mmol) were added to the stirring solution for 24 h to form FA-PEG-HZ-GO. FA-PEG-HZ-GO was lyophilized and characterized by 1H-NMR analysis.
Figure 1.
Synthesis of folate-contained, pH-sensitive ligands. Folate-contained, pH-sensitive ligands were synthesized by conjugating FA-PEG-COOH with GO through a hydrazone bond.
Abbreviations: FA, folate; PEG, polyethylene glycol; GO, glyceride oleate.
Preparation of CBP and PTX Co-Loaded LPNs
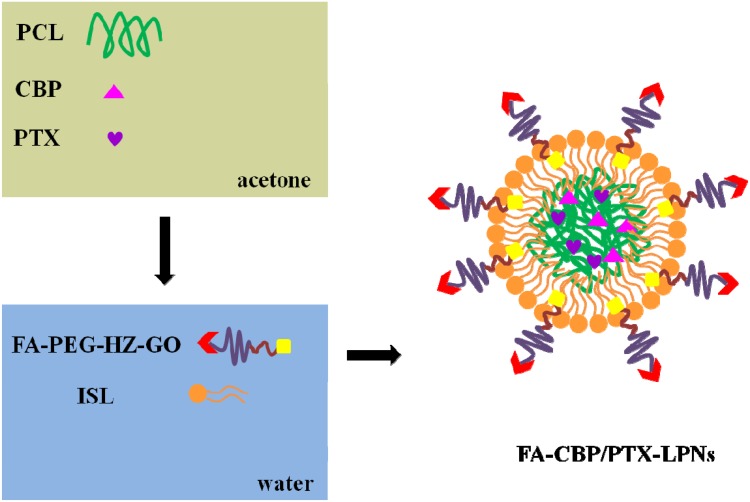
FA decorated, CBP and PTX co-loaded LPNs (FA-CBP/PTX-LPNs) were prepared by a one-step nanoprecipitation method (Figure 2).22 PCL (100 mg), CBP (10 mg), and PTX (10 mg) were dissolved in acetone (10 mL) to form the organic phase. FA-PEG-HZ-GO and ISL were dispersed in water, heated to 65–70°C to form a homogeneous dispersion. The organic phase was added dropwise to the dispersion which was stirred at 400 rpm. The HA-DOX/GA-LPHNs solution was washed for three times using a centrifugal filter with a molecular weight cut-off (MWCO) of 10 kDa to remove the acetone and free molecules and got FA-CBP/PTX-LPNs. FA decorated, single CBP or PTX loaded LPNs (FA-CBP-LPNs and FA-PTX-LPNs) were prepared by using one drug (CBP or PTX) only. FA decorated, blank LPNs (FA-LPNs) were prepared by using no drug. Undecorated CBP and PTX co-loaded LPNs (CBP/PTX-LPNs) were prepared by using PEG-DSPE instead of FA-PEG-HZ-GO.
Figure 2.
Schematic for nanoparticle assembly. FA decorated, CBP and PTX co-loaded LPNs (FA-CBP/PTX-LPNs) were prepared by a one-step nanoprecipitation method.
Abbreviations: FA, folate; CBP, carboplatin; PTX, paclitaxel; LPNs, lipid-polymer hybrid nanoparticles.
Characterization of LPNs
The average particle diameter, particle size distribution, and surface charge were determined by dynamic light scattering (DLS) technique using a Zetasizer Nano ZS (Malvern, UK).23 The morphology and size of the particles were observed using JEM-1010 transmission electron microscopy (JEOL, Tokyo, Japan). The encapsulation capacity of LPNs was determined by measuring the concentrations of CBP and PTX using a C-18 reverse-phase high-performance liquid chromatography (RP-HPLC) (Beckman Coulter, Brea, CA) at a flow rate of 1 mL/min.24 Tert-butyl methyl ether (5 mL) was added to the LPNs suspension and vortex for 1 min to extract the drugs and then redissolved in acetonitrile:water (10:90) solution. The solution (1 mL) was injected into the C-18 column, and CPT and PTX were detected at 227 nm after different retention times. The amounts of CBP and PTX were quantified by HPLC. The encapsulation efficiency (EE) was determined as: (Amount of entrapped drug/amount of total drug) × 100%.
In vitro Drug Release of LPNs
In vitro CBP and PTX release behaviors of LPNs were analyzed in acetate buffer (pH 5.5) or phosphate sodium buffer (PBS buffer) (pH 7.4) medium, respectively.25 FA-CBP/PTX-LPNs or CBP/PTX-LPNs were dispersed with buffer (5 mL) and sealed in a dialysis bag (MWCO: 2 kDa), which was immersed in the release medium (25 mL) shaking (100 rpm) at 37°C. At present time points, 2 mL of release medium was taken out and 2 mL of fresh medium were added. The amounts of CBP and PTX were quantified by HPLC.
Cellular Uptake of LPNs
Coumarin-6 (C6) was encapsulated in the FA-CBP/PTX-LPNs or CBP/PTX-LPNs and the uptake efficiency was evaluated on HeLa cells because C6 was a fluorescent marker that can be directly visualized and quantified.26 C6 was loaded into LPNs by adding C6 (1 mg/mL of the lipid phase) into the organic phase described in the “Preparation of CBP and PTX co-loaded LPNs” section. HeLa cells were seeded in 24-well plates (5×104 cells/well). When 80% confluence was achieved, C6 loaded FA-CBP/PTX-LPNs or CBP/PTX-LPNs were added and incubated for 4 h (pH 7.4, 37°C). Then, the cells were washed three times with PBS and photographed using fluorescence microscopy. After trypsinized, the cells were analyzed using a flow cytometer.27,28
In vitro Cytotoxicity and Synergistic Effect of LPNs
Cytotoxicity studies were carried out on HeLa cells.29 HeLa cells (103 cells/well in 96-well plates) were seeded in EMEM adding 10% of FBS (100 μL). After incubation for 24 h (37°C), FA-CBP/PTX-LPNs, FA-CBP-LPNs, FA-PTX-LPNs, CBP/PTX-LPNs, free CBP/PTX, and FA-LPNs were added and incubated in 5% CO2 for 72 hrs (37°C), and cell viability was measured by MTT assay based on the manufacturer’s manual. IC50 values were calculated from curves constructed by plotting cell viability (%) versus drug concentration (μM). The synergistic effect was determined by the Combination index (CI), the CI values were calculated through the Chou-Talalay method.30 The CI provides a quantitative value for synergy and is given by: CI50 = (D)a/(D50)a + (D)b/(D50)b. In this equation, a for CBP and b for PTX, (D)a and (D)b means the dose (concentration) of CBP and PTX in combination inhibited 50% of the cell growth; (D50)a and (D50)b means the dose (concentration) of CBP or PTX alone that inhibited 50% of the cell growth. CI<1, =1, and >1 indicate synergism, additive, and antagonism effect, respectively. CI values closer to zero represent increasing synergy.
In vivo Tissue Distribution and Anticancer Ability of LPNs
Cervical carcinoma xenograft was produced by injecting HeLa cells (5×106 cells in 200 µL of PBS) cells subcutaneously into the right armpit of BALB/c nude mice, which was grouped randomly (7 groups).31 FA-CBP/PTX-LPNs (contained 5 mg/kg CBP and 5 mg/kg PTX), FA-CBP-LPNs (contained 10 mg/kg CBP), FA-PTX-LPNs (contained 10 mg/kg PTX), CBP/PTX-LPNs (contained 5 mg/kg CBP and 5 mg/kg PTX), free CBP/PTX (contained 10 mg/kg CBP and 10 mg/kg PTX), FA-LPNs, and 0.9% saline were administered intravenously once every three days. The tumors and animal weights were measured. The tumor volume was calculated according to the equation: long axis × short axis2/2. At 1 h and 24 h post injection, mice were sacrificed. Tissues (tumor, heart, liver, spleen, lung and kidney) were collected, decomposed on heating in nitric acid, evaporated to dryness, and redissolved in acetonitrile: water (10:90) solution.32 The amounts of CBP and PTX were quantified by HPLC.
Statistical Analysis
Mean ± standard deviation (mean ± SD) was used to express the data. A post hoc test (S-N-K method) was performed following ANOVA. * P < 0.05 was considered as statistical significance and ** P < 0.01 as extreme statistical significance.
Results
Characterization of FA-PEG-HZ-GO
FA-PEG-HZ-GO was characterized by 1H-NMR: One peak at 7.1 ppm means the formation of HZ. Typical peaks of PEG were observed at 3.37–3.63 ppm, peaks at 7.77–8.10 were attributed to FA, and peaks at 1.29–1.33 belong to GO.
Characterization of LPNs
The particle diameter, size distribution, and surface charge of LPNs were characterized and summarized in Table 1. FA-decorated LPN formulations showed the sizes of about 170 nm, while undecorated CBP/PTX-LPNs had a diameter of 121.3 nm. Narrow size distributions were found for all the formulas (between 0.129 and 0.163). Positive surface charges of LPNs were observed, with over 80% of EE.
Table 1.
Characterization of LPNs
| Formulations | FA-CBP/PTX-LPNs | FA-CBP-LPNs | FA-PTX-LPNs | CBP/PTX-LPNs | FA-LPNs |
|---|---|---|---|---|---|
| Average diameter (nm) | 169.9 ± 5.6 | 171.3 ± 5.1 | 166.5 ± 5.5 | 121.3 ± 4.1 | 164.1 ± 4.8 |
| Particle size distribution | 0.151 ± 0.023 | 0.163 ± 0.025 | 0.147 ± 0.031 | 0.129 ± 0.018 | 0.136 ± 0.017 |
| Surface charge (mV) | 32.9 ± 3.1 | 34.1 ± 3.2 | 33.2 ± 2.9 | 21.3 ± 2.6 | 35.3 ± 2.7 |
| CBP EE (%) | 83.1 ± 2.1 | 82.6 ± 2.8 | N/A | 80.9 ± 2.5 | N/A |
| PTX EE (%) | 84.2 ± 2.7 | NA | 83.6 ± 2.6 | 82.8 ± 2.2 | N/A |
In vitro Drug Release of LPNs
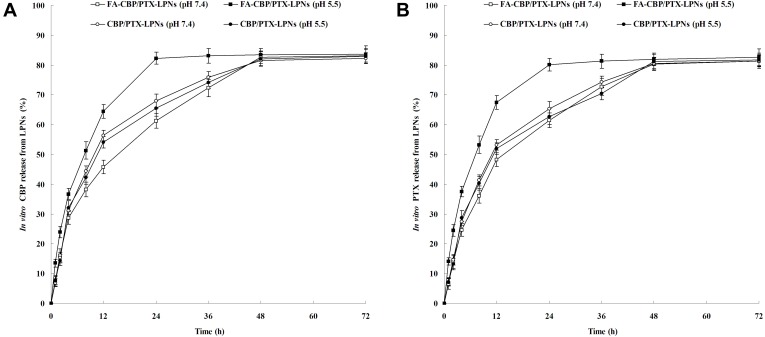
The drug release behaviors of FA-CBP/PTX-LPNs and CBP/PTX-LPNs in pH 7.4 and 5.5 are different (Figure 3). CBP/PTX-LPNs showed almost the same release pattern in the different pH values; however, FA-CBP/PTX-LPNs exhibited faster release at pH 5.5. FA-CBP/PTX-LPNs showed over 80% of CBP and PTX release at 24 h, while at pH 7.4 the time was delayed to 48 h.
Figure 3.
In vitro CBP (A) and PTX (B) release of LPNs. The drug release behaviors of FA-CBP/PTX-LPNs and CBP/PTX-LPNs were observed at pH 7.4 and 5.5. Data presented as mean ± SD (n=5).
Abbreviations: FA, folate; CBP, carboplatin; PTX, paclitaxel; LPNs, lipid-polymer hybrid nanoparticles.
Cellular Uptake of LPNs
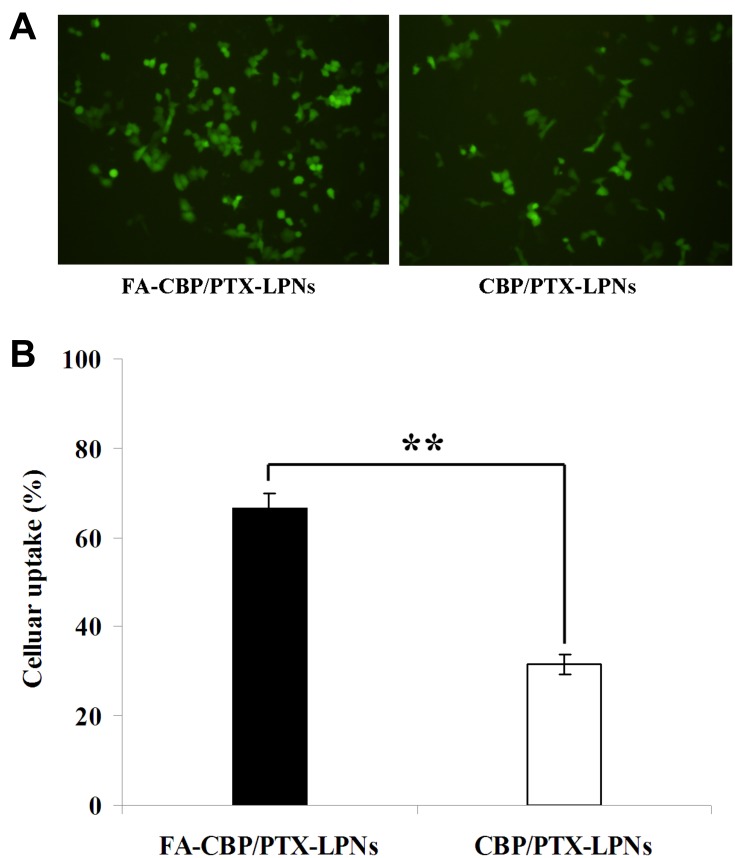
Figure 4 displays the cellular uptake efficiency results of FA-CBP/PTX-LPNs or CBP/PTX-LPNs. The HeLa cell uptake of FA-CBP/PTX-LPNs (66.7 ± 3.1%) was remarkably higher than CBP/PTX-LPNs (31.6 ± 2.2%) (P < 0.01).
Figure 4.
Cellular uptake efficiency of FA-CBP/PTX-LPNs and CBP/PTX-LPNs. (A) photographed using fluorescence microscopy and (B) analyzed using a flow cytometer. Data presented as mean ± SD (n=5), **P < 0.01.
Abbreviations: FA, folate; CBP, carboplatin; PTX, paclitaxel; LPNs, lipid-polymer hybrid nanoparticles.
In vitro Cytotoxicity and Synergistic Effect of LPNs
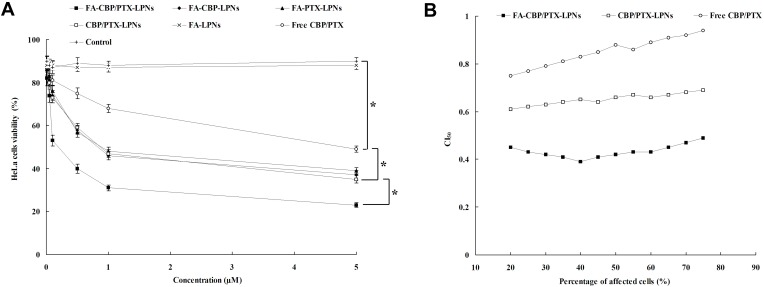
FA-CBP/PTX-LPNs showed remarkably higher cytotoxicity than CBP/PTX-LPNs (Figure 5A, P < 0.05), suggested that the use of FA contained pH-sensitive ligand could promote the delivery of the drugs encapsulated within nanoparticles.43 Significant improvements of cytotoxicity by CBP/PTX-LPNs in comparison to free CBP/PTX illustrated that the LPNs improved the drug delivery, thus gaining better efficiency than free drug solution. Evaluation of drug–drug interaction is important in combination with cancer chemotherapy, and combination index (CI) was introduced by Chou and Talalay for quantification of synergistic or antagonistic effect.44 FA-CBP/PTX-LPNs, CBP/PTX-LPNs, and free CBP/PTX showed CI50 value <1, showing synergy effects (Figure 5B).
Figure 5.
In vitro cytotoxicity (A) and synergistic effect (B) of LPNs evaluated on HeLa cells by MTT assay. Data presented as mean ± SD (n=5), *P < 0.05.
Abbreviation: LPNs, lipid-polymer hybrid nanoparticles.
In vivo Tissue Distribution and Anticancer Ability of LPNs
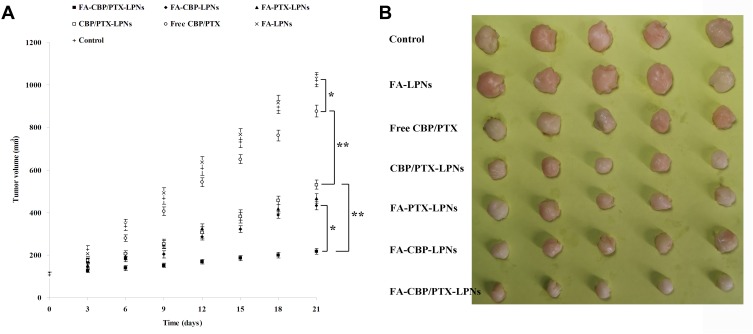
In vivo tissue distribution results are shown in Figure 6. At 1 h, LPNs showed less accumulation in the kidney and heart than free drugs (P < 0.05). At 24 h, the distribution of FA-CBP/PTX-LPNs in tumor was the highest, higher than CBP/PTX-LPNs and free CBP/PTX (P < 0.01). This phenomenon could be explained by the enhanced permeability and retention (EPR) effect on the tumor site that lets the LPNs accumulated easily in the tumor.45 The tumor tissue concentrations of LPNs kept up high at 24 h post administration, indicating the sustained-release behavior of the LPNs, which may attribute to the presence of PEG chain on the surface of particles.46 The most significant in vivo tumor inhibition efficiency was observed when FA-CBP/PTX-LPNs formula was administrated (Figure 7), which was better than FA-CBP-LPNs (P < 0.05) and CBP/PTX-LPNs (P < 0.01). CBP/PTX-LPNs illustrated better antitumor ability than free CBP/PTX (P < 0.01), the latter showed remarkable ability over the control group (P < 0.05). On day 21, the images of the tumor are taken and presented in Figure 7B. There was no obvious body weight loss in LPN groups. No animal death was observed in all the tested groups. These outcomes proved the system may be considered as a safe carrier to deliver the anti-cancer drugs. These could be explained by the LPNs constructed could exhibit high structural integrity, stability during usage, and sustained-release capability. FA-decorated pH-sensitive LPNs might be compatible with the lipid structured cell membrane, which allows the system to fuse to the cell membrane and deliver drugs to the tumor more efficiently.47
Figure 6.
In vivo tissue distribution of CBP (A, B) and PTX (C, D) investigated at 1 h (A, C) and 24 h (B, D) of administration. *P < 0.05 and **P < 0.01.
Abbreviations: CBP, carboplatin; PTX, paclitaxel.
Figure 7.
In vivo anti-tumor effect of the LPNs evaluated in terms of tumor volume (A) and tumor images (B). Data presented as mean ± SD (n=5), *P < 0.05 and **P < 0.01.
Abbreviation: LPNs, lipid-polymer hybrid nanoparticles.
Discussion
FA can bind to the folate receptor (FR) with high affinity and enter the FR-elevated malignant cells by receptor-mediated endocytosis, and meanwhile avoid being endocytosed by those normal cells that express a low level of FRs.33 Thus, in this study, FA was conjugated to the distal ends of FA-PEG-HZ-GO. PEG modification on the surface of nanoparticles could shield the surface from aggregation, opsonization, and phagocytosis, prolonging systemic circulation time.34 According to the pH difference between tumor tissues and normal tissues,35 pH-response FA-PEG-HZ-GO ligand was designed for the nanoparticle preparation.
FA-CBP/PTX-LPNs were prepared by a one-step nanoprecipitation method. The conventional two-step method was the most common method employed in the early phase of LPN development by mixing the preformed polymeric nanoparticles preformed lipid vesicles through electrostatic interactions.36 However, the main drawback of the two-step method was not efficient in terms of time and energy expenses. In contrast, the one-step method involves a single mixing of lipid with polymer to allow them self-assemble LPNs via nanoprecipitation. The size of prepared LPNs ranged from 121 to 170 nm, which could be efficiently internalized into cells.37 Size and drug loading efficiency did not change after co-loading with C6 dye. PCL was used as the polymer material to encapsulate the hydrophobic drugs. Over 80% of EE may prove the fine drug entrapment efficiency of these systems.38
FA-CBP/PTX-LPNs showed over 80% of CBP and PTX release at 24 h, while at pH 7.4 the time was delayed to 48 h. Wan et al observed similar release pattern for both decorated and undecorated nanoparticles, they argued that the modification of ligands on the nanoparticles’ surface did not change the release behavior of drugs.39 The same phenomenon was found in this research that similar release behavior was found in FA-CBP/PTX-LPNs and CBP/PTX-LPNs at pH 7.4. At pH 5.5, the drug release from CBP/PTX-LPNs had not been changed. On the contrary, FA-CBP/PTX-LPNs exhibited faster release at pH 5.5 than at pH 7.4. The results are in accordance with the observation of Yang et al, which indicated that an acidic environment could accelerate the breakdown of self-assemblies, further giving rise to faster release of the drugs.40
In the study carried out by Tang et al, the cellular uptake and cellular internalization mechanisms of nanoparticles in HeLa (high expression of FA receptor) cells were quantitatively detected by the fluorescence method.41 In the FA receptor-overexpressing HeLa cells, the internalization of FA-decorated nanoparticles was significantly more efficient than undecorated ones due to FA receptor-mediated endocytosis and cellular macropinocytosis involved in the cellular internalizations of nanoparticles in HeLa cells. Also, the pH-triggered payload release leads to the increased cumulation of drugs in the cells.42
Conclusion
In summary, FA-decorated, pH-sensitive LPNs were constructed for the co-delivery of CBP and PTX. FA-CBP/PTX-LPNs exhibited pH-responsive drug release, high cellular uptake efficiency, and prominent cytotoxicity. The high tumor distribution and remarkable antitumor efficiency of FA-CBP/PTX-LPNs further proved this system could be used as a promising tool for the targeted treatment of cervical cancer.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(1):64–84. [DOI] [PubMed] [Google Scholar]
- 2.Luo CL, Liu YQ, Wang P, et al. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed Pharmacother. 2016;82:595–605. doi: 10.1016/j.biopha.2016.05.029 [DOI] [PubMed] [Google Scholar]
- 3.Yuan YG, Gurunathan S. Combination of graphene oxide-silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int J Nanomedicine. 2017;12:6537–6558. doi: 10.2147/IJN.S125281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li F, Zhao C, Wang L. Molecular-targeted agents combination therapy for cancer: developments and potentials. Int J Cancer. 2014;134(6):1257–1269. doi: 10.1002/ijc.v134.6 [DOI] [PubMed] [Google Scholar]
- 5.Rosen VM, Guerra I, McCormack M, et al. Systematic review and network meta-analysis of bevacizumab plus first-line topotecan-paclitaxel or cisplatin-paclitaxel versus non-bevacizumab-containing therapies in persistent, recurrent, or metastatic cervical cancer. Int J Gynecol Cancer. 2017;27(6):1237–1246. doi: 10.1097/IGC.0000000000001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramzy L, Nasr M, Metwally AA, Awad GAS. Cancer nanotheranostics: a review of the role of conjugated ligands for overexpressed receptors. Eur J Pharm Sci. 2017;104:273–292. doi: 10.1016/j.ejps.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Meng X, Dong Y. Ursolic acid nanoparticles inhibit cervical cancer growth in vitro and in vivo via apoptosis induction. Int J Oncol. 2017;50(4):1330–1340. doi: 10.3892/ijo.2017.3890 [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Wang Z, Li C, et al. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed Pharmacother. 2018;106:275–284. doi: 10.1016/j.biopha.2018.06.137 [DOI] [PubMed] [Google Scholar]
- 9.Song Z, Shi Y, Han Q, Dai G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed Pharmacother. 2018;105:18–26. doi: 10.1016/j.biopha.2018.05.095 [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Cheng H, Han L, et al. Synergistic combination therapy of lung cancer using paclitaxel- and triptolide-coloaded lipid-polymer hybrid nanoparticles. Drug Des Devel Ther. 2018;12:3199–3209. doi: 10.2147/DDDT.S172199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng G, Zheng M, Yang B, Fu H, Li Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed Pharmacother. 2019;116:109006. doi: 10.1016/j.biopha.2019.109006 [DOI] [PubMed] [Google Scholar]
- 12.Luo M, Cheng W, Zeng X, Mei L, Liu G, Deng W. Folic acid-functionalized black phosphorus quantum dots for targeted chemo-photothermal combination cancer therapy. Pharmaceutics. 2019;11:5. doi: 10.3390/pharmaceutics11050242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamidu A, Mokrish A, Mansor R, et al. Modified methods of nanoparticles synthesis in pH-sensitive nano-carriers production for doxorubicin delivery on MCF-7 breast cancer cell line. Int J Nanomedicine. 2019;14:3615–3627. doi: 10.2147/IJN.S190830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Zhang N, Li C. Saccharide modified pharmaceutical nanocarriers for targeted drug and gene delivery. Curr Pharm Des. 2009;15(32):3826–3836. doi: 10.2174/138161209789649547 [DOI] [PubMed] [Google Scholar]
- 15.Abd Ellah NH, Abouelmagd SA. Surface functionalization of polymeric nanoparticles for tumor drug delivery: approaches and challenges. Expert Opin Drug Deliv. 2017;14(2):201–214. doi: 10.1080/17425247.2016.1213238 [DOI] [PubMed] [Google Scholar]
- 16.Pourjavadi A, Tehrani ZM, Moghanaki AA. Folate-conjugated pH-responsive nanocarrier designed for active tumor targeting and controlled release of gemcitabine. Pharm Res. 2016;33(2):417–432. doi: 10.1007/s11095-015-1799-7 [DOI] [PubMed] [Google Scholar]
- 17.Zhang G, Liu F, Jia E, Jia L, Zhang Y. Folate-modified, cisplatin-loaded lipid carriers for cervical cancer chemotherapy. Drug Deliv. 2016;23(4):1393–1397. doi: 10.3109/10717544.2015.1054052 [DOI] [PubMed] [Google Scholar]
- 18.Xu L, Bai Q, Zhang X, Yang H. Folate-mediated chemotherapy and diagnostics: an updated review and outlook. J Control Release. 2017;252:73–82. doi: 10.1016/j.jconrel.2017.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Tao R, Song M. Fabrication of self-assembled folate-biotin-quaternized starch nanoparticles as co-carrier of doxorubicin and siRNA. J Biomater Appl. 2017;32(5):587–597. doi: 10.1177/0885328217737187 [DOI] [PubMed] [Google Scholar]
- 20.Ma Z, Hu P, Guo C, et al. Folate-mediated and pH-responsive chidamide-bound micelles encapsulating photosensitizers for tumor-targeting photodynamic therapy. Int J Nanomedicine. 2019;14:5527–5540. doi: 10.2147/IJN.S208649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fathi M, Zangabad PS, Aghanejad A, Barar J, Erfan-Niya H, Omidi Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr Polym. 2017;172:130–141. doi: 10.1016/j.carbpol.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Shao Y, Luo W, Guo Q, Li X, Zhang Q, Li J. In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid-polymeric hybrid nano-system for leukemia therapy. Drug Des Devel Ther. 2019;13:2043–2055. doi: 10.2147/DDDT.S202818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji J, Zuo P, Wang YL. Enhanced antiproliferative effect of carboplatin in cervical cancer cells utilizing folate-grafted polymeric nanoparticles. Nanoscale Res Lett. 2015;10(1):453. doi: 10.1186/s11671-015-1162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Liu Y, Kim YJ, Mac J, Zhuang R, Wang P. Co-delivery of carboplatin and paclitaxel via cross-linked multilamellar liposomes for ovarian cancer treatment. RSC Adv. 2017;7(32):19685–19693. doi: 10.1039/C7RA01100H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang L, Chen G, Gong S. Carboplatin-complexed and cRGD-conjugated unimolecular nanoparticles for targeted ovarian cancer therapy. Macromol Biosci. 2017;17:5. doi: 10.1002/mabi.v17.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo S, Zhang Y, Wu Z, et al. Synergistic combination therapy of lung cancer: cetuximab functionalized nanostructured lipid carriers for the co-delivery of paclitaxel and 5-demethylnobiletin. Biomed Pharmacother. 2019;118:109225. doi: 10.1016/j.biopha.2019.109225 [DOI] [PubMed] [Google Scholar]
- 27.Shukla RS, Jain A, Zhao Z, Cheng K. Intracellular trafficking and exocytosis of a multi-component siRNA nanocomplex. Nanomedicine. 2016;12(5):1323–1334. doi: 10.1016/j.nano.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain A, Barve A, Zhao Z, et al. Targeted delivery of an siRNA/PNA hybrid nanocomplex reverses carbon tetrachloride‐induced liver fibrosis. Adv Therapeutic. 2019;2(8):1900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poon C, Duan X, Chan C, Han W, Lin W. Nanoscale coordination polymers codeliver carboplatin and gemcitabine for highly effective treatment of platinum-resistant ovarian cancer. Mol Pharm. 2016;13(11):3665–3675. doi: 10.1021/acs.molpharmaceut.6b00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10 [DOI] [PubMed] [Google Scholar]
- 31.Mo J, Wang L, Huang X, et al. Multifunctional nanoparticles for co-delivery of paclitaxel and carboplatin against ovarian cancer by inactivating the JMJD3-HER2 axis. Nanoscale. 2017;9(35):13142–13152. doi: 10.1039/C7NR04473A [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Yang C, Wang W, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep. 2016;6:21225. doi: 10.1038/srep21225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang WY, Cao YX, Zhou X, Wei B. Delivery of folic acid-modified liposomal curcumin for targeted cervical carcinoma therapy. Drug Des Devel Ther. 2019;13:2205–2213. doi: 10.2147/DDDT.S205787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. doi: 10.1016/j.addr.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong H, Pang L, Cong H, Shen Y, Yu B. Application and design of esterase-responsive nanoparticles for cancer therapy. Drug Deliv. 2019;26(1):416–432. doi: 10.1080/10717544.2019.1588424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85(3Pt A):427–443. doi: 10.1016/j.ejpb.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 37.Hong ST, Lin H, Wang CS, et al. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J Nanobiotechnology. 2019;17(1):89. doi: 10.1186/s12951-019-0519-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macedo LB, Nogueira-Librelotto DR, de Vargas J, Scheeren LE, Vinardell MP, Rolim CMB. Poly (ɛ-Caprolactone) nanoparticles with pH-responsive behavior improved the in vitro antitumor activity of methotrexate. AAPS Pharm Sci Tech. 2019;20(5):165. doi: 10.1208/s12249-019-1372-5 [DOI] [PubMed] [Google Scholar]
- 39.Wan X, Liu C, Lin Y, Fu J, Lu G, Lu Z. pH sensitive peptide functionalized nanoparticles for co-delivery of erlotinib and DAPT to restrict the progress of triple negative breast cancer. Drug Deliv. 2019;26(1):470–480. doi: 10.1080/10717544.2019.1576801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang T, Du G, Cui Y, et al. pH-sensitive doxorubicin-loaded polymeric nanocomplex based on β-cyclodextrin for liver cancer-targeted therapy. Int J Nanomedicine. 2019;14:1997–2010. doi: 10.2147/IJN.S193170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang H, Chen H, Jia Y, et al. Effect of inhibitors of endocytosis and NF-kB signal pathway on folate-conjugated nanoparticle endocytosis by rat Kupffer cells. Int J Nanomedicine. 2017;12:6937–6947. doi: 10.2147/IJN.S141407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia L, Jia N, Gao Y, et al. Multi-modulation of doxorubicin resistance in breast cancer cells by Poly(l-histidine)-based multifunctional micelles. Pharmaceutics. 2019;11:8. doi: 10.3390/pharmaceutics11080385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan S, Wang G. Redox-responsive and pH-sensitive nanoparticles enhanced stability and anticancer ability of erlotinib to treat lung cancer in vivo. Drug Des Devel Ther. 2017;11:3519–3529. doi: 10.2147/DDDT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Z, Li Z, Yan J, Wang P. Irinotecan and 5-fluorouracil-co-loaded, hyaluronic acid-modified layer-by-layer nanoparticles for targeted gastric carcinoma therapy. Drug Des Devel Ther. 2017;11:2595–2604. doi: 10.2147/DDDT.S140797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F, Li A, Liu H, Zhang H. Gastric cancer combination therapy: synthesis of a hyaluronic acid and cisplatin containing lipid prodrug coloaded with sorafenib in a nanoparticulate system to exhibit enhanced anticancer efficacy and reduced toxicity. Drug Des Devel Ther. 2018;12:3321–3333. doi: 10.2147/DDDT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Sun G, Zhang Z, Ou Y. Transcription activator, hyaluronic acid and tocopheryl succinate multi-functionalized novel lipid carriers encapsulating etoposide for lymphoma therapy. Biomed Pharmacother. 2017;91:241–250. doi: 10.1016/j.biopha.2017.04.104 [DOI] [PubMed] [Google Scholar]
- 47.Li S, Wang L, Li N, Liu Y, Su H. Combination lung cancer chemotherapy: design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed Pharmacother. 2017;95:548–555. doi: 10.1016/j.biopha.2017.08.090 [DOI] [PubMed] [Google Scholar]