Abstract
Disease recurrence (locoregional, distant) exerts a significant clinical impact on the survival of estrogen receptor–positive breast cancer patients. Many of these recurrences occur late, more than 5 years after original diagnosis, and represent a major obstacle to the effective treatment of this disease. Indeed, methods to identify patients at risk of late recurrence and therapeutic strategies designed to avert or treat these recurrences are lacking. Therefore, an international workshop was convened in Toronto, Canada, in February 2018 to review the current understanding of late recurrence and to identify critical issues that require future study. In this article, the major issues surrounding late recurrence are defined and current approaches that may be applicable to this challenge are discussed. Specifically, diagnostic tests with potential utility in late-recurrence prediction are described as well as a variety of patient-related factors that may influence recurrence risk. Clinical and therapeutic approaches are also reviewed, with a focus on patient surveillance and the implementation of extended endocrine therapy in the context of late-recurrence prevention. Understanding and treating late recurrence in estrogen receptor–positive breast cancer is a major unmet clinical need. A concerted effort of basic and clinical research is required to confront late recurrence and improve disease management and patient survival.
Recurrence 5 years or more after a diagnosis is commonly referred to as “late recurrence” and accounts for about one-half of all recurrences of estrogen receptor–positive (ER+) breast cancer (BC), whereas it is considerably less common in receptor-negative disease. Identifying who is at greatest risk for late recurrence and developing strategies to prevent it has emerged as a major unmet need (Figure 1 in Pan et al.) (1). To address this issue, an international workshop was convened in Toronto, Canada, in February 2018 that involved clinicians, trialists, scientists, and funders with expertise and/or interest in late recurrence (a list of participants is included as an Supplementary Appendix, available online). The goal of this workshop was to review current knowledge about the issue of late recurrence in this patient population and to make recommendations for future research strategies. This article summarizes the workshop discussions regarding our current understanding of the risk of late recurrence and related clinical issues. A companion article (2) discusses potential biomarkers of late recurrence and details research recommendations. Here, we begin by providing a working definition of late recurrence and by reviewing our current understanding of the biological factors contributing to late recurrence, the clinicopathologic features associated with late recurrence, and current therapeutic interventions to potentially prevent it.
Figure 1.
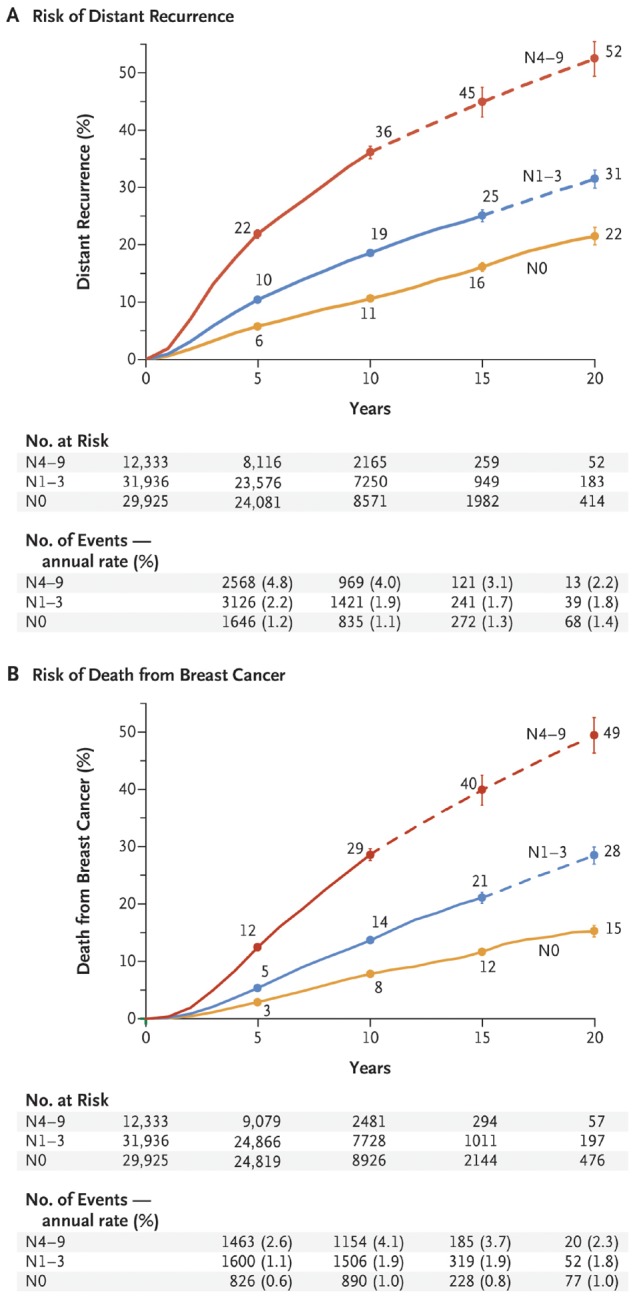
Association between pathological nodal status and the risk of distant recurrence or death from breast cancer (BC) during the 20-year study period. Shown are data regarding the risk of distant recurrence (A) and death from BC (B) among 74 194 women with estrogen receptor–positive T1 or T2 disease who were enrolled in 78 trials at year 0 and were scheduled to receive 5 years of endocrine therapy. (Data for another 10 200 women who enrolled in 10 trials after year 0 are not shown here.) The risk was calculated according to the patients’ pathological nodal status at the time of diagnosis: N0, N1–3, or N4–9. The number of events and annual rate are shown for the preceding period (eg, data for years 0–4 are shown at 5 years). The I bars indicate 95% confidence intervals. The dashed lines indicate that the event rate is for the whole 5-year period rather than for individual years, as is otherwise shown. The annual rate of death from BC was estimated by subtracting the death rate in women without recurrence from the rate in all women. From (1). Copyright © (2017) Massachusetts Medical Society. Reprinted with permission from the Massachusetts Medical Society and the Early Breast Cancer Trialists’ Collaborative Group.
Current Understanding of the Problem of Late Recurrence
Earlier diagnosis of BC as a result of screening, coupled with the use of more effective systemic adjuvant therapy to prevent recurrences, has significantly improved clinical BC outcomes, particularly during the first 5 to 10 years after diagnosis. Adjuvant chemotherapy and anti-HER2–directed therapy substantially reduce the risk of recurrence, with the greatest risk reduction noted within the first 5 years. Adjuvant endocrine treatment (ET) also reduces recurrence risk in patients with ER+ BC, with benefits observed within the first 5 years and also after completing a 5-year course due to “carryover effect” associated with tamoxifen in particular.
In the workshop, we defined late recurrence as that occurring more than 5 years after diagnosis and focused our deliberations on distant (metastatic) recurrence as opposed to local-regional recurrence or new ipsilateral or contralateral primary occurrences. Although the annual risk of distant recurrence is higher in the first 5 years of follow-up and is decreased by adjuvant chemotherapy and ET, as many as one-half of the life-threatening BC recurrences and deaths in patients with ER+ HER2− BC take place in the succeeding 15 or more years, often after completion of adjuvant hormonal therapy (1). There is an urgent, unmet need to identify factors beyond standard clinical and pathologic features that affect the risk of recurrence and inform treatment decisions in individual patients.
The risk of late recurrence raises the need for more aggressive or novel therapeutic approaches. However, these therapies are not without side effects, including potentially rare but life-threatening toxicities, and with important financial costs. Thus, as with all therapies, the goal is for clinicians to identify the majority of patients who will never recur and do not need additional therapy vs those who are at risk. This discrimination would be facilitated by enhanced understanding of why late recurrences occur and by the development of methods to identify individual patients who are at risk of late recurrence, with a focus on those at imminent risk (ie, within the next 1–2 years) for whom intervention may be effective in preventing recurrence.
Estimating Risk of Late Recurrence in Patients with ER+ Early-Stage BC
Traditional tumor-specific characteristics in patients with ER+ BC are significantly associated with clinical risk of recurrence out to at least 20 years postdiagnosis. In a patient-level meta-analysis, patients within each tumor diameter and nodal status (TN) category exhibited an appreciable risk of recurrence, with risk increasing with larger tumor size and nodal status (Table 1 [1]). For example, patients with T1N0 disease exhibited a 13% risk of distant recurrence from 5 to 20 years post diagnosis. This distant recurrence rate is in comparison with those patients with T1N1 and T2N1 cancers who had a risk of 20% and 26% between 5 and 20 years postdiagnosis and those with T1N2 and T2N2 cancers with a 34% and 41% risk, respectively. Among patients with T1N0 BCs, risk was higher with increasing tumor grade; risk of distant recurrence between 5 and 20 years postdiagnosis was 10% in those with low-grade tumors compared with 17% in those with high-grade tumors (1). It is possible that these data overestimate contemporary rates of late recurrence because of recent improvements in the treatment of ER+ BC, the implementation of more accurate tumor staging, and the possibility of suboptimal treatment adherence in earlier patient cohorts. However, they highlight the importance of characterizing the underlying mechanisms of late recurrence and the development of monitoring and treatment strategies for BC patients beyond 5 years postdiagnosis. Indeed, the clinical treatment score after 5 years (https://www.cts5-calculator.com/) is a clinically validated algorithm that integrates tumor characteristics, including size, grade, number of positive axillary lymph nodes, and age (at diagnosis), to provide an estimate of the risk of recurrence over the ensuing 5 years, which may facilitate providing an accurate estimate of late recurrence risk at 10 years for patients who are cancer free at 5 years (3,4).
Table 1.
Association of tumor size, nodal status, and grade with risk of recurrence in years 5 to 10 and 10 to 20*
| Women event free at 5 y Total No. | Annual rate of distant recurrence |
Cumulative risk from 5 to 20 y (%) | ||
|---|---|---|---|---|
| Variable | 5 to <10 y (%) | 10 to 20 y (%) | ||
| Nodal involvement | ||||
| N0 | 28 847 | 1.0 | 1.1 | 15 |
| N1–3 | 25 292 | 1.9 | 1.7 | 23 |
| N4–9 | 8784 | 3.9 | 2.8 | 38 |
| Tumor diameter (N0 only) | ||||
| T1a or T1b: ≤1.0 cm | 5527 | 0.5 | 0.8 | 10 |
| T1c: 1.1–2.0 cm | 13 875 | 0.8 | 1.1 | 14 |
| T2: 2.1–3.0 cm | 6700 | 1.5 | 1.4 | 19 |
| T2: 3.1–5.0 cm | 2745 | 1.7 | 1.4 | 20 |
| Tumor grade (T1N0 only) | ||||
| Low | 3524 | 0.4 | 0.8 | 10 |
| Moderate | 7363 | 0.7 | 1.0 | 13 |
| High | 3054 | 0.9 | 1.5 | 17 |
Data are for 62 923 patients with T1 or T2 ER+ disease with 0 to 9 positive nodes that were to receive 5 years of adjuvant ET and were disease free at 5 years. Most patients entered the study at diagnosis but some entered later, having already received 2 to 5 years of ET, and were randomly assigned to stop therapy at 5 years. P less than .001 for all subgroup comparisons. Modified from (1). ER+ = estrogen receptor positive; ET = endocrine treatment.
However, these standard clinicopathologic features, although prognostic, are far from ideal predictors of who is at risk of late recurrence and do not provide sufficient guidance to make treatment decisions at the individual level because a substantial number of patients in each risk group will not experience a relapse within the 20-year follow-up period. In this regard, several diagnostic tests and scores derived from analyses of the primary tumor have been developed to identify patients at risk of early or late recurrence vs patients for whom deescalation of treatment is appropriate (4). These tests include the immunohistochemical 4 (IHC4) protein test, 21-gene Recurrence Score (OncotypeDx), PAM50 intrinsic subtype (ProSigna), 12-gene Recurrence Score (EndoPredict), 2-component Breast Cancer Index (based on the molecular grade index and HOXB13: IL17BR), and 70-gene signature (Mammaprint) (5–17) (Table 2).
Table 2.
Univariate HRs and 95% CI indexes for all prognostic signatures in postmenopausal women according to nodal status during years 0 to 10*
| Signature | Patient group |
|||
|---|---|---|---|---|
| Node-negative disease (n = 591) |
Node-positive disease (n = 227) |
|||
| HR = (95% CI) | C index (95% CI) | HR = (95% CI) | C index (95% CI) | |
| CTS5 | 1.99 (1.58 to 2.50) | 0.721 (0.668 to 0.774) | 1.63 (1.20 to 2.21) | 0.640 (0.554 to 0.726) |
| IHC4 | 1.95 (1.55 to 2.45) | 0.725 (0.665 to 0.785) | 1.33 (0.99 to 1.78) | 0.601 (0.511 to 0.690) |
| RS | 1.69 (1.40 to 2.03) | 0.667 (0.585 to 0.750) | 1.39 (1.05 to 1.85) | 0.603 (0.513 to 0.693) |
| BCI | 2.46 (1.88 to 3.23) | 0.762 (0.704 to 0.820) | 1.67 (1.21 to 2.29) | 0.652 (0.566 to 0.739) |
| ROR | 2.56 (1.96 to 3.35) | 0.764 (0.707 to 0.821) | 1.58 (1.16 to 2.15) | 0.636 (0.552 to 0.719) |
| EPclin | 2.14 (1.71 to 2.68) | 0.765 (0.716 to 0.814) | 1.69 (1.29 to 2.22) | 0.671 (0.590 to 0.752) |
BCI = Breast Cancer Index; CI = confidence interval; CTS5 = Clinical Treatment Score-5 y; EPclin = EndoPredict clinical score; HR = hazard ratio; IHC4 = immunohistochemical 4 protein test; ROR = PAM 50 Risk of Recurrence Score; RS = Oncotype DX Recurrence Score. All HRs indicate a change of 1 SD. Adapted from (3).
Each of the six assays has been evaluated in at least one dataset and reported to have prognostic utility beyond the first 5 years for patients who have achieved that milestone on adjuvant ET without a recurrence (4,18,19). However, each of these studies has been a “prospective retrospective” study, applying the assay to archived tissue specimens collected previously from patients who participated in prospective trials. Of these assays, only the 2-component Breast Cancer Index was shown to be predictive of benefit from letrozole after a prior 5-year course of tamoxifen (19). Although the use of prospective-retrospective studies is an acceptable strategy to determine clinical utility, it has been suggested that at least 2 or more studies derived from different datasets showing similar, if not identical, results are required before the assay could be considered to achieve clinical utility (20). For some of the assays, efforts to validate the late prognostic effect have resulted in mixed results, and for others no validation studies have been reported. Thus, neither the American Society of Clinical Oncology nor the National Cancer Center Network suggests using the results of these assays in the primary tumor to guide decisions for patients who have reached 5 years without a recurrence.
Surveillance Imaging and Serologic Tests for Identification of Asymptomatic Late Recurrences
One potential approach to identifying patients at risk of late recurrence is to perform routine surveillance (radiographic or scintigraphic imaging and/or circulating liver- and bone-related enzymes or tumor marker tests) for an occult but impending recurrence in asymptomatic patients (21). These investigations would be performed serially to identify recurrent disease before it is clinically evident, with the hope that treatment initiation will either prevent subsequent symptomatic metastases or prolong overall survival (OS). However, it has been emphasized that simply demonstrating the appearance of asymptomatic metastases is not sufficient to support routine use of more intensive screening (21). Rather, doing so must result in improved clinical cancer outcomes, ideally improved OS or quality of life.
In this regard, five prospective, randomized clinical trials have compared outcomes for patients who have undergone intensive vs nonintensive screening. Because these studies were performed several decades ago, the diagnostic imaging was crude by today’s standards (chest X ray, liver ultrasound, and bone scan). A Cochrane review of these studies (22) found high-quality evidence of no effect of intensive screening on OS (hazard ratio [HR] = 0.98, 95% confidence interval [CI] = 0.84 to 1.15) and low-quality evidence of no effect on disease-free survival (DFS) (HR = 0.84, 95% CI = 0.71 to 1.00).
It is possible that intensive follow-up would have greater clinical utility with newer, higher-quality imaging modalities like computed tomography (CT) and positron emission tomography (PET), which offer greater sensitivity and specificity over conventional techniques, albeit with higher cost and radiation exposure. In a systematic review of almost 30 studies investigating detection of recurrence in symptomatic BC patients, PET in addition to conventional imaging exhibited higher specificity (93% vs 83%) and sensitivity (89% vs 79%) when compared with conventional imaging alone (23). PET/CT offered better sensitivity (95% vs 80%) and specificity (89 vs 77%) vs CT alone. The potential benefit of these modalities in the routine surveillance of asymptomatic BC patients has not been studied.
Serial assessment of tumor markers such as cancer antigen (CA)15-3 (the soluble moiety of the MUC-1 glycoprotein) and carcinoembryonic antigen (CEA) in the years following diagnosis may be useful for the detection of distant metastatic recurrence. Although preoperative serum levels of these tumor markers are prognostic for shorter DFS and OS in BC patients, they could potentially provide prognostic value for distant metastasis during patient follow-up. Gion et al. (24) and Mariani et al. (25) have explored trade-offs among sensitivity, specificity, and positive and negative predictive values when using CA15-3 and CEA to detect recurrences. The best diagnostic accuracy was obtained using both biomarkers jointly and combining two positivity criteria. However, their “optimal” criteria were associated with a sensitivity (58%) that was too low to be clinically useful. Furthermore, the positive predictive value of these markers was greatest during the first years after BC diagnosis, falling to less than 20–30% by 10–12 years of follow-up. In separate research, increases in CA15-3 in serially collected serum samples provided the earliest indication of a distant recurrence before clinical or radiological detection in up to 55% of BC patients; the addition of CEA measurements increased sensitivity for recurrence detection (26–28). However, development of appropriate assay thresholds and serum collection timelines will be required (29) if these markers are to be considered for use in the early detection of asymptomatic metastases.
Rather than improvement in OS, one argument for serial surveillance is the reassurance provided by a negative test. However, in the absence of clinical signs or symptoms of recurrence, the negative predictive value of a distant recurrence over the succeeding 6 months (a reasonable time period between testing) approaches 99% without the marker results (24,30). Indeed, preferences for intensive vs standard follow-up differ among patients, who may express either feelings of security associated with additional monitoring or increased anxiety over receiving the results (21).
The recent advent of high-sensitivity blood tests for circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA) may affect our ability to detect BC recurrences in a time frame that allows administration of effective and perhaps potentially curative therapy in asymptomatic patients without any evidence of systemic disease. However, although these new modalities provide provocative possibilities, it has not been established whether use of these modalities in asymptomatic patients leads to better patient outcomes (OS or metastasis-free survival) rather than simply moving forward the time of diagnosis of incurable metastases. These newer approaches are discussed in greater detail in our companion paper.
Taken together, these results are the basis for current American Society of Clinical Oncology and National Cancer Center Network guidelines that recommend that BC survivors undergo regular clinical assessment (history, physical examination) and breast imaging without other systemic imaging procedures or tumor marker assessment (12,31).
Endocrine Interventions That Could Potentially Reduce the Risk of Late Recurrence
The fundamental reason to more accurately identify those without vs those with a credible risk of recurrence is to determine who might be spared, or benefit from, further therapy to reduce or prevent the recurrence. In this regard, 5 years of adjuvant ET, consisting of tamoxifen, aromatase inhibitors (AIs), or some sequencing of both, considerably reduces the risk of disease recurrence (locoregional and distant) and death from BC (32,33). Recent research has focused on the potential for extended (defined as treatment beyond 5 years) adjuvant ET to have similar effects on the risk of late recurrence.
Extended Tamoxifen Therapy
Several trials (including Adjuvant Tamoxifen–To Offer More? [aTTom]; Adjuvant Tamoxifen Longer Against Shorter [ATLAS]; Eastern Cooperative Oncology Group [ECOG]; E4181/E5181, National Surgical Adjuvant Breast and Bowel Project [NSABP B-14], and a Scottish trial) have investigated the impact of continuing tamoxifen (vs placebo) for 5 years after an initial 5 years of treatment (Table 3) (35–39). In a meta-analysis of these trials, little effect was seen on risk of recurrence during years 5–9 (while treatment was being administered, odds ratio = 1.01, 95% CI = 0.79 to 1.29), consistent with a beneficial carryover effect of the first 5 years of treatment in patients receiving placebo during years 5–9. Reduced risk was seen beyond 10 years (odds ratio = 0.88, 95% CI = 0.77 to 1.01) (34), reflecting carryover of the effect of tamoxifen that was administered during years 5–10. In the largest of these, the ATLAS trial, the risk of recurrence at 15 years postdiagnosis was 25.1% vs 21.4% (P = .002) and of death was 15.0% vs 12.2% (P = .01) for patients receiving placebo vs extended tamoxifen, respectively (36).
Table 3.
Summary of trials examining impact of extended tamoxifen therapy
| Trial name | Median follow-up, y | Sample size | Length of treatment in control arm, y | Length of treatment in experimental arm, y | OR for recurrence | 95% CI | P |
|---|---|---|---|---|---|---|---|
| ATLAS | 7.6* | 6846 | 5 | 10 | 0.84 | 0.74 to 0.94 | .003 |
| aTTom | 9† | 6953‡ | 5 | 10 | 0.84 | 0.74 to 0.95 | .006 |
| ECOG E4181/E5181 | 9.6 | 140‖ | 5 | Indefinite | 0.33 | 0.15 to 0.70 | .004 |
| NSABP B-14 | 7 | 1172 | 5 | 10 | 1.18 | 0.80 to 1.72 | .41 |
| Scottish | 10 | 132§ | 5 | Indefinite | 0.93 | 0.46 to 1.92 | .85 |
Results for estrogen receptor (ER)-positive patients. Mean listed. ATLAS = Adjuvant Tamoxifen Longer Against Shorter; aTTom = Adjuvant Tamoxifen–To Offer More?; CI = confidence interval; ECOG E4141/E5181 = Eastern Cooperative Oncology Group; NSABP B-14 = National Surgical Adjuvant Breast and Bowel Project; OR = odds ratio; Scottish = Scottish adjuvant tamoxifen trial a randomized study updated to 15 years.
Estimated follow-up.
Whole cohort analyzed (40% ER positive, 60% ER untested).
ER level less than 20 fmol/mg on ligand-binding assay deemed ER negative and excluded.
Follow-up for ER-positive group is shorter than for the whole study population.
Modified from (34).
Extended AI Therapy
As with tamoxifen, several studies have examined potential benefits of extended adjuvant AI therapy in postmenopausal women after an initial 5 years with tamoxifen, tamoxifen for 2–3 years followed by an AI for the remainder of the first 5 years, or an AI for the full first 5 years (Table 4). However, the results are not as clearly positive as they are for extended tamoxifen. For example, in one study (MA17R), patients were randomly assigned to letrozole or placebo for 5 years after completing 4.5–6 years of adjuvant therapy with an AI, preceded in most cases by treatment with tamoxifen (41). At a median follow-up of 6.3 years from randomization, letrozole led to improved DFS (HR = 0.66, 95% CI = 0.48 to 0.91, P = .01). However, there was no statistically significant impact on distant recurrences (42 vs 53 recurrences) or OS (HR = 0.97, 95% CI = 0.73 to 1.28, P = .83).
Table 4.
Summary of trials examining impact of extended AI therapy*
| Trial name | Median follow-up, y | Control arm (No.) | Experimental arm (No.) | HR DFS (95% CI) | Prior ET |
|---|---|---|---|---|---|
| MA17R | 6.3 | Placebo (959) | Letrozole 5 y (959) | 0.66 (0.48 to 0.91) | Tamoxifen (0–6 y), AIs (4.5–6 y) |
| MA17 | 2.5 | Placebo (2577) | Letrozole 5 y (2572) | 0.58 (0.45 to 0.76) | Tamoxifen 5 y |
| DATA | 4.1 | Anastrozole 3 y (833) | Anastrozole 6 y (827) | 0.79 (0.62 to 1.02) | Tamoxifen 2–3 y |
| IDEAL | 6.6 | Letrozole 2.5 y (898) | Letrozole 5 y (903) | 0.96 (0.76 to 1.2) | Endocrine therapy 5 y |
| NSABP B-42 | 6.9 | Placebo (1964) | Letrozole 5 y (1959) | 0.85 (0.73 to 1.00) | Endocrine therapy 5 y |
Modified from (40). AI = aromatase inhibitor; CI = confidence interval; DATA = Different Durations of Adjuvant Anastrozole Therapy After 2 to 3 Years Tamoxifen Therapy in Breast Cancer; DFS = disease-free survival; ET = endocrine treatment; HR = hazard ratio; IDEAL = Duration of Extended Adjuvant Letrozole treatment; NSABP = National Surgical Adjuvant Breast and Bowel Project B-42.
Likewise, in a separate study (NSABP B-42), patients were randomly assigned to letrozole vs placebo after receiving 5 years of either an AI or up to 3 years of tamoxifen followed by an AI to complete 5 years. DFS and distant DFS (DDFS) were improved in the letrozole arm (HR = 0.85, P = .048; though this did not cross a predefined statistical significance level, and HR = 0.72, P = .03, respectively), but there was no effect on OS (HR = 1.15, P = .22) (42,43). In two other trials (the Investigation on the Duration of Extended Adjuvant Letrozole treatment [IDEAL] and Different Durations of Adjuvant Anastrozole Therapy After 2 to 3 Years Tamoxifen Therapy in Breast Cancer [DATA] trials), patients were either randomly assigned to 2.5 vs 5 years of letrozole after 5 years of tamoxifen, AI, or a combination of AI and tamoxifen (the IDEAL trial) or to 3 vs 6 years of anastrozole after 2–3 years of tamoxifen (the DATA trial). In the IDEAL trial, DFS, DDFS, and OS were similar in the two arms after a median of 6.6 years from randomization (44). In the DATA trial, a trend toward improved DFS (HR = 0.79, 95% CI = 0.62 to 1.02, P = .07) was observed for the extended treatment group, driven largely by a reduction in contralateral BCs and secondary non-BCs rather than differences in locoregional or distant recurrences (45).
A meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group including 22 192 patients enrolled in 11 randomized trials evaluating AI therapy (vs placebo) at 5 or more years after diagnosis indicated a 5-year reduction in any recurrence (distant, local, or new primary), distant recurrence, and BC mortality of 3.6% (P < .001), 1.5% (P = .008), and 0.8% (P = .05), respectively, for trials of an AI after 5 years of tamoxifen (n = 7483). For trials of an AI after 5–10 years of tamoxifen followed by AI, there was a 2.1% (P = .002) reduction in risk of any recurrence, but there was no beneficial effect on other endpoints (0.3% lower distant recurrence and 0.2% lower BC mortality, P = .09 and 0.45, respectively; N = 2304). Similarly, for those receiving extended AI after 5 years of AI alone, there was a modest reduction in risk of any recurrence (1.2%, P = .02), but no differences in other endpoints (n = 3322). When including all trials, the 5-year reduction in recurrence was 1.1% (P = .009) for node-negative disease (n = 10 620), 3.8% (P = .00003) for 1–3 positive axillary nodes (n = 6919), and 7.7% (P = .003) for four or more positive axillary nodes (n = 1621) (46). Taken together, these results suggest that extended adjuvant AI treatment is associated with potential modest beneficial effects on DFS and DDFS, with greatest benefit observed in higher-risk patients with positive axillary nodes. No effects on OS have been reported to date, although follow-up remains short for all these trials. Further, the major benefit appears to be reduction in risk of local-regional recurrence and contralateral BC, at least early on, with few distant recurrence events even in the shorter treatment groups.
These modest benefits of extended tamoxifen or AIs occur in the face of common side effects that reduce quality of life (climacteric and musculoskeletal symptoms) and uncommon serious toxicities (thrombosis and endometrial cancers with tamoxifen; osteoporosis and fracture with the AIs). Indeed, continuation of tamoxifen (vs placebo) from 5 to 10 years is associated with increased risk of endometrial cancer (HR = 1.74, P = .002) and pulmonary embolus (HR = 1.87, P = .01), translating to an absolute increase of 0.2% in risk of death from endometrial cancer or pulmonary embolism. In contrast, risk of ischemic heart disease (HR = 0.76, P = .02) and contralateral BC were lower (HR = 0.88, P = .05), with a 3% reduction in BC mortality and, ultimately, a net survival benefit of 2.7% in favor of extended tamoxifen (36). In MA17R, extended AI treatment was associated with increased fracture and new-onset osteoporosis risk (14% vs 9%, P = .001 and 11% vs 6%, P < .001) as well as greater rates of arthralgia and bone pain. However, extended AI (vs placebo, as opposed to tamoxifen in early adjuvant trials) was not associated with an increased risk of cardiovascular events in a recent meta-analysis (HR = 1.01, 95% CI = 0.85 to 1.20) (36,47). Consequently, the benefits of extending ET must be carefully measured against the increased risk of serious adverse toxicities. Furthermore, these potentially harmful side effects are accompanied by concerning rates of noncompliance. Several studies have documented that adherence to tamoxifen and AIs decreases at a constant rate over time in a clinical trial setting; in the DATA trial, compliance was 84% at 3 years and 66% at 6 years, and rates were 73.5% and 57.5% at 2.5 and 5 years in the IDEAL trial (44,45).
The use of extended ET is common, particularly in higher-risk patients, often determined by clinical and pathologic features such as larger tumors or those with lymph node involvement. Although DDFS and OS benefits of extended tamoxifen have been clearly demonstrated, they are modest. Benefits of AIs are less clear, although follow-up is short. It is a high priority to develop means, beyond standard clinical or pathologic features, that identify those at increased risk for recurrence and greatest likelihood of benefitting from extended treatment. Since completion of the workshop, some adjuvant trials involving CDK4/6 inhibitors have been initiated (reviewed in [48]). Results of these trials will be informative, and there may be a role for these agents in extended treatment and late-recurrence reduction.
Potentially Modifiable Host and Other Factors That May Contribute to Late Recurrence
Another possible approach to reduce the problem of late recurrence could involve the identification, and subsequent avoidance, of potentially modifiable host-related factors that might affect reactivation of cancer cells from dormancy (dormancy is discussed in detail in a companion manuscript in this issue). Potential exposures of interest include stimulation of growth factors by trauma and/or surgery, selected lifestyles including physical inactivity and obesity, use or nonuse of certain nonantineoplastic medications, and the development or existence of comorbidities (diabetes, immune disorders, stress) (Table 5). The limited evidence linking these factors to late recurrence is summarized briefly below.
Table 5.
Putative host factors that potentially modulate BC recurrence*
| Host factor | Characteristic |
|---|---|
| Surgery, trauma | Breast reconstruction, early or delayed |
| Lifestyle |
|
| Medications |
|
| Comorbid disease |
|
BC = breast cancer; RANK-L = RANK-ligand.
Trauma or Surgery
Numerous cytokines and growth and angiogenic factors that may contribute to exit from dormancy and tumor outgrowth are released during wound healing in response to trauma (surgery or accidental injury) events, which have been postulated to be associated with late recurrence (49–52). Most reports examining this relationship have focused on early or delayed breast reconstruction surgery. Initial observational studies suggested that risk of recurrence or mortality was lower after delayed breast reconstruction vs early or no reconstruction (53). Most, if not all, of these studies were retrospective, observational cohorts with no high-level randomized evidence to support the theory. The design of these studies may also have introduced a selection bias, in part, because events were analyzed from the time of BC diagnosis rather than the time of reconstruction (ie, patients who had experienced a distant recurrence would be less likely to have undergone reconstruction, leading to a spuriously lower risk in the reconstruction group). When recurrence in patients undergoing delayed tissue reconstruction vs not (controls) was compared from the time of reconstruction, using a matched time in controls (ie, a landmark analysis that may minimize the effect of time-related bias), there was an increased risk of recurrence in those undergoing reconstruction (HR = 2.08, 95% CI = 1.07 to 4.06) (54). However, in a retrospective analysis of patients enrolled in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) and Long-term Anastrozole versus Tamoxifen Treatment Effects (LATTE) trials, those who had trauma or surgery (excluding breast reconstruction) did not experience an increased risk of recurrence; the use of ET and the inclusion both of major and minor trauma or surgical events may have obscured any risk associated with these events (55). Based on available data, it is unclear whether surgery or trauma contributes to late recurrence in patients not on concurrent ET.
Lifestyle
Obesity at BC diagnosis has been associated with a higher risk of recurrence both before and after 5 years postdiagnosis (56–58). There is evidence that obesity-associated factors such as leptin (which affects JAK-STAT signaling), but not insulin or glucose, may mediate this association, potentially affecting exit from dormancy (56). Physical activity (either prediagnosis or postdiagnosis) has been consistently associated with better BC outcomes in observational studies. In one study, physical activity after completion of adjuvant ET (mean of 5.6 years after diagnosis) was inversely associated with subsequent BC mortality (ie, >21 metabolic equivalent (MET)-hours/week vs <2.8 MET-hours per week (HR = 0.44, 95% CI = 0.32 to 0.66) (59). Owing to the potential for a healthy-person bias in nonobese or active patients, it cannot be concluded that these associations are causal (or reversible). Intervention research in the late-recurrence setting similar to that ongoing in the adjuvant setting [eg, the Alliance for Clinical Trials in Oncology Breast Cancer Weight Loss trial, BWEL trial (60)] will be necessary to provide definitive evidence that changing lifestyle will lower risk of late BC recurrence. Other lifestyle-related factors including dietary composition, smoking and alcohol consumption, as well as psychological factors (such as social isolations or stress) that have been postulated to be associated with BC recurrence (but not specifically late recurrence) may also affect risk of late recurrence in patients with BC; however, data are sparse. At present, although it is preferable for any individual to pursue a healthy lifestyle, there are no data to support substituting such a strategy for pharmacologic adjuvant treatment, such as extended ET, or for adding lifestyle modification to extended ET in the contexts in which extended ET has proven efficacy.
Nonantineoplastic Medications
A variety of medications not directed toward neoplastic cells themselves may affect tumor dormancy. Perhaps the most relevant are agents used to modify bone (when used for nonneoplastic indications), such as bisphosphonates (zoledronic acid, ibandronate, clodronate) and the anti-RANK ligand agent, denosumab. The bisphosphonates have been linked to modestly reduced rates of disease recurrence in postmenopausal women with early-stage BC, but these small benefits were not confined to ER+ disease (61,62). Because dormancy may be particularly evident in bone, it has been suggested that bisphosphonates might prevent escape from dormancy by altering the bone microenvironment (63,64). However, there is little if any evidence that prolonged bisphosphonate therapy does, indeed, prevent late (as opposed to earlier) relapse. There is also no consistent evidence that denosumab is effective in the late-recurrence setting.
Several observational studies have suggested that other medications may also be associated with improved BC outcomes, including statins [HR for distant recurrence-free interval = 0.74, 95% CI = 0.56 to 0.93 (65)], aspirin [HR = for BC-specific mortality 0.42, 95% CI = 0.31 to 0.55 (66)], metformin [HR for all-cause mortality = 0.55, 95% CI = 0.44 to 0.70) (67)], and beta-blockers [HR = for BC death 0.50, 95% CI = 0.32 to 0.80 (68)]. These studies did not focus on associations with late vs early events, and they were subject to selection, allocation, and survival biases and cannot provide evidence of causality. Adjuvant trials of metformin [fully accrued (69)] and aspirin (accrual ongoing) will provide definitive evidence regarding the effectiveness of these agents both on early and late BC recurrence. Of concern, despite observational evidence suggesting a beneficial effect, a randomized trial of celecoxib (vs placebo) failed to identify an effect of the drug on risk of recurrence at any time (70).
In contrast, in a randomized trial, the synthetic estrogenic hormone tibolone increased the risk of recurrence in BC survivors, even when administered years after diagnosis (HR = 1.40, 95% CI = 1.14 to 1.70, P = .001), including among patients with ER+ cancer who were receiving adjuvant ET (71). These findings are hypothesized to be the result of proliferative effects exerted by tibolone on dormant luminal BC cells (72).
Comorbid Diseases
Diabetes has been associated with poor BC outcomes, including increased risk both of noncancer and BC-related deaths (73,74). Given the observational nature of this evidence, it is not clear that these associations, if real, are causal or coincidental, and it is also not clear whether the increased risk extends to late recurrences in ER+ BC.
Taken together, these host-related factors are of interest because many are potentially avoidable or modifiable. Standardized assessment of these factors in cohorts of patients with BC would provide valuable information regarding their relationships with dormancy and disease recurrence.
Conceptualization of Risk of Late Recurrence: Three Potential Scenarios
Given our current inability to identify individual patients who will develop late recurrences, it is useful to conceptualize three categories (fully described in our companion article, (2)) of patients in relation to “tumor dormancy” and its role in the development of late recurrence: 1) no dormant cells present, 2) dormant cells present that remain dormant, and 3) dormant cells present that have escaped dormancy. Although we use the term “dormancy” here, we recognize that other cellular mechanisms may contribute to early and/or late recurrence, and there is likely a continuum of dormancy including mixed cell populations that culminate in clinical development of metastases once a critical threshold is reached. Although it is not currently possible to place individual patients into these conceptual categories, emerging technologies (such as CTCs and ctDNA) may make this possible in the future. Importantly, from a theoretical perspective these categories can guide research and, once validated, may ultimately inform treatment decisions, notably decisions around extended adjuvant ET or introduction of novel therapies in the extended adjuvant setting.
In the face of a growing appreciation of the potential for late recurrence in ER+ BC, participants in this workshop reviewed the current data showing the absolute risk of recurrence varied from less than 1% to greater than 3% per year, with the annual risk remaining constant from 5 to 15 years postdiagnosis (1). Risk was dependent on traditional clinicopathologic characteristics, notably tumor size and nodal stage; emerging evidence suggests that immunohistochemical and gene-based tumor evaluation may help to refine this risk. The use of extended adjuvant ET, although endorsed by guidelines committees, was recognized to have only a modest impact on the risk of late distant recurrences. There was agreement that understanding and treating late recurrence in ER+ BC was a major, unmet clinical need.
To date, research has shown that routine surveillance for late recurrences may lead to early identification of asymptomatic metastases but has not led to improved BC outcomes. Existing research was largely conducted before the era of more sensitive CT-, PET-, and MRI-based imaging, and it is possible that routine imaging using these modalities may lead to greater detection of asymptomatic metastases; however, this approach was not expected to identify recurrences sufficiently early that intervention would lead to improved BC outcomes. Similarly, routine evaluation of tumor markers (eg, CEA, CA 15-3) does not appear to improve outcomes. There was a consensus that more refined approaches to detecting asymptomatic late recurrences that were potentially curable were urgently needed. Such approaches could include emerging CTC and ctDNA technologies; rigorous evaluation of these technologies will be required before they are used routinely in the clinical setting. These issues are discussed more fully in the companion article (2).
In parallel to this research, reflecting advances in understanding tumor dormancy, there was recognition that host factors including obesity, diabetes, and trauma or surgery may contribute to late recurrence by contributing to the escape of cancer cells from dormancy, and noncancer-targeted drugs may modify this risk. Although evidence supporting the contributions of each of these factors to late recurrence specifically (as opposed to an association with prognosis in general) is weak, further investigation of these potentially reversible or avoidable factors in future late-recurrence research was embraced by workshop participants.
The concept of tumor dormancy (and indolence), emerging technologies to identify escape from dormancy, and research priorities designed to prevent and/or treat late recurrence to improve cancer outcomes are discussed in the companion paper.
Funding
This work and the workshop described were funded by the Breast Cancer Research Foundation and the Hold’em for Life Charity.
Notes
Affiliations of authors: Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada (RJOD, DWC, SVB, VS); Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY (KK); Herbert Irving Comprehensive Cancer Center, Columbia University Irving Medical Center, New York, NY (KK); University of Michigan Rogel Cancer Center and the Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (DFH); Department of Medical Oncology, Institut Curie, PSL Research University, Paris, France (FCB); Division of Medical Oncology, Department of Medicine, University of Toronto, Toronto, ON, Canada (DWC); Human Oncology and Pathogenesis Program, and Breast Medicine Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY (SC); Weill-Cornell Medical College, New York, NY (SC); Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY (JOD); Ralph Lauren Centre for Breast Cancer Research, Royal Marsden Hospital, The Royal Marsden NHS Foundation Trust, Breast Cancer Now Research Centre, The Institute of Cancer Research, London, UK (MD); Department of Biostatistics, Dana-Farber Cancer Institute, Boston, MA; Harvard T.H. Chan School of Public Health, Boston, MA (RJG); University of Utah, Huntsman Cancer Institute, Salt Lake City, UT (NLH); Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, TX (FMB); Gemini Group, Ann Arbor, MI (JP); Division of Oncology, Department of Medicine, Stanford University School of Medicine, Stanford, CA (GWS); Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network Toronto, ON, Canada (SVB); Department of Radiation Oncology (SVB), and Department of Medical Biophysics (RJOD, SVB, VS), University of Toronto, Toronto, ON, Canada; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC (LAC); University of Vermont Medical Center, Larner College of Medicine, Burlington, VT (MCC); Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA (ADM); Applied Statistician, Markham, ON, Canada (ME); Division of Medical Oncology and Hematology, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, ON, Canada (KJJ); Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD (LAK); Lunenfeld-Tanenbaum Research Institute, Mt. Sinai Hospital, Sinai Health System, Toronto, ON, Canada (AEL, DS, JRW, PJG); Department of Medicine, University of Toronto, Toronto, ON, Canada (AEL, PJG); Orlando Health University of Florida Health Cancer Center, Orlando, FL (EPM); Canadian Cancer Trials Group, Queen's University, Kingston, ON, Canada (WRP); Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA (MMR); Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada (DS); Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Barts & The London School of Medicine and Dentistry, Queen Mary University of London, London, UK (MAT); McMaster University and Juravinski Cancer Centre, Hamilton, ON, Canada (TJW); The Johns Hopkins University School of Medicine and Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD (ACW); Departments of Medicine and Medical Oncology, Albert Einstein College of Medicine, Montefiore Medical Center, Albert Einstein Cancer Center, New York, NY (JAS).
P. J. Goodwin received research funding (in kind) support from Epic Sciences.
Supplementary Material
References
- 1. Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowling RJO, Sparano JA, Goodwin PJ, et al. Toronto Workshop on Late Recurrence in Estrogen–Receptor Positive Breast Cancer: Part 2: Approaches to Predict/Identify Late Recurrence, Research Directions. JNCI Cancer Spectr. 2019; pkz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dowsett M, Sestak I, Regan MM, et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol. 2018;36(19):1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sestak I, Regan M, Dodson A, et al. Integration of clinical variables for the prediction of late distant recurrence in patients with oestrogen receptor positive breast cancer treated with 5 years of endocrine therapy. In: San Antonio Breast Cancer Symposium, Philadelphia, PA: American Association for Cancer Research, Inc (AACR). 2017. [DOI] [PMC free article] [PubMed]
- 6. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29(32):4273–4278. [DOI] [PubMed] [Google Scholar]
- 7. Wolmark N, Mamounas EP, Baehner FL, et al. Prognostic impact of the combination of recurrence score and quantitative estrogen receptor expression (ESR1) on predicting late distant recurrence risk in estrogen receptor-positive breast cancer after 5 years of tamoxifen: results from NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-28 and B-14. J Clin Oncol. 2016;34(20):2350–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lænkholm A-V, Jensen M-B, Eriksen JO, et al. PAM50 risk of recurrence score predicts 10-year distant recurrence in a comprehensive Danish cohort of postmenopausal women allocated to 5 years of endocrine therapy for hormone receptor-positive early breast cancer. J Clin Oncol. 2018;36(8):735–740. [DOI] [PubMed] [Google Scholar]
- 9. Ma XJ, Hilsenbeck SG, Wang W, et al. The HOXB13: IL17BR expression index is a prognostic factor in early-stage breast cancer. J Clin Oncol. 2006;24(28):4611–4619. [DOI] [PubMed] [Google Scholar]
- 10. Muller BM, Keil E, Lehmann A, et al. The endopredict gene-expression assay in clinical practice—performance and impact on clinical decisions. PLoS One. 2013;8(6):e68252.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717–729. [DOI] [PubMed] [Google Scholar]
- 12. Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(10):1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krop I, Ismaila N, Andre F, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2017;35(24):2838–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25(2):339–345. [DOI] [PubMed] [Google Scholar]
- 15. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109(12):2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res. 2014;20(5):1298–1305. [DOI] [PubMed] [Google Scholar]
- 17. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2015;33(8):916–922. [DOI] [PubMed] [Google Scholar]
- 18. Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016;108(11):djw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst. 2013;105(14):1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simon RM, Paik S, Hayes DF.. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101(21):1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henry NL, Hayes DF, Ramsey SD, et al. Promoting quality and evidence-based care in early-stage breast cancer follow-up. J Natl Cancer Inst. 2014;106(4):dju034.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moschetti I, Cinquini M, Lambertini M, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2016;(5):CD001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pennant M, Takwoingi Y, Pennant L, et al. A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess. 2010;14(50):1–103. [DOI] [PubMed] [Google Scholar]
- 24. Gion M, Peloso L, Mione R, et al. Tumor markers in breast cancer monitoring should be scheduled according to initial stage and follow-up time: a prospective study on 859 patients. Cancer J. 2001;7(3):181–190. [PubMed] [Google Scholar]
- 25. Mariani L, Miceli R, Michilin S, et al. Serial determination of CEA and CA 15.3 in breast cancer follow-up: an assessment of their diagnostic accuracy for the detection of tumour recurrences. Biomarkers. 2009;14(2):130–136. [DOI] [PubMed] [Google Scholar]
- 26. Molina R, Jo J, Zanon G, et al. Utility of C-erbB-2 in tissue and in serum in the early diagnosis of recurrence in breast cancer patients: comparison with carcinoembryonic antigen and CA 15.3. Br J Cancer. 1996;74(7):1126–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molina R, Zanon G, Filella X, et al. Use of serial carcinoembryonic antigen and CA 15.3 assays in detecting relapses in breast cancer patients. Breast Cancer Res Treat. 1995;36(1):41–48. [DOI] [PubMed] [Google Scholar]
- 28. Tomlinson IP, Whyman A, Barrett JA, et al. Tumour marker CA15-3: possible uses in the routine management of breast cancer. Eur J Cancer. 1995;31A(6):899–902. [DOI] [PubMed] [Google Scholar]
- 29. Molina R, Barak V, van Dalen A, et al. Tumor markers in breast cancer—European Group on Tumor Markers recommendations. Tumor Biol. 2005;26(6):281–293. [DOI] [PubMed] [Google Scholar]
- 30. Keshaviah A, Dellapasqua S, Rotmensz N, et al. CA15-3 and alkaline phosphatase as predictors for breast cancer recurrence: a combined analysis of seven International Breast Cancer Study Group trials. Ann Oncol. 2006;18(4):701–708. [DOI] [PubMed] [Google Scholar]
- 31. Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961–965. [DOI] [PubMed] [Google Scholar]
- 32. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. [DOI] [PubMed] [Google Scholar]
- 33. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 34. Al-Mubarak M, Tibau A, Templeton AJ, et al. Extended adjuvant tamoxifen for early breast cancer: a meta-analysis. PLoS One. 2014;9(2):e88238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gray RG, Rea D, Handley K, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31(suppl 18):5.23169505 [Google Scholar]
- 36. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tormey DC, Gray R, Falkson HC; Eastern Cooperative Oncology Group. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. J Natl Cancer Inst. 1996;88(24):1828–1833. [DOI] [PubMed] [Google Scholar]
- 38. Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93(9):684–690. [DOI] [PubMed] [Google Scholar]
- 39. Stewart HJ, Prescott RJ, Forrest AP.. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst. 2001;93(6):456–462. [DOI] [PubMed] [Google Scholar]
- 40. Goldvaser H, AlGorashi I, Ribnikar D, et al. Efficacy of extended adjuvant therapy with aromatase inhibitors in early breast cancer among common clinicopathologically-defined subgroups: a systematic review and meta-analysis. Cancer Treat Rev. 2017;60:53–59. [DOI] [PubMed] [Google Scholar]
- 41. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wimmer K, Strobl S, Bolliger M, et al. Optimal duration of adjuvant endocrine therapy: how to apply the newest data. Ther Adv Med Oncol. 2017;9(11):679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schleicher SM, Dickler MN.. Extended adjuvant aromatase inhibitor therapy in post-menopausal women. Curr Breast Cancer Rep. 2017;9(4):236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL trial (BOOG 2006-05). J Natl Cancer Inst. 2018;110(1):40–48. [DOI] [PubMed] [Google Scholar]
- 45. Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 2017;18(11):1502–1511. [DOI] [PubMed] [Google Scholar]
- 46. Gray R, Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of prolonging adjuvant aromatase inhibitor therapy beyond five years on recurrence and cause-specific mortality: An EBCTCG meta-analysis of individual patient data from 12 randomised trials including 24,912 women. In: San Antonio Breast Cancer Symposium, Philadelphia, PA: American Association for Cancer Research, Inc (AACR). 2018. Abstract GS3-03.
- 47. Khosrow-Khavar F, Filion KB, Al-Qurashi S, et al. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2017;28(3):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pernas S, Tolaney SM, Winer EP, et al. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol. 2018;10:1758835918786451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coussens LM, Werb Z.. Inflammation and cancer. Nature. 2002;420(6917):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murthy BL, Thomson CS, Dodwell D, et al. Postoperative wound complications and systemic recurrence in breast cancer. Br J Cancer. 2007;97(9):1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Balkwill F, Mantovani A.. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. [DOI] [PubMed] [Google Scholar]
- 52. Tohme S, Simmons RL, Tsung A.. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holmich LR, During M, Henriksen TF, et al. Delayed breast reconstruction with implants after invasive breast cancer does not impair prognosis. Ann Plast Surg. 2008;61(1):11–18. [DOI] [PubMed] [Google Scholar]
- 54. Isern AE, Manjer J, Malina J, et al. Risk of recurrence following delayed large flap reconstruction after mastectomy for breast cancer. Br J Surg. 2011;98(5):659–666. [DOI] [PubMed] [Google Scholar]
- 55. Allawi Z, Cuzick J, Baum M.. Does trauma or an intercurrent surgical intervention lead to a short-term increase in breast cancer recurrence rates? Ann Oncol. 2012;23(4):866–869. [DOI] [PubMed] [Google Scholar]
- 56. Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30(2):164–171. [DOI] [PubMed] [Google Scholar]
- 57. Pritchard KI, Shepherd LE, Chapman JA, et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR therapy in the adjuvant treatment of early-stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol. 2011;29(29):3869–3876. [DOI] [PubMed] [Google Scholar]
- 58. Duggan C, Irwin ML, Xiao L, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol. 2011;29(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. [DOI] [PubMed] [Google Scholar]
- 60. Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer. 2017;3(1):37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. [DOI] [PubMed] [Google Scholar]
- 62. Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(18):2062–2081. [DOI] [PubMed] [Google Scholar]
- 63. Wilson C, Holen I, Coleman RE.. Seed, soil and secreted hormones: potential interactions of breast cancer cells with their endocrine/paracrine microenvironment and implications for treatment with bisphosphonates. Cancer Treat Rev. 2012;38(7):877–889. [DOI] [PubMed] [Google Scholar]
- 64. Gnant M. Targeting bone microenvironment: clinical implications. Breast. 2015;24(suppl 2):S49–S50. [DOI] [PubMed] [Google Scholar]
- 65. Borgquist S, Giobbie-Hurder A, Ahern TP, et al. Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1-98 study. J Clin Oncol. 2017;35(11):1179–1188. [DOI] [PubMed] [Google Scholar]
- 66. Fraser DM, Sullivan FM, Thompson AM, et al. Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study. Br J Cancer. 2014;111(3):623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang GH, Satkunam M, Pond GR, et al. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: a GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2018;27(6):627–635. [DOI] [PubMed] [Google Scholar]
- 68. Childers WK, Hollenbeak CS, Cheriyath P.. Beta-blockers reduce breast cancer recurrence and breast cancer death: a meta-analysis. Clin Breast Cancer. 2015;15(6):426–431. [DOI] [PubMed] [Google Scholar]
- 69. Goodwin PJ, Stambolic V, Lemieux J, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat. 2011;126(1):215–220. [DOI] [PubMed] [Google Scholar]
- 70. Coombes RC, Tovey H, Kilburn L, et al. A phase III multicentre double blind randomised trial of celecoxib versus placebo in primary breast cancer patients (REACT—Randomised EuropeAn celecoxib trial). In: San Antonio Breast Cancer Symposium, Philadelphia, PA: American Association for Cancer Research, Inc (AACR). 2017. Abstract No. GS3-03.
- 71. Kenemans P, Bundred NJ, Foidart JM, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol. 2009;10(2):135–146. [DOI] [PubMed] [Google Scholar]
- 72. Ogba N, Manning NG, Bliesner BS, et al. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 2014;16(6):489.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Campbell PT, Newton CC, Patel AV, et al. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jiralerspong S, Kim ES, Dong W, et al. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10):2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


