Abstract
Background
Risk-reducing salpingo-oophorectomy (RRSO) has been associated with approximately 50% breast cancer risk reduction among women with a pathogenic variant in BRCA1 or BRCA2 (BRCA1/2), a finding that has recently been questioned.
Methods
We estimated incidence rates of breast cancer and all cancers combined during 5 years of follow-up among participants selecting RRSO or ovarian cancer screening (OCS) among women with a BRCA1/2 pathogenic variant or strong breast and/or ovarian cancer family history. Ovarian or fallopian tube or peritoneal cancer incidence rates were estimated for the OCS group. Breast cancer hazard ratios (HRs) for time-dependent RRSO were estimated using Cox regression with age time-scale (4943 and 4990 women-years in RRSO and OCS cohorts, respectively). All statistical tests were two-sided.
Results
The RRSO cohort included 925 participants, and 1453 participants were in the OCS cohort (381 underwent RRSO during follow-up), with 88 incident breast cancers diagnosed. Among BRCA1/2 pathogenic variant carriers, a non-statistically significant lower breast cancer incidence was observed in the RRSO compared with the OCS cohort (HR = 0.86, 95% confidence interval = 0.45 to 1.67; P = .67). No difference was observed in the overall population or among subgroups stratified by prior breast cancer history or menopausal status. Seven fallopian tube and four ovarian cancers were prospectively diagnosed in the OCS cohort, and one primary peritoneal carcinoma occurred in the RRSO cohort.
Conclusions
These data suggest that RRSO might be associated with reduced breast cancer incidence among women with a BRCA1/2 pathogenic variant, although the effect, if present, is small. This evolving evidence warrants a thorough discussion regarding the impact of RRSO on breast cancer risk with women considering this intervention.
Hereditary breast and/or ovarian cancer is associated with statistically significantly increased risks of breast and ovarian cancer. Pathogenic variants in BRCA1 and BRCA2 (BRCA1/2) are associated with hereditary breast and ovarian cancer syndrome but do not account for all families with multiple cases of breast and/or ovarian cancer. The lifetime cumulative breast cancer risk is approximately 70% for female individuals with a BRCA1/2 pathogenic variant, whereas ovarian cancer risk approaches 40–50% for BRCA1 and 12–25% for BRCA2 [(1,2), reviewed in (3)]. For female individuals with a BRCA1/2 pathogenic variant, risk-reducing mastectomy (RRM) reduces breast cancer risk by approximately 90–95% (4). Risk-reducing salpingo-oophorectomy (RRSO) has been associated with reduced breast (approximately 50%) and ovarian and fallopian tube cancer incidence (approximately 80%), as well as reduced cancer-specific and overall mortality, and is considered the most effective option for ovarian cancer prevention in this setting [(5–9), reviewed in (3)]. However, recent studies showed that breast cancer risk was not reduced among women with a BRCA1 pathogenic variant (10) or those with BRCA1/2 pathogenic variant and a previous history of breast cancer (5) or when RRSO was performed after 50 years of age [reviewed in (3)]. A recent study, which excluded women with history of breast cancer, censored at RRM and considered RRSO as a time-dependent covariate, showed no breast cancer risk reduction associated with RRSO (11). Methodologic differences might explain some of the discrepancies between that study and prior studies; however, when data from the earlier studies were reanalyzed using RRSO as a time-dependent variable, breast cancer risk reduction persisted (12). Additionally, another recent study including women with a BRCA1/2 pathogenic variant without a cancer history showed that RRSO was associated with reduced breast cancer incidence before 50 years of age in BRCA2 (13). These conflicting data question the effect of RRSO on breast cancer risk among women with a BRCA1/2 pathogenic variant. Moreover, it is unclear whether RRSO reduces breast cancer risk among individuals at increased risk based on family history alone.
The Prospective Study of Risk-Reducing Salpingo-Oophorectomy and Longitudinal CA-125 Screening among Women at Increased Risk of Ovarian Cancer (Gynecologic Oncologic Group Protocol 0199 [GOG-0199]) was implemented in 2003. A primary study aim was to quantify the prospective incidence of ovarian, fallopian tube, and primary peritoneal (OFP) cancer; breast cancer; and all cancers and to evaluate cancer risk reduction associated with RRSO among women with a BRCA1/2 pathogenic variant and women at increased risk based solely on personal and family history.
Materials and Methods
Study Population
GOG-0199 was a multi-institution, prospective, two-cohort, nonrandomized study of women with a BRCA1/2 pathogenic variant or strong family history of breast and/or ovarian cancer with or without a personal history of breast cancer. Details of the study rationale, design, implementation, and accrual have been published (14). Women aged 30 years and older were eligible if they: carried a pathogenic variant in BRCA1 or BRCA2 or had a first-degree relative (FDR) or second-degree relative (SDR) with a BRCA1/2 pathogenic variant; had a personal and/or family history of at least two ovarian and/or breast cancers among FDRs or SDRs in the same lineage; were of Ashkenazi Jewish ancestry and had a personal history of breast cancer or had one FDR or two SDRs with breast and/or ovarian cancer; or reported a family history of breast and/or ovarian cancer that conferred no less than 20% probability for a pathogenic variant by BRCAPRO (15). Individuals with a prior history of OFP or bilateral oophorectomy were ineligible. At enrollment, participants elected immediate RRSO or ovarian cancer screening (OCS). Participants choosing OCS could have RRSO postenrollment, either electively or as clinically indicated.
Participants in the OCS cohort were screened according to the risk of ovarian cancer algorithm (ROCA) (16), with CA-125 measurements every 3 months and an annual transvaginal ultrasound. The ROCA score reflects the probability of harboring ovarian cancer (normal risk: <1%; intermediate risk: 1%–10%; and elevated risk: >10%). Additional follow-up, including repeat CA-125 measurements, transvaginal ultrasound, and/or clinical evaluation, was determined by the ROCA score. RRSO could be recommended as a result of the additional evaluations.
Participants electing RRSO underwent the protocol-defined procedure within 90 days of enrollment. Hysterectomy was performed electively per patient and physician discretion. Details of the surgical procedure and findings of the baseline RRSO have been reported (17). Participants in the RRSO cohort had CA-125 measurements and ROCA score calculations every 6 months. All study participants were followed for 5 years. Participants diagnosed with an OFP on study discontinued active screening but were followed for the remainder of the 5-year follow-up period for additional cancer diagnoses and vital status.
This protocol (NCT-00049049) was approved by institutional review boards at the National Cancer Institute, GOG, and 151 GOG institutions (United States and Australia).
Sociodemographic, Cancer History Information, and BRCA Status
We collected information on age at enrollment, body mass index (BMI), race, menopausal status, education, income, personal history of breast and other cancer, and family history of breast and ovarian cancer.
For participants who had undergone clinical germline BRCA1/2 testing, the test reports were verified by the study chair (MHG). Participants not previously tested, or whose test report could not be obtained, underwent research-based BRCA1/2 testing. In the absence of a clinical report and DNA for testing, the self-reported mutation status was used (NOCS = 2, NRRSO = 1). All participants with negative sequencing results, by either clinical or research testing, had research-based BRCA1/2 large re-arrangement testing. Nine individuals with unknown BRCA status (NOCS = 6, NRRSO = 3) were excluded from this analysis.
Cancer Cases
OFP cancers diagnosed in the RRSO cohort at the time of enrollment were reported previously (17). Differences in OFP cancer incidence were not formally compared between the two cohorts. Instead, the OFP cancer incidence rates after enrollment were calculated for participants in the OCS cohort. Breast and all other cancers diagnosed on study were reported by the participating study sites with a cancer update form every 6 months.
Statistical Analysis
Participants were followed from enrollment to occurrence of an off-study event (pregnancy, noncompliance with ROCA measurements, withdrawal from participation), end of 5-year prospective follow-up, loss to follow-up, or death.
For cancer incidence rate calculations, participants who enrolled in the OCS cohort and subsequently underwent RRSO contributed follow-up time to both cohorts. Contribution to the OCS cohort started on the date of entry and stopped on the day of surgery, and contribution to the RRSO cohort started the day after surgery. Participants who did not undergo RRSO contributed person-time and events to the OCS cohort only. To calculate incidence rates while on OCS in the age intervals 30–34.99, 35–39.99, ..., 65–69.99, and 70 years and older, the number of events was divided by the person-years in each interval. The cumulative risk of an event—for example from ages 30 to 70 years—was estimated assuming piecewise constant hazards. Variance estimates were obtained by applying the delta method to the piecewise exponential survival model [see (18), section 3.4.5] and assuming the hazard rate estimates in different intervals were uncorrelated (18). Cumulative risks were similarly calculated for the RRSO group (see Supplementary Material, available online, for details). These procedures do not require a proportional hazards assumption. The incidence rate of OFP cancer was estimated only for the OCS cohort.
Cox regression models were used to estimate the relative hazard of breast cancer for patients enrolled in RRSO compared with OCS. Models were specified with age as the time-scale, using age at enrollment as a covariate (survival times were left truncated and began at age at enrollment) and RRSO as a time-dependent covariate. Patients without the event of interest were censored on the date of last follow-up. A total of 4943 and 4990 women-years in RRSO and OCS cohorts, respectively, were included.
These methods were applied to calculate age-specific incidence rates and cumulative risks for OFP cancer, breast cancer, and all cancers combined. The all cancers and OFP cancer analyses included all evaluable participants. The sample for estimating breast cancer incidence rates excluded participants with previous bilateral breast cancer, unilateral breast cancer, and contralateral RRM or bilateral RRM prior to enrollment. For the calculation of incidence rates and cumulative risks and time-dependent breast cancer analyses, participants who underwent RRM during prospective follow-up were censored at the time of surgery.
We examined the differences in sociodemographic characteristics, BMI, BRCA1/2 status (BRCA1 pathogenic variant, BRCA2 pathogenic variant, noncarriers), personal cancer history, and family cancer history between the RRSO and OCS cohorts, using χ2 and t tests for categorical and continuous variables, respectively. Two-sided P values less than .05 were deemed statistically significant, with no adjustments for multiple testing. All data analyses were generated using SAS/STAT software, Version 9.4 (SAS Institute Inc., Cary, NC).
Results
Study Population
In the study, 2605 participants (1030 RRSO and 1575 OCS) were enrolled from June 16, 2003, to November 3, 2006. After exclusions (Figure 1), 925 and 1453 participants were in the RRSO and OCS cohort, respectively. RRSO participants had higher BMI and lower education levels; were older; and were more likely to be postmenopausal, have a BRCA1/2 pathogenic variant, and a personal history of breast cancer but were less likely to have a family history of breast cancer diagnosed before 50 years of age. There were no differences in family history of all breast cancer, male breast cancer, or ovarian cancer between the two groups (Table 1) or in parity or postmenopausal hormone, tamoxifen, or raloxifene use at baseline (data not shown).
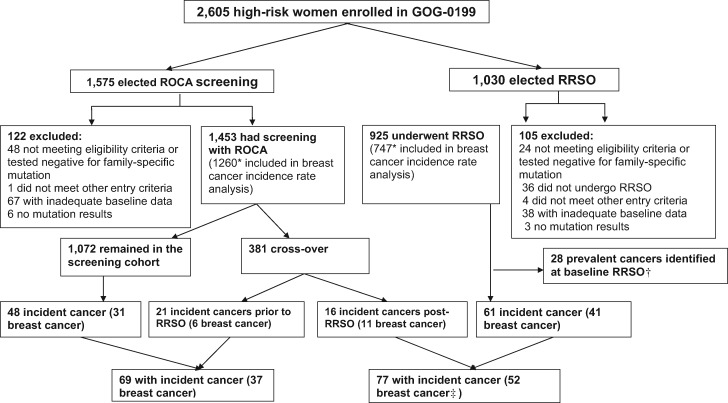
Figure 1.
CONSORT diagram, showing the number of participants included and number of incident cancer cases. RRSO = risk-reducing salpingo-oophorectomy; ROCA = risk of ovarian cancer algorithm.
*Participants with previous bilateral breast cancer, unilateral breast cancer, and contralateral risk-reducing mastectomy or bilateral risk-reducing mastectomy prior to enrollment were excluded from the breast cancer incidence rate estimates.
†Previously reported in (17).
‡One breast cancer was diagnosed in a participant with unilateral breast cancer and contralateral risk-reducing mastectomy prior to enrollment and was included in the overall cancer estimate.
Table 1.
Baseline characteristics of study participants*
| Characteristics | No. (%) RRSO (n = 925) | No. (%) OCS (n = 1453) | Total No. (%) (n = 2378) | P |
|---|---|---|---|---|
| Age, y | ||||
| 30–39 | 178 (19.2) | 403 (27.7) | 581 (24.4) | <.01 |
| 40–49 | 386 (41.7) | 503 (34.6) | 889 (37.4) | |
| 50–59 | 276 (29.8) | 399 (27.5) | 675 (28.4) | |
| 60–69 | 70 (7.6) | 126 (8.7) | 196 (8.2) | |
| ≥70 | 15 (1.6) | 22 (1.5) | 37 (1.6) | |
| Median (5th–95th percentile) | 47.5 (35.5–64.6) | 46.5 (32.9–65) | 46.9 (33.6–64.9) | |
| BMI | ||||
| Missing | 3 (0.3) | 9 (0.6) | 12 (0.5) | <.01 |
| ≤25 | 453 (49) | 863 (59.4) | 1316 (55.3) | |
| 26–30 | 246 (26.6) | 336 (23.1) | 582 (24.5) | |
| 31–35 | 119 (12.9) | 134 (9.2) | 253 (10.6) | |
| 36–40 | 72 (7.8) | 70 (4.8) | 142 (6) | |
| >40 | 32 (3.5) | 41 (2.8) | 73 (3.1) | |
| Median (5th–95th percentile) | 26.2 (19.9–39.4) | 24.7 (19.5–37.8) | 25.3 (19.6–38.6) | |
| Menopausal status | ||||
| Premenopausal | 535 (57.8) | 916 (63) | 1451 (61) | .01 |
| Menopausal | 390 (42.2) | 537 (37) | 927 (39) | |
| Race | ||||
| Asian | 7 (0.8) | 15 (1) | 22 (0.9) | .48 |
| Black | 29 (3.1) | 32 (2.2) | 61 (2.6) | |
| White | 882 (95.4) | 1396 (96.1) | 2278 (95.8) | |
| Other/Not specified | 7 (0.8) | 10 (0.7) | 17 (0.7) | |
| Highest level of schooling | ||||
| Missing | 11 (1.2) | 17 (1.2) | 28 (1.2) | .003 |
| 8 years or less | 4 (0.4) | 2 (0.1) | 6 (0.3) | |
| Some high school | 16 (1.7) | 15 (1) | 31 (1.3) | |
| High school graduate/GED | 100 (10.8) | 125 (8.6) | 225 (9.5) | |
| Some college or tech | 227 (24.5) | 297 (20.4) | 524 (22) | |
| College graduate or beyond | 567 (61.3) | 997 (68.6) | 1564 (65.8) | |
| Income | ||||
| Missing | 56 (6.1) | 92 (6.3) | 148 (6.2) | .67 |
| <$10 000 | 17 (1.8) | 23 (1.6) | 40 (1.7) | |
| $10 000 to $19 999 | 27 (2.9) | 28 (1.9) | 55 (2.3) | |
| $20 000 to $29 999 | 40 (4.3) | 54 (3.7) | 94 (4) | |
| $30 000 to $39 999 | 47 (5.1) | 69 (4.7) | 116 (4.9) | |
| $40 000 to $49 999 | 53 (5.7) | 102 (7) | 155 (6.5) | |
| $50 000 to $69 999 | 140 (15.1) | 217 (14.9) | 357 (15) | |
| $70 000 to $99 999 | 193 (20.9) | 299 (20.6) | 492 (20.7) | |
| ≥$100 000 | 352 (38.1) | 569 (39.2) | 921 (38.7) | |
| BRCA1/2 status | ||||
| Positive | 538 (58.2) | 367 (25.3) | 905 (38.0) | <.0001 |
| BRCA1 | 313 (33.8) | 206 (14.2) | 519 (21.8) | |
| BRCA2 | 223 (24.1) | 160 (11) | 383 (16.1) | |
| Both | 2 (0.2) | 1 (0.1) | 3 (0.1) | |
| Negative | 387 (41.8) | 1086 (74.7) | 1473 (61.9) | |
| Previous cancer history | ||||
| None | 376 (40.6) | 807 (55.5) | 1183 (49.7) | <.0001 |
| Breast cancer | 471 (50.9) | 533 (36.7) | 1004 (42.2) | |
| Other cancer | 38 (4.1) | 61 (4.2) | 99 (4.2) | |
| Both | 40 (4.3) | 52 (3.6) | 92 (3.9) | |
| Number of female relatives with breast cancer | ||||
| Missing | 3 (0.3) | 1 (0.1) | 4 (0.2) | .53 |
| 0 | 152 (16.4) | 254 (17.5) | 406 (17.1) | |
| 1 | 314 (33.9) | 460 (31.7) | 774 (32.5) | |
| 2 | 247 (26.7) | 418 (28.8) | 665 (28) | |
| ≥3 | 209 (22.6) | 320 (22) | 529 (22.2) | |
| Number of female relatives with breast cancer diagnosed <50 years of age | ||||
| Missing | 4 (0.4) | 1 (0.1) | 5 (0.2) | .03 |
| 0 | 412 (44.5) | 616 (42.4) | 1028 (43.2) | |
| 1 | 326 (35.2) | 580 (39.9) | 906 (38.1) | |
| 2 | 135 (14.6) | 209 (14.4) | 344 (14.5) | |
| ≥3 | 48 (5.2) | 47 (3.2) | 95 (4) | |
| Number of female relatives with ovarian cancer | ||||
| Missing | 4 (0.4) | 1 (0.1) | 5 (0.2) | .18 |
| 0 | 452 (48.9) | 710 (48.9) | 1162 (48.9) | |
| 1 | 289 (31.2) | 471 (32.4) | 760 (32) | |
| 2 | 138 (14.9) | 229 (15.8) | 367 (15.4) | |
| ≥3 | 42 (4.5) | 42 (2.9) | 84 (3.5) | |
| Number of male relatives with breast cancer | ||||
| Missing | 29 (3.1) | 26 (1.8) | 55 (2.3) | .06 |
| 0 | 861 (93.1) | 1369 (94.2) | 2230 (93.8) | |
| 1 | 28 (3) | 55 (3.8) | 83 (3.5) | |
| 2 | 7 (0.8) | 2 (0.1) | 9 (0.4) | |
| ≥3 | 0 | 1 (0.1) | 1 (0) |
BMI = body mass index; OCS = ovarian cancer screening; RRSO = risk-reducing salpingo-oophorectomy.
Breast Cancer Incidence Rates and Hazard Ratios (HRs)
After excluding participants who did not have breast tissue at enrollment, 2007 participants (RRSO = 747; OCS = 1260) were included in breast cancer–related analyses, and 316 who were initially enrolled in the OCS cohort underwent RRSO at a mean of 21 months after enrollment.
Of the participants included in this analysis, 88 intraductal and invasive breast cancer cases were diagnosed. Overall, the cumulative breast cancer risks from age 30 to 70 years were 0.29 (95% confidence interval [CI] = 0.20 to 0.39) for OCS and 0.35 (95% CI = 0.26 to 0.44) for RRSO (Table 2). The higher cumulative risk in the RRSO cohort was probably due to its larger number of BRCA1/2 pathogenic variant carriers. Among participants with a BRCA1/2 pathogenic variant, the cumulative breast cancer risks were 0.31 (95% CI = 0.16 to 0.46) for OCS and 0.20 (95% CI = 0.12 to 0.28) for RRSO from ages 30 to 50 years and 0.49 (95% CI = 0.24 to 0.75) for OCS and 0.42 (95% CI = 0.30 to 0.53) for RRSO from ages 30 to 70 years (Table 2). Although the cumulative incidences in BRCA1/2 pathogenic variant carriers were lower in the RRSO cohort, the differences were not statistically significant. The cumulative breast cancer risks were also estimated separately for BRCA1 and BRCA2 (listed in Table 2). In noncarriers, the cumulative risks from ages 30 to 70 years were 0.21 (95% CI = 0.12 to 0.30) for OCS and 0.30 (95% CI = 0.15 to 0.45) for RRSO. See Supplementary Table 1 (available online) for age-specific breast cancer incidence rates.
Table 2.
Cumulative risk for breast, OFP, and all cancers combined prospectively diagnosed during follow-up
| BRCA1/2 mutation status | Study cohort | Cumulative cancer risks* (95% CI) |
||
|---|---|---|---|---|
| Breast† | OFP‡ | All | ||
| Age 30 to 50 years | ||||
| Positive BRCA1/2 | OCS | 0.31 (0.16 to 0.46) | 0.14 (0.02 to 0.26) | 0.42 (0.27 to 0.57) |
| RRSO | 0.20 (0.12 to 0.28) | — | 0.20 (0.13 to 0.27) | |
| BRCA1 | OCS | 0.39 (0.29 to 0.49) | 0.26 (0.16 to 0.36) | 0.54 (0.45 to 0.63) |
| RRSO | 0.26 (0.20 to 0.32) | — | 0.23 (0.18 to 0.28) | |
| BRCA2 | OCS | 0.21 (0.08 to 0.34) | 0 | 0.30 (0.17 to 0.43) |
| RRSO | 0.12 (0.07 to 0.17) | — | 0.16 (0.13 to 0.19) | |
| Negative | OCS | 0.06 (0.02 to 0.11) | 0 | 0.12 (0.06 to 0.18) |
| RRSO | 0.11 (0.00 to 0.22) | — | 0.15 (0.04 to 0.26) | |
| All | OCS | 0.15 (0.08 to 0.22) | 0.03 (0.00 to 0.05) | 0.22 (0.15 to 0.30) |
| RRSO | 0.16 (0.10 to 0.22) | — | 0.17 (0.12 to 0.23) | |
| Age 30 to 70 years | ||||
| Positive BRCA1/2 | OCS | 0.49 (0.24 to 0.75) | 0.27 (0.08 to 0.46) | 0.65 (0.47 to 0.83) |
| RRSO | 0.42 (0.30 to 0.53) | — | 0.44 (0.34 to 0.53) | |
| BRCA1 | OCS | 0.67 (0.46 to 0.88) | 0.45 (0.31 to 0.59) | 0.84 (0.74 to 0.94) |
| RRSO | 0.49 (0.42 to 0.56) | — | 0.46 (0.40 to 0.52) | |
| BRCA2 | OCS | 0.39 (0.20 to 0.58) | 0 | 0.42 (0.27 to 0.55) |
| RRSO | 0.33 (0.24 to 0.42) | — | 0.41 (0.33 to 0.49) | |
| Negative | OCS | 0.21 (0.12 to 0.30) | 0.04 (−0.01 to 0.09) | 0.36 (0.26 to 0.45) |
| RRSO | 0.30 (0.15 to 0.45) | — | 0.44 (0.28 to 0.59) | |
| All | OCS | 0.29 (0.20 to 0.39) | 0.08 (0.02 to 0.13) | 0.44 (0.36 to 0.53) |
| RRSO | 0.35 (0.26 to 0.44) | — | 0.42 (0.34 to 0.51) | |
Cumulative risk denotes an estimate of the pure probability of developing the event over the age ranges specified. CI = confidence interval; OCS = ovarian cancer screening; OFP = ovarian/fallopian tube/peritoneal; RRSO = risk-reducing salpingo-oophorectomy.
Participants with bilateral breast cancer, unilateral breast cancer plus contralateral risk-reducing mastectomy, or bilateral risk-reducing mastectomy prior to enrollment were excluded from the breast cancer risk estimates. Participants who underwent risk-reducing mastectomy during prospective follow-up were censored at the time of risk-reducing surgery.
OFP cancer risk was not estimated for participants who had RRSO (indicated with —).
The overall breast cancer incidence RRSO-to-OCS hazard ratio was 1.04 (95% CI = 0.64 to 1.68; P = .88; Table 3). Among BRCA1/2 pathogenic variant carriers, a non-statistically significant lower breast cancer incidence was observed (HR = 0.86, 95% CI = 0.45 to 1.67; P = .67). No evidence of breast cancer risk reduction associated with RRSO was observed when stratified by prior history of breast cancer or menopausal status (Table 3).
Table 3.
Breast cancer incidence following RRSO and OCS
| Group | Breast cancers/Total* | Breast cancer cases |
RRSO-to-OCS HR (95% CI)‡ | P‡ | |
|---|---|---|---|---|---|
| OCS (n = 1260)† | RRSO (n = 747)† | ||||
| All participants | |||||
| Overall | 88/2007 | 48/1260 | 40/747 | 1.04 (0.64 to 1.68) | .88 |
| All pathogenic BRCA1/2 pathogenic variants | 52/713 | 20/295 | 32/418 | 0.86 (0.45 to 1.67) | .67 |
| BRCA1§ | 37/401 | 16/171 | 21/230 | 0.83 (0.39 to 1.79) | .64 |
| BRCA2§ | 15/310 | 4/123 | 11/187 | 0.98 (0.25 to 3.81) | .98 |
| Noncarriers | 36/1294 | 28/965 | 8/329 | 1.25 (0.64 to 2.44) | .52 |
| Participants with a prior history of breast cancer | |||||
| Overall | 27/775 | 13/422 | 14/353 | 0.62 (0.26 to 1.49) | .28 |
| All BRCA1/2 pathogenic variants | 14/281 | 4/86 | 10/195 | 0.45 (0.13 to 1.56) | .21 |
| BRCA1§ | 8/159 | 3/49 | 5/110 | 0.43 (0.10 to 1.86) | .26 |
| BRCA2§ | 6/121 | 1/36 | 5/85 | 0.42 (0.04 to 4.13) | .45 |
| Noncarriers | 13/494 | 9/336 | 4/158 | 0.80 (0.26 to 2.51) | .71 |
| Participants with no prior history of breast cancer | |||||
| Overall | 61/1232 | 35/838 | 26/394 | 1.33 (0.74 to 2.39) | .34 |
| All BRCA1/2 pathogenic variants | 38/432 | 16/209 | 22/223 | 1.15 (0.52 to 2.54) | .72 |
| BRCA1§ | 29/242 | 13/122 | 16/120 | 1.22 (0.50 to 3.00) | .66 |
| BRCA2§ | 9/189 | 3/87 | 6/102 | 1.09 (0.20 to 6.06) | .92 |
| Noncarriers | 23/800 | 19/629 | 4/171 | 1.56 (0.68 to 3.60) | .29 |
| Premenopausal | |||||
| Overall | 55/1272 | 31/826 | 24/446 | 1.03 (0.56 to 1.89) | .92 |
| All BRCA1/2 pathogenic variants | 37/507 | 17/250 | 20/257 | 0.84 (0.40 to 1.77) | .64 |
| BRCA1§ | 31/298 | 14/151 | 17/147 | 0.84 (0.37 to 1.91) | .68 |
| BRCA2§ | 6/207 | 3/98 | 3/109 | 0.73 (0.11 to 4.82) | .75 |
| Noncarriers | 18/765 | 14/576 | 4/189 | 1.43 (0.56 to 3.67) | .46 |
| Postmenopausal | |||||
| Overall | 33/735 | 17/434 | 16/301 | 1.13 (0.50 to 2.56) | .78 |
| All BRCA1/2 pathogenic variants | 15/206 | 3/45 | 12/161 | 0.97 (0.21 to 4.39) | .97 |
| BRCA1§ | 6/103 | 2/20 | 4/83 | 0.78 (0.09 to 6.80) | .82 |
| BRCA2§ | 9/103 | 1/25 | 8/78 | 1.17 (0.14 to 9.63) | .88 |
| Noncarriers | 18/529 | 14/389 | 4/140 | 1.19 (0.46 to 3.09) | .72 |
Participants who underwent risk-reducing mastectomy during prospective follow-up were censored at the time of risk-reducing surgery. HR = hazard ratio; OCS = ovarian cancer screening; RRSO = risk-reducing salpingo-oophorectomy.
Numbers reflect the original cohort enrollment.
Based on Cox regression model on age time-scale with RRSO as time-dependent covariate.
Two participants with pathogenic variants in both BRCA1 and BRCA2 were excluded.
OFP Cancer Incidence Rates
Seven fallopian tube cancers and four ovarian cancers were diagnosed prospectively in the OCS cohort—eight participants with a BRCA1 pathogenic variant and three noncarriers (Table 4)—and have been reported in detail elsewhere (16). The cumulative risk of OFP cancer from ages 30 to 70 years was 0.08 (95% CI = 0.02 to 0.13) in the entire OCS cohort, 0.27 (95% CI = 0.08 to 0.46) among individuals with a BRCA1/2 pathogenic variant, and 0.04 (95% CI = −0.01 to 0.09) among noncarriers (Table 2). See Supplementary Table 2 (available online) for age-specific incidence rates. One primary peritoneal carcinoma was diagnosed 18 months after RRSO at enrollment in a participant with a BRCA1 pathogenic variant.
Table 4.
OFP cancers prospectively diagnosed in participants initially enrolled in the OCS cohort*
| Case No. | Detected at elective RRSO | BRCA1/2 status | Age at diagnosis, y | Primary site | Optimal debulking | Histology | Stage | Grade | Vital status last follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No | Negative | 65 | Ovary | Yes | Serous adenocarcinoma | IIIC | 3 | Alive |
| 2 | No | BRCA1 | 42 | Fallopian tube | Yes | Undifferentiated carcinoma | IIA | 3 | Dead |
| 3 | No | Negative | 64 | Ovary | Yes | Serous adenocarcinoma | IIIC | 3 | Dead |
| 4 | No | BRCA1 | 49 | Ovary | Yes | Serous adenocarcinoma | IIIC | 3 | Dead |
| 5 | Yes | BRCA1 | 43 | Fallopian tube | Yes | Serous adenocarcinoma | IA | 3 | Alive |
| 6 | Yes | BRCA1 | 50 | Fallopian tube | Yes | Unspecified adenocarcinoma | IC | 3 | Alive |
| 7 | Yes | BRCA1 | 49 | Ovary | Yes | Serous adenocarcinoma | IC | 3 | Alive |
| 8 | Yes | BRCA1 | 46 | Ovary | Yes | Serous adenocarcinoma | IIIC | 2 | Dead |
| 9 | Yes | Negative | 59 | Fallopian tube | Yes | Serous adenocarcinoma | IA | 2 | Alive |
| 10 | No | BRCA1 | 48 | Ovary | Yes | Serous adenocarcinoma | IIIC | 2 | Dead |
| 11 | No | BRCA1 | 81 | Fallopian tube | Yes | Serous adenocarcinoma | IIIC | 3 | Dead |
OCS = ovarian cancer screening; OFP = ovarian/fallopian tube/peritonea; RRSO = risk-reducing salpingo-oophorectomy.
All Cancer Incidence Rates
Of the participants, 146 were diagnosed with at least one new cancer while on study. Four participants had two separate cancers. When estimating the overall rates, only the first cancer was considered.
The types and number of all cancers diagnosed during study follow-up are listed in Supplementary Table 3 (available online). The cumulative risk for all cancers from ages 30 to 70 years was 0.44 (95% CI = 0.36 to 0.53) for OCS and 0.42 (95% CI = 0.34 to 0.51) for RRSO (Table 2). Among participants with a BRCA1/2 pathogenic variant, the cumulative risk for all cancers up to age 70 years was 0.65 (95% CI = 0.47 to 0.83) for OCS and 0.44 (95% CI = 0.34 to 0.53) for RRSO. Among noncarriers, participants in the RRSO cohort appeared to have higher cumulative cancer risk (0.44, 95% CI = 0.28 to 0.59) than OCS participants (0.36, 95% CI = 0.26 to 0.45), with overlapping CIs. See Supplementary Table 4 (available online) for age-specific all cancer incidence rates.
Discussion
We report cumulative incidence rates of OFP cancer, breast cancer, and all cancers in women at increased breast and/or ovarian cancer risk who elected either RRSO or OCS. The RRSO-to-OCS HR for breast cancer was non-statistically significantly less than 1 among women with a BRCA1/2 pathogenic variant, and approximately 1 in the overall study population or when stratified by prior breast cancer history and menopausal status, indicating that RRSO was unlikely to be associated with a decrease in breast cancer risk. When GOG-0199 opened in 2003, limited data suggested that RRSO reduced breast cancer risk in women with a BRCA1/2 pathogenic variant (8). Subsequently, several studies showed that RRSO was associated with up to 50% reduction in breast cancer risk (5–7,9). However, recent reports have shown no overall association or only association with breast cancer diagnosed before age 50 years in women with a BRCA2 pathogenic variant (11,13). Our analysis followed the methodology suggested by one of these studies (11), with results showing a non-statistically significant trend toward a small breast cancer risk reduction with RRSO among women with a BRCA1/2 pathogenic variant. These results suggest that the effect of RRSO on breast cancer risk, if present, is small. Furthermore, postenrollment use of tamoxifen and raloxifene was evenly distributed in the two groups and thus was unlikely to have been an important confounder of the association between breast cancer risk and RRSO.
Previous observational studies reported an association between RRSO and reduced breast cancer risk among women with a family history (19,20). In our study, RRSO in women without a BRCA1/2 pathogenic variant was not associated with reduced breast cancer risk. The breast cancer incidence rate in this group, although elevated, was lower than that observed in women with a BRCA1/2 pathogenic variant. The RRSO-to-OCS hazard ratio for breast cancer was slightly higher than 1, without reaching statistical significance. This seemingly higher breast cancer risk among those without a BRCA1/2 pathogenic variant who elected RRSO may have been a reflection of a selection bias if participants who self-selected surgery had unrecognized higher risk (eg, stronger family history or prior breast biopsy) than those who selected OCS. Furthermore, this population was likely heterogeneous, potentially with a wide range of risk. In addition to BRCA1/2, multiple other genes have recently been implicated in hereditary breast cancer predisposition (21). The effect of RRSO on breast cancer risk related to these genes is unknown.
One primary peritoneal carcinoma developed among participants who underwent RRSO at enrollment, reinforcing prior observations that a small peritoneal cancer risk remains after RRSO (7,22–24). We also showed that, although the cumulative risk of OFP cancer for women with a family history of breast and/or ovarian cancer was slightly elevated (4% by age 70 years), it was much lower than BRCA1/2-related risk. Genetic testing for other hereditary ovarian cancer predisposition genes (25) was not performed; thus, it is possible that some of the participants who tested negative for a BRCA1/2 pathogenic variant had a hereditary predisposition because of other genes.
BRCA1/2 pathogenic variants have been reported to be associated with increased risks of melanoma and pancreatic cancer, although the absolute risks are small (26). We observed four and two such cancers, respectively, prohibiting reliable risk estimates. Similarly, the small number of other specific cancers precluded computing risk estimates. Finally, there is an ongoing debate regarding endometrial cancer risk related to BRCA1/2 pathogenic variants, especially the serous histologic subtype in BRCA1 (27,28). The small number of cases in this report (two serous adenocarcinoma in participants with a BRCA1 pathogenic variant and two endometrioid and two mucinous carcinomas in noncarriers) precluded a formal evaluation.
GOG-0199 had a large geographically and institutionally diverse study population and 5 years of prospective follow-up, and BRCA1/2 status was known for 99.6% of study participants (14). The study population is more representative of the general increased-risk population than that usually seen in a tertiary-referral setting. The population of women at increased risk in the absence of a BRCA1/2 pathogenic variant allowed for estimation of breast and ovarian cancer risks and exploration of potential risk reduction associated with RRSO.
Because prevalent OFP cancers in the RRSO cohort were excluded from the analysis, OFP incidence rates were not comparable for the RRSO and OCS cohorts. Our data do not permit conclusions regarding an OFP survival benefit for RRSO.
One study limitation was that RRSO was not randomly allocated; thus, selection bias may have masked potential small breast cancer risk-reduction benefits. For example, women who chose RRSO at enrollment might have had higher unmeasured risks than those who chose OCS. There were no apparent differences in overall breast or ovarian cancer family history, but participants in the RRSO cohort more frequently reported a personal breast cancer history and/or a family history of premenopausal breast cancer, which might suggest higher risk. Exploratory propensity score adjustments for potential confounding factors (BMI, menopausal status, education, income, BRCA status, history of cancer, and family history of breast cancer) did not meaningfully impact the results in Table 3. Another study limitation was that 371 women had no breast tissue at enrollment, which reduced anticipated statistical power. In addition, the study was designed with only 5 years of follow-up, which might have reduced the ability to detect a reduction in breast cancer risk associated with RRSO. Last, although RRM was treated as a censoring event, there is a potential for bias in the analysis if RRM is informative censoring. For example, if RRM was performed because there were higher estimates of breast cancer risk, censoring by RRM could bias the breast cancer incidence rate downward. In fact, approximately 17% of the RRSO group had RRM, compared with approximately 6% of the OCS group. If RRM censoring were informative, one would expect the bias to be in the direction of increased risk reducing effect of RRSO. This potential bias would suggest that the risk-reducing effects of RRSO might have been overestimated. If this were the case, it might have increased the estimated risk-reducing effect of RRSO among women with a previous history of breast cancer.
The data from our study provide an important increment to recent evidence (11,13) that RRSO might not be associated with reduced breast cancer risk. We observed a small, non-statistically significant breast cancer risk reduction in BRCA1/2 pathogenic variant carriers but not in noncarriers. These recent studies differed from those published earlier by virtue of more rigorous consideration of various methodologic biases, suggesting that those biases might have resulted in an apparent breast cancer risk reduction. This evolving evidence warrants a more cautious discussion regarding the impact of RRSO on breast cancer risk with women considering this intervention. We were also able to provide cumulative breast and ovarian cancer risk estimates for women at increased risk without a BRCA1/2 pathogenic variant. More data are needed to establish the most appropriate cancer risk management options in this population.
Funding
This study was supported by the Intramural (DCEG, CCR) and Extramural (DCTD/CTEP, DCP/CCOP) Research Programs of the National Cancer Institute. In addition, this study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office and the GOG Tissue Bank (CA 27469) and to the GOG Statistical and Data Center (CA 37517) as well as NRG Oncology Operations grant number U10 CA180868 and NRG SDMC grant U10 CA180822. KAP is a National Breast Cancer Foundation of Australia Practitioner Fellow.
Notes
Affiliations of authors: Clinical Genetics Branch (PLM) and Biostatistics Branch (MHGa) and Clinical Genetics Branch (MHGr), Division of Cancer Epidemiology and Genetics, and Environmental Epidemiology Branch (MES), National Cancer Institute, Rockville, MD; NRG Oncology, Statistical and Data Center, Roswell Park Cancer Institute, Buffalo, NY (AM, MP); Department of Biostatistics Unit, Massachusetts General Hospital, Boston, MA (SS); Department of GYN Oncology, MD Anderson Cancer Center, Houston, TX (KL); Department of Pathology, University of Maryland Medical Center, Baltimore, MD (OBI); Division of Gynecologic Oncology, NorthShore University Health System, Evanston, IL (GR); Department of Obstetrics and Gynecology, University of Chicago, Evanston, IL (GR); Division of Gynecologic Oncology, Ohio State University, Columbus, OH (DEC); Division of Gynecologic Oncology, University of North Carolina at Chapel Hill, Raleigh, NC (JB); Department of Gynecologic Oncology, Yale University, Norwalk, CT (TR); Gynecology and Clinical Genetics Services, Memorial Sloan Kettering Cancer Center, New York, NY (NDK); Division of Gynecologic Oncology, Medical College of Wisconsin, Milwaukee, WI (JSR); Division of Cancer Medicine, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia (K-AP); Sir Peter MacCallum Department of Oncology, The University of Melbourne, Parkville, VIC, Australia (K-AP); Department of Obstetrics & Gynecology, Women & Infants Hospital, Providence, RI (PAD); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine, Magee-Womens Hospital of UPMC, Pittsburgh, PA (ABO); OB/GYN, University of MS Medical Center, Jackson, MS (MRR); Department of OB/GYN, University of Oklahoma Health Sciences Center, Oklahoma City, OK (JLW).
The funders had no role in the design of the study, the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors declare no conflict of interest.
Present affiliations: Health Sciences Research, Mayo Clinic, Jacksonville, FL (MES); University of Pittsburgh School of Medicine, Magee-Womens Hospital of UPMC, Pittsburgh, PA (PLM); Northwell Health Cancer Institute, Lake Success, NY (NDK).
The following GOG member institutions participated in this study: Roswell Park Cancer Institute; University of Alabama at Birmingham; Duke University Medical Center; Walter Reed Army Medical Center; University of Minnesota Medical School; Mount Sinai School of Medicine; University of Mississippi Medical Center; Colorado Gynecologic Oncology Group PC; University of California at Los Angeles; University of Cincinnati; University of North Carolina School of Medicine; University of Iowa Hospitals and Clinics; University of Texas Southwestern Medical Center at Dallas; Indiana University School of Medicine; Wake Forest University School of Medicine; University of California Medical Center at Irvine; Tufts-New England Medical Center; Rush-Presbyterian–St. Luke’s Medical Center; Magee-Womens Hospital of the University of Pittsburgh Medical Center; University of New Mexico; The Cleveland Clinic Foundation; Washington University School of Medicine; Memorial Sloan-Kettering Cancer Center; Cooper Hospital/University Medical Center; Columbus Cancer Council; MD Anderson Cancer Center; University of Massachusetts Medical School; Fox Chase Cancer Center; Women’s Cancer Center; University of Oklahoma; University of Virginia Health Sciences Center; University of Chicago; Tacoma General Hospital; Thomas Jefferson University Hospital; Mayo Clinic; Case Western Reserve University; Tampa Bay Cancer Consortium; Gynecologic Oncology Network; Ellis Fischel Cancer Center; Fletcher Allen Health Care; Australia New Zealand Gynaecological Oncology Group; Yale University School of Medicine; University of Wisconsin Hospital; National Cancer Institute – Clinical Genetics Branch; The Hospital of Central Connecticut at New Britain General Hospital; and the Community Clinical Oncology Program.
Supplementary Material
References
- 1. Antoniou AC, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. [DOI] [PubMed] [Google Scholar]
- 3. Hartmann LC, Lindor NM.. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N Engl J Med. 2016;374(5):454–468. [DOI] [PubMed] [Google Scholar]
- 4. Ludwig KK, Neuner J, Butler A, Geurts JL, Kong AL.. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg. 2016;212(4):660–669. [DOI] [PubMed] [Google Scholar]
- 5. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rebbeck TR, Kauff ND, Domchek SM.. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346(21):1609–1615. [DOI] [PubMed] [Google Scholar]
- 9. Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. [DOI] [PubMed] [Google Scholar]
- 10. Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(5):pii. [DOI] [PubMed] [Google Scholar]
- 12. Chai X, Domchek S, Kauff N, Rebbeck T, Chen J.. RE: breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9):pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotsopoulos J, Huzarski T, Gronwald J, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greene MH, Piedmonte M, Alberts D, et al. A prospective study of risk-reducing salpingo-oophorectomy and longitudinal CA-125 screening among women at increased genetic risk of ovarian cancer: design and baseline characteristics: a Gynecologic Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2008;17(3):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Euhus DM, Smith KC, Robinson L, et al. Pretest prediction of BRCA1 or BRCA2 mutation by risk counselors and the computer model BRCAPRO. J Natl Cancer Inst. 2002;94(11):844–851. [DOI] [PubMed] [Google Scholar]
- 16. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the risk of ovarian cancer algorithm with frequent CA125 testing in women at increased familial risk - combined results from two screening trials. Clin Cancer Res. 2017;23(14):3628–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sherman ME, Piedmonte M, Mai PL, et al. Pathologic findings at risk-reducing salpingo-oophorectomy: primary results from Gynecologic Oncology Group Trial GOG-0199. J Clin Oncol. 2014;32(29):3275–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalbfleisch JD, Prentice RL.. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 19. Olson JE, Sellers TA, Iturria SJ, Hartmann LC.. Bilateral oophorectomy and breast cancer risk reduction among women with a family history. Cancer Detect Prev. 2004;28(5):357–360. [DOI] [PubMed] [Google Scholar]
- 20. Colditz GA, Kaphingst KA, Hankinson SE, Rosner B.. Family history and risk of breast cancer: Nurses’ Health Study. Breast Cancer Res Treat. 2012;133(3):1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen FC, van Overeem Hansen T, Sorensen CS.. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer. 2016;16(9):599–612. [DOI] [PubMed] [Google Scholar]
- 22. Piver MS, Jishi MF, Tsukada Y, Nava G.. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 1993;71(9):2751–2755. [DOI] [PubMed] [Google Scholar]
- 23. Schorge JO, Muto MG, Lee SJ, et al. BRCA1-related papillary serous carcinoma of the peritoneum has a unique molecular pathogenesis. Cancer Res. 2000;60(5):1361–1364. [PubMed] [Google Scholar]
- 24. Tobacman JK, Tucker MA, Kase R, Greene MH, Costa J, Fraumeni JF.. Intra-abdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;320(8302):795–797. [DOI] [PubMed] [Google Scholar]
- 25. Hall MJ, Obeid E, Daly MB.. Multigene panels to evaluate hereditary cancer risk: reckless or relevant? J Clin Oncol. 2016;34(34):4186–4187. [DOI] [PubMed] [Google Scholar]
- 26. Moran A, O’Hara C, Khan S, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11(2):235–242. [DOI] [PubMed] [Google Scholar]
- 27. Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2(11):1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Jonge MM, Mooyaart AL, Vreeswijk MP, et al. Linking uterine serous carcinoma to BRCA1/2-associated cancer syndrome: a meta-analysis and case report. Eur J Cancer. 2017;72:215–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.