Abstract
Background
The effects of dietary garcinol on diarrhea and intestinal barrier function associated with its modulation of gut microbiota in weaned piglets were investigated.
Method
One hundred forty four weaned piglets (Duroc × Yorkshire × Landrace) from 16 pens (9 piglets per pen) were randomly divided into four treatment groups: controls (CON) or those supplemented with 200 mg/kg (LOW), 400 mg/kg (MID), or 600 mg/kg (HIGH) diet garcinol. After 14-day trial, three piglets per pen were chosen to collect plasma, intestinal tissue and colonic digesta samples.
Results
We demonstrated for the first time that garcinol promoted growth performance, as increased average daily feed intake (ADFI) and decreased feed/gain ratio (F/G); and reduced diarrhea incidence (P < 0.05); and strengthened antioxidant capacity, as an increased antioxidative index (P < 0.05). Additionally, garcinol ameliorated intestinal barrier dysfunction, as an increased villus height to crypt depth ratio, increased zonula occludens protein 1 (ZO-1), occludin and claudin-1 expression in the jejunum and ileum (P < 0.05), and decreased intestinal permeability (P < 0.05); and reduced inflammation, as decreased cytokine interleukin (IL)-6, IL-10, IL-1β and tumor necrosis factor-α (TNF-α) levels in the mucosa of the jejunum and ileum, and NF-κB p65 translocation (P < 0.05). Moreover, garcinol inhibited the growth of most harmful bacteria in the gut, especially Escherichia coli, and increased the growth of the beneficial bacteria Lactobacillus.
Conclusion
This work provides a fundamental basis for the future development of garcinol-functional food use for improving diarrhea and intestinal barrier function in weaned piglets and for understanding the biological effects of garcinol and its potential as a functional feed additive.
Keywords: Diarrhea, Garcinol, Gut microbiota, Intestinal barrier function, Weaned piglets
Background
The weaning period is one of the most stressful phases in pig production [1]. During this time, piglets must rapidly adapt to a multitude of psychosocial and environmental stressors [2]. It has already been proven that in the process of early weaning, piglets suffer from reduced food intake, diarrhea, atrophy of villi in the small intestine and other symptoms, which affect the digestion and absorption of nutrients [3]. Many studies have demonstrated that the two important effects of weaning stress are the change in intestinal structure and function of piglets and the imbalance in the intestinal microbiota [4, 5]. Indeed, weaning induces an impairment in intestinal epithelial barrier function and results in the disruption of the intestinal microecological balance of piglets. Once the microecological balance is broken, the potential pathogens in the intestinal tract will invade and colonize, thus causing various diseases, especially inflammation and diarrhea.
Dietary interventions offer a viable and practical approach to mitigate intestinal barrier dysfunction at weaning [6]. An overwhelming consensus emerges among countless evidence showing that plant extracts have beneficial effects on improving the growth performance, antioxidant capacity, and immunity of livestock and poultry and preventing various diseases [7, 8]. Recently, it was reported that curcumin and resveratrol, antioxidative plant extracts, can regulate the gut microbiota of weaned piglets, downregulate the TLR4 signaling pathway, alleviate intestinal inflammation, and ultimately increase intestinal immune function [9]. As another excellent antioxidative plant extract, garcinol, the major component of Garcinia indica (G. indica) fruit rind, has been widely used in the dietary supplementation of rodents [10–12]. Numerous studies have revealed the effect of garcinol on antioxidant and anti-inflammatory properties [13, 14]. Previous studies in vivo have indicated that garcinol can eliminate free radicals and show protective antioxidation effects [15]. In addition, garcinol may inhibit the activation of NF-κB and suppress the expression of interleukin IL-1β and IL-6 in the spinal cord of rats [16]. Moreover, garcinol has bactericidal effects and changes the gut microbiota composition in mice [17]. Due to these efficacies, garcinol may be beneficial in improving the growth performance, diarrhea and gut health of weaning pigs.
Currently, most of the studies on garcinol have focused on the anti-inflammatory and antioxidant activities in model mice and human cells. It has long been reported that oxidative stress can damage the structure and function of the gut of an animal. However, the beneficial effects of garcinol on the enhancement of the intestinal barrier function and gut microbiota as well as the improvement of diarrhea in weaned pigs have rarely been reported. The purpose of this study was to investigate the effects of garcinol on the diarrhea, intestinal barrier function and gut microbiota of weaned piglets, thus providing a theoretical basis and experimental evidence for understanding the biological effects of garcinol and its potential as a functional feed additive.
Material and methods
Animals and experimental design
All animal protocols used in this study were in accordance with the Guidelines for the Care and Use of Animals for Research and Teaching and approved by the Animal Care and Use Committee of Huazhong Agricultural University. A total of 144 weaned pigs (Duroc × Landrace × Yorkshire) were randomly assigned to 4 dietary treatments with 4 replicate pens per treatment. Their initial body weight (BW) was 7.19 ± 0.28 kg. Control pigs (CON) were fed a basal diet, and the other animals were fed a basal diet supplemented with 200 mg/kg, 400 mg/kg or 600 mg/kg garcinol for a 14-day period (An independent experiment (unpublished) was carried out before this study to determine the most appropriate of six possible doses garcinol (100, 200, 300, 400, 500 or 600 mg/kg) on the performance of weaned piglets. It was found that the optimum effect on the growth performance of weaned piglets was obtained when garcinol was provided at 200-600 mg/kg of diet). The basal diet was formulated to based on (NRC, 2012) meet nutritional requirements. And the ingredients and composition of the basal diet are listed in Table 1. The garcinol was purchased from Xin Lu Biotechnology Company (Xi’an, China), and extracted from dried fruit rind of Garcinia indica with a purity of 98.1%, as measured by HPLC. All pigs were allowed ad libitum access to feed and water throughout the experimental period. The animals were kept in a temperature-controlled environment at 24~25 °C and alternating light and dark cycles with 12 h intervals. All experimental pigs were healthy during the feeding period. Weights were obtained on every pig and feed disappearance was recorded on d 0, 7, 14 and prior to slaughter to calculate average daily feed intake (ADG), average daily gain (ADFI), and feed/gain ratio (F/G). Fecal scores were monitored during the feeding trial and quantified using a scale ranging from 0 to 3 with 0 = normally shaped feces, 1 = shapeless (loose) feces, 2 = thick, liquid (soft) feces, and 3 = thin, liquid feces and watery diarrhea. A piglet with a score greater than 1 was regarded as having diarrhea. The incidence of diarrhea (%) was calculated as a percentage of the number of diarrheal piglets during the period divided by the total number of piglets. On d 0 at the beginning and d 14 at the end of the study, three randomly selected pigs from each pen within a treatment were selected for blood samples. The biochemical index of blood samples showed no difference among the treatments in the piglets at the start of the study (d 0). The blood samples from piglets of each treatment (n = 12) were collected from the jugular vein via 10 mL vacutainer tubes that contained EDTA as an anticoagulant. And the plasma samples were then centrifuged at 3500×g for 10 min at 4 °C. And then the piglets were sacrificed after anesthesia by i.m. injection with 50 mg/kg BW sodium pentobarbital. Immediately, a piece (2 cm-length) of the middle jejunum and distal ileum (2 cm-length) were collected (n = 12), rinsed with cold saline (NaCl 9 g/L, 4 °C), and fixed in paraformaldehyde. Other segments of the middle jejunum and distal ileum (5 cm-length) of the piglets (n = 12) were opened and thoroughly rinsed with sterile normal saline, and then the mucosa was collected by scraping with glass slides and immediately snap-frozen in liquid nitrogen and stored at − 80 °C.
Table 1.
Composition and nutrient levels of the basal diet (as fed basis)
| Ingredient | % | Nutrient level | |
|---|---|---|---|
| Extruded corn | 58.08 | DE, MJ/kg | 14.39 |
| Dehulled soybean meal | 20.00 | CP, % | 20.57 |
| Whey powder | 10.00 | Calcium, % | 0.85 |
| Fishmeal | 4.00 | Available P, % | 0.46 |
| Soybean oil | 3.00 | Lys, % | 1.70 |
| Calcium carbonate | 0.85 | Met, % | 0.66 |
| Dicalcium phosphate | 1.00 | Met + Cys, % | 0.97 |
| Sodium bicarbonate | 0.15 | Thr, % | 1.06 |
| Salt | 0.15 | Trp, % | 0.28 |
| L-Arg·HCl | 0.87 | ||
| DL -Met | 0.22 | ||
| Thr | 0.30 | ||
| Trp | 0.08 | ||
| Vitamin and mineral mix1 | 1.30 | ||
| Total | 100 |
1Provided per kilogram of diet: vitamin A, 2200 IU; vitamin D3, 220 IU; vitamin E, 16 IU; vitamin K3, 0.5 mg; vitamin C, 200 mg; thiamin, 1.5 mg; riboflavin, 4 mg; pyridoxine, 7 mg; cyanocobalamin, 0.02 mg; pantothenic acid, 12 mg; niacin, 30 mg; folic acid, 0.3 mg; biotin, 0.08 mg; Fe (FeSO4.H2O), 100 mg; Cu (CuSO4·5H2O), 6 mg; Mn (MnSO4·H2O), 4 mg; Zn (ZnSO4 ·H2O), 100 mg; I (Ca (IO3)2), 0.14 mg; Se (Na2SeO3), 0.30 mg; Co (CoSO4 ·7H2O), 0.15 mg. The carrier was zeolite
Analysis of serum biochemical indices and antioxidant properties
The levels of serum total protein (TP); albumin (ALB); blood urea nitrogen (BUN); total cholesterol (TCH); glucose (GLU); serum alanine aminotransferase (ALT); aspartate aminotransferase (AST) were analyzed by Zhongnan Hospital (Wuhan, Hubei, China); and the levels of glutathione peroxidase (GSH-Px), catalase (CAT) and superoxide dismutase (SOD), and total antioxidant capacity (T-AOC) and malondialdehyde (MDA) in plasma were determined using commercial kits according to the instructions of the manufacturer (Nanjing Jiancheng Bioengineering company, Jiangsu, China).
Analysis of intestinal morphology and permeability
The fixed jejunal and ileal segments were dehydrated, embedded in paraffin, and sectioned for intestinal morphology as described previously [18]. Briefly, sections of 5-μm thickness were deparaffinized in xylene, rehydrated, and stained with hematoxylin and eosin (H&E). Images were obtained using an Axio Scope A1 microscope (Zeiss, Germany). The villous height and crypt depth of each segment were measured with Image-Pro software (Media Cybernetics, Rockville, MD). A minimum of 9 villi from each sample were measured for each treatment. The mean villus height, crypt depth, and villus height to crypt depth ratio for each pig were used for analysis. The contents of diamine oxidase (DAO) and D-lactate and endothelin -1 (ET-1) and nitric oxide (NO) were measured using an instrument (Biochemical Analytical Instrument, Beckman CX4, Beckman Coulter Inc., Brea, CA).
Intestinal cytokines determined by ELISA
After grinding in liquid nitrogen, total protein was extracted from the jejunal and ileal mucosa samples with lysis buffer (KeyGEN, Nanjing, China), followed by clarification by centrifugation (3500×g for 10 min at 4 °C). The concentrations of IL-1β, IL-6, IL-10, and tumor necrosis factor-α (TNF-α) were determined by using ELISA kits (Nanjing Jiancheng Bioengineering company, Jiangsu, China).
Real-time PCR for the relative measurement of transcripts and quantification of microbiota in the jejunum and ileum
Total RNA was extracted from mucosal tissue powders (approximately 0.1 g, n = 12) using TRIzol reagent (100 mg tissue per 1 mL TRIzol; Invitrogen, Carlsbad, CA). The purity and the yield of the RNA were evaluated using a NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA). The integrity of RNA was determined by both agarose gel (1%) electrophoresis and spectrometry (A260/A280, Beckman DU-800; Beckman Coulter, Inc.)., and 1 μg of RNA was used to generate cDNA in a volume of 20 μL with a PrimeScript RT Reagent Kit (Takara, Dalian, China). Real-time PCR was performed in duplicate with SYBR Green master mix on a CFX Connect Detection system (Bio-Rad, Hercules, CA). Each 10-μL reaction included 5 μL iTaq Universal SYBR Green Supermix (Bio-Rad), 0.5 μL forward primer (10 μmol/L), 0.5 μL reverse primer (10 μmol/L), and 4 μL 20-fold diluted cDNA. The thermocycler protocol consisted of 10 min at 95 °C followed by 40 cycles of 30 s at 95 °C, 30 s at 60 °C, and 20 s at 72 °C. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin were used as housekeeping genes, and the primers for the real-time PCR in this study are listed in Table 2. The relative mRNA expression of the target genes Zonula occludens protein 1 (ZO-1), Occludin and anti-Claudin-1 was determined using the 2–ΔΔCt method, all data for each target transcript were normalized to the mRNA level of the GAPDH and β-actin gene.
Table 2.
PCR primer sequences used in this study
| Genes | Accession No. | Sequence (5’→3’) | Size, bp |
|---|---|---|---|
| ZO-1 | XM_021098896.1 | F:CCAACCATGTCTTGAAGCAGC | 215 |
| R:TGCAGGAGTGTGGTCTTCAC | |||
| Occludin | NC_010458.4 | F:TCAGGTGCACCCTCCAGATT | 169 |
| R:TATGTCGTTGCTGGGTGCAT | |||
| Claudin-1 | NC_010455.5 | F:AAGGACAAAACCGTGTGGGA | 247 |
| R:CTCTCCCCACATTCGAGATGATT | |||
| GAPDH | NM_001206359.1 | F:CCAAGGAGTAAGAGCCCCTG | 125 |
| R:AAGTCAGGAGATGCTCGGTG | |||
| β-actin | XM_003124280.4 | F:CTCCAGAGCGCAAGTACTCC | 153 |
| R:AATGCAACTAACAGTCCGCC |
ZO1 Zona occludens protein 1, GAPDH Glyceraldehyde 3-phosphate dehydrogenase
Digesta from jejunum and ileum samples (n = 12) were collected for the real-time PCR assay. The species-specific PCR primers are listed in Table 3. Real-time PCR was performed on an iCycler IQ real-time detection system using the iCycler optical system interface software version 2.3 (Bio-Rad, Veenen-daal, Netherlands) as previously described [19]. The reaction mixture (25 μL) consisted of 12.5 μL of a master mix (IQ SYBR Green Supermix; Bio-Rad), 0.2 μmol/L of each primer set, and 5 μL of template DNA. The amount of DNA in each treatment was determined, and the mean values were calculated. A standard curve was generated by using the serially diluted 16S rRNA gene amplicons obtained from Lactobacillus sp. The species-specific primer LAC1 and the primer Lab0677 [20] were used for the quantification of Lactobacillus sp. with the following conditions: an initial DNA denaturation step at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 30 s and primer annealing and extension at 60 °C for 1 min. Total Escherichia coli (E. coli) were quantified using the combination of the forward primer K88 AD and reserve primer K88 AD [21] and the following cycling program: after the initial denaturation 92 °C for 45 s, 40 cycles were applied at 50 °C for 45 s, and binding and extension at 75 °C for 45 s.
Table 3.
Primers used for Escherichia coli and Lactobacillus
Western blotting and immunoprecipitation
The mucosal scrapings were homogenized on ice in RIPA lysis buffer (Upstate; Temecula, CA) containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and phosphathase inhibitor cocktail 1 (Sigma-Aldrich, St. Louis, MO). After centrifugation at 4 °C and 14,000×g, the supernatants were collected for the assay. The primary antibodies used were: anti-ZO-1, anti-Occludin and anti-Claudin-1 antibodies (1:1,000 dilution; Abcam). For western blotting, the lysates were then resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Membranes processed with the antibodies of interest were treated with Restore™ Plus Western Blot Stripping Buffer (Pierce, Rockford, IL) for one hour or overnight and then exposed to anti-β-actin or anti-nucleolin (Sigma-Aldrich, St. Louis, MO) to assess the equal loading.
Microbial genomic DNA extraction
Total bacterial genomic DNA was extracted from colonic digesta (approximately 0.2 g) by using FastDNA SPIN extraction kits (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer’s instructions and stored at − 20 °C until further analysis. The quantity and quality of extracted DNA were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively. After genomic DNA quantity and quality testing, 30 ng genomic DNA was used to run PCR per reaction.
16S rDNA amplicon pyrosequencing and sequence analysis
Ten milliliters of colonic digesta samples from piglets (n = 12) were collected and stored at − 80 °C. PCR amplification of the bacterial 16S rRNA gene V-3V4 region was performed using the forward primer 338F (5′ -ACTCCTACGGGAGGCAGCA-3′) and the reverse primer 806R (5′ -GGACTACHVGGGTWTCTAAT-3′). Sample-specific 7-bp barcodes were incorporated into the primers for multiplex sequencing. The PCR components contained 5 μL Q5 reaction buffer (5×), 5 μL Q5 High-Fidelity GC buffer (5×), 0.25 μL Q5 High-Fidelity DNA Polymerase (5 U/μL), 2 μL (2.5 mmol/L) dNTPs, 1 μL (10 μmol/L) of each forward and reverse primer, 2 μL DNA Template, and 8.75 μL ddH2O. Thermal cycling consisted of initial denaturation at 98 °C for 2 min, followed by 25 cycles of denaturation at 98 °C for 15 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final extension for 5 min at 72 °C. The PCR amplicons were purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN) and quantified using a PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, the amplicons were pooled in equal amounts, and pair-end 2 × 300 bp sequencing was performed using an Illumina MiSeq platform with a MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
The Quantitative Insights Into Microbial Ecology (QIIME, v1.8.0) pipeline was employed to process the sequencing data, as previously described [22]. Briefly, raw sequencing reads with exact matches to the barcodes were assigned to respective samples and identified as valid sequences. The low-quality sequences were filtered through following criteria [23, 24]: sequences that had a length of < 150 bp, sequences that had average Phred scores of < 20, sequences that contained ambiguous bases, and sequences that contained mononucleotide repeats of > 8 bp. Paired-end reads were assembled using FLASH (v1.2.7) [25]. After chimera detection, the QIIME software (v1.8.0) USEARCH (v5.2.236) was used to check and remove chimeric sequences, and the remaining high-quality sequences were clustered into operational taxonomic units (OTUs) at 97% sequence identity by UCLUST (v1.2.22q) [26]. A representative sequence was selected from each OTU using default parameters. OTU taxonomic classification was conducted by BLAST searching the representative sequences set against the Greengenes Database [27] using the best hit [28]. An OTU table was further generated to record the abundance of each OTU in each sample and the taxonomy of these OTUs. OTUs containing < 0.001% of total sequences across all samples were discarded. To minimize the difference in sequencing depth across samples, an averaged, rounded rarefied OTU table was generated by averaging 100 evenly resampled OTU subsets under a 90% minimum sequencing depth for further analysis.
Statistical analyses
All the results from experiment were analyzed by using the one-way ANOVA, performed using the MIXED procedure of SAS (SAS Inst., Inc., Cary, NC, US). Replicate (n = 4) served as the experimental unit. Data were analyzed using the general linear model (GLM) with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The results in the tables are shown with the means ± SEM, and other figure results are presented with means ± SEM. Means are considered significantly different at P < 0.05.
Results
Effect of dietary garcinol on the performance, diarrhea rate and blood biochemical indices of weaned pigs
The growth performance and diarrhea of weaned pigs are shown in Table 4. Compared with the control group, dietary supplementation with garcinol significantly increased ADG (P < 0.05) and decreased F/G (P < 0.05) but had no effect on ADFI (P > 0.05). In addition, dietary garcinol significantly decreased the diarrhea rate compared with that in control group (P < 0.05). The F/G and diarrhea rates in the 200 and 400 mg/kg garcinol groups were significantly lower than those in the 600 mg/kg group. The blood indices of weaned pigs are shown in Table 5. Compared with the control group, dietary garcinol had no effect on TP, ALB, BUN, TCH, GLU, ALT and AST in plasma. Moreover, compared with the control group, garcinol significantly increased T-AOC, SOD, CAT and GSH-Px and decreased the MDA content (P < 0.05).
Table 4.
Effects of garcinol on growth performance and diarrhea rate of weaned piglets
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| Initial weight, kg | 7.21 | 7.19 | 7.18 | 7.20 | 0.03 |
| Final weight, kg | 11.35ab | 12.56a | 12.62a | 12.79a | 0.17 |
| ADG, g | 154.67b | 192.15a | 202.63a | 199.58a | 5.27 |
| ADFI, g | 349.53 | 350.46 | 356.72 | 358.68 | 11.56 |
| F/G | 2.26a | 1.97b | 1.96b | 2.02c | 0.05 |
| Diarrhea incidence | 10.34a | 7.25b | 6.54b | 4.67c | 0.70 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). ADFI Average daily feed intake, ADG Average daily gain, and F/G Feed/gain ratio. n = 12
Table 5.
Effects of garcinol on blood biochemical indices of weaned piglets
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| TP, g/L | 34.16 | 36.25 | 27.45 | 28.46 | 0.95 |
| ALB, g/L | 26.21 | 23.47 | 21.35 | 22.76 | 0.62 |
| BUN, U/L | 3.71 | 3.67 | 3.58 | 3.69 | 0.22 |
| TCH, U/L | 2.05 | 2.24 | 2.01 | 2.09 | 0.05 |
| GLU, mmol/L | 5.94 | 5.99 | 5.84 | 5.89 | 0.10 |
| ALT, U/L | 72.56 | 51.35 | 61.35 | 52.34 | 3.26 |
| AST, U/L | 65.21 | 69.35 | 78.45 | 72.53 | 2.76 |
| MDA, nmol/mL | 2.92a | 2.08b | 1.82b | 1.72b | 0.19 |
| T-AOC, U/mL | 1.94b | 2.54a | 2.69a | 2.72a | 2.21 |
| SOD, U/mL | 67.59b | 77.33a | 81.06a | 82.95a | 3.87 |
| CAT, U/mL | 20.91b | 22.47a | 24.09a | 24.37a | 1.42 |
| GSH- Px, U/mL | 872.4b | 896.1a | 911.6a | 932.75a | 43.56 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). TP, serum total protein; ALB Albumin, BUN Blood urea nitrogen, TCH Total cholesterol, GLU Glucose, ALT Serum alanine aminotransferase, AST Aspartate aminotransferase, MDA Malonaldehyde, GSH- Px Glutathione peroxidase, CAT Catalase, T-AOC Total antioxidative capacity, T-SOD Total superoxide dismutase. n = 12
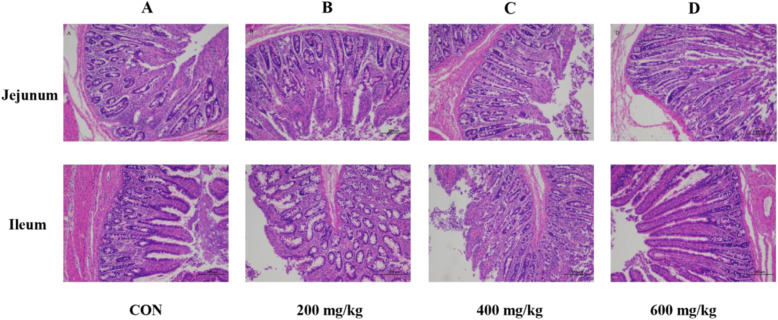
Effect of dietary garcinol on intestinal morphology of weaned pigs
As shown in Table 6 and Fig. 1, compared with the control group, dietary supplementation with garcinol significantly decreased the (P < 0.05) jejunal crypt depth and increased (P < 0.05) villus height to crypt depth ratio in both jejunum and ileum (Table 4). In addition, the villus height to crypt depth ratios in both the jejunum and ileum in the 200 and 400 mg/kg garcinol groups were significantly lower than those in the 600 mg/kg group (P < 0.05). For intestinal permeability (Table 7), compared to the control group, dietary garcinol significantly decreased the diamine oxidase (DAO) and D-lactate contents (P < 0.05). The DAO and D-lactate contents in the groups treated with 200 and 400 mg/kg garcinol were significantly lower than those in the 600 mg/kg group (P < 0.05).
Table 6.
Effects of garcinol on small intestine structure of weaned piglets
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| Villus height, μm | |||||
| Jejunum | 310.26a | 319.52b | 324.67b | 375.64c | 4.36 |
| Ileum | 267.63a | 346.57b | 342.27b | 398.43c | 9.24 |
| Crypt depth, μm | |||||
| Jejunum | 108.35a | 105.67b | 96.27b | 87.36b | 3.24 |
| Ileum | 90.83 | 92.67 | 87.67 | 88.59 | 2.89 |
| V/C | |||||
| Jejunum | 2.86a | 3.02b | 3.37b | 4.30c | 0.10 |
| Ileum | 2.95a | 3.74b | 3.91b | 4.49c | 0.12 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). V/C Villus height/Crypt depth. n = 12
Fig. 1.
Effects of garcinol on histopathological changes of jejunum and ileum with H&E staining (original magnification of 100×). Dietary treatments: a control (basal diet), b basal diet+ 200 mg/kg garcinol, c basal diet+ 400 mg/kg garcinol, d basal diet+ 600 mg/kg garcinol
Table 7.
Effects of garcinol on intestinal permeability in piglets
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| DAO, ng/mL | 39.46a | 10.76b | 9.56b | 5.67c | 2.79 |
| D-Lactate, μmol/mL | 55.26a | 39.47b | 32.79b | 27.64c | 1.98 |
| ET-1, ng/L | 130.67 | 110.47 | 95.26 | 91.58 | 5.04 |
| NO, μmol/L | 54.23 | 44.26 | 41.89 | 43.28 | 1.67 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). DAO Diamine oxidase, ET-1 Endothelin-1, NO Nitric oxide. n = 12
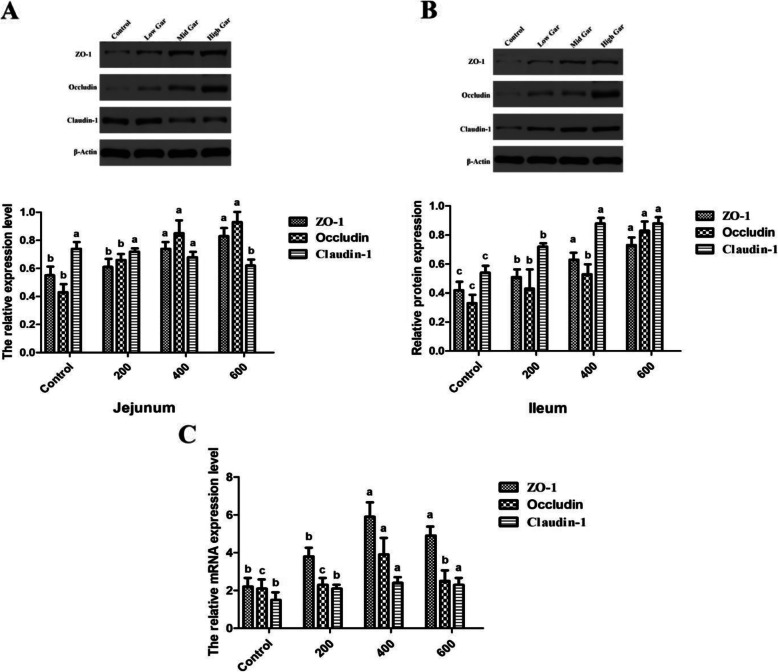
Effect of dietary garcinol on tight junctions in the intestinal mucosa
As shown in Fig. 2, for tight junction-related components, compared with the control group, dietary garcinol significantly increased (P < 0.05) ZO-1 and occludin protein expression in both the jejunal and ileal mucosa and claudin-1 in the ileum (Fig. 2a). The expression of ZO-1 and occludin in both jejunal and ileal mucosa and claudin-1 in the ileum of the 200 and 400 mg/kg garcinol groups was significantly lower than those in the 600 mg/kg group (P < 0.05). In addition, the expression of claudin-1 in the jejunum of 400 and 600 mg/kg garcinol groups was significantly lower than that in the control group (P < 0.05).
Fig. 2.
Effect of dietary garcinol on tight junctions in the intestinal mucosa. a Relative protein expression of ZO-1, occludin and claudin-1 in jejunum, b Relative protein expression of ZO-1, occludin and claudin-1 in ileum, c The relative mRNA expression of ZO-1, occludin and claudin-1 in jejunum. Values with no common superscripts means significant difference (P < 0.05). n = 12
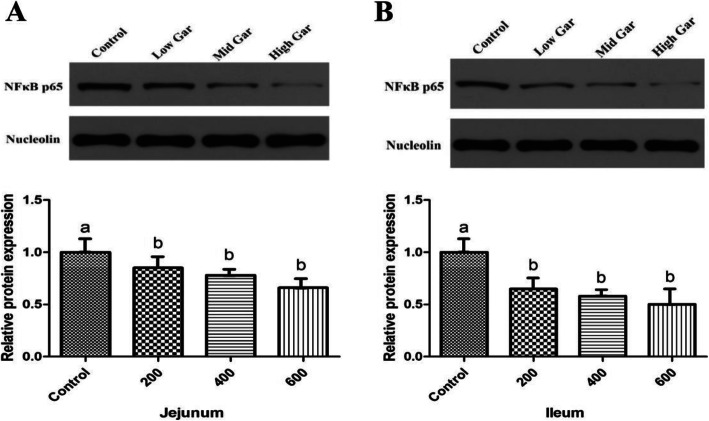
Effect of dietary garcinol on intestinal inflammation in weaned pigs
To evaluate the effects of garcinol on immune responses in the intestine of weaned pigs, the serum levels of immunoglobulin, cytokines IL-6, IL-10, IL-1β and TNF-α and NF-κB p65 translocation in the mucosa of the jejunum and ileum were determined (Tables 8, 9 and Fig. 3). There was no effect of dietary garcinol on the serum levels of immunoglobulin (P > 0.05). In addition, compared with the control group, dietary garcinol decreased the levels of IL-10 and significantly decreased (P < 0.05) the serum contents of IL-6, IL-1β and TNF-α. The levels of IL-6, IL-1β and TNF-α in the 200 and 400 mg/kg garcinol groups were significantly lower than those in the 600 mg/kg group (P < 0.05). Additionally, compared with CON, garcinol significantly decreased the NF-κB p65 protein content in both the jejunum and ileum (Fig. 3), and jejunal and ileal NF-κB p65 translocation in the 200 and 400 mg/kg garcinol groups was significantly lower (P < 0.05) than that in the 600 mg/kg group.
Table 8.
Effects of garcinol on serum immunoglobulin in weaned piglets
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| IgA, g/L | 1.29 | 1.24 | 1.28 | 1.26 | 0.03 |
| IgG, g/L | 2.59 | 3.74 | 3.95 | 3.82 | 0.24 |
| IgM, g/L | 0.42 | 0.37 | 0.36 | 0.40 | 0.02 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). IgA Immuneglobulin A, IgG Immuneglobulin G, IgM Immuneglobulin M. n = 12
Table 9.
Effects of garcinol on serum cytokines in weaned piglets
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| TNF-α, ng/L | 297.36a | 106.58b | 96.26b | 73.59c | 11.26 |
| IL-1β, ng/L | 17.56a | 14.56b | 11.23b | 8.95c | 0.79 |
| IL-6, ng/L | 45.72a | 25.62b | 20.67b | 14.58c | 1.85 |
| IL-10, ng/L | 157.92 | 192.34 | 210.53 | 219.57 | 6.15 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). TNF-α, tumor Necrosis Factor α; IL-1β, intermediate language 1 β; IL-6, intermediate language 6; IL-10, intermediate language 10. n = 12
Fig. 3.
Effect of dietary garcinol on NF-κB p65 expression in jejunum and ileum of weaned pigs. a Relative protein expression of NF-κB p65 in jejunum, b Relative protein expression of NF-κB p65 in ileum. Values with no common superscripts means significant difference (P < 0.05). n = 12
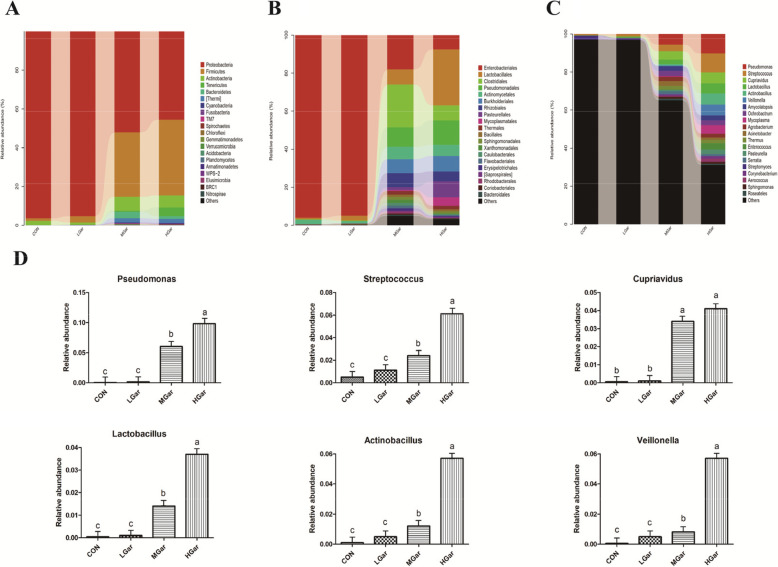
Effect of dietary garcinol on the diversity of gut microbiota
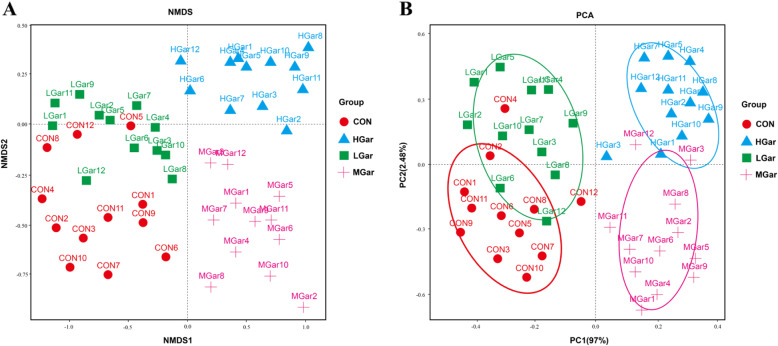
We then used high-throughput sequencing to perform 16S rRNA gene analysis in colonic digesta samples from 48 pigs. The results of the Chao index, Shannon index, ACE index and Simpson index are shown in Table 10. There were no significant differences between the 200 mg/kg garcinol and the control group, while all indices of the samples in the 400 and 600 mg/kg groups were significantly higher than those in the other groups (P < 0.05). The relative abundances of four different taxonomic levels are shown in Fig. 4 (phylum, order and genus). The results of phylum, order and genus analysis showed that the 200 mg/kg garcinol and control groups had a similar relative abundance, while the 400 and 600 mg/kg garcinol groups had a similar relative abundance. At the phylum level, 400 and 600 mg/kg garcinol decreased the relative abundance of Proteobacteria but increased the relative abundance of Firmicutes, Actinobacteria, Tenericutes and Bacteroidetes compared with the effects of 200 mg/kg garcinol and control treatments (Fig. 4a). At the order level, 400 and 600 mg/kg garcinol decreased the relative abundance of Enterobacteriales but increased the relative abundance of Lactobacillales, Clostridiales, Pseudomonadales, Actinomycetales, Burkholderiales, Pasteurellales and Mycoplasmatales (Fig. 4b). At the genus level, there was a significant change in 6 genera (Pseudomonas, Streptococcus, Cupriavidus, Lactobacillus, Actinobacillus and Veillonella) (Fig. 4c). However, most microbiota were not identified. The relative abundance of these 6 genera was significantly increased in the 400 and 600 mg/kg garcinol groups compared with those in the other groups (P < 0.05), while the relative abundance of Pseudomonas, Streptococcus, Lactobacillus, Actinobacillus and Veillonella in the 400 mg/kg garcinol group was significantly lower than that in the 600 mg/kg garcinol group (P < 0.05) (Fig. 4d). Enterobacteriales and Lactobacillales were significantly changed in the 400 and 600 mg/kg garcinol groups. We quantified these two important bacterial species in the jejunum and ileum digesta by using quantitative real-time PCR analysis. The results are shown in Table 11. The 200 mg/kg garcinol and control groups had a similar relative abundance of E. coli and Lactobacillus. Moreover, the number of Lactobacillus in pigs from the 400 and 600 mg/kg garcinol groups was significantly higher while the number of E. coli was significantly lower than those in the other groups (P < 0.05), and the number of Lactobacillus in the 600 mg/kg group was significantly higher than that in the 400 mg/kg garcinol group (P < 0.05). In addition, the results of both nonmetric multidimensional scaling (NMDS) and the principal component analysis (PCA) plot showed a significantly different composition of microbiota among the groups (P < 0.05) (Fig. 5).
Table 10.
Effect of garcinol on bacterial community alpha diversity in weaned piglet colon
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| Richness index | |||||
| Chao | 361.50a | 350.97a | 770.00b | 712.51b | 53.85 |
| Ace | 364.51a | 357.41a | 772.20b | 713.90b | 53.71 |
| Diversity index | |||||
| Shannon | 1.74a | 1.97a | 6.92b | 6.10b | 0.71 |
| Simpson | 0.31a | 0.35a | 0.97b | 0.96b | 0.10 |
The richness index (Chao and ACE) and diversity index (Shannon and Simpson) were calculated using the mothur program. Values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). n = 12
Fig. 4.
Effect of dietary garcinol on the composition of colonic microbiota in weaned piglets. a Graph represents the OTUs at different taxonomical levels: phylum (n = 12). b Graph represents the OTUs at different taxonomical levels: order (n = 12). c Graph represents the OTUs at different taxonomical levels: genus (n = 12). d The change in the relative abundance of genus Pseudomonas, Streptococcus, Cupriavidus, Lactobacillus, Actinobacillus and Veillonella. Metastats analysis was applied to identify the significantly differentially abundant genera among groups. Different letters above the bars denotes significantly differentially abundant genera among groups. (P < 0.05). n = 12
Table 11.
Quantitative real-time PCR analysis of total Escherichia coli, Lactobacillus in jejunum and ileum samples [log10 (copies/g wet weight)]a
| Items | Control group | The level of garcinol, mg/kg | SEM | ||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| Escherichia coli | |||||
| Jejunum | 8.55a | 8.17a | 7.23b | 7.24b | 0.86 |
| Ileum | 8.74a | 8.21a | 7.42b | 7.36b | 0.44 |
| Lactobacillus | |||||
| Jejunum | 7.69b | 8.03b | 8.64a | 8.56a | 0.68 |
| Ileum | 7.91b | 8.25b | 8.97a | 8.82a | 0.32 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). n = 12
Fig. 5.
Microbial structure analysis: a NMDS analysis, b PCA. n = 12
Discussion
Weaning, on the one hand, is a critical stage of gut development in mammals, while on the other hand, weaning stress can cause intestinal barrier dysfunction, inflammation, and gut microbiota disruption, accompanied by a reduction in the absorption capacity of the small intestine, eventually lead to the increasing of diarrhea [3, 5]. The weaning period is associated with impairment of cellular immunity, oxidative stress and an increased prevalence of gastrointestinal infection in many species [29]. Therefore, appropriate weaning nutrition appears to be essential for enhancing the gut health of animals. Previous studies have shown that some plant extracts (e.g., curcumin, resveratrol and essential oils) had beneficial effects on the performance and intestinal barrier function of mice and weaned piglets [9, 30, 31]. Currently, garcinol is regarded as one of the most promising plant extracts with effective antioxidant properties [32]. However, the effect and dose effect of dietary garcinol on growth performance, diarrhea, intestinal barrier function and gut microbiota are poorly understood. In this work, we demonstrated for the first time that as a natural antioxidant, garcinol efficiently improved growth performance and diarrhea, strengthened antioxidant capacity, ameliorated intestinal barrier dysfunction and inflammation, and altered the gut microbiota of weaning piglets.
First, the effect of dietary garcinol on growth performance, diarrhea and the blood biochemical index were measured. At the beginning of the study, we collected the blood samples and verified there were no differences among the piglets, and they were healthy at the start of the study. However, after 14-day period trial, the diarrhea incidence is high in control group, which may be associated with the weaning event [3, 5] Compared with the control group, dietary garcinol increased the ADG and F/G, suggesting that the growth performance was improved. In addition, dietary garcinol significantly decreased the diarrhea rate of weaned pigs. Previous studies have shown that the body gain and nutrient absorption of piglets, as well as the diarrhea rate are associated with oxidative stability, intestinal morphology and gut microbiota balance [33]. Then, we further analyzed the biochemical and antioxidative index in serum. Blood biochemical indices can reflect the growth or production performance of animals. The results showed that the antioxidant capacity was improved after treating with all doses of garcinol, while the levels of ALT, AST, BUN were not affected by garcinol. These findings suggested that garcinol supplementation did not have side effects on the hepatic and renal functions of weaned piglets. As an antioxidant additive, the effect of garcinol on the antioxidative index is consistent with other studies both in vitro and in vivo [16].
We further evaluated the effect of dietary garcinol on the intestinal morphology of weaned pigs. It has reported that weaning stress could cause intestinal injury with the reduced intestinal villi height, the deepened recess, the changes of intestinal morphology from dense and finger-like villi to smooth and tongue-like villi, and the reduced active absorption capacity of the small intestine [5]. Normal intestinal function depends on the local barrier, which prevents the translocation of bacteria and endotoxins contained within the intestinal lumen to extraintestinal sites, and largely prevents the occurrence of diarrhea [34]. Intestinal mucosa permeability is the principal basis for the diagnosis of the intestinal barrier dysfunction [34]. Serum D-lactate and DAO can be used as indicators of intestinal integrity of the gastrointestinal tract [35]. Furthermore, the tight junction is the most important structure of the epithelial barrier function. The tight junction structure between intestinal epithelial cells is closely related to the function of the intestinal physical barrier. Protecting the tight junction of the intestinal tract plays an important role in maintaining the polarity of intestinal epithelial cells and preventing material spillage in the epithelial cell gap [36]. ZO-1 protein is not only involved in the formation of tight junctions and the regulation of the permeability of epithelial cells but also involved in the regulation of the composition and function of the parietal cytoskeleton [37]. Occludin is a transmembrane protein closely associated with the integrity of the structure and the role of the intestinal epithelial barrier function [38]. This study showed that occludin protein expression was positively associated with intestinal epithelial barrier function. Interestingly, the results of the previous study also clearly indicated that dietary administration of garcinol at two dose levels markedly ameliorated DSS-induced ACF formation, morphological signs of mucosal damage, and subsequent inflammation [39]. In the present study, dietary garcinol improved the intestine structure of piglets. The villus height to crypt depth ratio and the expression of tight junction protein ZO-1, occludin and claudin-1 were increased, and intestinal permeability was decreased. There are no relevant studies on garcinol intestinal morphology; thus, these results may be the first to report the beneficial effects of garcinol. These effects may also be due to the antioxidant properties of garcinol, as reactive oxygen species (ROS) could be one of the main causes of intestinal injury and oxidative damage, leading to the decrease of growth performance and increase of diarrhea. Therefore, in this case, the improvement in intestine structure and permeability by dietary garcinol could be one of the reasons for the improvement of performance and diarrhea of weaned pigs.
Weaning-associated intestinal inflammation has been reported in weaned piglets [40]. Inflammatory cytokines play a paramount role in immune and inflammatory responses and in the regulation of intestinal barrier integrity, and are also closely related to the occurrence of diarrhea [41]. Therefore, we next tested the effect of dietary garcinol on the intestinal inflammation of weaned pigs. In the current study, dietary supplementation with garcinol decreased the cytokine IL-6, IL-10, IL-1β and TNF-α in the mucosa of jejunum and ileum and the NF-κB p65 protein content at all dosages, suggesting that garcinol ameliorated intestinal inflammation. Previous studies have indicated that controlling the release of intestinal pro-inflammatory cytokines may have potential benefits for alleviating gut disorders induced by weaning stress [42]. As an effective antioxidative plant extract, garcinol also shows anti-inflammatory effects. Studies in rodent models have shown the capability of garcinol in treating different oxidative and inflammatory situations and demonstrated that garcinol can reduce the expression of cytokines [39]. In addition, garcinol suppressed the expression of interleukin (IL)-1β and IL-6 in the spinal cord of rats [16]. The presented results are consistent with these observations. Furthermore, NF-κB plays a significant role in maintaining the normal physiological functions of the body. The classic NF-κB signaling pathway is associated with intestinal immune function, permeability and the occurrence of diarrhea [43]. Previous studies have indicated that the TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation, while another study showed that garcinol can inhibit the activation of NF-κB in vitro [43]. In the present study, dietary supplementation with garcinol at 3 dosages decreased NF-κB activity, suggesting that autoimmune and inflammation have been ameliorated. This effect could also be the reason for the improvement of intestinal permeability and the diarrhea rate of weaned piglets by dietary garcinol.
Recent studies have shown that plant extract can result in the intestinal flora shift and reduce inflammation, which in turn can improve the intestinal health of piglets [9]. Finally, we used high-throughput sequencing to perform 16S rRNA gene analysis in the colon of piglets. The large intestine supports a dynamic microenvironment that varies with age and environmental factors [34]. Any alteration in the physical or chemical properties of colonic contents has the potential to impact the resident bacterial population and potentially favor or inhibit the establishment of pathogenic species. In the current study, garcinol supplementation increased the relative abundance of genera Pseudomonas, Streptococcus, Cupriavidus, Lactobacillus, Actinobacillus and Veillonella, and increased the number of Lactobacillus species, while decreased the number of E. coli in jejunum and ileum. Most probiotics currently used belong to Lactobacillus, such as L. rhamnosus GG (LGG). These Lactobacillus strains promote weight gain; thus, garcinol supplementation promotes the weight gain of weaned piglets possibly through colonic microbiota, especially Lactobacillus. In addition, a previous study showed that Lactobacillus is a common probiotic that has been associated with the regulation of the immune system, prevention of diarrhea, and improvement of pig health [44]. Hence, it is plausible that garcinol supplementation could improve immune function in weaned piglets by increasing the relative abundance of Lactobacillus. In addition, as E. coli causes diarrhea, garcinol supplementation may improve the diarrhea rate by decreasing the number of E. coli species.
Nevertheless, this study has several limitations. The 4 replications and the length of the trial (14-day period) in this study may not be adequate for the evaluation of growth responses nor gut microbiota in weaned piglets. Generally, microflora require 4 to 6 weeks to stabilize after a change, so the variation of gut microbiota in this study may just be a short snapshot. In addition, the mechanism of gut microbiota alterations in response to garcinol treatment is superficial. Further research should focus on solving these problems.
Conclusion
In summary, the present study provides the first evidence that dietary supplementation with garcinol promoted growth performance, improved diarrhea, strengthened antioxidant capacity, ameliorated intestinal barrier dysfunction and inflammation, and altered gut microbiota of weaning piglets in 14-day trial without affecting hepatic and renal functions. These results provide a fundamental basis for the future development of garcinol-functional foods for improving diarrhea, intestinal barrier function and gut microbiota of weaned piglets, and a theoretical basis and experimental evidence for understanding the biological effects of garcinol and its potential as a functional feed additive.
Acknowledgments
The authors would like to thank members of their laboratory for helpful and constructive advice.
Abbreviations
- G. indica
Garcinia indica
- ADFI
Average daily feed intake
- ADG
Average daily gain
- ALB
Albumin
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BUN
Blood urea nitrogen
- CAT
Catalase
- DAO
Diamine oxidase
- ET-1
Endothelin-1
- F/G
Feed/gain ratio
- GLU
Glucose
- GSH-Px
Glutathione peroxidase
- IL
Interleukin
- MDA
Malondialdehyde
- NMDS
Nonmetric multidimensional scaling
- PCA
Principal component analysis
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- T-AOC
Total antioxidant capacity
- TCH
Total cholesterol
- TNF-α
Tumor necrosis factor-α
- TP
Total protein
- ZO1
Zonula occludens protein 1
Authors’ contributions
The authors’contributions are as follows: F. H. and T. W. contributed to the study design and data analyses; T. W. was the principal investigator and contributed to the study design and interpretation of the findings and wrote the manuscript; W. Y. contributed to the study design, subject briefings and data collection and analyses; J. L. contributed to the data analysesand version of the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was partly supported by National Key Research and Development Program (grant no. 2018YFD0500600), National Natural Science Foundation of China (grant no. 31572409), Hubei Provincial Natural Science Foundation of China (grant no. 2018CFA071).
Availability of data and materials
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics approval
All animal protocols used in this study were in accordance with the Guidelines for the Care and Use of Animals for Research and Teaching and approved by the Animal Care and Use Committee of Huazhong Agricultural University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s40104-024-01134-0"
Change history
12/2/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1186/s40104-024-01134-0
References
- 1.Madec F, Bridoux N, Bounaix S, Jestin A. Measurement of digestive disorders in the piglet at weaning and related risk factors. Prev Vet Med. 1998;35:53–72. [DOI] [PubMed] [Google Scholar]
- 2.Madec F, Josse J. Influence of environmental factors on the onset of digestive disorders of the weaned piglet. Annales De Recherches Vétérinaires. 1983;14:456. [PubMed] [Google Scholar]
- 3.Lallès JP, Boudry G, Favier C, Floc’H NL, Luron I, Montagne L, et al. Gut function and dysfunction in young pigs: physiology. Physiology. 2002;53:301–16. [Google Scholar]
- 4.Li XQ, Zhu YH, Zhang HF, Yue Y, Cai ZX, Lu QP, et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet Diarrhoea: intestinal microbiota and immune imbalances. PLoS One. 2012;7:e40666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith F, Clark JB. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peace RM, Campbell J, Polo J, Crenshaw J, Russell L, Moeser A. Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J Nutr. 2011;141:1312. [DOI] [PubMed] [Google Scholar]
- 7.Shah MA, Bosco SJD, Mir SA. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014;98:21–33. [DOI] [PubMed] [Google Scholar]
- 8.Fang CL, Sun H, ., Wu J, Niu HH, Feng J, . Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J Anim Physiol Anim Nutr 2014;98:680–685. [DOI] [PubMed] [Google Scholar]
- 9.Gan Z, Wei W, Li Y, Wu J, Zhao Y, Zhang L, et al. Curcumin and resveratrol regulate intestinal Bacteria and alleviate intestinal inflammation in weaned piglets. Molecules. 2019;24:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing Y, Ai Q, Lin L, Dai J, Jia M, Zhou D, et al. Protective effects of garcinol in mice with lipopolysaccharide/D-galactosamine-induced apoptotic liver injury ☆. Int Immunopharmacol. 2014;19:373–80. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T, Kohno H, Shimada R, Kagami S, Yamaguchi F, Kataoka S, et al. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats. Carcinogenesis. 2000;21:1183–9. [PubMed] [Google Scholar]
- 12.Yoshida K, Tanaka T, Hirose Y, Yamaguchi F, Kohno H, Toida M, et al. Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Cancer Lett. 2005;221:29–39. [DOI] [PubMed] [Google Scholar]
- 13.Hong J, Kwon SJ, Sang S, Ju J, Zhou JN, Ho CT, et al. Effects of garcinol and its derivatives on intestinal cell growth: inhibitory effects and autoxidation-dependent growth-stimulatory effects. Free Radic Biol Med. 2007;42:1211–21. [DOI] [PubMed] [Google Scholar]
- 14.Liao CH, Sang S, Liang YC, Ho CT, Lin JK. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 in downregulating nuclear factor-kappa B pathway by Garcinol. Mol Carcinog. 2004;41:140. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi F, Saito M, Ariga T, Yoshimura Y, Nakazawa H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48:2320. [DOI] [PubMed] [Google Scholar]
- 16.Wang YW, Zhang X, Chen CL, Liu QZ, Xu JW, Qian QQ, et al. Protective effects of Garcinol against neuropathic pain- evidence from in vivo and in vitro studies. Neurosci Lett. 2017;647:85. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee A, Yasmin T, Bagchi D, Stohs SJ, et al. Mol Cell Biochem. 2003;243:29–35. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Han F, Huang X, Rong Y, Yi H, Wang Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J Anim Sci. 2013;91:5614–25. [DOI] [PubMed] [Google Scholar]
- 19.Namkung H, Li MJ, Yu H, Cottrill M, Lange CFMD. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can J Anim Sci. 2004;84:697–704. [Google Scholar]
- 20.Su Y, Yao W, Perez-Gutierrez ON, Smidt H, Zhu WY. 16S ribosomal RNA-based methods to monitor changes in the hindgut bacterial community of piglets after oral administration of Lactobacillus sobrius S1. Anaerobe. 2008;14:78–86. [DOI] [PubMed] [Google Scholar]
- 21.Alexa P, Stouracova K, Hamrik J, Rychlik I. Gene typing of the colonisation factor K88 (F4) in enterotoxigenic Escherichia coli strains isolated from diarrhoeic piglets. Veterinarni Medicina-Praha. 2001;46:46–9. [Google Scholar]
- 22.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill SR, Mihai P, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–72. [DOI] [PubMed] [Google Scholar]
- 25.Tanja M, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. [DOI] [PubMed] [Google Scholar]
- 27.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altschul SF, Madden TL, SchäFfer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Xu C, Chen X, Cai X, Yang S, Sheng Y, et al. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–9. [DOI] [PubMed] [Google Scholar]
- 30.Li SY, Ru YJ, Liu M, Xu B, Péron A, Shi XG. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest Sci. 2012;145:119–23. [Google Scholar]
- 31.Soltan M. Effect of essential oils supplementation on growth performance, nutrient digestibility, health condition of Holstein male calves during pre-and post-weaning periods. Pak J Nutr. 2009;8:642–52. [Google Scholar]
- 32.Liu C, Ho PC-L, Wong FC, Sethi G, Wang LZ, Goh BC. Garcinol: current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015;362:8–14. [DOI] [PubMed] [Google Scholar]
- 33.Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–72. [DOI] [PubMed] [Google Scholar]
- 34.Qin L, Ji W, Wang J, Li B, Hu J, Wu X. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Food Funct. 2019;10:2359–71. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Xiao H, Ren W, Yin J, Tan B, Liu G, et al. Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS One. 2014;9:e100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber D, Balda MS, Matter K. Occludin modulates transepithelial migration of neutrophils. J Biol Chem. 2000;275:5773. [DOI] [PubMed] [Google Scholar]
- 37.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014: Elsevier;2014:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol-Gastroin Liver Physiol. 2011;300:G1054–G64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai ML, Chiou YS, Chiou LY, Ho CT, Pan MH. Garcinol suppresses inflammation-associated colon carcinogenesis in mice. Mol Nutr Food Res. 2014;58:1820–9. [DOI] [PubMed] [Google Scholar]
- 40.McCracken BA, Spurlock ME, Roos MA, Zuckermann FA, Gaskins HR. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J Nutr. 1999;129:613–9. [DOI] [PubMed] [Google Scholar]
- 41.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Huang J, Hou Y, Zhu H, Zhao S, Ding B, et al. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. 2008;100:552–60. [DOI] [PubMed] [Google Scholar]
- 43.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol-Gastroin Liver Physiol. 2004;286:G367–G76. [DOI] [PubMed] [Google Scholar]
- 44.Hou C, Zeng X, Yang F, Liu H, Qiao S. Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Animl Sci Biotechnol. 2015;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.