The global death toll from tuberculosis (TB) is an ongoing tragedy (1). Currently, clinicians in TB-prevalent resource-poor settings attempt to control the pandemic with clearly inadequate tools: bacillus Calmette-Guérin (BCG) vaccination, sputum smear microscopy, and antibiotic regimens of a minimum of 6 months, each of which has remained essentially unchanged for many decades (2). Although initial trials of novel vaccinations have been disappointing (3, 4), two recent vaccine trials have generated more promising results (5, 6). The M72/AS01E vaccine reduced the number of active cases by 50% in a phase 2b study (5), whereas a repeat BCG administration reduced sustained IFN-γ release assay conversion from 11.6% to 6.7% (6). Repeat BCG was not included in the first study, and so the relative efficacy cannot be defined. However, both studies demonstrate that a significant residual disease burden will persist, which can lead to ongoing transmission. Consequently, it is vital to consider what constitutes a protective immune response to Mycobacterium tuberculosis (Mtb) versus a pathological immune response that leads to cavitation and transmission (7). We review clinical and experimental observations that highlight the complexity of the host–pathogen interaction in human TB to develop an entirely new conceptual model that will inform future strategies.
The Sequence of Events in Human TB Infection
Humans and Mtb are believed to have coevolved for an estimated 70,000 years (8) and therefore have developed a highly complex host–pathogen interaction. Mtb is transmitted by aerosol, generated by a patient with pulmonary disease affecting the lung apices (Figure 1A). The initial site of implantation is the lung base, as described by Ghon in 1916 (9). The early immunological events at the lung base are clearly distinct from the late immunological events at the lung apex, as cavitation almost never happens at the site of initial implantation (10). After inhalation, an early inflammatory response to Mtb results, which can be extensive, with radiologically visible lung lesions and lymph node enlargement (the Ghon complex). However, in the vast majority of patients this self-resolves, only leaving postinflammatory calcification (Figure 1B). Primary progressive disease is associated with immunocompromise, such as HIV coinfection, newborn infants, or anti-TNF (tumor necrosis factor) treatment (11). Therefore, despite this extensive initial inflammation, the patient’s host immune response controls infection, and this individual is classified as having “latent” TB, with an approximately 1 in 10 lifetime risk of TB reactivation (2).
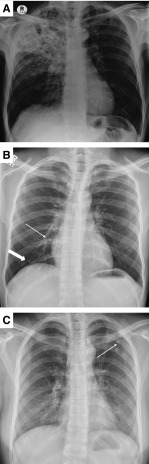
Figure 1.
Apical lung destruction in tuberculosis (TB). (A) Extensive right upper zone lung inflammation and cavitation in a patient with pulmonary tuberculosis. These patients are highly infectious and drive the pandemic. (B) Self-limiting initial infection. Mycobacterium tuberculosis has been inhaled to the lung base. The initial granulomatous response has left a calcified Ghon focus (broad arrow) and calcification in a mediastinal lymph node (narrow arrow). This initial infection has resolved, and the individual would be classified as having “latent” TB. (C) Apical lung scarring from self-resolved TB. A calcified area of fibrosis (arrow) demonstrates an area of TB infection that has progressed and then regressed without treatment.
The distinction between the events at the lung base and the lung apex is often not made, but evidently the immunological process at the two sites is different. Mtb must transfer from the lung bases to the lung apices, probably transported within infected macrophages that traffic through the initial granulomas (12, 13), although the mechanism for implantation in the lung apex is unknown. Insight into dissemination is limited, in part because of the experimental challenges studying the process. Clinically, the development of miliary TB in immunocompromise would suggest that Mtb initially disseminates widely throughout the body (14) and then is controlled at the onset of adaptive immunity in the majority of the implantation sites but not at the lung apices. Whether the hyperconservation of T-cell epitopes in Mtb (15) is related to facilitating dissemination, or alternatively causing immunopathology to permit subsequent exit from the lung via airways, is an open question.
After implantation, after a delay between months or many years (16), Mtb must generate a proinflammatory lesion that leads to tissue breakdown, access to the airway, exponential bacterial growth, and transmission (17). However, even after an extensive lesion develops, this should not be regarded as an inevitable sequence of events: in the preantibiotic era, one-third of “consumptives” would self-heal (18), and with surgery to collapse a cavity this increases to about 70% cure even in the absence of antibiotics (19). In high-incidence countries, areas of extensive pulmonary scarring from TB that have self-healed without diagnosis or treatment are frequently observed (Figure 1C). A radiologically visible lesion 0.5 cm in diameter would have contained approximately 65 million inflammatory cells, and yet this infected aggregate can often self-resolve. In addition, this flux between the host and pathogen has been confirmed by recent radionucleotide imaging studies demonstrating that in the same individual some lesions may progress while others regress (20). Therefore, a very fine balance exists between protection and progression in TB, determined at the local lesion level, and often the host immune response can bring extensive pulmonary infection under control.
Immunological Determinants of Protection versus Pathology
The host immune response is clearly needed to control Mtb infection. A deficiency of CD4-positive T cells, TNF-α, or IFN-γ/IL-12 signaling leads to disseminated TB in both human and animal studies (11), and the list of specific immunodeficiencies that can increase the risk of active TB continues to expand (21, 22). However, the fact that complete absence of a component of the immune response causes disease does not prove that an excess will increase protection, and these cases of specific immunodeficiencies can in fact be considered biological outliers (23). An alternative perspective is that a strong immune response can be as harmful as a weak immune response in TB. For this discussion, we characterize a strong immune response as increased cytokine secretion, augmented cellular infiltration, increased cell death, and high MMP (matrix metalloproteinase) production, whereas a weak immune response lacks these features.
The concept that an excessive host immune response can be harmful has been identified as long as the disease itself and was the subject of bitter disputes between Koch and Virchov (24). Koch’s treatment of patients with tuberculin to augment the host immune response to Mtb worsened pathology in more patients than those who benefitted (24). Cavitary pulmonary TB, which transmits infection, occurs most commonly between the age of 20 and 25 years (25), which could be regarded as the immunological prime and is certainly not a time of immunocompromise. In these seminal epidemiological studies, Comstock and colleagues demonstrated that the stronger the delayed-type hypersensitivity response to Mtb antigens as a child, the more likely individuals are to develop pulmonary disease as a young adult (25). The only data available for analysis were response to Mtb antigens and subsequent development of TB, and so the study indicates that a strong response associates with disease many years later.
Recently, further evidence that an excessive response increases the risk of TB has emerged from the cancer immunotherapy field. Treating patients with cancer with immune checkpoint inhibitors to globally activate the immune response improves outcomes in diverse malignancies (26) but also leads to active TB in a growing number of patients (27, 28). This phenomenon is entirely consistent with the hypersusceptibility of programmed death-1 knockout mice to Mtb infection; these mice die even more rapidly than IFN-γ–deficient mice (29, 30). The divergence is not due to increased bacterial load, as cyclophilin D–deficient mice with increased T-cell responses to Mtb and no increase in bacterial load also have worse outcome (31). These emerging lessons from novel medical interventions, which are providing unique insight into the function of the immune system in health and disease, further reinforce that an excessive response to Mtb is harmful.
Taken alongside the concept that either an insufficient or an excessive response may be harmful, the Mtb exposure intensity and strain virulence need to be considered (32, 33). Higher Mtb exposure levels are well documented to lead to active TB (34). The observation that Mtb T-cell epitopes are hyperconserved supports the notion that Mtb specifically benefits from the host immune response (15). Therefore, any conceptual model about the TB host–pathogen interaction needs to consider both the protective and the harmful effects of host immunity and the exposure level to Mtb.
Conceptual Models of Human Immunity to Mtb
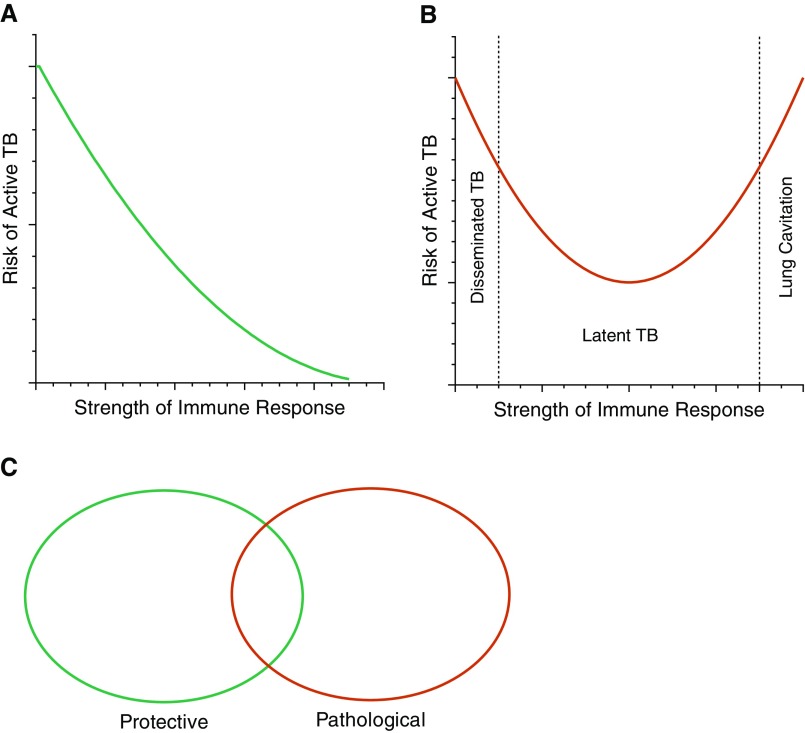
The simplest established model of the host–pathogen interaction is a linear one, whereby greater immunity leads to reduced disease incidence (Figure 2A). However, this model is inconsistent with epidemiological, clinical, and experimental observations outlined above, which demonstrate a strong immune response associates with increased incidence of cavitary pulmonary disease, and should be discarded.
Figure 2.
(A) Linear model of the host–pathogen interaction, proposing that the stronger the immune response, the greater the protection from tuberculosis (TB). This seems inconsistent with historical and emerging clinical observations. (B) U-shaped curve. Either an insufficient or an excessive immune response increases the risk of TB, with a central region of optimal protection. Although an insufficient response tends to lead to disseminated disease, an excessive response is required to drive cavitation and transmission. (C) Binary model. Some host immune response elements control Mycobacterium tuberculosis growth, whereas others cause pathology and transmission, and some are shared. The degree of overlap between pathology and protection is unknown.
Therefore, the concept of a U-shaped curve is emerging within the field (Figure 2B). Here, either an inadequate or an excessive immune response leads to TB. This model is improved by discriminating between different clinical presentations of TB, which are usually grouped together. A very weak response, such as advanced HIV infection, newborn infants, or IFN-γ deficiency, typically leads to disseminated disease, such as miliary TB or TB meningitis (35), whereas individuals with a strong immune response develop cavitary pulmonary TB (25). This distinction has significant implications. First, simply driving a greater immune response in the entire population may protect some individuals from disseminated disease but worsen disease in others, as it risks shifting a proportion of people with a controlling response into a cavitation response. In addition, the proposal that interventions that improve outcome for immunocompromise-associated TB will then also improve outcome in pulmonary disease (36) runs counter to clinical observations that pulmonary disease is caused by immune excess. In fact, interventions that improve immunodeficient TB may worsen immune-excess TB. Therefore, defining the characteristics of the immunological controllers, versus the two types of progressors, is a very pressing question to inform optimal strategies.
A third approach would be a binary model (Figure 2C). In this, some aspects of the host immune response to the pathogen are protective, whereas different responses drive pathology, and a central overlap occurs between the two processes. If these specific components could be determined, it would greatly advance vaccine development as augmenting the protective response, whereas suppressing the pathological response would then terminate the pandemic. However, because many of the effector molecules of the immune response, such as inflammatory cytokines, may be communal, it seems more likely that significant overlap exists, and separating processes into exclusively protective and pathological may be challenging.
A Three-Dimensional Model Considering Host and Pathogen Factors
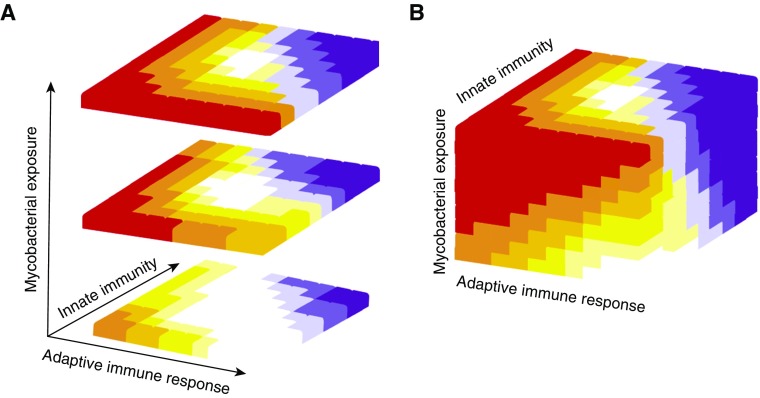
The models presented above do not consider intensity of Mtb exposure, which clearly determines risk (33, 34), nor do they differentiate between the host’s innate and adaptive immune response. An emerging concept is that innate memory may be just as important as adaptive memory (37–39), and so the two should be considered separately. A balance between innate and adaptive immunity is likely required, with either a gross deficit or excess of either harmful, and similarly a relatively strong innate response can compensate for a weak adaptive response and vice versa. Plotting the host immunological and mycobacterial elements into a model generates a more complex three-dimensional picture of optimal immunity to Mtb (Figure 3). Furthermore, this characterizes the likelihood of different forms of TB, such as disseminated TB associated with immunodeficiency (Figure 3, red), control (white) or cavitary pulmonary TB due to an excessive immune response (purple). The optimal immune response can be predicted to be an asymmetrical pyramid, whereby the disease likelihood increases as the Mtb exposure increases and the area of an optimal immune response to control infection declines.
Figure 3.
(A) Multidimensional model of tuberculosis (TB) risk. If the strength of the immune response is broken into innate and adaptive elements and then plotted against Mycobacterium tuberculosis exposure intensity, a more complex picture of risk of active TB emerges. Red represents disseminated TB, grading through progressively reduced risk to white indicating protection. An overexuberant immune response leads to lung inflammation, cavitation, and transmission (purple). (B) External appearance of TB risk matrix cube.
Within this model, each individual will start from a different position on the matrix, determined by diverse factors such as their genetic makeup and BCG vaccination. Age is an important determinant: newborn infants have poor immunity and have highest risk of disseminated TB (Figure 3, red zone); then children have a relative low risk (white zone) but after puberty enter the period of highest risk of pulmonary TB as young adults (purple zone). Other factors will also contribute to movement in the matrix, such as environmental mycobacterial exposure, nutritional status, comorbidities, medication, and intensity of Mtb exposure. The primary implication of this more-advanced model is that a novel intervention for TB may improve the chances of Mtb control for some individuals but conversely worsen it for others. A key consideration is that for Mtb to spread, it must cause extensive lung inflammation, tissue destruction, and cavitation from excessive immune responses.
Although this model appears to suggest a high risk of TB, it is essential to note that approximately 90% of Mtb-exposed individuals end life in the central white control zone (2), and only around 6% end in the purple zone, developing pulmonary TB and transmitting infection to continue the pandemic. A vaccination strategy that prevents pulmonary disease would break the cycle of transmission and end the TB pandemic. As many cases of disease are due to relatively recent transmission, such a vaccine could have a rapid impact (16).
Potential Mechanisms Driving Excessive Inflammation
The characterization of the immune response between protective and pathological elements opens the question about what events propel the immune response over the optimal “control” zone and into the “cavitary” zone. Hunter has argued that current models of TB are inconsistent with histological analysis of human pulmonary TB cases and has proposed that a progressive build-up of Mtb antigens leads to a sudden excessive inflammatory event (40). We have hypothesized that Mtb may drive an autoimmune/autoinflammatory response to exacerbate inflammation (41), and this suggestion has since been supported by bioinformatic analyses of gene expression signatures in TB and in infectious and autoimmune disease (42). Tangential evidence also arises from the association of immune checkpoint inhibition and active TB (27, 28), because the predominant side effects of these agents are autoimmune in nature (26).
If Mtb is driving an autoimmune process, the likely mechanisms and antigens involved are totally unknown. We suggest that one candidate mechanism is the presentation of lipids by CD1 molecules to tissue-resident innate-like T cells (43). Phospholipids are major components of mammalian and bacterial membranes, including those of Mtb. Although the lipid moieties of phospholipids derived from bacteria are structurally different from their mammalian counterparts, both mammalian and bacterial phospholipids can activate CD1d- and CD1b-restricted T cells, thus providing a mechanism for CD1-mediated T-cell activation by shared phospholipids (44, 45). Furthermore, stress-related lipids, such as host-derived cholesteryl esters that accumulate in TB lesions, are CD1c ligands that activate T cells (46). Mtb may induce an inflammatory milieu through upregulation of host-derived stress molecules, including CD1, provoking proinflammatory T-cell responses. Examples include rapid IL-17 production in response to mycobacteria by innate γδ cells (47, 48) and activation of pro-proinflammatory tissue-resident innate-like T cells such as γδ, mucosal-associated invariant T cells, natural killer cells, and innate lymphoid cells (49).
Thus, Mtb may drive local inflammation by upregulating host-derived stress ligands that provoke innate T-cell activation, and the resulting autoimmune inflammatory response may exacerbate pathology, a proposal reminiscent of recent findings in lung cancer (50). Furthermore, emerging evidence suggests an important role for B7-like members such as BTN (butyrophilin) and BTN-like molecules as self-ligands that regulate innate-like T cells such as tissue-resident γδ cells (43, 51). Interestingly, the expression of BTN and BTN-like molecules is significantly downregulated in inflammatory disorders and cancer (52). Hence, the inflammatory process in TB may cause further local T-cell dysregulation and perpetuate excessive pathology.
Along similar lines to this autoimmunity hypothesis, Divangahi and Sassetti have independently proposed that the development of active TB reflects primarily a loss of tolerance (53–55). Whether the inflammation is driven by autoimmunity or loss of tolerance is perhaps more a matter of semantics, as they could be regarded as the same fundamental process. Consequently, diverse research groups have reached a similar hypothesis from very different experimental approaches. One irrefutable conclusion is the urgent necessity to address the knowledge gap of what drives pathology versus protection in TB to inform new therapies (54).
Clues to Human TB Pathogenesis from Mycobacterium bovis
A significant unanswered question within the TB field is why Mtb and Mycobacterium bovis are so similar genetically, with 99.7% shared sequence identity, and yet the host tropism is very different (56). This suggests that comparison may shed light on Mtb pathogenicity. Only Mtb can cause lung cavitation and transmission in humans, whereas M. bovis can cause lymph node disease in humans but does not cause sufficient lung disease to spread to new hosts apart from in very occasional cases (57). Therefore, the key difference does not seem to be the ability to survive within the host macrophage but instead the ability to cause sufficient pulmonary inflammation to result in lung cavitation and transmission. M. bovis has multiple genetic regions of deletion relative to Mtb, and the primary difference when compared with Mtb is the loss of lipoprotein-related genes (56). Lipoproteins facilitate transport of hydrophobic lipids in solution, such as cholesterol and triglycerides. Consequently, one hypothesis would be that these lipoproteins are necessary to shuttle lipids from infected cells to uninfected cells, thereby amplifying the proinflammatory host immune response driven by CD1-responsive T cells as proposed above. These lipids could be pathogen derived, host stress lipids, or common to both. Alternatively, the divergence in lipid-related genes may be related to metabolism, as nutritional restriction by macrophages is emerging as a critical control mechanism of Mtb growth (58).
Investigating the Optimal Response to TB
The models of the host–pathogen interaction that we present suggest that a more detailed dissection of events is required to inform novel interventions. As Mtb and humans have coevolved for so long, correlation between experimental systems and human TB is essential. Furthermore, studying the correct compartment is key, as events in the periphery may not reflect those in the lung apex. The investigation of individuals who are recurrently exposed to Mtb but do not develop infection, and individuals who have spontaneously controlled disease, may provide unique insight into protective responses (59), if sufficient individuals can be identified. This approach may characterize innate profiles associated with resistance. However, these studies are inherently challenging, as it is difficult to differentiate individuals who have been infected and eradicated Mtb very early from the alternative groups with identical test results, never exposed or Mtb-infected but PPD false-negative. Furthermore, multiple mechanisms of innate resistance may exist, such as macrophage- and CD1-mediated early eradication processes (59) and trained innate immunity (60). Individuals who are IFN-γ release assay positive and then revert to negative may provide another group who may have successfully controlled Mtb (61). We propose that integration of clinical studies with advanced cellular models may be critical to dissect underlying processes (62). Although on one hand they can be dismissed as not reflecting the complexity of events in vivo, their primary advantage is their tractability to perform mechanistic studies in a human–Mtb system to confirm observations from clinical and animal studies.
Conclusions
The ongoing TB pandemic emphasizes the importance of reconsidering fundamental concepts of disease in light of historical and emerging data. Both clinical and experimental observations suggest that simple linear or U-shaped models of the optimal host immune response to Mtb are unlikely to be adequate, and a more nuanced multidimensional model is required. The three-parameter stratification of TB risk presented here is likely still an oversimplification, but it provides a framework within which to consider the optimal immune response to control Mtb infection. This permits dissection of the protective and pathological elements of the host immune response to inform strategies to reduce transmission in future studies. In addition, for previous studies, infants who have had a long-lasting increase to their immune response to Mtb (63) should be followed with the same intensity for adverse events after puberty as for potential early beneficial effects.
Supplementary Material
Footnotes
Supported by UK Medical Research Council grants MR/P023754/1 (P.E.) and MR/S024220/1 (S.M.), the Wessex Medical Research (L.B.T.), and Cancer Research UK grant 23562 (S.M.).
Author contributions: P.E. wrote the first draft and all authors sequentially edited the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201908-1506PP on October 28, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wallis RS, Maeurer M, Mwaba P, Chakaya J, Rustomjee R, Migliori GB, et al. Tuberculosis: advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis. 2016;16:e34–e46. doi: 10.1016/S1473-3099(16)00070-0. [DOI] [PubMed] [Google Scholar]
- 2.Dheda K, Barry CE, III, Maartens G. Tuberculosis. Lancet. 2016;387:1211–1226. doi: 10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. MVA85A 020 Trial Study Team. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dièye S, et al. MVA85A 030 Trial Investigators. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2015;3:190–200. doi: 10.1016/S2213-2600(15)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2018;379:1621–1634. doi: 10.1056/NEJMoa1803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. C-040-404 Study Team. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190:9–18. doi: 10.1164/rccm.201311-2106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brites D, Gagneux S. Co-evolution of Mycobacterium tuberculosis and Homo sapiens. Immunol Rev. 2015;264:6–24. doi: 10.1111/imr.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghon A. London: J. & A. Churchill; 1916. The primary lung focus of tuberculosis in children. [Google Scholar]
- 10.Elkington PT, Friedland JS. Permutations of time and place in tuberculosis. Lancet Infect Dis. 2015;15:1357–1360. doi: 10.1016/S1473-3099(15)00135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber HA, Harding JS, Hunt O, Altamirano CJ, Hulseberg PD, Stewart D, et al. Inflammatory dendritic cells migrate in and out of transplanted chronic mycobacterial granulomas in mice. J Clin Invest. 2011;121:3902–3913. doi: 10.1172/JCI45113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmail H, Riou C, Bruyn ED, Lai RP, Harley YXR, Meintjes G, et al. The immune response to Mycobacterium tuberculosis in HIV-1-coinfected persons. Annu Rev Immunol. 2018;36:603–638. doi: 10.1146/annurev-immunol-042617-053420. [DOI] [PubMed] [Google Scholar]
- 15.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738. doi: 10.1136/bmj.k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoder MA, Lamichhane G, Bishai WR. Cavitary pulmonary tuberculosis: the Holey Grail of disease transmission. Curr Sci. 2004;86:74–81. [Google Scholar]
- 18.Dubos R, Dubos J. The white plague: tuberculosis, man and society. New Brunswick, London: Rutgers University Press; 1987. [Google Scholar]
- 19.Sellors TH. The results of thoracoplasty in pulmonary tuberculosis. Thorax. 1947;2:216–223. doi: 10.1136/thx.2.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenaerts A, Barry CE, III, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 2015;264:288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boisson-Dupuis S, Ramirez-Alejo N, Li Z, Patin E, Rao G, Kerner G, et al. Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol. 2018;3:eaau8714. doi: 10.1126/sciimmunol.aau8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Barricarte R, Markle JG, Ma CS, Deenick EK, Ramírez-Alejo N, Mele F, et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci Immunol. 2018;3:eaau6759. doi: 10.1126/sciimmunol.aau6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karp CL, Wilson CB, Stuart LM. Tuberculosis vaccines: barriers and prospects on the quest for a transformative tool. Immunol Rev. 2015;264:363–381. doi: 10.1111/imr.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann SH. A short history of Robert Koch’s fight against tuberculosis: those who do not remember the past are condemned to repeat it. Tuberculosis (Edinb) 2003;83:86–90. doi: 10.1016/s1472-9792(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 25.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131–138. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 26.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkington PT, Bateman AC, Thomas GJ, Ottensmeier CH. Implications of tuberculosis reactivation after immune checkpoint inhibition. Am J Respir Crit Care Med. 2018;198:1451–1453. doi: 10.1164/rccm.201807-1250LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med. 2019;11:eaat2702. doi: 10.1126/scitranslmed.aat2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lázár-Molnár E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci USA. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzelepis F, Blagih J, Khan N, Gillard J, Mendonca L, Roy DG, et al. Mitochondrial cyclophilin D regulates T cell metabolic responses and disease tolerance to tuberculosis. Sci Immunol. 2018;3:eaar4135. doi: 10.1126/sciimmunol.aar4135. [DOI] [PubMed] [Google Scholar]
- 32.Merker M, Blin C, Mona S, Duforet-Frebourg N, Lecher S, Willery E, et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet. 2015;47:242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee RS, Proulx JF, Menzies D, Behr MA. Progression to tuberculosis disease increases with multiple exposures. Eur Respir J. 2016;48:1682–1689. doi: 10.1183/13993003.00893-2016. [DOI] [PubMed] [Google Scholar]
- 34.Saunders MJ, Wingfield T, Tovar MA, Baldwin MR, Datta S, Zevallos K, et al. A score to predict and stratify risk of tuberculosis in adult contacts of tuberculosis index cases: a prospective derivation and external validation cohort study. Lancet Infect Dis. 2017;17:1190–1199. doi: 10.1016/S1473-3099(17)30447-4. [Published erratum appears in Lancet Infect Dis 17:1117.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 36.Katsnelson A. Beyond the breath: exploring sex differences in tuberculosis outside the lungs. Nat Med. 2017;23:398–401. doi: 10.1038/nm0417-398. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172:176–190, e19. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Netea MG, van Crevel R. BCG-induced protection: effects on innate immune memory. Semin Immunol. 2014;26:512–517. doi: 10.1016/j.smim.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Hunter RL. Tuberculosis as a three-act play: a new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis (Edinb) 2016;97:8–17. doi: 10.1016/j.tube.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elkington P, Tebruegge M, Mansour S. Tuberculosis: an infection-initiated autoimmune disease? Trends Immunol. 2016;37:815–818. doi: 10.1016/j.it.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clayton K, Polak ME, Woelk CH, Elkington P. Gene expression signatures in tuberculosis have greater overlap with autoimmune diseases than with infectious diseases. Am J Respir Crit Care Med. 2017;196:655–656. doi: 10.1164/rccm.201706-1248LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol. 2018;19:1352–1365. doi: 10.1038/s41590-018-0253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatituri RV, Watts GF, Bhowruth V, Barton N, Rothchild A, Hsu FF, et al. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci USA. 2013;110:1827–1832. doi: 10.1073/pnas.1220601110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Rhijn I, van Berlo T, Hilmenyuk T, Cheng TY, Wolf BJ, Tatituri RV, et al. Human autoreactive T cells recognize CD1b and phospholipids. Proc Natl Acad Sci USA. 2016;113:380–385. doi: 10.1073/pnas.1520947112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mansour S, Tocheva AS, Cave-Ayland C, Machelett MM, Sander B, Lissin NM, et al. Cholesteryl esters stabilize human CD1c conformations for recognition by self-reactive T cells. Proc Natl Acad Sci USA. 2016;113:E1266–E1275. doi: 10.1073/pnas.1519246113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 48.Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maertzdorf J, Tönnies M, Lozza L, Schommer-Leitner S, Mollenkopf H, Bauer TT, et al. Mycobacterium tuberculosis invasion of the human lung: first contact. Front Immunol. 2018;9:1346. doi: 10.3389/fimmu.2018.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell. 2019;176:998–1013, e16. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell. 2016;167:203–218, e17. doi: 10.1016/j.cell.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lebrero-Fernández C, Wenzel UA, Akeus P, Wang Y, Strid H, Simrén M, et al. Altered expression of Butyrophilin (BTN) and BTN-like (BTNL) genes in intestinal inflammation and colon cancer. Immun Inflamm Dis. 2016;4:191–200. doi: 10.1002/iid3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Divangahi M, Khan N, Kaufmann E. Beyond killing Mycobacterium tuberculosis: disease tolerance. Front Immunol. 2018;9:2976. doi: 10.3389/fimmu.2018.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olive AJ, Sassetti CM. Tolerating the unwelcome guest; how the host withstands persistent Mycobacterium tuberculosis. Front Immunol. 2018;9:2094. doi: 10.3389/fimmu.2018.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, et al. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA. 2003;100:7877–7882. doi: 10.1073/pnas.1130426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans JT, Smith EG, Banerjee A, Smith RM, Dale J, Innes JA, et al. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. Lancet. 2007;369:1270–1276. doi: 10.1016/S0140-6736(07)60598-4. [DOI] [PubMed] [Google Scholar]
- 58.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. 2018;215:1135–1152. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simmons JD, Stein CM, Seshadri C, Campo M, Alter G, Fortune S, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol. 2018;18:575–589. doi: 10.1038/s41577-018-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khader SA, Divangahi M, Hanekom W, Hill PC, Maeurer M, Makar KW, et al. Bill and Melinda Gates Foundation Collaboration for TB Vaccine Discovery Innate Immunity Working Group18. Targeting innate immunity for tuberculosis vaccination. J Clin Invest. 2019;129:3482–3491. doi: 10.1172/JCI128877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews JR, Hatherill M, Mahomed H, Hanekom WA, Campo M, Hawn TR, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med. 2015;191:584–591. doi: 10.1164/rccm.201409-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elkington P, Lerm M, Kapoor N, Mahon R, Pienaar E, Huh D, et al. In vitro granuloma models of tuberculosis: potential and challenges. J Infect Dis. 2019;219:1858–1866. doi: 10.1093/infdis/jiz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tameris M, Geldenhuys H, Luabeya AK, Smit E, Hughes JE, Vermaak S, et al. The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS One. 2014;9:e87340. doi: 10.1371/journal.pone.0087340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.