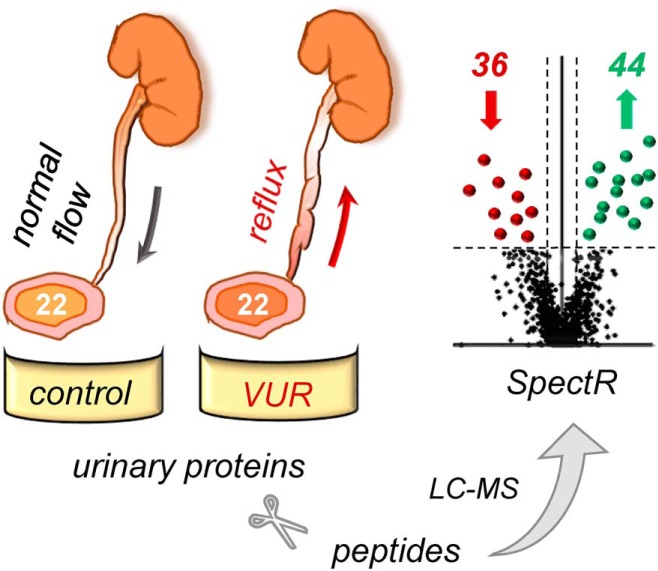
Recurrent urinary tract infections (UTIs) are a significant burden to the health care system, with little insight into underlying mechanisms. Urinary proteomes of children with recurrent infections and vesicoureteral reflux show differential expression of proteins involved in inflammation, acute phase response (APR), modulation of extracellular matrix (ECM), and carbohydrate metabolism. These altered proteins may represent candidate biomarkers to characterize susceptibility of infection and renal damage towards improving medical care for patients.
Keywords: Mass spectrometry, clinical proteomics, infectious disease, renal function or biology, urine analysis, acute phase response, urinary tract infection, vesicoureteral reflux
Graphical Abstract
Highlights
Urinary proteomes of patients with recurrent UTI, renal scarring, and VUR.
80 proteins differentially expressed, compared to healthy controls.
62 proteins may be indicative of susceptibility for UTI.
Altered acute phase response, extracellular matrix and carbohydrate metabolism.
Abstract
Recurrent urinary tract infections (UTIs) pose a significant burden on the health care system. Underlying mechanisms predisposing children to UTIs and associated changes in the urinary proteome are not well understood. We aimed to investigate the urinary proteome of a subset of children who have vesicoureteral reflux (VUR) and recurrent UTIs because of their risk of developing infection-related renal damage. Improving diagnostic modalities to identify UTI risk factors would significantly alter the clinical management of children with VUR. We profiled the urinary proteomes of 22 VUR patients with low grade VUR (1–3 out of 5), a history of recurrent UTIs, and renal scarring, comparing them to those obtained from 22 age-matched controls. Urinary proteins were analyzed by mass spectrometry followed by protein quantitation based on spectral counting. Of the 2,551 proteins identified across both cohorts, 964 were robustly quantified, as defined by meeting criteria with spectral count (SC) ≥2 in at least 7 patients in either VUR or control cohort. Eighty proteins had differential expression between the two cohorts, with 44 proteins significantly up-regulated and 36 downregulated (q <0.075, FC ≥1.2). Urinary proteins involved in inflammation, acute phase response (APR), modulation of extracellular matrix (ECM), and carbohydrate metabolism were altered among the study cohort.
Urinary tract infections (UTIs)1 pose a significant burden on the health care system, with annual societal cost of $3.5 billion in the United States (1). In select populations, UTIs can lead to pyelonephritis (kidney infections), causing renal scarring and long-term renal damage, the latter being exacerbated with repeated infections. Molecular mechanisms characterizing patients predisposed to recurrent UTIs have not been completely elucidated. Since the urinary proteome is a source of several host defense proteins, it may provide insights into better understanding the etiology of recurrent UTI.
The urinary tract has both mechanical and molecular host defense mechanisms (2–3). Molecular host defense encompasses innate and adaptive immune responses involving immune cell activity and soluble factors secreted into urine such as uromodulin. Mechanical defense includes physical or anatomic barriers, such as the glycosaminoglycan layer lining the urothelium, complete voiding to eliminate residual urine with bacteria, and valvular mechanisms which isolate the bladder from the kidneys during micturition. Vesicoureteral reflux (VUR) is a congenital condition where failure of this lattermost anatomic mechanical host defense causes backwards flow of urine to the kidneys from the bladder and contributes to incomplete urinary clearance. It is a common condition that often resolves without symptoms or intervention. However, during a UTI, VUR propagates the translocation of pathogenic bacteria from the bladder to the kidney, which can then lead to pyelonephritis and long-term sequalae such as renal scarring (4–5). Patients with VUR and a predisposition for recurrent UTIs are more likely to be at greater risk for long-term kidney damage and may merit earlier medical or surgical intervention (4–6). Although VUR itself is accurately diagnosed by current, albeit invasive, modalities, it is impossible to identify these patients who are at higher risk for UTI.
Because of the inability to determine the need for intervention, VUR patients are often overmanaged and overtreated both medically and surgically (6). Patients are placed on long-term daily antibiotic prophylaxis that can lead to antimicrobial resistance and associated side effects (6–10). Serial assessment for continued presence of VUR exposes patients to invasive catheterization and ionizing radiation. Surgical correction is undertaken for patients in whom the condition persists or for those who experience recurrent breakthrough UTIs despite antibiotic suppression (4–6). The availability of minimally invasive endoscopic intervention has increased the number of patients who undergo surgery, suggesting a trend of overtreatment without clear indication or need (11). Beyond the economic impact, these potentially unnecessary interventions present risks of over exposure to antibiotics, surgery and anesthesia, all of which can impact pediatric growth and development (6–10, 12, 13).
We hypothesized that there may be differences in host molecular mechanisms that distinguish VUR patients with recurrent UTIs and renal damage. If these differences become clinically relevant, the management of this population would dramatically change. To this aim, we performed a first-step pilot study comparing the urinary proteome of patients who underwent surgery for VUR with a history of recurrent UTI and renal scarring to that of healthy controls to determine if these cohorts can be distinguished by the urinary proteome. To categorize proteins that may be more characteristic of VUR and recurrent infection from those more likely indicative of renal damage, we compared proteomes to those from our previous study on patients with kidney damage resulting from ureteropelvic junction obstruction (UPJO). Furthermore, unlike prior studies, to reduce the potential bias of congenital kidney dysplasia often seen in higher grades of VUR, we focused only on children with lower grades of VUR (14–19).
By concentrating on a well-defined urologic cohort, this pilot study sought to gain insight into recurrent UTI to serve as a foundation for future prospective, longitudinal studies that will involve children with VUR but no history of UTI. Differences identified in this population could ultimately provide guidance in improving the management of patients with VUR, particularly toward the development of better non-invasive diagnostic tools. Additionally, this work may contribute broader impact to elucidating mechanisms of recurrent UTI in other affected populations.
EXPERIMENTAL PROCEDURES
Experimental Design and Statistical Rationale
All patients were identified from an Institutional Review Board (IRB)-approved protocol from the Boston Children's Hospital Pediatric Urinary Proteome Program Initiative (PUPPI) repository. VUR is graded from 1 to 5 (with 5 being the most severe), and we selected a cohort of 22 patients who underwent open surgical correction for low grade of VUR (grades 1–3), history of recurrent febrile UTIs, and radiographic renal damage. Patients with more severe grades (grades 4–5) were excluded due to the potential confounding factor of congenital renal dysplasia and kidney maldevelopment as the etiology of abnormal renal imaging (20). VUR grade was determined by voiding cystourethrogram (VCUG), and renal damage was defined as presence of renal cortical scarring on dimercaptosuccinic acid (DMSA) nuclear renal scan. Sample acquisition from VUR patients was by bladder catheterization via standard sterile technique on the day of surgery. None of the VUR patients had active infection as determined by urine culture.
A control cohort of 22 patients was additionally selected from PUPPI. Individuals in this group had no history of urologic conditions (VUR, UTI, or renal damage), or other comorbid conditions. Patients identified as healthy controls were undergoing procedures such as circumcision, hypospadias repair, lysis of labial adhesions removal, or inguinal hernia/hydrocele repair. Urine samples were obtained by voiding or crede maneuver. Due to the male predominance for these surgical procedures, there are a greater number of male samples available in the repository. Controls were selected to provide a similar age distribution to the VUR cohort, but gender and race distribution could not be achieved given limitations on available samples in the PUPPI repository. A pooled internal standard (PIS) was created from combining urine samples from 8 separate healthy controls. Aliquots of PIS were used as technical replicates for quality control of signal normalization between different MS acquisitions and of bioinformatics processing. Demographic categorical and continuous variables were compared using Fisher's exact test and Wilcoxon test, respectively, between the VUR and control groups for each cohort and between the VUR groups for the discovery versus validation cohorts (Table I).
Table I. Demographics of the VUR and Control Groups. Percentage values are listed with number in parentheses for the VUR and matched control groups for both the MS discovery (left) and validation (right) cohorts. The p value column reflects comparison between the VUR groups between the discovery and validation cohorts. The p value for comparison between the VUR and control groups within cohorts is listed below the comparison, where applicable. VUR, vesicoureteral reflux.
| MS discovery cohort |
Validation cohort |
p value | |||
|---|---|---|---|---|---|
| VUR | Control | VUR | Control | ||
| n | 22 | 22 | 20 | 20 | |
| Age (years) | |||||
| Median | 5.3 | 5.7 | 6.2 | 5.4 | 0.44 |
| Range | 1.1–22.2 | 1.0–22.0 | 1.2–14.0 | 0.4–14.0 | |
| p = 1.00 | p = 0.86 | ||||
| Gender | |||||
| Male | 13.6% (3) | 59.1% (13) | 20% (4) | 50% (10) | 0.40 |
| Female | 86.4% (19) | 40.9% (9) | 80% (16) | 50% (10) | 0.40 |
| p < 0.01 | p = 0.10 | ||||
| Reflux | 1.00 | ||||
| Unilateral | 40.9% (9) | 45.0% (9) | |||
| Bilateral | 59.1% (13) | 55.0% (11) | |||
| Reflux grade (Right) | 0.25 | ||||
| None | 18.2% (4) | 25.0% (4) | |||
| Grade 1 | 18.2% (4) | 0 | |||
| Grade 2 | 36.4% (8) | 40% (8) | |||
| Grade 3 | 27.3% (6) | 35% (7) | |||
| Reflux grade (Left) | 0.57 | ||||
| None | 22.7% (5) | 15.0% (3) | |||
| Grade 1 | 4.5% (1) | 0 | |||
| Grade 2 | 31.8% (7) | 45.0% (9) | |||
| Grade 3 | 40.9% (9) | 35.0% (7) | |||
| Grade 4 | 0 | 5.0% (1) | |||
| Antibiotic Prophylaxis | 95.5% (21) | 75% (15) | <0.01 | ||
| Renal Scarring (DMSA) | 1.00 | ||||
| Unilateral | 72.7% (16) | 75.0% (15) | |||
| Bilateral | 27.3% (6) | 25.0% (5) | |||
Urinary Protein Extraction
Samples were analyzed by urinalysis using a Siemens CLINITEK® status automated analyzer (Siemens, Tarrytown, NY) and confirmed there was no potential blood contamination or evidence of proteinuria. All samples were centrifuged at 4500 × g for 20 min to remove cellular debris, aliquoted, and frozen at −80 °C before protein purification compliant with downstream MS analysis (21). Urine aliquots were subsequently thawed, desalted, and concentrated on 10 kDa MW cutoff filters. Samples were reduced in 10 mm dithiothreitol (DTT, Sigma Aldrich, St. Louis, MO) and alkylated in 25 mm iodoacetamide (IAA, Sigma Aldrich). Extracted urinary proteins were quantified by micro BCA assay following manufacturer's instruction (ThermoFisher Scientific, Waltham, MA).
Peptide Isolation
Extracted urinary proteins from each individual were processed separately via solid-phase reversible sample-preparation (SRS) beads, as previously described (22). Incubation was performed in a benchtop thermomixer (ThermoMixer C, Eppendorf, Hamburg, Germany) simultaneously shaking at 1400 rpm with collection of supernatants after centrifugation at 5000 rpm for 30 s (Minispin, Eppendorf). Briefly, 10 mg of dry SRS beads (∼25 μl) was incubated with 0.01 m NaOH (150 μl) for 3 min at room temperature (RT) and then rinsed twice with 150 μl phosphate-buffered saline (PBS). Urinary proteins (80 μg) were dissolved in 100 μl of 0.5% SDS in PBS and incubated with the washed beads. Acetonitrile (100%, ACN, Thermo Fischer Scientific) was immediately added to reach a final concentration of 85% ACN to enable effective binding of proteins, as previously published (22). This mixture was incubated for 30 min at 1400 rpm at RT. Samples were centrifuged to remove unbound proteins, and 8 m urea in PBS (150 μl) was added. Excess reagent was removed by 8 m urea in PBS (150 μl) and 80% ACN in PBS (150 μl) (3 washes each). The beads were washed twice with 0.1 m ammonium bicarbonate (ABC, Sigma Aldrich) in heavy water (H218O) to label the glycosites for a separate prospective study and then placed in 150 μl of 0.1 m ABC in H218O for N-deglycosylation.
N-deglycosylation was performed by adding 2 μl of peptide-N-glycosidase F (PNGase F, New England Biolabs, Ipswich, MA) to each sample in 2.5:100 enzyme-to-protein ratio and incubated overnight (20 h) at 37 °C. Released N-glycans were collected in supernatant after centrifugation and in two subsequent washes with 0.1% formic acid (150 μl, FA, Sigma Aldrich) for additional analysis not assessed in this study. Proteins bound to the beads were resuspended in 150 μl of 50 mm ABC in unlabeled H2O. Trypsin (2 μg, Promega, Madison, WI) was added to beads in 1:40 enzyme-to-protein ratio, with a second 2 μg added after 4 h, and incubated at 37 °C for 7 h in total. Resulting peptides were collected in the supernatant after centrifugation. Samples were washed with 20% ACN (150 μl) and centrifuged, followed by wash and centrifugation with 70% ACN (150 μl). All supernatants were pooled for each sample and stored at −20 °C before MS analysis.
Mass Spectrometry Analysis and Database Search
Dried peptides were reconstituted with 400 μl 5% FA/5% ACN. An aliquot of each sample was transferred into HPLC vial and placed in the autosampler at 4 °C. Samples were individually analyzed on a Q-Exactive (ThermoFisher) coupled to a nanoflow UPLC system (Eksigent, Dublin, CA). The LC columns were packed in-house using Magic C18, 5 μm, 100 Å (Michrom BioResources/Bruker, Billrica, MA) into PicoTips (15 cm × 100 μm ID; New Objective, Woburn, MA). Peptides were separated using a linear LC gradient from 95% solvent A (0.1% FA in water) and 5% solvent B (0.1% FA in ACN) to 70% solvent A and 30% solvent B over 120 min, followed by a steeper gradient to reach 5% solvent A and 95% solvent B over 7 min.
Raw files were searched against Uniprot Homo sapiens and common contaminant databases (reviewed September 2015) using Proteome Discoverer 1.4 (ThermoFisher Scientific) and combination of Sequest and Mascot algorithms for database search (42,184 total entries). Tryptic peptides with a default charge states of 2+, 3+, and 4+ and up to one miscleaved site were considered. Mono-isotopic precursor mass tolerance was 10 ppm, and 0.02 Da search tolerance was permitted for fragment ion identification. Precursor and neutral loss peaks were removed in MS2 spectra. Carbamidomethylation of cysteine residues was set as fixed modification. Variable modifications were deamidation (N, N(18O), and Q) and oxidation (M). All data were searched against a combined target and decoy database. Confident peptide identifications were filtered at 1% false discovery rate (FDR) and identified proteins were matched by 2 or more spectra. Raw spectral data and Proteome Discoverer-generated MSF files have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD010469 (23).
Protein Quantification and Statistical Analysis
The SpectR Spectral Analysis R Package was developed in-house to provide comprehensive quantitative analysis of data based on spectral counts (SC). The package in R is provided as supplemental file. MSF files were re-searched to assign a SC score to each peptide and only unique peptides were extracted for quantitation. The detection threshold was defined as raw SC ≥2, as non-zero values in this range were indistinguishable from zero. The latter values were assigned as missing and replaced with NA until after normalization. For non-missing raw SC data, normalization was performed using square root sum. In brief, the square root was normalized to the sum of all square root data and then squared, as shown below for n non-zero spectral count values among all samples analyzed.
Reproducibility of the processing was assessed using the 1327 total proteins identified and quantified across the 5 technical replicates of PIS. Consistency of results was assessed based on pairwise comparisons of protein quantifications on peptides that were SC ≥2 (supplemental Table S1 and supplemental Fig. S1). Proteins that were identified with SC ≥2 in at least 7 patients in either VUR or control cohort were defined as reliably detected based on optimization of signal-to-noise ratio. This list of proteins was then subjected to permutation-based FDR to further filter the candidate protein lists for comparison. Non-parametric Wilcoxon testing was utilized to compare urinary protein abundances between VUR and control cohorts, and the resulting p values for each protein ID were adjusted for multiple testing via Benjamini-Hochberg correction. Positive false discovery rate (q-value) was controlled at 7.5%. Hierarchical clustering was performed using proteins meeting the above threshold. Fold change (FC) for each protein was determined using the ratio of the mean value in the VUR cohort to that in the control cohort. For ratios <1 where the mean value VUR was less than that of control, FC is expressed as −1/ratio to reflect down-regulation in VUR. The relationship of FC and q-value for each protein was visualized by volcano plot (Fig. 1).
Fig. 1.
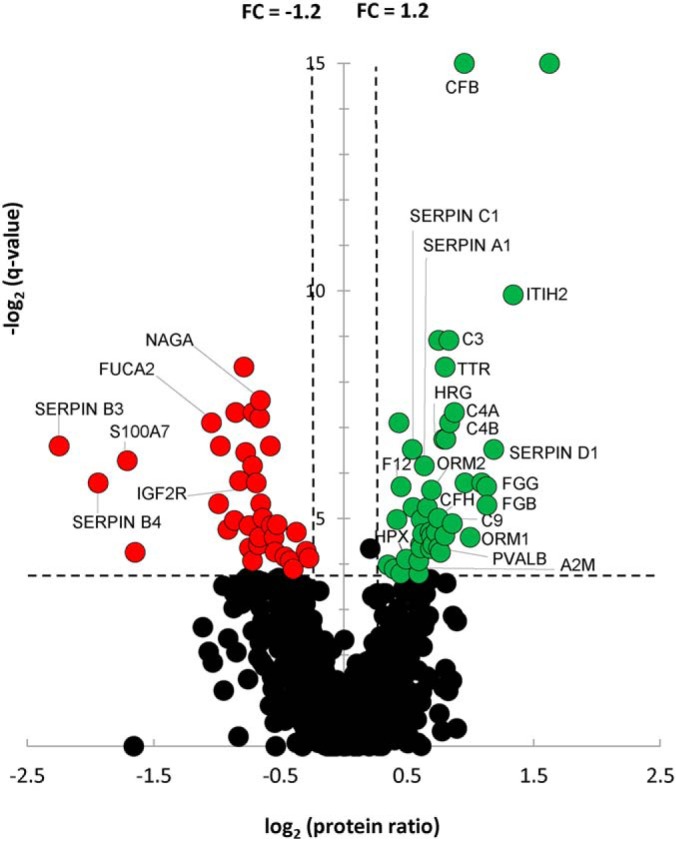
Volcano Plot of VUR to Control Cohort Ratios for All Quantified Proteins. Of the 964 robustly identified and quantified proteins in the VUR and control cohorts, 80 proteins were deemed differentially regulated candidate markers by meeting criteria of q-value <7.5% and FC ≥+1.2 (up-regulated in VUR; green) or ≤−1.2 (down-regulated in VUR; red). Specific candidate proteins of interest as determined by biological network analysis are indicated. FC, fold change.
Functional Pathway Analysis
In total, 80 proteins were differentially regulated in the VUR patients compared with the control cohort (q-value <0.075; FC ≥1.2); 44 proteins showed increase in abundance while 36 had decreased. These differentially-abundant urinary proteins were assessed via Functional Enrichment Analysis Tool (FunRich version 3.0) (26).
Comparison of the Urinary Proteome of the VUR and UPJO Patients
VUR data was compared with our previously published urinary proteome of pediatric ureteropelvic junction obstruction (UPJO) patients (750 proteins) and list of the candidate biomarkers for UPJO-associated renal damage (76 proteins) but unrelated to UTI (27). The comparison of VUR with UPJO data was performed to attempt segregating proteins that are more likely to be indicative of UTI from those that are associated with renal damage.
Validation of MS Results via Immunoaffinity-based Quantitation in Discovery and Independent Patient Cohorts
Alpha-1-acid glycoprotein (orosomucoid, ORM1) was selected for validation by enzyme-linked immunosorbent assay (ELISA R&D Systems, DAGP00, Minneapolis, MN). Initial verification was performed on remaining available urine from the original MS discovery cohort which included 19 VUR patients and 16 age-matched controls. A separate validation cohort of 20 VUR patients with similar disease phenotype and 20 age-matched controls was then tested (Table I). Urine aliquots (1 ml) were thawed and centrifuged at 4000 rpm for 10 min to remove insoluble particles. Samples were diluted 1:10 in HPLC grade water (Thermo Fisher) and analyzed in replicates following manufacturer's instructions. Optical density was measured at 450 nm with a correction at 540 nm using a FilterMax F3 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA). Readouts were normalized to total urinary protein amount as determined by Bradford assay (Bio-Rad, Hercules, CA) on a Beckman DU600 Spectrophotometer as previously described (28–31). Significant changes between ORM1 levels in VUR and control samples were assessed by Mann-Whitney-Wilcoxon test.
RESULTS
Altered Urinary Proteome in VUR Patients with UTI and Renal Scarring
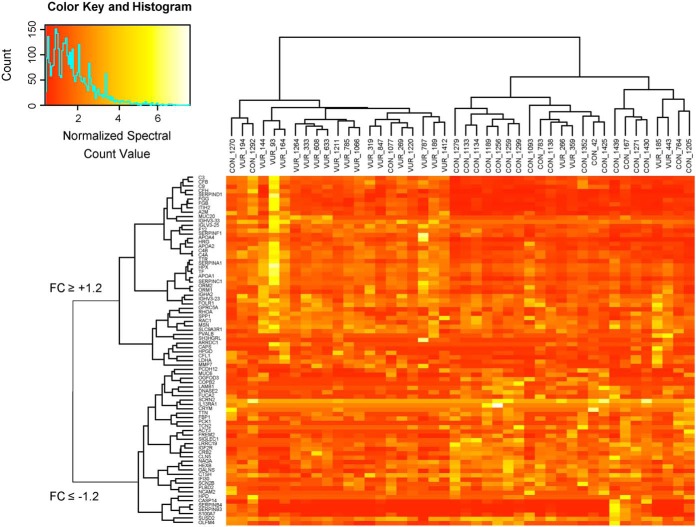
Demographics and clinical characteristics of the VUR and control groups in both the MS discovery and validation cohorts were not significantly different, as presented in Table I. In total 2551 proteins were detected in the urine of our VUR patients and healthy individuals. To strengthen the comparative analysis, only proteins that were identified with SC ≥2 in more than 30% of samples from disease and control cohort were considered, resulting in 964 proteins. The threshold settings were determined based on analysis of the technical PIS replicates and optimization of signal-to-noise ratio, as mentioned above. Criteria of q-value <7.5% and FC ≥1.2 were applied which resulted in 80 candidate protein biomarkers that may characterize the VUR patients with UTI and renal scarring based on urinary protein profiling (Fig. 1 and supplemental Table S2). Hierarchical clustering using these 80 proteins indicated near complete segregation of the two cohorts supporting a different expression profile of the proteomes in the VUR and healthy urine (Fig. 2).
Fig. 2.
Hierarchical Clustering of Differentially Regulated Proteins Demonstrates Segregation of the VUR and Control Cohorts. Eighty proteins met criteria of corrected q-value FDR <7.5% and FC ≥1.2. Hierarchical clustering utilized the normalized SC for these proteins in each sample, with color intensity based on SC value. The resulting dendrograms demonstrated segregation and clustering for the majority of the VUR and control samples (horizontal axis). The candidate markers also clustered and segregated based on whether they were up- or downregulated in the VUR cohort (vertical axis).
Functional Characterization of Differentially Expressed Proteins in VUR Patients
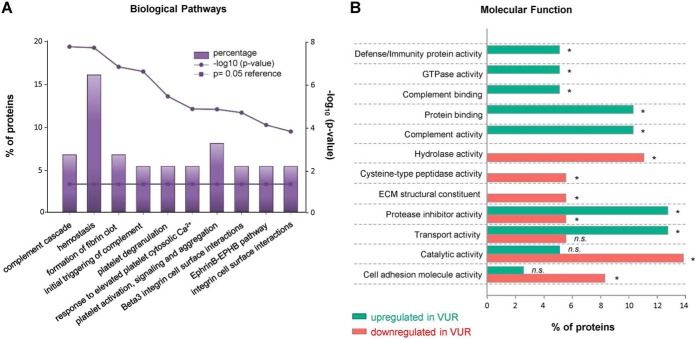
Significantly-altered proteins between VUR patient and control cohort were functionally assessed via FunRich. The proteins eligible for FunRich's functional enrichment are listed within supplemental Tables S3–S5. Regulation of complement cascade and hemostasis were the most significantly altered pathways in the VUR cohort compared with controls (Fig. 3A, supplemental Table S3). Over-representation of proteins involved in formation of the fibrin clot and activation of the platelet signaling was also observed. Extracellular matrix (ECM) proteins involved in integrin family cell surface interaction and EphrinB-EPHB pathway were significantly affected in disease cohort (Fig. 3A). Interestingly, many of acute phase response proteins that are known inflammatory plasma protein markers were significantly altered in the urine from the VUR patients compared with control. Proteins that were increased or decreased in abundance had distinctive molecular and biological functions (Fig. 3B). We observed an increase in proteins involved in complement binding/activation (C3, C4A, C9, CFH and CFB) and in immune defense mechanism (ORM1 and ORM2) (supplemental Table S4). On the contrary, proteins with hydrolase activity, cysteine-type peptidase (CP), and structural constituent of the ECM were downregulated (supplemental Table S5). Additionally, certain protease inhibitors (PIs) were specifically down- or up-regulated in the VUR patients. Serine protease inhibitors (serpins) SERPINB3 and SERPINB4 were downregulated while SERPINA1, SERPINC1 and SERPIND1 were up-regulated. Up-regulation of two additional PIs, alpha-2-macroglobulin (A2M) and inter-α trypsin inhibitor heavy chain 2 (ITIH2) was also observed. Four glycoprotein-binding proteins were significantly regulated: complement factor H (CFH) and histidine-rich glycoprotein (HRG) were up-regulated, while insulin-like growth factor 2 receptor (IGF2R) and SERPINA1 were downregulated.
Fig. 3.
Functional Characterization of VUR-regulated Proteins. A, Overrepresented biological pathways from all regulated proteins in the urine of the VUR patients. Percentage of proteins indicates the VUR-altered proteins over the entire proteins in the pathway (bars), while significance of the enrichment is represented by the p value (enrichment and reference 0.05 p value). B, Representative molecular function was separately assigned to the urinary proteins that were up- (green) or downregulated (red). Asterix represents p value <0.05. Functional enrichment was performed via FunRich version 3.0. ECM, extracellular matrix; n.s., not significant; VUR, vesicoureteral reflux.
Comparison of VUR and UPJO Urinary Proteomes Demonstrates Limited Numbers of Shared Varying Proteins
In a previous study, we focused on the discovery of putative urinary biomarkers of patients with renal damage caused by ureteropelvic junction obstruction (UPJO) (27). We hypothesized that overlap of proteins related to both VUR and UPJO cohorts will reveal biomarkers indicative of renal damage commonly associated with injury. Proteins specifically regulated in one of the examined patient cohort may be considered indicative of disease-specific clinical traits.
In the case of the UPJO patients, 76 proteins were significantly altered compared with the control cohort (27). This data set was intersected with 80 proteins that were significantly changed in urine of the VUR patients. Eighteen overlapping biomarkers that were altered in both UPJO and VUR patients suggest there is a shared host response to inflammation and tissue repair resulting from either obstructive or UTI-related kidney damage. The remaining 62 proteins were distinct candidate biomarkers that may be characteristic of the VUR group and potentially of recurrent infection. Disease-specific alteration of the proteins that belong to the same protein class was observed. For example, inter-alpha-trypsin inhibitor heavy chain 1 (ITIH1) and 2 (ITIH2) were specifically altered in the urine of the UPJO and VUR patients, respectively. Altered expression of S100P was solely observed in UPJO, while changes in S100A7 was VUR specific. Up-regulated serine protease inhibitors (SERPINA1, SERPINC1 and SERPIND1) in the VUR cohort were similarly regulated in UPJO patients. However, downregulated SERPINs (SERPINB3 and SERPINB4) were VUR specific.
ELISA Confirmation of ORM1 Levels in Discovery and Validation Cohorts
Levels of ORM1 were initially cross-validated by ELISA in 19 VUR and 16 controls of the original MS cohort (p < 0.01, supplemental Fig. 2). With this confirmation, an independent validation cohort of 20 VUR and 20 control patients were tested for ORM1. ELISA results confirmed significant up-regulation of ORM1 in the independent cohort of VUR patients compared with controls (p < 0.05, Fig. 4). This independent immunoaffinity assays mirrored the quantification in the MS-based proteomic approach, supporting reliability of our spectral count-based protein quantitation.
Fig. 4.
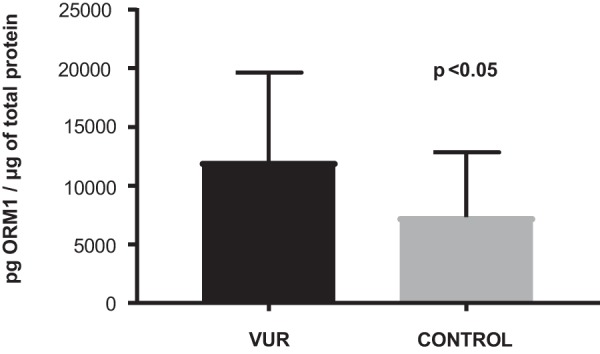
ELISA Quantification of ORM1 in ValidationCohort. A validation cohort consisting of VUR (n = 20) and control (n = 20) samples separate from the discovery cohort were tested. Mean levels with standard error are shown, demonstrating a statistically significant elevation of ORM1 levels in the VUR cohort by Mann-Whitney-Wilcoxon test. ORM, orosomucoid; VUR, vesicoureteral reflux.
DISCUSSION
We performed a comparative analysis of the urinary proteome in 22 healthy and 22 VUR patients with recurrent UTIs and renal scarring and identified 80 urinary proteins that were significantly altered in abundance. Unique to our study, we focused on a clinical cohort of children with low grade VUR (grade 1–3), history of recurrent UTIs, and radiographic confirmation of renal damage. Focusing on patients with low grade VUR favors discovery of proteins related to UTI and infection-induced renal damage as opposed to congenital renal dysplasia which is often observed in high grade VUR. This cohort enabled the identification of urinary proteins that may be related specifically to the VUR cohort (62 proteins) and a minor subpopulation of proteins that may be indicative of generalized renal damage (18 proteins). Functionally, these urinary proteins appeared to be involved in host defense mechanism and inflammation, including acute phase response and ECM remodeling, which are critical for clearing the pathogen and restoring homeostasis after resolving infection. With additional testing these candidate biomarkers may represent a pool of urinary proteins critical for distinguishing patients who are at higher risk for UTI and subsequent UTI-related renal scarring. Our discussion focuses on proteins that are known to directly or indirectly mediate inflammatory response and their putative role within the urine in the context of UTI.
Immunomodulatory acute phase proteins (APP) are mainly secreted from the liver and their serum concentration increases (positive APPs) or decreases (negative APPs) by at least 25% in response to bacterial infection (32–33). Release of APPs is a host defense response that mediates pathogen clearance by recruitment of neutrophils and macrophages and sequestering serum iron critical for bacterial growth, while it also limits tissue injury during inflammation and mediates restoration of homeostasis post infection (33–34). Serum alteration of APPs, such as C-reactive protein (CRP) or serum amyloid A (SAA) are rapid and large (32, 35–36), but the majority of APPs have a slower and moderate fluctuation occurring over days to weeks post infection. In our cohort numerous APPs that are altered in plasma during inflammation were also found altered in the urine of the VUR cohort. We investigated significantly-regulated urinary APPs, including those with moderate to low fold change.
Up-regulated urinary APPs are part of the complement system cascade (C3, C4-A, C4-B), involved in coagulation and fibrinolytic system (fibrinogen beta and gamma chains), protease inhibition (alpha-1-antitrypsin/SERPINA1) (37) and protein transport (hemopexin), or have more complex systemic function (ORM1, ORM2 and alpha-2-macroglobulin/A2M) (38). Notably, we did not detect significant alteration in urinary CRP and SAA, as their concentration peaks within the first couple of days of infection and normalizes after 1–2 weeks (36). The urine from our cohort was taken at a much later date when the children were not actively infected. A rapid increase in CRP levels activates the complement system (34, 39) and thus increase of complement system proteins in the urine from the VUR cohort suggests that CRP was elevated during the initial phase of UTI. Surprisingly, several APPs that have been reported as downregulated in serum after infection (i.e. negative APPs) were significantly increased in our VUR cohort urine, such as transthyretin (i.e. prealbumin), antithrombin-III (SERPINC1) and coagulation factor XII. Future longitudinal studies will be needed to confirm how these negative APPs change in the urine along the course of infection and resolution phase.
Using ELISA, we validated the quantitative MS up-regulation of ORM1 in a separate cohort of patients. ORM1 is a 44 kDa glycoprotein synthetized in the liver and secreted in plasma during stress conditions. As an APP ORM1 has broad immunomodulatory and anti-inflammatory function, but its role in systemic inflammatory response during UTI is less defined (40–42). ORM1 inhibits macrophage and neutrophil infiltration into injured tissue which may be reason for increased survival rates observed during systemic inflammation and septic shock (43). The immunosuppression triggered by ORM1, however, leads to increased susceptibility for opportunistic infections (44–45). Although, ORM1 is limited as a standalone marker of susceptibility to UTI, it could serve as a marker for detection and progression of subclinical renal damage. Diagnostic potential of urinary ORM1 in the future may be extended for evaluating a risk from recurrent UTI in the VUR patients as a preceding, early-stage event that can eventually lead to UTI-associated renal damage.
Secretion of APPs is often accompanied by changes in immunomodulatory peptides (46). These peptides can be directly involved in inflammatory response, or their constitutive expression may be required for maintaining a homeostatic environment. We closely investigated host-derived antimicrobial peptides (AMPs) and identified several AMPs in the urine. AMPs such as beta-defensin 1 (DEFB1), chathelicidin antimicrobial peptide (CAMP), and lysozyme (LYZ) were unaltered between cohorts, solely psoriasin (S100A7) was found to be significantly downregulated in VUR patients compared with controls. S100A7 is the only member of S100 family that is regulated in our VUR cohort, and it has been identified as a major host-defense protein in vaginal fluid that protects from E. coli infection (47). This suggests that down-regulation of S100A7 in the urine of VUR patients may be indicative of less effective host innate immunity and higher risk for UTI. As both ORM1 and S100A7 have been involved in modulating immune response to infection and host-defense mechanism (44, 48–50), there may be a functional dependence between S100A7 down- and ORM1/2- up-regulation that merits further investigation.
Inflammation is a tightly regulated process which coincides with complex changes of the cell extracellular matrix (ECM) (51–52). ECM composition and associated changes are critical for pathogen attachment to the cell, as well as recruitment of immune cells to the infected site (51, 53–54). Overrepresentation of regulated structural components of extracellular matrix (ECM), extracellular proteases (ECP) and inhibitors (ECPIs) was observed in VUR cohort (Fig. 3). ECPs are promiscuous enzymes with complex substrate specificity and poorly defined sequence cleavage preference (55). Nevertheless, they are critical components of ECM that maintain a healthy tissue environment and modulate signaling events during disease such as inflammation (56–57). We found significant alteration of protein-based ECPIs in the urine of VUR patients (Fig. 3B, supplemental Table S4 and S5). The change in abundance of these inhibitory proteins was not uniform, but rather isoform specific. Serpin peptidase inhibitors SERPINB3 and SERPINB4 were downregulated, while SERPINA1, SERPINC1 and SERPIND1 were up-regulated in the urine of VUR patients. This pattern is intriguingly correlated with the tissue-specific expression of up- and downregulated SERPINs. According to the RNA expression database (www.proteinatlas.org), up-regulated SERPINs are almost exclusively synthesized in the liver and gallbladder, whereas downregulated SERPINs originate from gastrointestinal tract, with a modest contribution from kidney and urinary bladder. At this stage, we are unable to distinguish the specific mechanism for the down-regulation of SERPINB3 and SERPINB4, but many children with VUR and/or UTI have concomitant functional constipation and many are on antibiotic therapy and it is unclear if these interactions are responsible for the observed down-regulation.
SERPINC1 (Antithrombin III), a negative APP, and SERPIND1 (Heparin cofactor 2) are the two major heparin-dependent thrombin inhibitors in plasma that play a critical role in preventing coagulation and thrombosis (58). More recent reports also suggest their involvement in inflammation (59). Interestingly, binding of SERPINs to their targeted proteases increase up to 1000-fold in a presence of glycosaminoglycan structures on the surface of host cells (60–61). This suggests a critical interplay of cell-surface glycan structures and ECPIs in regulation and fine-tuning activity of ECPs. As protein glycosylation is an abundant PTM on the cell surface proteins and soluble proteins found in urine (62–63), alteration in a single protein that binds or modifies glycan structure can have a broader, systemic impact. In our study, we identified two glucosidases that were significantly downregulated in the urine of the VUR patients: α-fucosidase 2 (FUCA2) and α-N-acetylgalactosaminidase (NAGA). FUCA2 is a plasma protein that cleaves α-1,6-linked fucose that is attached to the N-acetylglucosamine of carbohydrate moieties of glycoproteins. Interestingly, deficiency in α-fucosidase 1 (FUCA1) is a rare autosomal recessive disorder that along with other symptoms, is characterized by recurrent infections (64). In contrast to FUCA2, FUCA1 is primarily expressed in tissues, and its abundance is unaltered between VUR patient and control urine samples. What remains unclear is whether the observed decrease in FUCA2 in the urine of VUR patients can predispose them to develop UTI, similar to autosomal recessive FUCA1 deficiency disorder. The second glycosidase of interest, NAGA, is a lysosomal enzyme that hydrolyzes α-N-acetylgalactosamine residues from glycolipids and glycopeptides. Like FUCA, NAGA deficiency results in impaired glycan degradation and accumulation of complex glycan structures in tissue as well as their excessive secretion in the urine (65). Changes in enzymes that regulate glycan metabolism can be correlated to observable changes in glycan structures identified in the urine of VUR patients. Reports on a competitive advantage of pathogenic bacteria that bind fucosylated glycan structures in the gastrointestinal tract urges exploration of this relation in the bladder epithelium (66–69). In future studies it will be critical to correlate the function of these carbohydrates in the bladder and in the urine to those observed in the gut. Such studies will determine whether these structures provide any advantage for bacterial growth or pathogen adhesion to the surface of the host cells and may explain an increased susceptibility to infection observed in the VUR patients.
Although inflammatory response is beneficial for resolving infection, it damages surrounding tissues. Therefore, the inhibition of prolonged inflammatory response is critical for arresting further damage to the injured site. Proteins known to be involved in regulation of hemostasis and hemolysis were altered in the urine from the VUR patients compared with controls (Fig. 3A). Hemostasis is reported to be critical for the balance of host immune defense, being related to both innate and adaptive immune response (70). In contrast, hemolysis has been correlated with susceptibility to infection in various diseases and mouse models (71–73). It is well established that hemolysis-mediated release of iron can be beneficial for pathogen growth (74–75). A recent study additionally suggests that free heme decreases mobility of macrophages and prevents adequate pathogen clearance (76). To this end, disrupted hemostasis can have two detrimental outcomes which assist bacterial outgrowth: release of iron (77–78) that benefits bacterial replication, along with release of heme that may impair macrophage-mediated phagocytosis of pathogenic bacteria (76). As urinary samples from VUR patients have been obtained at minimum one month after infection, longitudinal follow up over the longer course of time is needed to further understand if quantitative changes of these urinary proteins are short term or remains altered over prolonged period.
We recognize that this study has limitations, including small sample size, the inherent clinical variability of patient selection and a single urinary proteome profiling after resolution of UTI. Controls were selected to provide a similar age distribution to the VUR cohort. Our VUR cohort was predominantly female, consistent with a known clinical preponderance of VUR, but the availability of female controls to allow for equal distribution was extremely limited. We have used our resources as the largest pediatric urologic department in the world to generate an extremely well characterized clinical cohort that minimizes variability by controlling for grade of VUR, recurrent UTI and renal scarring. Although no clinical cohort is completely homogeneous, we identified significant changes in proteins that can contribute to susceptibility to UTI and UTI-acquired renal damage in patients with low grade VUR. Whether these changes preceded or resulted from recurrent UTIs will be answered in follow-up longitudinal studies, using the information gained from this pilot study. These longitudinal studies will expand VUR patient cohorts to include asymptomatic VUR patients, and VUR patients with UTI but no renal scarring, as well as well-defined urine sampling over the longer course of recovery from a UTI. Together, this can not only aid in a finer separation of urinary biomarkers for susceptibility to infection from the ones that are indicative of renal damage because of recurrent UTIs, but also resolve temporal alteration of inflammatory markers after resolution of UTI.
CONCLUSIONS
To better characterize VUR patients with a history of recurrent UTIs and renal scarring, we compared their urinary proteome profiles with that of healthy controls. Eighty proteins were differentially expressed in the patients' urine. We identified 62 proteins that were solely altered in our chosen VUR cohort which may represent candidate biomarkers to characterize susceptibility of these patients to develop UTI, and 18 proteins that may more broadly represent renal injury. The former, for instance, include AMPs, protease inhibitors and proteins involved in glycoside catabolism. Further studies will be extended to distinct VUR cohorts in order to separate candidate biomarkers based on susceptibility to UTIs and renal scarring. Ultimately, larger cohorts and longitudinal studies utilizing these findings will improve the diagnosis, delivery and cost-effectiveness of care for VUR patients.
DATA AVAILABILITY
Raw spectral data and Proteome Discoverer-generated MSF files have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD010469 (https://www.ebi.ac.uk/pride/archive/projects/PXD010469).
Supplementary Material
Acknowledgments
We thank the Department of Urology at Boston Children's Hospital for their continued support.
Footnotes
* We acknowledge the following sources that supported this research: NIH/NIDDK R01DK096238 (R.S.L.), The Societies for Pediatric Urology Research Grant (R.S.L.), NIH/NIDDK K01 DK101632 (J.W.F.), and NIDDK/T32 DK060442 (P.S.C.). The authors declare that they have no conflicts of interest with the contents of this article.
 This article contains supplemental Figures and Tables.
This article contains supplemental Figures and Tables.
1 The abbreviations used are:
- UTI
- urinary tract infections
- VUR
- vesicoureteral reflux
- ECM
- extracellular matrix
- UPJO
- ureteropelvic junction obstruction
- VCUG
- voiding cystourethrogram
- DMSA
- dimercaptosuccinic acid
- PIS
- pooled internal standard
- APP
- acute phase proteins.
REFERENCES
- 1. Flores-Mireles A. L., Walker J. N., Caparon M., and Hultgren S. J. (2015) Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abraham S. N., and Miao Y. (2015) The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 15, 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neal D. E., Jr. (1999) Host defense mechanisms in urinary tract infections. Urol. Clin. North Am. 26, 677–686, vii [DOI] [PubMed] [Google Scholar]
- 4. American Urological Association Mangement and Screening of Primary Vesicoureteral Reflux in Children. http://www.auanet.org/guidelines/vesicoureteral-reflux-. (2010-reviewed-and-validity-confirmed-2017)
- 5. Peters C. A., Skoog S. J., Arant B. S. Jr.; Copp H. L., Elder J. S., Hudson R. G., Khoury A. E., Lorenzo A. J., Pohl H. G., Shapiro E., Snodgrass W. T., and Diaz M. (2010) Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J. Urol. 184, 1134–1144 [DOI] [PubMed] [Google Scholar]
- 6. Investigators R. T., Hoberman A., Greenfield S. P., Mattoo T. K., Keren R., Mathews R., Pohl H. G., Kropp B. P., Skoog S. J., Nelson C. P., Moxey-Mims M., Chesney R. W., and Carpenter M. A. (2014) Antimicrobial prophylaxis for children with vesicoureteral reflux. N. Engl. J. Med. 370, 2367–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uhari M., Nuutinen M., and Turtinen J. (1996) Adverse reactions in children during long-term antimicrobial therapy. Pediatr. Infect. Dis. J. 15, 404–408 [DOI] [PubMed] [Google Scholar]
- 8. Karpman E., and Kurzrock E. A. (2004) Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J. Urol. 172, 448–453 [DOI] [PubMed] [Google Scholar]
- 9. Williams G., and Craig J. C. (2011) Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst. Rev. 16, CD001534. [DOI] [PubMed] [Google Scholar]
- 10. Edmonson M. B., and Eickhoff J. C. (2017) Weight gain and obesity in infants and young children exposed to prolonged antibiotic prophylaxis. JAMA Pediatr. 171, 150–156 [DOI] [PubMed] [Google Scholar]
- 11. Lendvay T. S., Sorensen M., Cowan C. A., Joyner B. D., Mitchell M. M., and Grady R. W. (2006) The evolution of vesicoureteral reflux management in the era of dextranomer/hyaluronic acid copolymer: a pediatric health information system database study. J. Urol. 176, 1864–1867 [DOI] [PubMed] [Google Scholar]
- 12. Serafini G., and Zadra N. (2008) Anaesthesia for MRI in the paediatric patient. Curr. Opin. Anaesthesiol. 21, 499–503 [DOI] [PubMed] [Google Scholar]
- 13. Greenberg S. B. (2011) Rebalancing the risks of computed tomography and magnetic resonance imaging. Pediatr. Radiol. 41, 951–952 [DOI] [PubMed] [Google Scholar]
- 14. Kitao T., Kimata T., Yamanouchi S., Kato S., Tsuji S., and Kaneko K. (2015) Urinary biomarkers for screening for renal scarring in children with febrile urinary tract infection: pilot study. J. Urol. 194, 766–771 [DOI] [PubMed] [Google Scholar]
- 15. Drube J., Schiffer E., Lau E., Petersen C., Kirschstein M., Kemper M. J., Lichtinghagen R., Ure B., Mischak H., Pape L., and Ehrich J. H. (2012) Urinary proteome analysis to exclude severe vesicoureteral reflux. Pediatrics 129, e356–e363 [DOI] [PubMed] [Google Scholar]
- 16. Devuyst O., Knoers N. V., Remuzzi G., and Schaefer F.; Board of the Working Group for Inherited Kidney Diseases of the European Renal Association and European Dialysis and Transplant Association. (2014) Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet 383, 1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Good D. M., Zurbig P., Argiles A., Bauer H. W., Behrens G., Coon J. J., Dakna M., Decramer S., Delles C., Dominiczak A. F., Ehrich J. H., Eitner F., Fliser D., Frommberger M., Ganser A., Girolami M. A., Golovko I., Gwinner W., Haubitz M., Herget-Rosenthal S., Jankowski J., Jahn H., Jerums G., Julian B. A., Kellmann M., Kliem V., Kolch W., Krolewski A. S., Luppi M., Massy Z., Melter M., Neususs C., Novak J., Peter K., Rossing K., Rupprecht H., Schanstra J. P., Schiffer E., Stolzenburg J. U., Tarnow L., Theodorescu D., Thongboonkerd V., Vanholder R., Weissinger E. M., Mischak H., and Schmitt-Kopplin P. (2010) Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol. Cell. Proteomics 9, 2424–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pontillo C., and Mischak H. (2017) Urinary peptide-based classifier CKD273: towards clinical application in chronic kidney disease. Clin. Kidney J 10, 192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magalhaes P., Pejchinovski M., Markoska K., Banasik M., Klinger M., Svec-Billa D., Rychlik I., Rroji M., Restivo A., Capasso G., Bob F., Schiller A., Ortiz A., Perez-Gomez M. V., Cannata P., Sanchez-Nino M. D., Naumovic R., Brkovic V., Polenakovic M., Mullen W., Vlahou A., Zurbig P., Pape L., Ferrario F., Denis C., Spasovski G., Mischak H., and Schanstra J. P. (2017) Association of kidney fibrosis with urinary peptides: a path towards non-invasive liquid biopsies? Sci. Rep. 7, 16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters C., and Rushton H. G. (2010) Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J. Urol. 184, 265–273 [DOI] [PubMed] [Google Scholar]
- 21. Vaezzadeh A. R., Briscoe A. C., Steen H., and Lee R. S. (2010) One-step sample concentration, purification, and albumin depletion method for urinary proteomics. J. Proteome Res. 9, 6082–6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou H., Morley S., Kostel S., Freeman M. R., Joshi V., Brewster D., and Lee R. S. (2016) Universal Solid-phase reversible sample-prep for concurrent proteome and N-glycome characterization. J Proteome Res 15, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vizcaino J. A., Csordas A., del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., Xu Q. W., Wang R., and Hermjakob H. (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deleted in proof.
- 25. Deleted in proof.
- 26. Pathan M., Keerthikumar S., Ang C. S., Gangoda L., Quek C. Y., Williamson N. A., Mouradov D., Sieber O. M., Simpson R. J., Salim A., Bacic A., Hill A. F., Stroud D. A., Ryan M. T., Agbinya J. I., Mariadason J. M., Burgess A. W., and Mathivanan S. (2015) FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 15, 2597–2601 [DOI] [PubMed] [Google Scholar]
- 27. Froehlich J. W., Kostel S. A., Cho P. S., Briscoe A. C., Steen H., Vaezzadeh A. R., Lee R. S. (2016) Urinary proteomics yield pathological insights for ureteropelvic junction obstruction. Mol. Cell. Proteomics 15, 2607–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moses M. A., Wiederschain D., Loughlin K. R., Zurakowski D., Lamb C. C., and Freeman M. R. (1998) Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 58, 1395–1399 [PubMed] [Google Scholar]
- 29. Roy R., Wewer U. M., Zurakowski D., Pories S. E., and Moses M. A. (2004) ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J. Biol. Chem. 279, 51323–51330 [DOI] [PubMed] [Google Scholar]
- 30. Roy R., Louis G., Loughlin K. R., Wiederschain D., Kilroy S. M., Lamb C. C., Zurakowski D., and Moses M. A. (2008) Tumor-specific urinary matrix metalloproteinase fingerprinting: identification of high molecular weight urinary matrix metalloproteinase species. Clin. Cancer Res. 14, 6610–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pories S. E., Zurakowski D., Roy R., Lamb C. C., Raza S., Exarhopoulos A., Scheib R. G., Schumer S., Lenahan C., Borges V., Louis G. W., Anand A., Isakovich N., Hirshfield-Bartek J., Wewer U., Lotz M. M., and Moses M. A. (2008) Urinary metalloproteinases: noninvasive biomarkers for breast cancer risk assessment. Cancer Epidemiol. Biomarkers Prev. 17, 1034–1042 [DOI] [PubMed] [Google Scholar]
- 32. Kushner I. (1982) The phenomenon of the acute phase response. Ann. N.Y. Acad. Sci. 389, 39–48 [DOI] [PubMed] [Google Scholar]
- 33. Crispe I. N. (2016) Hepatocytes as immunological agents. J. Immunol. 196, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabay C., and Kushner I. (1999) Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 [DOI] [PubMed] [Google Scholar]
- 35. Kokubun M., Imafuku Y., Okada M., Ohguchi Y., Ashikawa T., Yamada T., and Yoshida H. (2005) Serum amyloid A (SAA) concentration varies among rheumatoid arthritis patients estimated by SAA/CRP ratio. Clin. Chim. Acta 360, 97–102 [DOI] [PubMed] [Google Scholar]
- 36. Kushner I., Broder M. L., and Karp D. (1978) Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J. Clin. Invest. 61, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koj A., and Regoeczi E. (1978) Effect of experimental inflammation on the synthesis and distribution of antithrombin III and alpha1-antitrypsin in rabbits. Br. J. Exp. Pathol. 59, 473–481 [PMC free article] [PubMed] [Google Scholar]
- 38. Bauer J., Tran-Thi T. A., Northoff H., Hirsch F., Schlayer H. J., Gerok W., and Heinrich P. C. (1986) The acute-phase induction of alpha 2-macroglobulin in rat hepatocyte primary cultures: action of a hepatocyte-stimulating factor, triiodothyronine and dexamethasone. Eur. J. Cell Biol. 40, 86–93 [PubMed] [Google Scholar]
- 39. Du Clos T. W. (2000) Function of C-reactive protein. Ann Med 32, 274–278 [DOI] [PubMed] [Google Scholar]
- 40. Arnaud P. a. G., E. (1982) Alpha-1 acid glycoprotein-structure, genetics and biological significance. In Marker Proteins in Inflammation (Allen R. C., Bienvenu J., Laurent P., and Suskind R. M., Ed.), pp. 157–169, Walter de Gruyter, Berlin [Google Scholar]
- 41. Logdberg L., and Wester L. (2000) Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim. Biophys. Acta 1482, 284–297 [DOI] [PubMed] [Google Scholar]
- 42. Fournier T., Medjoubi N. N., and Porquet D. (2000) Alpha-1-acid glycoprotein. Biochim. Biophys. Acta 1482, 157–171 [DOI] [PubMed] [Google Scholar]
- 43. Barroso-Sousa R., Lobo R. R., Mendonca P. R., Memoria R. R., Spiller F., Cunha F. Q., and Pazin-Filho A. (2013) Decreased levels of alpha-1-acid glycoprotein are related to the mortality of septic patients in the emergency department. Clinics 68, 1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakamura K., Ito I., Kobayashi M., Herndon D. N., and Suzuki F. (2015) Orosomucoid 1 drives opportunistic infections through the polarization of monocytes to the M2b phenotype. Cytokine 73, 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ongay S., Martin-Alvarez P. J., Neususs C., and de Frutos M. (2010) Statistical evaluation of CZE-UV and CZE-ESI-MS data of intact alpha-1-acid glycoprotein isoforms for their use as potential biomarkers in bladder cancer. Electrophoresis 31, 3314–3325 [DOI] [PubMed] [Google Scholar]
- 46. Kramer H. B., Lavender K. J., Qin L., Stacey A. R., Liu M. K., di Gleria K., Simmons A., Gasper-Smith N., Haynes B. F., McMichael A. J., Borrow P., and Kessler B. M. (2010) Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 6, e1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mildner M., Stichenwirth M., Abtin A., Eckhart L., Sam C., Glaser R., Schroder J. M., Gmeiner R., Mlitz V., Pammer J., Geusau A., and Tschachler E. (2010) Psoriasin (S100A7) is a major Escherichia coli-cidal factor of the female genital tract. Mucosal. Immunol. 3, 602–609 [DOI] [PubMed] [Google Scholar]
- 48. Raju M. S., V. J., Kamaraju R. S., Sritharan V., Rajkumar K., Natarajan S., Kumar A. D., and Burgula S. (2016) Continuous evaluation of changes in the serum proteome from early to late stages of sepsis caused by Klebsiella pneumoniae. Mol. Med. Rep. 13, 4835–4844 [DOI] [PubMed] [Google Scholar]
- 49. Lee E. G., Jung N. C., Lee J. H., Song J. Y., Ryu S. Y., Seo H. G., Han S. G., Ahn K. J., Hong K. S., Choi J., and Lim D. S. (2016) Tolerogenic dendritic cells show gene expression profiles that are different from those of immunogenic dendritic cells in DBA/1 mice. Autoimmunity 49, 90–101 [DOI] [PubMed] [Google Scholar]
- 50. Vandevyver S., Dejager L., Vandenbroucke R. E., and Libert C. (2014) An acute phase protein ready to go therapeutic for sepsis. EMBO Mol. Med. 6, 2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iijima J., Konno K., and Itano N. (2011) Inflammatory alterations of the extracellular matrix in the tumor microenvironment. Cancers 3, 3189–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arroyo A. G., and Iruela-Arispe M. L. (2010) Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 86, 226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Korpos E., Wu C., and Sorokin L. (2009) Multiple roles of the extracellular matrix in inflammation. Curr. Pharm. Des. 15, 1349–1357 [DOI] [PubMed] [Google Scholar]
- 54. Vaday G. G., and Lider O. (2000) Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 67, 149–159 [DOI] [PubMed] [Google Scholar]
- 55. Eckhard U., Huesgen P. F., Schilling O., Bellac C. L., Butler G. S., Cox J. H., Dufour A., Goebeler V., Kappelhoff R., Keller U. A. D., Klein T., Lange P. F., Marino G., Morrison C. J., Prudova A., Rodriguez D., Starr A. E., Wang Y., and Overall C. M. (2016) Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol. 49, 37–60 [DOI] [PubMed] [Google Scholar]
- 56. Musante L., Tataruch D., Gu D., Liu X., Forsblom C., Groop P. H., and Holthofer H. (2015) Proteases and protease inhibitors of urinary extracellular vesicles in diabetic nephropathy. J. Diabetes Res. 2015, 289734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hua Y., and Nair S. (2015) Proteases in cardiometabolic diseases: Pathophysiology, molecular mechanisms and clinical applications. Biochim. Biophys. Acta 1852, 195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salem H. H., and Thompson E. A. (1987) The role of heparin cofactor II in the modulation of hemostasis. Dev. Biol. Stand 67, 67–72 [PubMed] [Google Scholar]
- 59. Chen H., Davids J. A., Zheng D., Bryant M., Bot I., van Berckel T. J., Biessen E., Pepine C., Ryman K., Progulski-Fox A., Kesavalu L., Moyer R., McFadden G., and Lucas A. (2013) The serpin solution; targeting thrombotic and thrombolytic serine proteases in inflammation. Cardiovasc Hematol. Disord. Drug Targets 13, 99–110 [DOI] [PubMed] [Google Scholar]
- 60. Tollefsen D. M., Pestka C. A., and Monafo W. J. (1983) Activation of heparin cofactor II by dermatan sulfate. J. Biol. Chem. 258, 6713–6716 [PubMed] [Google Scholar]
- 61. Koide T. (2008) Antithrombin and Heparin Cofactor II: Structure and Functions. In Recent Advances in Thrombosis and Hemostasis 2008 (Tanaka K., D. E. W., Ikeda Y., Iwanaga S., Saito H., Sueishi K., Ed.), Springer, Tokyo [Google Scholar]
- 62. Zhou H., Froehlich J. W., Briscoe A. C., and Lee R. S. (2013) The GlycoFilter: a simple and comprehensive sample preparation platform for proteomics, N-glycomics and glycosylation site assignment. Mol. Cell. Proteomics 12, 2981–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Apweiler R., Hermjakob H., and Sharon N. (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1473, 4–8 [DOI] [PubMed] [Google Scholar]
- 64. Ashida H., and Kato T., K., Y. (2007) Degradation of Glycoproteins. In Comprehensive Glycoscience ( H., K., Ed.), 1 ed., Vol. 3, pp. 151–170, Elsevier Science [Google Scholar]
- 65. Michalski J. C., and Klein A. (1999) Glycoprotein lysosomal storage disorders: alpha- and beta-mannosidosis, fucosidosis and alpha-N-acetylgalactosaminidase deficiency. Biochim. Biophys. Acta 1455, 69–84 [DOI] [PubMed] [Google Scholar]
- 66. Stahl M., Friis L. M., Nothaft H., Liu X., Li J., Szymanski C. M., and Stintzi A. (2011) L-fucose utilization provides Campylobacter jejuni with a competitive advantage. Proc. Natl. Acad. Sci. U.S.A. 108, 7194–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hooper L. V., and Gordon J. I. (2001) Glycans as legislators of host-microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology 11, 1R–10R [DOI] [PubMed] [Google Scholar]
- 68. Snider T. A., Fabich A. J., Conway T., and Clinkenbeard K. D. (2009) E. coli O157:H7 catabolism of intestinal mucin-derived carbohydrates and colonization. Vet Microbiol 136, 150–154 [DOI] [PubMed] [Google Scholar]
- 69. Ruiz-Palacios G. M., Cervantes L. E., Ramos P., Chavez-Munguia B., and Newburg D. S. (2003) Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278, 14112–14120 [DOI] [PubMed] [Google Scholar]
- 70. Qu Z., and Chaikof E. L. (2010) Interface between hemostasis and adaptive immunity. Curr. Opin. Immunol. 22, 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Orf K., and Cunnington A. J. (2015) Infection-related hemolysis and susceptibility to Gram-negative bacterial co-infection. Front. Microbiol. 6, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Larsen R., Gozzelino R., Jeney V., Tokaji L., Bozza F. A., Japiassu A. M., Bonaparte D., Cavalcante M. M., Chora A., Ferreira A., Marguti I., Cardoso S., Sepulveda N., Smith A., and Soares M. P. (2010) A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2, 51ra71. [DOI] [PubMed] [Google Scholar]
- 73. Lin T., Maita D., Thundivalappil S. R., Riley F. E., Hambsch J., Van Marter L. J., Christou H. A., Berra L., Fagan S., Christiani D. C., and Warren H. S. (2015) Hemopexin in severe inflammation and infection: mouse models and human diseases. Crit. Care 19, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Caza M., and Kronstad J. W. (2013) Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front. Cell Infect. Microbiol. 3, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Skaar E. P. (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6, e1000949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martins R., Maier J., Gorki A. D., Huber K. V., Sharif O., Starkl P., Saluzzo S., Quattrone F., Gawish R., Lakovits K., Aichinger M. C., Radic-Sarikas B., Lardeau C. H., Hladik A., Korosec A., Brown M., Vaahtomeri K., Duggan M., Kerjaschki D., Esterbauer H., Colinge J., Eisenbarth S. C., Decker T., Bennett K. L., Kubicek S., Sixt M., Superti-Furga G., and Knapp S. (2016) Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat. Immunol. 17, 1361–1372 [DOI] [PubMed] [Google Scholar]
- 77. Skaar E. P., Humayun M., Bae T. DeBord K. L., and Schneewind O. (2004) Iron-source preference of Staphylococcus aureus infections. Science 305, 1626–1628 [DOI] [PubMed] [Google Scholar]
- 78. Martins R., and Knapp S. (2018) Heme and hemolysis in innate immunity: adding insult to injury. Curr. Opin. Immunol. 50, 14–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw spectral data and Proteome Discoverer-generated MSF files have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD010469 (https://www.ebi.ac.uk/pride/archive/projects/PXD010469).