Abstract
A better understanding of cancer biology has led to the development of molecular targeted therapy, which has dramatically improved the outcome of some cancer patients, especially when a biomarker of efficacy has been used for patients’ selection. In head and neck oncology, cetuximab that targets epidermal growth factor receptor is the only targeted therapy that demonstrated a survival benefit, both in the recurrent and in the locally advanced settings, yet without prior patients’ selection. We herein review the clinical development of targeted therapy in head and neck squamous cell carcinoma in light of the molecular landscape and give insights in on how innovative clinical trial designs may speed up biomarker discovery and deployment of new molecular targeted therapies. Given the recent approval of immune checkpoint inhibitors targeting programmed cell death-1 in head and neck squamous cell carcinoma, it remains to be determined how targeted therapy will be incorporated into a global drug development strategy that will inevitably incorporate immunotherapy.
Head and neck cancers represent a variety of cancers from different locations and with different histologies. The most frequent type of head and neck cancer is squamous cell carcinoma. Squamous cell carcinomas of the oral cavity, oropharynx, larynx, and hypopharynx are commonly grouped under the appellation of head and neck squamous cell carcinomas (HNSCC) because they usually share common etiologic factors, including alcohol and tobacco consumption. More recently, human papillomavirus (HPV) infection prevailed over known risk factors as an important etiologic factor for squamous cell carcinomas of the oropharynx (1). HPV is clinically significant only in oropharyngeal tumors. HPV prevalence was reported in 22.4%, 4.4%, and 3.5% of oropharynx, oral cavity, and larynx cancers, respectively (2). HPV-induced HNSCC activate distinct signaling pathways compared with HPV-negative tumors, raising the question of different therapeutic strategies for these two subtypes. HPV-related HNSCC has been shown to have a better prognosis than HNSCC that is not related to HPV (3).
HNSCC represents the sixth most common cancer worldwide, with an incidence of around 600 000 new cases per year (4). The overall mortality is high, reaching 40% to 50% (3). Small tumors without nodal involvement can be treated with single modality therapy (surgery or radiotherapy), whereas locally advanced tumors usually undergo multimodality treatments that involve surgery, radiotherapy, and chemotherapy. Patients experiencing a recurrence that is not amenable to surgery or radiotherapy have a limited overall survival (OS), with a median survival of less than 1 year (5).
Cetuximab is to date the only targeted therapy known to demonstrate an OS benefit in HNSCC, both in the locally advanced setting in combination with radiotherapy (6) and in the first-line recurrent and/or metastatic (R/M) setting in combination with chemotherapy (5). Cetuximab is a monoclonal antibody targeting the epidermal growth factor receptor (EGFR), for which no predictive biomarker of efficacy or resistance has been identified in HNSCC. A comprehensive genomic characterization of HNSCC reported by The Cancer Genome Atlas revealed multiple actionable molecular alterations differ slightly between HPV-negative and HPV-positive patients (7,8), potentially explaining the different natural histories and prognostics of these two entities (9). New concepts have emerged for molecular targeted therapies. First, oncogene addiction qualifies tumors for which the growth and survival can be impaired by the inhibition of a single oncogene (10). Another concept, synthetic lethality, occurs when the simultaneous perturbation of two genes results in cell death (11). If a tumor harbors a mutation in either of these two genes, a therapy could be efficient by targeting the other one. A more recent concept is collateral lethality, which concerns tumors for which a passenger deletion exposes cancer cells to specific therapeutic vulnerabilities (12). Despite our better understanding of HNSCC biology, no other molecular targeted agent besides cetuximab has been approved for HNSCC.
Immune checkpoint inhibitors targeting programmed cell death-1 were demonstrated to improve OS in the R/M setting not only after platinum failure (13–15) but also in first-line either as a single agent or combined with chemotherapy (16).
In this paper, we aim to review the clinical development of molecular targeted therapy in HNSCC and to discuss how it could be accelerated by exploiting the molecular features of the disease and innovative clinical trial designs.
Search Strategy and Selection Criteria
References for this review were identified through searches of PubMed with the search terms “head and neck cancer” and “trial” up to November 2018. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final references list was generated on the basis of originality and relevance to the broad scope of this review. A search of ongoing clinical trials of targeted therapy in HNSCC was performed using the National Cancer Institute website (https://www.clinicaltrials.gov/).
Clinical Development of Targeted Therapy in HNSCC
Targeting EGFR
Because most HNSCC highly express EGFR on the surface of tumor cells (17), therapies targeting EGFR have been extensively evaluated in this disease. Both monoclonal antibodies binding to the extracellular domain of EGFR like cetuximab and tyrosine kinase inhibitors (TKIs) binding to the intracellular kinase domain of EGFR, such as gefitinib, have shown to produce antitumor activity (18–20). Only cetuximab demonstrated an OS benefit in randomized trials (Table 1) (5,6). In a recurrent or metastatic setting, gefitinib and afatinib failed to improve OS when compared with standard treatments (22,24,29,30). Several reasons might explain why TKIs did not go to the market in HNSCC. The first reason is that overall response rates with TKIs were slightly lower as single agents than with monoclonal antibodies (18–20). The second reason is that TKIs are more toxic than monoclonal antibodies, with more diarrhea and transaminases increase due to the hepatic metabolism of TKIs. It cannot be excluded that part of the higher efficacy observed with cetuximab is also related to antibody-dependent cellular cytotoxicity (31,32). Last, TKIs are known to be less specific than antibodies (33).
Table 1.
Randomized clinical trials assessing molecular targeted therapies in recurrent and/or metastatic HNSCC targeting EGFR*
| Treatment arms | Systemic therapies given concomitantly | Phase | No. | ORR, % | PFS |
OS |
Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median, mo | HR | P | Median, mo | HR | P | |||||||
| Cetuximab | Cisplatin | — | III | 57 | 26 | 4.2 | 0.78 | NS | 9.2 | NA | — | (21) |
| Cisplatin | — | 60 | 10 | 2.7 | 8 | |||||||
| Cetuximab | Platinum | 5FU | III | 222 | 36 | 5.6 | 0.54 | <.001 | 10.1 | 0.9 | .04 | (5) |
| Platinum | 5FU | 220 | 20 | 3.3 | 7.4 | |||||||
| Panitumumab | Cisplatin | 5FU | III | 327 | 37 | 5.8 | 0.78 | .004 | 11.1 | 0.87 | NS | (22) |
| Cisplatin | 5FU | 330 | 26 | 4.6 | 9 | |||||||
| Panitumumab | Cisplatin | Docetaxel | II | 56 | 44 | 6.9 | 0.63 | .048 | 12.9 | 1.1 | — | (23) |
| Cisplatin | Docetaxel | 57 | 37 | 5.5 | 13.8 | NA | ||||||
| Zalutumumab | — | — | III | 191 | 6 | 2.2 | 0.63 | .001 | 6.7 | 0.77 | NS | (24) |
| BSC | — | — | 95 | 1 | 1.9 | 5.2 | ||||||
| Gefitinib | Docetaxel | — | III | 134 | 12.5 | 3.5 | 0.81 | NS | 7.3 | 0.93 | NS | (25) |
| Docetaxel | — | 136 | 6.2 | 2.1 | 6 | |||||||
| Gefitinib 500 mg | — | — | III | 167 | 8 | NA | NA | — | 6 | 1.12 | — | (26) |
| Gefitinib 250 mg | — | — | 158 | 3 | NA | NA | — | 5.6 | 1.22 | — | ||
| Methotrexate | — | — | 161 | 4 | NA | NA | — | 6.7 | — | — | ||
| Afatinib | — | — | III | 322 | 10 | 2.6 | 0.8 | .03 | 6.8 | 0.96 | NS | (27) |
| Methotrexate | — | — | 161 | 6 | 1.7 | 6 | ||||||
| Afatinib | — | — | II | 61 | 8 | 2.9 | 0.93 | NS | 8 | 1.06 | NS | (28) |
| Cetuximab | — | — | 60 | 10 | 3.3 | 10.5 | ||||||
BSC = best supportive care; EGFR = epidermal growth factor receptor; 5FU = 5-fluorouracil; HNSCC = head and neck squamous cell carcinoma; HR = hazard ratio; NA = not available; NS = not statistically significant; ORR = objective response rate; OS = overall survival; PFS = progression-free survival.
Panitumumab is another monoclonal antibody targeting EGFR that failed to demonstrate an OS benefit in R/M HNSCC in combination with chemotherapy, most likely because of clinical trial design issues including the lack of mandatory maintenance therapy (22). Other monoclonal antibodies targeting EGFR such as zalutumumab were not shown to improve OS, possibly because patients were allowed to receive prior cetuximab, which potentially could have induced secondary resistance (24).
Resistance to EGFR Inhibition
The efficacy of cetuximab as single agent in the R/M setting is limited, with an overall response rate of 13% and a median progression-free survival of 2.3 months (18). In combination with chemotherapy, the absolute OS benefit is 2.8 months in the first-line R/M setting (5). Extensive work has been undertaken to identify predictive biomarkers of the efficacy of cetuximab in HNSCC. Some activating EGFR mutations detected in non–small cell lung cancer predicting response to EGFR TKIs have not been reported in HNSCC (7). EGFR amplifications present in 11% of patients were not shown to predict the efficacy of cetuximab in combination with chemotherapy (29). Several mechanisms of resistance to EGFR inhibitors have been reported, including the EGFR variant III, whose existence and impact are debated (30); the reduced antibody-receptor interaction via the competition with other ligands such as EGF or transforming growth factorα; the activation of parallel resistance pathways such as ERBB2 (HER2) and MET amplifications; IGF1R, IGF1, and IGF2 overexpression; and the activation of downstream signals such as via RAS and PIK3CA mutations (34). Numerous clinical trials (NCT01488318, NCT01871311, NCT02277197, NCT02205398) have combined cetuximab with other molecular targeted therapies targeting resistance pathways such as MET, HGF, SRC, or ABL inhibitors with the aim of overcoming primary resistance to cetuximab. The validity of this strategy has not been demonstrated in randomized trials to date (Table 2) (37).
Table 2.
Randomized clinical trials assessing molecular targeted therapies beyond EGFR in recurrent and/or metastatic HNSCC*
| Targets | Treatment arms | Systemic therapies given concomitantly | Phase | No. | ORR, % | PFS |
OS |
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median, mo | HR | P | Median, mo | HR | P | |||||||
| EGFR/HER3 | Duligotuzumab | II | 59 | 12 | 4.2 | 1.23 | NS | 7.2 | 1.15 | NS | (35) | |
| Cetuximab | 62 | 14.5 | 4 | 8.7 | ||||||||
| HER3 | Patritumab | Platinum cetuximab | II | 44 | 36 | 5.6 | 1.11 | NS | NA | NA | — | (36) |
| Placebo | Platinum cetuximab | 43 | 28 | 5.5 | ||||||||
| PI3K | PX-866 | Cetuximab | II | 42 | 10 | 2.6 | 0.99 | NS | 6.9 | >1 | NS | (37) |
| Cetuximab | 41 | 7 | 2.6 | 8.4 | ||||||||
| Buparlisib | Paclitaxel | II | 79 | 39 | 4.6 | 0.65 | .01 | 10.4 | 0.72 | .04 | (38) | |
| placebo | Paclitaxel | 79 | 14 | 3.5 | 6.5 | |||||||
| EGFR/VEGFR | Vandetanib | Docetaxel | II | 15 | 13 | 2 | NA | — | 5.4 | NA | — | (39) |
| Docetaxel | 14 | 7 | 0.7 | 6 | ||||||||
| Sorafenib | Cetuximab | II | 26 | 8 | 3.2 | NA | — | 3 | NA | — | (40) | |
| Cetuximab | 26 | 8 | 5.7 | 9 | — | |||||||
| BCL-2 | AT-101 | Docetaxel | II | 22 | 9 | 3.5 | NA | — | 5 | NA | — | (41) |
| Docetaxel | 13 | 15 | 4.5 | 8.3 | ||||||||
| αvβ3 and αvβ5 integrins | Cilengitide qw | Cisplatin 5FU cetuximab | II | 62 | 47 | 6.4 | 1.03 | NS | 12.4 | 0.94 | NS | (42) |
| Cilengitide q2w | Cisplatin 5FU cetuximab | 60 | 27 | 5.6 | 1.55 | NS | 10.6 | 1.04 | NS | |||
| Cisplatin 5FU cetuximab | 62 | 36 | 5.7 | — | 11.6 | — | — | |||||
*EGFR = epidermal growth factor receptor; 5FU = 5-fluorouracil; HNSCC = head and neck squamous cell carcinoma; HR = hazard ratio; NA = not available; NS = not significant; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; qw = weekly; q2w = every 2 weeks.
Improving EGFR Targeting
Second-generation pan-HER TKIs such as afatinib were supposed to be able to overcome some of these resistance mechanisms, but they were not shown to improve efficacy as compared with cetuximab (Table 1) (28). However, some patients progressing on cetuximab did subsequently respond to afatinib and vice versa (28). The combination of cetuximab with a monoclonal antibody targeting HER3 (ie, patritumab) was also unsuccessful, even in an enriched patient population based on HER3 ligands expression (Table 2) (36).
Antibody-drug conjugates are empowered antibodies designed to exploit the targeting ability of monoclonal antibodies by linking them to cytotoxic agents (43). Trastuzumab-deruxtecan (DS-8201a) that targets HER2 was shown to produce antitumor activity in heavily pretreated patients beyond HER2-positive breast cancer in other HER2-positive cancers (44). Antibody-drug conjugates targeting EGFR such as depatuxizumab-mafodotin (ABT-414) are currently being investigated in glioblastoma, and ABBV-321 is currently being evaluated in HNSCC (NCT03234712) (45).
Actionable Molecular Alterations in HNSCC
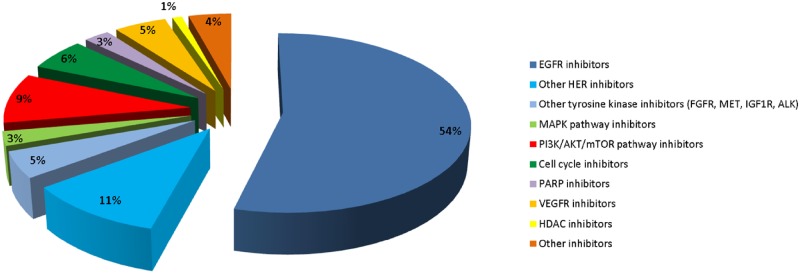
The tumor molecular landscape of HNSCC has been well characterized at the DNA and RNA levels (7). Gene expression profiling identified specific patterns in HNSCC, allowing a subgroup classification (46–49). Fifty to 100 driver molecular alterations were reported. Known hotspot mutations and/or amplifications for oncogenes or deep deletions and truncating mutations for tumor suppressor genes have been reported in different pathways (50). Of HNSCC, 24% have an alteration of a tyrosine kinase receptor, 9% on the downstream MAPK pathway, and 40% on the PI3K/AKT/mTOR pathway. Other altered pathways in HNSCC include the cell cycle pathway (62%), the DNA repair pathway (1.5%), and the epigenetics (22%) (Figure 1 and Table 3) (50). A recent review elegantly detailed the molecular landscape of HNSCC, highlighting the biological differences between HPV-positive and HPV-negative HNCCC (42). Particularly, in HPV-positive HNSCC compared with HPV-negative, substantial enrichment of somatic mutations were identified in PIK3CA (67% vs 43%), PTEN (23% vs 5%), or FGFR3 (17% vs 12%), and a lower percentage of CDKN2A alterations (6% vs 61%), respectively (51).
Figure 1.
Molecular targeted therapies evaluated in HNSCC clinical trials. Other inhibitors included ATR, aurora A, Bcl-2, BTK, CD44, CHEK1, EpCam, hedgehog, HSP90, JAK, MAPK, STAT3, and thrombospondin-1 inhibitors. ATR = ataxia telangiectasia and Rad3-related protein; Bcl-2 = B cell lymphoma 2; BTK = bruton tyrosine kinase; CD44 = cluster of differentiation 44; CHEK1 = checkpoint kinase 1; EpCAM = epithelial cell adhesion molecule; HNSCC = head and neck squamous cell carcinoma; HSP90 = heat shock protein 90; JAK = janus kinase; MAPK = mitogen-activated protein kinase; STAT3 = signal transducer and activator of transduction 3.
Table 3.
Main actionable molecular alterations in HNSCC
| Pathways | Actionable molecular alterations | Proportion of patients with actionable alterations* | Targeted therapy |
|---|---|---|---|
| Tyrosine kinase receptor | FGFR1/2/3 amplifications and mutations | 8% | FGFR inhibitors |
| MET amplifications and mutations | 1% | MET inhibitors | |
| IGF1R amplifications and mutations | 1% | IGF1R inhibitors | |
| EGFR amplifications | 11% | EGFR inhibitors | |
| ERBB2(HER2)/ERBB3/ERBB4 amplifications and mutations | 3% | Pan-HER inhibitors | |
| MAPK pathway |
|
9% |
|
| PI3K/AKT/mTOR pathway |
|
40% |
|
|
|
62% | CDK4/6 inhibitors |
|
|
1.5% | PARP inhibitors |
|
|
22% | HDAC inhibitors |
Only known hotspot mutations and amplifications for oncogenes as well as deep deletions and truncating mutations for tumor suppressor genes were taken into account. Data retrieved from http://www.cbioportal.org. HNSCC = head and neck squamous cell carcinoma.
The knowledge of potentially actionable molecular alterations has driven the clinical development of molecular targeted therapies accordingly, although it has mainly been conducted in unselected patient populations (Figure 2). The main actionable alterations in HNSCC can be classified in six major signaling pathways (Table 3).
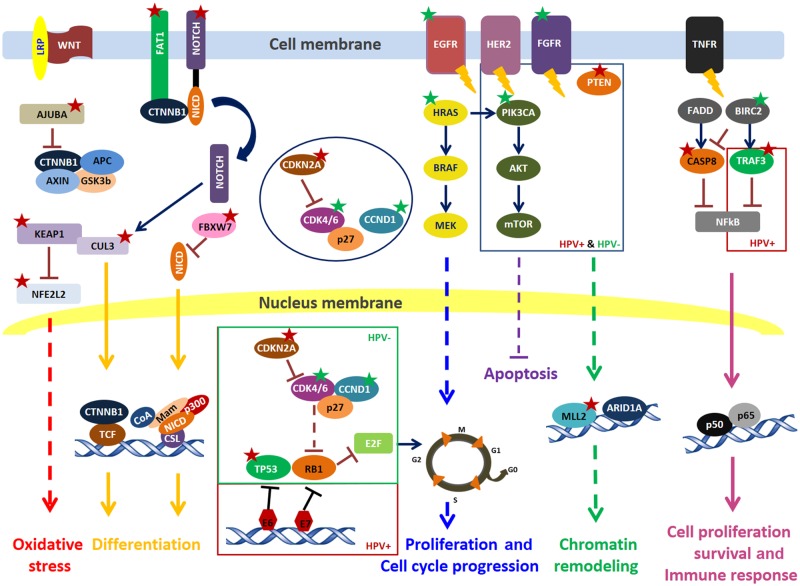
Figure 2.
Oncogenic signaling pathways involved in head and neck squamous cell carcinoma. Red star = loss of function; green star = activating alterations. AKT = PKB, protein kinase B; APC = adenomatous polyposis coli; ARID1A = AT-rich interactive domain-containing protein 1A; AT-rich domain = rich in adenine and thymine; BIRC2 = baculoviral inhibitor of apoptosis repeat-containing protein 2; BRAF = rapidly accelerated fibrosarcoma, protein B; CASP8 = caspase 8; CCND1 = cyclin D1; CDK4/6 = cyclin-dependent kinase 4 and 6; CDKN2A = cyclin-dependent kinase Inhibitor 2A; CSL = CBF1, centromere-binding factor 1; CTNNB1 = catenin beta-1 protein; CUL3 = cullin 3; E2F = E2 factor; EGFR = epidermal growth factor receptor; FADD = Fas-associated death domain; FBXW7 = F-box and tryptophan-aspartic acid dipeptide repeat domain-containing 7; FGFR = fibroblast growth factor receptor; GSK3b = glycogen synthase kinase 3 beta; HER2 = human epidermal growth factor receptor 2; HPV = human papilloma virus; HRAS = Harvey rat sarcoma viral; LRP = lipoprotein receptor-related protein; Mam = mastermind; MEK = MAPK/ERK Kinase; MLL2 = KMT2D, KMT2D = histone-lysine N-methyltransferase 2D; mTOR = mammalian target of rapamycin; NFE2L2 = nuclear factor, erythroid 2 like 2; NFkB = nuclear factor kappa-B; NICD = notch intracellular domain; PIK3CA = phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PTEN = phosphatase and tensin homolog; RB1 = retinoblastoma; TCF = T-cell factor; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor; TP53 = tumor protein 53; TRAF3 = TNF receptor-associated factor 3; WNT = wingless/int1.
Tyrosine Kinase Receptors
Druggable alterations of the proliferation pathway are mainly amplifications (6). In addition to EGFR amplifications, amplifications of other tyrosine kinase receptors were also reported, such as FGFR1-3 (8–10% amplifications), HER2 (3–4%), IGF-1R (0–2%), and MET amplifications (1–2%) (7,52). MET and HGF inhibitors are being investigated in combination with EGFR inhibitors (NCT01696955, NCT01332266, NCT02277184, NCT02277197). Although limited activity as single agents has been reported in an unselected patient population with figitumumab that targets IGF-1R (53), other IGF and IGF-1R inhibitors are being evaluated as single agents (NCT00872404) or in combination with cetuximab (NCT01427205, NCT00957853) in unselected patients. Interestingly, two patients with high FGFR1-3 mRNA expression in R/M HNSCC among 10 treated patients (20%) experienced an objective response with rogaratinib, an FGFR inhibitor (54).
FGFR mutations have infrequently been reported (2–3%) (7). Infigratinib is another pan-FGFR inhibitor that is being evaluated as a single agent in patients with FGFR-mutated HNSCC (NCT02706691).
MAPK Signaling Pathway
HRAS mutations were reported in 5% of HPV-negative patients according to The Cancer Genome Atlas (7). Transformation by HRAS mutations has been shown to be reversed by farnesyl transferase inhibition (55). Farnesyl transferase inhibitors are currently under investigation in HRAS-mutated patients. Whereas lonafarnib did not produce objective responses in unselected HNSCC patients (56), tipifarnib demonstrated encouraging results in HRAS-mutated HNSCC patients. In a phase II trial, among six patients with evaluable HNSCC, four heavily pretreated patients had a confirmed partial response (57).
PI3K/AKT/mTOR Pathway
Many components of the PI3K/AKT/mTOR pathway are frequently altered in HNSCC, with PIK3CA mutations/amplifications and PTEN losses occurring in approximately 30% and 10% of patients, respectively (7,58). PIK3CA alterations seem to be more common in HPV-positive HNSCC (7,59), but deregulation of this pathway is also common in HPV-negative patients, emphasizing the importance of this pathway independently of HPV infection (60). mTOR inhibitors and a new generation of small molecules targeting PI3K were largely evaluated in HNSCC, either as single agents (61,62), in combination with EGFR-targeting agents (63), chemotherapy (64–66), or both (67). Overall, these drugs were consistently evaluated without molecular selection, and limited activity was reported as single agents (61,62). In contrast, buparlisib that targets PI3K demonstrated an OS benefit in combination with paclitaxel as compared with paclitaxel alone, independent of PI3K/AKT/mTOR pathway alterations (Table 2) (38,68). In the latter study, patients with HPV-negative tumors, TP53 alterations, and low mutational load derived a greater benefit from the addition of buparlisib to paclitaxel (68). A phase III clinical trial is being launched.
Cell Cycle Pathway
At the cell cycle control level, CDKN2A inactivation (deletion and truncating mutation) usually coupled to CCND1 amplifications have been reported in more than 50% of HPV-negative HNSCC patients (7). CDK4/6 inhibitors are being evaluated in HNSCC (69), as well as WEE-1 inhibitors (70). Only one of these is a biomarker-driven trial with abemaciclib (CDK4/6 inhibitor) being investigated in an enriched patient population based on CDKN2A deletions and CCND1 and CDK6 amplifications (NCT03356223). Palbociclib and ribociclib, two other CDK4/6 inhibitors, are evaluated in combination with cetuximab in p16-negative HNSCC (NCT02499120, NCT02429089). Other cell cycle targets are being evaluated in clinical trials such as Aurora A inhibition in combination with cetuximab (NCT01540682). Similar to squamous cell carcinoma of the cervix, CDKN2A is not altered in HPV-positive HNSCC (71). The cell cycle pathway, however, remains altered with the inactivation of wild-type p53 and Rb1 via the E6 and E7 proteins, as well as focal amplification of E2F1 (7), supporting the clinical development of cell cycle inhibitors in this context. P53-targeting strategies are an interesting lead for investigation (72,73), although no HNSCC-specific trials with small molecules targeting p53 have been found in our research.
DNA Repair Pathway
Although homologous recombination deficiency has not clearly been reported in HNSCC, PARP inhibitors are currently under investigation in clinical trials as single agents (NCT02686008), in combination with (chemo)radiotherapy (NCT02308072, NCT01758731, NCT02229656, NCT01491139), chemotherapy alone (NCT01711541), and in combination with another DNA repair inhibitor targeting ATR (NCT03022409). The ATR inhibitor berzosertib is being evaluated in combination with chemoradiation (NCT02567422). Mutations on fanconi anemia or homologous recombination genes have been reported in HNSCC (∼20%) and are associated with poorer prognosis (74). However, no specific trial targeting fanconi anemia or homologous recombination mutated patients is currently ongoing.
Epigenetic Pathway
Disordered epigenetic regulation is an important feature of HPV-negative HNSCC. Inactivating mutations and deletions in KMT2C, KMT2D, ARID1A, and NSD1 occurs in more than 20% of HNSCC patients (7). Vorinostat, which is an HDAC inhibitor, is being investigated in combination with immunotherapy (NCT02538510) and capecitabine (NCT01267240).
Other Pathways
Tumor suppressor genes of the developmental pathways, in particular the WNT and NOTCH signaling pathways, are dysregulated. FAT1, AJUBA, FBXW7, and NOTCH1 are frequently mutated in HPV-negative HNSCC (7,8,75). Although NOTCH inhibitors exist, no clinical trial has been set up in HNSCC.
NFE2L2, a key transcription factor regulator of oxidative stress and its protein complex partners CUL3 and KEAP1, were also reported to be altered in HPV-negative patients. HPV-positive HNSCC are characterized by inactivating alterations (deletions and truncating mutations) of TRAF3, pinpointing deregulations in the NF-kB pathway (7). NF-kB inhibitors have been investigated in unselected patient populations in combination with radiotherapy (76,77) and in the R/M setting in combination with docetaxel in nonrandomized clinical trials (78).
Clinical Development Strategies
The development of targeted therapy has to be integrated into the standard-of-care treatment strategy of HNSCC patients. Although there are unmet medical needs both in the R/M and locally advanced settings, the clinical development of targeted therapy in each of these settings is challenging for different reasons.
R/M Setting
Most of the clinical development of targeted therapy in HNSCC has actually been performed in the R/M setting. The two main strategies in this setting are a chemo-additive strategy and development as a single agent. In the first case, the objective is to increase the efficacy of standard-of-care chemotherapy by overcoming primary and/or secondary resistance to treatment by adding a molecular targeted therapy, whereas in the latter case the objective is to get a new drug in the treatment armamentarium. Sequential strategies, such as maintenance therapy with molecular targeted therapy following initial chemotherapy, have not been developed in HNSCC, although they might be appealing given the different mechanism of action of molecular targeted therapy as compared with cytotoxic chemotherapy. The strategy to develop new drugs in the R/M setting is much easier from an ethical point of view. In addition, the toxicity and efficacy signals are not contaminated by the need to provide standard-of-care treatment. However, this strategy is associated with several challenges. First, the tumor biology is more complex in pretreated patients (79). The likelihood that patients will respond to molecular targeted therapy decreases with the advanced setting. As an example, HER2-targeting with trastuzumab decreases by half the recurrence risk in the adjuvant setting in HER2-positive breast cancer (80), as compared with a 20% decrease in death risk in the metastatic setting (81). Second, a substantial proportion of HNSCC patients are no longer able to swallow pills in the R/M setting (82), which may restrict the patient population for the development of oral-targeted therapy unless a liquid formulation that can be injected in a feeding tube is available.
Radiation-Based Combinations
The clinical development strategy of molecular targeted therapy in combination with radiotherapy has mostly been a chemo-additive strategy, which is expected, because the publication of the Meta-Analysis of Chemotherapy in Head and Neck Cancer established the benefit of concomitant platinum-based chemoradiation over radiotherapy alone in this patient population (83,84). Given the lack of data demonstrating the equivalence of chemoradiation and radiotherapy combined to EGFR inhibitors (Table 4) (88), chemoradiation remains the standard of care, while radiotherapy in combination with cetuximab is intended for patients with a contraindication to cisplatin. The chemotherapy-free trials therefore usually use at least an EGFR inhibitor. If a molecular targeted therapy is intended to be developed without chemotherapy and/or an EGFR inhibitor, the reirradiation setting is likely to be the most appropriate. As an example, bortezomib (proteasome inhibitor) was evaluated as a single agent in combination with reirradiation (76) and, in the curative setting, in combination with radiotherapy as well as cetuximab (77).
Table 4.
Randomized clinical trials of molecular targeted therapies combined to radiotherapy in locally advanced HNSCC*
| Treatment arms | Concomitant therapies | Comment | Phase | No. | ORR, % | DFS (or LDC) |
OS |
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (at y) | HR | P | % (at y) | HR | P | |||||||
| Cetuximab | RT | III | 211 | 74 | 50 (2y LDC) | 0.7 | .006 | 62 (2y) | 0.74 | .03 | (6) | |
| RT | 213 | 64 | 41 (2y LDC) | 55 (2y) | ||||||||
| Cetuximab | Cisplatin + RT | III | 444 | NA | 59 (3y) | 1.08 | NS | 76 (3y) | 0.95 | NS | (85) | |
| Cisplatin + RT | 447 | NA | 61 (3y) | 73 (3y) | ||||||||
| Cetuximab | RT | HPV+ | III | 168 | NA | 84 (2y) | 3.4 | .0007 | 89 (2y) | 5 | .001 | (86) |
| Cisplatin | RT | and low-risk | 166 | NA | 94 (2y) | 99 (2y) | ||||||
| Cetuximab | RT | HPV+ | III | 399 | NA | 67 (5y) | 1.72 | .0002 | 78 (5y) | 1.45 | NS | (87) |
| Cisplatin | RT | 406 | NA | 78 (5y) | 85 (5y) | |||||||
| Panitumumab | Accelerated RT | III | 159 | NA | 76 (2y) | 0.95 | NS | 88 (2y) | 0.89 | NS | (88) | |
| Cisplatin | Standard RT | 156 | NA | 73 (2y) | 85 (2y) | |||||||
| Panitumumab | Cisplatin + RT | II | 87 | 71 | 61 (2y) | 1.15 | NS | 69 (2y) | 1.63 | NS | (89) | |
| Cisplatin + RT | 63 | 82 | 65 (2y) | 78 (2y) | ||||||||
| Panitumumab | RT | II | 90 | NA | 41 (2y) | 1.73 | .03 | 63 (2y) | 1.59 | NS | (90) | |
| Cisplatin | RT | 61 | NA | 61 (2y) | 71 (2y) | |||||||
| Erlotinib | Cisplatin + RT | II | 99 | 70 | 74 (2y) | 0.9 | NS | NA | NA | — | (91) | |
| Cisplatin + RT | 105 | 63 | 70 (2y) | NA | NA | |||||||
| Gefitinib | Cisplatin + RT | II | 110 | 52 | 33 (2y LDC) | 0.92 | NS | NA | NA | — | (92) | |
| Placebo | Cisplatin + RT | 116 | 60 | 34 (2y LDC) | NA | NA | — | |||||
| Gefitinib | Maintenance post-RT | II | 111 | 59 | 29 (2y LDC) | 0.68 | NS | NA | NA | — | ||
| Placebo | 115 | 54 | 37 (2y LDC) | NA | NA | — | ||||||
| Lapatinib | Cisplatin + RT | Followed by maintenance | II | 34 | 65 | 55 (1.5y) | 0.74 | NS | 68 (1.5y) | 0.9 | NS | (93) |
| Placebo | Cisplatin + RT | 33 | 48 | 41 (1.5y) | 57 (1.5y) | |||||||
| Lapatinib | Cisplatin + RT | Followed by maintenance | III | 346 | NA | 57 (1.5y) | 1.1 | NS | 68 (1.5y) | 0.96 | NS | (94) |
| Placebo | Cisplatin + RT | 342 | NA | 58 (1.5y) | 66 (1.5y) | |||||||
*DFS = disease-free survival; HNSCC = head and neck squamous cell carcinoma; HR = hazard ratio; LDC = local disease control; NA = not available; NS = not statistically significant; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; RT = radiotherapy.
The evaluation of molecular targeted therapy in combination with radiotherapy is associated with several challenges. The first challenge is the combination by itself, because radiotherapy alone is associated with substantial toxicity. The chemo-additive strategy might lead to unacceptable toxicity even when molecular targeted therapies that often have no overlapping toxicities are combined with cisplatin. The addition of cetuximab to chemoradiation did not translate into improved survival (Table 4) (85), and the association of cetuximab with induction chemotherapy and chemoradiation was too toxic (95). Given the good prognosis of HPV-positive HNSCC, some molecular targeted therapies have been evaluated in combination with radiotherapy without cisplatin. Poorer outcomes have been reported with cetuximab and radiotherapy compared to chemoradiation in this population (Table 4) (86,87). Given these results, caution should be taken in the deescalation of chemoradiation to molecular targeted therapies in the future.
The second challenge is that the clinical development of molecular targeted therapy in combination with radiotherapy needs to go through a phase I trial because patients are treated with a curative intent. From a safety perspective, combining radiotherapy with anticancer agents not only increases acute toxicity compared to radiotherapy alone but also may produce chronic toxicity due to delayed or cumulative adverse effects on normal tissue. The Cancer Therapy Evaluation Program and the Radiation Research Program of the National Cancer Institute suggested that toxicity assessment for dose-limiting events spans the entire radiotherapy period and up to 30 days after completion of radiotherapy (96). It would be impractical from a dose-escalation perspective to wait for the late-phase toxicities because it would detrimentally slow patient accrual.
It is unclear whether new drugs should display a minimum threshold of clinical efficacy as a single agent in R/M HNSCC before being tested in combination with radiotherapy. Anticancer agents commonly used in combination with radiotherapy, including cisplatin, carboplatin, 5-fluorouracil (5FU), and cetuximab, have demonstrated single-agent efficacy in HNSCC patients. Some molecular targeted therapies have been studied in combination with radiotherapy despite lacking single-agent activity, such as bortezomib (proteasome inhibitor) or lapatinib (dual EGFR/HER2 inhibitor) (76,97).
Neoadjuvant Setting
Although the addition of docetaxel to neoadjuvant chemotherapy with cisplatin and 5FU (TPF regimen) before delivering definitive radiation-based treatment for locoregionally advanced HNSCC has demonstrated an OS benefit as compared with cisplatin and 5FU alone (98,99), several randomized phase III trials failed to demonstrate a benefit of neoadjuvant chemotherapy over standard chemoradiation (100–102). Although neoadjuvant therapy has not been shown to be able to improve patient outcomes as compared with radiation-based treatment alone, it is expected that the incorporation of molecular targeted therapy to neoadjuvant therapy will reach higher results than chemotherapy alone. The main strategy developed with molecular targeted therapy in this setting has been the combination of targeted therapy with a modified, less-toxic TPF-like regimen involving a platinum compound and a taxane. The only published randomized trial evaluating the addition of cetuximab to docetaxel and cisplatin did not demonstrate any improvement in progression-free survival or OS (103).
The main challenge of neoadjuvant strategies is the risk of compromising the radiation-based treatment that is the cornerstone of the treatment of locally advanced HNSCC patients. Toxicity occurring during the neoadjuvant part might indeed compromise the administration of radiotherapy or concomitant therapy.
Adjuvant Setting
No adjuvant systemic therapy administered either after exclusive radiation-based therapy or surgery has been demonstrated to improve patients’ outcome in localized HNSCC so far. Very few clinical trials have evaluated molecular targeted therapy in the adjuvant setting. The only two reported phase III trials have not met their primary endpoint (94,104). Both molecular targeted therapies inhibited the HER family (lapatinib and afatinib).
Development of drugs in the adjuvant setting is usually planned for high-risk patients who undergo (chemo)radiation as definitive treatment or in the adjuvant setting following surgery. Radiotherapy alone, and obviously chemoradiation and radiotherapy combined with cetuximab as well, are treatment regimens known to be hard to tolerate and are associated with acute and delayed toxicity. Patients might not be willing to undergo additional treatment following this. In addition, many patients might not be able to receive oral targeted therapy given their inability to swallow oral drugs. One of the reasons explaining the failure of the LUX HN2 trial that evaluated adjuvant afatinib (irreversible pan-HER inhibitor) after chemoradiation in locally advanced HNSCC has been the poor compliance with afatinib (104).
Innovative Clinical Trial Designs
It is striking to see that most of clinical trials evaluating molecular targeted therapies in HNSCC have been performed in unselected patient populations, although the molecular landscape of HNSCC has been well described for years now. Another difficulty in developing and conducting clinical trials in HNSCC is that patients with cancers from very distinct locations, such as the oropharynx, larynx, hypopharynx, and oral cavity, are lumped together, therefore diluting and missing any potential signal. Innovative clinical trials aiming at either identifying biomarkers of efficacy of molecular targeted therapies such as window-of-opportunity clinical trials or evaluating targeted therapies in enriched patient populations such as basket-and-umbrella trials are now becoming commonplace. These designs are key to speed up biomarker discovery and deployment of molecular targeted therapy in HNSCC, but they are associated with several challenges (Table 5).
Table 5.
Challenges for the clinical development of targeted therapy in HNSCC according to clinical setting*
| Specific considerations | Challenges |
|---|---|
| Recurrent and/or metastatic setting | |
|
|
| Combination with radiotherapy | |
|
|
| Adjuvant setting | |
|
|
| Neoadjuvant setting | |
| Tolerance and safety | Toxicity induced by neoadjuvant therapy might compromise completion of radiation-based therapy |
| Window-of-opportunity setting | |
|
|
*HNSCC = head and neck squamous cell carcinoma.
Window-of-Opportunity Clinical Trials
The goal of window-of-opportunity trials is not to demonstrate efficacy but to identify predictive and/or pharmacodynamic (PD) biomarkers of activity of a new drug. They especially apply to molecular targeted therapies that are supposed to produce antitumor activity only in the presence of the target. Windows of opportunities are situations in which drugs are given for a short period of time before a definitive treatment with the aim of collecting samples to identify biomarkers, without necessarily expecting efficacy. These windows of opportunity differ from neoadjuvant strategies, in which drugs are given with the primary objective of inducing tumor shrinkage for efficacy purposes. The main window of opportunity used in HNSCC has been before primary surgery. This setting is ideal for several reasons. First, the posttreatment sample, which is essential to identify PD biomarkers, is obviously easy to collect from the surgical specimen. Second, tumors at this stage have fewer molecular alterations than when they have been exposed to prior treatments. Window-of-opportunity trials before radiation-based therapy have been rarely performed, with the main drawback being the necessity of a posttreatment sample.
Window-of-opportunity trials have been rare, especially in HNSCC (105). Preoperative trials in HNSCC represent only 5% of all preoperative trials performed in oncology (105). Window-of-opportunity trials represented 8% of all trials of molecular targeted therapy conducted in HNSCC in our review.
Window-of-opportunity trials are challenging for several reasons. Because the primary objective of these trials is not efficacy, one must ensure that the participation of patients in these trials will not jeopardize the efficacy of the definitive treatment. The toxicity profile of drugs must be favorable and preferentially known for the tumor type of interest, although this was not always the case. The molecular targeted therapy, although given for a short period of time, sometimes produces antitumor activity. Tumor shrinkage might therefore lead to a surgical plan change or a down-staging of the tumor, which might result in undertreatment. Tattooing the original tumor region might help overcome this issue. The last challenge is the study design. Sample size calculation is tricky in these biomarker-finding trials without necessarily any relevant putative biomarker that would help set the null and the alternative hypotheses. A placebo or a no-treatment arm is highly desirable because controlled experimental conditions allow more rigorous investigation of biomarkers, but reinforces the necessity not to delay the definitive treatment. Potential biomarkers need to be correlated with biological or clinical activity. Because objective responses are not unexpectedly rare, taking the objective response as a continuous variable might be useful to assess the predictive or the PD value of potential biomarkers, because no surrogate biological characteristics of efficacy have been validated so far.
The inclusion of patients in these window-of-opportunity trials is challenging because patients know they can be cured with the planned definitive treatment without participating in the clinical trial. Finally, participation in such a trial often mandates additional imaging, blood sampling, and tumor biopsies.
Few window-of-opportunity clinical trials have been conducted in HNSCC evaluating mostly EGFR inhibitors (106–113), but also MEK (114), SRC (115), and STAT3 inhibitors (116). Predictive and/or PD biomarkers were identified in most of these trials. However, none of these biomarkers were further validated.
Umbrella Trials
Umbrella trials are precision medicine trials in which patients sharing the same disease condition are treated with different drugs depending on the molecular profile of their tumor. Umbrella trials are usually parallel phase II trials with as many treatment arms as the number of molecular subgroups. In these trials, a molecular profiling has to be performed before patients can be allocated to one or the other arm. In some trials, molecular profiling has to be performed on an on-purpose biopsy, whereas in others, analyses can be conducted on the primary tumor. The main advantage of these trials is that they are usually set up so all patients will be allocated to a treatment arm, even patients without identified druggable molecular alterations. It is of particular interest for arms for which the incidence of the molecular alteration is low. On the contrary, inclusion of patients can be hampered by the inability to perform the molecular analyses because of the difficulty of getting tumor tissue or because analyses have failed. One of the major points is establishment of a treatment algorithm before the trial opens. Each arm tests the efficacy of matched targeted therapy in an enriched subgroup of patients.
Two umbrella trials have recently started in R/M HNSCC. TRIUMPH is run by the Korean Cancer Study Group and involves a PI3K inhibitor, a dual EGFR/HER2 inhibitor, a CDK inhibitor, and an FGFR inhibitor given as single agents based on the results of targeted sequencing, and PD-L1, CD8+ TILs, and p16 protein expressions (NCT03292250). UPSTREAM, which is run by the European Organisation for Research and Treatment of Cancer, involves a pan-HER inhibitor, and a CDK inhibitor given as single agents based on the results of EGFR, HER2, and CCND1 amplifications, EGFR and RAS mutations, and PTEN and p16 protein expressions (NCT03088059). These trials are flexible and new treatment arms may open if new drugs become available for testing.
Basket Trials
Basket trials are precision medicine trials in which a specific drug is given to patients in a histology-agnostic way in patients harboring a (or several) molecular alteration(s) that are usually the target of the drug under investigation. These trials are usually parallel phase II trials with as many cohorts as there are different cancer types. These trials allow the evaluation of the efficacy of a molecular targeted therapy in multiple tumor types. These trials can be challenging if the incidence of the molecular alteration is low, resulting in screening many patients with ultimately only a few patients being treated. This kind of trial best fits into sequencing programs for which patients are screened for multiple molecular alterations. Algorithm-based precision medicine trials such as SHIVA01 (117) also include HNSCC patients who might benefit from molecular targeted therapy. As an example, one patient among the 11 HNSCC patients treated in SHIVA01 and harboring a PTEN inactivation experienced a 9-month disease stabilization with everolimus (mTOR inhibitor) (118).
Despite better knowledge of the biology both of HPV-positive and HPV-negative HNSCC, no other targets have been clinically validated beyond EGFR since the approval of cetuximab. So far, the main strategy for the clinical development of molecular targeted therapy in HNSCC has been a chemo-additive strategy, mostly using EGFR-targeting agents or the evaluation of novel molecular targeted therapies without molecular enrichment. However, innovative clinical trial designs have recently been developed that may boost the development of molecular targeted therapy in HNSCC, including window-of-opportunity clinical trials and precision medicine clinical trials such as umbrella and basket trials.
Some molecular targeted therapies might have failed in randomized trials just because their efficacy is confined to a subgroup of patients who had not been previously identified. Although no other molecular targeted therapy beside cetuximab has demonstrated an OS benefit, the experience in other cancers indicates that some may well have benefitted had they been tested in biomarker-selected populations. For example, the greatest successes of targeted agents to date are arguably all in enriched patient populations, such as trastuzumab in HER2-positive breast cancer or crizotinib in ALK-translocated non–small cell lung cancer (119,120). In addition, meta-analyses have clearly shown that enriched strategies in oncology correlate with improved outcomes in patients included in clinical trials with molecular targeted therapies (121).
Precision medicine trials using molecular selection to guide therapy also include umbrella and basket trials (122). The UPSTREAM and TRIUMPH umbrella trials are currently ongoing in R/M HNSCC and represent an elegant way to propose individualized molecular targeted therapy for all patients. Despite limitations of these precision medicine trials, when it comes to tissue provision, tumor heterogeneity, and low frequency of molecular alterations, physicians treating HNSCC patients should be encouraged to include their patients in precision medicine trials.
Window-of-opportunity clinical trials in HNSCC are recent and have been rare so far. These trials are key to identifying biomarkers of efficacy of molecular targeted therapy. Although they are challenging to conduct, they seem to be safe and provide clinical benefit in some patients. These trials represent powerful tools to identify predictive and/or PD biomarkers of efficacy of anticancer drugs that will need to be validated further in the clinical settings in which the drugs will be used. However, a close collaboration between medical oncologists and surgeons is clearly a prerequisite for successful and rapid patients’ accrual.
Finally, it remains to be determined what will be the place of molecular targeted therapy in HNSCC in the context of immunotherapy. Immune checkpoint inhibitors targeting programmed cell death-1 have been demonstrated to improve OS in the R/M setting (13,14,16). In addition, responses may sometimes be durable (123). These durable responses have now become the goal of what we would like to see achieved in all R/M HNSCC patients. Immunotherapy is now being evaluated earlier in the disease in the locally advanced setting. Clinical trials evaluating the combination of molecular targeted therapy with immunotherapy are emerging (NCT02499328, NCT02538510, NCT02501096, NCT03370276, NCT03082534). The future will tell whether molecular targeted therapy and immunotherapy benefit different patients with different molecular alterations, or whether there is space for combinations.
Notes
Affiliations of authors: Department of Drug Development and Innovation (D3i), Institut Curie, Paris & Saint-Cloud, France (PG, CMB, MK, EB, NT, CLT); AP-HP, Pitié-Salpêtrière Hospital, Department of Pharmacology, CIC-1421, CLIP2 Galilée, Paris, France (PG); Cancer Clinical Trial Centre, Austin Hospital, Heidelberg, Melbourne, Australia (HKG); Pharmacogenomics Unit, Institut Curie, Paris, France (IB); INSERM U900 Research Unit, Saint-Cloud, France (CLT); Paris-Saclay University, Paris, France (CLT).
CLT reports participation in advisory boards of BMS, MSD, Astra Zeneca, Roche, Merck Serono, Amgen, and Novartis, outside the submitted work. None of the other authors declare any conflict of interest.
All authors have contributed to the conception and writing of the manuscript.
References
- 1. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403.. [DOI] [PubMed] [Google Scholar]
- 3. D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 5. Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. [DOI] [PubMed] [Google Scholar]
- 6. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. [DOI] [PubMed] [Google Scholar]
- 7. The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–550. [DOI] [PubMed] [Google Scholar]
- 10. Weinstein IB, Joe A.. Oncogene addiction. Cancer Res. 2008;68(9):3077–3080. [DOI] [PubMed] [Google Scholar]
- 11. McLornan DP, List A, Mufti GJ.. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725–1735. [DOI] [PubMed] [Google Scholar]
- 12. Muller FL, Colla S, Aquilanti E, et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature. 2012;488(7411):337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. [DOI] [PubMed] [Google Scholar]
- 15. Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi.org/10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M.. KEYNOTE-048: phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ann Oncol. 2018;29(suppl 8). doi.org/10.1093/annonc/mdy424.045. [Google Scholar]
- 17. Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–7356. [PubMed] [Google Scholar]
- 18. Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171–2177. [DOI] [PubMed] [Google Scholar]
- 19. Cohen EEW, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21(10):1980–1987. [DOI] [PubMed] [Google Scholar]
- 20. Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL.. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22(1):77–85. [DOI] [PubMed] [Google Scholar]
- 21. Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA.. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(34):8646–8654. [DOI] [PubMed] [Google Scholar]
- 22. Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14(8):697–710. [DOI] [PubMed] [Google Scholar]
- 23. Wirth LJ, Dakhil S, Kornek G, et al. PARTNER: an open-label, randomized, phase 2 study of docetaxel/cisplatin chemotherapy with or without panitumumab as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2016;61:31–40. [DOI] [PubMed] [Google Scholar]
- 24. Machiels J-P, Subramanian S, Ruzsa A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 2011;12(4):333–343. [DOI] [PubMed] [Google Scholar]
- 25. Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2013;31(11):1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart JSW, Cohen EEW, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol. 2009;27(11):1864–1871. [DOI] [PubMed] [Google Scholar]
- 27. Machiels J-P, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583–594. [DOI] [PubMed] [Google Scholar]
- 28. Seiwert TY, Fayette J, Cupissol D, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25(9):1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Licitra L, Mesia R, Rivera F, et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol. 2011;22(5):1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas Koch D, Pickhard A, Gebel L, et al. Epidermal growth factor receptor variant III in head and neck squamous cell carcinoma is not relevant for targeted therapy and irradiation. Oncotarget. 2017;8(20):32668–32682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13(5):1552–1561. [DOI] [PubMed] [Google Scholar]
- 32. Monteverde M, Milano G, Strola G, et al. The relevance of ADCC for EGFR targeting: a review of the literature and a clinically-applicable method of assessment in patients. Crit Rev Oncol Hematol. 2015;95(2):179–190. [DOI] [PubMed] [Google Scholar]
- 33. Imai K, Takaoka A.. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6(9):714–727. [DOI] [PubMed] [Google Scholar]
- 34. Bertotti A, Sassi F.. Molecular pathways: sensitivity and resistance to anti-EGFR antibodies. Clin Cancer Res. 2015;21(15):3377–3383. [DOI] [PubMed] [Google Scholar]
- 35. Fayette J, Wirth L, Oprean C, et al. Randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN Study). Front Oncol. 2016;6:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harrington KJ, Forster MD, Le Tourneau C, et al. Randomized phase 2 trial of patritumab (P) or placebo (PBO) + cetuximab (C) + cisplatin (CIS) or carboplatin (CAR) for recurrent and/or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2018;36(suppl 15):6045. [Google Scholar]
- 37. Jimeno A, Shirai K, Choi M, et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann Oncol. 2015;26(3):556–561. [DOI] [PubMed] [Google Scholar]
- 38. Soulières D, Faivre S, Mesía R, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18(3):323–335. [DOI] [PubMed] [Google Scholar]
- 39. Limaye S, Riley S, Zhao S, et al. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol. 2013;49(8):835–841. [DOI] [PubMed] [Google Scholar]
- 40. Gilbert J, Schell MJ, Zhao X, et al. A randomized phase II efficacy and correlative studies of cetuximab with or without sorafenib in recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2015;51(4):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swiecicki PL, Bellile E, Sacco AG, et al. A phase II trial of the BCL-2 homolog domain 3 mimetic AT-101 in combination with docetaxel for recurrent, locally advanced, or metastatic head and neck cancer. Invest New Drugs. 2016;34(4):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leemans CR, Snijders PJF, Brakenhoff RH.. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269–282. [DOI] [PubMed] [Google Scholar]
- 43. Borcoman E, Le Tourneau C.. Antibody drug conjugates: the future of chemotherapy? Curr Opin Oncol. 2016;28(5):429–436. [DOI] [PubMed] [Google Scholar]
- 44. Iwata H, Tamura K, Doi T, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: long-term results of a large phase 1 study with multiple expansion cohorts. J Clin Oncol. 2018;36(suppl 15):2501. [Google Scholar]
- 45. Phillips AC, Boghaert ER, Vaidya KS, et al. Characterization of ABBV-221, a tumor-selective EGFR-targeting antibody drug conjugate. Mol Cancer Ther. 2018;17(4):795–805. [DOI] [PubMed] [Google Scholar]
- 46. Chung CH, Parker JS, Karaca G, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5(5):489–500. [DOI] [PubMed] [Google Scholar]
- 47. Belbin TJ, Singh B, Barber I, et al. Molecular classification of head and neck squamous cell carcinoma using cDNA microarrays. Cancer Res. 2002;62(4):1184–1190. [PubMed] [Google Scholar]
- 48. Walter V, Yin X, Wilkerson MD, et al. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One. 2013;8(2):e56823.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–881. [DOI] [PubMed] [Google Scholar]
- 50. cBioPortal for Cancer Genomics. http://www.cbioportal.org/. Accessed July 5, 2019.
- 51. Gillison ML, Akagi K, Xiao W, et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 2019;29(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dubot C, Bernard V, Sablin MP, et al. Comprehensive genomic profiling of head and neck squamous cell carcinoma reveals FGFR1 amplifications and tumour genomic alterations burden as prognostic biomarkers of survival. Eur J Cancer. 2018;91:47–55. doi.org/10.1016/j.ejca.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 53. Schmitz S, Kaminsky-Forrett M-C, Henry S, et al. Phase II study of figitumumab in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: clinical activity and molecular response (GORTEC 2008-02). Ann Oncol. 2012;23(8):2153–2161. [DOI] [PubMed] [Google Scholar]
- 54. Joerger M, Takahashi S, Sayehli CM, Slosarczyk S, Navarro Mendivil A.. Phase I experience with rogaratinib in patients with head and neck cancer selected based on FGFR mRNA overexpression. Ann Oncol. 29(suppl 8):viii372–viii399. [Google Scholar]
- 55. Chen X, Makarewicz JM, Knauf JA, Johnson LK, Fagin JA.. Transformation by Hras(G12V) is consistently associated with mutant allele copy gains and is reversed by farnesyl transferase inhibition. Oncogene. 2014;33(47):5442–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanrahan EO, Kies MS, Glisson BS, et al. A phase II study of lonafarnib (SCH66336) in patients with chemorefractory, advanced squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2009;32(3):274–279. [DOI] [PubMed] [Google Scholar]
- 57. Ho A, Chau N, Wong DJ, et al. Abstract LB-A10: preliminary results from a phase 2 proof of concept trial of tipifarnib in tumors with HRAS mutations. Mol Cancer Ther. 2018;17(suppl 1): LB-A10–LB-A10. [Google Scholar]
- 58. Sablin M-P, Dubot C, Klijanienko J, et al. Identification of new candidate therapeutic target genes in head and neck squamous cell carcinomas. Oncotarget. 2016;7(30):47418–47430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sewell A, Brown B, Biktasova A, et al. Reverse-phase protein array profiling of oropharyngeal cancer and significance of PIK3CA mutations in HPV-associated head and neck cancer. Clin Cancer Res. 2014;20(9):2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Geiger JL, Bauman JE, Gibson MK, et al. Phase II trial of everolimus in patients with previously treated recurrent or metastatic head and neck squamous cell carcinoma. Head Neck. 2016;38(12):1759–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grünwald V, Keilholz U, Boehm A, et al. TEMHEAD: a single-arm multicentre phase II study of temsirolimus in platin- and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO). Ann Oncol. 2015;26(3):561–567. [DOI] [PubMed] [Google Scholar]
- 63. Massarelli E, Lin H, Ginsberg LE, et al. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2015;26(7):1476–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dunn LA, Fury MG, Xiao H, et al. A phase II study of temsirolimus added to low-dose weekly carboplatin and paclitaxel for patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). Ann Oncol. 2017;28(10):2533–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fury MG, Sherman E, Ho AL, et al. A phase 1 study of everolimus plus docetaxel plus cisplatin as induction chemotherapy for patients with locally and/or regionally advanced head and neck cancer. Cancer. 2013;119(10):1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fury MG, Sherman E, Ho A, et al. A phase I study of temsirolimus plus carboplatin plus paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC). Cancer Chemother Pharmacol. 2012;70(1):121–128. [DOI] [PubMed] [Google Scholar]
- 67. Saba NF, Hurwitz SJ, Magliocca K, et al. Phase 1 and pharmacokinetic study of everolimus in combination with cetuximab and carboplatin for recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer. 2014;120(24):3940–3951. [DOI] [PubMed] [Google Scholar]
- 68. Soulières D, Licitra L, Mesía R, et al. Molecular alterations and buparlisib efficacy in patients with squamous cell carcinoma of the head and neck: biomarker analysis from BERIL-1. Clin Cancer Res. 2018;24(11):2505–2516. [DOI] [PubMed] [Google Scholar]
- 69. Michel L, Ley J, Wildes TM, et al. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016;58:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Méndez E, Rodriguez CP, Kao MC, et al. A phase I clinical trial of AZD1775 in combination with neoadjuvant weekly docetaxel and cisplatin before definitive therapy in head and neck squamous cell carcinoma. Clin Cancer Res. 2018;24(12):2740–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sánchez-Danés A, Blanpain C.. Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer. 2018;18(9):549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bykov VJN, Eriksson SE, Bianchi J, Wiman KG.. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. [DOI] [PubMed] [Google Scholar]
- 73. Castellanos MR, Pan Q.. Novel p53 therapies for head and neck cancer. World J Otorhinolaryngol Head Neck Surg. 2016;2(2):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Verhagen CVM, Vossen DM, Borgmann K, et al. Fanconi anemia and homologous recombination gene variants are associated with functional DNA repair defects in vitro and poor outcome in patients with advanced head and neck squamous cell carcinoma. Oncotarget. 2018;9(26):18198–18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sun W, Gaykalova DA, Ochs MF, et al. Activation of the NOTCH pathway in head and neck cancer. Cancer Res. 2014;74(4):1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kubicek GJ, Axelrod RS, Machtay M, et al. Phase I trial using the proteasome inhibitor bortezomib and concurrent chemoradiotherapy for head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2012;83(4):1192–1197. [DOI] [PubMed] [Google Scholar]
- 77. Argiris A, Duffy AG, Kummar S, et al. Early tumor progression associated with enhanced EGFR signaling with bortezomib, cetuximab, and radiotherapy for head and neck cancer. Clin Cancer Res. 2011;17(17):5755–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chung CH, Aulino J, Muldowney NJ, et al. Nuclear factor-kappa B pathway and response in a phase II trial of bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2010;21(4):864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Morris LGT, Chandramohan R, West L, et al. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform. JAMA Oncol. 2017;3(2):244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. [DOI] [PubMed] [Google Scholar]
- 81. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. [DOI] [PubMed] [Google Scholar]
- 82. Wilson JA, Carding PN, Patterson JM.. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg. 2011;145(5):767–771. [DOI] [PubMed] [Google Scholar]
- 83. Pignon JP, Bourhis J, Domenge C, Designé L.. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355(9208):949–955. [PubMed] [Google Scholar]
- 84. Pignon J-P, Le Maître A, Maillard E, Bourhis J, Mach NC, Collaborative Group.. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. [DOI] [PubMed] [Google Scholar]
- 85. Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Siu LL, Waldron JN, Chen BE, et al. Effect of standard radiotherapy with cisplatin vs accelerated radiotherapy with panitumumab in locoregionally advanced squamous cell head and neck carcinoma: a randomized clinical trial. JAMA Oncol. 2017;3(2):220–226. [DOI] [PubMed] [Google Scholar]
- 89. Mesía R, Henke M, Fortin A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):208–220. [DOI] [PubMed] [Google Scholar]
- 90. Giralt J, Trigo J, Nuyts S, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):221–232. [DOI] [PubMed] [Google Scholar]
- 91. Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol. 2013;31(11):1415–1421. [DOI] [PubMed] [Google Scholar]
- 92. Gregoire V, Hamoir M, Chen C, et al. Gefitinib plus cisplatin and radiotherapy in previously untreated head and neck squamous cell carcinoma: a phase II, randomized, double-blind, placebo-controlled study. Radiother Oncol. 2011;100(1):62–69. [DOI] [PubMed] [Google Scholar]
- 93. Harrington K, Berrier A, Robinson M, et al. Randomised phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur J Cancer. 2013;49(7):1609–1618. [DOI] [PubMed] [Google Scholar]
- 94. Harrington K, Temam S, Mehanna H, et al. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: a phase III, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2015;33(35):4202–4209. [DOI] [PubMed] [Google Scholar]
- 95. Specenier PM, Remenar E, Buter J, et al. TPF plus cetuximab induction chemotherapy followed by biochemoradiation with weekly cetuximab plus weekly cisplatin or carboplatin: a randomized phase II EORTC trial. Ann Oncol. 2017;28(9):2219–2224. [DOI] [PubMed] [Google Scholar]
- 96. Colevas AD, Brown JM, Hahn S, et al. Development of investigational radiation modifiers. J Natl Cancer Inst. 2003;95(9):646–651. [DOI] [PubMed] [Google Scholar]
- 97. Harrington KJ, El-Hariry IA, Holford CS, et al. Phase I study of lapatinib in combination with chemoradiation in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(7):1100–1107. [DOI] [PubMed] [Google Scholar]
- 98. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. [DOI] [PubMed] [Google Scholar]
- 99. Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–1704. [DOI] [PubMed] [Google Scholar]
- 100. Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–264. [DOI] [PubMed] [Google Scholar]
- 101. Cohen EEW, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hitt R, Grau JJ, López-Pousa A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25(1):216–225. [DOI] [PubMed] [Google Scholar]
- 103. Lee K-W, Koh Y, Kim S-B, et al. A randomized, multicenter, phase II study of cetuximab with docetaxel and cisplatin as induction chemotherapy in unresectable, locally advanced head and neck cancer. Oncologist. 2015;20(10):1119–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Burtness B, Haddad RI, Dinis J, et al. LUX-head and neck 2: randomized, double-blind, placebo-controlled, phase III trial of afatinib as adjuvant therapy after chemoradiation (CRT) in primary unresected, high/intermediate-risk, squamous cell cancer of the head and neck (HNSCC) patients (pts). J Clin Oncol. 2017;35(suppl 15):6001. [Google Scholar]
- 105. Marous M, Bièche I, Paoletti X, et al. Designs of preoperative biomarkers trials in oncology: a systematic review of the literature. Ann Oncol. 2015;26(12):2419–2428. [DOI] [PubMed] [Google Scholar]
- 106. Machiels J-P, Bossi P, Menis J, et al. Activity and safety of afatinib in a window preoperative EORTC study in patients with squamous cell carcinoma of the head and neck (SCCHN). Ann Oncol. 2018;29(4):985–991. [DOI] [PubMed] [Google Scholar]
- 107. Shayan G, Kansy BA, Gibson SP, et al. Phase Ib study of immune biomarker modulation with neoadjuvant cetuximab and TLR8 stimulation in head and neck cancer to overcome suppressive myeloid signals. Clin Cancer Res. 2018;24(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Temam S, Spicer J, Farzaneh F, et al. An exploratory, open-label, randomized, multicenter study to investigate the pharmacodynamics of a glycoengineered antibody (imgatuzumab) and cetuximab in patients with operable head and neck squamous cell carcinoma. Ann Oncol. 2017;28(11):2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Van Allen EM, Lui VWY, Egloff AM, et al. Genomic correlate of exceptional erlotinib response in head and neck squamous cell carcinoma. JAMA Oncol. 2015;1(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gross ND, Bauman JE, Gooding WE, et al. Erlotinib, erlotinib-sulindac versus placebo: a randomized, double-blind, placebo-controlled window trial in operable head and neck cancer. Clin Cancer Res. 2014;20(12):3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schmitz S, Hamoir M, Reychler H, et al. Tumour response and safety of cetuximab in a window pre-operative study in patients with squamous cell carcinoma of the head and neck. Ann Oncol. 2013;24(9):2261–2266. [DOI] [PubMed] [Google Scholar]
- 112. Del Campo JM, Hitt R, Sebastian P, et al. Effects of lapatinib monotherapy: results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br J Cancer. 2011;105(5):618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Thomas F, Rochaix P, Benlyazid A, et al. Pilot study of neoadjuvant treatment with erlotinib in nonmetastatic head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13(23):7086–7092. [DOI] [PubMed] [Google Scholar]
- 114. Uppaluri R, Winkler AE, Lin T, et al. Biomarker and tumor responses of oral cavity squamous cell carcinoma to trametinib: a phase II neoadjuvant window-of-opportunity clinical trial. Clin Cancer Res. 2017;23(9):2186–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bauman JE, Duvvuri U, Gooding WE, et al. Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI Insight. 2017;2(6):e90449.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sen M, Thomas SM, Kim S, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2(8):694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Le Tourneau C, Delord J-P, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–1334. [DOI] [PubMed] [Google Scholar]
- 118. Basse C, Morel C, Callens C, et al. Exploitation of precision medicine trials data: examples of long responders from the SHIVA01 trial. J Clin Oncol Precision Oncol. 2018;(2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shaw AT, Kim D-W, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 121. Schwaederle M, Zhao M, Lee JJ, et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J Clin Oncol. 2015;33(32):3817–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Le Tourneau C, Kamal M, Alt M, et al. The spectrum of clinical trials aiming at personalizing medicine. Chin Clin Oncol. 2014;3(2):13.. [DOI] [PubMed] [Google Scholar]
- 123. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. [DOI] [PubMed] [Google Scholar]