Abstract
Background
Liquid biopsies could improve diagnosis, prognostication, and monitoring of colorectal cancer (CRC). Mutation, chromosomal copy number alteration, and methylation analysis in circulating tumor DNA (ctDNA) from plasma or serum has gained great interest. However, the literature is inconsistent on preferred candidate markers, hampering a clear direction for further studies and clinical translation. This review assessed the potential of ctDNA analysis for clinical utility.
Methods
A systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines was conducted up to December 3, 2018, followed by methodological quality assessment. Primary endpoints were accuracy for detection, prognostication, and monitoring.
Results
Eighty-four studies were included. For CRC detection, sensitivity was 75% using ctDNA mutation analysis and up to 96% using copy number analysis. Septin 9 (SEPT9) hypermethylation analysis showed sensitivities of 100% and specificities of 97%. Regarding prognostication, ctDNA KRAS mutations were associated with oncological outcome and could predict response to anti–epidermal growth factor receptor therapy. For monitoring, sequential ctDNA KRAS mutation analysis showed promise for detection of relapses or therapy resistance.
Conclusions
This comprehensive overview of ctDNA candidate markers demonstrates SEPT9 methylation analysis to be promising for CRC detection, and KRAS mutation analysis could assist in prognostication and monitoring. Prospective evaluation of marker panels in clinical decision making should bring ctDNA analysis into practice.
Colorectal cancer (CRC) is the third most common cancer in the Western world (1,2) and the incidence is still rising (3). In recent decades, oncological outcomes have improved because of the implementation of screening programs, improvement of surgical procedures, and introduction of novel systemic regimens. However, CRC is still the second leading cause of cancer-related death (1,2). Further innovation is needed to improve diagnosis, patient-specific treatment selection, and disease monitoring.
The stage of disease at diagnosis is the most important prognostic factor for survival in CRC (4). It is therefore of utmost importance to detect CRC at an early stage, which requires improved screening approaches. The value of current screening methods is hampered by the low sensitivity of the fecal occult blood test (FOBT) and the invasive nature and costs of colonoscopy (5).
A second challenge concerns selection of the most suitable treatment, warranting better prognostic markers. The current decision process for systemic therapy is largely based on clinicopathological characteristics, leaving a substantial number of patients under- or overtreated. Genetic subtyping (6) and expression profiling (7) enhance patient selection. However, improved approaches are needed to further subclassify patients by their risk of recurrence and suitability for adjuvant therapies.
A third major area of interest is disease monitoring after initial curative treatment or during systemic therapy. Up to 40% of CRC patients will experience disease recurrence despite curatively intended treatment (8). Unfortunately, recurrences are often detected at advanced stages, excluding these patients from potentially curative rescue treatments. Current follow-up consists of serial carcinoembryonic antigen (CEA) measurements in serum, imaging, and colonoscopy (9). Unfortunately, the value of CEA for follow-up is limited by its low accuracy (10,11), with only marginal benefit observed when combined with computed tomography (CT) scans (12). The value of CT imaging is limited to the detection of large lesions, illustrated by a sensitivity of 11% for nodules smaller than 5 mm (13). Colonoscopy provides a high level of sensitivity (>95%) but can evaluate only endo luminal disease (5). These issues stress the urgent clinical need for a robust and noninvasive diagnostic marker facilitating CRC detection and prediction of treatment response.
Liquid biopsies are a rapidly developing field of research focused toward the analysis of cancer biomarkers isolated from nonsolid tissues. Various tumor-derived products can be detected in blood, including circulating tumor cells, circulating tumor DNA (ctDNA), circulating RNAs, exosomes, and tumor educated platelets (14–16). Of these tumor-derived products, ctDNA has been investigated most extensively and has shown promising accuracies for cancer detection (17–20). These DNA fragments originate from tumor cells and are released into the circulation through apoptosis, necrosis, and secretion (17). Accordingly, tumor-specific (epi-)genetic alterations such as driver mutations, chromosomal copy number alterations (CNAs), and methylation can be detected in ctDNA and could be of high value for cancer detection, prognostication, and treatment monitoring (17–20).
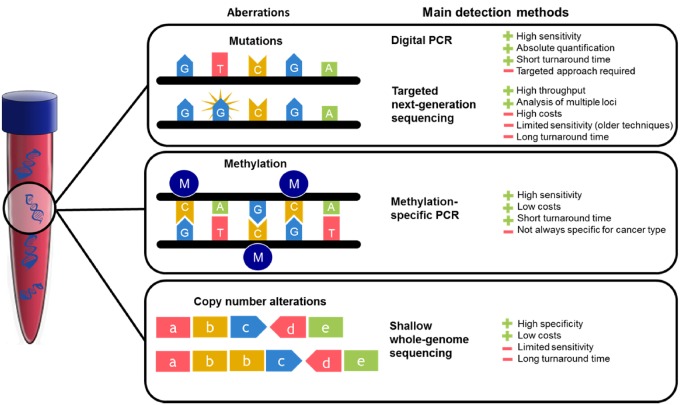
The primary challenge of ctDNA analysis is to detect tumor-derived molecules in a high background of cell-free DNA (cfDNA) from healthy cells. Currently, ctDNA detection techniques mainly revolve around real-time polymerase chain reaction (PCR) and sequencing approaches (14,15). Allele-specific quantitative PCR has a high sensitivity for ctDNA detection, with a detection limit of 0.014–0.004% (21). Emulsion PCR methods such as droplet digital PCR (ddPCR) and beads, emulsion, amplification, and magnetics are most sensitive, with a detection limit of 0.01–0.001% (22,23). The disadvantage of PCR-based methods is the limited number of foci that can be assessed, relying on the initial identification of patient-specific solid-tumor tissue alterations. Sequencing platforms including next-generation sequencing (NGS) allow for broader genomic coverage. However, this method is time consuming and expensive, hampering clinical implementation. An overview of the main methods to detect ctDNA is depicted in Figure 1.
Figure 1.
The three types of circulating tumor DNA aberrations covered in this review. For every DNA aberration, commonly used techniques to determine its presence in plasma or serum are depicted. PCR = polymerase chain reaction.
Several Food and Drug Administration (FDA)–approved assays are commercially available for ctDNA-based cancer diagnostics, including a PCR kit for detection of epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer patients (Cobas v2) (24) and a PCR assay measuring methylated SEPT9 in blood to detect CRC (Epi ProColon) (25). Copy number analysis of circulating DNA is currently routine diagnostic practice in several countries, including the Netherlands, for noninvasive prenatal testing (26). Numerous studies claim a potential clinical role for ctDNA, but the diverse and sometimes contradictory results and recommendations hamper widespread translation into daily practice of CRC patients. Therefore, the aim of this study is to systematically review the current literature on the potential role of ctDNA mutation, copy number, and methylation analysis for CRC diagnosis, prognostication, and monitoring.
Materials and Methods
Search Strategy
A systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (27). Systematic searches were performed in the bibliographic databases PubMed, Embase.com, and Clarivate Analytics/Web of Science up to December 3, 2018, by SB, NRS, and JCFK (Supplementary Table 1). The search query included indexed terms and free-text words for “DNA” and “variation” or “methylation” and “blood” or “serum” and “colorectal cancer.”
Study Selection
Screening and study selection was independently performed by three reviewers (JMM, NRS, SB). If necessary, articles were discussed to achieve consensus. All full-text articles in English, Dutch, French, German, or Russian on ctDNA mutation, copy number, or methylation analysis in the serum or plasma of CRC patients were considered eligible. Human studies assessing therapy-naive patients with a minimum age of 18 years that allowed determination of sensitivity were included. Literature reviews, case reports, and studies in which ctDNA analysis was performed in fewer than 10 CRC patients or in patients with hereditary CRC or inflammatory bowel disease were excluded. If overlapping data were reported, either the most recent study or that with the most complete data on our outcomes of interest was included.
Data Extraction
Primary outcomes were sensitivity and specificity of ctDNA analysis for CRC detection, subdivided according to several clinical settings: diagnosis, prognostication, and monitoring. Sensitivity was defined as the percentage of CRC patients in whom a specific ctDNA aberration was detected. Specificity was defined as the percentage of healthy control individuals without detected ctDNA. Additionally, the technical concordance was extracted, defined as the percentage of agreement between ctDNA and solid-tumor tissue analysis. Data on single mutations in sequencing panels were extracted if two or more studies reported this mutation.
Quality Assessment
Risk of bias assessment of all included studies was independently performed by three reviewers (JMM, NRS, SB). Risk of bias was scored as low, high, or unclear using the validated Quality Assessment of Diagnostic Accuracy Studies 2 tool (QUADAS-2) (28). Custom criteria were created, and agreement among reviewers was initially determined in a pilot of 10 studies. Disagreement was resolved by discussion with all reviewers present (JMM, NRS, SB). To ensure high-quality assessment of the described literature, articles were excluded from further analysis in case one domain was scored as “high” in combination with “unclear” or “high” risk at a second domain of the QUADAS-2. Review Manager 5 software (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for managing the QUADAS-2 results.
Results
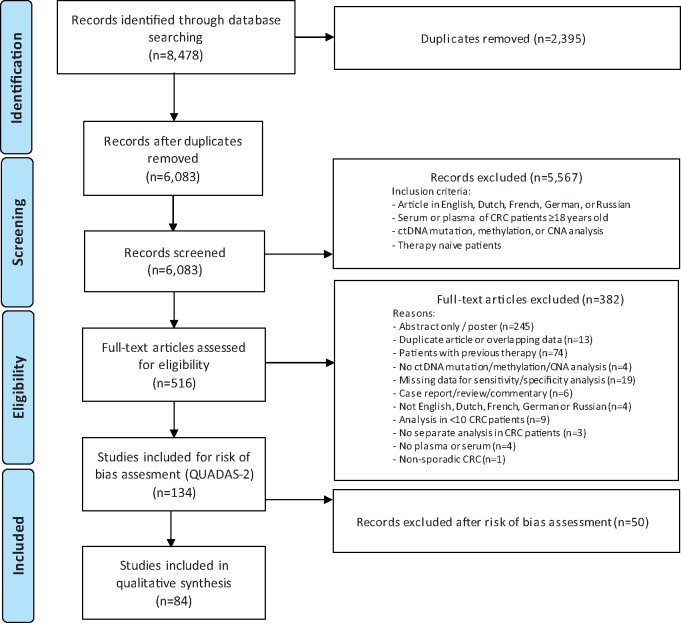
The search identified 8478 eligible abstracts. After removal of duplicates, 5567 studies were excluded by title and abstract screening. Subsequently, 382 articles were excluded by full-text evaluation, leaving 134 studies, all in English, for risk of bias assessment. Figure 2 depicts the study selection procedure.
Figure 2.
The Preferred Reporting Items for Systematic Reviews and Meta-analyses flowchart for inclusion of the studies. The risk of bias assessment using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) was incorporated in the flowchart. CNA = copy number alteration; CRC = colorectal cancer; ctDNA = circulating tumor DNA.
Fifty studies were excluded based on quality assessment using QUADAS-2, leading to the inclusion of 84 studies. The majority of studies (123 of 134) scored unclear or high risk of bias on at least one domain, mainly study design or index test. Only 11 studies scored low risk on all domains (29–39). Most papers scored low risk on applicability concerns, reference standard (histological assessment), and flow and timing. The main findings of the risk of bias assessment are depicted in Supplementary Figures 1, A and B (detailed overview, available online).
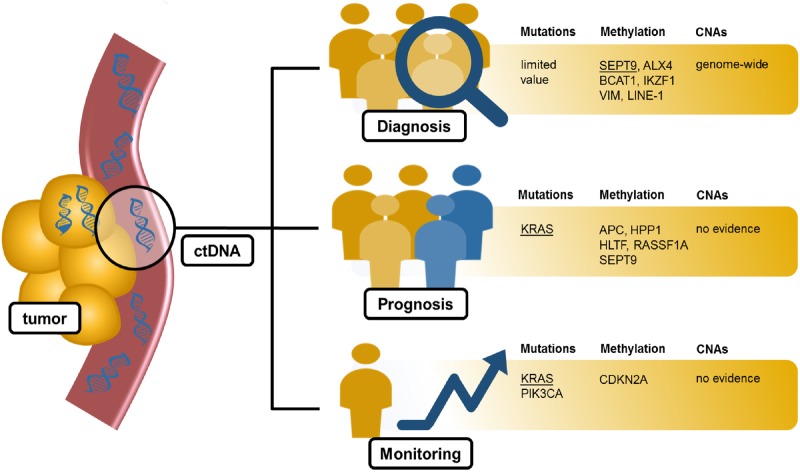
An overview of the clinical implications with the main markers of interest is provided in Figure 3.
Figure 3.
A graphical overview of the evidence for circulating tumor DNA (ctDNA) use in clinical practice. The most promising markers are presented for each clinical implication. Markers considered to be of special interest are underlined. Other markers depicted in the figure are promising but require further research. CNA = copy number alteration.
Accuracy of ctDNA Analysis for CRC Diagnosis
Current screening methods consist of FOBT and colonoscopy and have an overall sensitivity of 51% for individuals experiencing clinical symptoms and 19% at earlier stages (40). The present section describes ctDNA aberrations that could aid in CRC detection. Tables 1 and 2 present an overview of the identified candidate mutation and methylation markers (Table 1) and CNAs (Table 2) in ctDNA with sensitivities per stage, specificities, and concordance rates.
Table 1.
An overview of the sensitivity and specificity for CRC detection of all ctDNA mutation, hypermethylation, and hypomethylation markers included in this review*
| Marker | Sensitivity |
Specificity | Concordance with primary tumor | ||||
|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | Stage not reported | |||
| Mutation | |||||||
| APC (41–48) | 0–50% | 6–57% | 3–46% | 15–75% | 14–18% | NA | 16–100% |
| BRAF (34,35,42,45,48–53) | 50% | 0–9% | 33% | 3–29% | 2–12% | NA | 33–100% |
| ERBB2 (44,54) | NA | 5–9% | NA | NA | |||
| KRAS (32,34,35,41–46,48–53,55–64) | 0–67% | 3–46% | 5–50% | 5–73% | 8–71% | 70–100% | 25–100% |
| NRAS (35,52) | NA | 12% | NA | 100% | |||
| PIK3CA (44,45,48,49,51) | NA | 19% | 0–21% | NA | 0%–100% | ||
| tp53 (41–46,48) | 0–25% | 22–30% | 17–49% | 38–67% | 6–50% | 100% | 14–100% |
| Hypermethylation | |||||||
| AKAP12 (65) | NA | 48% | 92.0% | NA | |||
| ALX4 (66–70) | 75% | 83% | 82% | 100% | 29–83% | 66–99% | NA |
| 30% | 60% | ||||||
| APC (67,71–74) | 24% | 60% | 54% | 20–57% | 68–100% | 50% | |
| BCAT1 (31,75) | 21% | 62% | 68% | 81% | 57–65% | 95–97% | NA |
| BMP3 (67) | NA | 29% | 89% | NA | |||
| BNC1 (67) | NA | 12% | 87% | NA | |||
| BRCA1 (67) | NA | 25% | 78% | NA | |||
| CDH1 (72) | NA | 60% | 84% | NA | |||
| CDH4 (76) | NA | 70% | 100% | 83% | |||
| CDKN2A (55,67,71,77–79) | 15% | 50–67% | 50–67% | 10–75% | 9–61% | 70%–96% | 70–82% |
| CRABP1 (75,80) | NA | 50% | NA | NA | |||
| DAPK1 (72) | 50.0% | NA | 74% | 80% | |||
| DLC1 (81) | 36% | 48% | 42% | 91% | NA | ||
| ERCC1 (82) | 60% | NA | NA | 93% | 90% | ||
| EYA4 (80) | NA | 50% | NA | NA | |||
| FBN2 (83) | 9% | 7% | 8% | NA | 9% | NA | 8% |
| FGF5 (75) | NA | 85% | 83% | NA | |||
| FHIT (72,74) | NA | 20–50% | 84% | 40% | |||
| GATA5 (84) | 46% | 83% | 61% | NA | NA | ||
| GRASP (75) | NA | 54% | 93% | NA | |||
| HIC1 (67) | 6% | 99% | NA | ||||
| HLTF (67,85–89) | 8–20% | 15–16% | 9–16% | 24–47% | 11–30% | 96–100% | 41–42% |
| hMLH1 (67,77,89) | 27% | 0–24% | 25–27% | 12–40% | 16–29% | 100% | 33% |
| HPP1 (85,87–90) | 3–7% | 0–6% | 5–9% | 52–53% | 13–72% | NA | 56% |
| IKZF1 (31,75) | 28% | 41% | 55% | 94% | 48–68% | 95–99% | NA |
| IRF4 (75) | NA | 59% | 96% | NA | |||
| ITGA4 (84) | 24% | 54% | 37% | 81% | NA | ||
| LRR3CB (74) | NA | 15% | NA | 23% | |||
| MAL (80) | 50% | NA | NA | ||||
| MGMT (67,82) | 58% | NA | 6% | 95–99% | 94% | ||
| MLH1 (67,77,89,91) | NA | 45% | 57% | 33% | |||
| NELL1 (80) | NA | 33% | NA | NA | |||
| NDRG4 (67,92) | 54% | 56% | 9–55% | NA | NA | ||
| NEUROG1 (67,87) | 31% | 28% | 26% | 20% | 21–26% | NA | NA |
| NGFR (93) | 20% | 25% | 36% | 36% | 38% | 91.4% | NA |
| NPTX2 (67) | NA | 70% | 41% | NA | |||
| OSMR (67,94) | 74% | 77% | 11–75% | 86–93% | 79% | ||
| p73 (77) | NA | 25% | NA | NA | |||
| PCDH10 (36) | 71% | 54% | 63% | NA | 67% | ||
| PDX1 (75) | NA | 45% | 70% | NA | |||
| PHACTR3 (67) | NA | 15% | 94% | NA | |||
| PPENK (67) | NA | 10% | 96% | NA | |||
| RAR-β (67) | 25% | 30% | |||||
| RASSF1A (67,73) | 14% | 47% | 45% | 11–34% | 84–100% | NA | |
| RUNX3 (95) | 33% | 50% | 42% | 100% | NA | ||
| SDC2 (67,75) | NA | 24–59% | 84–94% | NA | |||
| SEPT9 (25,29,30,32,33,66,67,75, 80,93,94,96–107) | 14–84% | 50–100% | 38–100% | 68–100% | 24–96% | 73–97% | 80–88% |
| 20–57% | 52–70% | ||||||
| 64% | NA | ||||||
| SFRP1 (67) | 22% | 93% | |||||
| SFRP2 (67,84) | 42% | 71% | 20–54% | 72–82% | NA | ||
| SHOX2 (103) | NA | 44% | 21% | NA | NA | ||
| SMAD4 (72) | NA | 52% | 64% | NA | |||
| SOX21 (75) | NA | 80% | 50% | NA | |||
| SPG20 (67) | NA | 16% | 82% | NA | |||
| SST (67,80) | NA | 30–50% | 69% | NA | |||
| TAC1 (67,80) | NA | 50–53% | 53% | NA | |||
| TFPI1 (108) | NA | 7% | 98% | NA | |||
| TFPI2 (67) | 0% | 10% | 13% | 58% | 18% | 100% | NA |
| THBD (67) | NA | 10% | 99% | NA | |||
| TMEFF2 (66,93) | 5% | 22% | 47% | 45% | 30–71% | 90–95% | NA |
| VIM (67,109,110) | 50–52% | 55–67% | 40% | 86% | 18–71% | 60–93% | 78% |
| WIF1 (67) | NA | 10% | 96% | NA | |||
| WNT5A (67) | NA | 6% | 95% | NA | |||
| Hypomethylation | |||||||
| CBS (111) | NA | 56% | NA | NA | |||
| LINE-1 (112) | 63% | 68% | 66% | 90% | NA | ||
| Panels | |||||||
| Hypermethylation: ALX4 + BMP3 + NPTX2 + RARB + SDC2 + SEPT9 + VIM + female sex + age>66 (67) | 89% | NA | 91% | 73% | NA | ||
| Mutations: sequencing panel including TP53 + APC + KRAS (45) | NA | 100% | NA | NA | |||
| Mutations: APC + KRAS + TP53 (46) | 0% | 22% | 49% | 67% | 35% | 100% | 46% |
| Hypermethylation: APC + MGMT + RASSF2A + Wif-1 (86) | 87% | NA | 92% | NA | |||
| Hypermethylation: BCAT1 + IKZF1 (38) | 41% | 76% | 59% | 71% | 62% | 92% | NA |
| Hypermethylation: ALX4 + SEPT 9 + TMEFF 2 (66) | NA | 84% | 88% | NA | |||
The number of studies reporting a specific marker is represented next to the target gene. If possible, the sensitivity was presented separately for each disease stage. Concordance was defined as the percentage of agreement between ctDNA analysis and mutation or methylation analysis in the primary tumor. CRC = colorectal cancer; ctDNA = circulating tumor DNA; NA = not available, for when no data were available in a specific category.
Table 2.
An overview of the sensitivity and specificity for CRC detection of all analyzed potential ctDNA markers
| Sensitivity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | Overall | Specificity | ||||
| Detection of any CNA (37,39,113) |
41% |
73% |
56% |
66–87% | |||||
| Chr | Arm | Locus | Gene | 50–100% | 45–100% | 45–91% | 58–100% | 49–96% | |
| Copy number gains | |||||||||
| 1 | q | 20% | 33% | 9% | 0% | 17% | 100% | ||
| 1 | p | 20% | 17% | 0% | 9% | 100% | |||
| 2 | q | 20% | 17% | 9% | 0% | 13–19% | 100% | ||
| 2 | p | 20% | 33% | 9% | 0% | 16–17% | 100% | ||
| 3 | q | 0% | 0% | 9% | 100% | ||||
| 4 | q | 40% | 17% | 0% | 4% | 100% | |||
| 5 | q | 0% | 17% | 0% | 4–19% | 100% | |||
| 5 | p | 20% | 17% | 18% | 0% | 17–18% | 100% | ||
| 6 | p | 21.1 | CCND3 | 0% | 15% | 4% | NA | ||
| 6 | q | 0% | 9% | 0% | 4% | 100% | |||
| 6 | p | 20% | 50% | 18% | 0% | 26% | 100% | ||
| 7 | q | 21.2 | CDK6 | 0% | 5% | 10% | 4% | NA | |
| 7 | q | 34 | BRAF | 0% | 5% | 15% | 4% | NA | |
| 7 | q | 0% | 9% | 0% | 4% | 100% | |||
| 7 | p | 0% | 33% | 9% | 0% | 9% | 100% | ||
| 8 | p | 11.21 | KAT6A | NA | 20% | NA | |||
| 8 | q | 23.1 | RSPO2 | 0% | 0% | 5% | 40% | 11% | NA |
| 8 | q | 24.21 | MYC | 0% | 35% | 9% | NA | ||
| 8 | p | 11.21 | IKBKB | 0% | 20% | 4% | NA | ||
| 8 | q | 0% | 18% | 0% | 9% | 100% | |||
| 9 | q | 0% | 9% | 0% | 4% | 100% | |||
| 9 | p | NA | 28% | NA | |||||
| 10 | q | 0% | 33% | 0% | 13% | 100% | |||
| 10 | p | 0% | 33% | 36% | 0% | 13–30% | 100% | ||
| 11 | q | 13.3 | CCND1 | 0% | 20% | 4% | NA | ||
| 12 | p | 13.33 | KDM5A | 0% | 15% | 4% | NA | ||
| 12 | p | 12.1 | KRAS | 0% | 15% | 4% | NA | ||
| 12 | p | 0% | 33% | 9% | 100% | 22% | 100% | ||
| 13 | q | 12.13 | CDK8 | 0% | 30% | 8% | NA | ||
| 13 | q | 13.1 | BRCA2 | 0% | 30% | 8% | NA | ||
| 13 | q | 34 | IRS2 | 0% | 5% | 25% | 8% | NA | |
| 13 | 0% | 27% | 100% | 22% | 100% | ||||
| 15 | 20% | 17% | 9% | 0% | 13% | 100% | |||
| 17 | 0% | 33% | 45% | 0% | 30% | 100% | |||
| 17 | p | NA | 13% | NA | |||||
| 18 | 20% | 0% | 4% | 100% | |||||
| 19 | 0% | 33% | 55% | 100% | 39% | 100% | |||
| 19 | q | NA | 28% | NA | |||||
| 19 | p | NA | 16% | NA | |||||
| 20 | q | 13.2 | AURKA | 0% | 5% | 20% | 13% | NA | |
| 20 | q | 11.23 | SRC | 0% | 5% | 45% | 13% | NA | |
| 20 | 20% | 0% | 18% | 0% | 13% | 100% | |||
| 20 | p | NA | 16% | NA | |||||
| 21 | 0% | 17% | 0% | 4% | 100% | ||||
| 22 | 20% | 17% | 18% | 0% | 17% | 100% | |||
| Copy number losses | |||||||||
| 1 | p | 0% | 9% | 0% | 4–16% | 100% | |||
| 2 | p | 0% | 9% | 0% | 4% | 100% | |||
| 3 | q | 0% | 9% | 0% | 4% | 100% | |||
| 3 | p | 0% | 18% | 0% | 9–13% | 100% | |||
| 4 | q | 0% | 9% | 0% | 4% | 100% | |||
| 4 | p | 20% | 33% | 18% | 0% | 22% | 100% | ||
| 5 | q | 0% | 9% | 0% | 4% | 100% | |||
| 5 | p | 20% | 33% | 18% | 0% | 22% | 100% | ||
| 6 | p | NA | 17% | 0% | 4–16% | 100% | |||
| 6 | q | NA | 28% | NA | |||||
| 7 | q | 20% | 0% | 4–13% | 100% | ||||
| 7 | p | 0% | 9% | 0% | 4% | 100% | |||
| 8 | q | 0% | 17% | 0% | 0% | 4% | 100% | ||
| 8 | p | 20% | 50% | 45% | 100% | 25–43% | 100% | ||
| 9 | q | 0% | 33% | 18% | 100% | 22% | 100% | ||
| 9 | p | 20% | 50% | 27% | 0% | 30% | 100% | ||
| 10 | q | 0% | 33% | 0% | 9% | 100% | |||
| 10 | p | 0% | 17% | 0% | 4% | 100% | |||
| 11 | q | 0% | 17% | 9% | 0% | 9% | 100% | ||
| 11 | p | 0% | 33% | 18% | 0% | 17% | 100% | ||
| 12 | p | NA | 13% | NA | |||||
| 12 | q | 20% | 0% | 0% | 0% | 4–13% | 100% | ||
| 12 | p | 20% | 33% | 0% | 0% | 13% | 100% | ||
| 14 | 0% | 17% | 0% | 0% | 4% | 100% | |||
| 14 | q | NA | 25% | NA | |||||
| 14 | p | NA | 13% | NA | |||||
| 15 | 20% | 17% | 0% | 9% | 100% | ||||
| 16 | 20% | 83% | 9% | 0% | 13–26% | 100% | |||
| 17 | p | 13.1 | AURKB | 0% | 20% | 4% | NA | ||
| 17 | p | 13.1 | TP53 | 0% | 5% | 25% | 8% | NA | |
| 17 | 20% | 17% | 9% | 0% | 17% | 100% | |||
| 18 | q | 22.2 | SOCS6 | 0% | 30% | 8% | NA | ||
| 18 | 0% | 33% | 55% | 0% | 39% | 100% | |||
| 19 | 80% | 66% | 9% | 100% | 39% | 100% | |||
| 20 | 0% | 33% | 9% | 0% | 13% | 100% | |||
| 21 | 0% | 18% | 0% | 9% | 100% | ||||
| 22 | 40% | 17% | 36% | 0% | 30% | 100% | |||
The number of studies reporting on a specific marker is represented next to the target gene. If possible, the sensitivity was presented separately for each disease stage. CNA = copy number alteration; CRC = colorectal cancer; NA = not available, no data were available in a specific category.
In general, the analysis of ctDNA mutations showed a limited sensitivity of up to 57% in stage I–III disease, although a higher sensitivity of 75% was found in stage IV CRC using analysis of APC mutations. Detection of CRC by use of ctDNA copy number analysis showed promising sensitivities up to 96% but was described by only three studies. Analysis of SEPT9 hypermethylation resulted in high sensitivities (up to 100%) and specificities up to 97%. The methylation markers adenomatous polyposis coli (APC), vimentin (VIM), branched chain amino acid transaminase 1 (BCAT1), Aristaless-like homeobox 4 (ALX4), IKAROS family zinc finger 1 (IKZF1), and LINE-1 showed potential but were described by a limited number of studies (n < 5).
Mutation Marker Candidates
The mutational landscape of CRC is very heterogeneous, but several well-studied hot-spot mutations in genes with a crucial role in the progression of adenoma to carcinoma are known (6). Inactivating mutations in the tumor-suppressor gene APC are present in 30–70% of sporadic CRC (114). KRAS and BRAF mutations are found in 30% and 10% of CRC, respectively (114). The presence of these mutations is both a reflection of tumor biology (qualitative information) and tumor burden (quantitative information). Detection of these mutations is therefore an attractive approach for cancer diagnosis.
KRAS
For diagnostic purposes, point mutations of the KRAS gene were most frequently evaluated (n = 25 articles), resulting in sensitivities between 0 and 73% for stage I–IV CRC (32,34,35,41–46,49–53,55–64,115). Fourteen studies reported a sensitivity of more than 30% using various detection methods (32,34,35,41,42,44,53,55,56,59,61,63,64,115). The largest and most recently published studies found sensitivities between 32% and 41% in patients with stage I–IV CRC using ddPCR or Intplex allele-specific PCR in plasma (34,62,64). Two recently published studies using ddPCR to analyze ctDNA from plasma (n = 150 patients) (64) and allele-specific PCR on ctDNA from serum (n = 50 patients) (62) found sensitivities of 41% and 32%, respectively, that increased to 48% and 53% in stage IV CRC. KRAS mutations were rarely detected in ctDNA from healthy control individuals, illustrated by specificities ranging between 70% and 100% (32,46,49,58–60,62). Technical concordance between ctDNA and solid-tumor tissue analysis was heavily influenced by the analytical platform and ranged between 25% and 100% (32,34,35,42–44,46,49,50,52,56,57,59,61–64,115). Higher concordance rates (>60%) were reported by recent studies using ddPCR in plasma (34,35,62,64). In summary, the use of KRAS mutation analysis in ctDNA is hampered by low sensitivities of less than 50% for detection of CRC despite relatively good specificities and concordance rates.
BRAF
Detection of CRC by BRAF mutation analysis in ctDNA was evaluated in 10 studies, all reporting relatively low sensitivities of 0–50% independently of the technique used (34,35,42,45,49–53,115). The largest cohort study on BRAF ctDNA analysis found a BRAF mutation in only one of the 115 CRC patients using nested-PCR in serum (50), and a recent study in 97 locally advanced rectal cancer patients reported BRAF ctDNA mutations in the plasma of only two patients using ddPCR (34). Another recent study in 21 stage IV CRC patients reported a higher sensitivity of 29% for detection of BRAF mutations using an NGS panel of 90 oncogenes in plasma (45). None of these studies provided data to determine specificity. Concordance rates varied heavily among studies, but the only two studies evaluating BRAF mutations with ddPCR found a concordance of 100% (35,53). Nevertheless, because of the low frequency of BRAF mutations in ctDNA of CRC patients, analysis of this aberration is not suitable for large-scale CRC screening.
APC
Four of eight studies investigating APC mutations in ctDNA reported sensitivities greater than 35% for CRC diagnosis using various detection methods (41,42,44–47,115,116). In the largest cohort (n = 133 patients), a sensitivity of 8% was found for detection of stage I–IV CRC and 15% for stage IV disease using a MassArray assay in plasma (43). A recent study showed a comparable sensitivity of 18% using an NGS panel in plasma of stage I–IV patients (44). A specificity of 100% was reported by only one study using single-strand conformation polymorphism-PCR for ctDNA detection in serum (46). The concordance for detection of APC mutations ranged from 16% to 100% (42–44,46,47,115). Four of the six studies describing concordance reported rates lower than 50% (43,44,46,47), none of them describing ddPCR. The low sensitivity makes APC an unattractive marker for CRC detection.
Copy Number Alterations
Aneuploidy, an abnormal number of chromosomes, is a common causal event in CRC. Several CNA patterns have been identified, including deletions of both arms of chromosome 17 and 18 in 56% and 66% of CRC patients, respectively (6). Analysis of copy numbers uses a genomewide approach so does not rely on detecting nucleotide-specific changes that may occur below the detection threshold in a cfDNA sample. Furthermore, large (>3 Mb) or high-level (≥4 copies) CNAs are absent in healthy individuals, allowing a high level of specificity (117).
So far, a limited number of studies have investigated the use of ctDNA for CRC detection. The three included studies on CNAs in blood of CRC patients are the most recent and reported inconsistent results using shallow whole-genome sequencing methods (Table 2) (39,113,118). Depending on the study, detection of CNAs was described on the level of a whole chromosome, chromosome arm, and/or a specific gene. One study reported copy number gains or losses across the whole genome in the plasma of 96% of stage I–IV CRC patients and 100% of stage IV CRC patients (113). Other studies reported lower sensitivities of 49% (39) and 56% (118) for detection in plasma of stage I–IV CRC patients. When focusing on CNAs of specific chromosomes, copy number losses on chromosome 18q and both gains and losses on chromosome 19 were found in the plasma of 39% of CRC patients (113). Furthermore, a specificity of 66–87% was reported (113,118). Because studies did not provide data to determine CNA concordance, this is not reported in Table 2. In summary, the analysis of genomewide CNAs is a promising method for noninvasive CRC detection but requires more research.
Methylation Marker Candidates
Hypermethylation in promotor regions of genes associated with tumorigenesis is a common phenomenon in CRC that mainly occurs in CpG islands, concentrated regions of DNA sequences susceptible to methylation. Fifteen percent of sporadic colorectal tumors are characterized by high methylation levels, referred to as CpG island methylation phenotype (119). However, CpG island methylation phenotype–negative tumors also have recurrent patterns of DNA methylation, which could allow methylation to be exploited for CRC detection (120).
SEPT9
Hypermethylation of the SEPT9 promotor region was frequently investigated in large cohorts. Most of the 23 studies (25,29,30,32,33,66,67,75,80,93,94,96–107) that analyzed SEPT9 hypermethylation by various methods demonstrated it to be among the most accurate candidate markers, reporting sensitivities greater than 50% for stage I–IV CRC (25,30,32,33,66,75,93,94,96–105,107). The analysis of SEPT9 hypermethylation in ctDNA in plasma using quantitative methylation-specific PCR (qMSP) showed sensitivities of 61–62% in three recent large cohorts (n = 98, n = 123, and n = 187 patients) (94,104,105). Several other large-cohort studies showed potential for a commercially available test using qMSP for analysis of SEPT9 hypermethylation in plasma, reporting sensitivities between 73% and 87% for stage I–IV CRC (30,33,97,99,101,102,107). The sensitivity gradually increased with higher stages and was reported to be 100% in stage IV CRC patients in several studies (30,99,101). In most recent studies, specificities of 82–95% were found (29,67,94,99,104). The few studies describing concordance reported rates of approximately 80% (32,94,97). Overall, detection of hypermethylated SEPT9 seems promising for CRC detection considering its high accuracy.
CDKN2A (p16)
All six studies evaluating cyclin-dependent kinase inhibitor 2A (CDKN2A) hypermethylation in ctDNA of CRC patients used MSP. The most recent study used qMSP and reported a sensitivity of 9% for stage I–IV CRC detection (67). Other studies published in the past decade did not find specificities exceeding 35% (71,77,78). One study reported a specificity of 96% (67). Concordance rates of 70% and 82% were described in two studies (78,79). Taken together, only a limited number of studies provided an overall picture of the potential value of CDKN2A hypermethylation analysis in ctDNA for CRC detection. Detection of hypermethylated CDKN2A by MSP does not show potential for CRC detection considering its low sensitivity.
HLTF
All six studies on helicase-like transcription factor (HLTF) hypermethylation analysis for the purpose of CRC detection used qMSP and described large cohorts of more than 100 patients (67,85–89). The most recent study found a sensitivity of 11% for analysis in plasma (67), which was supported by the majority of other studies describing sensitivities of less than 20% (67,85–88). Two studies reported specificities (96% and 100%) (67,89), and two studies reported concordance rates (41% and 42%) (86,88). Taken together, this candidate marker is not considered to be of value for CRC detection because of the low observed sensitivities.
Other Candidate Methylation Markers
Several less frequently described candidate markers presented in Table 1 showed high sensitivities, supporting their further investigation. Of particular interest for further validation are (studies with highest reported sensitivity) across stages I–IV: ALX4 [sensitivity 83%, specificity 70% (68)], APC [sensitivity 57%, specificity 86% (72)], BCAT1 [sensitivity 65%, specificity 97% (75)], IKZF1 [sensitivity 68%, specificity 95 (76)], and VIM [sensitivity 71%, specificity not reported (109)]. Furthermore, hypomethylation of LINE-1 [sensitivity 66%, specificity 90% (112)] and cystathionine-beta-synthase (CBS) [sensitivity 56%, specificity not reported (111)] are of interest and require further study.
Marker Panels
The simultaneous analysis of multiple ctDNA mutation, copy number, and/or hypermethylation markers potentially results in higher accuracy for CRC detection. Most evidence arises from studies evaluating panels of hypermethylation markers. Combined analysis of APC, O-6-methylguanine-DNA methyltransferase (MGMT), Ras association domain family member 2 (RASSF2A), and WNT inhibitory factor 1 (Wif-1) hypermethylation was evaluated in 243 stage I–II CRC patients and demonstrated a sensitivity of 87% and a specificity of 92% (86). In a more recent study (n = 193 patients), a panel of the plasma hypermethylation markers ALX4, bone morphogenetic protein 3 (BMP3), neuronal pentraxin 2 (NPTX2), retinoic acid receptor beta (RARB), syndecan 2 (SDC2), SEPT9, and VIM analyzed with MSP showed a sensitivity of 91% for stage I–IV and 89% for stage I–II CRC using a multifactorial model accounting for sex and age (67). This study reported a specificity of 73%. The largest described panel was an NGS panel of 90 oncogenes including the most common CRC mutations. With this panel, one to six mutations were found in all 21 studied CRC patients (sensitivity 100%) without providing information on specificity (45). None of the studies reported technical concordance rates for these panels. Overall, the use of marker panels for CRC detection resulted in high accuracy.
ctDNA for Prognostication and Treatment Selection in CRC
Pre therapeutic Analysis
Pre- as well as post-therapeutic ctDNA analysis have the potential to improve clinical decision making. Quantification of ctDNA before treatment could serve as a prognosticator because of a strong correlation with tumor burden. In the included studies, ctDNA analysis in therapy-naive patients allowed profiling of mutation patterns and detection of KRAS mutations before anti-EGFR therapy. Additionally, the presence of ctDNA was correlated with clinicopathological parameters (Figure 3), supporting its use in treatment planning. None of the included studies reported on detection of posttherapeutic ctDNA CNAs.
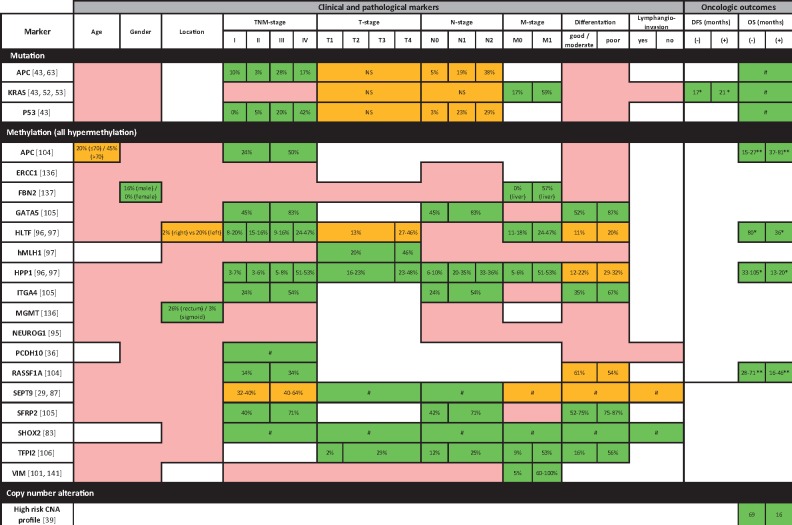
Figure 4.
Associations between the presence of preoperative circulating tumor DNA and clinicopathological variables and oncologic outcomes. The percentage of patients with a positive marker is represented for the categories of the variables. Green: all studies reporting on the specific marker found statistically significant associations; orange: part of the studies found statistically significant and part found statistically nonsignificant (NS) associations; pink: all studies found statistically NS associations. The overall (OS) and disease-free survival (DFS) is presented for patients with a positive (+) and a negative (−) marker. # = no percentage of patients or median or mean OS or DFS provided, * = median, ** = mean. TNM = tumor (T), nodes (N), and metastases (M).
Quantitative analysis showed that ctDNA mutations in KRAS, APC, and TP53 genes (41,46) and hypermethylation of multiple genes (APC, GATA binding protein 5 (GATA5), HLTF, hyperpigmentation, progressive, 1 (HPP1), integrin subunit alpha 4 (ITGA4), protocadherin 10 (PCDH10), Ras association domain family member 1 (RASSF1A), SEPT9, short stature homeobox 2 (SHOX2), and secreted frizzled-related protein 2 (SFPR2) are frequently present in patients with late-stage CRC (29,36,46,73,84,89,103,106). The detection of KRAS mutations (60) and hypermethylation of the HLTF, HPP1, tissue factor pathway inhibitor 2 (TFPI2), SEPT9, SHOX2, and VIM genes (89,103,108,109) in ctDNA was associated with the presence of distant metastases. Accordingly, the presence of ctDNA as detected by mutation [90-gene NGS panel (45), KRAS, APC, tumor protein P53 (TP53) (46,60)], copy number (113), or hypermethylation analysis [APC, HLTF, HPP1, RASSF1A (73,89,90)] was associated with worse progression-free and overall survival. Qualitative ctDNA analysis showed that presence of KRAS mutations in ctDNA could predict the effectiveness of targeted therapies, illustrated by an absence of clinical response to anti-EGFR therapy in stage IV CRC patients with KRAS mutations detected in pretherapeutic blood samples (61).
Post therapeutic Analysis
The detection of ctDNA after therapy could qualify patients for additional therapies by indicating residual disease or recurrence. The studies included in this review showed that the posttherapeutic detection of ctDNA mutations was correlated with poor oncologic outcome and, accordingly, may reflect (residual) tumor load after tumor resection. The detection of ctDNA using an NGS panel of 90 oncogenes after start of systemic treatment was found to be an independent risk factor for poor survival in 21 stage IV CRC patients (45). In seven CRC patients, the postoperative presence of driver gene mutations in plasma ctDNA, as detected by an 85-gene NGS panel, was associated with a poor prognosis (44). Another study (n = 60 patients) demonstrated that the persistence of serum KRAS mutations after surgery was associated with an increased risk of recurrence (59).
Postoperative ctDNA hypermethylation was found to be associated with poor oncologic outcome. In 79 CRC patients, SEPT9 methylation levels dropped to barely detectable amounts after surgery in all patients except those with distant metastases or positive resection margins (103). In another study (n = 16 patients), the two patients with methylated SEPT9 in postoperative ctDNA both presented with a recurrence during follow-up (104). Furthermore, in a study describing 82 CRC patients, postoperative detection of SEPT9 hypermethylation in plasma was associated with increased mortality (107). Several other methylation markers were proposed as indicators of residual disease. Postoperative detection of HPP1 hypermethylation was associated with poor survival in 337 CRC patients (90). Elevated VIM methylation plasma levels were associated with residual disease after surgery in patients with colorectal liver metastases, whereas CEA levels had returned to normal levels after surgery (110). Another proposed method to detect residual disease is combined analysis of plasma BCAT1 and IKZF1 hypermethylation. Tumor resection resulted in reduced methylation levels of these genes with complete elimination of the signal in 10 of 26 patients (31). Taken together, postoperative presence of ctDNA suggests residual disease. However, included studies consist of small cohorts and clinical validation is warranted.
ctDNA for CRC Monitoring
Monitoring of disease by serial liquid biopsies to assess treatment response and detect recurrences during follow-up is a promising and valuable companion to current detection methods. Quantitative detection of ctDNA levels potentially allows early detection of recurrences (121). Qualitative analysis of ctDNA mutations and CNAs could find therapeutic targets and help detect therapy resistance (121).
Six studies evaluated the potential of ctDNA analysis during follow-up after surgery or during systemic treatment of CRC patients (45,55,59–61,115), all of which had small sample sizes. Five studies reported data on ctDNA mutation analysis (45,59–61,115) and one study investigated a combination of hypermethylation and mutation markers (55). No articles reported on CNAs for the use of CRC patient monitoring.
An increase in ctDNA levels, as detected by an NGS panel of 90 oncogenes, could detect resistance to chemotherapy (45). Additionally, quantitative analysis of KRAS mutations allowed detection of recurrences with 100% sensitivity in patients with KRAS-positive solid tumors (60,61,115) and improved monitoring compared with current diagnostic modalities (61). In three of seven metastatic CRC patients with a recurrence, reappearance of plasma KRAS mutations was detected before a diagnosis could be made using conventional methods. Moreover, in eight patients with acquired resistance during anti-EGFR therapy, KRAS mutations were detectable in plasma 3 months before disease progression was seen on CT scans (61). Furthermore, newly diagnosed KRAS and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations were found up to 4 months before radiological progression in two stage IV CRC patients receiving systemic therapy (115). The combined analysis of KRAS mutations and CDKN2A methylation analysis in plasma of CRC patients increased diagnostic accuracy (55). At this moment, however, conclusions of all studies are hampered by small sample sizes.
Discussion
Analysis of ctDNA in peripheral blood samples, so-called liquid biopsies, has the potential to realize early-stage detection of CRC and serve as a prognostic, predictive, and monitoring tool. The present systematic review is the first to evaluate the use of the most promising types of ctDNA analysis in a clinical setting. To date, the highest accuracy for CRC detection has been obtained by SEPT9 hypermethylation analysis, especially in combined panels. For diagnostic purposes, analysis of single ctDNA mutations does not yet allow for clinical decision making. For the purposes of prognostication and disease monitoring, the most robust results were obtained by consecutive sampling and subsequent KRAS mutation ctDNA analysis. The analysis of CNAs could be promising for clinical use as well but is still in its infancy.
The present findings provide a starting point for implementation of ctDNA analysis into the clinic by setting out promising candidate markers. The high sensitivities of up to 100% and specificities of up to 97% of SEPT9 methylation ctDNA analysis suggest a diagnostic role for this candidate marker. Even higher sensitivities could theoretically be obtained in combination with other promising methylation markers such as APC, VIM, BCAT1, ALX4, IKZF1, and LINE-1. Cancer detection through copy number analysis in ctDNA has great potential for CRC detection, with sensitivities up to 96% and specificities up to 100%. However, only a small number of included studies reported on CNAs in ctDNA, hampering solid conclusions. In contrast, analysis of single-gene ctDNA mutations showed disappointing sensitivities of less than 50% with highly variable specificities so is unlikely to increase the accuracy of current screening methods. The low sensitivities are probably due to the relatively low proportion of cfDNA fragments carrying the tumor-specific mutation, described as the variant allele frequency, or due to the absolute number of mutant DNA molecules in the sample (17,122,123).
For prognostication and disease monitoring, mutation ctDNA analysis is considered the most valuable. For prognostication, pre- and posttherapeutic analyses alike of KRAS and APC mutations provided information on tumor load (quantitative analysis) and allowed molecular profiling (qualitative analysis) to guide treatment decisions by determining the indication for (neo-)adjuvant therapies (19,20,124). Owing to correlation with oncologic outcomes, ctDNA detection after tumor resection suggests the presence of residual disease undetectable with conventional methods (17). This potentially enables accurate identification of patients for adjuvant systemic therapies. Additionally, the presence of KRAS mutations in ctDNA could predict treatment response to anti-EGFR therapy (61). The detection of ctDNA at higher stages could result from increased shedding of ctDNA or occult micrometastases (17). For monitoring purposes, consecutive analysis during follow-up showed high accuracy for detection of recurrences in patients with known pre-therapeutic detectable KRAS mutations (55,59–61,115). Additionally, KRAS mutation analysis in ctDNA allows repeated analysis of tumor mutations to identify acquired resistance (61) and emerging potential therapeutic targets (121). In this way, ctDNA analysis could guide tailored treatment. None of the included studies investigated CNAs for monitoring of CRC. Theoretically, serial copy number analysis could be useful as well because it does not target a specific genomic site but measures across the entire genome.
Clinical implementation of liquid biopsies for population-based screening also has high potential. Limitations of current studies are the small cohorts and poorly defined or absent healthy control individuals. Moreover, there is a lack of studies focusing on detection of precursor lesions. Before widespread implementation for screening, adequately powered validation studies comparing ctDNA with the FOBT and colonoscopy are essential. The current literature on liquid biopsies for CRC mainly consists of nonrandomized retrospective studies, with only a few markers tested in validation cohorts. A technical issue is the mutational heterogeneity observed in CRC. Accurate mutation monitoring requires expensive panel-based NGS approaches to test many genes before start of therapy and subsequent consecutive analyses of specific mutations. For this process, multiple-gene testing and highly robust assays for individual mutations are warranted, impeding widespread use. However, large-scale whole-genome mutation analysis in blood as a liquid biopsy will be feasible in the near future, enabling not only monitoring of recurrences but also evaluation of clonal evolution to adjust therapeutic approaches. Cost-effectiveness analysis and clinical validation in prospective trials are currently ongoing.
To our knowledge, this is the first systematic review assessing candidate mutation, CNA, and methylation markers in blood samples for clinical use in CRC patients. These approaches could not only complement each other but also be combined to achieve higher accuracy (125). In line with the present review, the value of methylation analysis for CRC detection is supported by a systematic review reporting hypermethylation of the APC, neurogenin 1 (NEUROG1), RASSF1A, RASSF2A, SDC2, SEPT9, tachykinin precursor 1 (TAC1), and thrombomodulin (THBD) genes in ctDNA to be detectable in early-stage CRC patients (20). However, in contrast to the low sensitivities reported for ctDNA mutation analysis, in a recent review the use of KRAS and APC mutation analysis in ctDNA was advocated for early CRC detection, with particular interest in APC mutations because of their presence in precursor lesions (126). Notably, this review was not performed systematically and no quality assessment was performed, impeding the authors’ conclusions.
Unfortunately, it was not possible to conduct a meta-analysis because of the variability of methods. Furthermore, the use of other liquid biopsy substrates such as circulating RNAs or circulating tumor cells was beyond the scope of this study. The focus on ctDNA was chosen because it has been investigated most extensively and is proposed as the most promising reproducible method for CRC detection with high accuracy (17–19). We included analysis of copy numbers because this is a promising, novel, and relatively simple method to detect ctDNA (15,127). Moreover, we did not include studies on other ctDNA sources currently being explored, such as urine, stool, and saliva (128,129). Similarly, other noninvasive approaches to genetic diagnosis of CRC were also omitted despite widespread clinical use. For example, the FDA-approved Cologuard (Exact Sciences) test analyzes mutations and methylation changes in DNA from stool (130). However, because stool is not a source of ctDNA, it was not covered by the scope of this study.
Translation of ctDNA into clinical daily practice is still awaited. The use of ctDNA for therapy guidance has already been suggested for locally advanced rectal cancer patients (131) and could help clinicians decide whether additional intervention is required after local excision of early-stage rectal cancer (132). A prospective comparison of current guidelines for adjuvant treatment with a novel approach based on residual ctDNA should be carried out and is currently being planned for advanced rectal cancer patients (Dynamic-Rectal study— ACTRN12617001560381). Prospective combined analysis of (epi-) genomic markers integrated with other biomarker substrates such as proteomics or metabolomics could facilitate cancer detection with higher accuracy (133). Such innovative blood tests should be designed using an “-omics” approach (134), opening up potential combinations of other candidate biomarkers. Furthermore, implementation of ctDNA analysis is promoted by novel detection methods that are being developed at a rapid pace. Techniques that are currently too expensive for routine use, such as personalized ctDNA sequencing, might become feasible within years (135). However, the use of highly sensitive and specific single-locus assays such as ddPCR, which is currently a more straightforward and cost-effective method, are still expected to be relevant (136,137), particularly for repeated measurements in patients with known tumor mutations in a tissue-guided manner (18). Finally, collaboration between academia and industrial partners is becoming increasingly important for the transition of biomarkers into the clinic, but a solid cost-effectiveness analysis is key for this purpose (138).
In conclusion, the present overview of literature proposes ctDNA analysis of methylation panels including SEPT9 as the most valuable option for CRC detection. The use of liquid biopsies for disease monitoring seems even more promising. KRAS mutation analysis appears of particular interest for prognostication and monitoring of CRC patients to provide treatment guidance and tailored therapies. CNAs can be detected in the blood of CRC patients at various stages. Owing to its genomewide rather than gene-specific approach, copy number analysis could potentially be useful as a companion for early detection or monitoring. However, more research is needed. Creation of approaches combining various types of ctDNA analyses could further enhance accuracy. Prospective studies, preferably in a randomized setting in which clinical decisions depend on ctDNA results of the currently proposed candidate markers, should provide the definitive evidence to bring ctDNA analysis to clinical practice.
Funding
This work was supported by intramural funding from the VUmc-CCA Foundation, Edli Foundation, and the Weijerhorst Foundation. This research did not receive any other specific grants from funding agencies in the public or commercial sectors.
Notes
Affiliations of authors: Department of Surgery, Cancer Center Amsterdam (SB, NRS, JMM, GK, JBT), Department of Pathology, Cancer Center Amsterdam (JJB, NCvG, RDMS, BY) and Medical Information Specialist/Literature Researcher Medical Library (JCK), Amsterdam UMC, Vrije Universiteit Amsterdam, Vrije Universiteit, Amsterdam, the Netherlands.
Supplementary Material
References
- 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Brouwer NPM, Bos A, Lemmens V, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143(11):2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner H, Kloor M, Pox CP.. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. [DOI] [PubMed] [Google Scholar]
- 5. McLoughlin RM, O'Morain CA.. Colorectal cancer screening. World J Gastroenterol. 2006;12(42):6747–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Böhm B, Schwenk W, Hucke HP, et al. Does methodic long-term follow-up affect survival after curative resection of colorectal carcinoma? Dis Colon Rectum. 1993;36(3):280–286. [DOI] [PubMed] [Google Scholar]
- 9. Werkgroep Colorectaal Carcinoom. Richtlijn Colorectaal Carcinoom http://www.oncoline.nl/colorectaalcarcinoom. Accessed January 11, 2019.
- 10. Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001;47(4):624–630. [PubMed] [Google Scholar]
- 11. Rockall TA, McDonald PJ.. Carcinoembryonic antigen: its value in the follow-up of patients with colorectal cancer. Int J Colorectal Dis. 1999;14(1):73–77. [DOI] [PubMed] [Google Scholar]
- 12. Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311(3):263–270. [DOI] [PubMed] [Google Scholar]
- 13. Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16(2):327–333. [DOI] [PubMed] [Google Scholar]
- 14. Normanno N, Cervantes A, Ciardiello F, et al. The liquid biopsy in the management of colorectal cancer patients: current applications and future scenarios. Cancer Treat Rev. 2018;70:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Heitzer E, Haque IS, Roberts CES, et al. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2018;20(2):71–88. [DOI] [PubMed] [Google Scholar]
- 16. Best M, Sol N, Kooi I, et al. mRNA-sequencing of tumour-educated platelets allows for multiclass liquid biopsy-based diagnosis of cancer. J Extracell Vesicles. 2015;4:169–170. [Google Scholar]
- 17. Wan JC, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–238. [DOI] [PubMed] [Google Scholar]
- 18. Ignatiadis M, Lee M, Jeffrey SS.. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015;21(21):4786–4800. [DOI] [PubMed] [Google Scholar]
- 19. Nordgård O, Tjensvoll K, Gilje B, et al. Circulating tumour cells and DNA as liquid biopsies in gastrointestinal cancer. Br J Surg. 2018;105(2):e110–e120. [DOI] [PubMed] [Google Scholar]
- 20. Rasmussen SL, Krarup HB, Sunesen KG, et al. Hypermethylated DNA as a biomarker for colorectal cancer: a systematic review. Colorectal Dis. 2016;18(6):549–561. [DOI] [PubMed] [Google Scholar]
- 21. Thierry AR, Mouliere F, El Messaoudi S, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014;20(4):430–435. [DOI] [PubMed] [Google Scholar]
- 22. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sausen M, Phallen J, Adleff V, et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun. 2015;6:7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jenkins S, Yang JC, Ramalingam SS, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(7):1061–1070. [DOI] [PubMed] [Google Scholar]
- 25. Warren JD, Xiong W, Bunker AM, et al. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Schendel RV, van El CG, Pajkrt E, et al. Implementing non-invasive prenatal testing for aneuploidy in a national healthcare system: global challenges and national solutions. BMC Health Serv Res. 2017;17(1):670.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 29. Chen CH, Yan SL, Yang TH, et al. The relationship between the methylated septin-9 DNA blood test and stool occult blood test for diagnosing colorectal cancer in Taiwanese people. J Clin Lab Anal. 2017;31(1). doi: 10.1002/jcla.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin P, Kang Q, Wang X, et al. Performance of a second-generation methylated SEPT9 test in detecting colorectal neoplasm. J Gastroenterol Hepatol. 2015;30(5):830–833. [DOI] [PubMed] [Google Scholar]
- 31. Pedersen SK, Symonds EL, Baker RT, et al. Evaluation of an assay for methylated BCAT1 and IKZF1 in plasma for detection of colorectal neoplasia. BMC Cancer. 2015;15:654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Danese E, Minicozzi AM, Benati M, et al. Comparison of genetic and epigenetic alterations of primary tumors and matched plasma samples in patients with colorectal cancer. PLoS One. 2015;10(5):e0126417.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song L, Jia J, Yu H, et al. The performance of the mSEPT9 assay is influenced by algorithm, cancer stage and age, but not sex and cancer location. J Cancer Res Clin Oncol. 2017;143(6):1093–1101. [DOI] [PubMed] [Google Scholar]
- 34. Sclafani F, Chau I, Cunningham D, et al. KRAS and BRAF mutations in circulating tumour DNA from locally advanced rectal cancer. Sci Rep. 2018;8(1):1445.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sefrioui D, Beaussire L, Perdrix A, et al. Direct circulating tumor DNA detection from unpurified plasma using a digital PCR platform. Clin Biochem. 2017;50(16–17):963–966. [DOI] [PubMed] [Google Scholar]
- 36. Danese E, Minicozzi AM, Benati M, et al. Epigenetic alteration: new insights moving from tissue to plasma - the example of PCDH10 promoter methylation in colorectal cancer. Br J Cancer. 2013;109(3):807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu JF, Kang Q, Pan YM, et al. A novel method to detect early colorectal cancer based on chromosome copy number variation in plasma. Cell Physiol Biochem. 2018;45(4):1444–1454. [DOI] [PubMed] [Google Scholar]
- 38. Symonds EL, Pedersen SK, Baker RT, et al. A blood test for methylated BCAT1 and IKZF1 vs. a fecal immunochemical test for detection of colorectal neoplasia. Clin Transl Gastroenterol. 2016;7(1):e137.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li J, Dittmar RL, Xia S, et al. Cell-free DNA copy number variations in plasma from colorectal cancer patients. Mol Oncol. 2017;11(8):1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, et al. A novel hypothesis on the sensitivity of the fecal occult blood test: results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115(11):2410–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsieh JS, Lin SR, Chang MY, et al. APC, K-ras, and p53 gene mutations in colorectal cancer patients: correlation to clinicopathologic features and postoperative surveillance. Am Surg. 2005;71(4):336–343. [PubMed] [Google Scholar]
- 42. Lilleberg SL, Durocher J, Sanders C, et al. High sensitivity scanning of colorectal tumors and matched plasma DNA for mutations in APC, TP53, K-RAS, and BRAF genes with a novel DHPLC fluorescence detection platform. Ann N Y Acad Sci. 2004;1022:250–256. [DOI] [PubMed] [Google Scholar]
- 43. Lin JK, Lin PC, Lin CH, et al. Clinical relevance of alterations in quantity and quality of plasma DNA in colorectal cancer patients: based on the mutation spectra detected in primary tumors. Ann Surg Oncol. 2014;21(suppl 4):S680–S686. [DOI] [PubMed] [Google Scholar]
- 44. Sun X, Huang T, Cheng F, et al. Monitoring colorectal cancer following surgery using plasma circulating tumor DNA. Oncol Lett. 2018;15(4):4365–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamauchi M, Urabe Y, Ono A, et al. Serial profiling of circulating tumor DNA for optimization of anti-VEGF chemotherapy in metastatic colorectal cancer patients. Int J Cancer. 2018;142(7):1418–1426. [DOI] [PubMed] [Google Scholar]
- 46. Wang JY, Hsieh JS, Chang MY, et al. Molecular detection of APC, K- ras, and p53 mutations in the serum of colorectal cancer patients as circulating biomarkers. World J Surg. 2004;28(7):721–726. [DOI] [PubMed] [Google Scholar]
- 47. Diehl F, Schmidt K, Durkee KH, et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 2008;135(2):489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim ST, Lira M, Deng SB, et al. PIK3CA mutation detection in metastatic biliary cancer using cell-free DNA. Oncotarget. 2015;6(37):40026–40035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kidess E, Heirich K, Wiggin M, et al. Mutation profiling of tumor DNA from plasma and tumor tissue of colorectal cancer patients with a novel, high-sensitivity multiplexed mutation detection platform. Oncotarget. 2015;6(4):2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pu X, Pan Z, Huang Y, et al. Comparison of KRAS/BRAF mutations between primary tumors and serum in colorectal cancer: biological and clinical implications. Oncol Lett. 2013;5(1):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li X, Yang T, Li CS, et al. Surface enhanced raman spectroscopy (SERS) for the multiplex detection of Braf, Kras, and Pik3ca mutations in plasma of colorectal cancer patients. Theranostics. 2018;8(6):1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beranek M, Sirak I, Vosmik M, et al. Carrier molecules and extraction of circulating tumor DNA for next generation sequencing in colorectal cancer. Acta Medica (Hradec Kralove). 2016;59(2):54–58. [DOI] [PubMed] [Google Scholar]
- 53. Sakai K, Tsurutani J, Yamanaka T, et al. Extended RAS and BRAF mutation analysis using next-generation sequencing. PLoS One. 2015;10(5):e0121891.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Loree JM, Bailey AM, Johnson AM, et al. Molecular landscape of ERBB2/ERBB3 mutated colorectal cancer. J Natl Cancer Inst. 2018;110(12):1409–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frattini M, Gallino G, Signoroni S, et al. Quantitative and qualitative characterization of plasma DNA identifies primary and recurrent colorectal cancer. Cancer Lett. 2008;263(2):170–181. [DOI] [PubMed] [Google Scholar]
- 56. Kuo YB, Chen JS, Fan CW, et al. Comparison of KRAS mutation analysis of primary tumors and matched circulating cell-free DNA in plasmas of patients with colorectal cancer. Clin Chim Acta. 2014;433:284–289. [DOI] [PubMed] [Google Scholar]
- 57. Liu P, Liang H, Xue L, et al. Potential clinical significance of plasma-based KRAS mutation analysis using the COLD-PCR/TaqMan((R)) -MGB probe genotyping method. Exp Ther Med. 2012;4(1):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Perrone F, Lampis A, Bertan C, et al. Circulating free DNA in a screening program for early colorectal cancer detection. Tumori. 2014;100(2):115–121. [DOI] [PubMed] [Google Scholar]
- 59. Ryan BM, Lefort F, McManus R, et al. A prospective study of circulating mutant KRAS2 in the serum of patients with colorectal neoplasia: strong prognostic indicator in postoperative follow up. Gut. 2003;52(1):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shin SJ, Chun SM, Kim TI, et al. Feasibility of multiplexed gene mutation detection in plasma samples of colorectal cancer patients by mass spectrometric genotyping. PLoS One. 2017;12(5):e0176340.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yamada T, Iwai T, Takahashi G, et al. Utility of KRAS mutation detection using circulating cell-free DNA from patients with colorectal cancer. Cancer Sci. 2016;107(7):936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kloten V, Ruchel N, Bruchle NO, et al. Liquid biopsy in colon cancer: comparison of different circulating DNA extraction systems following absolute quantification of KRAS mutations using Intplex allele-specific PCR. Oncotarget. 2017;8(49):86253–86263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ono Y, Sugitani A, Karasaki H, et al. An improved digital polymerase chain reaction protocol to capture low-copy KRAS mutations in plasma cell-free DNA by resolving ‘subsampling’ issues. Mol Oncol. 2017;11(10):1448–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tian F, Liao Y, Zhang Y.. Variations in transrenal DNA and comparison with plasma DNA as a diagnostic marker for colorectal cancer. Int J Biol Markers. 2017;32(4):e434–e440. [DOI] [PubMed] [Google Scholar]
- 65. Liu W, Guan M, Su B, et al. Rapid determination of AKAP12 promoter methylation levels in peripheral blood using methylation-sensitive high-resolution melting (MS-HRM) analysis: application in colorectal cancer. Clin Chim Acta. 2010;411(13–14):940–946. [DOI] [PubMed] [Google Scholar]
- 66. He Q, Chen HY, Bai EQ, et al. Development of a multiplex MethyLight assay for the detection of multigene methylation in human colorectal cancer. Cancer Genet Cytogenet. 2010;202(1):1–10. [DOI] [PubMed] [Google Scholar]
- 67. Rasmussen SL, Krarup HB, Sunesen KG, et al. Hypermethylated DNA, a circulating biomarker for colorectal cancer detection. PLoS One. 2017;12(7):e0180809.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ebert MP, Model F, Mooney S, et al. Aristaless-like homeobox-4 gene methylation is a potential marker for colorectal adenocarcinomas. Gastroenterology. 2006;131(5):1418–1430. [DOI] [PubMed] [Google Scholar]
- 69. Herbst A, Rahmig K, Stieber P, et al. Methylation of NEUROG1 in serum is a sensitive marker for the detection of early colorectal cancer. Am J Gastroenterol. 2011;106(6):1110–1118. [DOI] [PubMed] [Google Scholar]
- 70. Salehi R, Atapour N, Vatandoust N, et al. Methylation pattern of ALX4 gene promoter as a potential biomarker for blood-based early detection of colorectal cancer. Adv Biomed Res. 2015;4(1):252.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Erdem B, Kucukyildirim S, Saglar E, et al. Promoter hypermethylation of p16 and APC in gastrointestinal cancer patients. Turk J Gastroenterol. 2014;25(5):512–517. [DOI] [PubMed] [Google Scholar]
- 72. Pack SC, Kim HR, Lim SW, et al. Usefulness of plasma epigenetic changes of five major genes involved in the pathogenesis of colorectal cancer. Int J Colorectal Dis. 2013;28(1):139–147. [DOI] [PubMed] [Google Scholar]
- 73. Matthaios D, Balgkouranidou I, Karayiannakis A, et al. Methylation status of the APC and RASSF1A promoter in cell-free circulating DNA and its prognostic role in patients with colorectal cancer. Oncol Lett. 2016;12(1):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kondratov AG, Nekrasov KA, V LL, et al. Comparative analysis of epigenetic markers in plasma and tissue of patients with colorectal cancer. Biopolym Cell. 2014;30(2):129–134. [Google Scholar]
- 75. Mitchell SM, Ho T, Brown GS, et al. Evaluation of methylation biomarkers for detection of circulating tumor DNA and application to colorectal cancer. Genes (Basel) 2016;7(12):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miotto E, Sabbioni S, Veronese A, et al. Frequent aberrant methylation of the CDH4 gene promoter in human colorectal and gastric cancer. Cancer Res. 2004;64(22):8156–8159. [DOI] [PubMed] [Google Scholar]
- 77. Kim JW, Park HM, Choi YK, et al. Polymorphisms in genes involved in folate metabolism and plasma DNA methylation in colorectal cancer patients. Oncol Rep. 2011;25(1):167–172. [PubMed] [Google Scholar]
- 78. Sakamoto J, Fujiya M, Okamoto K, et al. Immunoprecipitation of nucleosomal DNA is a novel procedure to improve the sensitivity of serum screening for the p16 hypermethylation associated with colon cancer. Cancer Epidemiol. 2010;34(2):194–199. [DOI] [PubMed] [Google Scholar]
- 79. Zou HZ, Yu BM, Wang ZW, et al. Detection of aberrant p16 methylation in the serum of colorectal cancer patients. Clin Cancer Res. 2002;8(1):188–191. [PubMed] [Google Scholar]
- 80. Liu Y, Chew MH, Tham CK, et al. Methylation of serum SST gene is an independent prognostic marker in colorectal cancer. Am J Cancer Res. 2016;6(9):2098–2108. [PMC free article] [PubMed] [Google Scholar]
- 81. Wu PP, Zou JH, Tang RN, et al. Detection and clinical significance of DLC1 gene methylation in serum DNA from colorectal cancer patients. Chin J Cancer Res. 2011;23(4):283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shalaby SM, El-Shal AS, Abdelaziz LA, et al. Promoter methylation and expression of DNA repair genes MGMT and ERCC1 in tissue and blood of rectal cancer patients. Gene. 2018;644:66–73. [DOI] [PubMed] [Google Scholar]
- 83. Hibi K, Mizukami H, Saito M, et al. FBN2 methylation is detected in the serum of colorectal cancer patients with hepatic metastasis. Anticancer Res. 2012;32(10):4371–4374. [PubMed] [Google Scholar]
- 84. Zhang X, Song YF, Lu HN, et al. Combined detection of plasma GATA5 and SFRP2 methylation is a valid noninvasive biomarker for colorectal cancer and adenomas. World J Gastroenterol. 2015;21(9):2629–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Herbst A, Wallner M, Rahmig K, et al. Methylation of helicase-like transcription factor in serum of patients with colorectal cancer is an independent predictor of disease recurrence. Eur J Gastroenterol Hepatol. 2009;21(5):565–569. [DOI] [PubMed] [Google Scholar]
- 86. Lee BB, Lee EJ, Jung EH, et al. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res. 2009;15(19):6185–6191. [DOI] [PubMed] [Google Scholar]
- 87. Philipp AB, Nagel D, Stieber P, et al. Circulating cell-free methylated DNA and lactate dehydrogenase release in colorectal cancer. BMC Cancer. 2014;14:245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Philipp AB, Stieber P, Nagel D, et al. Prognostic role of methylated free circulating DNA in colorectal cancer. Int J Cancer. 2012;131(10):2308–2319. [DOI] [PubMed] [Google Scholar]
- 89. Wallner M, Herbst A, Behrens A, et al. Methylation of serum DNA is an independent prognostic marker in colorectal cancer. Clin Cancer Res. 2006;12(24):7347–7352. [DOI] [PubMed] [Google Scholar]
- 90. Herbst A, Vdovin N, Gacesa S, et al. Methylated free-circulating HPP1 DNA is an early response marker in patients with metastatic colorectal cancer. Int J Cancer. 2017;140(9):2134–2144. [DOI] [PubMed] [Google Scholar]
- 91. Grady WM, Rajput A, Lutterbaugh JD, et al. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61(3):900–902. [PubMed] [Google Scholar]
- 92. Xiao W, Zhao H, Dong W, et al. Quantitative detection of methylated NDRG4 gene as a candidate biomarker for diagnosis of colorectal cancer. Oncol Lett. 2015;9(3):1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lofton-Day C, Model F, Devos T, et al. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54(2):414–423. [DOI] [PubMed] [Google Scholar]
- 94. Yuan P, Cheng XJ, Wu XJ, et al. OSMR and SEPT9: promising biomarkers for detection of colorectal cancer based on blood-based tests. Transl Cancer Res. 2016;5(2):131–139. [Google Scholar]
- 95. Zheng Y, Zhang Y, Huang X, et al. Analysis of the RUNX3 gene methylation in serum DNA from esophagus squamous cell carcinoma, gastric and colorectal adenocarcinoma patients. Hepatogastroenterology. 2011;58(112):2007–2011. [DOI] [PubMed] [Google Scholar]
- 96. Ahlquist DA, Taylor WR, Mahoney DW, et al. The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin Gastroenterol Hepatol. 2012;10(3):272–277 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63(2):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Potter NT, Hurban P, White MN, et al. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem. 2014;60(9):1183–1191. [DOI] [PubMed] [Google Scholar]
- 99. Song L, Peng X, Li Y, et al. The SEPT9 gene methylation assay is capable of detecting colorectal adenoma in opportunistic screening. Epigenomics. 2017;9(5):599–610. [DOI] [PubMed] [Google Scholar]
- 100. Tänzer M, Balluff B, Distler J, et al. Performance of epigenetic markers SEPT9 and ALX4 in plasma for detection of colorectal precancerous lesions. PLoS One. 2010;5(2):e9061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tóth K, Sipos F, Kalmár A, et al. Detection of methylated SEPT9 in plasma is a reliable screening method for both left- and right-sided colon cancers. PLoS One. 2012;7(9):e46000.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Johnson DA, Barclay RL, Mergener K, et al. Plasma Septin9 versus fecal immunochemical testing for colorectal cancer screening: a prospective multicenter study. PLoS One. 2014;9(6):e98238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bergheim J, Semaan A, Gevensleben H, et al. Potential of quantitative SEPT9 and SHOX2 methylation in plasmatic circulating cell-free DNA as auxiliary staging parameter in colorectal cancer: a prospective observational cohort study. Br J Cancer. 2018;118(9):1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fu B, Yan P, Zhang S, et al. Cell-free circulating methylated SEPT9 for noninvasive diagnosis and monitoring of colorectal cancer. Dis Markers. 2018;2018:6437104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Xie L, Jiang XY, Li Q, et al. Diagnostic value of methylated septin9 for colorectal cancer detection. Front Oncol. 2018;8:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim H, Lee JK, Hong YJ, et al. Detection of methylated SEPT9 in Korean colorectal cancer patients: comparison with previous studies. Clin Lab. 2018;64(9):1573–1579. [DOI] [PubMed] [Google Scholar]
- 107. Song L, Guo S, Wang J, et al. The blood mSEPT9 is capable of assessing the surgical therapeutic effect and the prognosis of colorectal cancer. Biomarkers Med. 2018;12(9):961–973. [DOI] [PubMed] [Google Scholar]
- 108. Hibi K, Goto T, Shirahata A, et al. Detection of TFPI2 methylation in the serum of colorectal cancer patients. Cancer Lett. 2011;311(1):96–100. [DOI] [PubMed] [Google Scholar]
- 109. Shirahata A, Sakuraba K, Goto T, et al. Detection of vimentin (VIM) methylation in the serum of colorectal cancer patients. Anticancer Res. 2010;30(12):5015–5018. [PubMed] [Google Scholar]
- 110. Li M, Chen WD, Papadopoulos N, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol. 2009;27(9):858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Xue G, Lu CJ, Pan SJ, et al. DNA hypomethylation of CBS promoter induced by folate deficiency is a potential noninvasive circulating biomarker for colorectal adenocarcinomas. Oncotarget. 2017;8(31):51387–51401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nagai Y, Sunami E, Yamamoto Y, et al. LINE-1 hypomethylation status of circulating cell-free DNA in plasma as a biomarker for colorectal cancer. Oncotarget. 2017;8(7):11906–11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Molparia B, Oliveira G, Wagner JL, et al. A feasibility study of colorectal cancer diagnosis via circulating tumor DNA derived CNV detection. PLoS One. 2018;13(5):e0196826.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Armaghany T, Wilson JD, Chu Q, et al. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 115. Kim ST, Lee WS, Lanman RB, et al. Prospective blinded study of somatic mutation detection in cell-free DNA utilizing a targeted 54-gene next generation sequencing panel in metastatic solid tumor patients. Oncotarget. 2015;6(37):40360–40369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lin PC, Lin JK, Lin CH, et al. Clinical relevance of plasma DNA methylation in colorectal cancer patients identified by using a genome-wide high-resolution array. Ann Surg Oncol. 2015;22(suppl 3):S1419–S1427. [DOI] [PubMed] [Google Scholar]
- 117. Feuk L, Carson AR, Scherer SW.. Structural variation in the human genome. Nat Rev Genet. 2006;7(2):85–97. [DOI] [PubMed] [Google Scholar]
- 118. Xu JF, Yang L, Ma XY, et al. Chromosome copy number variation analysis to detect early colorectal cancer with liquid biopsy. Clin Gastroenterol Hepatol. 2017;15(1):e46–e47. [Google Scholar]
- 119. Juo YY, Johnston FM, Zhang DY, et al. Prognostic value of CpG island methylator phenotype among colorectal cancer patients: a systematic review and meta-analysis. Ann Oncol. 2014;25(12):2314–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Freitas M, Ferreira F, Carvalho S, et al. A novel DNA methylation panel accurately detects colorectal cancer independently of molecular pathway. J Transl Med. 2018;16(1):45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Greaves M, Maley CC.. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Vaughn CP, Zobell SD, Furtado LV, et al. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosom Cancer. 2011;50(5):307–312. [DOI] [PubMed] [Google Scholar]
- 123. de Cuba EM, Snaebjornsson P, Heideman DA, et al. Prognostic value of BRAF and KRAS mutation status in stage II and III microsatellite instable colon cancers. Int J Cancer. 2016;138(5):1139–1145. [DOI] [PubMed] [Google Scholar]
- 124. Ocaña A, Díez-González L, García-Olmo DC, et al. Circulating DNA and survival in solid tumors. Cancer Epidemiol Biomarkers Prev. 2016;25(2):399–406. [DOI] [PubMed] [Google Scholar]
- 125. Bachet JB, Bouche O, Taieb J, et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: the AGEO RASANC prospective multicenter study. Ann Oncol. 2018;29(5):1211–1219. [DOI] [PubMed] [Google Scholar]
- 126. Howell JA, Khan SA, Knapp S, et al. The clinical role of circulating free tumor DNA in gastrointestinal malignancy. Transl Res. 2017;183:137–154. [DOI] [PubMed] [Google Scholar]
- 127. Mouliere F, Chandrananda D, Piskorz AM, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. 2018;10(466). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Janku F, Vibat CR, Kosco K, et al. BRAF V600E mutations in urine and plasma cell-free DNA from patients with Erdheim-Chester disease. Oncotarget. 2014;5(11):3607–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Peng M, Chen C, Hulbert A, et al. Non-blood circulating tumor DNA detection in cancer. Oncotarget. 2017;8(40):69162–69173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–1297. [DOI] [PubMed] [Google Scholar]
- 131. Tie J, Cohen JD, Wang Y, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut. 2019;68(4):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Borstlap WA, Tanis PJ, Koedam TW, et al. A multi-centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer. 2016;16:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359(6378):926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hasin Y, Seldin M, Lusis A.. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Strickler JH, Loree JM, Ahronian LG, et al. Genomic landscape of cell-free DNA in patients with colorectal cancer. Cancer Discov. 2018;8(2):164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Denis JA, Guillerm E, Coulet F, et al. The role of BEAMing and digital PCR for multiplexed analysis in molecular oncology in the era of next-generation sequencing. Mol Diagn Ther. 2017;21(6):587–600. [DOI] [PubMed] [Google Scholar]
- 137. Baker M. . Digital PCR hits its stride. Nat Methods. 2012;9(6):541–544. [Google Scholar]
- 138. Asadullah K, Busch A, Gottwald M, et al. Industry-academia collaborations for biomarkers. Nat Rev Drug Discov. 2015;14(12):805–806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.