Abstract
Late disease recurrence (more than 5 years after initial diagnosis) represents a clinical challenge in the treatment and management of estrogen receptor-positive breast cancer (BC). An international workshop was convened in Toronto, Canada, in February 2018 to review the current understanding of late recurrence and to identify critical issues that require future study. The underlying biological causes of late recurrence are complex, with the processes governing cancer cell dormancy, including immunosurveillance, cell proliferation, angiogenesis, and cellular stemness, being integral to disease progression. These critical processes are described herein as well as their role in influencing risk of recurrence. Moreover, observational and interventional clinical trials are proposed, with a focus on methods to identify patients at risk of recurrence and possible strategies to combat this in patients with estrogen receptor-positive BC. Because the problem of late BC recurrence of great importance, recent advances in disease detection and patient monitoring should be incorporated into novel clinical trials to evaluate approaches to enhance patient management. Indeed, future research on these issues is planned and will offer new options for effective late recurrence treatment and prevention strategies.
We convened an international workshop that involved clinicians, trialists, scientists, and funders with expertise and/or interest in addressing the problem of late recurrence in estrogen receptor-positive (ER+) breast cancer (BC). The workshop was held in Toronto, Canada, on February 15–16, 2018, and its goal was to review current knowledge about the issue of late recurrence and to make recommendations for future research strategies (a list of participants is included as an Supplementary Appendix, available online). This article summarizes the workshop discussions regarding the biology underlying late recurrence with a focus on tumor dormancy, potential approaches to identify late recurrences before they are clinically apparent, and key research directions. A companion article (2) describes the current understanding of the problem of late recurrence as well as clinical considerations around late recurrence. Here, we review diagnostic testing approaches to predict or identify late recurrence and propose a framework for approaching potential research directions, including prognostic biomarkers and therapeutic interventions. Herein, we define late recurrence as recurrence 5 or more years after diagnosis, which corresponds to the minimum recommended course of adjuvant endocrine therapy (ET) and accounts for approximately one-half of all recurrences.
Late Recurrences in ER+ Breast Cancer: A Question of Tumor Dormancy
To diagnose and treat late recurrences in patients with ER+ BC, it is essential to consider the biology of these events. There were two competing models discussed at the workshop: indolency vs dormancy. In the former, preexisting disseminated micrometastases in distant organs from the breast might be growing at a constant, but very slow, rate so that their clinical appearance occurs much later after diagnosis and treatment of the primary cancer. In the latter, preexisting micrometastases in distant organs are dormant or remain in a balanced equilibrium of cellular turnover for prolonged periods of time. At some point, some of these are “tipped” into rapid growth, or in other words, escape or exit dormancy to appear as clinically apparent metastases.
Overall, the attendees of the workshop favored the dormancy model because the indolence model may relate more to relatively early recurrences, for example, those that occur in the first few years after diagnosis. Different rates of tumor growth would explain the diversity among the time to first recurrence—some within months, whereas others may be years later. In theory, these are the types of cancer recurrence in which adjuvant chemotherapy given soon after diagnosis has the greatest benefit. It was felt that these cancers are overrepresented by ER- negative tumors, regardless of human epidermal growth factor receptor 2 (HER2 or ERBB2) status, as well as ER+ cancers with high proliferative thrust (so-called luminal B BCs).
The dormancy model might itself be split into two separate models. In one, a single cell or a small accumulation of cancer cells has exited the active cell cycle and exists in a resting phase (G0). In other words, they are quiescent. In the second, a cluster of malignant cells making up a micrometastasis is in equilibrium between proliferation and programmed cell death. This scenario might be termed “balanced cell proliferation and death.” These two models are supported by preclinical studies and may not be mutually exclusive, either between patients or even within a single individual (2–4).
These models raise the issue of what generates dormancy, and why and how metastases escape it. Metastatic dormancy may result from a complex relationship between the genetic status of the systemic tumor cells and their microenvironment, including both the local and systemic status of the immune system and other systemic host factors, such as the presence of nutrients, growth, and angiogenic factors. The epigenetic state of systemic tumor cells may also play a role, given the possibility that epigenetic reprogramming may be required for these cells to adapt and thrive in foreign microenvironments. Dormant cancer cells often resemble normal stem cells, which also undergo periods of dormancy followed by activation and self-renewal (5,6). Malignant cells exhibiting markers of stemness are frequently found in primary BC as well as distant metastases. In one study, 65% of disseminated tumor cells (DTCs) within the bone marrow of patients exhibited a stem cell-like phenotype (7). Cells exhibiting these stem cell properties as well as specific surface markers have also been detected in the blood of patients with BC (8).
Alterations in any or all of the factors discussed above may potentially contribute to escape from dormancy, and the mechanisms of escape from dormancy appear to be multi-factorial (4). The complexity of these interactions is evidenced by variability in metastatic latency, as described above among various intrinsic subtypes of BCs (Figure 1). One mechanism may be a subsequent genetic “hit” that was not necessary for establishment of a micrometastases in a foreign site (9) but that induces a quiescent group of cells to reenter the cell cycle or tips a group of cells in balanced cell proliferation and death towards a state favoring growth over equilibrium. For example, p38 MAPK signaling is associated with maintenance of quiescence in breast and other cancer cells (4,10,11). Conversely, escape from dormancy is associated with activation of intracellular pathways associated with proliferation and growth such as the Akt, extracellular signal-regulated kinase 1/2, and Src signaling networks (12–14), or Nuclear Receptor Subfamily 2 Group F Member 1, an orphan nuclear receptor of the retinoic acid receptor family (15).
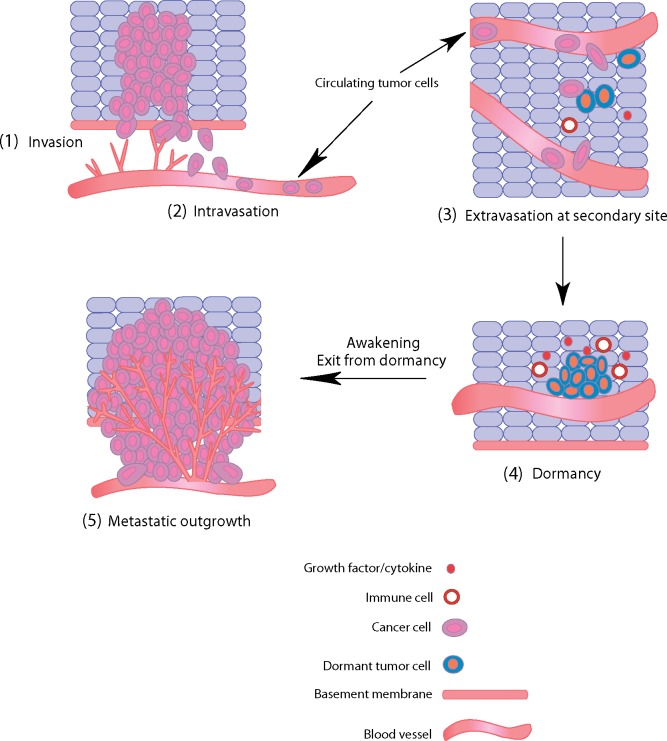
Figure 1.
Metastatic spread of human breast cancer. Metastatic spread and tumor outgrowth at a distant site require the completion of a number of critical steps, each involving complex interactions between cancer cells and the local microenvironment. Cells at the primary site invade tissue architecture and spread through the basement membrane (1: invasion), after which they enter blood vessels (2: intravasation). These cells then enter the circulation, where they must survive in the blood vessels before adhering to a vessel wall at a distant site. Cancer cells in the bloodstream can now be detected by various methods and enumerated to provide prognostic information. After adhering to a vessel wall, cancer cells then extravasate and enter normal tissue at a secondary site (3: extravasation). Interactions between cancer cells and the local microenvironment, which may include various growth factors, cytokines, and immune cells, may lead to the induction of dormancy for long periods of time (4: dormancy). Later, changes in these same factors, or the presence of new molecules, can induce an exit from dormancy, leading to full metastatic outgrowth and disease recurrence (5: metastatic outgrowth).
Alternatively, or in concert with new genetic changes, alterations in host factors may also play a role in escape from dormancy. For example, it has long been proposed that the immune system may play a critical role in tumor dormancy through immunosurveillance. In preclinical models, both the innate and adaptive immune systems detect and eliminate cancer cells as a means of anticancer defense (3,16). If this mechanism is disturbed, some cancer cells may emerge as resistant to elimination, or masked from detection, and initiate the development of a macrometastatic lesion.
Although this theory is appealing, it is likely that the interactions between the immune system and dormant cancer cells are more complex. In certain contexts, inflammation may have a paradoxical effect by stimulating emergence from dormancy, for instance through the action of neutrophil-derived factors (17). Moreover, there is no evidence that immunosuppressed patients, such as those with acquired immune deficiency or solid organ transplants, have either a higher risk of BCs or a higher risk of subsequent recurrences (early or late) following diagnosis (18,19). Indeed, tolerance to components of the immune system is an important part of evolution of multi-cellular species; otherwise, the human race would be plagued with auto-immune diseases. However, recent studies have identified both check-points that control the immune response as well as pharmaceutical agents to inhibit them (20–22). These immune checkpoint inhibitors have resulted in exceptional responses and even cures in metastatic melanoma, non-small lung cancer, colorectal and bladder cancers, among other malignancies. Recently, activity with checkpoint inhibitors in metastatic BC (MBC) has been reported, but these trials have been primarily conducted in patients with ER- and HER2-negative (“triple negative”) MBC (23–25). Such therapeutic approaches might be an exciting strategy to enhance or activate immune surveillance if they can be instituted safely in the future, but their relevance in ER+ BC is currently unclear.
Tumor dormancy may also be induced by the absence of sufficient nutrients and oxygen to support the level of disseminated cell proliferation necessary for tumor growth. This state of angiogenic impairment limits the ability of cancer cells to grow beyond micrometastases, because cells can proliferate but lack the blood supply required for full metastatic outgrowth (26,27). Exit from angiogenic dormancy, or the angiogenic switch, can be stimulated either locally within the microenvironment or systemically via alterations in circulating factors (28). In particular, vascular endothelial growth factor is implicated in the stimulation of angiogenesis, and placenta growth factor may play a role in metastatic outgrowth in the bone (29–31). However, although initially met with great enthusiasm, anti-angiogenic therapy with bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has resulted in minimal benefit in the metastatic or adjuvant settings in BC (32–34). These disappointing results may suggest that either the hypothesis of tumor-induced neo-angiogenesis is incorrect or that this particular agent is insufficient to prevent it. Furthermore, relevant to the issue of late recurrence in ER+ BC, subset analyses of several trials have suggested that if bevacizumab is effective at all in BC, the benefit is limited to patients with ER-negative disease (35).
The most common metastatic site for patients with ER+ BC is bone. This observation suggests that bone exhibits unique properties that allow disseminated BC cells to survive and colonize this tissue microenvironment. Cancer cells that enter the bone localize to specific niches and often exhibit distinct interactions with the normal stroma. In particular, tumor cells are often found in regions of bone containing cells of osteoblast lineage where they interact via specific proteins and ligands to establish colonization and dormancy (36,37) (Figure 2). In preclinical models, escape from dormancy in the bone is a complex process characterized by paracrine interactions between the cancer cells and osteoblast and osteoclast activation (4,36,38). Therefore, therapies designed to reduce metastatic outgrowth in the bone, such as bisphosphonates or RANK ligand inhibitors, represent potentially effective disease management strategies (39–42). The use of adjuvant bisphosphonates has provided some benefit (42,43), but effects are limited to postmenopausal women and not necessarily to those with ER+ disease. Likewise, evidence that adjuvant use of the RANK ligand inhibitor denosumab reduces risk of recurrence (early vs late) in ER+ postmenopausal BC patients receiving aromatase inhibitor therapy is not convincing (44).
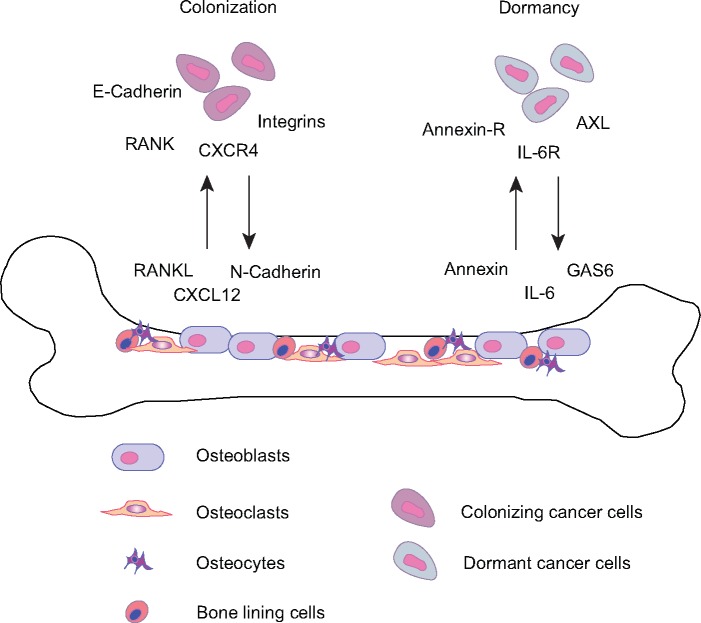
Figure 2.
Colonization of bone by breast cancer (BC) cells. BC cells frequently metastasize to the bone where a series of complex interactions between tumor cells and normal cells in the microenvironment mediate colonization and dormancy. For example, RANKL and CXCL12 are produced by normal cells and attract tumor cells to home towards the bone and initiate bone colonization. Cadherins and integrins are also implicated in this process. Conversely, annexin, IL-6, and GAS6 are secreted by normal cells within the bone niche and engage their cognate receptors on tumor cells to enable survival within the bone microenvironment and subsequent dormancy. RANK-L: RANK Ligand, CXCL12: C-X-C motif chemokine 12, also known as stromal cell-derived factor 1 (SDF1), IL-6: interleukin-6, GAS6: growth arrest-specific-6.
Taken together, these considerations suggest that the (epi)genetic makeup of the cancer cells themselves as well as the levels of numerous immune, inflammatory, and angiogenic factors may be responsible for dormancy and that alterations in them may account for release from dormancy and late recurrences of ER+ BCs. Therefore, understanding these factors may provide potential diagnostic and therapeutic opportunities for the prevention of disease recurrence. However, the field is limited by the lack of prospectively conducted clinical research of patients who have achieved 5 or more years without recurrence, and further research is required to determine if a patient with a previously diagnosed ER+ BC can safely discontinue all therapy, should continue on extended ET, or should add or switch to a completely novel therapeutic.
Theoretical Classification of Dormancy Status in Patients with ER+ BC
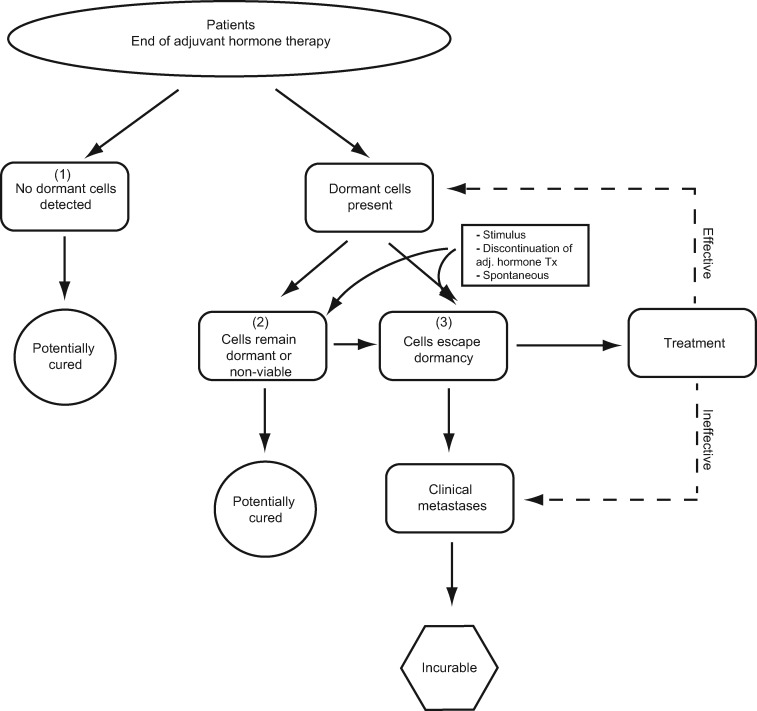
We propose that patients with a previously diagnosed ER+ invasive BC and who reach 5 or more years without recurrence may be divided into one of three theoretical categories (Figure 3): 1) no dormant cells present, 2) cells present, but still dormant, and 3) cells present that have escaped dormancy. It is important to note that patients may move between categories over time, for example, from category 2 to category 3, as cells exit dormancy. At present these categories are theoretical, because adequately validated tests to detect residual cancer cells are not yet available. Although definitive classification into these categories is not currently possible for any individual patient, in the sections below we suggest how future tests based on DTCs, circulating tumor cells (CTCs), and/or ctDNA may someday enable categorization. Although we use the term “dormancy” here and describe three distinct categories, we recognize that other cellular mechanisms may contribute to early and/or late recurrence, and there is likely a continuum of dormancy including mixed cell populations that culminate in clinical development of metastases once a critical threshold is reached.
Figure 3.
Theoretical classification of dormancy status in patients with estrogen receptor-positive breast cancer and possible outcomes. Three categories are possible: Those with 1) no dormant cells present, 2) cells present, but still dormant, and 3) cells present that have escaped dormancy; although patients may move between categories over time. Adj = adjuvant; Tx = treatment.
Category 1: No Dormant Cells Present
These patients do not have any evidence of distant, dormant, or active malignancy, such as DTCs detectable in bone marrow, CTCs, or ctDNA (see below). These patients would have a high chance of being cured and thus spared additional therapy.
Category 2: Cells Present but Still Dormant
These patients have some sort of potentially detectable evidence of viable cells present in distant organs. Evidence might include DTCs, CTCs, or ctDNA, with tumor cell or DNA biomarker tests suggesting the detected cells are viable but still dormant. These patients are likely at risk of recurrence, but the absolute annual risk and time course of risk are unknown and need to be quantified. Therapeutic strategies might be to continue current therapy, because it appears to be “working,” switch to or add something that could be effective but still has low toxicity if patients are currently on ET, or consider initiating therapy (endocrine or other) if patients are not on treatment. A subcategory of this group might include patients who have detectable DTCs or CTCs, but if the markers were available, these cells could be shown to be short-lived terminally differentiated bulk cells with no evidence of “stemness” and no viable potential. These patients would have no or very low chance of recurrence and could safely stop ET or any other therapy designed to prevent late distant recurrence.
Category 3: Cells Present, Have Escaped Dormancy
These patients exhibit some detectable preclinical marker indicative of recurrence (DTCs, CTCs, ctDNA), and theoretical tumor biomarker tests, if available, would demonstrate that these cells have escaped dormancy. These patients are at high risk of distant recurrence in a relatively short time. The challenge is to both detect tumor cells or DNA and determine that tumor cells are no longer dormant. If this can be accomplished, one might consider treating these patients as if they had established metastases in the hopes of returning cells to dormancy or preventing clinical metastases. At present, palliation is the evidence-supported goal of therapy for MBC, because prior and outdated prospective randomized trials have suggested no benefit in early vs later treatment of patients with occult but impending relapse (45). Therefore, one should emphasize inclusion of such patients in clinical trials, either to readdress the issue of switching to (or initiating) an existing standard therapy, such as a different ET, or to ask important questions of novel therapies, such as CDK4/6 inhibitors, which are proven effective in the metastatic setting (46,47). One could also consider using the DTC-CTC-ctDNA characterization results to provide “Precision Targeted Therapy” or modern immunotherapy within a matching-type trial.
Although it is appealing to consider these three categories, at present the technology to identify them with any certainty is in its infancy. Nonetheless, we propose that studies using modern and emerging technologies attempting to do so, as well as interventional trials directed towards each group, are imperative, including possible trials evaluating the benefit of reduced or extended adjuvant ET vs completion of the standard course for the low-risk patients in group 1. It is worth noting that we may find that tissue-based or blood-based biomarkers associated with risk of late relapse are best measured at the time of diagnosis and that later emergence of detectable tumor-associated abnormalities reflects relapse and inferior actionability; all of these possibilities need to be explored.
New Technologies for Identifying Potential Exit From Dormancy and Predicting Late Recurrence
Disseminated Tumor Cells
Identification of DTCs, or indication of their presence, has been the focus of substantial translational research over the last several decades (Table 1). Several investigators have reported that up to 30% of patients with newly diagnosed BC have bone marrow micrometastases at the time of initial diagnosis. A pooled analysis of these data demonstrates that having bone marrow micrometastases is associated with higher rates of recurrence, but the risk is not absolute (48,49).
Table.
1. Methods for identifying potential exit from dormancy with potential utility in predicting late recurrence*
| Assay | Method | Advantages | Limitations |
|---|---|---|---|
| DTCs | Detection of cytokeratin-positive cells in bone marrow aspirates by ICC |
|
|
| Circulating tumor antigens | Detection of tumor-associated proteins (CA15-3, CA27.29, CEA, CA125) in blood |
|
|
| CTCs | Enumeration of EpCAM-positive cells (CellSearch), or enrichment-free multiparametric detection of cells (EPIC Sciences), in blood |
|
|
| ctDNA | Targeted (PCR-based) or nontargeted (genome or exome sequencing), methylation analysis detection of DNA in blood |
|
|
CEA = carcinoembryonic antigen; CTC = circulating tumor cell; ctDNA = circulating tumor DNA; DTC = disseminated tumor cell; EpCAM = epithelial cell adhesion molecule; FDA = Food and Drug Administration; ICC = immunocytochemistry; PCR = polymerase chain reaction.
Furthermore, in one study, bone marrow micrometastases were identified in 15% of patients who were at least 3 years from initial diagnosis (50). The risk of distant recurrence over the succeeding 24 months was 21% compared with 7% for those who did not have detectable bone marrow micrometastases (P < .001). In another report, the incidence of bone marrow metastases remained high (>16%) at 2–3 years after diagnosis but declined to 6% at the last date evaluated (51).
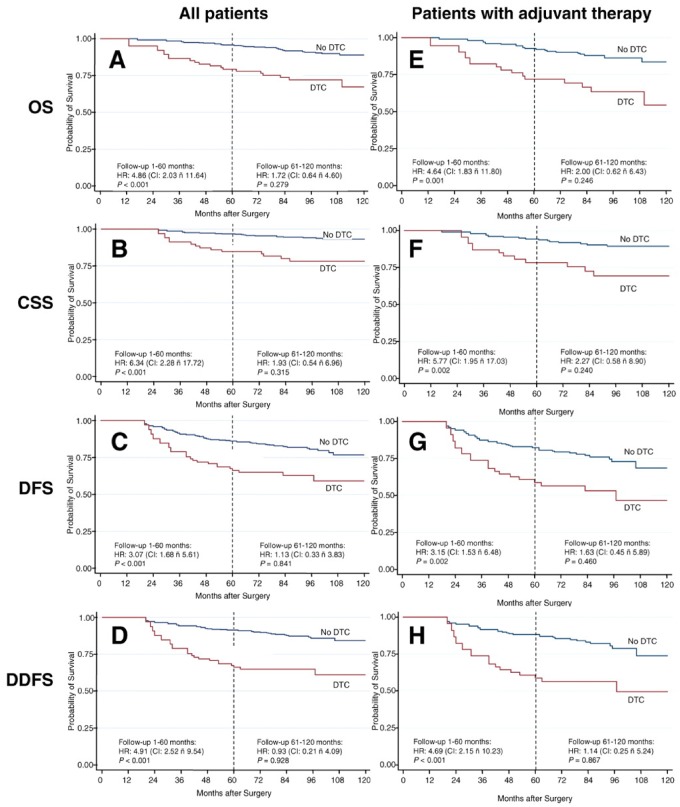
Furthermore, work by Janni and colleagues (52) demonstrated that the hazard ratio (HR) for distant recurrence for those with bone marrow micrometastases vs those without was greater than fourfold 5 years after initial diagnosis, which represented approximately 2–3 years after the bone marrow was analyzed (Figure 4). However, the risk of distant recurrence for those with positive bone marrow in the succeeding 2–3 years after bone marrow analysis was only approximately 40%. Furthermore, after this time, the presence of previously positive bone marrow was no longer associated with higher risk of recurrence compared with those with negative bone marrows (although both groups continued to develop distant recurrences). Specifically, there was no overall survival (OS) disadvantage for patients with DTC during the follow-up period from 6–10 years (after initial diagnosis). These data, combined with the invasive nature of obtaining bone marrow aspirates, has resulted in DTC evaluation being omitted from routine follow-up programs. Consequently, detection of tumor cells in blood became the focus of ongoing research aimed at identifying biomarkers of residual disease and distant recurrence in patients with cancer.
Figure 4.
Patient outcomes according to the presence or absence of disseminated tumor cells (DTCs) in bone marrow. Kaplan–Meier plots of long-term survival and outcome according to the presence or absence of DTCs in bone marrow. Vertical dotted lines indicate the cutoff point at 5 years of follow-up used in the piecewise Cox regression modeling. A–D, All patients in the study. E–H, Patients receiving adjuvant systemic treatment. CI = confidence interval; HR = hazards ratio. CSS = cancer-specific survival; DFS = disease free survival; DDFS = distant disease free survival; OS = overall survival. ñ = “to”. Reprinted from (52). Copyright©(2011) with permission from AACR.
Circulating Biomarkers: “Liquid Biopsies”
Evaluation of circulating markers, or so-called liquid biopsies, for prognosis in BC patients has also been an active area of research (Table 1). This has been a priority area of interest given the greater convenience and patient compliance with obtaining peripheral blood compared with bone marrow. Most studies to date have addressed circulating tumor-associated proteins, tumor cells, and cell-free tumor DNA. However, most of these have been studied in patients at or relatively near the time of initial diagnosis, with few data available regarding patients who are 5 or more years without recurrence.
Circulating Tumor-Associated Antigens
Circulating tumor-associated antigens are proteins, including products of the MUC1 gene (detected by commercially available CA15-3 or CA27.29 assays) as well as carcinoembryonic antigen and the CA125 antigen (53). These markers are elevated in approximately 75%, 50%, and 25%, respectively, of patients with MBC. Several studies have demonstrated that in asymptomatic patients being followed after primary diagnosis and treatment, a rising circulating tumor biomarker measured serially over time has a positive predictive value of 75–100% for subsequent detectable metastases, depending on the criteria to determine “rising” and the succeeding follow-up period. These markers are also fraught with false-positive findings, often associated with inflammatory but benign conditions of liver, gastrointestinal tract, and lung and mesothelial tissues (53). Only a single small study has randomly assigned such patients to having serial circulating tumor markers evaluated vs routine follow-up and failed to demonstrate any OS benefit for the former (54). Neither the American Society of Clinical Oncology nor the National Cancer Center Network guidelines recommend monitoring patients in this situation with these markers at present (45).
Circulating Tumor Cells
CTCs are elevated in 25–50% of patients with MBC (55). The desire to identify non-MBC patients who are at risk of disease recurrence has led to the evaluation of CTCs as a prognostic marker in the operable setting (Table 2). The detection of CTCs before initiation of chemotherapy (using a threshold of ≥1 CTC per 7.5 mL blood expressing the epithelial cell adhesion molecule [EpCAM] protein) is consistently associated with reduced distant disease-free survival and OS (56–59).
Table 2.
CTC detection in newly diagnosed breast cancer cohorts
| Study | No. of patients | Disease stage | Detection rate + | Predicts DFS? | Predicts OS? |
|---|---|---|---|---|---|
| REMAGUS02 (56,85,86) | 115 | II–III | 23% | Yes (HR 2.4) | Yes (HR 3.0) |
| GEPARQUATTRO (87,88) | 213 | I–III | 22% | Yes (HR 2.1) | Yes (HR 3.0) |
| NEOALTTO (89)* | 51 | I–III | 11% | NA | NA |
| NEOZOTAC (90) | 95 | I–III | 18% | NA | NA |
| MD Anderson (91) | 57 | I–III† | NA | Yes (HR 5.3) | Yes (HR 7.0) |
| MD Anderson (92) | 77 | III (T4d) | 55% | No | No |
| MD Anderson (93) | 63 | III (T4d) | NA | Yes (HR 4.2) | No |
| BEVERLY-1 and 2 (94) | 137 | III (T4d) | 35% | Yes (HR 2.8) | Yes (HR 4.3) |
| JBCRG-07 (95) | 34 | I-III | NA | Yes (HR 1.01) | NA |
| IMENEO (59)‡ | 2156 | I-III | 25% | Yes | Yes (HR 1.1) |
22.5 mL blood assessed. CTC = circulating tumor cell; DFS = disease free-survival; HR = Hazard Ratio; OS = overall survival; Distant metastasis free survival; LRFI = Local recurrence-free interval; + detection defined as presence of at least 1 CTC/7.5 mL blood by the CellSearch assay that detects EpCAM-expressing cells.
Triple negative tumors.
Meta-analysis.
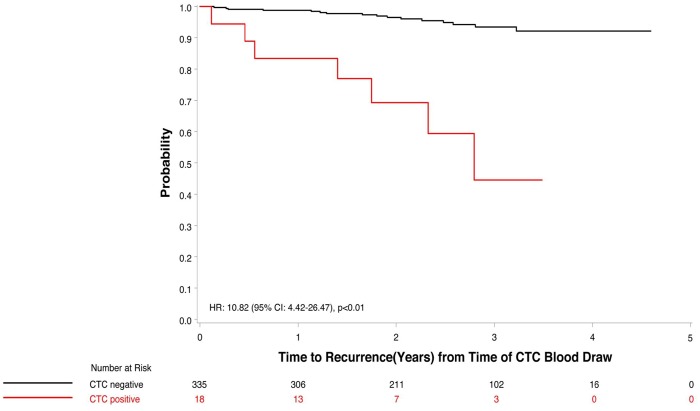
Sparano and colleagues (60) have recently reported a pilot study in which they evaluated the presence of CTCs, measured using the EpCAM-based CellSearch technology, in over 350 patients who had participated in an adjuvant clinical trial conducted by the Eastern Cooperative Oncology Group and were 4.5–7.5 years post-diagnosis without recurrence. Most, but not all, of these patients had ER+ disease (60). Eighteen (5%) of these patients had detectable CTCs (defined as ≥1 CTC per 7.5 mL whole blood), and the presence of CTCs was associated with a 10.82-fold higher risk of recurrence (95% confidence interval [CI] = 4.42 to 26.47, P < .01) (Figure 5). In multivariable models, the relative risk for distant recurrence after a median follow-up of 2.6 years was 13.1 (95% CI = 4.7 to 36.3) (60). However, as with bone marrow micrometastases, the presence of CTCs was not an absolute indicator of rapid recurrence. The recurrence rates over the succeeding 2–3 years of follow-up in the CTC-positive and CTC-negative groups were approximately 26% and 3%, respectively. Notably, no imaging was performed at the time of CTC evaluation, and thus it is not clear whether those with positive CTCs had asymptomatic metastatic disease at the time of the blood draw or subsequently developed metastatic disease.
Figure 5.
Circulating tumor cell positivity and time to recurrence in patients with hormone receptor-positive breast cancer. CI = confidence interval; CTC = circulating tumor cell; HR = hazard ratio. Modified and reproduced with permission from (60). Copyright©(2018) American Medical Association. All rights reserved.
Similar, but less striking, results were reported by Janni et al. (61) for CTCs measured using CellSearch 5 years after chemotherapy in patients enrolled in the SUCCESS A trial. In patients with HR+ disease, the presence of CTCs (>1 CTC per 7.5 cc) was a statistically significant prognostic factor for recurrence-free survival in both univariate (HR = 5.14, 95% CI = 1.47 to 18.03, P = .01) and multivariable analyses, adjusted for age, tumor stage, nodal stage, grade, histological type, and HER2 status (HR = 5.95, 95% CI = 1.14 to 31.16, P = .04) (61).
As with the bone marrow findings, the salient increased risk of recurrence for HR+ patients with at least one CTC supports the theory that CTC detection might serve as a potential method of identifying BC patients who at least have dormant disease (group 2, Figure 3). Furthermore, CTC enumeration may identify those who have escaped dormancy (Figure 3, group 3), with a very high risk of disease recurrence after completion of 5 years of ET. However, the short follow-up in these studies (2.6 years post CTC evaluation in the Sparano study) and the fact that the positive predictive value for distant recurrence is far from 100% may indicate that many of these patients still have dormant disease. Moreover, as was seen with randomized surveillance vs regular care studies in the past (using imaging and less sophisticated blood-based tests), early identification may not confer improved outcome (45).
To date, CTC studies in BC have used EpCAM-based CellSearch technology and relied on context-specific thresholds for prognostic utility (>5 CTC per 7.5 cc blood in the metastatic setting, >1 CTC per 7.5 cc in early BC patients). Other technologies use approaches that are not EpCAM based, which allow for enumeration of cells that may exhibit loss of EpCAM expression, thus possibly providing a more comprehensive evaluation of CTC numbers. The use of these newer technologies, potentially coupled with validated biomarkers present on CTCs, may provide enhanced prognostic and predictive information. For example, the EPIC Sciences CTC assay that characterizes an androgen receptor splice variant (AR-V7) has been used in castration-resistant prostate cancer to predict treatment benefit (62).
Circulating Tumor DNA
The detection of circulating cell free tumor DNA (ctDNA) has also been investigated for its prognostic potential in BC patients. In addition, analysis of ctDNA can yield information on the (epi)genetic composition of the tumor including clonal heterogeneity and possible actionable mutations (63). In the metastatic setting, several studies have demonstrated an association between the detection of ctDNA and poor outcome (64–66). In particular, mutations in the gene encoding the estrogen receptor (ER) (ESR1) found in ctDNA from ER+ BC patients are associated with worse outcome and resistance to ET (aromatase inhibitors and tamoxifen) (64,67). Furthermore, the ESR1 mutation rate appears to be higher in ctDNA than in metastatic tissue biopsies, suggesting ctDNA may overcome limitations in sampling and capture heterogeneity better (67,68). Although these results support the utility of ctDNA analysis in monitoring patients and possibly guiding treatment decisions, the field is relatively new and several issues regarding preanalytical and analytical factors remain unclear (69,70).
Importantly, although ctDNA is increasingly utilized for identification of actionable alterations and treatment planning in metastatic cancer, the clinical utility of ctDNA in early-stage disease for detecting minimal residual disease and for monitoring recurrence is not yet established (69,71). Nevertheless, preliminary studies have suggested that ctDNA may be present in up to 50% of patients with localized BC (stages I–III) (72), and ctDNA detection has demonstrated utility in the prediction of disease recurrence in at least some patient cohorts (72–75). However, one must be concerned whether the presence of ctDNA in patients with ER+ BC who have remained disease free for several years has the same clinical relevance as it might earlier in the disease course or in established metastatic disease. Currently, there are no data regarding determination of prognosis of ctDNA and late relapse in BC. For example, if the model of dormancy based on balanced cell proliferation and death is valid, it is possible that ctDNA may be present from such cells but may not portend immediate, or even high, risk of recurrence. Likewise, the presence of CTCs may also not signify high risk of recurrence in the context of balanced cell proliferation and death.
Although ctDNA technologies are currently robust enough to detect genomic alterations in patients with advanced metastatic disease, more sensitive technologies to detect minimally residual disease are still evolving. Planning to use such sensitive ctDNA technologies for clinical decision-making, such as possible therapy escalation, also raises concerns about the potential for false positives.
Future Directions
All present at the workshop were enthusiastic about initiating new investigations into the issue of late relapse in patients with ER+ BC who are 5 or more years from diagnosis without recurrence. However, discussions surrounding what exactly the most important research questions are as well as assay development and validation (tissue-based multi-factorial assays that risk-stratify patients based on tumor characteristics at diagnosis, bone marrow analyses or CTCs, ctDNA, and other circulating factors that can be measured serially to identify dynamic changes associated with exit from dormancy or impending recurrence) were robust. These discussions raised issues of different study designs that could be developed, how such studies would be funded, and whether the research questions could be addressed using existing resources (eg, biospecimens from ongoing clinical trials).
Diagnostic Studies
Analytical Issues Regarding Liquid Biopsies
A first goal is development of better diagnostics to determine how likely a patient is to be in one of the three categories identified (Figure 3) so that clinical strategies could be followed accordingly. Further goals include generation of interventions that can be used to prevent late recurrences, either those likely to occur within a few months of the time of testing or those that may occur in the distant future.
Assay Development and Validation
Issues surrounding potential variability among various platforms to enrich and enumerate DTCs and CTCs need to be addressed in pilot studies. Likewise, the technology to identify and quantify ctDNA is changing rapidly, as is the increasing understanding of the importance of careful attention to preanalytical issues such as the proper fixative in the collection tube and time to specimen processing.
Other issues include establishment of clinically validated thresholds for recurrence and the value of serial liquid biopsies to increase the positive and negative predictive values for true impending recurrence and later in response to therapy. Future studies should ideally include imaging at the time of liquid biopsies, especially if they appear to be providing an indication of impending relapse. Importantly, as in the past, prospective trials will be necessary to evaluate whether DTC, CTC, or ctDNA positivity can identify patients who will benefit from change in therapy. These trials will need to address each of the different categories (Figure 3) so that proper clinical utility can be established in each use context.
Finally, it is possible that DTC, CTC, and ctDNA may provide complementary information regarding late recurrences. This possibility is especially likely in regards to characterization of tumor cells or DNA as well as quantitation of the detected analytes. For example, several studies have demonstrated the ability to phenotype both DTCs and CTCs for expression of important proteins, such as ER, HER2, Ki67, markers of apoptosis, androgen receptor, and others (76). Similarly, cellular expression of a variety of genes at the RNA level is now technically feasible (77–80).
It is hoped that such evaluations might provide insight into whether detected cancer cells are still in, or have escaped from, dormancy. Furthermore, beyond quantification of ctDNA, determination of specific genomic (or epigenomic) abnormalities that might be exploited for treatment with targeted agents or provide an indication for the use of checkpoint inhibitors offers an exciting opportunity. As another example, identification of an ESR1 ligand-binding domain mutation might suggest a change in therapeutic strategy from aromatase inhibition to a selective estrogen receptor downregulator such as fulvestrant or a novel oral agent in development.
Moreover, recent studies have demonstrated the technical ability to isolate individual DTCs and CTCs and perform genomic analyses, offering the possibility of assessing genomic and phenotypic intra-patient heterogeneity and leading to informed combination therapy in the future (81–83).
Observational Studies
Prospective cohort studies of ER+ BC patients at high risk for late recurrence (based on clinicopathologic characteristics), with serial measurements (every 6–12 months) of blood and host factors (ideally combined with imaging when abnormalities are identified) and rigorous ascertainment of recurrences, will provide important insights into factors that precede late recurrence, leading to more accurate identification of patients at risk of late recurrence who could be enrolled in intervention studies evaluating agents with the goal of preventing disease relapse. A major outcome of these studies would be the identification of patients not at risk of recurrence or not at risk within a specified time frame so that these patients would not be subject to immediate interventions. These studies would also provide valuable information regarding the timeline between appearance of a marker of late recurrence (eg, DTCs, CTC, ctDNA, or tumor markers) and the appearance of clinically or radiographically apparent metastases.
The use of such assays that do not require “real-time” analysis would allow more cost-effective research because it is then possible to conduct nested case-control analyses at a later date (ie, focused on all patients with recurrence and a matched subset of patients without recurrence). This approach also avoids ethical issues that might arise if CTCs are found in real time and no intervention is provided (alternatively, this concern may be avoided if these patients are offered participation in an intervention trial), but it precludes the conduct of contemporaneous imaging studies to determine whether clinical metastases are present at the time of first detection of CTCs or ctDNA.
Such studies could also collect host factor information, with a focus on a parsimonious group of factors most likely to be associated with late recurrence. These data may provide insight into patient-related factors that could contribute to exit from dormancy, as discussed in the companion article.
One key design issue is the consideration of whether the patient is currently on ET when biomarkers are examined. The extent to which tumor-related factors change when ET is stopped or altered, whether assays performed while on or at completion of ET predict future recurrence, and whether these assays can be used to guide immediate treatment decisions (to stop, change, or continue ET) is not clear.
Interventional Studies
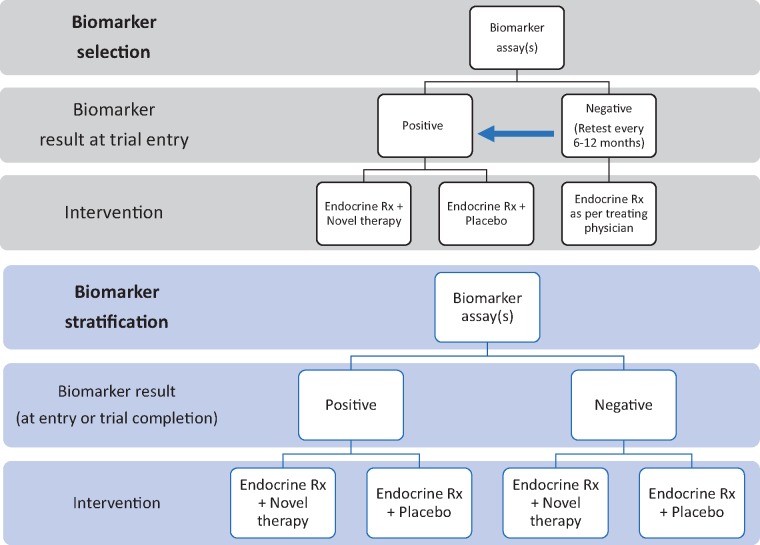
Potential interventional trial strategies are summarized in Figure 6, which include a “biomarker stratification” trial, in which all patients are randomly assigned to a novel therapeutic intervention, or a “biomarker selection” trial, in which only patients with a positive integral biomarker are selected for randomization to a novel therapeutic intervention. A clinical and regulatory framework for such an approach is exemplified by regulatory approval for anti-androgenic agents (apalutamide and enzalutamide) for the treatment of nonmetastatic castration-resistant prostate cancer based on the endpoint of metastasis-free survival, with selection of the high-risk population based on a rising serum prostate-specific antigen level (84).
Figure 6.
Schemas of hypothetical clinical trials including circulating tumor cells and/or other “liquid biopsy” assays as for testing novel treatment intervention to prevent metastasis.
With regard to the biomarker stratification trial, a major advantage is that it would allow cross-platform comparison of several integrated biomarkers, with the goal of selecting one or more that may best predict therapeutic benefit from the intervention and subsequently serve as an integral biomarker to select for treatment. Furthermore, this type of trial would provide valuable information pertaining to patients in each category of dormancy status and how often patients progress from the low-(1 and 2) to the high-risk (3) categories. A major disadvantage of this design is that it requires treatment of all patients selected for high risk based on clinicopathologic features alone, resulting in overtreatment of the vast majority of trial participants.
A major advantage with the biomarker selection design is that only patients at highest risk would be included, reducing the number of trial participants and enhancing the likelihood of success by selection of patients at highest risk of recurrence. Major disadvantages include the need to screen a very large number of patients to identify the biomarker-enriched population and the need to rely on one biomarker, or a panel of biomarkers, that may not have a robust evidence base to support its use and may not be the optimal biomarker for these purposes.
A biomarker selection trial could also include serial assays to screen high-risk populations at multiple time -points after diagnosis but before recurrence rather than screen at a single time-point, or evaluation of assays as an intermediate pharmacodynamic biomarker of drug response. Both trial designs could also provide an opportunity to identify biomarkers that distinguish cells that remain in dormancy vs those that have exited dormancy and have the potential to identify clinically detectable and incurable distant metastasis.
Funding, Existing Resources, Navigator
In addition to prospectively collecting tissue and/or blood to test the prognostic and/or predictive utility of a tissue-based biomarker, samples that have already been collected and stored in a repository can serve as a precious resource. In 2018, the National Cancer Institute’s National Cancer Trials Network (NCTN) Navigator went live (https://navigator.ctsu.org/navigator/login). The advantage of using NCTN Navigator is that the specimens are procured in the context of a clinical trial, with available information about treatment and clinical outcome. Researchers can also query based on specific clinical trials and patient characteristics. Sample availability is currently limited to phase III NCTN trials for which the primary outcome has been publicly reported. Potential associated fees and timeline of concept review and tissue procurement are detailed on the Navigator website. In addition to NCTN Navigator, there were discussions of the potential utility of identifying accessible tumor tissue and/or blood from smaller NCTN trials that are not on NCTN Navigator as well as in non-NCTN repositories that have annotated clinical information. Most of the biospecimens that are potentially available through Navigator, however, include primary tumor biospecimens or blood specimens obtained at or relatively soon after diagnosis and not blood specimens obtained 5 or more years after diagnosis in patients who were cancer-free and subsequently relapsed.
Conclusions
In this workshop, we detailed the potential underlying mechanisms for the development of late recurrence, a significant clinical issue for patients with early-stage ER+ BC. Although it was recognized that the biology associated with dormancy escape may be complex and due to various processes, we categorized a patient’s disease status into three groups to frame the risk of late recurrence and consideration for therapeutic intervention: 1) no dormant cells present, 2) cells present, but still dormant, and 3) cells present that have escaped dormancy. Though there remains enthusiasm about the possible clinical utility of blood-based methods, such as CTCs and ctDNA, the role of these assays remains unclear in the early-stage BC setting, including if these markers can precisely discriminate a patient’s late recurrence risk into one of these categories; this highlights the need for additional rigorous research on this topic.
As discussed in the companion article, there is a pressing need to identify reliable markers of late recurrence risk beyond standard clinical and pathologic features. Clinical implications include not only deescalating therapy in patients for whom no dormant cells are present but also modifying therapy in the hopes of preventing relapse in those at immediate risk. We discussed optimizing the preanalytic, analytic, and post-analytic considerations of the various tissue-based platforms in development. In addition, different observational and interventional designs were detailed, with discussions about discriminating the prognostic and predictive capacity of an assay (or combination of assays) and maximizing research opportunities to understand risk in distinct patient populations, such as those still on anti-estrogen therapy compared with those no longer receiving treatment.
In conclusion, there is considerable potential for decreasing the alarming risk of late recurrence in nonmetastatic ER+ BC. Although there may be a number of mechanisms at play, including host factors described in the companion article (2), we hope to continue moving beyond a one-size- fits-all approach and thoughtfully stop, continue, or modify therapy based on reliable estimates of the likelihood of recurrence. Our hope is that international collaborations, such as those discussed in this workshop, will help achieve this goal and ultimately improve patient outcome.
Funding
This work and the workshop described were funded by the Breast Cancer Research Foundation and the Hold’em for Life Charity.
Notes
Affiliations of authors: Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada (RJOD, DWC, SVB, VS); Departments of Medicine and Medical Oncology, Albert Einstein College of Medicine, Montefiore Medical Center, Albert Einstein Cancer Center, New York, NY (JAS); Lunenfeld-Tanenbaum Research Institute, Mt. Sinai Hospital, Sinai Health System, Toronto, ON, Canada (PJG, AEL, DS, JRW); Department of Medicine, University of Toronto, Toronto, ON, Canada (PJG, AEL); Department of Medical Oncology, Institut Curie, PSL Research University, Paris, France (FCB); Division of Medical Oncology, Department of Medicine, University of Toronto, Toronto, ON, Canada (DWC); Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center; Breast Medicine Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY (SC); Weill-Cornell Medical College, New York, NY (SC); Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY (JOD); Ralph Lauren Centre for Breast Cancer Research, Royal Marsden Hospital, The Royal Marsden NHS Foundation Trust, Breast Cancer Now Research Centre, The Institute of Cancer Research, London, UK (MD); Department of Biostatistics, Dana-Farber Cancer Institute, Boston, MA (RJG); Harvard T.H. Chan School of Public Health, Boston, MA (RJG); University of Utah, Salt Lake City, UT (NLH); Huntsman Cancer Institute, Salt Lake City, UT (NLH); Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX (FMB); Gemini Group, Ann Arbor, MI (JP); Division of Oncology, Department of Medicine, Stanford University School of Medicine, Stanford, CA (GWS); Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Barts & The London School of Medicine and Dentistry, Queen Mary University of London, London, UK (MAT); Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada (SVB); Department of Radiation Oncology, University of Toronto, Toronto, ON, Canada (SVB); Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada (RJOD, SVB, VS); Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC (LAC); University of Vermont Medical Center, Larner College of Medicine, Burlington, VT (MCC); Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA (ADM); Applied Statistician, Markham, ON, Canada (ME); Division of Medical Oncology and Hematology, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, ON, Canada (KJJ); Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD (LAK); Orlando Health University of Florida Health Cancer Center, Orlando, FL (EPM); Canadian Cancer Trials Group, Queen's University, Kingston, ON, Canada (WRP); Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA (MMR); Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada (DS); McMaster University and Juravinski Cancer Centre, Hamilton, ON, Canada (TJW); The Johns Hopkins University School of Medicine and Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD (ACW); Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY; Herbert Irving Comprehensive Cancer Center, Columbia University Irving Medical Center, New York, NY (KK); University of Michigan Rogel Cancer Center, and Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (DFH).
P. J. Goodwin received research funding (in kind) support from Epic Sciences.
Supplementary Material
References
- 1.Dowling RJO, Kalinsky K, Hayes DF, et al. Toronto workshop on late recurrence in estrogen receptor-positive breast cancer: part 1: Late recurrence: Current understanding, clinical considerations. J Natl Cancer Inst Cancer Spectrum. 2019. doi:10.1093/jncics/pkz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hensel JA, Flaig TW, Theodorescu D.. Clinical opportunities and challenges in targeting tumour dormancy. Nat Rev Clin Oncol. 2013;10(1):41–51. [DOI] [PubMed] [Google Scholar]
- 3. Dittmer J. Mechanisms governing metastatic dormancy in breast cancer. Semin Cancer Biol. 2017;44:72–82. [DOI] [PubMed] [Google Scholar]
- 4. Gomis RR, Gawrzak S.. Tumor cell dormancy. Mol Oncol. 2017;11(1):62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trumpp A, Essers M, Wilson A.. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10(3):201–209. [DOI] [PubMed] [Google Scholar]
- 6. Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12(19):5615–5621. [DOI] [PubMed] [Google Scholar]
- 8. Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 10. Harper KL, Sosa MS, Entenberg D, et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature. 2016;540(7634):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aguirre-Ghiso JA, Liu D, Mignatti A, et al. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12(4):863–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeh AC, Ramaswamy S.. Mechanisms of cancer cell dormancy—another hallmark of cancer? Cancer Res. 2015;75(23):5014–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Touny LH, Vieira A, Mendoza A, et al. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J Clin Invest. 2014;124(1):156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borgen E, Rypdal MC, Sosa MS, et al. NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Res. 2018;20(1):120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malladi S, Macalinao DG, Jin X, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165(1):45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coghill AE, Engels EA, Schymura MJ, et al. Risk of breast, prostate, and colorectal cancer diagnoses among HIV-infected individuals in the United States. J Natl Cancer Inst. 2018;110(9):959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raaschou P, Soderling J, Turesson C, et al. Tumor necrosis factor inhibitors and cancer recurrence in Swedish patients with rheumatoid arthritis: a nationwide population-based cohort study. Ann Intern Med. 2018;169(5):291–299. [DOI] [PubMed] [Google Scholar]
- 20. Postow MA, Callahan MK, Wolchok JD.. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Topalian SL, Drake CG, Pardoll DM.. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108. [DOI] [PubMed] [Google Scholar]
- 24. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort a of the phase 2 keynote-086 study. Ann Oncol. 2018;5(1):74–82 [DOI] [PubMed] [Google Scholar]
- 26. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. [DOI] [PubMed] [Google Scholar]
- 27. Baeriswyl V, Christofori G.. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–337. [DOI] [PubMed] [Google Scholar]
- 28. Castano Z, Tracy K, McAllister SS.. The tumor macroenvironment and systemic regulation of breast cancer progression. Int J Dev Biol. 2011;55(7–9):889–897. [DOI] [PubMed] [Google Scholar]
- 29. Ping YF, Bian XW.. Concise review: contribution of cancer stem cells to neovascularization. Stem Cells. 2011;29(6):888–894. [DOI] [PubMed] [Google Scholar]
- 30. Zhao D, Pan C, Sun J, et al. VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene. 2015;34(24):3107–3119. [DOI] [PubMed] [Google Scholar]
- 31. Laurent J, Hull EF, Touvrey C, et al. Proangiogenic factor PlGF programs CD11b(+) myelomonocytes in breast cancer during differentiation of their hematopoietic progenitors. Cancer Res. 2011;71(11):3781–3791. [DOI] [PubMed] [Google Scholar]
- 32. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. [DOI] [PubMed] [Google Scholar]
- 33. Miller KD, O’Neill A, Gradishar W, et al. Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer (E5103). J Clin Oncol. 2018;36(25):2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayes DF. Bevacizumab treatment for solid tumors: boon or bust? JAMA. 2011;305(5):506–508. [DOI] [PubMed] [Google Scholar]
- 35. Nahleh ZA, Barlow WE, Hayes DF, et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res Treat. 2016;158(3):485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Croucher PI, McDonald MM, Martin TJ.. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16(6):373–386. [DOI] [PubMed] [Google Scholar]
- 37. Gao XL, Zhang M, Tang YL, et al. Cancer cell dormancy: mechanisms and implications of cancer recurrence and metastasis. Onco Targets Ther. 2017;10:5219–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shupp AB, Kolb AD, Mukhopadhyay D, et al. Cancer metastases to bone: concepts, mechanisms, and interactions with bone osteoblasts. Cancers (Basel). 2018;10(6):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. [DOI] [PubMed] [Google Scholar]
- 40. Llombart A, Frassoldati A, Paija O, et al. Immediate administration of zoledronic acid reduces aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012;12(1):40–48. [DOI] [PubMed] [Google Scholar]
- 41. Coleman R, Gnant M, Morgan G, et al. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104(14):1059–1067. [DOI] [PubMed] [Google Scholar]
- 42. Group EBCTC. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. [DOI] [PubMed] [Google Scholar]
- 43. Heeke A, Nunes MR, Lynce F.. Bone-modifying agents in early-stage and advanced breast cancer. Curr Breast Cancer Rep. 2018;10(4):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coleman RE, Finkelstein D, Barrios CH, et al. Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D-CARE study. J Clin Oncol. 2018;36(suppl 15):501–505. [Google Scholar]
- 45. Henry NL, Hayes DF, Ramsey SD, et al. Promoting quality and evidence-based care in early-stage breast cancer follow-up. J Natl Cancer Inst. 2014;106(4):dju034.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. [DOI] [PubMed] [Google Scholar]
- 47. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. [DOI] [PubMed] [Google Scholar]
- 48. Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342(8):525–533. [DOI] [PubMed] [Google Scholar]
- 49. Hartkopf A, Brucker SY, Taran FA, et al. International pooled analysis of the prognostic impact of disseminated tumor cells from the bone marrow in early breast cancer: results from the PADDY study. In: San Antonio Breast Cancer Symposium Philadelphia, PA: American Association for Cancer Research, Inc. (AACR); 2018. Abstract GS5-07.
- 50. Wiedswang G, Borgen E, Karesen R, et al. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res. 2004;10(16):5342–5348. [DOI] [PubMed] [Google Scholar]
- 51. Janni W, Rack B, Schindlbeck C, et al. The persistence of isolated tumor cells in bone marrow from patients with breast carcinoma predicts an increased risk for recurrence. Cancer. 2005;103(5):884–891. [DOI] [PubMed] [Google Scholar]
- 52. Janni W, Vogl FD, Wiedswang G, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse—a European pooled analysis. Clin Cancer Res. 2011;17(9):2967–2976. [DOI] [PubMed] [Google Scholar]
- 53. Stearns V, Yamauchi H, Hayes DF.. Circulating tumor markers in breast cancer: accepted utilities and novel prospects. Breast Cancer Res Treat. 1998;52(1–3):239–259. [DOI] [PubMed] [Google Scholar]
- 54. Kokko R, Hakama M, Holli K.. Follow-up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Res Treat. 2005;93(3):255–260. [DOI] [PubMed] [Google Scholar]
- 55. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–414. [DOI] [PubMed] [Google Scholar]
- 56. Bidard FC, Belin L, Delaloge S, et al. Time-dependent prognostic impact of circulating tumor cells detection in non-metastatic breast cancer: 70-month analysis of the REMAGUS02 study. Int J Breast Cancer. 2013;2013:130470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Janni WJ, Rack B, Terstappen LW, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res. 2016;22(10):2583–2593. [DOI] [PubMed] [Google Scholar]
- 58. Bidard FC, Proudhon C, Pierga JY.. Circulating tumor cells in breast cancer. Mol Oncol. 2016;10(3):418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bidard FC, Michiels S, Riethdorf S, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst. 2018;110(6):560–567. [DOI] [PubMed] [Google Scholar]
- 60. Sparano J, O’Neill A, Alpaugh K, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(12):1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Janni W, Rack BK, Fasching PA, et al. Persistence of circulating tumor cells in high risk early breast cancer patients five years after adjuvant chemotherapy and late recurrence: results from the adjuvant SUCCESS A trial. J Clin Oncol. 2018;36(15):515.29267131 [Google Scholar]
- 62. Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2(11):1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stover DG, Parsons HA, Ha G, et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol. 2018;36(6):543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Reinert T, Saad ED, Barrios CH, et al. Clinical implications of ESR1 mutations in hormone receptor-positive advanced breast cancer. Front Oncol. 2017;7:26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199–1209. [DOI] [PubMed] [Google Scholar]
- 66. Chandarlapaty S, Chen D, He W, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016;2(10):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Angus L, Beije N, Jager A, et al. ESR1 mutations: moving towards guiding treatment decision-making in metastatic breast cancer patients. Cancer Treat Rev. 2017;52:33–40. [DOI] [PubMed] [Google Scholar]
- 68. Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7(1):11579.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J Clin Oncol. 2018;36(16):1631–1641. [DOI] [PubMed] [Google Scholar]
- 70. Corcoran RB, Chabner BA.. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754–1765. [DOI] [PubMed] [Google Scholar]
- 71. Ossandon MR, Agrawal L, Bernhard EJ, et al. Circulating tumor DNA assays in clinical cancer research. J Natl Cancer Inst. 2018;110(9):929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Olsson E, Winter C, George A, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015;7(8):1034–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Coombes RC, Armstrong A, Ahmed S, et al. Early detection of residual breast cancer through a robust, scalable and personalized analysis of circulating tumour DNA (ctDNA) antedates overt metastatic recurrence. In: San Antonio Breast Cancer Symposium Philadelphia, PA: American Association for Cancer Research, Inc. (AACR); 2018. Abstract P4-01-02.
- 75. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7(302):302ra133.. [DOI] [PubMed] [Google Scholar]
- 76. Paoletti C, Muniz MC, Thomas DG, et al. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer. Clin Cancer Res. 2015;21(11):2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jack RM, Grafton MM, Rodrigues D, et al. Ultra-specific isolation of circulating tumor cells enables rare-cell RNA profiling. Adv Sci. 2016;3(9):1600063.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lang JE, Ring A, Porras T, et al. RNA-seq of circulating tumor cells in stage II-III breast cancer. Ann Surg Oncol. 2018;25(8):2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kwan TT, Bardia A, Spring LM, et al. A digital RNA signature of circulating tumor cells predicting early therapeutic response in localized and metastatic breast cancer. Cancer Discov. 2018;8(10):1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Keup C, Mach P, Aktas B, et al. RNA profiles of circulating tumor cells and extracellular vesicles for therapy stratification of metastatic breast cancer patients. Clin Chem. 2018;64(7):1054–1062. [DOI] [PubMed] [Google Scholar]
- 81. Paoletti C, Cani AK, Larios JM, et al. Comprehensive mutation and copy number profiling in archived circulating breast cancer tumor cells documents heterogeneous resistance mechanisms. Cancer Res. 2018;78(4):1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paoletti C, Schiavon G, Dolce EM, et al. Circulating biomarkers and resistance to endocrine therapy in metastatic breast cancers: correlative results from AZD9496 oral SERD phase I trial. Clin Cancer Res. 2018;24(23):5860–5872. [DOI] [PubMed] [Google Scholar]
- 83. Scher HI, Jendrisak A, Graf R, et al. CTC phenotype classifier to identify mCRPC patients (pts) with high genomic instability CTCs and to predict failure of androgen receptor signaling (AR Tx) and taxane (T) systemic therapies. J Clin Oncol. 2016;34(15):5044. [Google Scholar]
- 84. Beaver JA, Kluetz PG, Pazdur R.. Metastasis-free survival—a new end point in prostate cancer trials. N Engl J Med. 2018;378(26):2458–2460. [DOI] [PubMed] [Google Scholar]
- 85. Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14(21):7004–7010. [DOI] [PubMed] [Google Scholar]
- 86. Bidard FC, Mathiot C, Delaloge S, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21(4):729–733. [DOI] [PubMed] [Google Scholar]
- 87. Riethdorf S, Muller V, Loibl S, et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant “geparquattro.” Clin Cancer Res. 2017;23(18):5384–5393. [DOI] [PubMed] [Google Scholar]
- 88. Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–2645. [DOI] [PubMed] [Google Scholar]
- 89. Azim HA Jr, Rothe F, Aura CM, et al. Circulating tumor cells and response to neoadjuvant paclitaxel and HER2-targeted therapy: a sub-study from the NeoALTTO phase III trial. Breast. 2013;22(6):1060–1065. [DOI] [PubMed] [Google Scholar]
- 90. Onstenk W, Kraan J, Mostert B, et al. Improved circulating tumor cell detection by a combined EpCAM and MCAM cellsearch enrichment approach in patients with breast cancer undergoing neoadjuvant chemotherapy. Mol Cancer Ther. 2015;14(3):821–827. [DOI] [PubMed] [Google Scholar]
- 91. Hall C, Karhade M, Laubacher B, et al. Circulating tumor cells after neoadjuvant chemotherapy in stage I-III triple-negative breast cancer. Ann Surg Oncol. 2015;22(suppl 3):S552–S558. [DOI] [PubMed] [Google Scholar]
- 92. Mego M, Giordano A, De Giorgi U, et al. Circulating tumor cells in newly diagnosed inflammatory breast cancer. Breast Cancer Res. 2015;17(1):2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hall CS, Karhade M, Laubacher BA, et al. Circulating tumor cells and recurrence after primary systemic therapy in stage III inflammatory breast cancer. J Natl Cancer Inst. 2015;107(11): djv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pierga JY, Bidard FC, Autret A, et al. Circulating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol. 2017;28(1):103–109. [DOI] [PubMed] [Google Scholar]
- 95. Ueno T, Masuda N, Kamigaki S, et al. A multicenter phase II trial of neoadjuvant letrozole plus low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer (JBCRG-07): therapeutic efficacy and clinical implications of circulating endothelial cells. Cancer Med. 2018;7(6):2442–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Thery L, Meddis A, Cabel L, et al. Circulating tumor cells in early breast cancer. J Natl Cancer Inst Cancer Spectrum. 2019;3(2): pkz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.