Abstract
Cannabis has been considered as a therapeutic strategy to control intractable epilepsy. Several cannabis components, especially cannabidiol (CBD), induce antiseizure effects. However, additional information is necessary to identify the types of epilepsies that can be controlled by these components and the mechanisms involved in these effects. This review presents a summary of the discussion carried out during the 2nd Latin American Workshop on Neurobiology of Epilepsy entitled “Cannabinoid and epilepsy: myths and realities.” This event was carried out during the 10th Latin American Epilepsy Congress in San José de Costa Rica (September 28, 2018). The review focuses to discuss the use of CBD as a new therapeutic strategy to control drug‐resistant epilepsy. It also indicates the necessity to consider the evaluation of unconventional targets such as P‐glycoprotein, to explain the effects of CBD in drug‐resistant epilepsy.
Keywords: cannabidiol, cannabis, drug‐resistant epilepsy, P‐glycoprotein
Key points.
Conflicting results exist about the use of artisanal cannabis to control drug‐resistant epilepsy
Cannabidiol is a multitarget drug that represents a new hope to control drug‐resistant epilepsy
Cannabidiol may act on unconventional central and peripheral targets to control drug‐resistant epilepsy
1. INTRODUCTION
Epilepsy is a neurological disease characterized by the presence of spontaneous and recurrent seizures.1 About 50 million people worldwide suffer epilepsy.2 Despite the development of new antiepileptic medications over the last decades, 30% of patients with epilepsy continue having seizures, resulting in the named drug‐resistant epilepsy. Drug‐resistant epilepsy is associated with comorbid psychiatric and psychological disorders, severe economic and social impairments, and high risk of suicide as well as sudden unexpected death (SUDEP).3, 4 The high prevalence of drug‐resistant epilepsy has generated the search of new solutions using old drugs.
2. IS CANNABIS AN OPTION FOR EPILEPSY?
Cannabis, one of the oldest plants that humans grow, was used in the Middle East to control nightly seizures around 1800 BC.5 Experimental evidence obtained during the 1970s‐1980s indicates that “phytocannabinoids” obtained from cannabis exerted anticonvulsant effects in experimental models of both, acute seizures6, 7, 8, 9 and epilepsy.10, 11, 12, 13, 14
During the last decade, the use of cannabis extracts has been of great interest in the control of drug‐resistant epilepsy, mainly in children with severe (catastrophic) epileptic syndromes, that is, neurological syndromes associated with seizures difficult to control and cognitive dysfunction such as Dravet (pathogenic variants in the sodium channel gene SCN1A) or Lennox‐Gastaut syndromes. Clinical evidence supports that pediatric and adult patients with refractory epileptic disorders may achieve a significant improvement with the administration of cannabis (Table 1). However, other studies indicate that the effectiveness of phytocannabinoids as antiseizure therapy is contradictory.15, 16 Based on the information described above, the National Academy of Science, Engineering and Medicine of the USA indicates that at present, the evidence to support the use of cannabinoids in epilepsy is insufficient.17
Table 1.
Summary of the clinical studies evaluating the efficacy of cannabinoids in epilepsy and drug‐resistant seizures
| Type of study | Type of epilepsy | Number of subjects, and age | Drugs | Doses | Treatment duration | Results | References |
|---|---|---|---|---|---|---|---|
| Combination of cannabinoids | |||||||
| Open‐label, uncontrolled clinical trial | DRE, diverse etiology |
n = 46 1‐20 y |
CBD/THC (20:1) in enriched cannabis oil, oral administration | Tritiated administration starting with 2‐5 mg/kg/d sublingually and up to 50 mg/kg/d, plus THC (<1.35 mg/kg/d) | 12 wk | ≥50% reduction in the seizure frequency of 26 patients (56%) | 127 |
| Open‐label, uncontrolled clinical trial | Dravet syndrome with DRE |
n = 19 1‐18 y |
CBD/THC (50:1) in enriched cannabis oil, oral administration | Tritiated administration starting with 2‐5 mg/kg/d and up to 50 mg/kg/d, plus THC (0.27 mg/kg/d) | 20 wk | ≥50% reduction in the seizure frequency of 12 patients (63%) | 128 |
| Retrospective cohort study | DRE, diverse etiology |
n = 74 1‐18 y |
CBD/THC (20:1) in enriched cannabis oil, oral administration |
Two CBD groups:
|
12‐48 wk |
≥50% reduction in the seizure frequency in: 34 patients (46%) 4 patients (5%) |
62 |
| Retrospective cohort study | DRE, diverse etiology |
n = 75 30 d‐18 y |
Oral cannabis extracts:
|
Variable, not specified | 4‐96 wk | ≥50% reduction in the seizure frequency of 25 patients (33%) | 129 |
| Case series | DRE of diverse etiology |
n = 18 19‐50 y |
Inhalated marijuana, smoked (n = 15) Oral marijuana (n = 2) Inhalated marijuana, vaporized (n = 1) |
2.05 ± 1.87 g/d | 4‐220 wk | Decrease of seizure frequency and severity | 130 |
| Cannabidiol | |||||||
| Open‐label, expanded‐access study | Lennox‐Gastaut syndrome and Dravet syndrome |
n = 152 1‐51 y |
CBD oil, oral administration | Tritiated administration starting with 2‐10 mg/kg/d and up to 25 mg/kg/d | 144 wk | ≥50% reduction in the seizure frequency of 25 patients (49%) | 131 |
| Open‐label extension trial | Dravet syndrome |
n = 264 2‐55 y |
CBD oil, oral administration | Tritiated administration starting with 2.5 mg/kg/d and up to 30 mg/kg/d | 48 wk | ≥50% reduction in the seizure frequency of 41 patients (40%) | 53 |
| Open‐label, expanded‐access study | DRE, diverse etiology |
n = 100 >1 y |
CBD oil, oral administration | Tritiated administration starting with 5 mg/kg/d and up to 50 mg/kg/d | 12‐48 wk |
≥50% reduction in the seizure frequency of 57 patients (57%) Children responded to lower dosages |
132 |
| Prospective open‐label cohort study | DRE, diverse etiology |
n = 40 <18 y |
CBD oil, oral administration | Tritiated administration starting with 2‐5 mg/kg/d and up to 25 mg/kg/d | 12 wk | Clinical improvement of 7 patients (17.5%, according to physician) or 12 patients (30%, according to caregivers) | 133 |
| Double‐blind, randomized placebo controlled trial | Lennox‐Gastaut syndrome |
n = 225 2‐55 y |
CBD oil, oral administration (n = 149) Placebo (n = 76) |
Two CBD groups, tritiated administration starting with 2.5 mg/kg/d:
|
14 wk |
≥50% reduction of seizure frequency of:
And 11 patients (14%) of the placebo group |
52 |
| Open‐label, expanded‐access study | CDKL5 deficiency disorder and Aicardi syndrome, Dup15q syndrome, Doose syndrome |
n = 46 1‐30 y |
CBD oil, oral administration | Tritiated administration starting with 2‐5 mg/kg/d and up to 25 mg/kg/d | 48 wk | ≥50% reduction in the seizure frequency of 26 patients (57%) | 61 |
| Retrospective cohort study | DRE, diverse etiology |
n = 108 <18 y |
|
|
|
≥50% reduction in seizure frequency of:
|
134 |
| Open‐label, uncontrolled clinical trial | DRE, diverse etiology |
n = 26 1‐17 y |
CBD oil, oral administration | Tritiated administration starting with 5 mg/kg/d and up to 25 mg/kg/d | 16‐212 wk | ≥50% reduction in the seizure frequency of 7 patients (26.9%) at the end of the study | 135 |
| Open‐label, uncontrolled clinical trial | DRE, diverse etiology |
n = 132 >1 y |
CBD oil, oral administration | Tritiated administration starting with 5 mg/kg/d and up to 50 mg/kg/d | 12‐48 wk | About 50% of the participants achieved ≥50% reduction in seizure frequency | 136 |
| Double‐blind, randomized placebo controlled trial | Lennox‐Gastaut syndrome |
n = 171 2‐55 y |
CBD oil, oral administration (n = 86) Placebo (n = 85) |
Tritiated administration starting with 2‐5 mg/kg/d and up to 20 mg/kg/d | 14 wk |
≥50% reduction in the seizure frequency of: CBD, 38 patients (44%) Placebo, 20 patients (24%) |
52 |
| Double‐blind, randomized placebo controlled trial | Dravet syndrome |
n = 120 2‐18 y |
CBD oil, oral administration (n = 61) Placebo (n = 59) |
20 mg/kg/d | 14 wk |
≥50% reduction in seizure frequency of: CBD, 22 patients (43%) Placebo, 15 patients (27%) |
137 |
| Case series | Febrile Infection‐Related Epilepsy Syndrome (FIRES) |
n = 5 Children, age not specified |
CBD oil, oral administration | 15‐20 mg/kg/d | 48 wk | Reduction in seizure frequency and severity | 138 |
| Case series | Refractory seizures in Sturge‐Weber syndrome |
n = 5 1 mo‐45 y |
CBD oil, oral administration | Tritiated administration starting with 5 mg/kg/d and up to 25 mg/kg/d | 14‐80 wk | ≥50% reduction in seizure frequency of 3 patients (60%) with bilateral brain involvement | 139 |
| Open‐label, uncontrolled clinical trial | Epilepsy of diverse etiology |
n = 48 1‐30 y |
CBD oil, oral administration | Tritiated administration starting with 2‐5 mg/kg/d and up to 50 mg/kg/d | 4 wk | ≥50% reduction in seizure frequency of 20 patients (41.7%), with improvement in memory and other cognitive functions | 140 |
| Case series | Brain tumor‐related epilepsy |
n = 3 17‐40 y |
CBD oil, oral administration | Tritiated administration starting with 5 mg/kg/d and up to 50 mg/kg/d | 8‐44 wk | Reduction in seizure frequency and severity of 2 patients | 141 |
| Double‐blind, randomized placebo controlled trial | Focal seizures, DRE |
n = 186 18‐71 y |
Transdermal gel (CBD 4.2%), local administration Placebo |
Two CBD groups:
|
12 wk | CBD and placebo showed similar effect | 142 |
| Open‐label, uncontrolled clinical trial | DRE, diverse etiology |
n = 137 1‐30 y |
CBD oil, oral administration | Tritiated administration starting with 2‐5 mg/kg/d and up to 25‐50 mg/kg/d | 12 wk | ≥50% reduction in seizure frequency of 51 patients (37%) | 143 |
| Open‐label, expanded‐access study | TSC and DRE |
n = 18 2‐31 y |
CBD oil, oral administration | Tritiated administration starting with 5 mg/kg/d and up to 25‐50 mg/kg/d | 24‐48 wk | ≥50% reduction in seizure frequency of 4 patients (50%) at the end of the study | 144 |
| Double‐blind, randomized placebo controlled trial | Temporal lobe epilepsy with secondarily generalized seizures |
n = 15 14‐49 y |
CBD, capsules for oral administration (n = 8) Placebo (n = 8) |
200‐300 mg/d | 8‐18 wk |
Clinical improvement in:
|
45, 145 |
| Cannabidivarin | |||||||
| Double‐blind, randomized placebo controlled trial | Focal epilepsy, DRE |
n = 32 18‐65 y |
CBDV (GWP42006), oral administration Placebo |
800 mg/d | 2 wk | Results not published | 146 |
| Double‐blind, randomized placebo controlled trial | Focal epilepsy, DRE |
n = 162 18‐65 y |
CBDV (GWP42006), oral administration Placebo |
Tritiated administration starting with 800 and up to 1600 mg/d | 8 wk | Seizure frequency decrease 40% in both groups | 147 |
| Tetrahydrocannabidiol | |||||||
| Case series | Epilepsy and MR |
n = 5 Children, age not specified |
THC isomers, route of administration not described | Up to 4 mg/d | 3‐7 wk | Clinical improvement in 2 patients (40%) | 148 |
| Case series | Epilepsy and MR |
n = 6 8‐14 y |
THC oil, oral administration | Up to 0.12 mg/kg/d | Not specified | Clinical improvement in 4 patients (67%) | 149 |
Abbreviations: AEDs, Antiepileptic drugs; artisanal CBD oil, ten different CBD products; CBD, cannabidiol; CBDV, cannabidivarin; DRE, drug‐resistant epilepsy; mo, months; MR, mental retardation; OCE, oral cannabis extracts; pCB, phytocannabinoid; SD, standard deviation; THC, Δ9‐tetrahydrocannabidiol; THCA, tetrahydrocannabinolic acid; TSC, tuberous sclerosis complex; wk, weeks; y, years.
The conflicting results about cannabis failure or success in epilepsy can be explained by several circumstances. First of all, the pharmacokinetics and pharmacodynamics of cannabinoids depend on the formulation and route of administration. Indeed, some effects of cannabinoids are explained by pharmacokinetic interactions such as inhibition or induction of enzymes involved in drug metabolism.18 Other important issue is that the bioavailability of cannabinoids applied by oral administration in liquid formulations augments with the fed state, especially with high‐fat meals.19
On the other hand, the effects of cannabis on specific types of epilepsy are unknown. Concerning this issue, few studies exist about the effects of cannabis extracts in temporal lobe epilepsy, the most common drug‐resistant epileptic syndrome in adults (see below). The influence of clinical factors (age of patient, gender, etc) in the efficacy of cannabis has not been considered in clinical studies. Other factor that can modify the effects of cannabis is its coadministration with other drugs, especially antiseizure drugs. Few data exist about the pharmacokinetic and pharmacodynamic interaction between cannabis and other drugs.20 However, no information exists about the pharmacokinetic and pharmacodynamic interaction between cannabis and antiseizure drugs.
An important condition that explains the contradictory effects of cannabis on epilepsy is that this plant contains more than 480 compounds, including noncannabinoids such as prenylated flavonoids, stilbenoids derivatives, and lignanammides.21 The content of the different chemical components in cannabis depends on each species. Cannabis ruderalis contains the lowest concentrations of Δ9‐tetrahydrocannabidiol (THC).22 European Cannabis sativa contains more cannabidiol (CBD) than THC, whereas Asian Cannabis indica has more THC than CBD.23
Although artisanal cannabis is considered a “miracle therapy,” at present there are not regulations to maintain the quality and purity of the drug during the obtaining procedure. Artisanal cannabis oil may contain abiotic (dust, fertilizers) and biotic (ie, insect, fungi, bacteria) contaminants, heavy metals, pesticides, etc,24 a situation that represents a high risk to the health of patients. Unfortunately, the evaluation of the effects of artisanal cannabis is difficult, has yielded controversial results, and lacks controlled clinical studies.25
There is an apparent disregard for long‐term use of cannabis. Long‐term cannabis administration augments the risk of addiction and is associated with side effects such as chronic bronchitis. It also enhances the possibility to present psychosis and schizophrenia in persons with a predisposition to such disorders. Adolescents are more vulnerable to the side effects of chronic cannabis use as there is altered brain development, cognitive impairment, poor academic outcomes, etc26. According to this information, it is evident the necessity to obtain more information concerning the beneficial effects of cannabis oil in the control of drug‐resistant epilepsy and stablish standardized procedures to obtain homogeneous products. In addition, it is essential to elucidate the contribution of each compound in the therapeutic effects induced by cannabis.
3. IS CANNABIDIOL A NEW HOPE FOR DRUG‐RESISTANT EPILEPSY?
At present, there are studies indicating that some cannabis products may induce antiepileptic effects. These products are THC, CBD, Δ9‐tetrahydrocannabivarin, cannabidivarin, and Δ9‐tetrahydrocannabinolic acid.27, 28 The main phytocannabinoids evaluated with this purpose are THC and CBD. THC is an active ingredient of cannabis plant that induces psychoactive effects, augments oxidative stress, and produces mitochondrial dysfunction in the brain, conditions that increase the risk to stroke and brain damage.29, 30 For these reasons, low interest exists about THC as an antiseizure drug.
Cannabidiol is the most abundant phytocannabinoid in cannabis. It has a terpenophenolic structure and hydroxyl groups in carbons 1 and 3.31 CBD shares lipophilic characteristics with the rest of the cannabinoids, lacks psychoactive effects,32 and induces neuroprotective effects.33
The metabolism of CBD comprises oxidation and hydroxylation through different enzymes of the cytochrome P450 family (CYP450) (Figure 1).34, 35, 36 In vitro studies using human liver microsomes revealed that 6α‐hydroxylation of CBD is mediated by CYP3A4 and CYP2C19 isoforms, 6β‐hydroxylation is induced by CYP3A4, whereas 7‐hydroxylation is mediated by CYP2C19.35 Glucuronosyltransferase and sulfotransferases enzymes are also involved in the metabolism of CBD.36, 37 At present, more than fifty metabolites from CBD have been identified in urine.34, 38 The most abundant metabolites are 7‐carboxy‐cannabidiol (7‐COOH‐CBD), 7‐hydroxy‐cannabidiol (7‐OH‐CBD), and 6‐hydroxy‐cannabidiol (6‐OH‐CBD). Although the biological activity of many of CBD metabolites is unknown,36, 39 preclinical studies suggest that 7‐OH‐CBD obtained from humans induces anticonvulsant effects in mice.40
Figure 1.
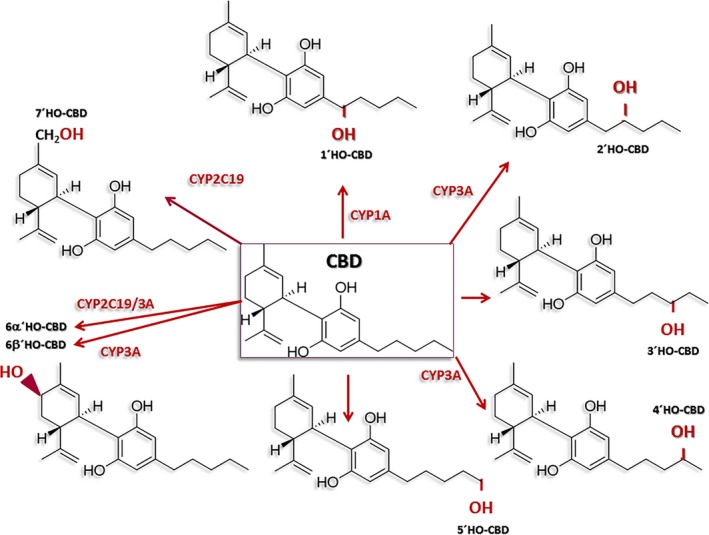
Schematic diagram indicating the different enzymes of the cytochrome P450 family (CYP450) involved in the metabolism of cannabidiol (CBD)
On the other hand, CBD is a potent inhibitor of CYP1A1, CYP2B6, CYP2D6, and CYP2C19 with a subsequent reduction in the metabolism of some drugs. This condition explains the increases in the serum levels of THC, topiramate, rufinamide, clobazam, and N‐desmethylclobazam when they are coadministered with CBD.41, 42, 43 This effect is more evident when the drugs are oral administered.44 These studies lead to suggest that CBD augments the effects of antiseizure drugs. This idea is supported by the early observational clinical study carried out by Cunha et al,45 who described for the first time that the chronic administration of CBD reduced the seizure activity in seven of eight patients with drug‐resistant temporal lobe epilepsy. During the CBD treatment, the patients received the administration of the antiseizure drugs prescribed before the study. According to this information, it is evident the necessity of clinical studies focused to determine the effects of CBD in different experimental models of drug‐resistant epilepsy and its pharmacokinetic interactions with other drugs.
Several studies support that CBD could be effective in the control of epilepsy.46 Results obtained from experimental models reveal that CBD reduces the seizure activity47, 48, 49 and delays the epileptogenesis process, effects associated with neuroprotection.50, 51 CBD in oral solution (Epidiolex®) is considered a therapy to control seizures associated with the Lennox‐Gastaut syndrome,52 Dravet syndrome,53 and infantile spasms.54 Indeed, the US Food and Drug Administration (FDA) recently approved Epidiolex for the control of seizures associated with Lennox‐Gastaut syndrome and Dravet syndrome, in children (2 years of age and older) and adults.55
The antiepileptic, anxiolytic, antipsychotic, and neuroprotective effects induced by CBD lead to suggest that it is an excellent candidate to control drug‐resistant epilepsy and comorbid disorders.56 This notion is supported by results obtained from experimental models of temporal lobe epilepsy, a neurological disorder with a high prevalence of drug resistance and comorbid psychiatric symptoms.57 CBD induces neuroprotection, decreased neuronal excitability, and avoids cell death in the hippocampus of animals with temporal lobe epilepsy.50, 58 However, the effects of CBD in other types of drug‐resistant epilepsy are not conclusive due to the presence of subjects who do not respond to the treatment.59, 60, 61, 62
Clinical data in humans indicate that CBD‐rich extracts are more effective to reduce the seizure frequency when compared with purified CBD. In addition, CBD‐rich extracts reduce the seizure activity with a significantly lower average daily dose, supporting a higher potency when compared with purified CBD. These effects are associated with side effects such as appetite alterations, nausea, diarrhea and other gastrointestinal alterations, sleepiness, weight changes, and fatigue, among others.63 It is known that THC augments the analgesic effects of CBD.64 On the other hand, CBD potentiates or reduces some effects induced by THC.65 However, it is unknown whether the THC and CBD interaction facilitates or reduces the seizure activity.
4. CONVENTIONAL EFFECTS THAT EXPLAIN THE ANTIEPILEPTIC EFFECTS OF CANNABIDIOL
Different targets are involved in the mechanisms by which CBD induces antiepileptic effects. Computational analysis and ligand displacement assays revealed that CBD augments the levels of endocannabinoids (anandamide) as result of the inhibition of the fatty acid‐binding proteins (FABPs) that mediate the anandamide transport to its catabolite enzyme (fatty acid amide hydrolase [FAAH]).43 On the other hand, experiments indicate that CBD is a very low‐affinity ligand at CB1 and CB2 receptors inducing antagonism.66 It acts as agonist on D2 (partial agonist)67 and 5‐HT1A receptors.68, 69 CBD is an agonist of TRPV1 channels27 and activator of TRPV2 channels.70 It is an allosteric modulator of mu‐ and delta‐opioid receptors.71 CBD induces resting‐state blockage of sodium channels and blocks the voltage‐gated potassium channel subunit Kv2.1.72 Studies indicate that CBD produces neuroprotection by activation of CB2 and adenosine A2 receptors.73 In experimental models of pain, CBD induces analgesic effects through the activation of 5HT1A and TRPV1 channels.74, 75 Using blood‐brain barrier (BBB) modeled with human brain microvascular endothelial cell and astrocyte co‐cultures, it was found that CBD induces neuroprotection in ischemic stroke by activation of PPARγ and 5‐HT1A receptors.76 CBD injected into the dorsolateral periaqueductal gray of rats exerts anxiolytic‐like effects through the activation of 5‐HT1A receptors (Figure 2).77
Figure 2.
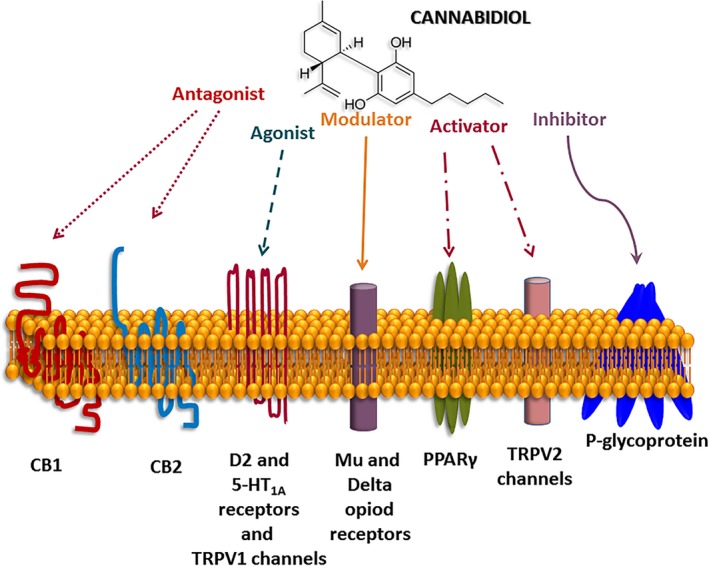
Mechanisms of action of cannabidiol (CBD) on different receptors, channels, and P‐glycoprotein transporter
CBD has been suggested to play a critical role in the glutamatergic neurotransmission. However, the findings are controversial. CBD induces antidepressant‐like effects associated with enhanced serotonin and glutamate neurotransmission in a mouse model of depression.78 Other studies indicate that CBD reduces the overactivity of NMDA receptors through the antagonism of the sigma 1 receptors (σ1R).79 CBD also reduces glutamate release and protects from convulsive activity in an experimental model of seizures induced by cocaine.80 The effect of CBD on the glutamatergic neurotransmission is relevant because excess of extracellular levels of glutamate is associated with recurrent seizures and chronic epilepsy.81 Indeed, the blockage of NMDA receptors can prevent and in some cases reverse certain pathological conditions associated with neurological disorders, including epilepsy.82, 83
High glutamatergic neurotransmission, neuroinflammation, and oxidative stress are interconnected phenomena that occur in the brain of subjects with epilepsy. Interestingly, the increase in the oxidative stress and neuroinflammation associated with drug‐resistant epilepsy can be reverted when the seizure activity decreases as result of the surgical resection of the epileptic foci.84, 85, 86
Seizure‐induced neuroinflammation is a condition associated with the increase of cytokines such as interleukin (IL)‐1β, tumor necrosis factor (TNF), transforming growth factor (TGF)‐β, and danger signals such as High Mobility Group Box 1 (HMGB1). The activation of cytokines may underlie hyperexcitability and neurotoxicity, a situation that facilitates the epileptic activity.87, 88, 89 Concerning this issue, it is described that IL‐1β induced in activated astrocytes and microglia contributes to the occurrence of seizure activity.90, 91 IL‐1β and TNF produce excitatory effects by enhancement of Ca2+ influx and extracellular levels of glutamate with a subsequent production of hydroxyl radicals. TNF also modifies the glutamate subunit receptor composition of neurons and augments the glutamate release from microglia. In astrocytes, TNF enhances Ca2+ mobilization with a subsequent cyclooxygenase enzyme‐2 (COX‐2) activation, prostaglandin‐2 synthesis, and glutamate release.92, 93
Oxidative stress is a condition detected during epileptogenesis and chronic epilepsy. It is a consequence of mitochondrial dysfunction and increased activity of nicotinamide adenine dinucleotide phosphate oxidase (NOX), xanthine oxidase, and inducible nitric oxide synthase (iNOS) that result in the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Oxidant stress facilitates inflammation through the induction of COX‐2 gene expression and ictogenic cytokines.93, 94 The high glutamate release and NMDA receptor activation in chronic epilepsy can also facilitate oxidative mechanisms and neurotoxicity.95, 96
Experimental evidence supports that CBD represents a novel strategy to reduce oxidative stress, excitotoxicity, and neuroinflammation in neurodegenerative disorders. CBD decreases oxidative stress, mitochondrial dysfunction and reactive oxygen species generation, effects associated with reduced neuroinflammation.97 CBD also augments microglial phagocytosis by the modification of TRPV channel activity.98 It induces anti‐inflammatory effects by decreasing the plasma levels of prostaglandin E2, the production of free radicals, and the activity of COX‐1/COX‐2.99, 100 CBD reduces neuronal damage, astrogliosis, excitotoxicity, and neuroinflammation in experimental models of ischemia.101 However, the immune effect of CBD can vary depending on the concentrations administered as well as the type and/or magnitude of stimulus.102
5. CANNABIDIOL MAY ACT ON UNCONVENTIONAL CENTRAL AND PERIPHERAL TARGETS TO CONTROL DRUG‐RESISTANT EPILEPSY
P‐glycoprotein is a BBB efflux transporter that limits drug accumulation in the brain. Its overexpression at the luminal side of the BBB is associated with drug‐resistant epilepsy because it results in a low penetration of antiseizure drugs into the brain.103, 104 P‐glycoprotein is also overexpressed in astrocytes and neurons in brain tissue obtained from patients and animals with drug‐resistant epilepsy.105, 106
The enhanced extracellular levels of glutamate produced in the brain of subjects with drug‐resistant epilepsy107 represent a mechanism that facilitates the overexpression of P‐glycoprotein in a COX‐2‐dependent manner.108 P‐glycoprotein overexpression in cells of the BBB can also result from chronic oxidative stress or prolonged neuroinflammation.109, 110 According to several experimental evidence, the administration of inhibitors of P‐glycoprotein function or expression represents a potential therapeutic strategy to control drug‐resistant epilepsy.81, 111 Concerning this issue, the use of celecoxib, a specific COX‐2 inhibitor, reverts the P‐glycoprotein overexpression and facilitates brain delivery of antiseizure drugs in animals with epilepsy.112 The administration of P‐glycoprotein inhibitors such as verapamil induces encouraging effects in patients with drug‐resistant epilepsy. However, these drugs may induce significant side effects that restrict their clinical application.113 Experimental evidence also supports that a better control of drug‐resistant epilepsy could be obtained if antiseizure drugs are associated with P‐glycoprotein inhibitors.111, 114, 115
Studies indicate that CBD down‐regulates the protein and mRNA expression of P‐glycoprotein and inhibits its efflux function in trophoblast cell lines.116 CBD also interacts with a specific site of the P‐glycoprotein interfering with the ATPase activity stimulated by substrates and consequently decreasing the energy required for their transport in Caco‐2 and LLC‐PK1/MDR1 cells.117 The inhibitory effect of CBD on P‐glycoprotein is evident after prolonged, but not short‐term exposure, in cells CEM/VLB100 that overexpress this transporter.118
It is important to mention that CBD is not a substrate of P‐glycoprotein. In mice, experiments revealed that the overexpression of this transporter at BBB does not limit the brain uptake of CBD.119 This condition associated with the inhibitory effect of P‐glycoprotein at BBB plus its anticonvulsant and neuroprotective effects suggests that CBD can be an attractive adjunctive therapy to control drug‐resistant epilepsy. However, further studies are necessary to demonstrate that the exposure to CBD blocks the activity of P‐glycoprotein and/or reverts its overexpression in neurons and astrocytes in brain tissue obtained from subjects with drug‐resistant epilepsy.
The overexpression of P‐glycoprotein in neurons is associated with high membrane depolarization, a condition that may facilitate the epileptiform activity.120 Interestingly, P‐glycoprotein overexpression is also induced in cardiomyocytes of subjects submitted to repetitive convulsive seizures. This condition is related with electrocardiographic (ECG) alterations and SUDEP as consequence of a depolarizing role in cardiomyocytes.121 If CBD is able to revert the P‐glycoprotein overexpression and its depolarizing condition in heart, it may represent a novel strategy to reduce SUDEP in drug‐resistant epilepsy. However, further experiments are necessary to support this hypothesis.
In addition to the pharmacological mechanisms previously described, CBD also induces epigenetic changes associated with neuroprotective effects. Concerning this issue, it is known that iron accumulation in brain regions is a condition that contributes to neurodegeneration. CBD restores the basal levels of hippocampal dynamin‐1‐like protein (DNM1L), caspase 3, and synaptophysin in animals with cell damage subsequent to iron loading.122 These effects are also associated with reversion of the iron‐induced mitochondrial deoxyribonucleic acid (mtDNA) deletions, the decreased epigenetic modulation of mtDNA, as well as restauration of the mitochondrial ferritin levels and succinate dehydrogenase activity.123 According to these studies, CBD induces epigenetic effects that results in the restauration of the normal cellular function and neuroprotection. Epigenetic effects induced by CBD hold promise as a future therapeutic strategy for drug‐resistant epilepsy. However, further research is essential to determine side effects induced by CBD when applied chronically, alone or combined with other antiseizure drugs inducing epigenetic effects such as valproic acid.124
On the other hand, the repeated coadministration of CBD with THC induces histone 3 acetylation (H3K9/14ac) in the ventral tegmental area, an epigenetic effect related with addiction processes.125 These effects should be considered when apply CBD chronically because H3K9 acetylation is significantly augmented as result of seizure activity, a condition associated with the activation of TLR4 and subsequent inflammation process.126
6. CONCLUSIONS
In the modern era of highly effective and specific therapies targeted to treat different disorders, efforts have to focus to identify the real composition of the used extracts of phytocannabinoids. Concerning CBD, it is evident that it induces therapeutic effects that can be applied to control drug‐resistant epilepsy. However, it is necessary to identify the types of epilepsy responsive to the beneficial effects of CBD. In addition, it is essential to know its pharmacokinetic, side effects as well as the cellular and molecular effects induced by its repetitive and long‐term administration, alone and associated with antiseizure drugs.
Other important issue to consider is the evaluation of unconventional targets and mechanisms of action of CBD and other cannabinoids to control drug‐resistant epilepsy and reduce fatal complications such as SUDEP. Several studies support that CBD decreases the expression and function of P‐glycoprotein in different cell types. If CBD is able to diminish the overexpression of this transporter in the brain of patients with drug‐resistant epilepsy, it could be used as adjunctive therapy to better biodistribution and CNS access of antiseizure drugs.
Finally, a scientific validation of the antiepileptic properties of the different cannabinoids and their metabolites as well as terpenes (alone and in combination) would benefit in the control of different types of epilepsy. This validation should consider the genetic background to understand the patients’ response to cannabinoids.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The present study is consistent with the Journal's guidelines for ethical publication.
ACKNOWLEDGMENTS
This study was supported by the Neurobiology Commission of ILAE and the National Council for Science and Technology (CONACyT, grants A3‐S‐26782 and 261481).
Rocha L, Frías‐Soria CL, Ortiz JG, Auzmendi J, Lazarowski A. Is cannabidiol a drug acting on unconventional targets to control drug‐resistant epilepsy? Epilepsia Open. 2020;5:36–49. 10.1002/epi4.12376
REFERENCES
- 1. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 Neurological Disorders Collaborator Group . Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryvlin P, Nashef L, Tomson T. Prevention of sudden unexpected death in epilepsy: a realistic goal? Epilepsia. 2013;54(Suppl 2):23–8. [DOI] [PubMed] [Google Scholar]
- 4. Granata T, Marchi N, Carlton E, Ghosh C, Gonzalez‐Martinez J, Alexopoulos AV, et al. Management of the patient with medically refractory epilepsy. Expert Rev Neurother. 2009;9:1791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman D, Sirven JI. Historical perspective on the medical use of cannabis for epilepsy: Ancient times to the 1980s. Epilepsy Behav. 1980s;70:298–301. [DOI] [PubMed] [Google Scholar]
- 6. Chesher GB, Jackson DM, Malor RM. Interaction of delta9‐tetrahydrocannabinol and cannabidiol with phenobarbitone in protecting mice from electrically induced convulsions. J Pharm Pharmacol. 1975;27:608–9. [DOI] [PubMed] [Google Scholar]
- 7. Izquierdo I, Orsingher OA, Berardi AC. Effect of cannabidiol and of other Cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia. 1973;28:95–102. [DOI] [PubMed] [Google Scholar]
- 8. Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol. 1982;83:293–8. [DOI] [PubMed] [Google Scholar]
- 9. Ten Ham M, Loskota WJ, Lomax P. Acute and chronic effects of beta9‐tetrahydrocannabinol on seizures in the gerbil. Eur J Pharmacol. 1975;31:148–52. [DOI] [PubMed] [Google Scholar]
- 10. Karler R, Turkanis SA. Cannabis and epilepsy. Adv Biosci. 1978;22–23:619–41. [DOI] [PubMed] [Google Scholar]
- 11. Boggan WO, Steele RA, Freedman DX. 9 ‐Tetrahydrocannabinol effect on audiogenic seizure susceptibility. Psychopharmacologia. 1973;29:101–6. [DOI] [PubMed] [Google Scholar]
- 12. Corcoran ME, McCaughran JA, Wada JA. Acute antiepileptic effects of 9‐tetrahydrocannabinol in rats with kindled seizures. Exp Neurol. 1973;40:471–83. [DOI] [PubMed] [Google Scholar]
- 13. Turkanis SA, Smiley KA, Borys HK, Olsen DM, Karler R. An electrophysiological analysis of the anticonvulsant action of cannabidiol on limbic seizures in conscious rats. Epilepsia. 1979;20:351–63. [DOI] [PubMed] [Google Scholar]
- 14. Colasanti BK, Lindamood C, Craig CR. Effects of marihuana cannabinoids on seizure activity in cobalt‐epileptic rats. Pharmacol Biochem Behav. 1982;16:573–8. [DOI] [PubMed] [Google Scholar]
- 15. Neale M. Efficacy and safety of cannabis for treating children with refractory epilepsy. Nurs Child Young People. 2017;29:32–7. [DOI] [PubMed] [Google Scholar]
- 16. Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy‐related neuroprotection. Epilepsy Behav. 2017;70:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med. 2018;49:7–11. [DOI] [PubMed] [Google Scholar]
- 18. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60:1586–92. [DOI] [PubMed] [Google Scholar]
- 20. Anderson GD, Chan LN. Pharmacokinetic drug interactions with tobacco, cannabinoids and smoking cessation products. Clin Pharmacokinet. 2016;55:1353–68. [DOI] [PubMed] [Google Scholar]
- 21. Pollastro F, Minassi A, Fresu LG. Cannabis phenolics and their bioactivities. Curr Med Chem. 2018;25:1160–85. [DOI] [PubMed] [Google Scholar]
- 22. Gloss D. An overview of products and bias in research. Neurotherapeutics. 2015;12:731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McPartland JM. Cannabis systematics at the levels of family, genus, and species. Cannabis Cannabinoid Res. 2018;3:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russo EB. Current therapeutic cannabis controversies and clinical trial design issues. Front Pharmacol. 2016;7:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sulak D, Saneto R, Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017;70:328–33. [DOI] [PubMed] [Google Scholar]
- 26. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017;70:313–8. [DOI] [PubMed] [Google Scholar]
- 28. Hill AJ, Mercier MS, Hill TDM, Glyn S, Jones N, Yamasaki Y, et al. Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol. 2012;167:1629–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosales‐Corral S, Hernández L, Gallegos M. Cannabinoids in neuroinflammation, oxidative stress and neuro excitotoxicity. Pharm Anal Acta. 2015;6:346 10.4172/2153-2435.1000346 [DOI] [Google Scholar]
- 30. Wolff V, Schlagowski A‐I, Rouyer O, Charles A‐L, Singh F, Auger C, et al. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: a potential mechanism involved in cannabis‐related stroke. Biomed Res Int. 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palazzoli F, Citti C, Licata M, Vilella A, Manca L, Zoli M, et al. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC‐MS/MS) method for the determination of cannabidiol (CBD), Δ9 ‐tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J Pharm Biomed Anal. 2018;150:25–32. [DOI] [PubMed] [Google Scholar]
- 32. Croxford JL. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17:179–202. [DOI] [PubMed] [Google Scholar]
- 33. Fernández‐Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harvey DJ, Mechoulam R. Metabolites of cannabidiol identified in human urine. Xenobiotica. 1990;20:303–20. [DOI] [PubMed] [Google Scholar]
- 35. Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89:165–70. [DOI] [PubMed] [Google Scholar]
- 36. Ujváry I, Hanuš L. Human metabolites of cannabidiol: A review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 2016;1:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus‐Zawada A, et al. Characterization of human hepatic and extrahepatic UDP‐glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37:1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harvey DJ, Samara E, Mechoulam R. Urinary metabolites of cannabidiol in dog, rat and man and their identification by gas chromatography‐mass spectrometry. J Chromatogr. 1991;562:299–322. [DOI] [PubMed] [Google Scholar]
- 39. Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose‐ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whalley BJ, Stott C, Gray RA, Jones NA. The human metabolite of cannabidiol, 7‐hydroxy cannabidiol, but not 7‐carboxy cannabidiol, is anticonvulsant in the maximal electroshock seizure threshold test (MEST) in mouse. American Epilepsy Society Meeting. 1.435, Abstract, 2017, p 1.
- 41. Gaston TE, Bebin EM, Cutter GR, Cutter GR, Liu Y, Szaflarski JP. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58:1586–92. [DOI] [PubMed] [Google Scholar]
- 42. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug‐drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51. [DOI] [PubMed] [Google Scholar]
- 43. Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, et al. Fatty acid‐binding proteins (FABPs) are intracellular carriers for Δ9‐tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015;290:8711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, et al. Randomized, double‐blind, placebo‐controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9‐tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27:799–810. [DOI] [PubMed] [Google Scholar]
- 45. Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–85. [DOI] [PubMed] [Google Scholar]
- 46. Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373:1048–58. [DOI] [PubMed] [Google Scholar]
- 47. Jones NA, Glyn SE, Akiyama S, Hill TDM, Hill AJ, Weston SE, et al. Cannabidiol exerts anti‐convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–52. [DOI] [PubMed] [Google Scholar]
- 48. Vilela LR, Lima IV, Kunsch ÉB, Pinto HPP, de Miranda AS, Vieira ÉLM, et al. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: Pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. 2017;75:29–35. [DOI] [PubMed] [Google Scholar]
- 49. Patra PH, Barker‐Haliski M, White HS, Whalley BJ, Glyn S, Sandhu H, et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2019;60:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Do Val‐da Silva RA, Peixoto‐Santos JE, Kandratavicius L, De Ross JB, Esteves I, De Martinis BS, et al. Protective effects of cannabidiol against seizures and neuronal death in a rat model of mesial temporal lobe epilepsy. Front Pharmacol. 2017;8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hosseinzadeh M, Nikseresht S, Khodagholi F, Maghsoudi N. Cannabidiol post‐treatment alleviates rat epileptic‐related behaviors and activates hippocampal cell autophagy pathway along with antioxidant defense in chronic phase of pilocarpine‐induced seizure. J Mol Neurosci. 2016;58:432–40. [DOI] [PubMed] [Google Scholar]
- 52. Thiele EA, Marsh ED, French JA, Mazurkiewicz‐Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet (London, England). 2018;391:1085–96. [DOI] [PubMed] [Google Scholar]
- 53. Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, et al. Long‐term cannabidiol treatment in patients with Dravet syndrome: An open‐label extension trial. Epilepsia. 2019;60:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hussain SA, Dlugos DJ, Cilio MR, Parikh N.A phase‐2 study of pharmaceutical grade synthetic cannabidiol oral solution for treatment of refractory infantile spasms. AES Annual Meeting. 2017, p Abst. 3.278. [DOI] [PubMed]
- 55. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. 2018. Available from https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm (accessed 15 Jul 2019).
- 56. Crippa JA, Guimarães FS, Campos AC, Zuardi AW. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakahara S, Adachi M, Ito H, Matsumoto M, Tajinda K, Erp TGM. Hippocampal pathophysiology: Commonality shared by temporal lobe epilepsy and psychiatric disorders. Neurosci J. 2018;2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khan AA, Shekh‐Ahmad T, Khalil A, Walker MC, Ali AB. Cannabidiol exerts antiepileptic effects by restoring hippocampal interneuron functions in a temporal lobe epilepsy model. Br J Pharmacol. 2018;175:2097–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3:CD009270 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rosenberg EC, Tsien RW, Whalley BJ, et al. Cannabinoids and epilepsy. Neurotherapeutics. 2015;12:747–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Devinsky O, Verducci C, Thiele EA, Laux LC, Patel AD, Filloux F, et al. Open‐label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–7. [DOI] [PubMed] [Google Scholar]
- 62. Tzadok M, Uliel‐Siboni S, Linder I, Kramer U, Epstein O, Menascu S, et al. CBD‐enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure. 2016;35:41–4. [DOI] [PubMed] [Google Scholar]
- 63. Pamplona FA, da Silva LR, Coan AC. Potential clinical benefits of CBD‐rich cannabis extracts over purified CBD in treatment‐resistant epilepsy: Observational data meta‐analysis. Front Neurol. 2018;9:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. King KM, Myers AM, Soroka‐Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, et al. Single and combined effects of Δ9 ‐tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy‐induced neuropathic pain. Br J Pharmacol. 2017;174:2832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karniol IG, Carlini EA. Pharmacological interaction between cannabidiol and delta 9‐tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. [DOI] [PubMed] [Google Scholar]
- 66. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9‐tetrahydrocannabinol, cannabidiol and delta9‐tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Seeman P. Cannabidiol is a partial agonist at dopamine D2 High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6:e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5‐HT1a receptors. Neurochem Res. 2005;30:1037–43. [DOI] [PubMed] [Google Scholar]
- 69. Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5‐hydroxytryptamine1A receptor‐dependent mechanism. Stroke. 2005;36:1077–82. [DOI] [PubMed] [Google Scholar]
- 70. Pumroy RA, Samanta A, Liu Y, Hughes TET, Zhao S, Yudin Y, et al. Molecular mechanism of TRPV2 channel modulation by cannabidiol. Elife. 2019;8;48792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu‐ and delta‐opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:354–61. [DOI] [PubMed] [Google Scholar]
- 72. Ghovanloo M‐R, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ. Inhibitory effects of cannabidiol on voltage‐dependent sodium currents. J Biol Chem. 2018;293:16546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Castillo A, Tolón MR, Fernández‐Ruiz J, Romero J, Martinez‐Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic‐ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37:434–40. [DOI] [PubMed] [Google Scholar]
- 74. Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA. Cannabidiol inhibits paclitaxel‐induced neuropathic pain through 5‐HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014;171:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hind WH, England TJ, O’Sullivan SE. Cannabidiol protects an in vitro model of the blood‐brain barrier from oxygen‐glucose deprivation via PPARγ and 5‐HT1A receptors. Br J Pharmacol. 2016;173:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic‐like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology. 2008;199:223–30. [DOI] [PubMed] [Google Scholar]
- 78. Linge R, Jiménez‐Sánchez L, Campa L, Pilar‐Cuéllar F, Vidal R, Pazos A, et al. Cannabidiol induces rapid‐acting antidepressant‐like effects and enhances cortical 5‐HT/glutamate neurotransmission: role of 5‐HT1A receptors. Neuropharmacology. 2016;103:16–26. [DOI] [PubMed] [Google Scholar]
- 79. Rodríguez‐Muñoz M, Onetti Y, Cortés‐Montero E, Garzón J, Sánchez‐Blázquez P. Cannabidiol enhances morphine antinociception, diminishes NMDA‐mediated seizures and reduces stroke damage via the sigma 1 receptor. Mol Brain. 2018;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gobira PH, Vilela LR, Gonçalves BDC, Santos RPM, de Oliveira AC, Vieira LB, et al. Cannabidiol, a Cannabis sativa constituent, inhibits cocaine‐induced seizures in mice: Possible role of the mTOR pathway and reduction in glutamate release. Neurotoxicology. 2015;50:116–21. [DOI] [PubMed] [Google Scholar]
- 81. Bankstahl JP, Hoffmann K, Bethmann K, Löscher W. Glutamate is critically involved in seizure‐induced overexpression of P‐glycoprotein in the brain. Neuropharmacology. 2008;54:1006–16. [DOI] [PubMed] [Google Scholar]
- 82. Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system‐too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. [DOI] [PubMed] [Google Scholar]
- 83. Ghasemi M, Schachter SC. The NMDA receptor complex as a therapeutic target in epilepsy: a review. Epilepsy Behav. 2011;22:617–40. [DOI] [PubMed] [Google Scholar]
- 84. López J, González ME, Lorigados L, Morales L, Riverón G, Bauzá JY. Oxidative stress markers in surgically treated patients with refractory epilepsy. Clin Biochem. 2007;40:292–8. [DOI] [PubMed] [Google Scholar]
- 85. Lorigados Pedre L, Gallardo JM, Morales Chacón LM, Vega García A, Flores‐Mendoza M, Neri‐Gómez T, et al. Oxidative stress in patients with drug resistant partial complex seizure. Behav Sci (Basel, Switzerland). 2018;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lorigados Pedre L, Morales Chacón LM, Pavón Fuentes N, Robinson Agramonte M, Serrano Sánchez T, Cruz‐Xenes R, et al. Follow‐up of peripheral IL‐1β and IL‐6 and relation with apoptotic death in drug‐resistant temporal lobe epilepsy patients submitted to surgery. Behav Sci (Basel, Switzerland). 2018;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 2010;89:34–42. [DOI] [PubMed] [Google Scholar]
- 89. Lorigados Pedre L, Morales Chacón LM, Orozco Suárez S, Pavón Fuentes N, Estupiñán Díaz B, Serrano Sánchez T, et al. Inflammatory mediators in epilepsy. Curr Pharm Des. 2013;19:6766–72. [DOI] [PubMed] [Google Scholar]
- 90. Akin D, Ravizza T, Maroso M, Carcak N, Eryigit T, Vanzulli I, et al. IL‐1β is induced in reactive astrocytes in the somatosensory cortex of rats with genetic absence epilepsy at the onset of spike‐and‐wave discharges, and contributes to their occurrence. Neurobiol Dis. 2011;44:259–69. [DOI] [PubMed] [Google Scholar]
- 91. Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49(Suppl 2):24–32. [DOI] [PubMed] [Google Scholar]
- 92. Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. [DOI] [PubMed] [Google Scholar]
- 93. Terrone G, Balosso S, Pauletti A, Ravizza T, Vezzani A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology. 2019;107742 [Epub ahead of print]. 10.1016/j.neuropharm.2019.107742. [DOI] [PubMed] [Google Scholar]
- 94. Feng L, Xia Y, Garcia GE, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase‐2 expression induced by interleukin‐1, tumor necrosis factor‐alpha, and lipopolysaccharide. J Clin Invest. 1995;95:1669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Küçükkaya B, Haklar G, Yalçin AS. NMDA excitotoxicity and free radical generation in rat brain homogenates: application of a chemiluminescence assay. Neurochem Res. 1996;21:1535–8. [DOI] [PubMed] [Google Scholar]
- 96. Shaw CA, Bains JS. Synergistic versus antagonistic actions of glutamate and glutathione: the role of excitotoxicity and oxidative stress in neuronal disease. Cell Mol Biol (Noisy‐le‐grand). 2002;48:127–36. [PubMed] [Google Scholar]
- 97. Vallée A, Lecarpentier Y, Guillevin R, Vallée J‐N. Effects of cannabidiol interactions with Wnt/β‐catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin (Shanghai). 2017;49:853–66. [DOI] [PubMed] [Google Scholar]
- 98. Hassan S, Eldeeb K, Millns PJ, Bennett AJ, Alexander SPH, Kendall DA. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br J Pharmacol. 2014;171:2426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non‐psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. [DOI] [PubMed] [Google Scholar]
- 100. Ruhaak LR, Felth J, Karlsson PC, Rafter JJ, Verpoorte R, Bohlin L. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa . Biol Pharm Bull. 2011;34:774–8. [DOI] [PubMed] [Google Scholar]
- 101. Ceprián M, Jiménez‐Sánchez L, Vargas C, Barata L, Hind W, Martínez‐Orgado J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology. 2017;116:151–9. [DOI] [PubMed] [Google Scholar]
- 102. Karmaus PWF, Wagner JG, Harkema JR, Kaminski NE, Kaplan BLF. Cannabidiol (CBD) enhances lipopolysaccharide (LPS)‐induced pulmonary inflammation in C57BL/6 mice. J Immunotoxicol. 2013;10:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tang F, Hartz AMS, Bauer B. Drug‐resistant epilepsy: Multiple hypotheses, few answers. Front Neurol. 2017;8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Höcht C, Lazarowski A, Gonzalez NN, Auzmendi J, Opezzo JAW, Bramuglia GF, et al. Nimodipine restores the altered hippocampal phenytoin pharmacokinetics in a refractory epileptic model. Neurosci Lett. 2007;413:168–72. [DOI] [PubMed] [Google Scholar]
- 105. Lazarowski A, Ramos AJ, Garcia‐Rivello H, Brusco A, Girardi E. Neuronal and glial expression of the multidrug resistance gene product in an experimental epilepsy model. Cell Mol Neurobiol. 2004;24:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Marchi N, Hallene KL, Kight KM, Cucullo L, Moddel G, Bingaman W, et al. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med. 2004;2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet (London, England). 1993;341:1607–10. [DOI] [PubMed] [Google Scholar]
- 108. Bauer B, Hartz AMS, Pekcec A, Toellner K, Miller DS, Potschka H. Seizure‐induced up‐regulation of P‐glycoprotein at the blood‐brain barrier through glutamate and cyclooxygenase‐2 signaling. Mol Pharmacol. 2008;73:1444–53. [DOI] [PubMed] [Google Scholar]
- 109. Hong H, Lu Y, Ji Z‐N, Liu G‐Q. Up‐regulation of P‐glycoprotein expression by glutathione depletion‐induced oxidative stress in rat brain microvessel endothelial cells. J Neurochem. 2006;98:1465–73. [DOI] [PubMed] [Google Scholar]
- 110. Roberts DJ, Goralski KB. A critical overview of the influence of inflammation and infection on P‐glycoprotein expression and activity in the brain. Expert Opin Drug Metab Toxicol. 2008;4:1245–64. [DOI] [PubMed] [Google Scholar]
- 111. Robey RW, Lazarowski A, Bates SE. P‐glycoprotein–a clinical target in drug‐refractory epilepsy? Mol Pharmacol. 2008;73:1343–6. [DOI] [PubMed] [Google Scholar]
- 112. van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, et al. COX‐2 inhibition controls P‐glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010;58:404–12. [DOI] [PubMed] [Google Scholar]
- 113. Nicita F, Spalice A, Raucci U, Iannetti P, Parisi P. The possible use of the L‐type calcium channel antagonist verapamil in drug‐resistant epilepsy. Expert Rev Neurother. 2016;16:9–15. [DOI] [PubMed] [Google Scholar]
- 114. Lazarowski A, Czornyj L, Lubienieki F, Girardi E, Vazquez S, D'Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48(Suppl 5):140–9. [DOI] [PubMed] [Google Scholar]
- 115. Narayanan J, Frech R, Walters S, Patel V, Frigerio R, Maraganore DM. Low dose verapamil as an adjunct therapy for medically refractory epilepsy – an open label pilot study. Epilepsy Res. 2016;126:197–200. [DOI] [PubMed] [Google Scholar]
- 116. Feinshtein V, Erez O, Ben‐Zvi Z, Erez N, Eshkoli T, Sheizaf B, et al. Cannabidiol changes P‐gp and BCRP expression in trophoblast cell lines. PeerJ. 2013;1:e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhu H‐J, Wang J‐S, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, et al. Characterization of P‐glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther. 2006;317:850–7. [DOI] [PubMed] [Google Scholar]
- 118. Holland ML, Panetta JA, Hoskins JM, Bebawy M, Roufogalis BD, Allen JD, et al. The effects of cannabinoids on P‐glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol. 2006;71:1146–54. [DOI] [PubMed] [Google Scholar]
- 119. Brzozowska N, Li KM, Wang XS, Booth J, Stuart J, McGregor IS, et al. ABC transporters P‐gp and Bcrp do not limit the brain uptake of the novel antipsychotic and anticonvulsant drug cannabidiol in mice. PeerJ. 2016;4:e2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Auzmendi JA, Orozco‐Suárez S, Bañuelos‐Cabrera I, González‐Trujano ME, Calixto González E, Rocha L, et al. P‐glycoprotein contributes to cell membrane depolarization of hippocampus and neocortex in a model of repetitive seizures induced by pentylenetetrazole in rats. Curr Pharm Des. 2013;19:6732–8. [DOI] [PubMed] [Google Scholar]
- 121. Auzmendi J, Buchholz B, Salguero J, Cañellas C, Kelly J, Men P, et al. Pilocarpine‐induced status epilepticus is associated with P‐Glycoprotein induction in cardiomyocytes, electrocardiographic changes, and sudden death. Pharmaceuticals (Basel). 2018;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. da Silva VK, de Freitas BS, da Silva DA, Nery LR, Falavigna L, Ferreira RDP, et al. Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: Implications for neuroprotection. Mol Neurobiol. 2014;49:222–33. [DOI] [PubMed] [Google Scholar]
- 123. da Silva VK, de Freitas BS, Dornelles VC, Kist LW, Bogo MR, Silva MC, et al. Novel insights into mitochondrial molecular targets of iron‐induced neurodegeneration: reversal by cannabidiol. Brain Res Bull. 2018;139:1–8. [DOI] [PubMed] [Google Scholar]
- 124. Navarrete‐Modesto V, Orozco‐Suárez S, Feria‐Romero IA, Rocha L. The molecular hallmarks of epigenetic effects mediated by antiepileptic drugs. Epilepsy Res. 2019;149:53–65. [DOI] [PubMed] [Google Scholar]
- 125. Todd SM, Zhou C, Clarke DJ, Chohan TW, Bahceci D, Arnold JC. Interactions between cannabidiol and Δ9‐THC following acute and repeated dosing: Rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur Neuropsychopharmacol. 2017;27:132–45. [DOI] [PubMed] [Google Scholar]
- 126. Hu Q‐P, Mao D‐A. Histone deacetylase inhibitor SAHA attenuates post‐seizure hippocampal microglia TLR4/MYD88 signaling and inhibits TLR4 gene expression via histone acetylation. BMC Neurosci. 2016;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hausman‐Kedem M, Menascu S, Kramer U. Efficacy of CBD‐enriched medical cannabis for treatment of refractory epilepsy in children and adolescents ‐ An observational, longitudinal study. Brain Dev. 2018;40:544–51. [DOI] [PubMed] [Google Scholar]
- 128. McCoy B, Wang L, Zak M, Al‐Mehmadi S, Kabir N, Alhadid K, et al. A prospective open‐label trial of a CBD/THC cannabis oil in Dravet syndrome. Ann Clin Transl Neurol. 2018;5:1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49–52. [DOI] [PubMed] [Google Scholar]
- 130. Ladino LD, Hernández‐Ronquillo L, Téllez‐Zenteno JF. Medicinal marijuana for epilepsy: a case series study. Can J Neurol Sci. 2014;41:753–8. [DOI] [PubMed] [Google Scholar]
- 131. Laux LC, Bebin EM, Checketts D, Chez M, Flamini R, Marsh ED, et al. Long‐term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox‐Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res. 2019;154:13–20. [DOI] [PubMed] [Google Scholar]
- 132. Szaflarski JP, Hernando K, Bebin EM, Gaston TE, Grayson LE, Ampah SB, et al. Higher cannabidiol plasma levels are associated with better seizure response following treatment with a pharmaceutical grade cannabidiol. Epilepsy Behav. 2019;95:131–6. [DOI] [PubMed] [Google Scholar]
- 133. Chen KA, Farrar M, Cardamone M, Gill D, Smith R, Cowell CT, et al. Cannabidiol for treating drug‐resistant epilepsy in children: the New South Wales experience. Med J Aust. 2018;209:217–21. [DOI] [PubMed] [Google Scholar]
- 134. Porcari GS, Fu C, Doll ED, Carter EG, Carson RP. Efficacy of artisanal preparations of cannabidiol for the treatment of epilepsy: Practical experiences in a tertiary medical center. Epilepsy Behav. 2018;80:240–6. [DOI] [PubMed] [Google Scholar]
- 135. Sands TT, Rahdari S, Oldham MS, Caminha Nunes E, Tilton N, Cilio MR. Long‐term safety, tolerability, and efficacy of cannabidiol in children with refractory epilepsy: Results from an expanded access program in the US. CNS Drugs. 2019;33:47–60. [DOI] [PubMed] [Google Scholar]
- 136. Szaflarski JP, Bebin EM, Cutter G, DeWolfe J, Dure LS, Gaston TE, et al. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open‐label add‐on prospective study. Epilepsy Behav. 2018;87:131–6. [DOI] [PubMed] [Google Scholar]
- 137. Devinsky O, Cross JH, Wright S. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;377:699–700. [DOI] [PubMed] [Google Scholar]
- 138. Gofshteyn JS, Wilfong A, Devinsky O, Bluvstein J, Charuta J, Ciliberto MA, et al. Cannabidiol as a potential treatment for febrile infection‐related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol. 2017;32:35–40. [DOI] [PubMed] [Google Scholar]
- 139. Kaplan EH, Offermann EA, Sievers JW, Comi AM. Cannabidiol treatment for refractory seizures in Sturge‐Weber syndrome. Pediatr Neurol. 2017;71:18–23.e2. [DOI] [PubMed] [Google Scholar]
- 140. Rosenberg EC, Louik J, Conway E, Devinsky O, Friedman D. Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open‐label clinical study with cannabidiol. Epilepsia. 2017;58:e96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Warren PP, Bebin EM, Nabors LB, Szaflarski JP. The use of cannabidiol for seizure management in patients with brain tumor‐related epilepsy. Neurocase. 2017;23:287–91. [DOI] [PubMed] [Google Scholar]
- 142. Zynerba Pharmaceuticals . Zynerba Pharmaceuticals announces top‐line results from Phase 2 STAR 1 Trial of ZYN002 in adult epilepsy patients with focal seizures. 2017;1–4.
- 143. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurol. 2016;15:270–8. [DOI] [PubMed] [Google Scholar]
- 144. Hess EJ, Moody KA, Geffrey AL, Pollack SF, Skirvin LA, Bruno PL, et al. Cannabidiol as a new treatment for drug‐resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1617–24. [DOI] [PubMed] [Google Scholar]
- 145. Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21:417S–27S. [DOI] [PubMed] [Google Scholar]
- 146. GW Pharmaceuticals .A study of GWP42006 in people with focal seizures – part A. 2015. Available from https://clinicaltrials.gov/ct2/show/NCT02369471 (accessed 15 Jul 2019).
- 147. GW Pharmaceuticals . GW Pharmaceuticals announces preliminary results of Phase 2a study for its pipeline compound GWP42006. 2018. Available from http://ir.gwpharm.com/news-releases/news-release-details/gw-pharmaceuticals-announces-preliminary-results-phase-2a-study (accessed 15 Jul 2019).
- 148. Davis JP, Ramsey HH. Anti‐epileptic action of marijuana‐active substances. Fed Pro. 1949;8:284–5. [Google Scholar]
- 149. Lorenz R. On the application of cannabis in paediatrics and epileptology. Neuro Endocrinol Lett. 2004;25:40–4. [PubMed] [Google Scholar]


