Abstract
Background
The 18-year-old age limit for inclusion in clinical trials constitutes a hurdle for adolescents and young adults (AYAs) with cancer. We analyzed the impact of this age barrier on the access of AYAs to cancer trials and novel therapies.
Methods
ClinicalTrials.gov was searched to identify all the trials including patients with 10 malignancies relevant for AYAs (January 2007 to July 2018). The trials were categorized as pediatric (patients <18 y), adult (≥18 y), and transitional (including adult and pediatric patients). Transitional trials with a lower limit between 12 and 18 years and an upper limit younger than 40 years were considered AYA-specific.
Results
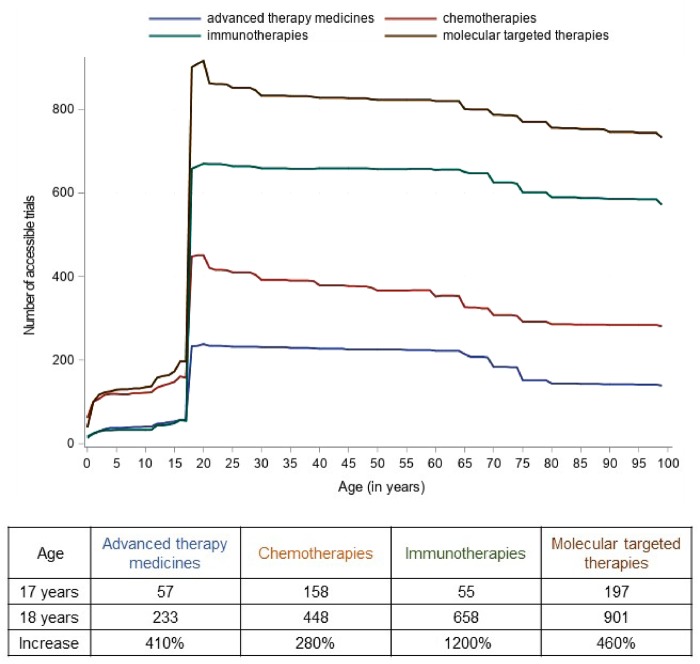
Of 2764 identified trials, 2176 were included: 79% adult, 19% transitional, 2% pediatric. Five trials were AYA-specific. The proportion of academic trials was higher for transitional (69%; 288 of 421) than for adult trials (48%; 832 of 1718) (P < .0001). The total number of new trials increased over the years (156 in 2007; 228 in 2017); however, the number of transitional trials remained stable. The availability of trials increased with age, with a major increase at age 18 years: at age 17 years, 20% (442 of 2176) of trials were potentially accessible vs 95% (2075 of 2176) at 18 years. For trials investigating targeted therapies, this increase was 460% (197 trials available at age 17 years; 901 at 18 years) and for immunotherapies, 1200% (55 at age 17 years; 658 at 18 years).
Conclusions
AYAs have limited access to cancer trials and innovative therapies, with no improvement over the last decade. The 18-years-old age limit continues to be a major hurdle. Our findings are consistent with the internationally supported idea that age inclusion criteria in oncological trials should be changed.
Cancer occurring in adolescents and young adults (AYAs) aged 15–30 years is 2.7 times more common than cancer occurring in children (<15 years), yet it is much less common than cancer in older age groups (1). The distribution of cancer types is unique: Hodgkin and non-Hodgkin lymphoma, melanoma, germ cell tumors, soft-tissue and bone sarcomas, leukemia, central nervous system tumors, thyroid cancer, and other carcinomas account for 95% of the cancers in this age group (1,2).
AYAs with cancer have been historically managed within pediatric or adult health-care facilities, traditionally split with a strict 18-year-old age limit that artificially separates childhood from adulthood. Over recent years, there has been an increasing awareness in the oncology research community that this dichotomy is far from optimal and that it has led to several shortfalls among AYA cancer patients when compared to their pediatric and adult counterparts. AYA cancer patients have differentiated tumor type epidemiology (2,3) and biology (4,5), are recruited remarkably less frequently than pediatric patients into clinical trials (6), and have globally worse outcomes (7,8). Additionally, they have specific psychosocial features that distinguish them from pediatric and adult patients (9).
Within this landscape, AYA oncology is a young discipline that has emerged to address the specific needs of this patient population. There seems to be a general consensus about the need for AYA oncology units and AYA specialists (7,10,11). However, although improvements in the survival for several cancer types in AYAs are being observed both in Europe and the United States, survival does not appear to be improving to the same extent in AYAs as in children or older adults for several cancers (12,13). Furthermore, some works correlate the smaller proportion of AYA patients enrolled in clinical trials with their globally worse survival progress (14,15). Part of the lack of clinical trial availability in AYAs has been hypothesized to be related to age barrier inclusion criteria in pediatric and adult trials (16). This has gathered substantial social attention (17,18); there are several multistakeholder initiatives ongoing to change the paradigm and ensure that all AYAs have access to clinical trials, overcoming the 18-year-old barrier (6,16,19–21).
After observing the increased awareness and interest in AYA oncology, we hypothesized an accelerated increase in AYA-specific research over the last decade. To test this hypothesis, we performed a meta-research analysis investigating clinical trials that include relevant AYA malignancies with three aims: 1) to analyze the number of clinical trials available for AYAs, overall and depending on tumor types, assessing changes in available trials over time; 2) to analyze the impact of the 18-year-old barrier; and 3) to analyze the access of AYAs to targeted therapies and immunotherapies.
Methods
The study methodology complies with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-analysis) statement and guidelines whenever applicable to the meta-research context (22).
Trial Selection
The Clinicaltrials.gov (23) database was searched to identify all clinical trials (early and late phase; postmarketing authorization studies excluded) with a study start date within the last decade (between January 2007 and July 2018) and recruiting patients with one of the most relevant tumor types for AYAs.
The decision on which tumor types to include was made by expert consensus, taking into account the prevalence among AYAs, particularly in the range between 14 and 24 years old (>5% in that range) (2), and the relative prevalence among children and adults. The study start was selected as January 2007, after the release of two landscape-changing policies. First, the European pediatric medicines regulation EC 1901/2006 in December 2006, which mandated that the marketing-authorization applications for a new medicinal product (or a new indication) must include the results of studies conducted in the pediatric population in compliance with a pediatric investigation plan (24,25). Second, the setup of a trial registration policy by the World Health Organization in 2006, which entailed a global network of clinical trial registers to increase transparency and accountability in the conduct of clinical trials (26,27).
All clinical trials investigating the clinical impact of medical interventions and including patients with one of the selected tumor types (Hodgkin lymphoma, anaplastic large cell lymphoma [ALCL], melanoma, extracranial germ cell tumors [GCTs], medulloblastoma, thyroid cancer, Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, and synovial sarcoma) were included. The search was performed in July 2018, using the following terms: “[Tumor type] + investigational studies + study start from 01/01/2007 + early phase 1 or Phase 1 or Phase 2 or Phase 3.”
Cross-tumoral trials were included, as long as they allowed for inclusion of at least one of the selected tumor types. Trials investigating psychosocial or behavioral interventions, nonconventional therapies (acupuncture, hypnosis, homeopathy, herbal therapy, etc.), supportive care (anti-emetic drugs, antibiotics, pain management, etc.), or other types of research (biology studies, such as sequencing studies not inherently linked to drug access, health-care organization, etc.) were excluded.
Data Extraction
The trial screening on clinicaltrials.gov was performed by one investigator (TdR). Eligibility of all identified trials was assessed by one of the investigators (TdR, JP, MT, MGA). All excluded trials and all trials with uncertainties regarding any of the manually coded variables were reviewed by at least two investigators. In case of discrepancy, a decision was made by consensus of the four investigators.
The collected variables per trial included the National Clinical Trial (NCT) number, title, status, availability of results tumor types included in each trial, experimental interventions (name and category), sponsor, location, age, phase, sample size, study design (randomization, number of centers, blinding), and start and completion date. A standardized data extraction form was prepared by three investigators (TdR, JP, AN) and used by the investigators to capture the data.
Definition of Trial Characteristics
The trials were divided in three main age groups: pediatric (patients <18 years), adult (≥18 years), and transitional (with both pediatric, lower limit <18 years, and adult population, upper limit >18 years). The subgroup of transitional trials with a lower limit between 12 and 18 years and an upper limit younger than 40 years were considered AYA-specific trials.
The definitions of other trial variables are shown in Supplementary Methods (available online).
Analysis
Median and interquartile range (IQR) were used to describe quantitative data. Percentages were used to describe qualitative data; the χ2 or the Fisher exact test was used for statistical comparisons when appropriate. Percentages may not always total 100% because of rounding error.
SAS software v.9.4 (SAS, Cary, NC) was used to perform the analysis and to plot the results.
Results
Trial Design and Characteristics
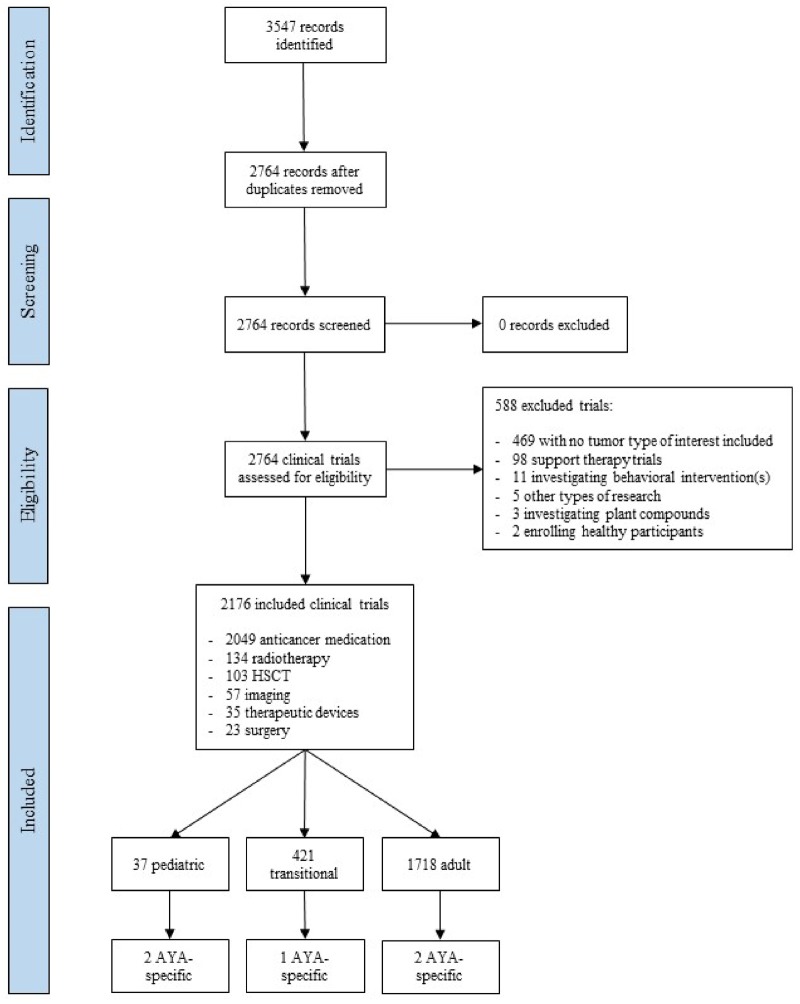
The search resulted in 2764 trials; 2176 (79%) were included (Figure 1). Out of the 588 (21%) excluded trials, the reasons for exclusion were as follows: no tumor type of interest included (469 trials), support therapy trials (98 trials), behavioral interventions (11 trials), other types of research (5 trials), plant compounds (3 trials), and enrollment of healthy participants (2 trials). Most included trials were exclusively for adults (1718, 79%), 37 (2%) were pediatric, and 421 (19%) were transitional. Out of the latter group, 5 of 421 (1.2%; 0.2% of the total number of included trials) were AYA-specific trials: 2 academic trials for Hodgkin lymphoma (age limit 18–30 years and 18–24 years); 1 academic trial for sarcoma (12–30 years); and 2 industry trials for melanoma (12–17 years). The main characteristics of the 2176 included trials are shown in Table 1; a detailed overview of the five AYA-specific trials can be found in Supplementary Table 1 (available online). Complete data for the primary endpoint (age limit) and for most variables were available for all trials; unknown data were only found for the variables “Status,” “Sponsor,” “Location,” and “Number of centers,” in less than 5% of the trials.
Figure 1.
PRISMA flow diagram of this meta-research study showing the number of clinical trials identified and their categories. Pediatric trial: upper inclusion age limit <18 years. Adult trial: lower inclusion age limit ≥18 years. Transitional trial: lower limit <18 years and upper limit >18 years. AYA-specific: lower limit between 12 and 18 years and upper limit <40 years. Of note, the investigational interventions add up to more than the total of included trials because some trials investigated more than one intervention. AYA = adolescents and young adults; HSCT = Hematopoietic stem cell transplantation.
Table 1.
Main characteristics of the trials according to age groups
| Global characteristic | Total No. (%) | Pediatric No. (%) | Transitional No. (%) | Adult No. (%) | P |
|---|---|---|---|---|---|
| Number of trials | 2176 | 37 | 421 | 1718 | — |
| Status | .090 | ||||
| Ongoing | 1063 (49%) | 24 (65%) | 226 (54%) | 813 (47%) | |
| Not yet recruiting | 57 (3%) | — | 14 (3%) | 43 (3%) | |
| Closed | 873 (40%) | 11 (30%) | 147 (35%) | 715 (42%) | |
| Withdrawn | 79 (4%) | 2 (5%) | 15 (4%) | 62 (4%) | |
| Unknown status | 104 (5%) | — | 19 (5%) | 85 (5%) | |
| Published results among closed trials* (n = 873) | 329 (38%) | 5 (46%) | 58 (39%) | 266 (37%) | .760 |
| Sponsor | <.0001 | ||||
| Industry | 1033 (48%) | 17 (46%) | 132 (31%) | 884 (52%) | |
| Academic | 1140 (52%) | 20 (54%) | 288 (69%) | 832 (48%) | |
| Unknown | 3 (0%) | — | 1 (0%) | 2 (0%) | |
| Location | <.0001 | ||||
| North America | 1324 (61%) | 13 (35%) | 291 (69%) | 1020 (59%) | |
| Europe | 364 (17%) | 5 (14%) | 41 (10%) | 318 (19%) | |
| Asia | 130 (6%) | 4 (11%) | 22 (5%) | 104 (6%) | |
| Intercontinental | 259 (12%) | 13 (35%) | 48 (11%) | 198 (12%) | |
| Other | 30 (1%) | 2 (5%) | 6 (1%) | 22 (1%) | |
| Unknown | 69 (3%) | — | 13 (3%) | 56 (3%) | |
| Number of centers | <.0001 | ||||
| Monocentric | 1084 (50%) | 13 (35%) | 176 (42%) | 895(52%) | |
| Multicentric, single country | 618 (28%) | 8 (22%) | 139 (33%) | 471 (27%) | |
| Multicentric, international | 378 (17%) | 16 (43%) | 84 (20%) | 278 (16%) | |
| Unknown | 96 (4%) | — | 22 (5%) | 74 (4%) | |
| Trial design | |||||
| Focus of trial | <.0001 | ||||
| Single tumor type | 1245 (57%) | 13 (35%) | 171 (41%) | 1061 (62%) | |
| >1 tumor type | 931 (43%) | 24 (65%) | 250 (59%) | 657 (38%) | |
| Phase | |||||
| Early phase | 1084 (50%) | 22 (60%) | 190 (45%) | 872 (51%) | .086 |
| Phase 2 | 926 (43%) | 12 (32%) | 189 (45%) | 725 (42%) | |
| Late phase | 166 (8%) | 3 (8%) | 42 (10%) | 121 (7%) | |
| Randomization of phase 2 trials (n =926) | <.0001‡ | ||||
| Single arm | 659 (71%) | 7 (58%) | 135 (71%) | 517 (71%) | |
| Multiple arms, not randomized | 93 (10%) | 4 (33%) | 26 (14%) | 63 (9%) | |
| Randomized | 174 (19%) | 1 (8%) | 28 (15%) | 145 (20%) | |
| Blinding | 93 (4%) | — | 15 (4%) | 78 (5%) | .291 |
| Sample size among closed trials (n=873): Median (IQR)† | — | ||||
| Early phase | 20.5 (10–36) | 15 (7–57) | 22 (9–34) | 20 (11–37) | |
| Phase 2 | 25 (10–50) | NA | 32 (18–71) | 25 (10–48) | |
| Late phase | 250.5 (96–520) | NA | 318 (166–711) | 250 (80–437) | |
Percentages may not always total 100% because of rounding error. IQR = interquartile range.
Among the ongoing trials, 40 trials have results; 1 trial with unknown status also has results.
Numbers will be presented if the subgroup contains at least five closed trials. There are only two closed pediatric phase 2 and two closed pediatric late-phase trials.
Fisher exact test.
Regarding the status of the trials, 49% (1063) were ongoing, 3% (57) not yet recruiting, 40% closed (873), 4% (79) withdrawn, and 5% (104) unknown, with no statistically significant differences among the age groups (pediatric, transitional, adult) (P = .09), that is, with status homogeneity. Among closed trials, 38% (329 of 873) had published results reported in clinicaltrials.gov, also with no differences among the three age groups (P = .76).
The proportion of academic trials was statistically significantly higher for transitional trials (69%, 288 of 421) than for adult trials (48%, 832 of 1718) (P< .0001). Most trials were conducted in North America (1324, 61%), and only 12% (259) were intercontinental. Pediatric trials were more frequently intercontinental (35%, 13 of 37) compared to transitional and adult trials (11%, 48 of 421 and 12%, 198 of 1718, respectively) (P< .0001). The majority of trials were monocentric (1084, 50%), except for the pediatric trials, which had a statistically significantly higher proportion of multicentric-international trials (P < .0001). The proportion of monocentric trials was similar across location and type of the trials. Late-phase trials were less often monocentric.
The focus of the trials was on one tumor type exclusively in the majority of adult trials (62%, 1061 of 1718) and on more than one tumor type in pediatric (65%, 24 of 37) and transitional trials (59%, 250 of 421) (P < .0001).
Early phase and phase 2 trials were more frequent than late-phase trials: 1084 (50%) and 926 (43%) vs 166 (8%), respectively. The proportion of early-phase, phase 2, and late-phase trials was similar when comparing academic to industry trials. Among the 926 phase 2 trials, only 174 (19%) were randomized. Most trials (2083, 96%) were open label. No pediatric trials performed blinding. The median sample size for the closed trials was 20.5 (10–36) for early-phase, 25 (10–50) for phase 2, and 250.5 (96–520) for late-phase trials.
Differences Between Tumor Types
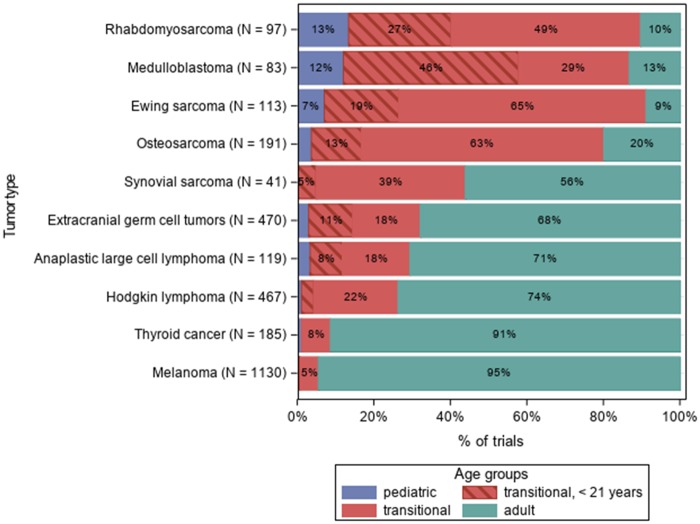
The proportion of trials by inclusion age and tumor type is shown in Figure 2. The tumors considered similar in adult and pediatric populations showed a disparate proportion of transitional trials: 84% and 76% for Ewing sarcoma and osteosarcoma, respectively; 44% for synovial sarcoma; 25%, 26%, and 29% for Hodgkin lymphoma, ALCL, and extracranial GCT, epidemiologically typical AYA tumors. For adult tumors that rarely present in adolescents (melanoma and thyroid cancer), the lowest proportion of transitional trials (5% and 8%, respectively) were shown. For pediatric tumors rarely present in the adult population (rhabdomyosarcoma and medulloblastoma), the proportion was 76% and 75%, respectively.
Figure 2.
Number of trials by tumor type.
Evolution Over Time and Duration of the Trials
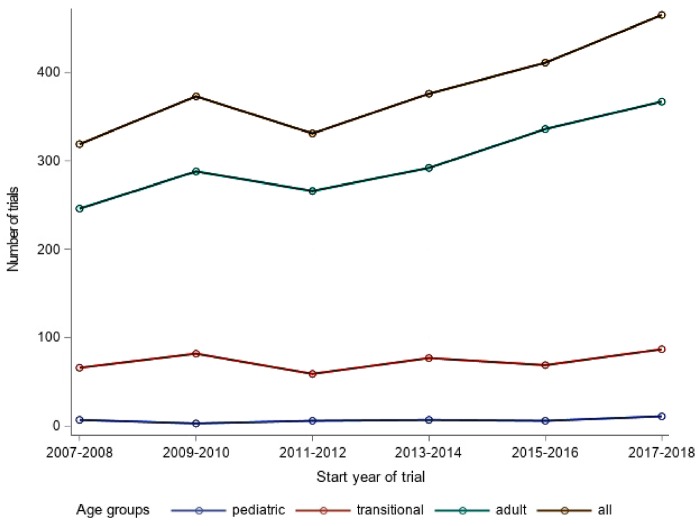
The total number of new trials increased over the years of the study from 156 in 2007 to 228 in 2017. Whereas the number of new pediatric and transitional trials remained stable, the number of new adult trials increased from 114 in 2007 to 183 in 2017 (Figure 3).
Figure 3.
Evolution over time. The values shown refer to 2-year periods each, as indicated in the x-axis. Of note, for projecting the missing months of 2018 (search performed in July), the included months (January–July) were standardized.
The median duration of the closed trials was 33 months (IQR = 22–49 months) for early-phase trials, 35 months (IQR = 21–50 months) for phase 2 trials, and 34 months (IQR = 19–58 months) for late-phase trials.
The 18-Year-Old Barrier
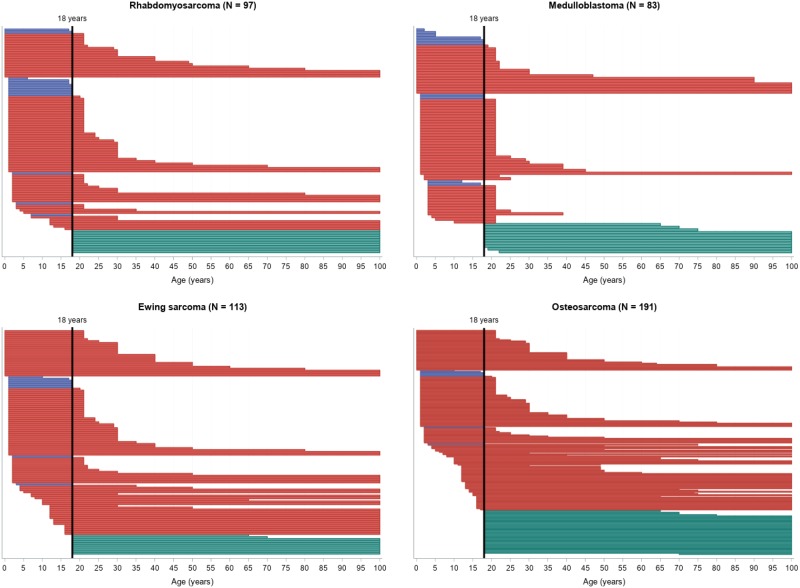
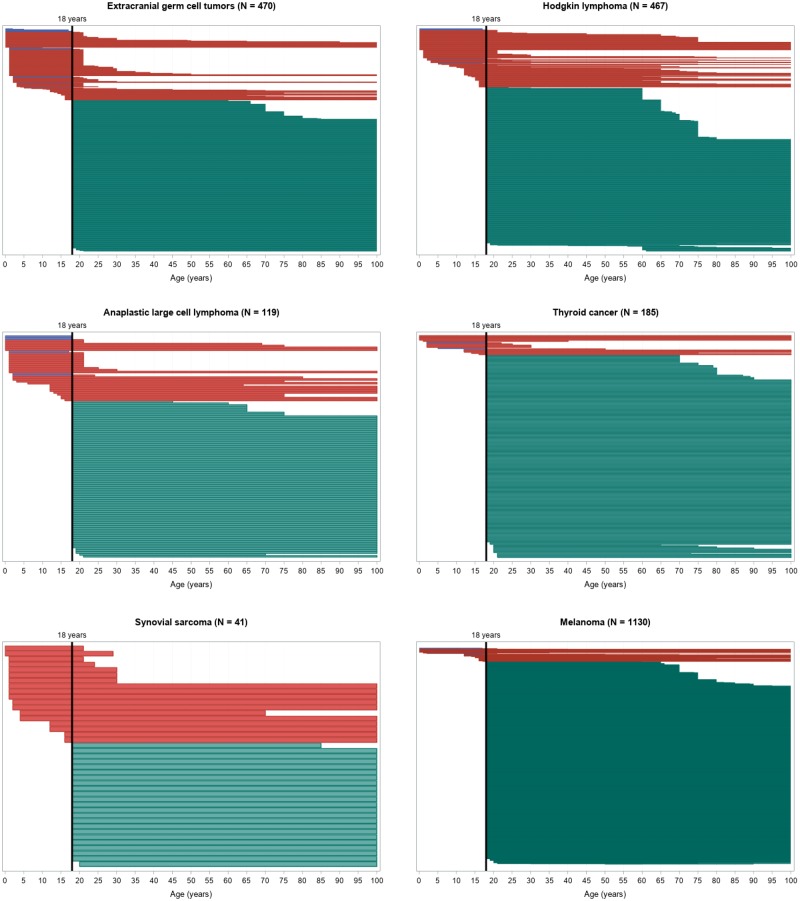
For most tumor types, the number of potentially accessible trials was lower for younger AYAs, especially younger than 18 years, as shown in Figure 4. The number of trials theoretically accessible for a patient at age 17 years was 442 of 2176 vs 2075 of 2176 at 18 years.
Figure 4.
Swimmer-like plot for the range of age inclusion. Each bar represents one trial: blue bars correspond to pediatric trials, red bars to transitional, and green bars to adult trials. Note that the length and location of each bar (in relation to the x-axis) depends on the range of the allowed inclusion age for that particular trial. For example, a trial that enrolls patients from 18 years of age to 100 years of age would start at the highlighted, vertical 18-year line and end at the right margin of the plot. The width of the bars is proportional to the number of trials included for each tumor type for visibility reasons.
Figure 4.
Continued
Seventeen-year-old patients would have had access to the following trials: 16 of 185 (9%) for thyroid cancer, 58 of 1130 (5%) for melanoma, 145 of 470 (31%) for extracranial GCT, 120 of 467 (26%) for Hodgkin lymphoma, 34 of 119 (29%) for ALCL, 101 of 113 (89%) for Ewing sarcoma, 151 of 191 (79%) for osteosarcoma, 66 of 83 (80%) for medulloblastoma, 18 of 41 (44%) for synovial sarcoma, and 83 of 97 (86%) for rhabdomyosarcoma. Eighteen-year-old patients would have been eligible for 170 of 185 (92%) thyroid cancer trials, 1100 of 1130 (97%) melanoma trials, 450 of 470 (96%) extracranial GCT trials, 445 of 467 (95%) Hodgkin lymphoma trials, 110 of 119 (92%) ALCL trials, 105 of 113 (93%) Ewing sarcoma trials, 183 of 191 (96%) osteosarcoma trials, 71 of 83 (86%) medulloblastoma trials, 40 of 41 (98%) synovial sarcoma trials, and 84 of 97 (87%) rhabdomyosarcoma trials. The difference in the number of potentially accessible trials for 17- vs 18-year-olds is not as remarkable for tumors with higher incidence in the pediatric population (Ewing, medulloblastoma, and rhabdomyosarcoma), because most of them are transitional trials (215 of 293, 73%), with 27% (59 of 215) having an upper-limit inclusion age of 21 years.
Access to Novel Therapies
The experimental interventions of interest included anticancer medication in 2049 of 2176 (94%) trials, radiotherapy in 134 (6%), hematopoietic stem cell transplantation in 103 (5%), imaging in 57 (3%), therapeutic devices in 35 (2%), and surgery in 23 (1%). Among medications, the investigational drugs were chemotherapies (483 of 2049, 24%), molecular targeted therapies (945, 46%), immunotherapies (680, 33%), and advanced therapy medicines (239, 11%), which are medicines for human use that are based on genes, tissues, or cells, including cell therapies and viruses (28).
For all four medication categories, there was a prominent gap in the access to drugs for patients younger than 18 years vs 18 years and older, as shown in Figure 5. Patients aged 18 years would have had access to 2.8× more trials investigating chemotherapies than patients aged 17 years; 4.1× more for advanced therapy medicines; 4.6× more for molecular targeted therapies; and 12× more for immunotherapies. Similar relevant increases remain when melanoma trials (the largest group of trials) are removed from the analysis.
Figure 5.
Access to anticancer medicines according to age.
Discussion
Our study shows that AYAs have limited access to cancer trials and innovative therapies, with no improvement over the last decade. Our findings suggest that the 18-year-old age limit continues to be a major obstacle.
There is an increasing awareness of the importance of addressing the specific needs of the AYA population that has led to the development of AYA oncology (10,29). For instance, a simple search in PubMed (with the terms “adolescents” or “adolescent” in the title and “cancer” in the title or abstract) reveals a considerable increase in the number of publications over the last 20 years, from 31 in 1998 to 318 in 2017.
Despite this increased awareness about AYA, there has been no improvement in the number of trials that allow the inclusion of the AYA population younger than 18 years of age during the last decade. Although the total number of cancer trials is increasing year by year, the number of trials potentially accessible to children and adolescents remains stable. Among the reviewed malignancies, less than 20% of the trials were transitional (ie, crossed the 18-year-old inclusion frontier), and only 5 out of more than 2000 trials were AYA-specific, even though most tumor types analyzed were AYA-specific. The low proportion of AYA-specific trials remains even if the melanoma trials are not taken into account (0.28%; 3 of 1046). This strengthens the concept that the main stakeholders have been advocating for, namely, to lower the inclusion age for adult clinical trials, particularly for those tumors with relevant incidence among the AYA population or for those with the most unmet needs (6,16,19). Interestingly, our study suggests that academic sponsors are more prone to widen age inclusion criteria, with only 31% of transitional trials having industry sponsors or co-sponsors. The proportion of different phase trials was similar when comparing academic to industry trials, thus this was not a confounding factor. The 18-year-old limit continues to be a major hurdle for AYAs with cancer to access new drugs; the stakeholders involved in drug development (pediatric and adult academia, industry, regulators, parents, and patients) need to work together to change this situation. This joint effort is ongoing in Europe within the Fostering Age Inclusive Research trial initiative of the ACCELERATE platform (30), which includes parents and patients’ representatives (16). In the United States, the US Food and Drug Administration (FDA) has recently produced guidance for the industry to support inclusion of adolescents in adult trials from early phase and beyond (31). A positive example of the ongoing efforts can be seen in the tropomyosin receptor kinase (TRK) inhibitors setting. The first trial for larotrectinib (LOXO-TRK-14001, NCT02122913), a phase 1, adult-only trial, started in December 2014. Nine months later, a phase 2 trial (NAVIGATE, NCT02576431) followed, allowing inclusion from 12 years of age; shortly after, a pediatric phase 1–2 trial (SCOUT, NCT02637687) allowing inclusion up to 21 years opened. These three trials led to accelerated approval by the FDA in 2018, evaluating safety in 176 patients, including 44 pediatric patients (32). Moreover, the first trial to open for LOXO-195, a second-generation TRK inhibitor, allows inclusion of patients starting at 1 month of age (NCT03215511).
A good example of the unmet needs for adolescents is given by melanoma (16), a typically adult tumor that is, however, responsible for 5–10% of the cancers in the age group of 14–18 years. According to our study, whereas a 17-year-old patient with melanoma would have had potential access to 58 (5%) of the reviewed melanoma trials, 1 year later (aged 18 years), that same patient would have had access to 1100 (97%) trials. In fact, even for typical AYA tumors such as Hodgkin lymphoma, with the highest prevalence (>20%) occurring in adolescents (14–18 years), a 17-year-old patient could only have enrolled in 120 (26%) of the reviewed Hodgkin lymphoma trials. At the age of 18 years, that Hodgkin lymphoma patient would have had theoretical access to almost the quadruple (445, 95%) number of trials.
This gap is further seen regarding access to anticancer medicines. Whereas AYAs 18 years and older may have accessed the vast majority of investigational drugs, the access is much more restricted for patients younger than 18 years. For instance, an 18-year-old patient would have had access to almost 5 times more trials investigating molecular targeted therapies and to 12 times more trials investigating immunotherapies than a 17-year-old patient (7 times when taking out the melanoma trials).
The gap in the availability of trials and new drugs is not only a quantitative problem. Opening trials indiscriminately for children and AYAs will hardly solve the problem; addressing unmet needs and refining which trials need to be opened for AYAs will. Specially challenging are the rare-condition settings in this population. As an example, the occurrence of carcinomas in AYAs with cancer predisposition syndromes (eg, breast cancer and Breast cancer gene mutation, colorectal cancer and Adenomatous polyposis coli gene) opens the question of how to manage this particular adolescent population.
Furthermore, in addition to the reduced number of trials and drugs available to AYAs, it seems that this access is delayed when comparing the opening of trials in adults vs in children and adolescents (16,33). Although it is beyond the scope of this work, further research will allow measuring the delay more accurately.
Another aspect to consider is that the emerging molecular profiling platforms could play a major role in improving drug access for the AYA population in the upcoming years. However, not all sequencing platforms are inherently linked to drug access, and hence, new difficulties may arise for patients younger than 18 years, if genomic-driven drug access is only available on adult-based early-phase trials (34). Meanwhile, there are several ongoing genomic profiling initiatives for childhood and adult cancers that are reshaping the landscape of personalized medicine: the NCI-MATCH, NCI-COG Pediatric MATCH, INFORM, and SPECTA platforms, among others (35–37). Although recruitment of AYA patients to these platforms might be fragmentary, some promising projects such as the recently opened SPECTA-AYA are aiming specifically to recruit AYAs.
Other remarkable findings of our study, not specific to the AYA topic but nonetheless worth mentioning, are the small number of closed trials that have published results (<40%); the high proportion of monocentric trials (50%), which can be an indicator of insufficient collaboration in the clinical research community; the lack of research for nonmedication treatments, with only 1% of the trials investigating surgical aspects and 6% radiotherapy aspects, in spite of both still being crucial treatment modalities; and the unequal access to trials and innovative treatments across the globe, with almost two-thirds of the trials being conducted exclusively in North America [although there are signs that the North American research hegemony may be shifting (38)].
We acknowledge the limitations of the study. First, clinicaltrials.gov was the only source used for the search. This registry is being increasingly used by investigators to assess research practices; nevertheless, conducting valid analyses requires an understanding of both the capabilities and limitations of the database, as pointed out by Tse et al. (39). A major issue to be considered is that incentives for reporting trials have changed over time. Our study search is limited to trials starting from 2007, notably after systematic registration of clinical trials started being promoted in 2005 (40). This included two key policy changes for clinical trial reporting on clinicaltrials.gov: the 2005 requirement by the International Committee of Medical Journal Editors to register all clinical trials as a condition for publication of results (40) and the 2007 requirement by the FDA Amendments Act to register non-phase 1 clinical trials of drug and biological products, as well as nonfeasibility trials of device products (41). However, we acknowledge that the dynamic nature of clinicaltrials.gov needs to be taken into account when interpreting the results of the study. Other limiting aspects include the possible underrepresentation of trials conducted out of Western countries and the lack of data regarding willingness and/or ability of specific groups of patients to participate in trials (39,42).
In spite of these shortfalls, clinicaltrials.gov remains the largest publicly available trial database, and we believe it to be better suited for the proposed research question, as it takes most registered trials into account. Using MedLine or Scopus would have limited the search to trials with published results (370 of 2176, 17%).
Second, the trial search was limited to a number of solid tumor types. The 10 selected tumor types are a worthy representation of the complex AYA cancer reality. Adapting the classification used by Gaspar et al. (16), we aimed to include tumors considered similar in the adult and pediatric population (Ewing sarcoma, osteosarcoma, synovial sarcoma, ALCL, Hodgkin lymphoma, and extracranial GCT, the latter two epidemiologically typical AYA tumors); adult tumors rarely present in adolescents (melanoma and thyroid cancer); and pediatric tumors rarely present in the adult population (rhabdomyosarcoma and medulloblastoma). Other tumors such as diffuse large B-cell lymphoma or acute leukemias were left out; conversely, it can be argued that melanoma and thyroid cancer are not representative of the unmet needs for AYA patients, because they usually have a good prognosis in this age group and are treated predominantly with surgery. Nonetheless, this arguably subjective selection of tumor types is unlikely to interfere with the aim of this meta-research study, a study of research itself: to describe research practices in order to improve them (43,44). Albeit the inherent limitations of meta-research, the performed review of more than 2000 clinical trials is the largest study of AYA trials conducted to date.
Promising changes have been proposed by ongoing international multistakeholder initiatives, such as the European ACCELERATE platform and the US FDA recommendations to improve access of novel anticancer drugs to adolescents (6,16,31). Some of these proposals include the following: in adult early-phase trials that have study mechanisms of action relevant to adolescents, the inclusion age limit should be lowered to 12 years; for AYA cancers present in both pediatric and adult populations with similar biology, no upper or lower age limit criteria should be fixed for phase 2 and 3 trials; where relevant, adolescents should be included in pediatric phase 1, 2, and 3 trials and, reversely, young adults with pediatric malignancies should be offered enrollment in pediatric phase 2 and 3 trials (16). While changing the age barrier is crucial for improving trial availability for AYA patients, it only ameliorates one part of a complex problem. Other factors will need to be addressed as well, such as service configuration and/or place-of-care factors and recruitment methods (institutional and/or structural barriers), and developmental factors specific to young people, for instance, acceptability of studies (patient-related barriers) (45). Developing more AYA-driven trials will hopefully help overcome these obstacles.
In conclusion, the increasing interest and advocacy for AYA oncology has not yet sufficiently translated into clinical research. AYAs have limited and delayed access to cancer trials and innovative therapies, with no improvement over the last decade. The 18-year-old age barrier continues to be a major hurdle. The findings of this study are consistent with the ongoing initiatives by the research community to discuss age inclusion criteria in oncological clinical trials, based on scientific rationale (epidemiology and targets) rather than by age categories without biological basis. Hopefully, this study will contribute to improve awareness of all stakeholders on breaking the age barrier in clinical research and ultimately help improve clinical care for AYAs with cancer.
Funding
This publication was supported by Fonds Cancer from Belgium and the European Organisation for Research and Treatment of Cancer Cancer Research Fund (ECRF).
Notes
Affiliations of authors: Medical Department (TdR, MT, JP) and Statistics Department (AN), European Organisation for Research and Treatment of Cancer HQ, Brussels, Belgium; Pediatric Oncology Department, Hospital Donostia, San Sebastian, Spain (MGA); Clinical Research Unit, Hospital Niño Jesús, Madrid, Spain (LM); Department of Oncology for Child and Adolescent, Gustave Roussy Cancer Campus, Villejuif, France (NG); Medical Oncology Department, Institut de Cancérologie des Hospices Civils de Lyon, Université Lyon 1, Lyon, France (JP).
The authors thank Ms Sarah Kelly for language editing.
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Bleyer A, O’Leary M, Barr R, Ries L.. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age. Washington, DC: National Cancer Institute, NIH Pub. No. 06-5767; 2006. https://seer.cancer.gov/archive/publications/aya/. Accessed April 2, 2019.
- 2. Desandes E, Stark DP.. Epidemiology of adolescents and young adults with cancer in Europe. Prog Tumor Res. 2016;43:1–15. [DOI] [PubMed] [Google Scholar]
- 3. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F.. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017; 18(12):1579–1589. [DOI] [PubMed] [Google Scholar]
- 4. van der Graaf WTA, Orbach D, Judson IR, Ferrari A.. Soft tissue sarcomas in adolescents and young adults: a comparison with their paediatric and adult counterparts. Lancet Oncol. 2017;18(3):e166–e175. [DOI] [PubMed] [Google Scholar]
- 5. Cha S, Lee J, Shin J-Y, et al. Clinical application of genomic profiling to find druggable targets for adolescent and young adult (AYA) cancer patients with metastasis. BMC Cancer. 2016;16(1):170.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chuk MK, Mulugeta Y, Roth-Cline M, Mehrotra N, Reaman GH.. Enrolling adolescents in disease/target-appropriate adult oncology clinical trials of investigational agents. Clin Cancer Res. 2017;23(1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tai E, Buchanan N, Westervelt L, Elimam D, Lawvere S.. Treatment setting, clinical trial enrollment, and subsequent outcomes among adolescents with cancer: a literature review. Pediatrics. 2014;133(suppl):S91–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen I, Pasalic D, Fischer-Valuck B, et al. Disparity in outcomes for adolescent and young adult patients diagnosed with pediatric solid tumors across 4 decades. Am J Clin Oncol. 2016;41(5):471–475. [DOI] [PubMed] [Google Scholar]
- 9. Frederick NN, Mack JW.. Adolescent patient involvement in discussions about relapsed or refractory cancer with oncology clinicians. Pediatr Blood Cancer. 2018;65(4):e26918. doi:10.1002/pbc.26918. [DOI] [PubMed] [Google Scholar]
- 10. Stark D, Bielack S, Brugieres L, et al. Teenagers and young adults with cancer in Europe: from national programmes to a European integrated coordinated project. Eur J Cancer Care (Engl). 2016;25(3):419–427. [DOI] [PubMed] [Google Scholar]
- 11. Desandes E, Brugières L, Molinié F, et al. Adolescent and young adult oncology patients in France: heterogeneity in pathways of care. Pediatr Blood Cancer. 2018;65(9):e27235.. [DOI] [PubMed] [Google Scholar]
- 12. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009–1016. [DOI] [PubMed] [Google Scholar]
- 13. Trama A, Botta L, Foschi R, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population-based data from EUROCARE-5. Lancet Oncol. 2016;17(7):896–906. [DOI] [PubMed] [Google Scholar]
- 14. Bleyer A, Tai E, Siegel S.. Role of clinical trials in survival progress of American adolescents and young adults with cancer-and lack thereof. Pediatr Blood Cancer. 2018;65(8):e27074.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roth ME, O’Mara AM, Seibel NL, et al. Low enrollment of adolescents and young adults onto cancer trials: insights from the Community Clinical Oncology Program. J Oncol Pract. 2016;12(4):e388–e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaspar N, Marshall LV, Binner D, et al. Joint adolescent-adult early phase clinical trials to improve access to new drugs for adolescents with cancer: proposals from the multi-stakeholder platform-ACCELERATE. Ann Oncol. 2018;29(3):766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accelerate. Mixed media: childhood and adolescent cancer in the UK press. https://www.accelerate-platform.eu/work-programme/ongoing/working-group-fair/opinion/mixed-media-childhood-adolescent-cancer-uk-press/. Accessed October 1, 2018.
- 18. Scott C. Children dying of cancer because they’re excluded from drug trials DailyMail.com. June 25, 2018. https://www.dailymail.co.uk/health/article-5884939/Tragedy-children-dying-cancer-drug-companies-wont-include-trials.html. Accessed October 1, 2018.
- 19. Gore L, Ivy SP, Balis FM, et al. Modernizing clinical trial eligibility: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Minimum Age Working Group. J Clin Oncol. 2017;35(33):3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis LE, Janeway KA, Weiss AR, et al. Clinical trial enrollment of adolescents and young adults with sarcoma. Cancer. 2017;123(18):3434–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vassal G, Rousseau R, Blanc P, et al. Creating a unique, multi-stakeholder paediatric oncology platform to improve drug development for children and adolescents with cancer. Eur J Cancer. 2015;51(2):218–224. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed September 2, 2018.
- 24.Regulation (Ec) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on Medicinal Products for Paediatric Use; 2006. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf. Accessed September 2, 2018.
- 25. Vassal G, Geoerger B, Morland B.. Is the European Pediatric Medicine Regulation working for children and adolescents with cancer? Clin Cancer Res. 2013;19(6):1315–1325. [DOI] [PubMed] [Google Scholar]
- 26. Gülmezoglu AM, Pang T, Horton R, Dickersin K.. WHO facilitates international collaboration in setting standards for clinical trial registration. Lancet. 2005;365(9474):1829–1831. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO clinical trials initiative to protect the public. Bulletin of the World Health Organization 2016. http://www.who.int/bulletin/volumes/84/1/who_news0106/en/. Accessed October 2, 2018. [PMC free article] [PubMed]
- 28. European Medicines Agency. Advanced therapy medicinal products: overview. https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview. Accessed June 28, 2019.
- 29. Saloustros E, Stark DP, Michailidou K, et al. The care of adolescents and young adults with cancer: results of the ESMO/SIOPE survey. ESMO Open. 2017;2(4):e000252.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Accelerate Platform. FAIR Trials. https://www.accelerate-platform.org/fair-trials/. Accessed August 7, 2019.
- 31. U.S. Department of Health and Human Services. Considerations for the inclusion of adolescent patients in adult oncology clinical trials guidance for industry. June 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM609513.pdf. Accessed July 15, 2019.
- 32.FDA approves larotrectinib for solid tumors with NTRK gene fusions. December 14, 2018. https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0. Accessed June 28, 2019.
- 33. Neel DV, Shulman DS, DuBois SG.. Timing of first-in-child trials of FDA-approved oncology drugs. Eur J Cancer. 2019;112:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewin J, Siu LL.. Cancer genomics: the challenge of drug accessibility. Curr Opin Oncol. 2015;27(3):250–257. [DOI] [PubMed] [Google Scholar]
- 35. Allen CE, Laetsch TW, Mody R, et al. Target and agent prioritization for the Children’s Oncology Group—National Cancer Institute Pediatric MATCH trial. J Natl Cancer Inst. 2017;109(5):djw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Worst BC, van Tilburg CM, Balasubramanian GP, et al. Next-generation personalised medicine for high-risk paediatric cancer patients? The INFORM pilot study. Eur J Cancer. 2016;65:91–101. [DOI] [PubMed] [Google Scholar]
- 37.European Organisation for Research and Treatment of Cancer. SPECTA Projects. https://www.eortc.org/specta/specta-projects/. Accessed April 3, 2019.
- 38. Drain PK, Parker RA, Robine M, Holmes KK.. Global migration of clinical research during the era of trial registration. PLoS One. 2018;13(2):e0192413.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tse T, Fain KM, Zarin DA.. How to avoid common problems when using ClinicalTrials.gov in research. BMJ. 2018;361:k1452.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351(12):1250–1251. [DOI] [PubMed] [Google Scholar]
- 41. Gov.info. Food and Drug Administration Amendments Act of 2007. Public Law. 2007:1–156. https://www.gpo.gov/fdsys/. Accessed July 15, 2019. [Google Scholar]
- 42. Durham TA. How did these data get here? Recommendations for the analysis of data from ClinicalTrials.gov. Ther Innov Regul Sci. 2018:2–3. doi:10.1177/2168479018811825. [DOI] [PubMed] [Google Scholar]
- 43. Ioannidis JPA, Fanelli D, Dunne DD, Goodman SN.. Meta-research: evaluation and improvement of research methods and practices. PLoS Biol. 2015;13(10):e1002264.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ioannidis J. Meta-research: why research on research matters. PLoS Biol. 2018;16(3):e2005468.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fern LA, Taylor RM.. Enhancing accrual to clinical trials of adolescents and young adults with cancer. Pediatr Blood Cancer. 2018;65(9):e27233.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.