Abstract
Background
Platinum‐based chemotherapy is the standard of care as first‐line treatment for recurrent or metastatic nasopharyngeal carcinoma (RM‐NPC); however, the prognosis of patients with RM‐NPC remains poor. The aim of this study was to evaluate the role of anti‐epidermal growth factor receptor (anti‐EGFR) antibody plus chemotherapy for RM‐NPC.
Methods
RM‐NPC patients who received first‐line chemotherapy plus an anti‐EGFR antibody were recruited from Sun Yat‐Sen University Cancer Center between July 2007 and November 2017. Survival analyses were performed using the Kaplan‐Meier method with a log‐rank test. A Cox proportional hazards model was used for the multivariate analyses.
Results
A total of 203 patients were enrolled in the present study. The median follow‐up time was 34.3 months (interquartile range: 19.7‐66.5 months). The median progression‐free survival (PFS) was 8.9 months (95% CI: 7.7‐10.0 months) and the median overall survival (OS) was 29.1 months (95% CI: 23.5‐34.6 months). The 1‐, 3‐, and 5‐year PFS and OS rates were 35.5% and 79.6%, 15.2% and 42.5%, and 11.6% and 23.6%, respectively. The objective response rate (ORR) was 67.5% and the disease control rate (DCR) was 91.1%. The multivariate analysis identified the following prognostic factors for PFS: anti‐EGFR agent (P = .010), recurrence/metastasis sequence (P = .016), KPS (P = .017), and combined chemotherapy regimen (P = .015). Independent risk factors for OS included age >43 years (P = .002), Karnofsky performance score ≤80 (P < .001), and higher level of baseline Epstein‐Barr virus (EBV) DNA (P = .008). Leukopenia was the most common adverse event (AE) in this cohort (any grade, 84.2%; grades 3‐4, 43.4%).
Conclusions
Anti‐EGFR antibody plus chemotherapy achieved promising antitumor activity with a tolerable toxicity profile in RM‐NPC. Thus, randomized clinical trials are warranted to compare the efficacy of chemotherapy with or without anti‐EGFR antibody in these patients.
Keywords: anti‐epidermal growth factor receptor, first‐line treatment, monoclonal antibody, palliative chemotherapy, recurrent or metastatic nasopharyngeal carcinoma
Anti‐EGFR monoclonal antibody plus chemotherapy achieved promising response rate, progression‐free survival, and overall survival with a tolerable toxicity profile for recurrent or metastatic nasopharyngeal carcinoma.
1. INTRODUCTION
Nasopharyngeal carcinoma (NPC) is an endemic tumor in the eastern and southeastern regions of Asia. Radiotherapy with or without concurrent chemotherapy is the standard of care for patients with early or locally advanced stage disease. With the development of radiation techniques and systemic treatment, the overall survival (OS) of NPC has improved in recent decades.1 However, most patients eventually develop locoregional recurrence and/or distant metastasis.2, 3, 4 For those with recurrence or metastatic NPC (RM‐NPC), the prognosis remains extremely poor, with a median OS ranging from 15 to 29 months.5 Therefore, new systemic treatment strategies are urgently required to optimize clinical outcomes.
Epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein and a member of the erbB family of tyrosine kinase receptors. In addition, EGFR is involved in the regulation of cellular proliferation, differentiation, and survival. Thus, EGFR represents one of the most attractive targets for cancer therapy due to the fact that many solid tumors, including NPC, display EGFR overexpression.6, 7 In particular, EGFR overexpression is observed in more than 90% of NPC and is associated with a poor prognosis.8, 9 Moreover, anti‐EGFR monoclonal antibodies (mAbs) were found to inhibit the activation of EGFR downstream signaling pathways by blocking its extracellular association with its ligands.10 Therefore, anti‐EGFR mAbs are considered to be a promising agent for NPC.
Cetuximab (CTX) and nimotuzumab (NTZ) are two major anti‐EGFR mAbs that have frequently been used for the treatment of NPC. Several studies have indicated that treatment with CTX or NTZ enhances the efficacy of chemoradiotherapy for locoregionally advanced NPC.11, 12, 13, 14, 15 However, the antitumor activity of anti‐EGFR mAbs for RM‐NPC has rarely been reported.16, 17 The aim of the present study was to evaluate the antitumor efficacy and safety of anti‐EGFR mAbs (CTX or NTZ) plus palliative chemotherapy as a first‐line treatment for RM‐NPC.
2. MATERIALS AND METHODS
2.1. Study population
We recruited consecutive patients from Sun Yat‐Sen University Cancer Center between July 2007 and November 2017, who met the following criteria: (a) histologically confirmed NPC; (b) metastatic or recurrent disease following primary standard treatment, or primarily metastasis; (c) no history of previous systemic chemotherapy for recurrent or metastatic disease; (d) received palliative chemotherapy plus anti‐EGFR mAbs; (e) received at least one cycle of anti‐EGFR mAbs; (f) ability to be evaluated with complete clinical data; and (g) received study treatment outside of anti‐EGFR therapy clinical trials as indicated by the medical records. Figure 1 illustrates the process of patient selection.
Figure 1.
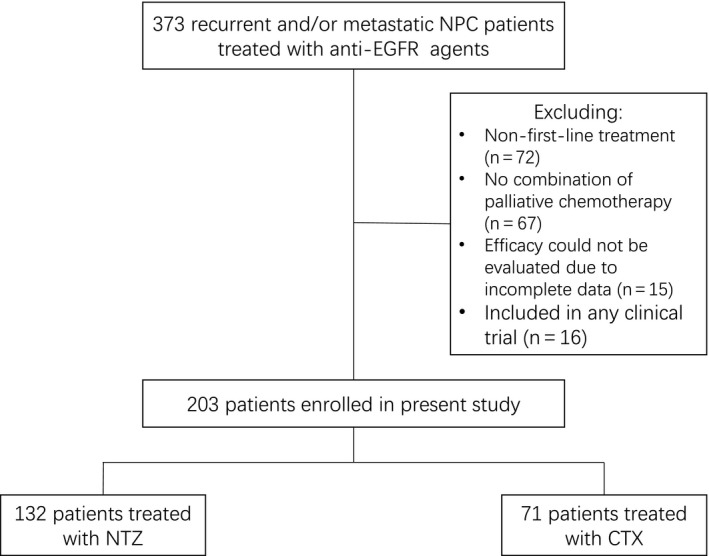
Diagram illustrating the patient selection process. NPC, nasopharyngeal carcinoma; EGFR, anti‐epidermal growth factor receptor; NTZ, nimotuzumab; CTX, cetuximab
The institutional review board of Sun Yat‐sen University Cancer Center approved this retrospective study and waived the need for informed consent (written or verbal).
2.2. Data collection
The collected data contained: (a) gender, age, smoking status, Karnofsky performance score (KPS),18 and Epstein‐Barr virus (EBV) DNA count before the administration of anti‐EGFR agents; (b) pathological histology, recurrence/metastasis sequence (synchronous or metachronous with respect to the primary diagnosis of NPC); (c) type of anti‐EGFR agent (NTZ or CTX) combined chemotherapy regimens; (d) imaging information for the evaluation of treatment efficacy; and (e) survival status and time point.
The plasma EBV DNA concentration prior to treatment with the anti‐EGFR agent was measured using a real‐time quantitative polymerase chain reaction as previously described by our institution.19 According to the EBV DNA concentration, four groups were defined by magnitudes of 10 and another group was established for unknown levels. All adverse events (AEs) were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0.
2.3. Treatment
All patients received palliative chemotherapy plus an anti‐EGFR agent as first‐line treatment. Palliative chemotherapy included four common regimens: (a) taxane plus cisplatin/nedaplatin/carboplatin and fluorouracil (TPF); (b) taxane plus cisplatin/nedaplatin/carboplatin (TP); (c) fluorouracil plus cisplatin/nedaplatin/carboplatin (PF); and (d) gemcitabine plus cisplatin/nedaplatin/carboplatin (GP). Anti‐EGFR agents included the intravenous administration of NTZ or CTX prior to chemotherapy. Given the retrospective nature of this study, the treatment regimens were directly extracted from the electronic patient records. Treatment decisions were made according to the discretion of the treating physicians and the patients' desire, which were considered to be based on factors including, but not limited to, the patient's economic situation, complications, the patient's physical condition, and the doctor's preference. The dosage, administration, and modification of these drugs were determined according to the locally approved formulary information of the treating physicians.
2.4. Endpoints and statistical analysis
The primary endpoint was progression‐free survival (PFS), which was defined as the time from the initiation of first‐line therapy to the date of disease progression or death from any cause, whichever came first. Secondary endpoints included OS and the tumor response. OS was defined as the time from the beginning of first‐line therapy to the date of death due to any cause. The tumor response was assessed by regular imaging per RECIST version 1.1, which consisted of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). All medical imaging was independently reviewed by the first and second authors of this study. In case of any discrepancies, the final decision was made by a full discussion involving the corresponding author(s). The objective response rate (ORR) was defined as the proportion of patients achieving a complete or partial response, and disease control was defined as the proportion of CR + PR + SD. To explore the prognostic factors, interventions, including anti‐EGFR mAbs and chemotherapy regimens, were included in the multivariate analysis as important confounding factors together with baseline characteristics.
A Chi‐square test was used to distinguish the distributional differences of the categorical variables. The Kaplan‐Meier method with a log‐rank test was used to calculate and compare the cumulative survival rates. A Cox proportional hazards model was used for the multivariate analysis and to estimate the hazard ratio (HR) with 95% confidence intervals (CIs). Statistical analyses were performed using SPSS version 21.0 (Chicago, IL, USA). A threshold two‐sided P value of less than 0.05 was considered significant.
3. RESULTS
3.1. Patient demographic characteristics
A total of 373 RM‐NPC patients treated with anti‐EGFR agents were screened and 203 patients were finally included in this study. The baseline characteristics of the total patients are listed in Table 1 and the baseline characteristics of the patients in each of the different chemotherapy regimens are listed in Table S1. The median age was 43 years (range: 12‐72 years). The primary pathological histology consisted of undifferentiated non‐keratinized carcinoma (n = 187, 92.1%). Other types of pathological histology consisted of non‐keratosis (n = 3, 1.5%), differentiated non‐keratosis (n = 6, 3.0%), squamous carcinoma (n = 3, 1.5%), and unknown type (n = 4, 2.0%). A total of 100 (49.3%) patients were initially diagnosed with distant metastases (synchronous metastasis), and 103 (50.7%) patients experienced recurrence or metastasis secondary to the initial treatment (metachronous metastasis). A total of 132 (65.0%) patients received NTZ, and 71 (35.0%) patients received CTX. More patients received TP (n = 84, 41.4%) as a combined chemotherapy regimen.
Table 1.
The baseline characteristics of patients
| Characters | Patients (%) |
|---|---|
| Gender | |
| Male | 168 (82.8) |
| Female | 35 (17.2) |
| Age | |
| ≤43 y | 98 (48.3) |
| >43 y | 105 (51.7) |
| Smoke | |
| Yes | 65 (32.0) |
| No | 138 (68.0) |
| Anti‐EGFR agent | |
| Nimotuzumab | 132 (65.0) |
| Cetuximab | 71 (35.0) |
| Pathological histology | |
| Undifferentiated non‐keratosis | 187 (92.0) |
| Others† | 16 (8.0) |
| Recurrence/Metastasis sequence | |
| Synchronous | 100 (49.3) |
| Metachronous | 103 (50.7) |
| Karnofsky Performance Score (KPS) | |
| >80 | 173 (85.2) |
| ≤80 | 30 (14.8) |
| Baseline Epstein‐Barr virus DNA level (copies/mL) | |
| <10E3 | 26 (12.8) |
| ≥10E3 and < 10E4 | 32 (15.8) |
| ≥10E4 and < 10E5 | 57 (28.1) |
| ≥10E5 | 67 (33.0) |
| Unknown | 21 (10.3) |
| Combined chemotherapy regimen | |
| TPF | 47 (23.2) |
| TP | 84 (41.4) |
| PF | 24 (11.8) |
| GP | 37 (18.2) |
| Others‡ | 11 (5.4) |
Abbreviations: EGRF, epidermal growth factor receptor; TPF, taxane plus cisplatin/nedaplatin/carboplatin and fluorouracil; TP, taxane plus cisplatin/nedaplatin/carboplatin; PF, fluorouracil plus cisplatin/nedaplatin/carboplatin; GP, gemcitabine plus cisplatin/nedaplatin/carboplatin.
Other pathological histology types contained non‐keratosis, differentiated non‐keratosis, squamous carcinoma, and unknown type.
Other chemotherapy regimens included pemetrexed + cisplatin/nedaplatin, pemetrexed + gemcitabine, gemcitabine + capecitabine/S‐1, gemcitabine + oxaliplatin, and gemcitabine + vincristine.
3.2. Survival analysis and tumor response
The cutoff date for the data was 31 October 2018. The median follow‐up time was 34.3 months (interquartile range: 19.7‐66.5 months). During the follow‐up period, 145 (71.4%) patients displayed progressive disease and 115 (56.7%) patients died after undergoing first‐line palliative treatment (survival curves are shown in Figure 2). The 1‐, 3‐, and 5‐year PFS rates were 35.5%, 15.2%, and 11.6%, respectively. In contrast, the 1‐, 3‐, and 5‐year OS rates were 79.6%, 42.5%, and 23.6%, respectively.
Figure 2.
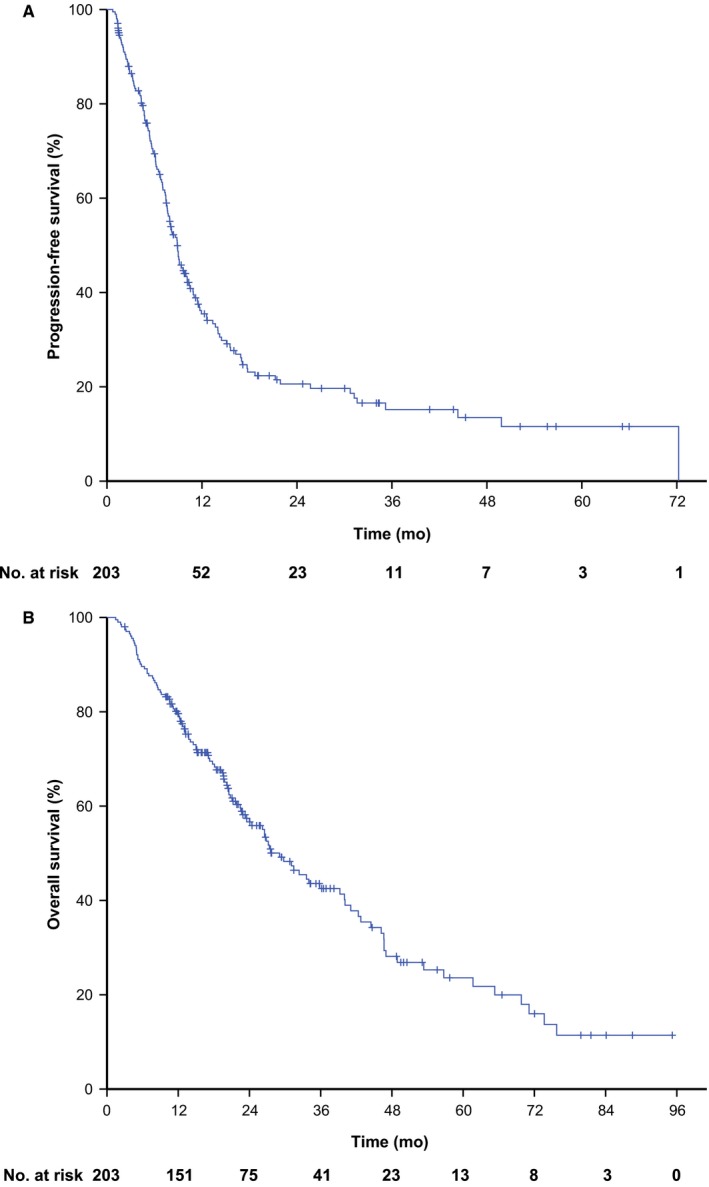
Survival curves of recurrent or metastatic nasopharyngeal carcinoma treated with anti‐epidermal growth factor receptor monoclonal antibody plus palliative chemotherapy as first‐line therapy. A, progression‐free survival curve; B, overall survival curve
The median PFS was 8.9 months (95% CI: 7.7‐10.0 months) and the median OS was 29.1 months (95% CI: 23.5‐34.6 months). Specifically, the median PFS and OS of the patients with different characteristic are listed in Table 2. Moreover, patients with a higher level of EBV DNA exhibited a longer median survival time. Among the 203 patients, eight (3.9%) achieved CR, 129 (63.6%) had PR, 48 (23.6%) had SD, and 18 (8.9%) had PD as the best response, respectively. The ORR was 67.5%, and the disease control rate (DCR) was 91.1%.
Table 2.
Univariate and multivariate analyses of progression‐free survival and overall survival
| characters | Progression‐free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Median months (95% CI) | P‐uni | P‐multi | HR (95% CI) | Median months (95% CI) | P‐uni | P‐multi | HR (95% CI) | |
| Gender | ||||||||
| Male | 8.9 (7.7‐10.2) | .80 | .81 | 1 | 29.8 (23.8‐35.8) | .74 | .21 | 1 |
| Female | 8.9 (6.7‐11.1) | 1.058 (0.663‐1.689) | 26.5 (6.8‐46.2) | 1.384 (0.838‐2.285) | ||||
| Age | ||||||||
| ≤43 y | 10.0 (8.4‐11.5) | .071 | .060 | 1 | 34.0 (20.6‐47.4) | .096 | .002 | 1 |
| >43 y | 8.0 (6.8‐9.2) | 1.398 (0.986‐1.984) | 27.2 (19.2‐35.2) | 1.861 (1.248‐2.775) | ||||
| Smoke | ||||||||
| Yes | 8.6 (7.2‐10.1) | .61 | NA | NA | 29.1 (19.5‐38.7) | .62 | NA | NA |
| No | 9.0 (7.3‐10.7) | 27.4 (19.4‐35.3) | ||||||
| Anti‐EGFR agent | ||||||||
| NTZ | 7.9 (6.1‐9.7) | .054 | .010 | 1 | 27.2 (18.6‐35.7) | .21 | .15 | 1 |
| CTX | 9.7 (7.7‐11.7) | 0.623 (0.435‐0.891) | 32.4 (19.6‐45.1) | 0.736 (0.485‐1.118) | ||||
| Pathological histology | ||||||||
| Undifferentiated non‐keratosis | 8.9 (7.8‐9.9) | .62 | NA | NA | 27.6 (21.8‐33.3) | .51 | NA | NA |
| Others† | 9.7 (2.0‐17.4) | 34.8 (0.0‐114.8) | ||||||
| Recurrence/Metastasis sequence | ||||||||
| Synchronous | 10.0 (8.2‐11.7) | .039 | .016 | 1 | 31.1 (23.3‐38.9) | .33 | NA | NA |
| Metachronous | 7.9 (6.8‐9.0) | 1.629 (1.094‐2.424) | 26.8 (17.7‐36.0) | |||||
| Karnofsky Performance Score (KPS) | ||||||||
| >80 | 9.1 (7.7‐10.4) | .007 | .017 | 1 | 33.6 (22.4‐44.8) | <.001 | <.001 | 1 |
| ≤80 | 5.4 (3.0‐7.7) | 1.803 (1.114‐2.919) | 11.8 (1.2‐22.4) | 2.749 (1.682‐4.496) | ||||
| Baseline Epstein‐Barr virus DNA level (copies/mL) | ||||||||
| <10E3 | 15.5 (6.7‐24.4) | .051 | .26 | 1 | 61.7 (32.3‐91.0) | .009 | .008 | 1 |
| ≥10E3 and <10E4 | 10.9 (7.9‐13.8) | 1.420 (0.723‐2.790) | 46.7 (37.5‐55.8) | 1.715 (0.739‐3.979) | ||||
| ≥10E4 and <10E5 | 8.2 (6.6‐9.9) | 1.709 (0.917‐3.184) | 26.5 (19.9‐33.2) | 2.285 (1.098‐4.756) | ||||
| ≥10E5 | 7.3 (5.5‐9.1) | 1.919 (1.041‐3.536) | 20.5 (16.8‐24.3) | 3.445 (1.684‐7.047) | ||||
| Unknown | 12.6 (0.0‐29.3) | 1.244 (0.589‐2.629) | 26.2 (4.7‐47.7) | 2.944 (1.329‐6.519) | ||||
| Combined chemotherapy regimen | ||||||||
| TPF | 9.7 (6.5‐12.9) | .082 | .015 | 1 | 40.0 (28.4‐51.6) | .16 | .082 | 1 |
| TP | 8.2 (6.4‐9.9) | 1.896 (1.212‐2.966) | 24.0 (18.3‐29.7) | 1.915 (1.145‐3.203) | ||||
| PF | 6.6 (5.8‐7.5) | 1.636 (0.866‐3.093) | 21.7 (8.9‐34.5) | 2.037 (1.100‐3.775) | ||||
| GP | 12.6 (7.1‐18.1) | 0.935 (0.511‐1.709) | 31.5 (22.5‐40.5) | 1.652 (0.860‐3.175) | ||||
| Others‡ | 7.7 (5.8‐9.5) | 1.542 (0.737‐3.226) | 48.9 (6.1‐91.7) | 0.987 (0.374‐2.605) | ||||
Abbreviations: NTZ, Nimotuzumab; CI, confidence interval; CTX, Cetuximab; GP, gemcitabine plus cisplatin/nedaplatin/carboplatin; HR, hazard ratio; PF, fluorouracil plus cisplatin/nedaplatin/carboplatin; P‐uni, P value for univariate analysis; P‐multi, P value for multivariate analysis; TP, taxane plus cisplatin/nedaplatin/carboplatin; TPF, taxane plus cisplatin/nedaplatin/carboplatin and fluorouracil.
Other pathological histology types contained non‐keratosis, differentiated non‐keratosis, squamous carcinoma, and unknown type.
Other chemotherapy regimens included pemetrexed + cisplatin/nedaplatin, pemetrexed + gemcitabine, gemcitabine + capecitabine/S‐1, gemcitabine + oxaliplatin, and gemcitabine + vincristine.
3.3. Prognostic analysis
Univariate and multivariate analyses of the PFS and OS are presented in Table 2. The univariate analysis revealed that recurrence/metastasis sequence and KPS had a significant effect on the PFS. The anti‐EGFR agent (P = .054) and baseline level of EBV DNA (P = .051) were associated with a potential effect. The multivariate analysis identified four independent prognostic factors for PFS, including the anti‐EGFR agent (P = .010), recurrence/metastasis sequence (P = .016), KPS (P = .017), and combined chemotherapy regimen (P = .015) (corresponding hazard ratios are listed in Table 2, cumulative hazard curves are shown in Figure 3 A‐D). Age (P = .060) was a potential prognostic factor.
Figure 3.
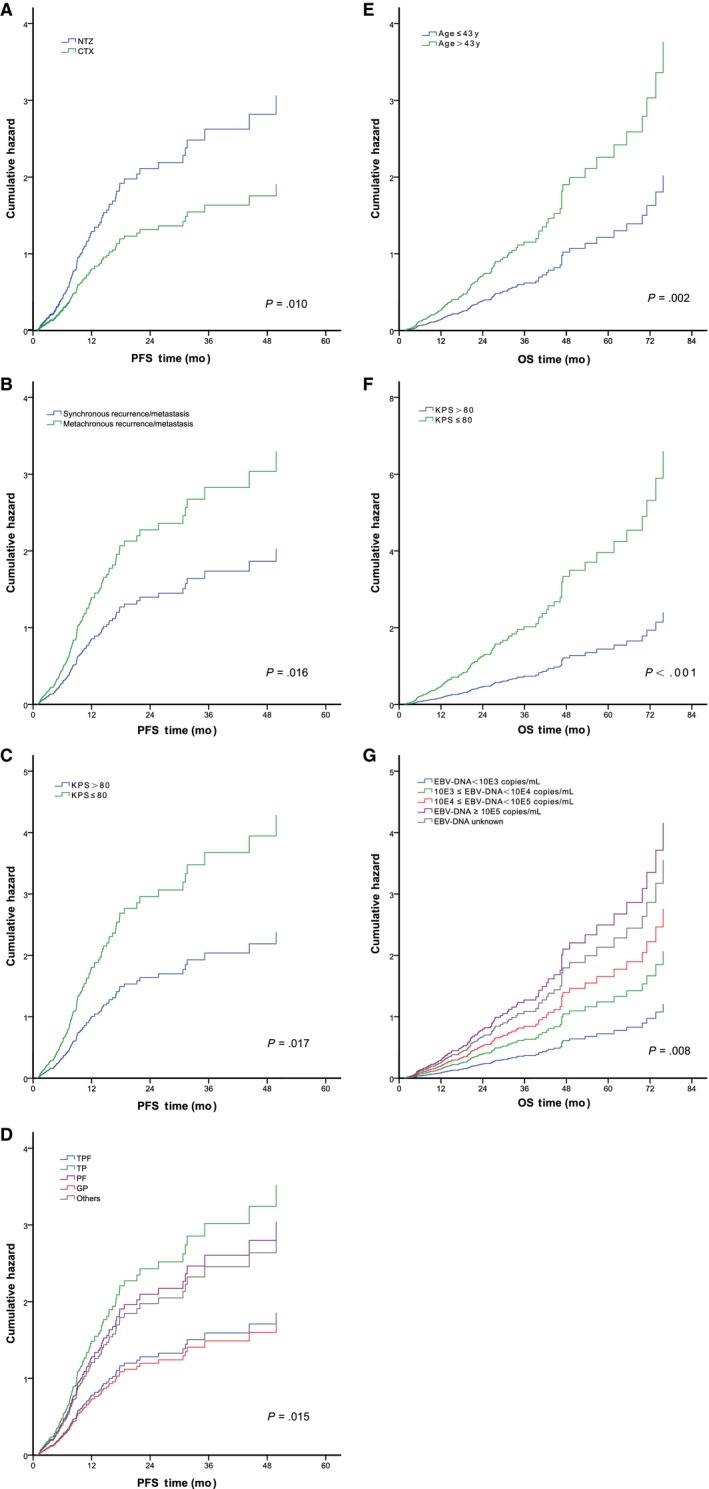
Cumulative hazard curves of the independent risk factors identified by multivariate analyses for progression‐free survival and overall survival, respectively. A, type of anti‐EGFR agent for PFS; B, recurrence/metastasis sequence for PFS; C, KPS for PFS; D, chemotherapy regimen for PFS; E, age for OS; F, KPS for OS; and G, EBV DNA level for OS. EGFR, anti‐epidermal growth factor receptor; PFS, progression‐free survival; OS, overall survival; KPS, Karnofsky performance score; EBV, Epstein‐Barr virus; NTZ, Nimotuzumab; CTX, Cetuximab; TPF, taxane plus cisplatin/nedaplatin/carboplatin and fluorouracil; TP, taxane plus cisplatin/nedaplatin/carboplatin; PF, fluorouracil plus cisplatin/nedaplatin/carboplatin; GP, gemcitabine plus cisplatin/nedaplatin/carboplatin; Other chemotherapy regimens included pemetrexed + cisplatin/nedaplatin, pemetrexed + gemcitabine, gemcitabine + capecitabine/S‐1, gemcitabine + oxaliplatin, and gemcitabine + vincristine
For the OS, the multivariate analysis confirmed that an older age (age > 43 years) (P = .002), poor KPS (KPS ≤ 80) (P < .001), and higher level of baseline EBV DNA (P = .008) were independent risk factors (Table 2; cumulative hazard curves are shown in Figure 3E‐G). A combined chemotherapy regimen was a potential prognostic factor (P = .082).
3.4. Toxicity analysis
Common treatment‐related AEs are summarized in Table 3. A total of 192 patients (94.6%) experienced at least one AE, among whom 121 patients were treated with NTZ (121/132, 91.7%) and 71 patients were treated with CTX (71/71, 100%). The most common AE was leukopenia (n = 171, 84.2%) followed by decreased appetite (n = 135, 66.5%) and nausea (n = 123, 60.6%). With the exception of severe hematologic toxicity, including grades 3‐4 leukopenia (n = 88, 43.4%) and thrombocytopenia (n = 23, 11.3%), other grades 3‐4 AEs were rare (occurrence rate < 5%). Compared with patients who received NTZ, the patients treated with CTX were more likely to suffer from mucosal inflammation (CTX vs NTZ: 31.0% vs 13.6%), weight loss (CTX vs NTZ: 42.3% vs 23.5%), rash (CTX vs NTZ: 33.8% vs 2.3%), fever (CTX vs NTZ: 40.9% vs 23.5%), ALT elevation (CTX vs NTZ: 53.5% vs 29.5%), and AST elevation (CTX vs NTZ: 45.1% vs 24.2%). The cases of grades 3‐4 toxicity were comparable between the patients treated with CTX and NTZ, except for rash (CTX vs NTZ: 4.2% vs 0.0%). For the chemotherapy regimens, patients treated with GP were more likely to have thrombocytopenia (75.7%), including grades 3‐4 thrombocytopenia (32.4%), compared to other regimens (detailed in Table S2).
Table 3.
Common treatment‐related adverse events
| Adverse events | No (%) | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) | Grade 3 + 4 (%) | All grade (%) |
|---|---|---|---|---|---|---|---|
| Leukopenia | |||||||
| Total | 32 (15.8) | 21 (10.3) | 62 (30.5) | 69 (34.0) | 19 (9.4) | 88 (43.4) | 171 (84.2) |
| NTZ group | 25 (18.9) | 13 (9.9) | 45 (34.1) | 38 (28.8) | 11 (8.3) | 49 (37.1) | 107 (81.1) |
| CTX group | 7 (9.8) | 8 (11.3) | 17 (23.9) | 31 (43.7) | 8 (11.3) | 39 (55.0) | 64 (90.2) |
| Thrombocytopenia | |||||||
| Total | 120 (59.1) | 29 (14.3) | 31 (15.3) | 13 (6.4) | 10 (4.9) | 23 (11.3) | 83 (40.9) |
| NTZ group | 80 (60.6) | 17 (12.9) | 19 (14.4) | 10 (7.6) | 6 (4.5) | 16 (12.1) | 52 (39.4) |
| CTX group | 40 (56.4) | 12 (16.9) | 12 (16.9) | 3 (4.2) | 4 (5.6) | 7 (9.8) | 31 (43.6) |
| Vomiting | |||||||
| Total | 124 (61.1) | 68 (33.5) | 9 (4.4) | 2 (1.0) | 0 (0.0) | 2 (1.0) | 79 (38.9) |
| NTZ group | 83 (62.9) | 45 (34.1) | 2 (1.5) | 2 (1.5) | 0 (0.0) | 2 (1.5) | 49 (37.1) |
| CTX group | 41 (57.7) | 23 (32.4) | 7 (9.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (42.3) |
| Nausea | |||||||
| Total | 80 (39.4) | 101 (49.7) | 20 (9.9) | 2 (1.0) | 0 (0.0) | 2 (1.0) | 123 (60.6) |
| NTZ group | 52 (39.4) | 65 (49.3) | 13 (9.8) | 2 (1.5) | 0 (0.0) | 2 (1.5) | 80 (60.6) |
| CTX group | 28 (39.4) | 36 (50.7) | 7 (9.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 43 (60.6) |
| Mucosal inflammation | |||||||
| Total | 163 (80.3) | 24 (11.8) | 14 (6.9) | 2 (1.0) | 0 (0.0) | 2 (1.0) | 40 (19.7) |
| NTZ group | 114 (86.4) | 11 (8.3) | 6 (4.5) | 1 (0.8) | 0 (0.0) | 1 (0.8) | 18 (13.6) |
| CTX group | 49 (69.0) | 13 (18.3) | 8 (11.3) | 1 (1.4) | 0 (0.0) | 1 (1.4) | 22 (31.0) |
| Decreased appetite | |||||||
| Total | 68 (33.5) | 120 (59.1) | 15 (7.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 135 (66.5) |
| NTZ group | 48 (36.3) | 74 (56.1) | 10 (7.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 84 (63.7) |
| CTX group | 20 (28.2) | 46 (64.8) | 5 (7.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 51 (71.8) |
| Diarrhea | |||||||
| Total | 175 (86.2) | 24 (11.8) | 2 (1.0) | 2 (1.0) | 0 (0.0) | 2 (1.0) | 28 (13.8) |
| NTZ group | 116 (87.8) | 14 (10.6) | 1 (0.8) | 1 (0.8) | 0 (0.0) | 1 (0.8) | 16 (12.2) |
| CTX group | 59 (83.1) | 10 (14.1) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 1 (1.4) | 12 (16.9) |
| Nephrotoxicity | |||||||
| Total | 163 (80.3) | 38 (18.7) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 40 (19.7) |
| NTZ group | 102 (77.3) | 29 (21.9) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (22.7) |
| CTX group | 61 (85.9) | 9 (12.7) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (14.1) |
| Hypotension | |||||||
| Total | 169 (83.3) | 34 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 34 (16.7) |
| NTZ group | 114 (86.4) | 18 (13.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 18 (13.6) |
| CTX group | 55 (77.5) | 16 (22.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 16 (22.5) |
| Weight loss | |||||||
| Total | 142 (70.0) | 45 (22.1) | 16 (7.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 61 (30.0) |
| NTZ group | 101 (76.5) | 23 (17.4) | 8 (6.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 31 (23.5) |
| CTX group | 41 (57.7) | 22 (31.0) | 8 (11.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (42.3) |
| Rash | |||||||
| Total | 176 (86.6) | 20 (9.9) | 4 (2.0) | 3 (1.5) | 0 (0.0) | 3 (1.5) | 27 (13.4) |
| NTZ group | 129 (97.7) | 3 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.3) |
| CTX group | 47 (66.2) | 17 (24.0) | 4 (5.6) | 3 (4.2) | 0 (0.0) | 3 (4.2) | 24 (33.8) |
| Fever | |||||||
| Total | 143 (70.4) | 48 (23.7) | 12 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 60 (29.6) |
| NTZ group | 101 (76.5) | 27 (20.5) | 4 (3.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 31 (23.5) |
| CTX group | 42 (59.1) | 21 (29.6) | 8 (11.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 29 (40.9) |
| ALT elevation | |||||||
| Total | 126 (62.1) | 62 (30.5) | 8 (3.9) | 7 (3.5) | 0 (0.0) | 7 (3.5) | 77 (37.9) |
| NTZ group | 93 (70.5) | 33 (25.0) | 2 (1.5) | 4 (3.0) | 0 (0.0) | 4 (3.0) | 39 (29.5) |
| CTX group | 33 (46.5) | 29 (40.8) | 6 (8.5) | 3 (4.2) | 0 (0.0) | 3 (4.2) | 38 (53.5) |
| AST elevation | |||||||
| Total | 139 (68.4) | 54 (26.6) | 5 (2.5) | 5 (2.5) | 0 (0.0) | 5 (2.5) | 64 (31.6) |
| NTZ group | 100 (75.8) | 25 (18.9) | 4 (3.0) | 3 (2.3) | 0 (0.0) | 3 (2.3) | 32 (24.2) |
| CTX group | 39 (54.9) | 29 (40.9) | 1 (1.4) | 2 (2.8) | 0 (0.0) | 2 (2.8) | 32 (45.1) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTX, cetuximab; NTZ, nimotuzumab.
4. DISCUSSION
Patients with RM‐NPC have very poor survival outcomes due to therapeutic resistance. Therefore, novel treatment strategies are required to optimize clinical outcomes. To our knowledge, this is the largest cohort study that has evaluated the antitumor activity of an anti‐EGFR mAb plus chemotherapy as a first‐line treatment for RM‐NPC. Our findings indicate that anti‐EGFR mAbs plus chemotherapy achieved a promising response rate, PFS, and OS for RM‐NPC.
The first head‐to‐head randomized phase III clinical trial conducted by our group20 established the standard first‐line chemotherapy regimen for RM‐NPC based on the fact that GP achieved a longer PFS than PF (median: 7.0 vs 5.6 months, respectively). The response rates for GP and PF were 64% and 42% (P < .001), respectively. While these findings were statistically significant, clinically mild improvements indicate that the efficacy of chemotherapy alone has reached a plateau. It has been recognized that patients with RM‐NPC are likely to harbor platinum‐resistant tumor clones, which may be partially due to the activation of the EGFR signaling pathway.21, 22 Therefore, it is rational that blocking the EGFR pathway could resensitize these tumor clones to chemotherapy and delay disease progression.6, 8, 9 Moreover, Chan et al17 conducted a phase II trial, which included 60 RM‐NPC patients who had been heavily treated with platinum‐based chemotherapy for recurrent or metastatic disease. These patients were treated with cetuximab plus carboplatin, which yielded a median PFS of 81 days, ORR of 11.7%, and DCR of 60%. Recently, Zhao et al16 conducted a single arm, phase II study to evaluate the role of NTZ plus PF as a first‐line treatment for 35 patients with RM‐NPC. The median PFS was 7.0 months, median OS was 16.3 months, and ORR was 71.4%. However, since both trials enrolled a very limited number of patients, there is limited confidence in the data interpretation. The efficacy of treatment with anti‐EGFR mAbs plus chemotherapy must be validated in a larger population. Therefore, we conducted this retrospective study with 203 RM‐NPC patients treated with either CTX or NTZ plus palliative chemotherapy as first‐line therapy. The median PFS was 8.9 months (95% CI: 7.7‐10.0 months) and the median OS was 29.1 months (95% CI: 23.5‐34.6 months). The ORR and DCR were 67.5% and 91.1%, respectively. The survival outcomes of the above mentioned studies and our study are summarized in Table 4.
Table 4.
Survival outcomes comparison between other previous studies and our present study
| Author | Year of case | Patients number | Arms/Cohort | Therapeutic regimen | PFS | OS | ORR | DCR |
|---|---|---|---|---|---|---|---|---|
| Zhang20 | 2012‐2015 | 362 | Arm 1 | GP | 7.0 mo | 29.1 mo | 64% | 90% |
| Arm 2 | PF | 5.6 mo | 20.9 mo | 42% | 86% | |||
| Chan17 | Published in 2005 | 60 | Single arm | CTX + Carboplatin | 81 d | NA | 11.2% | 61% |
| Zhao16 | 2012‐2015 | 35 | Single arm | NTZ + PF | 7.0 mo | 16.3 mo | 71.4% | 85.7% |
| Present study | 2007‐2017 | 203 | Whole cohort | CTX/NTZ + palliative chemotherapy | 8.9 mo | 29.1 mo | 67.5% | 91.1% |
Abbreviations: CTX, Cetuximab; DCR, disease control rate; GP, gemcitabine plus cisplatin; NA, not applicable; NTZ, Nimotuzumab; OS, overall survival; ORR, objective response rate; PF, fluorouracil plus cisplatin; PFS, progression‐free survival.
Using univariate and multivariate analyses, several prognostic factors were confirmed for the PFS and OS, respectively. In addition, NTZ treatment, metachronous metastasis, and poor KPS were found to be independent risk factors for PFS. CTX was associated with a longer PFS than those with NTZ, but not for OS, which may be due to the impact of post‐progression treatment. Therefore, head‐to‐head randomized studies are required to confirm whether there is a real difference regarding the efficacy of CTX and NTZ. The poor PFS of metachronous metastasis compared to synchronous metastasis may be attributed to greater therapeutic resistance.23, 24, 25 We also found that while the combined chemotherapy regimen was an independent prognostic factor for PFS, the optimal chemotherapy regimens were not determined in the current study. Recently, a meta‐analysis showed that the triple combination regimen was associated with the best short‐term efficacy but failed to improve the patient prognosis among the four commonly used first‐line chemotherapy regimens (PF, GP, TP, and triplet combination regimen) for RM‐NPC.26
An age older than 43 years, KPS ≤ 80, and a higher level of baseline EBV DNA were found to be independent risk factors for OS. Patients older than 43‐year‐old had a shorter OS than young patients. Moreover, age was a common influential factor for the outcomes of many metastatic cancers, including breast cancer,27 colon cancer,28 and NPC patients with bone metastases.29 Thus, these findings indicate that older patients may be associated with poor treatment tolerance. In addition, poor KPS was identified as an independent risk factor for OS, even if there was no effect on PFS. This finding is consistent with the results of Zheng et al,24 who found that multimodal treatment could improve the survival of metastatic NPC patients who exhibited a good performance status. Patients with favorable KPS had increased opportunity for posterior‐line therapy. As an important prognostic factor for NPC,1, 30, 31 EBV DNA also exhibited a positive correlation with OS risk in patients with RM‐NPC. With an increased level of EBV DNA, the OS risk increased monotonously.
The overall toxicity profile was tolerable in this study. Severe toxicity was primarily associated with hematology, but could be managed by medical intervention. Moderate mucosal inflammation, rash, fever, and liver functional damage were more common in patients treated with CTX than those treated with NTZ, which may be related to the drug properties of these two mAbs. Greater thrombocytopenia was observed in patients with GP, which was primarily attributed to gemcitabine.
Despite these findings, our study has several limitations: (a) since this was a retrospective nonrandomized study, there are potential confounding factors, including the chemotherapy regimens and anti‐EGFR therapies, which may affect the interpretation of the results. However, we attempted to compensate for this deficiency by performing multivariate analyses; (b) the first‐line palliative chemotherapy regimens were variable in this study. We are unable to reveal the most suitable chemotherapy regimens that can be combined; (c) we lacked information regarding EGFR expression in our cohort, which is due to the retrospective nature of this study, technical challenges of the tissue sampling process, and the difficulty of conducting robust interlaboratory quality assurance of EGFR expression; (d) no head‐to‐head comparison of efficacy was made between treatment with anti‐EGFR mAbs plus chemotherapy and chemotherapy alone in this study; and (e) due to the inherent limitations of this retrospective study in terms of AE reporting, we have only listed AE items that could be obtained from the patient medical records and laboratory results.
In conclusion, the anti‐EGFR mAbs (CTX or NTZ) combined with palliative chemotherapy achieved promising antitumor activity with a tolerable toxicity profile as a first‐line treatment for RM‐NPC. Thus, further studies are urgently required to verify our findings and to compare the safety and efficacy of anti‐EGFR mAbs plus chemotherapy with chemotherapy alone for the treatment of RM‐NPC.
FUNDING AND ACKNOWLEDGMENTS
This study was funded by the Natural Science Foundation of Guangdong Province (No. 2018A030310236); the Outstanding Young Talents Program of Sun Yat‐sen University Cancer Center (16zxyc04); the Central Basic Scientific Research Fund for Colleges‐Young Teacher Training Program of Sun Yat‐sen University (17ykpy81). We sincerely appreciate Yidu Cloud Corporation (Beijing, China) for data retrieval and management.
CONFLICT OF INTEREST
There are none potential conflict of interest.
AUTHOR CONTRIBUTIONS
Chen Chen: Conceptualization, data curation, formal analysis, methodology, investigation, writing–original draft, and writing–review and editing. Yixin Zhou: Conceptualization, data curation, formal analysis, methodology, investigation, writing–original draft, and writing–review and editing. Xuanye Zhang: Conceptualization, data curation, formal analysis, methodology, investigation, writing–original draft, and writing–review and editing. Sha Fu: Formal analysis, methodology, and writing–review and editing. Zuan Lin: Methodology and writing–review and editing. Wenfeng Fang: Analysis and writing–review and editing. Yunpeng Yang: Analysis and writing–review and editing. Yan Huang: Analysis and writing–review and editing. Hongyun Zhao: Analysis and writing–review and editing. Shaodong Hong: Conceptualization, data curation, formal analysis, supervision, writing–original draft, and writing–review and editing. Li Zhang: Conceptualization, data curation, formal analysis, supervision, writing–original draft, and writing–review and editing.
Supporting information
Chen C, Zhou Y, Zhang X, et al. Anti‐epidermal growth factor receptor monoclonal antibody plus palliative chemotherapy as a first‐line treatment for recurrent or metastatic nasopharyngeal carcinoma. Cancer Med. 2020;9:1721–1732. 10.1002/cam4.2838
Chen Chen, Yixin Zhou and Xuanye Zhang should be considered joint first author.
Shaodong Hong and Li Zhang should be considered joint senior author.
Contributor Information
Shaodong Hong, Email: hongshd@sysucc.org.cn.
Li Zhang, Email: zhangli6@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012‐1024. [DOI] [PubMed] [Google Scholar]
- 2. Tian Y‐M, Liu M‐Z, Zeng L, et al. Long‐term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity‐modulated radiotherapy. Head Neck. 2019;41(5):1246‐1252. [DOI] [PubMed] [Google Scholar]
- 3. Sun X, Su S, Chen C, et al. Long‐term outcomes of intensity‐modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398‐403. [DOI] [PubMed] [Google Scholar]
- 4. Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 2018;77:16‐21. [DOI] [PubMed] [Google Scholar]
- 5. Prawira A, Oosting SF, Chen TW, et al. Systemic therapies for recurrent or metastatic nasopharyngeal carcinoma: a systematic review. Br J Cancer. 2017;117:1743‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369‐385. [DOI] [PubMed] [Google Scholar]
- 7. Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958‐2970. [PubMed] [Google Scholar]
- 8. Ma X, Huang J, Wu X, et al. Epidermal growth factor receptor could play a prognostic role to predict the outcome of nasopharyngeal carcinoma: a meta‐analysis. Cancer Biomark. 2014;14:267‐277. [DOI] [PubMed] [Google Scholar]
- 9. Ma BBY, Poon TCW, To KF, et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma–a prospective study. Head Neck. 2003;25:864‐872. [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21‐26. [DOI] [PubMed] [Google Scholar]
- 11. Wang F, Sun Q, Jiang C, et al. Additional induction chemotherapy to concurrent chemotherapy and intensity‐modulated radiotherapy with or without nimotuzumab in first‐line treatment for locoregionally advanced nasopharyngeal carcinoma: a propensity score matched analysis. J Cancer. 2018;9:594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng H, Tang L‐L, Liu XU, et al. Anti‐epidermal growth factor receptor therapy concurrently with induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Cancer Sci. 2018;109:1609‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao JJ, Zhang LL, Gao TS, et al. Comparing treatment outcomes of concurrent chemoradiotherapy with or without nimotuzumab in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer Biol Ther. 2018;19:1102‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. You R, Hua Y‐J, Liu Y‐P, et al. Concurrent chemoradiotherapy with or without anti‐EGFR‐targeted treatment for stage II‐IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and long follow‐up. Theranostics. 2017;7:2314‐2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma BBY, Kam MKM, Leung SF, et al. A phase II study of concurrent cetuximab‐cisplatin and intensity‐modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012;23:1287‐1292. [DOI] [PubMed] [Google Scholar]
- 16. Zhao C, Miao J, Shen G, et al. Anti‐Epidermal Growth Factor Receptor (EGFR) monoclonal antibody combined with cisplatin and 5‐fluorouracil in patients with metastatic nasopharyngeal carcinoma after radical radiotherapy: a multicentre, open‐label, phase II clinical trial. Ann Oncol. 2019;30(4):637‐643. [DOI] [PubMed] [Google Scholar]
- 17. Chan ATC, Hsu M‐M, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23:3568‐3576. [DOI] [PubMed] [Google Scholar]
- 18. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187‐193. [DOI] [PubMed] [Google Scholar]
- 19. Shao JY, Zhang Y, Li YH, et al. Comparison of Epstein‐Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res. 2004;24:4059‐4066. [PubMed] [Google Scholar]
- 20. Zhang LI, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open‐label, phase 3 trial. Lancet. 2016;388:1883‐1892. [DOI] [PubMed] [Google Scholar]
- 21. Ma L, Zhang G, Miao XB, et al. Cancer stem‐like cell properties are regulated by EGFR/AKT/beta‐catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma. FEBS J. 2013;280:2027‐2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wykosky J, Fenton T, Furnari F, Cavenee WK. Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin J Cancer. 2011;30:5‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fandi A, Bachouchi M, Azli N, et al. Long‐term disease‐free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. 2000;18:1324‐1330. [DOI] [PubMed] [Google Scholar]
- 24. Zheng W, Zong J, Huang C, et al. Multimodality treatment may improve the survival rate of patients with metastatic nasopharyngeal carcinoma with good performance status. PLoS ONE. 2016;11:e0146771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tian Y‐M, Zeng L, Wang F‐H, et al. Prognostic factors in nasopharyngeal carcinoma with synchronous liver metastasis: a retrospective study for the management of treatment. Radiat Oncol. 2013;8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma S‐X, Zhou T, Huang Y, et al. The efficacy of first‐line chemotherapy in recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta‐analysis. Ann Transl Med. 2018;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Largillier R, Ferrero J‐M, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonsalves WI, Wolpert J, Tashi T, et al. Assessment of prognostic factors after primary tumor resection in metastatic colon cancer patients: a Veteran's Affairs Central Cancer Registry (VACCR) analysis, 1995–2008. J Surg Oncol. 2012;106:486‐490. [DOI] [PubMed] [Google Scholar]
- 29. Chen C, Wu J‐B, Jiang H, et al. A prognostic score for nasopharyngeal carcinoma with bone metastasis: development and validation from multicenter. J Cancer. 2018;9:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. You R, Liu Y‐P, Lin M, et al. Relationship of circulating tumor cells and Epstein‐Barr virus DNA to progression‐free survival and overall survival in metastatic nasopharyngeal carcinoma patients. Int J Cancer. 2019;145(10):2873‐2883. [DOI] [PubMed] [Google Scholar]
- 31. Liu M‐Z, Fang S‐G, Huang W, et al. Clinical characteristics and prognostic value of pre‐retreatment plasma epstein‐barr virus DNA in locoregional recurrent nasopharyngeal carcinoma. Cancer Med. 2019;8(10):4633‐4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


