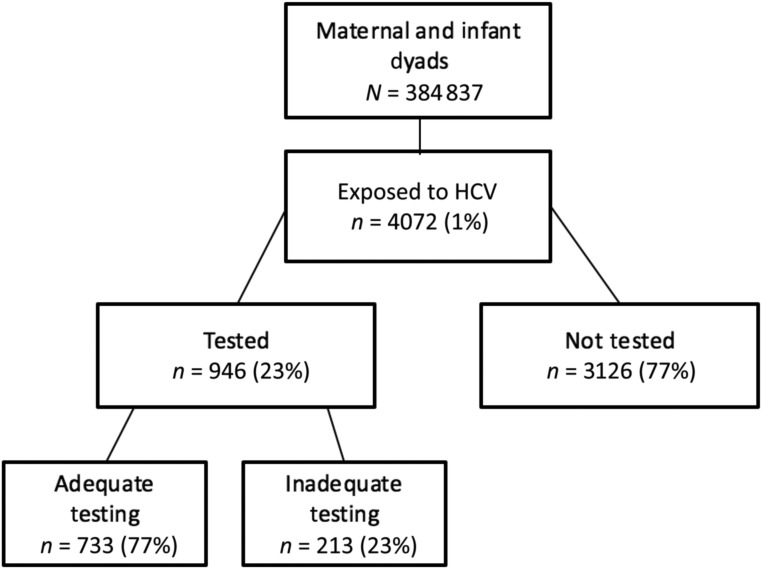
Among infants perinatally exposed to HCV, testing occurred in <1 in 4 infants overall.
Abstract
Video Abstract
BACKGROUND:
Hepatitis C virus (HCV) prevalence doubled among pregnant women from 2009 to 2014, reaching 3.4 per 1000 births nationwide. Infants exposed to HCV may acquire HCV by vertical transmission. National guidelines recommend that infants exposed to HCV be tested; however, it is unclear if these recommendations are being followed. Our objectives were to determine if infants exposed to HCV were tested and to determine hospital- and patient-level factors associated with differences in testing.
METHODS:
In this retrospective cohort study of infants exposed to HCV who were enrolled in the Tennessee Medicaid program, we used vital statistics–linked administrative data for infants born between January 1, 2005, and December 31, 2014. Infants were followed until 2 years old. Multilevel logistic regression was used to assess the association of HCV testing and hospital- and patient-level characteristics.
RESULTS:
Only 23% of 4072 infants exposed to HCV were tested. Infants whose mothers were white versus African American (96.6% vs 3.1%; P <.001), used tobacco (78% vs 70%; P <.001), and had HIV (1.3% vs 0.4%; P = .002) were more likely to be tested. Infants exposed to HCV who had a higher median of well-child visits (7 vs 6; P <.001) were more likely to be tested. After accounting for maternal and infant characteristics and health care use patterns, African American infants were less likely to undergo general testing (adjusted odds ratio 0.32; 95% confidence interval, 0.13–0.78).
CONCLUSIONS:
Testing occurred in <1 in 4 infants exposed to HCV and less frequently among African American infants. Public health systems need to be bolstered to ensure that infants exposed to HCV are tested for seroconversion.
What’s Known on This Subject:
Hepatitis C virus (HCV) prevalence is rising among pregnant women in the United States. Infants born to mothers with HCV should be tested because the virus can be acquired by vertical transmission.
What This Study Adds:
Less than 1 in 4 infants exposed to HCV were tested overall, with disparities in testing noted in African Americans and in those residing in a rural area. Efforts are needed to ensure that infants who are exposed to HCV are tested.
Hepatitis C virus (HCV) is the most common blood-borne infection in the United States, affecting an estimated 2.4 million currently.1 Reported new HCV infections are on the rise, especially in rural areas among young adults, particularly white young adults with a history of injection drug use.2,3 As new HCV infections have risen among young adults in the United States, rates of HCV infections have also increased among pregnant women. From 2009 to 2014, HCV infections in women with live births nearly doubled in the United States, reaching 3.4 per 1000 births.4 The rate of vertical transmission of HCV is estimated to be ∼3% to 6% but can be as high as 11% if the mother is coinfected with HIV.5,6 Because vertical transmission is the most common route of infection for children,7,8 the rapid rise of HCV infections among pregnant women in the United States is an emerging public health concern for children. Current estimates suggest perinatal HCV exposure affects an estimated 40 000 children born annually in the United States, resulting in ∼2700 to 4000 new HCV infections.7,9
Despite the rapid rise of HCV infections among pregnant women and data demonstrating the cost-effectiveness of universal HCV screening during pregnancy,10 there are limited data evaluating the testing of infants exposed to HCV. National guidelines recommend that infants exposed to HCV be tested with an HCV antibody at 18 months of age or an HCV RNA polymerase chain reaction (PCR) starting at 1 to 2 months. The few published studies on this subject suggest that infants exposed to HCV are commonly not tested for HCV, with testing rates ranging from 16% to 68%.11–15 These data, however, are limited by the small numbers generated from studies of a single city11 or single tertiary care facility.14,15 Other studies are limited by a relatively short study period11,15 or only followed a specific maternal population, such as infants of women with opioid use disorder.13
To address the gaps in the existing literature, the objectives of this study were (1) to determine what proportion of infants exposed to HCV were tested for HCV in a large population-based cohort, (2) to evaluate if testing was adequate according to national guidelines, and (3) to determine if hospital- and patient-level factors were associated with the performance of testing.
Methods
This retrospective cohort study included mother–infant dyads for infants who were born in Tennessee from January 1, 2005, to December 31, 2014, and who were enrolled in TennCare (Tennessee’s Medicaid program). Infants were followed through 2 years of age, until December 31, 2016. Data were obtained from merged TennCare and birth certificate records.16,17 This study was approved by the Institutional Review Boards of Vanderbilt University Medical Center and the Tennessee Department of Health.
Cohort
Mother–infant dyads were included if the mother was between 15 and 44 years of age at the time of delivery and was enrolled in TennCare at least 30 days before delivery and if the infant was enrolled in TennCare within 30 days of birth and had continued enrollment until 2 years of age with no greater than 30 days of a noncontinuous period of enrollment during this time. Infants who died during the 2-year follow-up period were excluded.
Maternal HCV status was obtained from birth certificates and the following International Classification of Diseases, Ninth Revision, Clinical Modification codes from the mother’s delivery hospitalization: 070.41, 070.44, 070.51, 070.54, 070.70, and 070.71.
Outcome
The primary outcome of interest was HCV testing among infants perinatally exposed during the first 24 months. HCV testing was determined by using Current Procedural Terminology codes for HCV antibody (86803 and 86804), HCV RNA (87520, 87521, and 87522), and HCV genotype (87902) testing. The secondary outcome was adequate HCV testing per current national testing guidelines (Supplemental Table 4), which was defined as either HCV antibody testing performed at or after 18 months of age or HCV RNA testing performed at or after 2 months of age.18–20
Covariates
Covariates associated with HCV testing were chosen a priori on the basis of the literature and clinical relevance. We hypothesized that mothers with a younger age and less education would be less likely to have infants who underwent testing. We speculated that infants with birth defects or infants who were admitted to the NICU would be more likely to have additional follow-up visits and would therefore have higher HCV testing rates. Similarly, we hypothesized that higher rates of health care use for both the mother and the child would translate into a higher likelihood of HCV testing. Maternal covariates included the following: maternal age, race, ethnicity, educational attainment, maternal gravidity and parity, tobacco use, maternal ICU admission, and maternal coinfections of hepatitis B or HIV. Infant covariates included the following: gestational age at birth, birth weight, classification as small for gestational age (<10th percentile in weight at birth), sex, breastfeeding at the time of discharge, NICU admission, infant seizures, birth injuries, and congenital disorders (cleft lip, cleft palate, confirmed trisomy 21, congenital hernia, gastroschisis, heart disease, hypospadias, limb reduction, omphalocele, and spina bifida). Hospital- and provider-level factors included the following: hospital, county of residence, and health care use, defined as the number of maternal prenatal visits and the number of well-child visits. Maternal county of residence was classified according to the 2013 Rural-Urban Continuum Code (RUCC)21 as urban (RUCC 1, 2, or 3), rural adjacent (RUCC 4, 6, or 8), or rural remote (RUCC 5, 7, or 9; Supplemental Table 5).
Data Analysis
Descriptive statistics were used to compare both the infants exposed to HCV with the nonexposed infants and the HCV-tested populations with the nontested populations. These were presented as the frequency (percentage) for categorical variables and the median (interquartile range) for continuous variables. χ2 tests and Wilcoxon rank tests were used to compare categorical and continuous variables, respectively. The primary model was a multilevel, multivariable logistic regression model constructed to evaluate whether the following factors were associated with HCV testing of the infant: maternal age, maternal race, rurality, maternal education, maternal parity, number of maternal prenatal visits, maternal tobacco use, maternal coinfection with hepatitis B or HIV, NICU admission, gestational age, small for gestational age, infant sex, presence of a congenital birth defect or neonatal disorder identified at birth (such as seizures or birth injury), and number of well-child visits. This regression model accounted for random effects at the birth hospital level. The intraclass correlation coefficient was calculated to determine how much of the variability in testing was accounted for by clustering at the hospital level. Next, a similar model was constructed to evaluate the secondary outcome of adequate testing for HCV, as previously defined.
A series of supplemental analyses were conducted to test the robustness of our study assumptions. First, the level of missing data was evaluated. Overall, 11.3% of observations had missing data. Each of the covariates was missing for <0.5% of observations, except for the number of prenatal visits, which was missing for 10.1% of observations. We conducted a supplemental analysis using multiple imputation with 11 iterations to account for this missing data (Supplemental Table 6). Next, given that prenatal visits had greater levels of missing data, we performed a supplemental analysis excluding prenatal visits as a covariate (Supplemental Table 7). Statistical significance was set at P < .05 for all tests. Statistical analyses were conducted by using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) and Stata version 15.1 (Stata Corp, College Station, TX).
Results
Among 384 837 mother–infant dyads born in Tennessee and enrolled in TennCare from 2005 to 2014, a total of 4072 (1.1%) mothers had an HCV infection during pregnancy. Mothers who were HCV-positive, compared with mothers who were HCV-negative, were more likely (P <.001) to be white than African American (92.9% vs 6.4%), more likely to have higher gravidity (2 vs 1), more likely to use tobacco (72% vs 29%), more likely to be hepatitis B-positive (2.5% vs 0.2%), and more likely to be HIV-positive (0.6% vs 0.2%). Infants exposed to HCV, compared with infants not exposed to HCV, were more likely (P <.001) to have a lower birth weight (median of 3027 vs 3204 g), more likely to be small for their gestational age (21% vs 14%), and more likely to be admitted to the NICU (11.5% vs 6.9%). HCV-negative mother–infant dyads were more likely to have more prenatal visits (median of 11 vs 10; P <.001), and infants were more likely to be breastfed (54% vs 33%; P <.001; Table 1).
TABLE 1.
Maternal and Infant Characteristics Stratified by HCV-Negative and HCV-Positive Mother–Infant Pairs
| Maternal Characteristics | Mother HCV-Negative, N = 380 765 | Mother HCV-Positive, N = 4072 | P |
|---|---|---|---|
| Age, median (IQR), y | 23 (20–27) | 26 (23–30) | <.001 |
| Education, median (IQR), y | 12 (11–13) | 12 (11–12) | <.001 |
| Race, % (n) | <.001 | ||
| White | 66.3 (251 859) | 92.9 (3775) | — |
| African American | 32.4 (123 071) | 6.4 (261) | — |
| Other | 1.3 (4982) | 0.6 (26) | — |
| Ethnicity, % (n) | <.001 | ||
| Hispanic | 3.3 (12 546) | 1.3 (52) | — |
| Non-Hispanic | 96.7 (367 865) | 98.7 (4012) | — |
| Residence rurality, % (n) | <.001 | ||
| Urban | 73.8 (280 465) | 68.6 (2783) | — |
| Rural adjacent | 21.5 (81 696) | 25.6 (1039) | — |
| Rural remote | 4.7 (17 947) | 5.8 (236) | — |
| Pregnancy characteristics | |||
| Gravidity, median (IQR) | 1 (0–2) | 2 (1–3) | <.001 |
| Parity, median (IQR) | 1 (0–2) | 1 (0–2) | <.001 |
| Admitted to ICU, % (n) | 0.1 (370) | 0.3 (12) | <.001 |
| Tobacco use, % (n) | 29 (110 286) | 72 (2916) | <.001 |
| Maternal infections | |||
| Hepatitis B, % (n) | 0.2 (724) | 2.5 (103) | <.001 |
| HIV, % (n) | 0.2 (673) | 0.6 (24) | <.001 |
| Infant characteristics | |||
| Gestational age at birth, median (IQR), wk | 39 (38–39) | 39 (37–39) | <.001 |
| Birth wt, median (IQR), g | 3204 (2863–3515) | 3027 (2665–3372) | <.001 |
| Small for gestational age (<10th percentile), % (n) | 14 (51 707) | 21 (871) | <.001 |
| Sex, % (n) | .83 | ||
| Male | 51 (194 404) | 51 (2072) | — |
| Female | 49 (186 358) | 49 (2000) | — |
| Admitted to NICU, % (n) | 6.9 (26 222) | 11.5 (467) | <.001 |
| Congenital or neonatal disorder,a % (n) | 0.4 (1440) | 0.4 (17) | .69 |
| Breastfed infant, % (n) | 54 (196 178) | 33 (1310) | <.001 |
| Health care use | |||
| Prenatal visit No., median (IQR) | 11 (9–14) | 10 (7–13) | <.001 |
| Well-child visit No., median (IQR) | 6 (4–7) | 6 (4–8) | <.001 |
Data may not sum up to 100% because of missing data. IQR, interquartile range; —, not applicable.
Congenital or neonatal disorder included birth injury, cleft lip, cleft palate, confirmed trisomy 21, congenital hernia, gastroschisis, heart disease, hypospadias, limb reduction, omphalocele, seizures, and spina bifida.
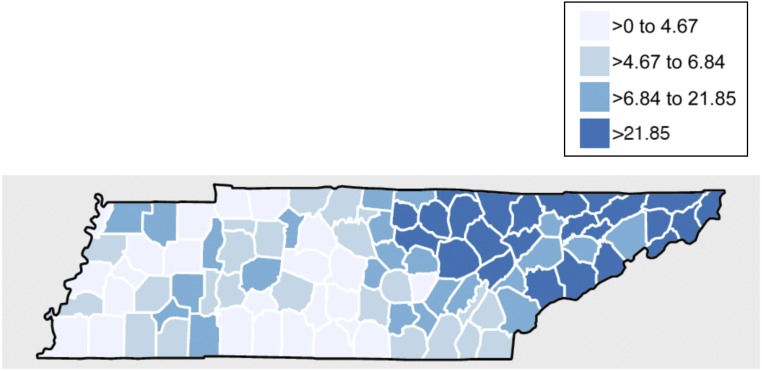
The prevalence of infants exposed to HCV rose each year, from 5.1 per 1000 live births in 2005 to 22.7 per 1000 live births in 2014 (P <.001). Overall, 92.9% of mothers who were HCV-positive were white, compared with 6.4% who were African American and 0.6% who were of other races. Although rates of HCV remained relatively constant for mothers who were African American or other races, rates of HCV grew sharply for white mothers (Supplemental Fig 3). There was significant county variation in HCV exposure rates, with the highest rates of perinatal HCV exposure noted in the eastern, predominately Appalachian, region of Tennessee (Fig 1).
FIGURE 1.
Number of infants exposed to HCV per 1000 live births in Tennessee by county, 2005–2014.
Overall, 946 (23%) infants exposed to HCV underwent any HCV testing in the first 24 months of life (Fig 2), with a slight year-to-year variation ranging from 18% to 26%. The majority (57.3%) of tests performed were HCV antibody tests, compared with 39% that were HCV RNA PCR tests and 3.7% that were HCV genotyping tests (Supplemental Fig 4). Most tested infants (70%) underwent only 1 test to evaluate for HCV infection. The number of tests, however, varied, with an outlier of 1 child undergoing 13 HCV tests. There was significant county variation in testing rates, with lower rates of HCV testing in western Tennessee (Supplemental Fig 5). Of the infants exposed to HCV who were tested, 733 (18%) met our definition for adequate testing. Three hundred fifty-four (48%) of these adequately tested children had HCV antibody testing at or after 18 months of age, 298 (41%) had HCV RNA PCR testing at or after 2 months of age, and 81 (11%) had both (Supplemental Fig 4).
FIGURE 2.
Testing of infants exposed to HCV.
Among infants exposed to HCV, maternal educational attainment, parity, and the number of prenatal visits were similar among infants who were tested and infants who were not tested. However, infants exposed to HCV who were tested were more likely to be born to mothers who used tobacco (78% vs 70%; P <.001) or had HIV coinfection (1.3% vs 0.4%; P <.001). In addition, infants exposed to HCV who were born at a lower gestational age (38 vs 39 weeks) or a lower birth weight (2960 vs 3040 g), were admitted to a NICU (14% vs 11%), or had more well-child visits (median of 7 vs 6) were also more likely to be tested (P <.001; Table 2). Among infants exposed to HCV, adequate HCV testing was significantly more likely with the following covariates: white race (96.5% vs 3.1% vs 0.4%; P <.001), urban residence (73% vs 22.1% vs 4.9%; P = .02), maternal tobacco use (78% vs 70%; P <.001), maternal HIV coinfection (1.2% vs 0.5%; P = .01), lower birth weight (2954 vs 3037 g; P <.001), small for gestational age (24% vs 21%; P = .048), NICU admission (15% vs 11%; P =.001), and more well-child checks (median of 7 vs 6; P <.001), which is similar to factors associated with any HCV testing (Supplemental Table 8).
TABLE 2.
Maternal and Infant Characteristics Among Mother–Infants Dyads Infected With and Exposed to HCV Stratified by Whether Infants Exposed to HCV Were Tested for HCV
| Maternal Characteristics | Not Tested for HCV, N = 3126 | Tested for HCV, N = 946 | P |
|---|---|---|---|
| Age, median (IQR), y | 26 (23–30) | 26 (23–30) | .57 |
| Education, median (IQR), y | 12 (11–12) | 12 (11–12) | .02 |
| Race, % (n) | <.001 | ||
| White | 91.8 (2862) | 96.6 (913) | — |
| African American | 7.4 (232) | 3.1 (29) | — |
| Other | 0.7 (23) | 0.3 (3) | — |
| Ethnicity, % (n) | .71 | ||
| Hispanic | 1.3 (41) | 1.2 (11) | — |
| Non-Hispanic | 98.7 (3077) | 98.8 (935) | — |
| Residence rurality, % (n) | .16 | ||
| Urban | 67.8 (2111) | 71.1 (672) | — |
| Rural adjacent | 26.2 (816) | 23.6 (223) | — |
| Rural remote | 6 (186) | 5.3 (50) | — |
| Pregnancy characteristics | |||
| Gravidity, median (IQR) | 2 (1–3) | 2 (1–3) | .10 |
| Parity, median (IQR) | 1 (0–2) | 1 (0–2) | .02 |
| Admitted to ICU, % (n) | 0.3 (a) | 0.4 (a) | .41 |
| Tobacco use, % (n) | 70 (2180) | 78 (736) | <.001 |
| Maternal infections | |||
| Hepatitis B, % (n) | 2.7 (83) | 2.1 (20) | .35 |
| HIV, % (n) | 0.4 (12) | 1.3 (12) | .002 |
| Infant characteristics | |||
| Gestational age at birth, median (IQR), wk | 39 (37–39) | 38 (37–39) | <.001 |
| Birth wt, median (IQR), g | 3040 (2680–3380) | 2960 (2580–3320) | <.001 |
| Small for gestational age (<10th percentile), % (n) | 21 (657) | 23 (214) | .26 |
| Sex, % (n) | .9 | ||
| Male | 51 (1589) | 51 (483) | — |
| Female | 49 (1537) | 49 (463) | — |
| Admitted to NICU, % (n) | 11 (332) | 14 (135) | .002 |
| Congenital or neonatal disorder,b % (n) | 0.4 (13) | 0.4 (a) | .98 |
| Breastfed infant, % (n) | 34 (1018) | 32 (292) | .25 |
| Health care use | |||
| Prenatal visit No., median (IQR) | 10 (7–13) | 10 (7–13) | .01 |
| Well-child visit No., median (IQR) | 6 (4–7) | 7 (6–9) | <.001 |
Data may not sum up to 100% because of missing data. IQR, interquartile range; —, not applicable.
Values <10 were suppressed.
Congenital or neonatal disorder included the following: birth injury, cleft lip, cleft palate, confirmed trisomy 21, congenital hernia, gastroschisis, heart disease, hypospadias, limb reduction, omphalocele, seizures, and spina bifida.
After accounting for maternal and infant characteristics, health care use patterns, and birth hospital, African American infants exposed to HCV were less likely to undergo testing compared with white infants exposed to HCV (adjusted odds ratio [aOR] 0.32; 95% confidence interval [CI], 0.13–0.78). Infants exposed to HCV residing in rural counties adjacent to metropolitan areas were also less likely to be tested (aOR 0.73; 95% CI, 0.58–0.92). Infants exposed to HCV who attended a greater number of well-child visits (aOR 1.29; 95% CI, 1.24–1.33), whose mothers used tobacco (aOR 1.41; 95% CI, 1.14–1.74), or who had HIV exposure (aOR 7.85; 95% CI, 2.82–21.84) were more likely to be tested for HCV. Additionally, infants exposed to HCV with a higher gestational age (aOR 0.95; 95% CI, 0.91–0.99) and whose mothers had a greater number of previous births (aOR 0.93; 95% CI, 0.86–0.99) had lower odds of HCV testing (Table 3). Results were similar for adequate HCV testing (Table 3) and in supplemental analyses (Supplemental Tables 6 and 7).
TABLE 3.
Unadjusted and Adjusted Characteristics Associated With Any and Adequate HCV Testing Among Infants Who Were Exposed
| Any Testing, Unadjusted Odds Ratio (95% CI) | Any Testing, aOR (95% CI) | Adequate Testing,a Unadjusted Odds Ratio (95% CI) | Adequate Testing,a aOR (95% CI) | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age (per 1-y increase) | 1.00 (0.99–1.02) | 1.01 (0.99–1.03) | 1.00 (0.98–1.01) | 1.00 (0.98–1.02) |
| African American (versus white) | 0.39 (0.26–0.58) | 0.32 (0.13–0.78) | 0.42 (0.27–0.65) | 0.36 (0.14–0.97) |
| Other race (versus white) | 0.41 (0.12–1.37) | 0.21 (0.00–13.33) | 0.57 (0.17–1.89) | 0.46 (0.01–24.64) |
| Education (per 1-y increase) | 0.93 (0.87–0.99) | 0.92 (0.85–1.00) | 0.92 (0.86–0.99) | 0.91 (0.83–0.99) |
| Rural adjacent (versus urban) | 0.86 (0.72–1.02) | 0.73 (0.58–0.92) | 0.78 (0.64–0.94) | 0.66 (0.51–0.85) |
| Rural remote (versus urban) | 0.84 (0.61–1.17) | 0.67 (0.43–1.03) | 0.76 (0.52–1.09) | 0.69 (0.43–1.11) |
| Pregnancy characteristics | ||||
| Maternal parity (per 1-U increase) | 0.95 (0.90–1.00) | 0.93 (0.86–1.00) | 0.93 (0.88–0.99) | 0.93 (0.86–1.01) |
| Tobacco use | 1.52 (1.28–1.81) | 1.41 (1.14–1.74) | 1.52 (1.26–1.84) | 1.53 (1.21–1.94) |
| Maternal infections | ||||
| Hepatitis B | 0.79 (0.48–1.30) | 0.83 (0.47–1.47) | 0.83 (0.49–1.43) | 0.87 (0.46–1.64) |
| HIV | 3.33 (1.49–7.45) | 7.85 (2.82–21.84) | 2.76 (1.20–6.32) | 5.40 (1.92–15.24) |
| Infant characteristics | ||||
| Small for gestational age (<10th percentile) | 1.11 (0.93–1.32) | 0.93 (0.76–1.15) | 1.21 (1.00–1.46) | 0.99 (0.79–1.24) |
| Gestational age at birth (per 1-wk increase) | 0.95 (0.92–0.98) | 0.95 (0.91–0.99) | 0.97 (0.94–1.01) | 0.98 (0.93–1.02) |
| Female sex | 0.99 (0.86–1.15) | 1.01 (0.85–1.19) | 1.05 (0.89–1.23) | 1.03 (0.86–1.24) |
| Congenital disorder | 1.02 (0.33–3.13) | 1.18 (0.35–3.99) | 0.98 (0.28–3.41) | 1.18 (0.31–4.50) |
| NICU admission | 1.40 (1.13–1.74) | 1.05 (0.78–1.41) | 1.46 (1.15–1.83) | 1.16 (0.84–1.59) |
| Health care use | ||||
| Prenatal visits (per 1-visit increase) | 0.98 (0.96–0.99) | 0.98 (0.96–1.00) | 0.98 (0.97–1.00) | 0.99 (0.97–1.01) |
| Well-child visits (per 1-visit increase) | 1.27 (1.23–1.31) | 1.29 (1.24–1.33) | 1.27 (1.23–1.31) | 1.27 (1.23–1.32) |
Variability in testing was accounted for by clustering at the hospital level. The intraclass correlation coefficient was 0.052 (95% CI, 0.022–0.117) for general testing and 0.041 (95% CI, 0.014–0.113) for adequate testing, suggesting that a small percentage of the total variance in both general and adequate testing is accounted for by the clustering. Characteristics were adjusted for the other covariates included in this table.
Adequate testing is defined as either HCV antibody testing performed at or after 18 mo or HCV RNA PCR testing performed at or after 2 mo of age.
Discussion
In a state disproportionately affected by the rise of HCV among women with live births, testing of infants exposed to HCV occurred for <1 in 4 at-risk infants overall and for only 1 in 10 African American infants. Furthermore, infants exposed to HCV residing in rural counties adjacent to metropolitan areas were also less likely to undergo testing, which is concerning given the rapidly rising rates of HCV among young adults in rural communities. Assuming a 3% to 6% vertical transmission rate among the 4072 women with live births who were identified as having HCV,5,6 an estimated 122 to 244 children in the state of Tennessee are presumed to have been infected with the virus during the study period, with 94 to 187 children not identified because of a lack of testing.
The increased probability of testing with perinatal HIV exposure, a lower gestational age, and more well-child checks could be due to more exposure to the health care system and potentially increased access to specialist care. The decreased likelihood of testing of infants whose mothers resided in rural areas could represent issues with transportation, a lack of provider education on testing recommendations, or less overall availability of testing. Higher parity may result in a lower likelihood of testing because mothers may have a false sense of security because of the relatively low vertical transmission rate and potentially because of having other children who were not perinatally infected.
Unfortunately, there are currently no recommended medical interventions to lower the risk of vertical transmission during pregnancy.7,22 Of infants who acquire HCV, 20% will have an acute resolving infection, 50% will develop a chronic asymptomatic infection, and 30% will develop a chronic active infection.23 Given these risks and the possibility of treatment before adulthood, infants infected with HCV need to be managed, tested, and identified so effective treatment can be implemented as soon as possible. There are several national organizations with recommendations on the testing of infants who are exposed. All organizations recommend HCV antibody as a first-line evaluation starting at 18 months of age. Antibody testing before 18 months of age is unreliable because of passively acquired or transplacental acquisition of maternal antibody, which can persist up to 18 months; this can lead to false-positive antibody test results. Opinions on the timing of initial and repeat HCV RNA testing vary somewhat18–20,24 (Supplemental Table 4). Before 1 to 2 months of age, HCV RNA testing is not recommended given the low sensitivity early in a child’s life and the potential for false-negatives due to intermittent viremia.25
There are a few studies that also found inadequate testing of infants exposed to HCV throughout the United States.11–15 Taken together, these findings suggest there is an urgent need to ensure adequate testing of infants exposed to HCV. Universal HCV screening of pregnant women could enhance detection of infants who are exposed. In addition, building data systems that ensure that maternal laboratory results are included in the child’s medical record and augmenting provider and patient education on national guidelines for HCV testing among infants exposed to HCV, particularly in at-risk groups such as African Americans and those who live in rural areas, may improve appropriate testing of infants who are exposed.
Given that there has been a substantial improvement in treatment options for HCV,26 pregnancy should serve as an opportunity to identify women who are HCV-positive and connect them to treatment after delivery. This strategy facilitates timely identification of infants exposed to HCV, and it also potentially eliminates the risk of vertical transmission in subsequent pregnancies. Current strategies to identify women who are HCV-positive during pregnancy by using a risk-based screening approach have been evaluated in the literature and have suggested failures in HCV identification.27–29 Moreover, there is evidence in the literature that indicates that universal screening in pregnancy can be feasible and performed at an acceptable cost.10,30 The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America now recommend that all pregnant women be tested for HCV infection, preferably when prenatal care is initiated.31 In addition, some states, such as Kentucky, have adopted universal HCV screening during pregnancy, which may be preferable to risk-based screening, particularly in communities with a high prevalence of HCV infections.
Despite recognition among public health officials and clinicians caring for adults that HCV has become epidemic, there appears to be less awareness among those caring for infants. For instance, infant testing remained low throughout our study period despite increased identification of mothers. Importantly, infants exposed to HCV do not show any clinical signs of exposure, and maternal risk factors for HCV may not be identified or communicated. In addition, even among clinicians considering the possibility of mother-to-child HCV infection, the lack of changes in policies and guidelines for the approach to mothers and infants affected by HCV may impede testing. Centers, particularly those in high HCV prevalence settings, should consider standardizing their approach to pregnant women and infants to ensure the appropriate identification and treatment of HCV.
There are limitations to this study, as with any study involving secondary data analyses of administrative data. First, given that HCV testing among pregnant women in Tennessee is not universal but risk-based, it is possible infants exposed to HCV were not identified.22 In this case, our prevalence estimates may be underestimated. In addition, our reliance on administrative and vital records data may have resulted in misclassification bias due to errors of omission or commission. Our inclusion criteria requiring pregnant women to be enrolled in TennCare for at least 30 days before delivery may exclude women who receive no prenatal care, a population that may also be at risk for HCV infection and poor follow-up. The rate of HCV infection in a given county in Tennessee may not necessarily represent the burden of disease, but rather the initiative in that county to identify disease. Furthermore, because maternal HCV positivity was obtained from birth certificates and billing data and not laboratory data, HCV positivity could be indicative of a past infection or a false-positive test and not necessarily an active infection during pregnancy. This study also only included births financed by Medicaid, which represents approximately half of all births in Tennessee; therefore, our study may not be generalizable in other populations.
Conclusions
HCV infection is a growing public health problem affecting maternal and child health. HCV testing among known infants exposed to HCV was poor, with fewer than 1 in 4 infants being tested, and was worse among African American infants and those with a rural residence. Furthermore, even among infants who were tested, testing was often inadequate. Strategies to improve provider and patient education on HCV and the targeting of at-risk populations could improve the care of those affected by or exposed to HCV.32–34
Acknowledgments
We thank the Division of TennCare of the Tennessee Department of Finance and Administration, which provided the data. We also thank the Tennessee Department of Health, Office of Health Statistics for providing vital records data.
Glossary
- aOR
adjusted odds ratio
- CI
confidence interval
- HCV
hepatitis C virus
- PCR
polymerase chain reaction
- RUCC
Rural-Urban Continuum Code
Footnotes
Dr Lopata had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Patrick had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, conceptualized and designed the study, analyzed the data, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Dudley had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and reviewed and revised the manuscript; Ms McNeer and Dr Dupont analyzed the data, performed the statistical analysis, and reviewed and revised the manuscript; Drs Wester, Cooper, Carlucci, and Espinosa conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Division of TennCare of the Tennessee Department of Finance and Administration.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute On Drug Abuse of the National Institutes of Health under awards K23DA038720 (awarded to Dr Patrick) and R01DA045729 (awarded to Dr Patrick). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Espinosa has received grant support from Gilead. Gilead did not support any aspect of conceptualization or development of this article; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69(3):1020–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zibbell JE, Iqbal K, Patel RC, et al. ; Centers for Disease Control and Prevention (CDC) . Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458 [PMC free article] [PubMed] [Google Scholar]
- 3.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419 [DOI] [PubMed] [Google Scholar]
- 4.Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C virus infection among women giving birth - Tennessee and United States, 2009-2014. MMWR Morb Mortal Wkly Rep. 2017;66(18):470–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59(6):765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173–177 [DOI] [PubMed] [Google Scholar]
- 7.Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(2):109–113 [DOI] [PubMed] [Google Scholar]
- 8.Wilson CB, Nizet V, Maldonado YA, Remington JS, Klein JO. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant, 8th ed Philadelphia, PA: Elsevier/Saunders; 2015 [Google Scholar]
- 9.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714 [DOI] [PubMed] [Google Scholar]
- 10.Chaillon A, Rand EB, Reau N, Martin NK. Cost-effectiveness of universal hepatitis C virus screening of pregnant women in the United States. Clin Infect Dis. 2019;69(11):1888–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to test and identify perinatally infected children born to hepatitis C virus-infected women. Clin Infect Dis. 2016;62(8):980–985 [DOI] [PubMed] [Google Scholar]
- 12.Watts T, Stockman L, Martin J, Guilfoyle S, Vergeront JM. Increased risk for mother-to-infant transmission of hepatitis C virus among Medicaid recipients - Wisconsin, 2011-2015. MMWR Morb Mortal Wkly Rep. 2017;66(42):1136–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein RL, Sabharwal V, Wachman EM, et al. Perinatal transmission of hepatitis C virus: defining the cascade of care. J Pediatr. 2018;203:34–40.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chappell CA, Hillier SL, Crowe D, Meyn LA, Bogen DL, Krans EE. Hepatitis C virus screening among children exposed during pregnancy. Pediatrics. 2018;141(6):e20173273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towers CV, Fortner KB. Infant follow-up postdelivery from a hepatitis C viral load positive mother. J Matern Fetal Neonatal Med. 2019;32(19):3303–3305 [DOI] [PubMed] [Google Scholar]
- 16.Patrick SW, Dudley J, Martin PR, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135(5):842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maalouf FI, Cooper WO, Slaughter JC, Dudley J, Patrick SW. Outpatient pharmacotherapy for neonatal abstinence syndrome. J Pediatr. 2018;199:151–157.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack CL, Gonzalez-Peralta RP, Gupta N, et al. ; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition . NASPGHAN practice guidelines: diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr. 2012;54(6):838–855 [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics, Committee on Infectious Diseases In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2015 Report of the Committee on Infectious Diseases, 30th ed Elk Grove Village, IL: American Academy of Pediatrics; 2015 [Google Scholar]
- 20.Centers for Disease Control and Prevention. Hepatitis C questions and answers for health professionals. 2017. Available at: https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed January 1, 2019
- 21.US Department of Agriculture Economic Research Service. Documentation. 2018. Available at: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation/#referencedate. Accessed February 18, 2019.
- 22.American College of Obstetricians and Gynecologists ACOG practice bulletin No. 86: viral hepatitis in pregnancy. Obstet Gynecol. 2007;110(4):941–956 [DOI] [PubMed] [Google Scholar]
- 23.European Paediatric Hepatitis C Virus Network Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41(1):45–51 [DOI] [PubMed] [Google Scholar]
- 24.American Association for the Study of Liver Diseases ; Infectious Diseases Society of America. HCV in children. Available at: https://www.hcvguidelines.org/unique-populations/children. Accessed January 10, 2020
- 25.Polywka S, Pembrey L, Tovo PA, Newell ML. Accuracy of HCV-RNA PCR tests for diagnosis or exclusion of vertically acquired HCV infection. J Med Virol. 2006;78(2):305–310 [DOI] [PubMed] [Google Scholar]
- 26.American Association for the Study of Liver Diseases ; Infectious Diseases Society of America. Initial treatment of adults with HCV infection. Available at: https://www.hcvguidelines.org/treatment-naive. Accessed January 10, 2020
- 27.El-Kamary SS, Hashem M, Saleh DA, et al. Reliability of risk-based screening for hepatitis C virus infection among pregnant women in Egypt. J Infect. 2015;70(5):512–519 [DOI] [PubMed] [Google Scholar]
- 28.Krans EE, Zickmund SL, Rustgi VK, Park SY, Dunn SL, Schwarz EB. Screening and evaluation of hepatitis C virus infection in pregnant women on opioid maintenance therapy: a retrospective cohort study. Subst Abus. 2016;37(1):88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waruingi W, Mhanna MJ, Kumar D, Abughali N. Hepatitis C virus universal screening versus risk based selective screening during pregnancy. J Neonatal Perinatal Med. 2015;8(4):371–378 [DOI] [PubMed] [Google Scholar]
- 30.Selvapatt N, Ward T, Bailey H, et al. Is antenatal screening for hepatitis C virus cost-effective? A decade’s experience at a London centre. J Hepatol. 2015;63(4):797–804 [DOI] [PubMed] [Google Scholar]
- 31.American Association for the Study of Liver Diseases ; Infectious Diseases Society of America. HCV in Pregnancy. Available at: https://www.hcvguidelines.org/unique-populations/pregnancy. Accessed January 10, 2020
- 32.Coughlin SS. “Test, listen, cure” (TLC) hepatitis C community awareness campaign. JMIR Res Protoc. 2015;4(1):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51(3):729–733 [DOI] [PubMed] [Google Scholar]
- 34.Koman D. Increasing hepatitis C virus knowledge through an evidence-based educational intervention. Gastroenterol Nurs. 2018;41(2):95–102 [DOI] [PubMed] [Google Scholar]