Abstract
Purpose
To validate the 2018 revised FIGO cervical cancer staging system for stage III patients with a cohort from China.
Patients and Methods
Patients with stage III cervical cancer (FIGO 2018) treated with definitive radiotherapy at our institute were reviewed. Each patient was evaluated with both the 2014 and 2018 staging systems. Disease-free survival (DFS) was calculated with the Kaplan-Meier method. Receiver operative characteristic (ROC) curves for the predictive accuracy of DFS in patients with cervical cancer according to different FIGO staging systems were created.
Results
Between January 2008 and December 2014, a total of 586 patients with FIGO stage IIIC cervical cancer (2018) were treated with definitive radiotherapy at our institute. The 3-year DFS for patients according to FIGO stage (2014) were as follows: IB2 73.2%, IIA 63.7%, IIB 66.7%, IIIA 64.7%, and IIIB 59.6% (P=0.580). The 3-year DFS according to FIGO stage (2018) were IIIA 79.9%, IIIB 70.4%, IIIC1 66.3% and IIIC2 29.8% (P<0.001). The AUC values for DFS were 0.552 (95% CI: 0.503–0.600, P=0.037) and 0.623 (95% CI: 0.575–0.671, P<0.001) for the 2014 and 2018 FIGO staging systems, respectively.
Conclusion
The 2018 FIGO staging system of cervical cancer showed more distinction within stages and better predictive accuracy for DFS than the preceding staging system in patients with stage III disease from China.
Keywords: cervical cancer, FIGO 2018 staging system, validation, China
Introduction
Cervical cancer is one of the most prevalent cancers for women in China.1 The former International Federation of Gynecology and Obstetrics (FIGO) staging system of cervical cancer which is mainly based on characteristics of primary tumors, does not take the characteristics of positive lymph nodes into consideration.2 However, many studies have identified that pelvic or para-aortic lymph node metastasis is associated with poor survival outcomes.3–6
In 2018, The FIGO revised the 2014 FIGO staging system of cervical cancer.7 The revised staging system defined patients with regional lymph node metastasis as stage IIIC. Patients with stage IIIC cervical cancer were further divided into two substages, stage IIIC1 included patients with pelvic lymph node metastasis only and stage IIIC2 consisted of patients with para-aortic lymph node metastasis.
After the new staging system was released, Matsuo K et al performed a study that validated the 2018 FIGO staging system with data from The Surveillance, Epidemiology, and End Result Program (SEER).8 However, the data in the SEER program are mainly from developed countries. The characteristics of patients and tumors are usually quite different between developed and developing countries.7 Due to a lack of effective cancer screening measures, patients in developing countries are more prone to suffer from advanced disease than those in developed countries. According to global epidemiology, in 2012, approximately 527,600 patients suffered cervical cancer and 265,700 patients died.9 In low- and middle- income countries, cervical cancer is more common. China contributes approximately 100,000 new cases each year, accounting for nearly 20% of all new cases in the world.1 Thus, validation of the new staging system with data from developing countries such as China is urgently needed and of great significance.
To the best of our knowledge, this study is the first to validate the 2018 FIGO staging system of cervical cancer for stage III patients with a cohort from a developing country.
Materials and Methods
Patients Selected
The Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH) reviewed and approved this protocol. We reviewed the clinical data of all patients with cervical cancer treated with definitive radiotherapy or chemoradiotherapy at our institute from January 2008 to December 2014. The inclusion criteria were as follows: histologically confirmed cervical cancer; FIGO stage III (FIGO 2018, Table 1);7 and no evidence of distant metastasis before treatment. As described in our previous articles,10 lymph node metastasis was diagnosed with imaging. Lymph nodes with a short-axis diameter longer than 1 cm on CT or MRI, or positive on PET/CT were considered as lymph nodes metastasis.
Table 1.
FIGO Staging of Cervical Cancer (2018)
| Stage | Description |
|---|---|
| I | The carcinoma is strictly confined to the cervix (extension to the uterine corpus should be disregarded) |
| IA | Invasive carcinoma that can be diagnosed only by microscopy, with maximum depth of invasion <5mma |
| IA1 | Measured stromal invasion <3mm in depth |
| IA2 | Measured stromal invasion ≥3mm and <5mm in depth |
| IB | Invasive carcinoma with measured deepest invasion ≥5 mm (greater than Stage IA), lesion limited to the cervix uterib |
| IB1 | Invasive carcinoma ≥ 5mm depth of stromal invasion, and < 2cm in greatest dimension |
| IB2 | Invasive carcinoma ≥ 2cm and < 4cm in greatest dimension |
| IB3 | Invasive carcinoma ≥ 4cm in greatest dimension |
| II | The carcinoma invades beyond the uterus, but has not extended onto the lower third of the vagina or to the pelvic wall |
| IIA | Involvement limited to the upper two-thirds of the vagina without parametrial involvement |
| IIA1 | Invasive carcinoma < 4cm in greatest dimension |
| IIA2 | Invasive carcinoma ≥ 4cm in greatest dimension |
| IIB | With parametrial involvement but not to the pelvic wall |
| III | The carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/or causes hydronephrosis or nonfunctioning kidney and/or involves pelvic and/or para-aortic lymph nodesc |
| IIIA | The carcinoma involves the lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or nonfunctioning kidney (unless known to be due to another cause) |
| IIIC | Involvement of pelvic and/or para-aortic lymph nodes, irrespective of tumor size and extent (with r and p notations)c |
| IIIC1 | Pelvic lymph node metastasis only |
| IIIC2 | Para-aortic lymph node metastasis |
| IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. (A bullous edema, as such, does not permit a case to be allotted to Stage IV) |
| IVA | Spread to adjacent pelvic organs |
| IVB | Spread to distant organs |
Notes: Reproduced from Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.7 When in doubt, the lower staging should be assigned. aImaging and pathology can be used, where available, to supplement clinical findings with respect to tumor size and extent, in all stages. bThe involvement of vascular/lymphatic spaces dose not change the staging. The lateral extent of the lesion is no longer considered. cAdding notations of r (imaging) and p (pathology) to indicate the findings that are used to allocate the case to stage IIIC. Example: If imaging indicates pelvic lymph node metastasis, the stage allocation would be Stage IIIC1r, and if confirmed by pathologic findings, it would be Stage IIIC1p. The type of imaging modality of pathology technique used should always be documented.
Evaluation of the FIGO Stage
For the 2014 FIGO staging system, the patient’s stage was mainly based on gynecologic examination by two experienced gynecological oncologists.2
For the 2018 FIGO staging system, except for the gynecologic examinations, the information from imaging examinations was also taken into consideration. If positive regional lymph nodes, including pelvic and para-aortic lymph nodes were identified from the imaging examinations, the patient would be grouped into stage IIICr. Table 17 shows the detailed information of the 2018 FIGO staging system.
Radiotherapy
Definitive chemoradiotherapy or radiotherapy was performed for enrolled patients in our study. As described in our previous study,5,11 the clinical target volume (CTV) included the primary tumor, cervix, uterus, parametrium, part of the vagina (depending on the extent of the primary tumor) and pelvic lymph node region (including common iliac, internal iliac, external iliac, obturator and presacral lymph nodes). In patients with stage IIIC2 and positive common iliac lymph nodes, the para-aortic lymphatic drainage area also contributed to the CTV. The planning clinical target volume (PCTV) was defined as the CTV plus a 7–10 mm margin. At least 95% of the PCTV received a dose of 50.4 Gy in 28 fractions. Positive lymph nodes were defined as the gross tumor volume (GTVnd), and margins of 5 mm were added to GTVnd to form the planning gross tumor volume (PGTVnd). A dose of 60.2 Gy in 28 fractions was prescribed to at least 95% of the PGTVnd with simultaneous integrated boost (SIB).
All patients received intracavity brachytherapy with 192Ir radioactive source. A dose of 30 Gy in 5 fractions was delivered to point A in patients receiving 2D brachytherapy. For patients who underwent 3D brachytherapy, at least 90% of the high-risk CTV (HRCTV) received a dose of 30 Gy in 5 fractions.
Chemotherapy
Cisplatin (30–40 mg/m2) was used as the first line concurrent chemotherapy regimen. No patients received neoadjuvant or adjuvant chemotherapy in our study.
Methodology and Statistical Analyses
Disease free survival (DFS) was chosen as the end point of our study. DFS was defined as the time from the date of the start of treatment to the date of disease progression (local recurrence or distant metastasis) or last follow-up. The Kaplan-Meier method was used to calculate DFS.
Each case in our study group was evaluated with both the 2014 and 2018 FIGO staging systems. The Log rank test was used to examine the significant difference in survival curves between the different stages. Receiver operative characteristic (ROC) curves for DFS in patients with cervical cancer according to different FIGO staging systems were created. The values of area under the curve (AUC) were calculated and compared between the two different FIGO staging systems.
All statistical analyses were performed with SPSS 23.0. A two-sided P value of <0.05 was defined as statistically significant.
Results
Patients and Tumor Characteristics
Between January 2008 and December 2014, a total of 1433 patients with histologically confirmed cervical cancer were treated with radiotherapy at our institute. After restaging with the 2018 FIGO staging system, five hundred and eighty-six patients were grouped as FIGO stage III. The number of patients by stage in the 2018 FIGO staging system were as follows: IIIA, 31 (5.3%); IIIB, 142 (24.2%); IIIC1, 325 (55.5%); and IIIC2, 88 (15.0%). Squamous cell carcinoma was the major histological pattern (519/586, 88.6%). Most of the patients received concurrent chemoradiotherapy (483/586, 82.4%). Detailed information on the patients and tumor characteristics is shown in Table 2. The 586 eligible patients were also evaluated with the 2014 FIGO staging system. Thirty-three (5.5%), 25 (4.3%), 254 (43.3%), 46 (7.8%) and 229 (39.1%) patients were considered as FIGO stage (2014) IB2, IIA, IIB, IIIA and IIIB, respectively (Table 3).
Table 2.
Characteristics of Patients with FIGO Stage III (2018)
| Characteristics | No. (100%) |
|---|---|
| Total | 586 |
| Age (years old) | |
| Median: 50 | |
| <30 | 9 (1.5%) |
| 30–39 | 43 (7.3%) |
| 40–49 | 223 (38.1%) |
| 50–59 | 210 (35.8%) |
| 60–69 | 80 (13.7%) |
| ≥70 | 21 (3.6%) |
| FIGO stage | |
| IIIA | 31 (5.3%) |
| IIIB | 142 (24.2%) |
| IIIC1 | 325 (55.5%) |
| IIIC2 | 88 (15.0%) |
| Histology | |
| Scc | 519 (88.6%) |
| non-Scc | 54 (9.2%) |
| unclear | 13 (2.2%) |
| Tumor size | |
| <4cm | 135 (23.1%) |
| ≥4cm | 432 (73.7%) |
| unclear | 19 (3.2%) |
| CCT | |
| yes | 483 (82.4%) |
| No | 103 (17.6%) |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; Scc, squamous cell carcinoma; CCT, concurrent chemotherapy.
Table 3.
The Number of Patients Regarding Different FIGO Stage
| Stage | FIGO2014 System | FIGO2018 System |
|---|---|---|
| Total | 586 | 586 |
| IB2 | 32 (5.5%) | n/a |
| IIA | 25 (4.3%) | n/a |
| IIB | 254 (43.3%) | n/a |
| IIIA | 46 (7.8%) | 31 (5.3%) |
| IIIB | 229 (39.1%) | 142 (24.2%) |
| IIIC1 | n/a | 325 (55.5%) |
| IIIC2 | n/a | 88 (15.0%) |
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Comparation of the Two FIGO Staging Systems
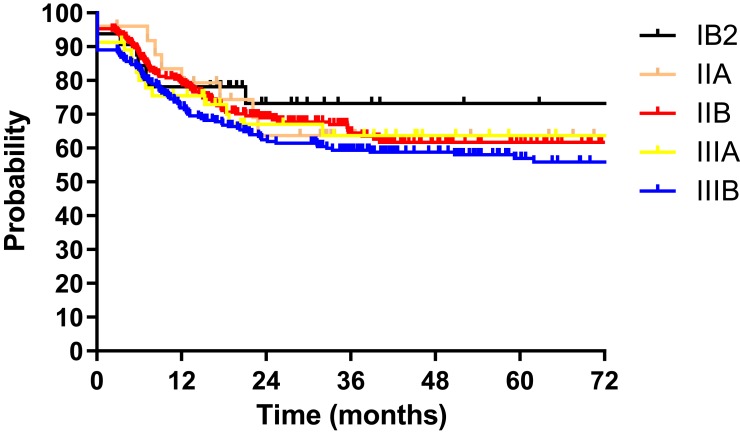
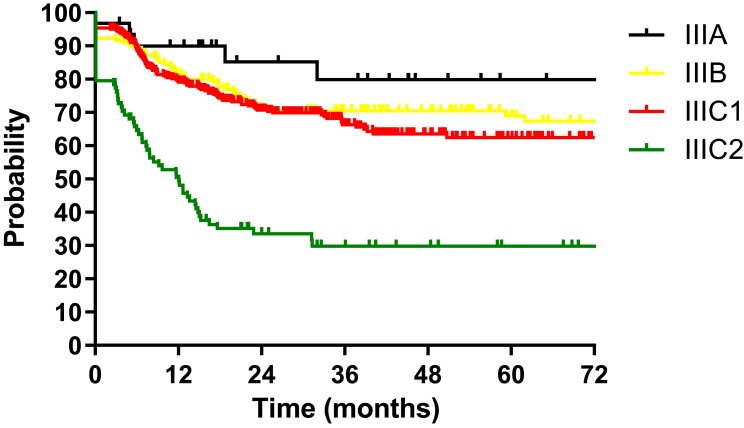
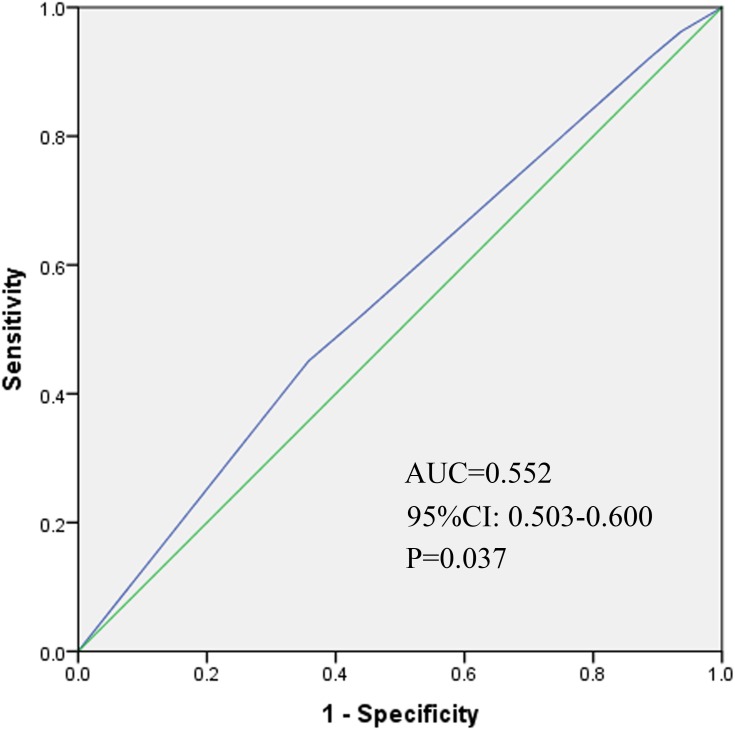
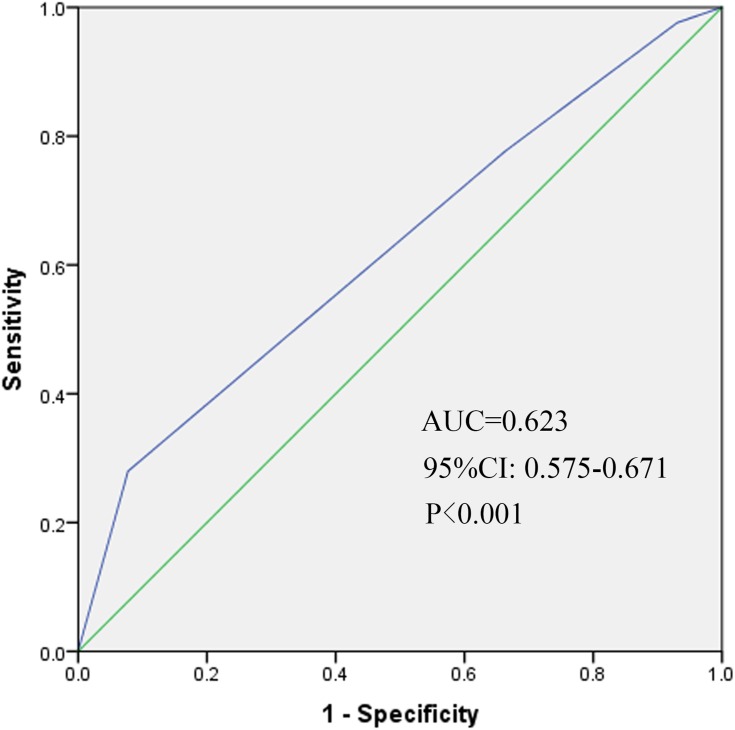
The median follow-up duration of all patients was 28.5 months (range: 1.9–124.9 months). The 3-year DFS for patients according to FIGO stage (2014) were as follows: IB2 73.2%, IIA 63.7%, IIB 66.7%, IIIA 64.7%, and IIIB 59.6% (P=0.580, Figure 1). The 3-year DFS according to FIGO stage (2018) were IIIA 79.9%, IIIB 70.4%, IIIC1 66.3% and IIIC2 29.8% (P<0.001, Figure 2). We also depicted ROC curves of predictive accuracy for DFS in the two different FIGO staging systems (Figures 3 and 4). The AUC values for DFS were 0.552 (95% CI: 0.503–0.600, P=0.037) and 0.623 (95% CI: 0.575–0.671, P<0.001) for the 2014 and 2018 FIGO staging systems, respectively.
Figure 1.
Disease free survival (DFS) for 586 patients with cervical cancer regarding FIGO stage (2014).
Figure 2.
Disease free survival (DFS) for 586 patients with cervical cancer regarding FIGO stage (2018).
Figure 3.
Receiver operative characteristic (ROC) curve of the predictive accuracy for DFS of patients by FIGO stage (2014) AUC = 0.552 (95% CI: 0.503–0.600, P=0.037).
Figure 4.
Receiver operative characteristic (ROC) curve of the predictive accuracy for DFS of patients by FIGO stage (2018) AUC = 0.623 (95% CI: 0.575–0.671, P<0.001).
Discussion
The purpose of this study was to validate the 2018 revised FIGO staging system of cervical cancer for stage III patients with a cohort from China. The DFS for patients according to both the 2014 and 2018 FIGO staging systems were calculated and compared between the different stages with the Log rank test. Significant differences were found between stages in the 2018 FIGO staging system (P<0.001, Figure 2). However, the 2014 FIGO staging system did not show distinctive survival differences between groups (P=0.580, Figure 1). To further compare the predictive accuracy of DFS in the two staging systems, ROC curves were created (Figures 3 and 4). The AUC values for the 2014 and 2018 FIGO staging systems were 0.552 (95% CI: 0.503–0.600, P=0.037) and 0.623 (95% CI: 0.575–0.671, P<0.001), respectively. Compared with the preceding staging system, the 2018 FIGO staging system showed more distinction within groups and better predictive accuracy for DFS.
Except for the ability of distinction, an effective cancer staging system should also be monotonic.12 This means that the survival curves of different stages should not cross each other. In the 2014 FIGO staging system, the survival curves were not well separated, and crossed over each other several times (Figure 1). With the new revised staging system, few crossings were seen among the survival curves.
Another characteristic of an effective staging system is homogenous within groups, which means that patients within the same stage should have little variation in prognosis.12 Previous studies have identified significant relationships among the characteristics of primary tumor, positive lymph nodes and survival outcomes.4,13,14 In the study of JCOG0806-A,13 patients with a tumor size ≤ 2 cm had more favorable 5-year OS than those with tumor size > 2 cm (95.8% vs 91.9%, P<0.05). Our previous study also identified tumor size as a significant factor for OS, DFS and LC.4 In addition to tumor size, the characteristics of positive lymph nodes are also an important factor for survival. A study from China showed significant relationships between number of positive LN, LN-volume, LN-diameter and survival outcomes.14 However, stage IIIC in the 2018 FIGO staging system does not take the characteristics of primary tumor and positive lymph nodes into consideration. Patients with stage IIIC might have great heterogeneity, which might be a limitation of the 2018 FIGO staging system.
Matsuo K and colleagues have already validated this new staging system with data from the SEER database.8 However, this validation could not represent the real world of cervical cancer since the SEER database usually records data from developed countries, and cervical cancer mainly occurs in developing countries. China is the largest developing country, contributing approximately 20% of all new cervical cancer cases in the world each year.1,9 Without Chinese patients, validation is unconvincing. From this perspective, our study is valuable and can further reinforce previous evidence.
Our study successfully validated the 2018 revised FIGO staging system of cervical cancer for stage III patients with a cohort from China. However, there are still several limitations. First, this was a retrospective study, and all study cases were from a single institution. Moreover, due to a lack of clinical records of patients with cervical cancer who underwent surgery, patients with stage IIIC who received surgery were not included in our study. The number of patients with stage IIIC may be lower than the actual situation.
Conclusion
The 2018 FIGO staging system of cervical cancer showed more distinction within groups and better predictive accuracy for DFS than the preceding staging system in patients with stage III cervical cancer from China.
Acknowledgment
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (grant number 2016YFC0105207).
Ethics Statement
The study was approved by Peking Union Medical College Hospital Ethics Committee. The study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrolment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [published Online First: Epub Date]|. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Oncology FCoG. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri, Int J Gynaecol Obstet. 2014;125(2):97–98. [published Online First: Epub Date]|. doi: 10.1016/j.ijgo.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 3.Narayan K, Fisher RJ, Bernshaw D, Shakher R, Hicks RJ. Patterns of failure and prognostic factor analyses in locally advanced cervical cancer patients staged by positron emission tomography and treated with curative intent. Int J Gynecol Cancer. 2009;19(5):912–918. [published Online First: Epub Date]|. doi: 10.1111/IGC.0b013e3181a58d3f [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Meng Q, Hou X, et al. Efficacy and toxicity of image-guided intensity-modulated radiation therapy combined with dose-escalated brachytherapy for stage IIB cervical cancer. Oncotarget. 2017;8(61):102965–102973. [published Online First: Epub Date]|. doi: 10.18632/oncotarget.22434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Zhang F, Hu K, Hou X. Image-guided, intensity-modulated radiation therapy in definitive radiotherapy for 1433 patients with cervical cancer. Gynecol Oncol. 2018;151:444–448. [published Online First: Epub Date]|. doi: 10.1016/j.ygyno.2018.09.024 [DOI] [PubMed] [Google Scholar]
- 6.Singh AK, Grigsby PW, Dehdashti F, Herzog TJ, Siegel BA. FDG-PET lymph node staging and survival of patients with FIGO stage IIIb cervical carcinoma. Int J Radiat Oncol Biol Phy. 2003;56(2):489–493. [published Online First: Epub Date]|. doi: 10.1016/s0360-3016(02)04521-2 [DOI] [PubMed] [Google Scholar]
- 7.Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. [published Online First: Epub Date]|. doi: 10.1002/ijgo.12611 [DOI] [PubMed] [Google Scholar]
- 8.Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2018. [published Online First: Epub Date]|. doi: 10.1016/j.ygyno.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. [published Online First: Epub Date]|. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Liu X, Meng Q, Zhang F, Hu K. Nomogram for predicting para-aortic lymph node metastases in patients with cervical cancer. Arch Gynecol Obstet. 2018;298(2):381–388. [published Online First: Epub Date]|. doi: 10.1007/s00404-018-4829-y [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Wang W, Meng Q, Zhang F, Hu K. Extended-field intensity-modulated radiation therapy combined with concurrent chemotherapy for cervical cancer with para-aortic lymph nodes metastasis. Jpn J Clin Oncol. 2018;49(3):263–269. [published Online First: Epub Date]|. doi: 10.1093/jjco/hyy184 [DOI] [PubMed] [Google Scholar]
- 12.Fibla JJ, Cassivi SD, Decker PA, et al. Validation of the lung cancer staging system revisions using a large prospective clinical trial database (ACOSOG Z0030). Eur J Cardiothorac Surg. 2013;43(5):911–914. [published Online First: Epub Date]|. doi: 10.1093/ejcts/ezs520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato T, Takashima A, Kasamatsu T, et al. Clinical tumor diameter and prognosis of patients with FIGO stage IB1 cervical cancer (JCOG0806-A). Gynecol Oncol. 2015;137(1):34–39. [published Online First: Epub Date]|. doi: 10.1016/j.ygyno.2015.01.548 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Wei LC, Zhang Y, et al. The prognosis and risk stratification based on pelvic lymph node characteristics in patients with locally advanced cervical squamous cell carcinoma treated with concurrent chemoradiotherapy. Int J Gynecol Cancer. 2016;26(8):1472–1479. [published Online First: Epub Date]|. doi: 10.1097/IGC.0000000000000778 [DOI] [PubMed] [Google Scholar]