Abstract
Background:
The long-term benefits of local therapy in metastatic renal cell carcinoma (mRCC) have been widely documented. In this context, single fraction gamma knife radiosurgery (SF-GKRS) is routinely used in the management of brain metastases. However, SF-GKRS is not always feasible due to volumetric and regional constraints. We intend to illustrate how a dose-volume adaptive hypofractionated GKRS technique based on two concurrent dose prescriptions termed rapid rescue radiosurgery (RRR) can be utilized in this particular scenario.
Case Description:
A 56-year-old man presented with left-sided hemiparesis; the imaging showed a 13.1 cc brain metastasis in the right central sulcus (Met 1). Further investigation confirmed the histology to be a metastatic clear cell RCC. Met 1 was treated with upfront RRR. Follow-up magnetic resonance imaging (MRI) at 10 months showed further volume regression of Met 1; however, concurrently, a new 17.3 cc lesion was reported in the boundaries of the left frontotemporal region (Met 2) as well as a small metastasis (<1 cc) in the left temporal lobe (Met 3). Met 2 and Met 3 underwent RRR and SF-GKRS, respectively.
Results:
Gradual and sustained tumor ablation of Met 1 and Met 2 was demonstrated on a 20 months long follow- up. The patient succumbed to extracranial disease 21 months after the treatment of Met 1 without evidence of neurological impairment post-RRR.
Conclusion:
Despite poor prognosis and precluding clinical factors (failing systemic treatment, eloquent location, and radioresistant histology), RRR provided optimal tumor ablation and salvage of neurofunction with limited toxicity throughout follow-up.
Keywords: Brain metastases, Engel score, Hypofractionated gamma knife radiosurgery, Karnofsky performance status, Metastatic renal cell carcinoma, Recursive partitioning analysis, Single-dose gamma knife radiosurgery

INTRODUCTION
Despite promising advances in the fields of neurosurgery and radiotherapy in the era of targeted therapy and immunotherapy, the prognosis of metastatic renal cell carcinoma (mRCC) remains poor. The overall benefits of systemic treatment can significantly be precluded by the presence of brain metastases. Microsurgery is not always feasible due to preexisting regional physioanatomical constraints and/or the extent of intra- and extra-cranial metastatic activity. Due to the radioresistant nature of renal cell tumors, the clinician is often obliged to deliver focal high dose radiation, such as single fraction gamma knife radiosurgery (SF-GKRS). However, SF-GKRS may lead to severe toxicity in lesions >8–10 cc, especially in the eloquent brain. We present the case of a patient with mRCC and two large, critically located, brain metastases treated in next to emergency setting with a gamma knife based dose-volume adaptive technique coined rapid rescue radiosurgery (RRR). To the best of our knowledge, this is the first reported case of mRCC brain lesions treated with a staged dose-volume adaptive procedure structured on two concurrent prescription doses, at least in Scandinavia.
CASE REPORT
A previously healthy 56-year-old man developed gradual left- side hemiparesis, more accentuated in the lower limb (July 2016). A brain computerized tomography (CT)-scan and complementary magnetic resonance imaging (MRI) showed a solitary metastatic lesion (Met 1) in the right central sulcus (11.95 cc) with extensive perilesional edema [Figure 1a and Table 1]. The investigative tumor screening, including a CT- scan of the thorax and abdomen, revealed a suspect primary tumor in the left kidney as well as multiple, disseminated metastatic lesions in the lungs, liver, peri-aortic abdominal nodes, and left adrenal gland. A thrombotic mass positioned within the boundaries of the left renal vein was also reported. The subsequent biopsy revealed a clear cell renal cancer, Fuhrman Grade 2, as the primary. Due to the underlying extension of metastatic activity, nephrectomy was not indicated. Because of his relatively young age and previous healthy condition, the patient was accepted for upfront RRR- treatment of Met 1, structured on three separate fractions (GKRS 1–3) over a 7-day period [Tables 1 and 2]. This strategy aimed to sustain the patient’s performance status by avoiding further neurological deterioration while providing a window to start first-line targeted therapy (sunitinib). The rationale behind RRR can be found elsewhere in the literature.[26-28,31]
Figure 1:
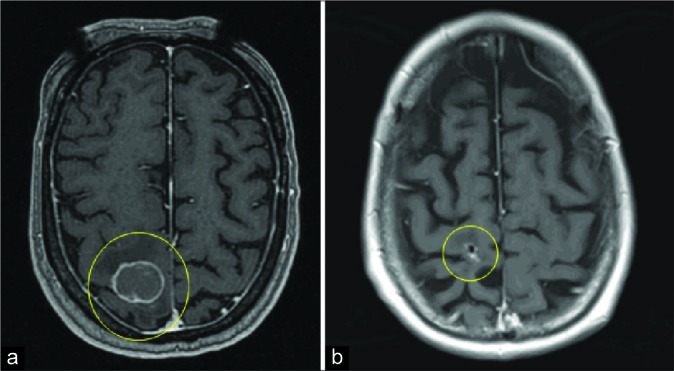
Tumor volume dynamics of Met 1 (T1-weighted MRI with gadolinium, axial cross-sections). (a) Magnetic resonance imaging (MRI) at GKRS 1. (b) Last follow up MRI at 20 months.
Table 1:
Tumor volume dynamics of Met 1 throughout treatment (GKRS1-GKRS3) and during follow-up: 93% tumor volume reduction between GKRS1 and last follow-up MRI (20 months).

Table 2:
Peripheral dose (Gy - % isodose line) and 10Gy-volume prescriptions (%) at each GKRS for Met 1 (RRR nr 1) and Met 2 (RRR nr 2).
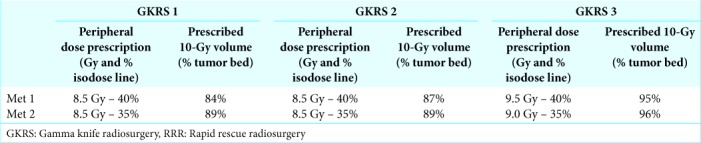
Follow-up MRI at 1, 3, and 6 months confirmed subsequent tumor volume reduction of Met 1 with ensuing significant improvement of the patient’s motor function [Table 1]. Notably, a discrete, nonsymptomatic adverse radiation effect (ARE) around Met 1 was reported at 3 months, however, this subsequently resolved at 6 and 10 months. Despite the positive evolution of Met 1, the corresponding follow-up MRI at 10 months demonstrated a new, large metastatic lesion (17.3 cc) with extensive perilesional edema within the confinements of the left frontotemporal region, threatening the area of Broca and the insular region (Met 2). A parasagittal micrometastasis in the posterior boundaries of the left temporal lobe was also reported (Met 3). Despite further thoracic disease progression on anti-PD1 treatment (nivolumab), the patient’s clinical condition remained stable (Karnofsky Performance Scale [KPS] 90, recursive partitioning analysis [RPA] Class II); it was, therefore, decided to treat Met 2 with RRR and Met 3 with SF-GKRS [Tables 2 and 3] before switching systemic treatment to axitinib.
Table 3:
Tumor volume dynamics of Met 2 and Met 3 throughout treatment (GKRS1-GKRS3) and during follow-up. Met 2 treated with RRR and Met 3 with single fraction GKRS (22 Gy at the 65% isodose line): 81% tumor volume reduction of Met 2 between GKRS1 and last follow-up MRI.
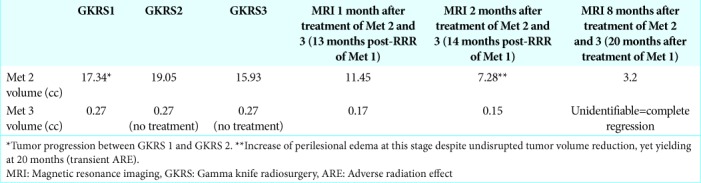
Met 2 decreased in volume by 15% between GKRS 1 and GKRS 3. Follow-up MRI at 14 months after RRR-treatment of Met 1 (=2 months after RRR-treatment of Met 2) showed further tumor volume reduction of Met 2 while Met 1 and Met 3 remained unchanged [Tables 1-3]. However, edema increment around Met 2 was also reported, suggesting an additional focal (yet asymptomatic) ARE. Due to further radiological evidence of extracranial disease progression (CT-scan thorax and abdomen, October 2017), low- tolerance to axitinib and the patient’s own choice, further systemic therapy was altogether disrupted 14 months post- RRR of Met 1. Last follow-up MRI (20 months after RRR of Met 1) showed almost complete ablation of all GKRS-treated lesions and a reduction in perifocal edema surrounding Met 2, without major corticosteroid intervention [Figures 1a-b and 2a-b]. Despite remaining free from motor-sensory deficits, epileptic seizures (Engel score of 1) or cognitive impairment, the patient promptly deteriorated at this stage due to extracranial disease progression (KPS 50, RPA 3). The patient succumbed to his disease 21 months post-RRR-treatment of Met 1.
Figure 2:

Tumor volume dynamics of Met 2 (T1-weighted MRI with gadolinium, axial cross-sections). (a) Magnetic resonance imaging (MRI) at GKRS 1. (b) Last follow-up MRI 8 months post-RRR to Met 2 (20 months post-RRR Met 1).
DISCUSSION
Background
Brain metastases occur in approximately 3.5–17% of patients with mRCC and are associated with significant morbidity and poor survival (median overall survival <11 months).[21] These neoplasms often require acute/ subacute intervention to avoid severe neurological deterioration secondary to tumor enlargement (solid or cystic) and/or metastatic hemorrhage, which may ultimately prevent further systemic treatment and limit survival. Through a retrospective analysis involving 117 patients with mRCC, our group reported the crucial impact of local metastatic therapy (including brain stereotactic radiation) on overall survival.[32] Similar conclusions can be found in the medical literature.[5,22,32] In the context of large brain metastases, safe microsurgical resection followed by radiation to the surgical cavity by LINAC-based instruments or gamma knife remains the best therapeutic option in many centers;[2,6,8,14,22,34] however, as in our patient, this approach is often precluded by the presence of complex variables such as tumor volume, tumor growth kinetics, patterns of infiltration, degree of regional neurofunction, underlying meta-/synchronous metastatic activity, RPA-surrogate factors/comorbidity, and intrinsic stress responses conditioning tumor radioresistance.[5,8,16,21,38] In the realm of brain radiotherapy, focal high dose radiation remains the cornerstone of treatment for unresected radioresistant metastases, such as mRCC brain lesions.[13,21,22,24] In the latter context, SF-GKRS is a well-established modality able to achieve optimal local control rates.[3,15,16,18,20,22,25,35,38] However, as demonstrated here, in the context of metastases larger than 8–10 cc and/or occurring in a critical location, ARE-development secondary to SF-GKRS remains a concern.[1] Matters become more complicated with the presence of tumor bed cysts, extensive edema and underlying hemorrhage, as often seen in mRCC.
Staged schedules
Aiming to decrease the risk of SF-GKRS associated ARE, different groups have explored alternative schedules with the aim of reducing toxicity whilst optimising local control; in this framework, volume-staged hypofractionation has become a vivid subject of discussion.[1,26-30,36] Yamamoto et al. conducted a three-stage gamma knife treatment study on 78 patients with lesions >10 cc; a static peripheral dose of 10 Gy was delivered on the 50% isodose line at each fraction (2 weeks interval) resulting in high rates of local control; the overall median survival time was of 8.3 months (with survival rates of 55.1% and 35.2% at 6 and 12 months, respectively). Of note, the incidence of neurological deterioration and neurological death was reported at 12.8% and 7.7%, respectively.[36] Another study by Angelov et al. involved 54 patients undergoing a two-stage radiosurgical intervention for lesions >20 mm; the median doses were 15 Gy at both fractions with approximately 1 month interval between fractions.[1] Median tumor volumes were reported at 10.5 cm3 and 7.0 cm3 at first and second treatment, respectively. Local control rates at 3 and 6 months were 95% and 88%, respectively, while the overall rate of ARE was 11%. Median tumor volume reduction between the first fraction and the first follow-up imaging (3 months post-treatment) was 54.9%. Roh et al. reported similar tumor volume evolution on a case with a large mRCC brain lesion treated with hypofractionated GKRS, suggesting the crucial role of interval imaging in radiosurgery planning and treatment outcome. However, as illustrated by these studies and the present case report, hypofractionated regimens do not fully exclude the risk of ARE-evolvement. In our view, staged techniques require the inclusion of ‘tailored’ bio-mathematical models controling key aspects of intratumoral ‘behaviour’ post radiation and perilesional toxicity.
Modulation of two concurrent prescription doses at each fraction: RRR
RRR is a ‘double blade’ staged technique based on the concurrent adaption of two different dose prescriptions:
A BED (biological effective dose) plotted peripheral dose conceived to control dose dissipation to the circumscribing healthy tissues while ensuring steep intratumoural dose escalation
A 10Gy baseline ablative prescription set to cover 70% to 90% of the tumor bed (previously coined baseline ablative isodose line or BAIL).[27]
Both prescriptions are surrogate to tumor volume dynamics and change concurrently at each fraction; the Leksell GammaPlanÒ software plays a fundamental role in that respect.
The inclusion of underlying radiobiological parameters and inherent physio-mathematical variables are quintessential for triggering key microenvironmental events involving vascular damage and antitumor immunity. The rationale of the procedure can be found elsewhere.[26-28,30]
Further aspects
In this particular case, the positive clinical and radiographic evolution of Met 1 and Met 2 post-RRR were similar to previous reports from other authors and Sinclair et al.[24,26-30] Unfortunately, a deeper analysis of the kinetic effectiveness of RRR compared to single fraction treatments or other oligo- staged approaches remains complex and will continue to be a source of bias; indeed, although a few groups have studied the specific effects of stereotactic hypofractionated radiotherapy on tumor kinetics in renal histology,[33,37] to the best of our knowledge, there are no or few studies comparing the ablative dynamics of brain lesions post single fraction versus post hypofractionation. Nonetheless, based on (i) the volumetric data post treatment of this case [Tables 1-3] (ii) our institutional experience,[26-30] and (iii) the available medical literature,[1,24,32,36] we suggest that, in the context of this case, the expeditious and lasting ablative effects triggered by this double prescription technique extended survival.[24,26-30]
Moreover, the fact that our patient developed an ARE at the site of Met 1 and Met 2 despite a hypofractionated approach, suggests that SF-GKRS would have led to greater perilesional edema and possibly substantial radionecrosis resulting in further neurologic damage. Several studies seem to support the latter proposal.[9,10,17]
As illustrated in this report, progressive extracranial disease remains a major problem for many patients with brain metastatic disease. Promising therapeutic breakthroughs in the field of medical uro-oncology are reshaping the management of mRCC; however, the long-term effects of most agents, such as checkpoint, multitargeted tyrosine kinase, and mammalian target of rapamycin inhibitors require much improvement, particularly in the context of concurrent metastatic disease in the central nervous system. The latter might require a better understanding of key concurrent antitumoral mechanisms aiming to optimize the use of radiation such as the modulation of T-cell expansion, the regulation of postradiation blood-brain barrier permeability and the use of “scavenging” agents targeting angiogenesis and endothelial cell proliferation to further “downturn” tumor radioresistance.[4,7,11,12,16,19,21,23,27,31,38]
CONCLUSION
In this case of mRCC brain disease, RRR proved effective in producing sustained ablation while limiting toxicity. We can cautiously assume that RRR had a positive impact over local tumor control and overall survival based on the following variables: (i) degree of local response of Met 1 and Met 2 to RRR, (ii) Time of survival (21 months) despite limited response / tolerance to systemic treatment (iii) known median overall survival in these cases (<11 months), and (iv) stable KPS- and neurological status throughout follow-up.
RRR should be considered in the acute/subacute management of mRCC brain metastases, particularly in those requiring a more tailored approach due to local anatomo-functional constraints. Yet, as illustrated here, overall metastatic activity remains a major challenge in many cases; thus, further studies on the synergetic role of radiation and specific antitumoral therapies are of interest and warranted.
Footnotes
How to cite this article: Sinclair G, Stenman M, Benmakhlouf H, Johnstone P, Wersäll P, Lindskog M, et al. Adaptive radiosurgery based on two simultaneous dose prescriptions in the management of large renal cell carcinoma brain metastases in critical areas: Towards customization. Surg Neurol Int 2020;11:21.
Contributor Information
Georges Sinclair, Email: georges.sinclair@gmail.com.
M. Stenman, Email: maria.stenman@igp.uu.se.
H. Benmakhlouf, Email: hamza.benmakhlouf@gmail.com.
P. Johnstone, Email: philippa.johnstone1@nhs.net.
P. Wersäll, Email: peter.wersall@sll.se.
M. Lindskog, Email: magnus.lindskog@igp.uu.se.
M. A. Hatiboglu, Email: azizhatiboglu@yahoo.com.
U. Harmenberg, Email: ulrika.harmenberg@sll.se.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Angelov L, Mohammadi AM, Bennett EE, Abbassy M, Elson P, Chao ST, et al. Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg. 2018;129:366–82. doi: 10.3171/2017.3.JNS162532. [DOI] [PubMed] [Google Scholar]
- 2.Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al. Updates in the management of brain metastases. Neuro Oncol. 2016;18:1043–65. doi: 10.1093/neuonc/now127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco AI, Teh BS, Amato RJ. Role of radiation therapy in the management of renal cell cancer. Cancers (Basel) 2011;3:4010–23. doi: 10.3390/cancers3044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botta GP, Granowicz E, Costantini C. Advances on immunotherapy in genitourinary and renal cell carcinoma. Transl Cancer Res. 2017;6:17–29. doi: 10.21037/tcr.2017.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SY, Yoo S, You D, Jeong IG, Song C, Hong B, et al. Prognostic factors for survival of patients with synchronous or metachronous brain metastasis of renal cell carcinoma. Clin Genitourin Cancer. 2017;15:717–23. doi: 10.1016/j.clgc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Connolly EP, Mathew M, Tam M, King JV, Kunnakkat SD, Parker EC, et al. Involved field radiation therapy after surgical resection of solitary brain metastases--mature results. Neuro Oncol. 2013;15:589–94. doi: 10.1093/neuonc/nos328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza NM, Fang P, Logan J, Yang J, Jiang W, Li J. Combining radiation therapy with immune checkpoint blockade for central nervous system malignancies. Front Oncol. 2016;6:212. doi: 10.3389/fonc.2016.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalhaug A, Haukland E, Nieder C. Leptomeningeal carcinomatosis from renal cell cancer: Treatment attempt with radiation and sunitinib (case report) World J Surg Oncol. 2010;8:36. doi: 10.1186/1477-7819-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donovan EK, Parpia S, Greenspoon JN. Incidence of radionecrosis in single-fraction radiosurgery compared with fractionated radiotherapy in the treatment of brain metastasis. Curr Oncol. 2019;26:e328–33. doi: 10.3747/co.26.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frischer JM, Fraller A, Mallouhi A, Vogl UM, Baier F, Ertl A, et al. Evaluation of dose-staged gamma knife radiosurgical treatment method for high-risk brain metastases. World Neurosurg. 2016;94:352–9. doi: 10.1016/j.wneu.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 11.Heravi M, Tomic N, Liang L, Devic S, Holmes J, Deblois F, et al. Sorafenib in combination with ionizing radiation has a greater anti-tumour activity in a breast cancer model. Anticancer Drugs. 2012;23:525–33. doi: 10.1097/CAD.0b013e32834ea5b3. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim N, Yu Y, Walsh WR, Yang JL. Molecular targeted therapies for cancer: Sorafenib mono-therapy and its combination with other therapies (review) Oncol Rep. 2012;27:1303–11. doi: 10.3892/or.2012.1675. [DOI] [PubMed] [Google Scholar]
- 13.Ippen FM, Mahadevan A, Wong ET, Uhlmann EJ, Sengupta S, Kasper EM. Stereotactic radiosurgery for renal cancer brain metastasis: Prognostic factors and the role of whole-brain radiation and surgical resection. J Oncol. 2015;2015:636918. doi: 10.1155/2015/636918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen CA, Chan MD, McCoy TP, Bourland JD, deGuzman AF, Ellis TL, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg. 2011;114:1585–91. doi: 10.3171/2010.11.JNS10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kano H, Iyer A, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Outcome predictors of gamma knife radiosurgery for renal cell carcinoma metastases. Neurosurgery. 2011;69:1232–9. doi: 10.1227/NEU.0b013e31822b2fdc. [DOI] [PubMed] [Google Scholar]
- 16.Kasibhatla M, Steinberg P, Meyer J, Ernstoff MS, George DJ. Radiation therapy and sorafenib: Clinical data and rationale for the combination in metastatic renal cell carcinoma. Clin Genitourin Cancer. 2007;5:291–4. doi: 10.3816/CGC.2007.n.007. [DOI] [PubMed] [Google Scholar]
- 17.Lesueur P, Lequesne J, Barraux V, Kao W, Geffrelot J, Grellard JM, et al. Radiosurgery or hypofractionated stereotactic radiotherapy for brain metastases from radioresistant primaries (melanoma and renal cancer) Radiat Oncol. 2018;13:138. doi: 10.1186/s13014-018-1083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: The current evidence. Cancer Treat Rev. 2014;40:48–59. doi: 10.1016/j.ctrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Rao M, Poiret T, Nava S, Meng Q, von Landenberg A, et al. Mesothelin as a novel biomarker and immunotherapeutic target in human glioblastoma. Oncotarget. 2017;8:80208–22. doi: 10.18632/oncotarget.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo CH, Huang WY, Chao HL, Lin KT, Jen YM. Novel application of stereotactic ablative radiotherapy using cyberknife® for early-stage renal cell carcinoma in patients with pre-existing chronic kidney disease: Initial clinical experiences. Oncol Lett. 2014;8:355–60. doi: 10.3892/ol.2014.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maria B, Antonella V, Michela R, Silvana G, Anita S, Anna Maria A, et al. Multimodality treatment of brain metastases from renal cell carcinoma in the era of targeted therapy. Ther Adv Med Oncol. 2016;8:450–9. doi: 10.1177/1758834016659825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mennitto A, Verzoni E, Grassi P, Ratta R, Fucà G, Procopio G. Multimodal treatment of advanced renal cancer in 2017. Expert Rev Clin Pharmacol. 2017;10:1395–402. doi: 10.1080/17512433.2017.1386552. [DOI] [PubMed] [Google Scholar]
- 23.Rao M, Zhenjiang L, Meng Q, Sinclair G, Dodoo E, Maeurer M, editors. Oncoimmunology: A Practical Guide for Cancer Immunotherapy. Switzerland: Springer International Publishing AG; 2018. Mutant epitopes in cancer; pp. 41–67. [Google Scholar]
- 24.Roh H, Kim J, Chong K, Yoon WK, Kwon TH, Kim JH. Unexpected daily changes in tumor volume during fractionated gamma knife radiosurgery for solitary intraventricular metastatic renal cell carcinoma: A case report. Stereotact Funct Neurosurg. 2019;97:44–8. doi: 10.1159/000497152. [DOI] [PubMed] [Google Scholar]
- 25.Shuto T, Matsunaga S, Suenaga J, Inomori S, Fujino H. Treatment strategy for metastatic brain tumors from renal cell carcinoma: Selection of gamma knife surgery or craniotomy for control of growth and peritumoral edema. J Neurooncol. 2010;98:169–75. doi: 10.1007/s11060-010-0170-4. [DOI] [PubMed] [Google Scholar]
- 26.Sinclair G, Bartek J, Jr, Martin H, Barsoum P, Dodoo E. Adaptive hypofractionated gamma knife radiosurgery for a large brainstem metastasis. Surg Neurol Int. 2016;7:S130–8. doi: 10.4103/2152-7806.176138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinclair G, Benmakhlouf H, Brigui M, Maeurer M, Dodoo E. The concept of rapid rescue radiosurgery in the acute management of critically located brain metastases: A retrospective short-term outcome analysis. Surg Neurol Int. 2018;9:218. doi: 10.4103/sni.sni_480_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinclair G, Benmakhlouf H, Martin H, Brigui M, Maeurer M, Dodoo E. The role of radiosurgery in the acute management of fourth ventricle compression due to brain metastases. Surg Neurol Int. 2018;9:112. doi: 10.4103/sni.sni_387_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinclair G, Benmakhlouf H, Martin H, Maeurer M, Dodoo E. Adaptive hypofractionated gamma knife radiosurgery in the acute management of brainstem metastases. Surg Neurol Int. 2019;10:14. doi: 10.4103/sni.sni_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinclair G, Martin H, Fagerlund M, Samadi A, Benmakhlouf H, Doodo E. Adaptive hypofractionated gamma knife radiosurgery in the acute management of large thymic carcinoma brain metastases. Surg Neurol Int. 2017;8:95. doi: 10.4103/sni.sni_391_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Stenman M, Sinclair G, Paavola P, Wersäll P, Harmenberg U, Lindskog M. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005-2014. Radiother Oncol. 2018;127:501–6. doi: 10.1016/j.radonc.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Sun MR, Brook A, Powell MF, Kaliannan K, Wagner AA, Kaplan ID, et al. Effect of stereotactic body radiotherapy on the growth kinetics and enhancement pattern of primary renal tumors. AJR Am J Roentgenol. 2016;206:544–53. doi: 10.2214/AJR.14.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel J, Ojerholm E, Hollander A, Briola C, Mooij R, Bieda M, et al. Intracranial control after cyberknife radiosurgery to the resection bed for large brain metastases. Radiat Oncol. 2015;10:221. doi: 10.1186/s13014-015-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wowra B, Siebels M, Muacevic A, Kreth FW, Mack A, Hofstetter A. Repeated gamma knife surgery for multiple brain metastases from renal cell carcinoma. J Neurosurg. 2002;97:785–93. doi: 10.3171/jns.2002.97.4.0785. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M, Higuchi Y, Serizawa T, Kawabe T, Nagano O, Sato Y, et al. Three-stage gamma knife treatment for metastatic brain tumors larger than 10 cm3: A 2-institute study including re-analyses of earlier results using competing risk analysis. J Neurosurg. 2018;129:77–85. doi: 10.3171/2018.7.GKS181392. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Kang SK, Wang L, Touijer A, Hricak H. Distribution of renal tumor growth rates determined by using serial volumetric CT measurements. Radiology. 2009;250:137–44. doi: 10.1148/radiol.2501071712. [DOI] [PubMed] [Google Scholar]
- 38.Zingg D, Riesterer O, Fabbro D, Glanzmann C, Bodis S, Pruschy M. Differential activation of the phosphatidylinositol 3’-kinase/Akt survival pathway by ionizing radiation in tumor and primary endothelial cells. Cancer Res. 2004;64:5398–406. doi: 10.1158/0008-5472.CAN-03-3369. [DOI] [PubMed] [Google Scholar]


