Abstract
Plasma cell mucositis (PCM) is an unusual plasma cell proliferative disorder of the upper aerodigestive tract. It is a rare disease, and its etiology is not yet known with variable clinical features. Symptoms include dysphagia, oral pain, and swelling. We described a case of PCM involving the tongue of a 14-year-old man. In the first place, several diagnostic hypotheses were proposed, most of them discarded for incompatibility with blood and laboratory tests. This disease rarely manifests itself on the tongue, especially in young patients with no comorbidities. The management of PCM is mainly aimed at reducing the symptoms, and in our report, the treatment involved the use of systemic prednisone with an improvement of the quality of life. At 1-year follow-up, there was no recurrence of the disease. Many therapeutic treatments are able to stabilize but not able to induce a complete remission. PCM is considered an uncommon benign disorder with a favorable prognosis and should be considered in the differential diagnosis with other inflammatory or neoplastic conditions.
1. Introduction
Plasma cell mucositis (PCM) is an unusual plasma cell proliferative disorder of the upper aerodigestive tract [1]. In the past, this pathology has been reported under various names. The name indicated the anatomical structure involved together with the component of plasma cells, such as idiopathic plasmacytosis of the gingiva, plasma cell vulvitis, oral papillary plasmacytosis, or mucous membrane plasmacytosis of the upper aerodigestive tract [2]. It is a rare disease, and its etiology is not yet known; it is considered a benign condition of adults, and there are no correlations in literature with the development of plasma cell neoplasm. Clinical features are an intensely erythematous mucosa with papillomatous, cobblestone, nodular, or velvety surface changes. Symptoms include dysphagia, oral pain, sore throat, and pharyngitis [2]. PCM can be treated with corticosteroids, administrated topically, systemically, or intralesionally. Usually, the treatment is not resolutive; however, the main outcome is the improvement of the symptoms. Generally, PCM patients have a previous history of an autoimmune or immunologically mediated disease like Sjögren syndrome or possible autoimmune hepatitis; however, these are not present in all cases and no single disease is consistently associated.
2. Case Report
We described a case of PCM involving the tongue of a 14-year-old man. The patient was referred to the Oral Pathology Unit at the Faculty of Dentistry, Magna Graecia University of Catanzaro, in August 2019. The referring practitioner suspected squamous cell carcinoma. His past medical history was not relevant, and the patient was a nonsmoker and nonalcohol user. The patient had previously used a nocturnal bite to control bruxism. He reported a burning sensation in his mouth, local dysgeusia, and pain on the tip and on the right lingual border for 1 year. The patient also referred that he was unable to eat or drink any hot or spicy foods due to burning and pain on his tongue. He stated persistent hoarseness, sore throat, and difficulty sleeping due to continuous oral pain.
Extraoral examination was unremarkable, while intraoral examination revealed the presence of an ulcer and intense erythema of the tongue (Figures 1 and 2) and gingival edema and erythema (Figure 3); however, the other parts of the oral mucosa was clinically normal. In the first place, several diagnostic hypotheses were proposed, most of them discarded for incompatibility with full blood count, serum B12 and folate, urea and electrolytes, liver function tests, glucose, anti-nuclear antibody, and syphilis IgG that were normal or negative. The patient underwent an incisional biopsy under local anesthesia. The specimen was stored in a tube containing formalin 10% and sent to a laboratory for histopathological analysis. Microscopically, a large area of ulceration of the coating epithelium subtended by dense plasma cell infiltrate was observed (Figures 4 and 5). CD20 and CD3 showed focal positivity for B and T cells, respectively. The plasma cell infiltrate was positive for CD138 (Figures 6 and 7) and showed kappa light chain restriction (Figures 8 and 9) with a kappa/lambda ratio of 20 : 1 (Figures 10 and 11). The final histopathological diagnosis was “plasmacytosis of the mucous membranes with restriction for the kappa chains.” Initial pharmacotherapy with prednisone began with 50 mg/day for two weeks, and the patient referred an improvement of his conditions (reduction of pain and swelling). He repeated this treatment one month later, and he experienced a partial regression of the lesions. At 1-year follow-up, there was no regression of the disease and no recurrence of the lesions (Figure 12).
Figure 1.
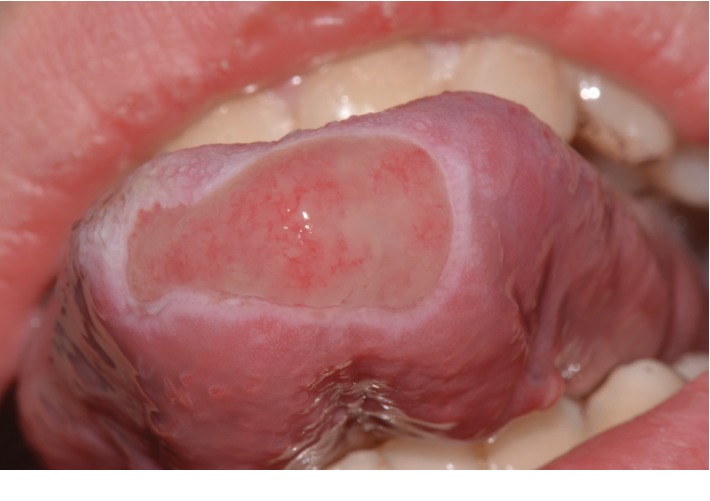
Ulceration on the tip of the tongue.
Figure 2.
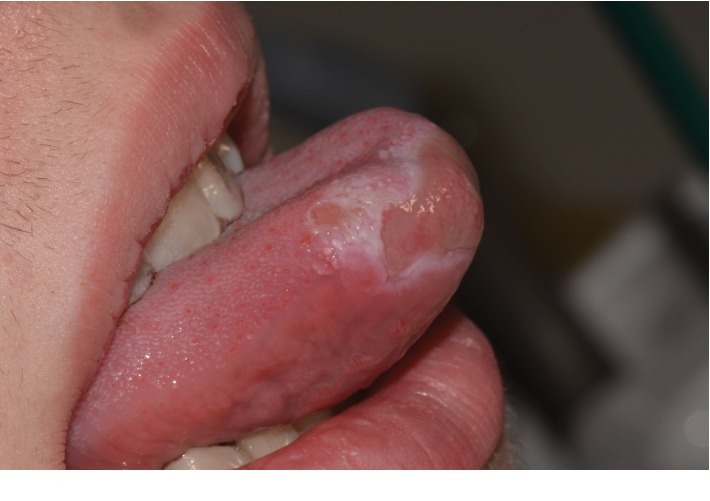
Lateral view of the tongue.
Figure 3.
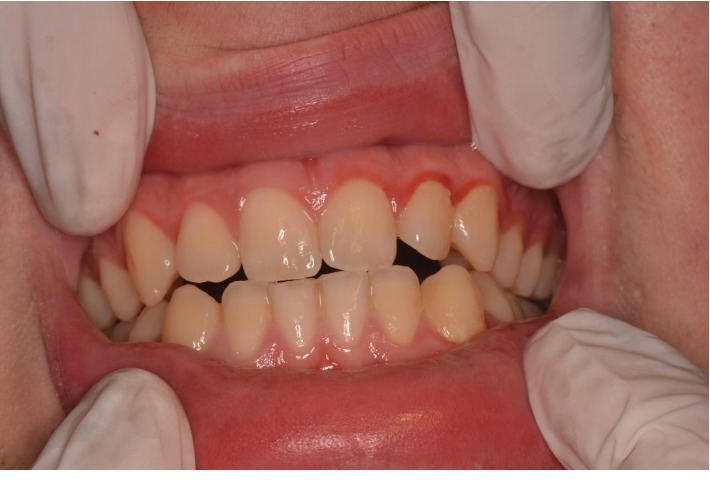
Gingival edema and erythema.
Figure 4.
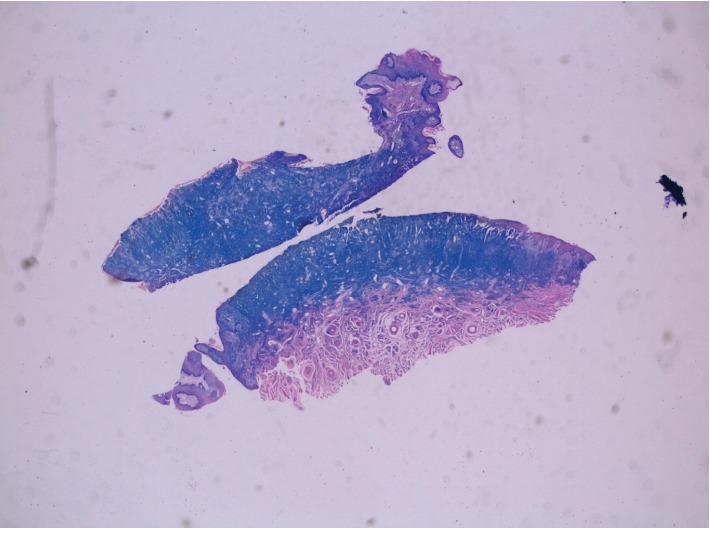
Medium-power hematoxylin and eosine-stained biopsy sample from the tongue mucosa showing a dense polyclonal plasmacytic inflammatory infiltrate throughout the connective tissue.
Figure 5.
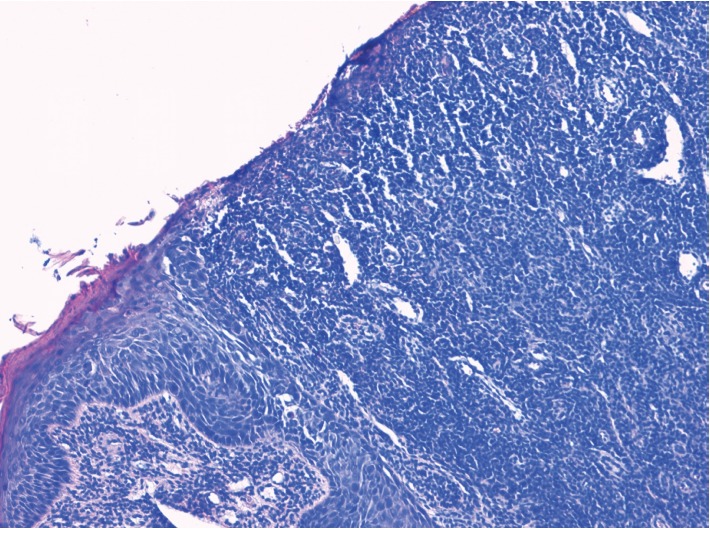
Histological examination of a biopsy taken from the tongue showed a denuded surface epithelium with the superficial and mid dermis demonstrating a dense infiltration of plasma cells.
Figure 6.
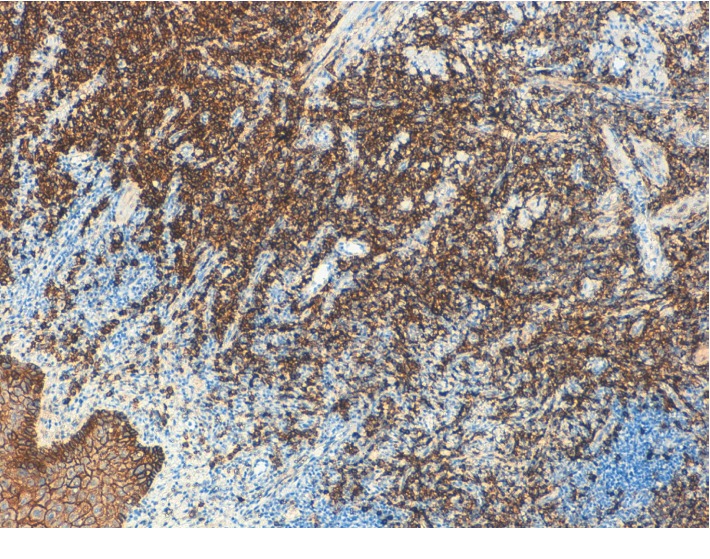
CD138. A high-power view shows a monomorphic population of mature plasma cells (×10).
Figure 7.
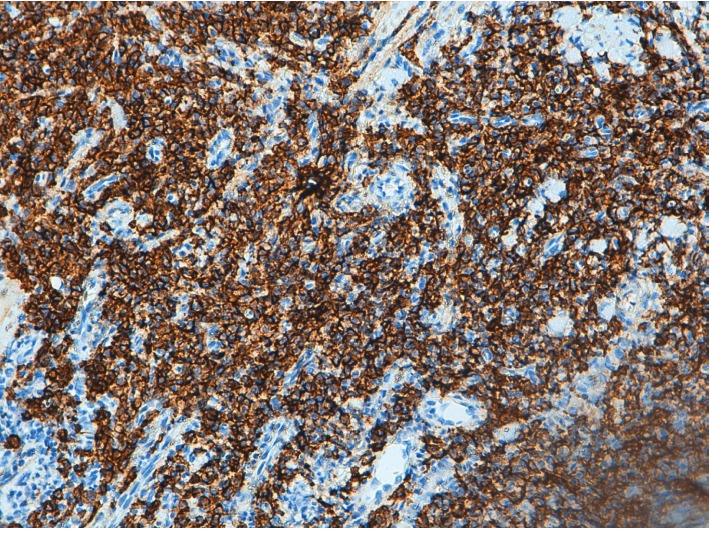
CD138. A high-power view shows a monomorphic population of mature plasma cells (×20).
Figure 8.
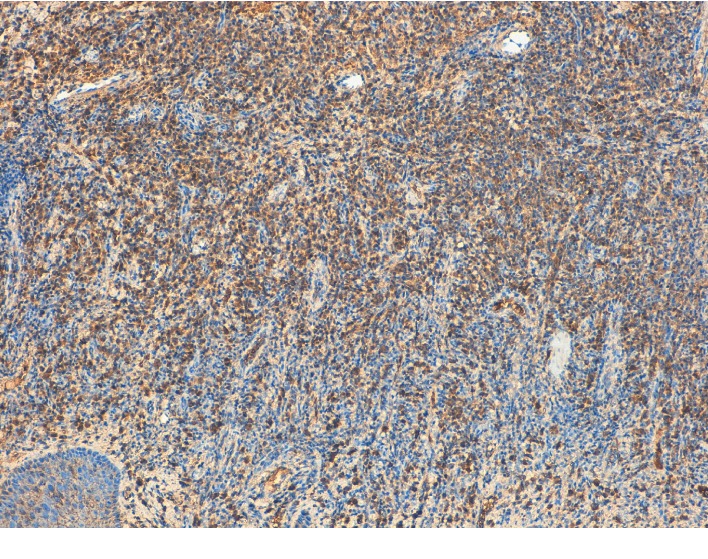
Plasma cells showing kappa light chain restriction (×10).
Figure 9.
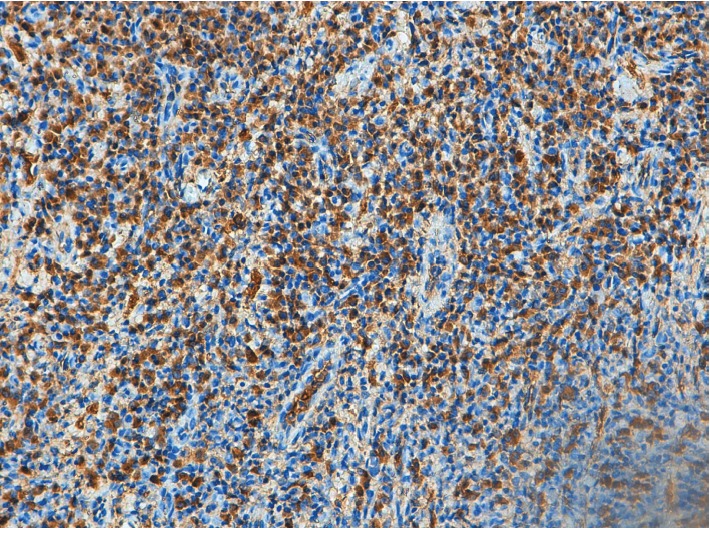
Plasma cells showing kappa light chain restriction (×20).
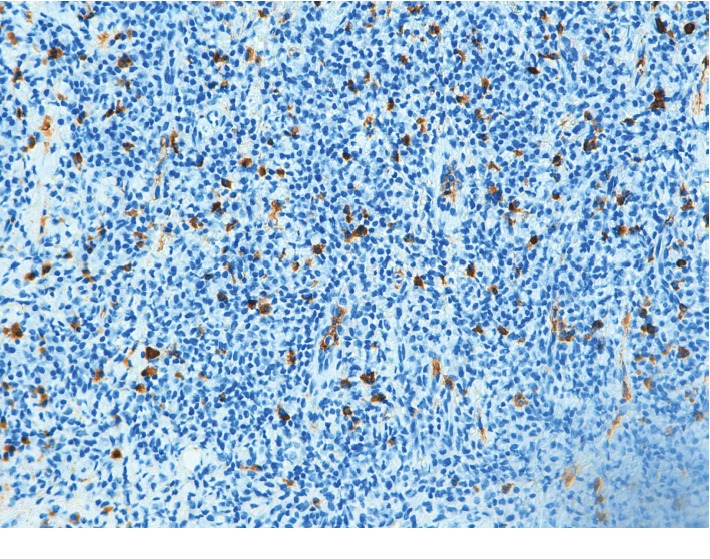
Figure 10.
Plasma cells showing lambda light chain restriction (×10).
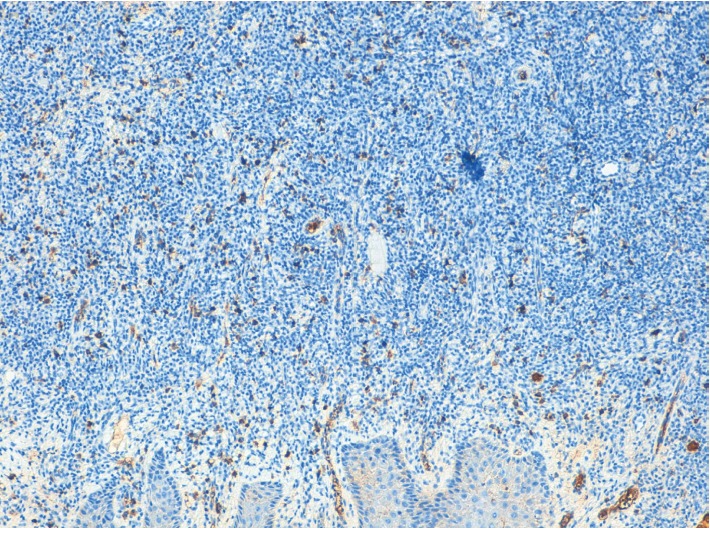
Figure 11.
Plasma cells showing lambda light chain restriction (×20).
Figure 12.
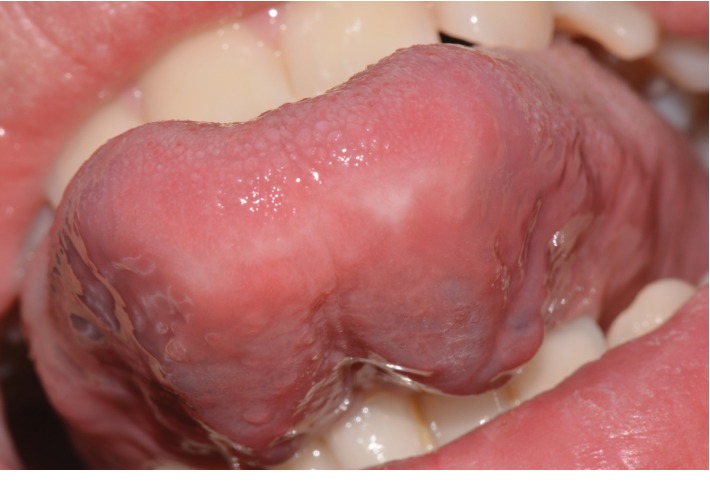
Follow-up at 1 year.
3. Discussion
Plasma cell mucositis (PCM) is an idiopathic disorder histologically characterized by dense infiltrates of lymphocytes and plasma cells in submucosa. Usually, the lesions are mainly observed on the mucosal surfaces, vulva and penis, gingiva, lips, tongue, buccal mucosa, epiglottis, and larynx.
A review of the English-language literature was performed. The keywords “plasma cell mucositis” and “oral” were entered in the search fields of PubMed. 103 results were identified. Another research was performed including the keywords “plasmacytosis” and “oral.” 40 results were identified. The research was conducted by considering the articles published until November 2019. Excluded were cases that were not about the oral cavity, not in English, not human study, and not compatible with the PCM diagnosis or report lacking immunohistochemical analysis. According to the exclusion criteria, we selected 26 studies [1–26] that included 45 cases of PCM. We analysed patients' age, gender, symptoms, lesion location, treatment, and follow-up. The principal features and data pertaining the selected cases are compiled in Table 1. Age data were available in all selected studies, and the average age was 56.06 ± 16.33 years. The youngest patient was 13 years old [17] and the oldest 83 years old [20]. All studies included in our review reported the gender of the subjects. The analysis of the data collected suggested a male predilection with a male-to-female ratio of 1.25 : 1 (45 cases: 25 M, 20 F). A graph of the age and gender distribution is reported in Figure 13. Rarely the lesions are isolated, and in 45 cases reviewed, the oral cavity zones most affected by the lesions were the gingiva (20 cases), the lips (14 cases), the upper aerodigestive tract (13 cases), the palate (12 cases), the buccal mucosa (11 cases), and the tongue (8 cases). In our review, the symptoms most present are typical of inflammation with a particular interest of the oropharynx; the main symptoms are erythema (13 cases), pain (10 cases), swelling (9 cases), sore throat (7 cases), dysphonia, and dysphagia (6 cases). The analysis of the treatment evidenced 24 cases (53.3%) treated with corticosteroids in different forms (systemic, topical, and injections), and in 10 cases of these, there was no recurrence of the disease.
Table 1.
Review of PCM.
| Study/year of publication | Age∗ | Gender | Symptoms | Location of the lesions | Treatment | Follow-up |
|---|---|---|---|---|---|---|
| Poswillo et al. [7]/1967 | 37 | F | Redness, swelling | Gingiva | Resection, oral hygiene programme | AWD, 2 years |
| 39 | F | Redness, hypertrophy | Gingiva | Resection | Not available | |
| 21 | F | Hyperplastic red lesion, hypertrophy, erythema | Maxillary labial vestibule, gingiva | Resection, oral hygiene programme | Not available | |
|
| ||||||
| White et al. [3]/1986 | 47 | F | Dysphonia, sore throat | Lips, mouth, tongue, supraglottic larynx | Resection, prednisone | AWD, 9 years (f/u reported by Ferreiro et al. [1]) |
|
| ||||||
| Timms et al. [8]/1988 | 70 | F | Hoarseness, cough, stridor | Gingiva, supraglottic, hard palate | CO2 laser, prednisolone, topical steroid spray | AWD, 1 year |
|
| ||||||
| Timms and Sloan [9]/1991 | 32 | F | Hoarseness, sore throat | Gingiva, false cord, mucobuccal | Systemic and topical steroids | Marginal improvement, no f/u epiglottis |
|
| ||||||
| Ferreiro et al. [1]/1994 | 60 | F | Dysphonia, dysphagia | Supraglottic larynx | CO2 laser, prednisone, antibiotics, beclomethasone (Vanceril) spray | AWD, 1 year |
| 41 | M | Dysphonia, stridor | Supraglottic, glottic larynx, nose, pharynx | Antibiotics | AWD, 7 years; tracheostomy | |
| 62 | F | Dysphonia, stridor, dry eyes, dysphagia | Supraglottic, glottic larynx, trachea | Not available | AWD, 16 years; tracheostomy | |
| 54 | M | Dysphonia | Supraglottic, glottic larynx | Resection | AWD, 1 year | |
| 40 | M | Dysphonia, sore mouth | Lips, tongue, palate, pharynx, supraglottic & glottic larynx | Prednisone, isotretinoin (Accutane), CO2 laser | AWD, 15 years; sleep apnea | |
| 67 | M | Sore mouth | Lips, mouth, tongue, palate | Prednisone | AWD, 3 years | |
| 61 | M | Unknown | Lips through larynx | Not available | Not available | |
| 56 | M | Unknown | Nose, palate, pharynx | Resection | Not available | |
|
| ||||||
| Van de Kerkhof and Van Baar [26]/1995 | 80 | F | Pain, soreness, erythema, swelling | Lips | Betamethasone dipropionate | Partial symptomatic relief, f/u not available |
| 57 | F | Sore throat | Gingiva, supraglottic larynx | Topical steroids | Gingival improvement, no f/u | |
|
| ||||||
| Khan et al. [5]/1997 | 69 | M | Hoarseness, dysphagia, hemoptysis | Left faucial pillar, hypopharynx, epiglottis, larynx | Beclomethasone spray, Corsodyl mouthwash | No recurrence, 20 months f/u |
|
| ||||||
| Noorily [25]/1997 | 67 | M | Induration, erythema, crusting | Lower lip | Resection, primary closure | Not available |
|
| ||||||
| Smith et al. [4]/1999 | 59 | M | Swelling, hoarseness, sore throat, erythema | Soft palate, gingiva, oropharynx, nasopharynx | None | Asymptomatic, 6 months f/u |
|
| ||||||
| Kaur et al. [24]/2001 | 47 | M | Swelling, erythema, inflammation | Upper lip | Triamcinolone acetonide injections | No recurrence, 3 months f/u |
|
| ||||||
| Bharti and Smith [10]/2003 | 42 | F | Pain, dysphagia | Buccal mucosa, palate | Topical and systemic antifungals, corticosteroids | Partial symptomatic relief, no regression of the disease, no f/u |
|
| ||||||
| Heinemann et al. [23]/2006 | 61 | F | Pain, ulcerations, erythema, erosions | Tongue, lips, buccal mucosa, vulvae | Prednisolone, cyclosporin | Complete remission, 6 months f/u |
|
| ||||||
| Solomon et al. [2]/2008 | 60 | F | Pain, sore throat, erosions, swelling, erythema | Gingiva, lips, ventral tongue, hard palate | Prednisone | Disease remission, f/u not available |
|
| ||||||
| Senol et al. [11]/2008 | 46 | M | Inflammation, maceration, pruritic, eczema | Oral commissures, gingivobuccal mucosa, toe-webs, groins, preputium, perineum, umbilicus | Prednisolone | No recurrence, 1 year f/u |
|
| ||||||
| Najarian et al. [12]/2008 | 56 | M | Pain, impetigo, bleeding | Lower lip | Topical and systemic glucocorticosteroid, cryotherapy | No recurrence, 6 months f/u |
|
| ||||||
| Pepper et al. [13]/2009 | 77 | M | Atrophy, hyperkeratosis, erosion, pain | Commissure, lips, cheek | Tacrolimus, methotrexate, betamethasone mouthwash, CO2 laser, radiotherapy | Onset of squamous cell carcinoma, partial resolution of mucosal plasmacytosis |
|
| ||||||
| Puvanendran et al. [22]/2012 | 74 | M | Ulceration, erythema | Uvula, hypopharyngeal wall | Resection | Lesion resolved, 6 months f/u |
|
| ||||||
| Gupta et al. [21]/2014 | 72 | M | Difficulty in swallowing, soreness, burning sensation, sore throat, ulcerations, swelling, bleeding gums, erosions | Buccal mucosa, palate, tongue, pharynx, gingiva | Topical corticosteroids (betamethasone 1 mg, triamcinolone acetonide gel 0.1%), prednisolone, topical antifungals | Partial symptomatic relief, no regression of the disease, no f/u |
|
| ||||||
| Madhavarajan and Tighe [15]/2015 | 63 | M | Pain, ulceration | Commissure, buccal mucosa, gingiva | No treatment | Spontaneous resolving, 6 months f/u |
|
| ||||||
| Cottom et al. [20]/2015 | 54 | F | Erythema | Soft palate | Not available | Not available |
| 51 | M | Erythema, ulceration, erosions | Border of tongue | Not available | Not available | |
| 50 | M | Inflammation | Gingiva | Not available | Not available | |
| 67 | M | Pain, bleeding, erosions | Gingiva | Not available | Not available | |
| 83 | F | Erosions | Gingiva | Not available | Not available | |
| 65 | M | Inflammation | Gingiva, hard palate | Not available | Not available | |
| 72 | M | Inflammation | Gingiva | Not available | Not available | |
|
| ||||||
| Galvin et al. [14]/2016 | 68 | F | Erythema, ulceration | Alveolar ridges, palate, buccal mucosa, gingiva | Oral fluconazole, chlorhexidine mouthwash, betamethasone cream and tablets | AWD, 7 years |
| 61 | F | Sore mouth, ulceration, erythema, edema | Gingiva, buccal mucosa | Prednisolone, adalimumab | No recurrence, 18 months | |
| 69 | M | Oropharynx congestion | Gingiva, dorsum and borders of the tongue, soft palate, uvula | Mycophenolate mofetil, prednisolone | Partial symptomatic relief, no regression of the disease, no f/u | |
|
| ||||||
| Trehan et al. [19]/2016 | 39 | M | Ulceration, dysphagia, erosions | Lips, buccal mucosa, gingiva | Prednisolone | Good response, f/u not available |
|
| ||||||
| Arun et al. [17]/2017 | 13 | M | Swelling, bleeding, erythema, | Upper lip, gingiva | Triamcinolone acetonide injections, | No recurrence, 1 year f/u |
|
| ||||||
| Liu et al. [18]/2017 | 18 | F | Swelling, bleeding, erosions, pain | Lips | Methylprednisolone, dapsone, hydroxychloroquine, antibiotics | Symptoms decreasing, 6 months f/u |
|
| ||||||
| Gasparro et al. [6]/2019 | 78 | F | Pain, erythema, ulcerations, erosions | Buccal mucosa | Injectable platelet-rich fibrin (i-PRF) | No complete healing, inflammation reduction, 6 months f/u |
|
| ||||||
| Shanahan et al. [16]/2019 | 62 | F | Dry mouth, ulceration, swelling, dysphagia, hoarseness | Soft palate, buccal mucosa | Prednisolone, dapsone, mycophenolate mofetil | No recurrence of symptoms, 1 year f/u |
AWD: alive with disease; F: female; f/u: follow-up; M: male. ∗Age at presentation (years).
Figure 13.
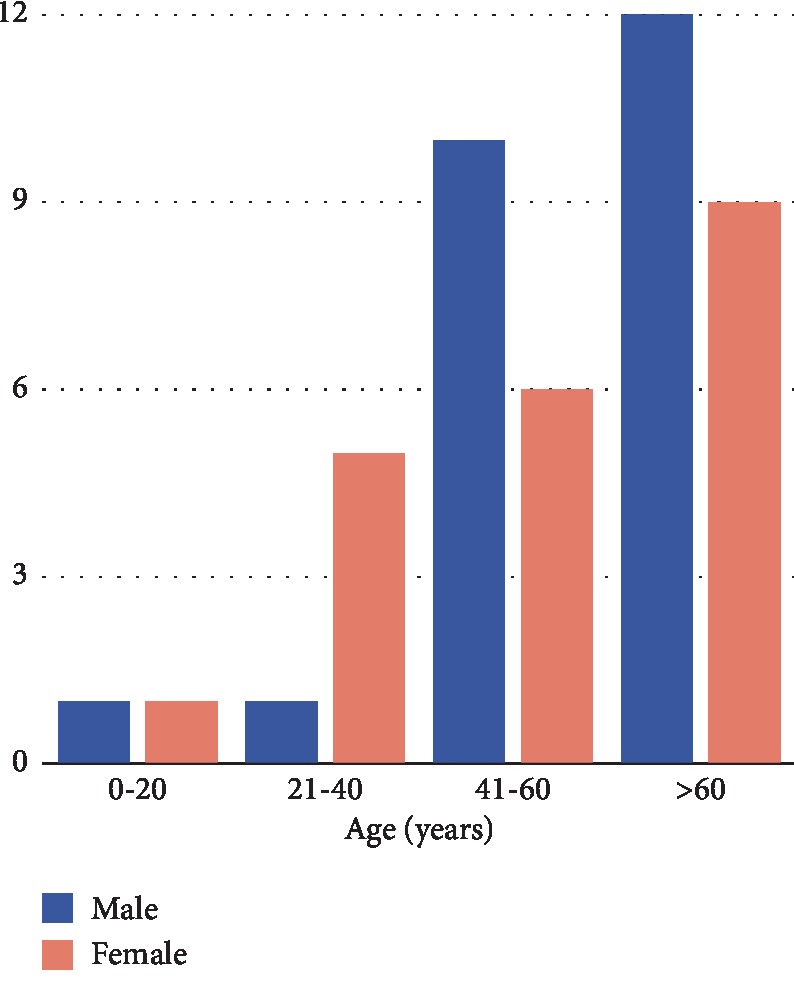
Distribution of age and sex in the 45 cases of PCM of the oral cavity reviewed.
Plasma cell mucositis (PCM) is classified as idiopathic and rarely manifests itself on the tongue, especially in young patients with no comorbidities. Despite the fact that plasma cell mucositis often involves the oral and genital mucosa [3], there was no genital involvement in this circumstance. Since it is a very rare pathology, in the diagnosis of PCM, it is important to exclude other pathologies with similar pathological features, such as erosive lichen planus, mucous membrane pemphigoid, sarcoidosis, allergic gingivostomatitis, extramedullary plasmacytoma, rhinoscleroma, pemphigus, erythroplasia, squamous cell carcinoma, and fungal infections [4, 5]. Lichen planus is a chronic-relapsing inflammatory disease, with cutaneous and mucous involvement. It can be distinguished in oral lichen planus (OLP) and oral lichenoid drug reactions (OLDRs). The lesions may cause pain and, generally, appear as white patches with erosive aspects. A typical OLP model is identified with bilateral and symmetrical lesions; moreover, a hyperkeratosis is observed in the lower epidermis and in the upper dermis [27]. Pemphigoid of the mucous membranes affects mainly older individuals, as well as the plasma cell mucositis. Unlike the PCM, it presents itself with erosions to the mucous membranes of variable gravity that are mainly located in the oral cavity [5]. Sarcoidosis is a rare acquired systemic granulomatous disease. The respiratory system is more interested, but in a smaller number of cases, there is the involvement of the oral and perioral mucosa. Oral lesions can be solitary or multiple; they appear as a well-delimited and occasionally ulcerated red swelling [28]. Allergic gingivostomatitis is the result of an immunologic injury reaction; it is a complex disease which can have more than one etiologic factor [29]. Extramedullary plasmacytoma is a tumor that rarely affects the head and neck, especially the upper airways, and the lesions are generally polypoidal in appearance [5]. Primary Non-Hodgkin's Lymphoma (NHL) can rarely arise from lymphoid tissue of the tongue and give manifestations that mimic PCM [30].
Several times, PCM lesions can enter into differential diagnosis with squamous cell carcinoma (SCC) [4–22]; in literature, there are cases in which a differential diagnosis was made with oral carcinoma and there is a case report where a SCC arose from a mucosal plasmacytosis [13]. Therefore, a correct diagnosis and adequate management of the disease are essential.
The management of PCM is mainly aimed at reducing the symptoms; in fact, the patient treatment involved the use of systemic prednisone 50 mg/day to improve his quality of life.
However, in literature, there are some reported cases where the use of PRF injections is associated with cortisone therapy [6]. The benefit of PRF is to allow a continuous release of growth factors, inducing neoangiogenesis and fibroblast activations. Therefore, PRF creates an optimal scaffold for the tissue healing process, with the advantage of low cost and easy to prepare [31].
Although many therapeutic treatments are able to stabilize the disease, they are not able to induce a complete remission. The condition is generally of long-standing duration and the disease has a significant impact on the patient's quality of life. The disease treatment is mainly targeted to the management of symptoms. PCM is considered an uncommon benign disorder with a favorable prognosis. The distinctions between the conditions that are present in the oral cavity with the histologic finding of a dense submucosal plasma cell infiltrate are not well documented in oral pathology literature.
4. Conclusion
It is important that PCM is recognized in the dental community, because diagnosis is dependent on clinical pathologic correlation. Only close communication between specialists in several disciplines prevented inappropriate treatment of a patient with an extremely rare condition. Nevertheless, it is very important to differentiate the PCM disease from other neoplastic conditions in order to achieve a better clinical management of the patients, so it is necessary to investigate this disease in depth.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ferreiro J. A., Egorshin E. V., Olsen K. D., Banks P. M., Weiland L. H. Mucous membrane plasmacytosis of the upper aerodigestive tract. A clinicopathologic study. The American Journal of Surgical Pathology. 1994;18(10):1048–1053. doi: 10.1097/00000478-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Solomon L. W., Wein R. O., Rosenwald I., Laver N. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2008;106(6):853–860. doi: 10.1016/j.tripleo.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 3.White J. W., Jr., Olsen K. D., Banks P. M. Plasma cell orificial mucositis: report of a case and review of the literature. Archives of Dermatology. 1986;122(11):1321–1324. doi: 10.1001/archderm.122.11.1321. [DOI] [PubMed] [Google Scholar]
- 4.Smith M. E., Crighton A. J., Chisholm D. M., Mountain R. E. Plasma cell mucositis: a review and case report. Journal of Oral Pathology & Medicine. 1999;28(4):183–186. doi: 10.1111/j.1600-0714.1999.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 5.Khan N. A., McKerrow W. S., Palmer T. J. Mucous membrane plasmacytosis of the upper aerodigestive tract. A case report with effective treatment. The Journal of Laryngology & Otology. 1997;111(3):293–295. doi: 10.1017/s0022215100137132. [DOI] [PubMed] [Google Scholar]
- 6.Gasparro R., Adamo D., Masucci M., Sammartino G., Mignogna M. D. Use of injectable platelet-rich fibrin in the treatment of plasma cell mucositis of the oral cavity refractory to corticosteroid therapy: a case report. Dermatologic Therapy. 2019;32(5, article 13062) doi: 10.1111/dth.13062. [DOI] [PubMed] [Google Scholar]
- 7.Poswillo D. Plasmacytosis of the gingiva. British Journal of Oral Surgery. 1967;5(3, article S0007117X67800611):194–202. doi: 10.1016/S0007-117X(67)80061-1. [DOI] [PubMed] [Google Scholar]
- 8.Timms M. S., Sloan P., Pace Balzan A. Idiopathic plasmacytosis of the oral and supraglottic mucosa. The Journal of Laryngology & Otology. 1988;102(7):646–648. doi: 10.1017/s0022215100105997. [DOI] [PubMed] [Google Scholar]
- 9.Timms M. S., Sloan P. Association of supraglottic and gingival idiopathic plasmacytosis. Oral Surgery, Oral Medicine, Oral Pathology. 1991;71(4):451–453. doi: 10.1016/0030-4220(91)90428-F. [DOI] [PubMed] [Google Scholar]
- 10.Bharti R., Smith D. R. Mucous membrane plasmacytosis: a case report and review of the literature. Dermatology Online Journal. 2003;9(5):p. 15. [PubMed] [Google Scholar]
- 11.Senol M., Ozcan A., Aydin N. E., Hazneci E., Turan N. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. International Journal of Dermatology. 2008;47(3):265–268. doi: 10.1111/j.1365-4632.2008.03385.x. [DOI] [PubMed] [Google Scholar]
- 12.Najarian D. J., Rao B. K., Pappert A. S. A case of mucous membrane plasmacytosis successfully treated with cryotherapy. Dermatology Online Journal. 2008;14(2):p. 6. [PubMed] [Google Scholar]
- 13.Pepper T., Shekar K., Singh M., Brennan P. A. Squamous cell carcinoma arising in mucosal plasmacytosis. British Journal of Oral and Maxillofacial Surgery. 2010;48(3):208–210. doi: 10.1016/j.bjoms.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Galvin S., Bowe C., Regan E. M. O., Conlon N., Flint S. R., Healy C. M. Circumorificial plasmacytosis/plasma cell orificial mucositis: a case series and a review of the literature. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2016;122(3):e77–e81. doi: 10.1016/j.oooo.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Madhavarajan S., Tighe J. Orofacial plasmacytosis: a management conundrum. British Journal of Oral and Maxillofacial Surgery. 2015;53(4):399–402. doi: 10.1016/j.bjoms.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Shanahan D., Shipley D., Staines K. Plasma cell mucositis. Ear, Nose, & Throat Journal. 2019;(article 145561319849001) doi: 10.1177/0145561319849001. [DOI] [PubMed] [Google Scholar]
- 17.Arun K., Ambooken M., Varghese S. S., Varghese T., Mathew J. J. A rare case of plasma cell mucositis in a young patient. Journal of Indian Society of Periodontology. 2017;21(1):63–65. doi: 10.4103/jisp.jisp_335_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R.-F., Chen C. B., Kuo T. T., Chung W. H. Idiopathic lymphoplasmacellular mucositis of the lips: a case report and review of the literature. Journal of Cutaneous Pathology. 2017;44(9):776–780. doi: 10.1111/cup.12967. [DOI] [PubMed] [Google Scholar]
- 19.Trehan P., Pang E., Khirwadkar N., Alsharqi A., Parslew R. A rare cause of mucositis. Clinical and Experimental Dermatology. 2016;41(8):951–952. doi: 10.1111/ced.12987. [DOI] [PubMed] [Google Scholar]
- 20.Cottom H., Mighell A. J., High A., Bateman A. C. Are plasma cell-rich inflammatory conditions of the oral mucosa manifestations of IgG4-related disease? Journal of Clinical Pathology. 2015;68(10):802–807. doi: 10.1136/jclinpath-2014-202814. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S. R., Gupta R., Saran R. K., Krishnan S. Plasma cell mucositis with gingival enlargement and severe periodontitis. Journal of Indian Society of Periodontology. 2014;18(3):p. 379. doi: 10.4103/0972-124X.134583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puvanendran M., Lieder A., Issing W. Plasma Cell Mucositis of Oro- and Hypopharynx: A Case Report. Case Reports in Otolaryngology. 2012;2012:3. doi: 10.1155/2012/304136.304136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinemann C., Fischer T., Barta U., Michaelides A., Elsner P. Plasma cell mucositis with oral and genital involvement–successful treatment with topical cyclosporin. Journal of the European Academy of Dermatology and Venereology. 2006;20(6):739–740. doi: 10.1111/j.1468-3083.2006.01467.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaur C., Thami G. P., Sarkar R., Kanwar A. J. Plasma cell mucositis. Journal of the European Academy of Dermatology and Venereology. 2001;15(6):566–567. doi: 10.1046/j.1468-3083.2001.00277.x. [DOI] [PubMed] [Google Scholar]
- 25.Noorily A. D. Plasma cell orificial mucositis. Otolaryngology - Head and Neck Surgery. 1997;116(3):416–417. doi: 10.1016/s0194-5998(97)70287-0. [DOI] [PubMed] [Google Scholar]
- 26.Van de Kerkhof P. C. M., Van Baar H. M. J. Co-occurrence of plasma cell orificial mucositis and plasmoacanthoma. Dermatology. 1995;191(1):52–55. doi: 10.1159/000246489. [DOI] [PubMed] [Google Scholar]
- 27.Giudice A., Liborio F., Averta F., Barone S., Fortunato L. Oral lichenoid reaction: an uncommon side effect of rituximab. Case Reports in Dentistry. 2019;2019:3. doi: 10.1155/2019/3154856.3154856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolokotronis A. E., Belazi M. A., Haidemenos G., Zaraboukas T. K., Antoniades D. Z. Sarcoidosis: oral and perioral manifestations. Hippokratia. 2009;13(2):119–121. [PMC free article] [PubMed] [Google Scholar]
- 29.Paul R. E., Hoover D., Dunlap C., Gier R., Alms T. An immunologic investigation of atypical gingivostomatitis. Journal of Periodontology. 1978;49(6):301–306. doi: 10.1902/jop.1978.49.6.301. [DOI] [PubMed] [Google Scholar]
- 30.Guastafierro S., Falcone U., Celentano M., Cappabianca S., Giudice A., Colella G. Primary mantle-cell non-Hodgkin’s lymphoma of the tongue. International Journal of Hematology. 2008;88(2):206–208. doi: 10.1007/s12185-008-0142-z. [DOI] [PubMed] [Google Scholar]
- 31.Fortunato L., Barone S., Bennardo F., Giudice A. Management of facial pyoderma gangrenosum using platelet-rich fibrin: a technical report. Journal of Oral and Maxillofacial Surgery. 2018;76(7):1460–1463. doi: 10.1016/j.joms.2018.01.012. [DOI] [PubMed] [Google Scholar]


