Abstract
Rationale: Chronic azithromycin is commonly used in cystic fibrosis based on short controlled clinical trials showing reductions in pulmonary exacerbations and improved FEV1. Long-term effects are unknown.
Objectives: Examine pulmonary outcomes among chronic azithromycin users compared with matched controls over years of use and consider combined azithromycin use in cohorts using chronic inhaled tobramycin or aztreonam.
Methods: This retrospective cohort study used the U.S. cystic fibrosis Foundation Patient Registry. Incident chronic azithromycin users were compared with matched controls by FEV1% predicted rate of decline and rates of intravenous antibiotic use to treat pulmonary exacerbations. Propensity score methods were utilized to address confounding by indication. Predefined sensitivity analyses based on lung function, Pseudomonas aeruginosa (PA) status, and follow-up time intervals were conducted.
Measurements and Main Results: Across 3 years, FEV1% predicted per-year decline was nearly 40% less in those with PA using azithromycin compared with matched controls (slopes, −1.53 versus −2.41% predicted per yr; difference: 0.88; 95% confidence interval [CI], 0.30–1.47). This rate of decline did not differ based on azithromycin use in those without PA. Among all cohorts, use of intravenous antibiotics was no different between azithromycin users and controls. Users of inhaled tobramycin and azithromycin had FEV1% predicted per-year decline of −0.16 versus nonusers (95% CI, −0.44 to 0.13), whereas users of inhaled aztreonam lysine and azithromycin experienced a mean 0.49% predicted per year slower decline than matched controls (95% CI, −0.11 to 1.10).
Conclusions: Results from this study provide additional rationale for chronic azithromycin use in PA-positive patients to reduce lung function decline.
Keywords: azithromycin, cystic fibrosis, long-term outcomes, tobramycin, Pseudomonas aeruginosa
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic azithromycin is one of the most common therapies used by people with cystic fibrosis in the United States. The long-term health impact of this therapy, which has been used for many years, is largely unknown.
What This Study Adds to the Field
Using the U.S. CF Foundation Patient Registry, we find that those with Pseudomonas aeruginosa infection had slower decline in FEV1% predicted when using chronic azithromycin. We do not find this benefit in those without P. aeruginosa infection and do not find an impact on the need for intravenous antibiotics. Users of inhaled tobramycin appear to have less benefit from chronic azithromycin than those not using inhaled tobramycin.
Azithromycin (AZM) is a macrolide antibiotic that has proven health benefits as a chronic therapy in a variety of lung diseases through both antimicrobial and postulated immunomodulatory effects (1–6). Perhaps the most frequent users of chronic AZM therapy are those with cystic fibrosis (CF), a genetic condition affecting several organ systems. People with CF have impaired mucociliary clearance complicated by chronic, robust inflammation and persistent bacterial infection in the airways (7). Largely based on health benefits observed in people with diffuse panbronchiolitis who were treated with erythromycin (8–10), therapeutic trials and subsequent controlled clinical trials were conducted in people with CF to determine whether AZM would improve lung function and to assess other measures of pulmonary or general health (11–15). These studies clearly demonstrated health gains for at least six months. Initial studies focused on people with CF who had persistent airway infection with Pseudomonas aeruginosa (PA). Later studies included people without PA and found a reduced risk of acute pulmonary exacerbation (PEx) but no significant impact on lung function measured by spirometry (e.g., FEV1) (12, 16, 17).
Based on supportive study results and endorsement in clinical care guidelines, approximately 70% of people with CF and PA airway infection in the United States are currently prescribed chronic AZM as part of a maintenance therapy regimen (18). Fewer but still significant numbers of those without PA are also prescribed this drug. Although AZM is one of the most commonly prescribed chronic pulmonary medications, little is known about the long-term health impacts of macrolide therapy in CF. Specifically, virtually no data are available to judge the durability of health gains observed in the pivotal clinical trials, most of which lasted six months (19). Recent single-center reports with relatively small numbers of patients suggest that health gains from chronic AZM may be lost beyond the first year of use (20, 21).
Estimating the long-term effects of AZM (and virtually any chronic CF therapy) is more challenging than may be immediately recognized because clinical use of such interventions is confounded by indication bias based on disease status and other factors. In addition, prospective clinical trials of necessary duration (i.e., multiple years) are not easily justified or feasible in a rare disease like CF. However, a robust U.S. CF Foundation Patient Registry (CFFPR) exists and captures both therapy use and key clinical outcome measures at more than 130 CF care centers (22). The size of this registry (nearly 30,000 patients, ≥95% of the U.S. patient population), the formal validation of key data variables (22), the longstanding use of AZM in the United States, and the careful analytical methods allow one to address indication bias in considering the long-term impact of this common medication. In doing so, we applied predefined analytic methods to adjust for confounding and indication bias in registry data and analyzed the relationship between initiating chronic AZM use and pulmonary outcomes including decline in lung function measured by FEV1% predicted (FEV1pp) and the use of intravenous antibiotics to treat acute PEx. Furthermore, to build on related research, we examined pulmonary outcomes in cohorts using inhaled tobramycin (TOB) or inhaled aztreonam lysine (AZLI), hypothesizing lesser gains when chronic AZM and TOB use were combined (23–25). Some results of these studies have been reported previously in the form of an abstract (26).
Methods
Population and Variables
Four prespecified, retrospective, CFFPR cohort analyses were conducted to compare outcomes among chronic AZM users with control participants who were matched by propensity score (PS) to PA-positive (cohort 1) and PA-negative (cohort 2) patients with a period of incident chronic AZM use. Separately, PS-matched cohort analyses were conducted to compare outcomes among patients with chronic use of inhaled TOB (cohort 3) or inhaled AZLI (cohort 4). The study was approved by the Seattle Children’s Hospital Institutional Review Board and the CF Foundation Patient Registry Committee.
For all cohorts, exclusion criteria included age younger than 6 years (to improve quality of spirometry data), AZM initiation after age 40 years (extreme phenotype), any respiratory culture positivity for nontuberculous mycobacteria, solid organ transplantation, and monotherapy ivacaftor CFTR modulator usage at baseline or during follow-up. For inclusion, antibiotics were summarized into age-quarters. Because AZM use is common and can be used sporadically, we defined the groups of interest as low and high AZM use to differentiate outcomes related to chronic use. High AZM was defined as at least 2 age-quarters per year indicating AZM use. Low AZM was defined as no more than 1 quarter per year reporting any use with at least 2 age-quarters with nonmissing data per year. For cohorts 1 and 2, incident chronic AZM was defined by at least 2 years of low AZM followed by 3 consecutive years of high AZM, and comparison controls were identified by 5 consecutive years satisfying low AZM criteria (Figure E1A in the online supplement). PA-positive respiratory culture was defined as a positive respiratory culture in ≥3 age-quarters in the initial 2-year period for each person (8 quarters) with at least 2 age-quarters of nonmissing culture results per year (Figure E1A) and PA negative otherwise (27).
Indicated use of either inhaled TOB (any formulation, including generic) or AZLI for at least 2 age-quarters in a 12-month reporting period determined cohorts 3 and 4, respectively. Any 12-month period with both TOB and AZLI use was excluded. Eligible periods were a minimum of 1-year follow-up (Figure E1B).
PS Matching
PSs were computed by logistic regression with 14 variables that are well recognized as potentially affecting the outcomes of interest: age, baseline FEV1pp, sex, mutation class, race/ethnicity, body mass index, CF-related diabetes, CF liver disease, number of PEx in the previous 12 months, pancreatic enzyme use, dornase alfa use, hypertonic saline use, insurance category, and calendar year at baseline. Consistent with guidelines, these variables were identified by the investigators before analyses and were based on complete observations (see online supplement for details) (28). Exact matching occurred for age groupings and FEV1pp calculated using the Global Lung Index equations (29). An indicator of any CFTR modulator use during follow-up was added to the PS model to account for imbalance in this time-varying confounder. PS distributions were visually inspected, and baseline demographics for unmatched and matched cohorts were summarized to evaluate PS performance.
Statistics
Trend in lung function over time since baseline (start of chronic AZM use or start of chronic inhaled TOB or AZLI) was estimated and compared between AZM users and matched controls by linear mixed-effects models with random intercepts and slopes. This approach was applied to all FEV1pp measurements obtained during clinic visits and limited to maximum FEV1pp in a year (yearly max).
The rate ratio of PEx treated by intravenous antibiotics per year was estimated and compared by negative binomial generalized estimating equations models (to accommodate overdispersion). For the incident chronic AZM cohorts, PEx-free survival was analyzed using Kaplan-Meier curves and Cox proportional hazards regression. To compare with the study of PA-positive incident chronic AZM, post hoc sensitivity analyses restricted inhaled TOB and AZLI to those meeting PA-positive criteria and with at least 2 years of follow-up. Analyses were conducted in SAS version 9.4 (SAS Institute) and R version 3.4.0 (30), with the RStudio environment. R packages used include haven, MatchIT (31), ggplot, lme4, and geepack.
Results
Chronic AZM and Controls
Among 22,338 persons with CF aged 6 to 40 years in the CFFPR between 2006 and 2016 with at least 5 years of follow-up, 8,177 had a qualified 5 consecutive years defined as initiating chronic AZM or control. Selection of cohorts 1 and 2 is shown in Figure E2A. After exclusions, 1,474 PA-positive and 3,241 PA-negative patients remained (characteristics of unmatched cohorts provided in Table E1). PA status was intermediate or unknown for 1,786 participants, and they were excluded. PS matching across 14 predefined variables reduced the samples to 752 and 814 among PA-positive and PA-negative cohorts, respectively (evenly split between AZM and control). PS models discriminated between AZM and control subgroups with sufficient score overlap to allow for matching (Figure E3).
Baseline characteristics of incident chronic AZM-matched groups are summarized in Table 1. Among the PA-positive groups, the mean age at baseline was 18, and the mean best FEV1pp in the prior 6 months was 82%; 53% were female, 89% reported dornase alfa use, and 48% reported hypertonic saline use at baseline. PA-negative groups were younger (mean age, 13 yr), with mean best FEV1pp in the prior 6 months of 92%.
Table 1.
Cohort Demographics for Incident Chronic Use of Azithromycin by PA Status, Matched
| PA Positive, Matched |
PA Negative, Matched |
|||
|---|---|---|---|---|
| Control (n = 376) | AZM (n = 376) | Control (n = 407) | AZM (n = 407) | |
| Best 6-mo FEV1pp, mean (SD)* | 81.5 (21.8) | 81.9 (21.2) | 92.7 (17.8) | 92.1 (17.1) |
| Baseline age, mean (SD)* | 18.4 (7.5) | 18.16 (7.3) | 13.4 (4.8) | 13.3 (4.7) |
| Any lumacaftor/ivacaftor use | 24 (6.4) | 23 (6.1) | 21 (5.2) | 23 (5.7) |
| PE prior 12 mo | ||||
| 0 | 214 (56.9) | 209 (55.6) | 287 (70.5) | 284 (69.8) |
| 1–2 | 141 (37.5) | 141 (37.5) | 112 (27.5) | 109 (26.8) |
| ≥3 | 21 (5.6) | 26 (6.9) | 8 (2.0) | 14 (3.4) |
| Sex, M | 177 (47.1) | 178 (47.3) | 197 (48.4) | 194 (47.7) |
| Nonwhite or Hispanic | 53 (14.1) | 48 (12.8) | 0.14 (0.35) | 0.15 (0.36) |
| Mutation class | ||||
| 1–3 | 320 (85.1) | 316 (84.0) | 325 (79.9) | 317 (77.9) |
| 4–5 | 13 (3.5) | 14 (3.7) | 17 (4.2) | 25 (6.1) |
| Other | 43 (11.4) | 46 (12.2) | 65 (16.0) | 65 (16.0) |
| Baseline year in 2011–2014† | 172 (45.7) | 167 (44.4) | 209 (51.4) | 197 (48.4) |
| Dornase alfa | 333 (88.6) | 339 (90.2) | 352 (86.5) | 353 (86.7) |
| Pancreatic enzymes | 335 (89.1) | 329 (87.5) | 359 (88.2) | 347 (85.3) |
| CF liver disease | 39 (10.4) | 33 (8.8) | 22 (5.4) | 27 (6.6) |
| CF-related diabetes | 70 (18.6) | 62 (16.5) | 31 (7.6) | 32 (7.9) |
| Hypertonic saline | 182 (48.4) | 179 (47.6) | 205 (50.4) | 212 (52.1) |
| Insurance | ||||
| Private | 254 (67.6) | 248 (66.0) | 281 (69.0) | 273 (67.1) |
| Medicare | 6 (1.6) | 10 (2.7) | 2 (0.5) | 3 (0.7) |
| Medicaid | 102 (27.1) | 104 (27.7) | 104 (25.6) | 109 (26.8) |
| No insurance | 14 (3.7) | 14 (3.7) | 20 (4.9) | 22 (5.4) |
| BMI percentile (aged <20 yr), mean (SD) | 47.9 (26.5) | 46.7 (27.6) | 49.0 (27.6) | 47.8 (26.4) |
| BMI (aged ≥20 yr), mean (SD) | 21.9 (3.1) | 22.2 (3.5) | 23.2 (4.2) | 23.3 (4.2) |
Definition of abbreviations: AZM = azithromycin; BMI = body mass index; CF = cystic fibrosis; FEV1pp = FEV1% predicted; PA = Pseudomonas aeruginosa; PE = pulmonary exacerbation events.
Data provided as n (%) unless otherwise specified.
Exact matching within age groups (6–35, 36–40 yr) and within groups by best FEV1pp in the past 6 mo (<60%, 60% to <80%, 80% to <90%, 90% to <100%, ≥100%).
Reference group: baseline year 2007–2010.
Decline in FEV1pp
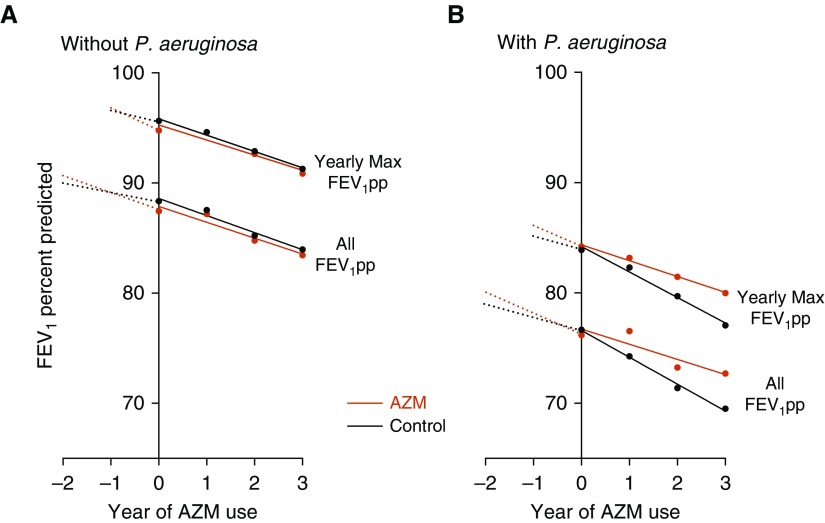
The decline in FEV1pp per year across 3 years among PA-positive patients initiating chronic AZM was significantly less compared with matched controls (slopes, −1.53 vs. −2.41% predicted per year [pp/yr]; difference, 0.88; 95% confidence interval [CI], 0.30–1.47; P = 0.003) (Figure 1A and Table E3). As a sensitivity analysis, the difference in slope of the yearly max FEV1pp was 0.81 (95% CI, 0.33–1.28; P = 0.001). This represents a 37% slower rate of decline associated with AZM use.
Figure 1.
(A and B) FEV1% predicted (FEV1pp) over 3 years (using all data and, separately, only yearly maximum) after incident chronic azithromycin (AZM) versus control among those without Pseudomonas aeruginosa (A) and those with P. aeruginosa (B). Estimates for all data were derived from the model allowing individual trends per year.
Among PA-negative patients, decline in FEV1pp per year across 3 years in those initiating chronic AZM was similar to matched controls (slopes, −1.46 vs. −1.70 pp/yr; difference, 0.24; 95% CI, −0.32 to 0.79; P = 0.40). Yearly max FEV1pp difference in slope was 0.23 (95% CI, −0.25 to 0.70; P = 0.35) (Figure 1B and Table E3). FEV1pp decline for the complete, unmatched cohort is also reported Table E3 and shows similar results.
Using the PS-matched cohorts, we also considered the trend in FEV1pp in the 2 years before the point of matching at initiation of chronic AZM (Figure 1, dotted lines). The AZM groups had faster rates of decline before initiating AZM compared with the matched population that did not start AZM.
Need for intravenous antibiotics to treat acute pulmonary exacerbations
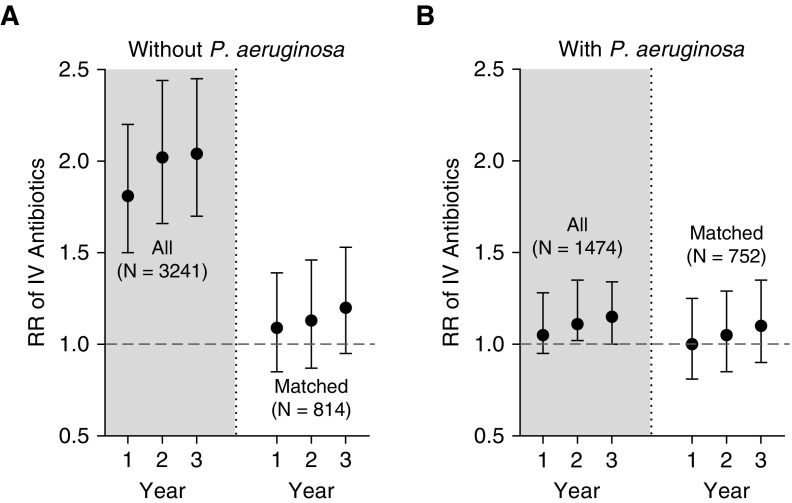
In PS-matched cohorts (Figure 2 unshaded regions), similar rates of intravenously treated PEx occurred in the first year (PA-positive AZM users: relative risk [RR], 1.004; 95% CI, 0.81–1.25; and PA-negative AZM users: RR, 1.09; 95% CI, 0.85–1.39). This RR increased to slightly higher rates of intravenously treated PEx in chronic AZM patients at 3 years after baseline, but the CIs are fairly wide around these estimates (PA-positive AZM users: RR, 1.11; 95% CI, 0.9–1.35; and PA-negative AZM users: RR, 1.20; 95% CI, 0.95–1.53) (Figures 2A and 2B). Similarly, no difference was observed in time to first PEx (PA-positive AZM users: hazard ratio, 1.12; 95% CI, 0.94–1.34; and PA-negative AZM users: hazard ratio, 1.08; 95% CI, 0.89–1.30).
Figure 2.
Relative risk (RR) of pulmonary exacerbations treated with intravenous (IV) antibiotics among those with incident chronic use of azithromycin (AZM) versus controls. Shaded regions show all identified patients (not propensity score matched), and unshaded areas show the propensity score–matched cohorts. (A) Those without Pseudomonas aeruginosa and (B) those with P. aeruginosa. Figure represents high AZM users relative to low AZM users, and RR > 1.0 indicates greater use of IV antibiotics.
Note that shaded regions of Figure 2 represent the unmatched data, showing greater risk of PEx among AZM users versus controls. This result underscores the anticipated indication bias for AZM use because the PS-matched analysis (unshaded regions) shows no increased risk.
AZM Use among Chronic Inhaled TOB or AZLI Users
Among 21,793 patients in the CFFPR during 2010–2016 who were aged 6 years or older with any TOB or AZLI use, 9,658 and 2,888, respectively, had a qualified window (Figure E2B), with criteria as illustrated in Figure E1B. After exclusions, 7,990 and 2,449 remained in the TOB and AZLI cohorts, respectively (characteristics of unmatched cohorts provided in Table E2). PS matching reduced cohort sizes to 3,902 for TOB (1,951 high AZM and 1,951 low AZM) and 872 for AZLI (436 high AZM and 436 low AZM).
Baseline characteristics of matched groups for inhaled antibiotics are described in Table 2. Among inhaled TOB pairs, mean age was lower and mean FEV1pp was higher compared with matched AZLI pairs.
Table 2.
Cohort Demographics for Users of Inhaled TOB or AZLI, Matched (≥1-Year Follow-up)
| Inhaled TOB |
Inhaled AZLI |
|||
|---|---|---|---|---|
| Low AZM (n = 1,951) | High AZM (n = 1,951) | Low AZM (n = 436) | High AZM (n = 436) | |
| Follow-up years, mean (SD) | 2.46 (1.53) | 2.68 (1.60) | 2.06 (1.22) | 2.29 (1.26) |
| Baseline age, mean (SD)* | 16.7 (10.26) | 16.8 (9.89) | 23.0 (13.6) | 23.2 (13.6) |
| Best 6-mo FEV1pp, mean (SD)* | 84.1 (21.4) | 84.2 (21.0) | 75.8 (24.4) | 75.0 (24.9) |
| Any lumacaftor/ivacaftor use | 258 (13.2) | 266 (13.6) | 65 (14.9) | 62 (14.2) |
| PE prior 12 mo | ||||
| 0 | 1,220 (62.5) | 1,202 (61.6) | 231 (53.0) | 221 (50.7) |
| 1–2 | 631 (32.3) | 643 (33.0) | 170 (39.0) | 184 (42.2) |
| ≥3 | 100 (5.1) | 106 (5.4) | 35 (8.0) | 31 (7.1) |
| Sex, M | 994 (50.9) | 1,016 (52.1) | 195 (44.7) | 184 (42.2) |
| Nonwhite or Hispanic | 335 (17.2) | 342 (17.5) | 49 (11.2) | 56 (12.8) |
| Mutation class | ||||
| 1–3 | 1,562 (80.1) | 1,594 (81.7) | 357 (81.9) | 350 (80.3) |
| 4–5 | 85 (4.4) | 71 (3.6) | 22 (5.0) | 31 (7.1) |
| Other | 278 (14.2) | 264 (13.5) | 57 (13.1) | 55 (12.6) |
| Baseline year in 2013–2016† | 702 (36.0) | 666 (34.1) | 202 (46.3) | 189 (43.3) |
| Dornase alfa | 1,687 (86.5) | 1,720 (88.2) | 375 (86.0) | 384 (88.1) |
| Pancreatic enzymes | 1,716 (88.0) | 1,731 (88.7) | 392 (89.9) | 401 (92.0) |
| CF liver disease | 154 (7.9) | 141 (7.2) | 45 (10.3) | 35 (8.0) |
| CF-related diabetes | 240 (12.3) | 225 (11.5) | 101 (23.2) | 93 (21.3) |
| Hypertonic saline | 1,005 (51.5) | 1,058 (54.2) | 262 (60.1) | 293 (67.2) |
| Insurance | ||||
| Private | 1,146 (58.7) | 1,174 (60.2) | 295 (67.7) | 285 (65.4) |
| Medicare | 66 (3.4) | 66 (3.4) | 21 (4.8) | 26 (6.0) |
| Medicaid | 638 (32.7) | 604 (31.0) | 89 (20.4) | 96 (22.0) |
| Other/no insurance | 101 (5.2) | 107 (5.5) | 31 (7.1) | 29 (6.7) |
| BMI percentile (aged <20 yr), mean (SD) | 49.4 (27.4) | 49.5 (26.8) | 48.8 (27.4) | 50.9 (26.1) |
| BMI (aged ≥20 yr), mean (SD) | 22.6 (3.9) | 22.8 (3.4) | 23.1 (4.0) | 22.8 (3.7) |
Definition of abbreviations: AZLI = aztreonam lysine; AZM = azithromycin; BMI = body mass index; CF = cystic fibrosis; FEV1pp = FEV1 percentage predicted; PE = pulmonary exacerbation events; TOB = tobramycin.
Data provided as n (%) unless otherwise specified.
Exact matching within age groups (6–35, 36–40 yr) and within groups for best FEV1pp in the past 6 mo (<60%, 60% to <80%, 80% to <90%, 90% to <100%, ≥100%).
Reference group: baseline year 2010–2012.
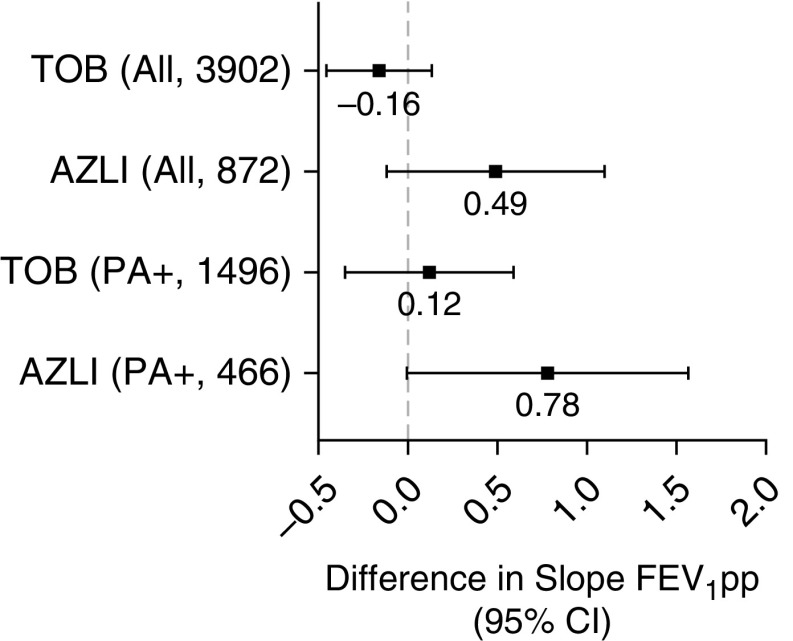
Lung function change and use of intravenous antibiotics among matched groups of TOB or AZLI users are summarized in Table 3 and represented in Figure 3. Among TOB users, AZM use did not associate with slower decline in FEV1pp/year (difference in slope, −0.16 FEV1pp/yr; 95% CI, −0.44 to 0.13) (Table 3 and Figure 3). Fewer people were identified as using AZLI alone, but in this group, AZM use generally associated with slower rate of decline in lung function (difference in slope, 0.49; 95% CI, −0.11 to 1.10; using yearly max FEV1pp, difference was 0.70; 95% CI, 0.01–1.39). AZM use was not associated with intravenous antibiotic treatment for PEx in either group. Notably, not all users of chronic inhaled antibiotics met our microbiological criteria for PA-positive status. Among those who did, outcomes were similar (Table E4).
Table 3.
FEV1 Rate of Change and Pulmonary Exacerbation Outcomes among Cohorts 3 and 4: Chronic Inhaled Antipseudomonal Tobramycin or Aztreonam Lysine for Low and High Azithromycin Usage
| TOB (n = 3,906) | AZLI (n = 872) | |
|---|---|---|
| FEV1pp, slope/yr (all data) | ||
| Low AZM | −1.73 | −2.21 |
| High AZM | −1.88 | −1.72 |
| High AZM − low AZM | −0.16 | 0.49 |
| SE for high AZM − low AZM | 0.15 | 0.31 |
| 95% CI | −0.44 to 0.13 | −0.11 to 1.10 |
| P value | 0.287 | 0.109 |
| FEV1pp, slope/yr (yearly maximum) | ||
| Low AZM | −2.69 | −3.05 |
| High AZM | −2.75 | −2.35 |
| High AZM − low AZM | −0.06 | 0.70 |
| SE for high AZM − low AZM | 0.15 | 0.35 |
| 95% CI | −0.35 to 0.24 | 0.01–1.39 |
| P value | 0.706 | 0.049 |
| Intravenous antibiotic–treated PEx | ||
| Low AZM, mean PEx rate in 12 mo | 0.68 | 0.85 |
| High AZM, mean PEx rate in 12 mo | 0.68 | 0.83 |
| Rate ratio | 1.00 | 0.98 |
| 95% CI | 0.91–1.11 | 0.82–1.18 |
| P value | 0.952 | 0.838 |
Definition of abbreviations: AZLI = aztreonam lysine; AZM = azithromycin; CI = confidence interval; FEV1pp = FEV1% predicted; PEx = pulmonary exacerbations; TOB = tobramycin.
Bold highlights the difference in high versus low AZM.
Figure 3.
Difference in FEV1% predicted (FEV1pp) slopes between high and low azithromycin among concomitant users of chronic inhaled TOB or chronic inhaled AZLI. Data shown for all individuals with ≥1 years of follow-up and, separately, among only those meeting the definition of Pseudomonas aeruginosa positive. Mean estimates and 95% confidence intervals are shown. Positive numbers reflect slower rate of decline in FEV1pp. AZLI = aztreonam lysine; CI = confidence interval; PA+ = Pseudomonas aeruginosa positive; TOB = tobramycin.
Discussion
Through this careful analysis of a retrospective data registry, we observe for the first time that CF patients who are chronically infected with PA experience a significantly slower loss in lung function over a 3-year period after initiation of chronic AZM compared with similar patients who do not use this therapy. Those with PA infection were the initial population studied in clinical trials of AZM and those for whom the greatest health benefits have been reported (14). Prospective placebo-controlled clinical trials in those with PA demonstrated that AZM use for 6 months improved FEV1 and lowered the risk of PEx (14). In our retrospective analyses of those meeting our definition of PA positive, we found a 37% reduced rate of decline in FEV1pp over a 3-year period in chronic users of AZM compared with a similar group of controls. This pattern of apparent clinical benefit is also reflected in yearly max FEV1pp as an alternative to all FEV1 data. Reducing the rate of decline in FEV1 is seen as an important goal linked to disease progression and eventual morbidity or mortality in CF (32). The AZM group, before initiating this therapy, was experiencing faster decline in FEV1pp than the comparative control group. If anything, this finding strengthens our interpretation that chronic AZM is beneficial in those with PA infection and reduces the likelihood that our results are due to unmeasured confounding or indication bias.
The beneficial impact on PEx that was reported in clinical trials was not seen in our study; however, there are important differences in how these data were collected. The trial by Saiman and colleagues defined PEx events by hospitalization or ≥7 days of oral quinolone antibiotics (14). The CFFPR has collected high-quality data on intravenous antibiotic use for many years, and our analyses are limited to only patients treated with intravenous therapy. Use of oral antibiotics outside of clinical encounters and, at times, without direct knowledge of CF providers can be more difficult to track and report, even in a registry as robust as the CFFPR. We cannot comment, from these analyses, on the sustained impact that AZM may have on the use of oral or inhaled antibiotic therapy to treat acute pulmonary worsening.
In those not chronically infected with PA at baseline, we do not observe the association between AZM use and preserved lung function over 3 years of medication use. Prospective clinical trials of 6-month duration in those without PA similarly found no significant impact on FEV1 but measured a reduced risk of acute PEx or use of oral antibiotics (12, 16). When considering AZM use for 3 years, we did not find a reduced risk of PEx among AZM users who were not chronically infected with PA. Again, our analyses are limited to the use of intravenous antibiotics, and this pivotal trial similarly found no difference in the use of intravenous antibiotics based on AZM use versus placebo. Our analyses of a 3-year observational period are retrospective but able to collect many more intravenous treatment events. Altogether, our findings indicate that chronic AZM may have little to no impact on the risk of severe PEx requiring intravenous antibiotics regardless of PA status.
Retrospective analyses and in vitro work have provided some evidence of a suspected adverse drug interaction between AZM and TOB in the CF population chronically infected with PA (23–25, 33, 34). For this reason, we extended our work to consider the impact of chronic AZM in those prescribed chronic inhaled TOB and compared these results with those of patients prescribed chronic inhaled AZLI. Consistent with our hypothesis of a selective drug interaction, the subgroup of patients prescribed chronic inhaled TOB and using AZM did not have a slower rate of decline in FEV1pp. This result was unchanged when we considered only users of TOB who met the PA-positive definition and is unlike our findings when considering incident AZM in all PA-positive patients regardless of inhaled antibiotic use. Those prescribed inhaled AZLI had evidence of a slower rate of decline based on sensitivity analysis or when restricted to people meeting the PA-positive definition (22% slower decline in mean FEV1pp [P value not significant]; 23% slower in yearly max FEV1pp [P < 0.05]; 31% slower when restricted to PA-positive definition [P = 0.05]). We recognize that this analysis was not focused on incident or first AZM use and that the baseline characteristics of the TOB and AZLI cohorts differed. Patients are increasingly cycling between TOB and AZLI, and such data were necessarily excluded from our analyses, which may limit generalizability (35). Nonetheless, our results are consistent with a growing body of evidence supporting the hypothesis of a selective drug interaction between AZM and TOB, but this question may not be fully addressed until the ongoing prospective clinical trial testing this theory is completed (ClinicalTrials.gov identifier NCT02677701).
Indication bias for AZM use based on clinical disease is the greatest challenge when trying to measure long-term therapeutic effectiveness using data collected through a patient registry. This challenge is readily apparent in our analyses, as demonstrated by baseline health indexes and the trends in lung function and use of intravenous antibiotics. Without careful analytic methods to match users and nonusers of AZM, AZM use associates with worse health outcomes compared with nonusers. This result is clearly demonstrated in the unmatched data shown in Figure 2. Once strict matching methods are applied through PSs using more than 12 key clinical variables, highly similar comparative populations are organized, and clinical benefit associated with AZM long-term use in those with chronic PA becomes apparent. This indication bias for treatment use in those with more advanced or aggressive disease would bias toward the null in our consideration of improved health outcomes associated with AZM use. If residual confounding persists, then it would be likely to reduce our positive findings.
We also acknowledge several potential weaknesses or limitations in this approach. We applied a conservative PS-matching algorithm that utilized clinical variables believed to associate with health status and risk of decline. This approach produced comparative cohorts that were closely balanced at baseline; indeed, this analytic approach is often referred to as simulated randomization because it allows one to utilize a large data set and develop matched, balanced populations when considering the impact of an intervention. Although effective in addressing indication bias for medication use, this approach comes with two key costs—drastic reduction in the size of the analytic data sets and disproportionate elimination of those with larger effects—that can mute the overall observation. In developing our statistical analysis plan, we intentionally prioritized the need to control for indication bias, even at the expense of a large analytic data set that may be more generalizable. One could use a less conservative matching algorithm if concerns for indication bias were lower. We reported results from unmatched analyses for comparison (see the online supplement).
Some have argued for alternative analytic methods, such as instrumental variables, when approaching similar research questions (36). Although such methods are useful to address unmeasured confounding, one must be able to identify a variable that reliably predicts a practice pattern (e.g., prescription of AZM) but does not affect the outcome measure (e.g., preservation of lung function). The most commonly applied variable is the care center where patients are treated, which has been used successfully in studies outside and, more recently, within CF (36). We considered this method for researching the long-term effects of AZM but believe strongly that care center fails both requirements as an instrumental variable in this scenario. For several reasons, including dissimilar provider preferences within a center, not all patients at one center will be prescribed AZM based on a set of equally applied criteria. In addition, aspects of a center’s care practice that are not linked to AZM use are likely to affect our outcome measures of interest—namely, preservation of lung function and the decision to treat with intravenous antibiotics. We conducted post hoc analyses to test the impact of care center as a random effect and observed that the addition of care center did not diminish but rather modestly strengthened the association between AZM use and slower rate of decline in those with PA–positive infection (difference, 0.93 pp/yr; P = 0.002). This approach accounts for correlated FEV1 outcomes within care centers. That said, a potential for unmeasured confounding or residual indication bias is a recognized limitation in our approach, even when including a large number of variables in the PS model. PS weighting (rather than matching) could have incorporated more of those patients left out; however, we felt PS matching best addressed the research question at hand and provided distinct cohorts of patients that could be summarized concisely.
Registry-based studies such as this one are complicated by missing or incorrect data and a need to prespecify complex algorithms to define high, low, and chronic medication use and microbiology using moving windows of time. We chose definitions that allowed for limited missing data, and we conducted a number of sensitivity analyses to assess impact of definition or subset on outcomes. We were motived by the need for high confidence in AZM use status while preserving as many participants as possible for analyses. The decision to use high versus low rates of AZM use rather than categorical always versus never use was important to maintain an adequately large data set but may also bias toward the null when considering the impact of AZM on health outcomes. Less than complete adherence to prescribed AZM may also reduce the effect size. Both factors increase confidence in our positive finding (i.e., slower decline in FEV1pp in those with PA) but may have limited other analyses. The PA-negative group was younger and healthier, and this may also be important when interpreting our findings.
In summary, these data add to the existing literature supporting chronic AZM therapy in people with CF chronically infected with PA. Our findings significantly reinforce prior observations from clinical trials reporting no link between AZM use and use of intravenous antibiotics. We are unable to address how years of AZM use may affect the use of acute oral or inhaled therapy, and we find no significant impact on lung function decline in people not infected with PA. A reduction in the rate of decline in lung function (FEV1pp) in those infected with PA by 37% is notable, and we are pleased to find such benefit when considering long-term use of a common treatment option. We cannot extrapolate what may occur beyond three years but see this finding as a significant contribution to results from studies generally limited to several months in length. The association between inhaled TOB use and an apparent loss of effect with AZM is concerning and consistent with ongoing work to better understand this potential adverse drug interaction.
Few would argue against the importance of studying long-term clinical impacts of chronic therapies and how drug combinations may interact in real-world use. When the strengths and limitations of robust patient registries are understood, these tools can fundamentally enable this type of research, further justifying the significant effort required to establish and maintain such registries.
Supplementary Material
Acknowledgments
Acknowledgment
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct the study and both NIH and Gilead Sciences for financial support. In addition, the authors would like to thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Footnotes
Supported by NHLBI/NIH grant 5R01HL124053, Gilead Sciences Investigator Sponsored Research (D.P.N. and S.L.H.), and NIDDK/NIH grant 5P30DK089507.
Author Contributions: D.P.N., K.O.-D., R.S., and S.L.H. were responsible for the concept, design, data acquisition, analyses, interpretation, and manuscript preparation. M.S. critically contributed to analyses, interpretation, and manuscript review. J.D.C., C.H.G., and C.L.R. contributed importantly to data interpretation, manuscript preparation, and final approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201906-1206OC on October 29, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19–26. doi: 10.1016/S2213-2600(15)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171.e1–1178.e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 4.Herath SC, Normansell R, Maisey S, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2018;10:CD009764. doi: 10.1002/14651858.CD009764.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly C, Chalmers JD, Crossingham I, Relph N, Felix LM, Evans DJ, et al. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst Rev. 2018;3:CD012406. doi: 10.1002/14651858.CD012406.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeersch K, Gabrovska M, Aumann J, Demedts IK, Corhay JL, Marchand E, et al. Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalization (BACE): a multicenter, randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2019;200:857–868. doi: 10.1164/rccm.201901-0094OC. [DOI] [PubMed] [Google Scholar]
- 7.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oda H, Kadota J, Kohno S, Hara K. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest. 1994;106:1116–1123. doi: 10.1378/chest.106.4.1116. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa Y, Ninomiya H, Koga H, Tanaka M, Kinoshita M, Tokunaga N, et al. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Respir Dis. 1992;146:196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Kondo A, Tamura M, Izumi T, Ina Y, Noda M. Long-term therapeutic effects of erythromycin and new quinolone antibacterial agents on diffuse panbronchiolitis [in Japanese] Nihon Kyobu Shikkan Gakkai Zasshi. 1990;28:1305–1313. [PubMed] [Google Scholar]
- 11.Jaffé A, Francis J, Rosenthal M, Bush A. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet. 1998;351:420. doi: 10.1016/S0140-6736(05)78360-4. [DOI] [PubMed] [Google Scholar]
- 12.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 13.Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais JP. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax. 2006;61:895–902. doi: 10.1136/thx.2005.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Macrolide Study Group. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 15.Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet. 2002;360:978–984. doi: 10.1016/s0140-6736(02)11081-6. [DOI] [PubMed] [Google Scholar]
- 16.Saiman L, Mayer-Hamblett N, Anstead M, Lands LC, Kloster M, Goss CH, et al. AZ0004 Macrolide Study Team. Open-label, follow-on study of azithromycin in pediatric patients with CF uninfected with Pseudomonas aeruginosa. Pediatr Pulmonol. 2012;47:641–648. doi: 10.1002/ppul.21601. [DOI] [PubMed] [Google Scholar]
- 17.Ratjen F, Saiman L, Mayer-Hamblett N, Lands LC, Kloster M, Thompson V, et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest. 2012;142:1259–1266. doi: 10.1378/chest.12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 19.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD002203. doi: 10.1002/14651858.CD002203.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samson C, Tamalet A, Thien HV, Taytard J, Perisson C, Nathan N, et al. Long-term effects of azithromycin in patients with cystic fibrosis. Respir Med. 2016;117:1–6. doi: 10.1016/j.rmed.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Willekens J, Eyns H, Malfroot A. How long should we maintain long-term azithromycin treatment in cystic fibrosis patients? Pediatr Pulmonol. 2015;50:103–104. doi: 10.1002/ppul.22981. [DOI] [PubMed] [Google Scholar]
- 22.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 23.Somayaji R, Russell R, Cogen JD, Goss CH, Nick SE, Saavedra MT, et al. Oral azithromycin use and the recovery of lung function from pulmonary exacerbations treated with intravenous tobramycin or colistimethate in adults with cystic fibrosis. Ann Am Thorac Soc. 2019;16:853–860. doi: 10.1513/AnnalsATS.201811-773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols DP, Happoldt CL, Bratcher PE, Caceres SM, Chmiel JF, Malcolm KC, et al. Impact of azithromycin on the clinical and antimicrobial effectiveness of tobramycin in the treatment of cystic fibrosis. J Cyst Fibros. 2017;16:358–366. doi: 10.1016/j.jcf.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nick JA, Moskowitz SM, Chmiel JF, Forssén AV, Kim SH, Saavedra MT, et al. Azithromycin may antagonize inhaled tobramycin when targeting Pseudomonas aeruginosa in cystic fibrosis. Ann Am Thorac Soc. 2014;11:342–350. doi: 10.1513/AnnalsATS.201310-352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odem-Davis K, Ma S, Somayaji R, Cogen J, Heltshe SL, Nichols D. Long-term azithromycin use: rate of FEV1 decline and risk of exacerbation in CF patients receiving inhaled antipseudomonal antibiotics [abstract 539] Pediatr Pulmonol. 2018;53:354. [Google Scholar]
- 27.Crull MR, Somayaji R, Ramos KJ, Caldwell E, Mayer-Hamblett N, Aitken ML, et al. Changing rates of chronic Pseudomonas aeruginosa infections in cystic fibrosis: a population-based cohort study. Clin Infect Dis. 2018;67:1089–1095. doi: 10.1093/cid/ciy215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 29.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the Global Lung Function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available from: https://www.R-project.org/
- 31.Ho D, Imai K, King G, Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236. [Google Scholar]
- 32.Szczesniak R, Heltshe SL, Stanojevic S, Mayer-Hamblett N. Use of FEV1 in cystic fibrosis epidemiologic studies and clinical trials: a statistical perspective for the clinical researcher. J Cyst Fibros. 2017;16:318–326. doi: 10.1016/j.jcf.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols DP, Caceres S, Caverly L, Fratelli C, Kim SH, Malcolm K, et al. Effects of azithromycin in Pseudomonas aeruginosa burn wound infection. J Surg Res. 2013;183:767–776. doi: 10.1016/j.jss.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klingel M, Stanojevic S, Tullis E, Ratjen F, Waters V. The effect of oral azithromycin on the response to pulmonary exacerbations treated with intravenous tobramycin in children with cystic fibrosis. Ann Am Thorac Soc. 2019;16:861–867. doi: 10.1513/AnnalsATS.201811-774OC. [DOI] [PubMed] [Google Scholar]
- 35.Nichols DP, Durmowicz AG, Field A, Flume PA, VanDevanter DR, Mayer-Hamblett N. Developing inhaled antibiotics in cystic fibrosis: current challenges and opportunities. Ann Am Thorac Soc. 2019;16:534–539. doi: 10.1513/AnnalsATS.201812-863OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanDyke RD, McPhail GL, Huang B, Fenchel MC, Amin RS, Carle AC, et al. Inhaled tobramycin effectively reduces FEV1 decline in cystic fibrosis: an instrumental variables analysis. Ann Am Thorac Soc. 2013;10:205–212. doi: 10.1513/AnnalsATS.201209-082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.