Abstract
Background
Talazoparib is a poly(adenosine diphosphate-ribose) polymerase inhibitor that causes death in cells with breast cancer susceptibility gene 1 or 2 (BRCA1/2) mutations.
Methods
EMBRACA (NCT01945775) was a randomized phase III study comparing efficacy, safety, and patient-reported outcomes (PROs) of talazoparib (1 mg) with physician’s choice of chemotherapy (PCT: capecitabine, eribulin, gemcitabine, vinorelbine) in locally advanced or metastatic breast cancer with a germline BRCA1/2 (gBRCA1/2) mutation. Prespecified patient subgroups were analyzed for progression-free survival, objective response, clinical benefit, duration of response, and safety. PROs were evaluated in hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) or triple-negative breast cancer (TNBC) subgroups.
Results
Of 431 patients, 287 were randomly assigned to talazoparib and 144 to PCT. Prespecified subgroup analyses showed prolonged progression-free survival with talazoparib (HR+/HER2−: hazard ratio = 0.47, 95% confidence interval = 0.32 to 0.71; TNBC: hazard ratio = 0.60, 95% confidence interval = 0.41 to 0.87) and greater objective response rate (odds ratio = 1.97 to 11.89), clinical benefit rate (odds ratio = 2.05 to 7.77), and duration of response with talazoparib in all subgroups. PROs in HR+/HER2− and TNBC subgroups showed consistent overall improvement and delay in time to definitive clinically meaningful deterioration with talazoparib vs PCT. Across subgroups, common adverse events included anemia, fatigue, and nausea with talazoparib and neutropenia, fatigue, and nausea with PCT. Seven patients (2.4%) receiving talazoparib had grade II alopecia and 22.7% had grade I alopecia.
Conclusions
Across all patient subgroups with gBRCA-mutated advanced breast cancer, talazoparib demonstrated clinically significant superiority in outcomes compared with PCT.
Cancer cells with deleterious mutations in breast cancer susceptibility gene 1 or 2 (BRCA1/2) have a defective homologous recombination DNA repair mechanism, making them highly dependent on poly(adenosine diphosphate-ribose) polymerase (PARP) function (1–3). In cells with a BRCA1/2 mutation, PARP inhibition results in cell death due to a buildup of irreparably damaged DNA (1–3). Talazoparib, a potent PARP inhibitor, not only blocks enzymatic activity but also traps PARP at sites of DNA damage, a mechanism that might be more effective at inducing cancer cell death than enzymatic inhibition alone (4–7). Talazoparib was recently approved by the US Food and Drug Administration, European Medicines Agency, and in other countries (8,9).
In phase II and III clinical trials, talazoparib was shown to provide clinical benefit to patients with germline BRCA (gBRCA)-mutated locally advanced or metastatic breast cancer (10,11). The phase III EMBRACA study compared the efficacy, safety, and patient-reported outcomes (PROs) of talazoparib with physician’s choice of trial-specified chemotherapy (PCT: capecitabine, eribulin, gemcitabine, or vinorelbine). Primary results in the intent-to-treat (ITT) population showed a significantly prolonged progression-free survival (PFS) with talazoparib vs PCT (hazard ratio [HR] = 0.54, 95% confidence interval [CI] = 0.41 to 0.71) and a manageable safety profile (12). In the PRO-evaluable population, statistically significant delay in time to definitive clinically meaningful deterioration (TTD) and statistically significant overall improvement in multiple patient-reported cancer-related and breast cancer-specific symptoms, functions, and global health status/quality of life (GHS/QoL) favoring talazoparib were observed, with none statistically significantly favoring PCT (13).
Efficacy endpoints were previously reported in a limited number of patient subgroups (12). However, it is also of interest to analyze multiple efficacy endpoints based on other patient factors, including age, race, geographic region, Eastern Cooperative Oncology Group performance status (ECOG PS), measurable disease, disease-free interval (DFI) (<12 vs ≥12 months), bone-only disease, prior neoadjuvant or adjuvant therapy, capecitabine, and prior hormonal therapy. This analysis expanded on the preliminary analysis (12) by further evaluating outcomes across multiple efficacy, safety, and PRO endpoints and in a broad spectrum of relevant prespecified patient subgroups (>15 additional subgroups examined).
Methods
Study Design
Detailed study information has been published previously (12,13). Briefly, EMBRACA was an open-label, randomized, multinational phase III study (NCT01945775) comparing the efficacy, safety, and PROs of oral talazoparib (1 mg once daily) with PCT (capecitabine, eribulin, gemcitabine, or vinorelbine) in patients with gBRCA-mutated locally advanced or metastatic breast cancer. Patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer were excluded from this study. Additional study details are provided in the Methods section of the Supplementary Materials (available online). The data cutoff date was September 15, 2017.
Endpoints and Study Assessments
The primary endpoint was radiographic PFS by blinded independent central review using Response Evaluation Criteria in Solid Tumors version 1.1. Imaging was performed every 6 weeks during the initial part of the study (12,13). Secondary and exploratory efficacy endpoints included overall survival, objective response, clinical benefit at 24 weeks (defined as complete response, partial response, or stable disease for ≥24 weeks), and duration of response (DOR). Secondary and exploratory endpoints are further detailed in the Supplementary Materials (available online).
PROs (13) were assessed as exploratory endpoints using the European Organisation for Research and Treatment of Cancer QoL Questionnaire-Core 30 (EORTC QLQ-C30) and breast cancer module (QLQ-BR23) at baseline, the beginning of each treatment cycle (every 3 weeks), and the end of treatment (see Methods in the Supplementary Materials, available online). In this study, PROs for breast cancer hormonal subtypes (hormone receptor-positive [HR+]/human epidermal growth factor receptor 2-negative [HER2−] and triple-negative breast cancer [TNBC]) were analyzed separately.
Patient Subgroups
The following prespecified patient subgroups, including differences between subgroups, were assessed: age (<50 vs ≥50 years), race, geographic region, ECOG PS, centrally tested BRCA mutation type, hormone receptor status (HR+/HER2− or TNBC), prior history of central nervous system (CNS) metastases (yes vs no), measurable disease (yes vs no), visceral disease (yes vs no), DFI (<12 vs ≥12 months), prior neoadjuvant or adjuvant therapy (yes vs no), prior platinum-based chemotherapy (yes vs no), prior capecitabine (yes vs no), prior hormonal therapy (yes vs no), and prior cytotoxic regimens for locally advanced or metastatic breast cancer (yes vs no).
Statistical Analyses
Statistical methodology has been described previously (12,13). The current analyses conducted in prespecified clinically relevant subgroups characterized baseline and demographic factors as well as efficacy (prespecified for PFS/objective response), safety, and PRO endpoints. The definitive clinically meaningful deterioration for all TTD analyses for each arm was defined as at least a 10-point change from baseline threshold with no subsequent observation less than a 10-point change from baseline (14).
Results
Patients
A total of 431 patients were included (ITT population: talazoparib, n = 287; PCT, n = 144). Protocol-specified PCTs included capecitabine (44%), eribulin (40%), gemcitabine (10%), and vinorelbine (7%) (Supplementary Figure 1, available online). Baseline characteristics were generally balanced, although more patients in the talazoparib arm were younger than 50 years of age, had an ECOG greater than 0, or had a DFI of less than 12 months (Table 1), and some differences were seen among the two breast cancer subgroups (HR+/HER2− or TNBC; Supplementary Table 1, available online).
Table 1.
Baseline characteristics (ITT population)
| Baseline characteristics | Talazoparib (n = 287) | Overall PCT (n = 144) |
|---|---|---|
| Female, % | 98.6 | 97.9 |
| Age, y | ||
| n | 287 | 144 |
| Mean (SD) | 47.5 (11.6) | 49.4 (12.1) |
| Median | 45.0 | 50.0 |
| Min, max | 27.0, 84.0 | 24.0, 88.0 |
| Age category, no. (%) | ||
| <50 y | 182 (63.4) | 67 (46.5) |
| ≥50 to <65 y | 78 (27.2) | 67 (46.5) |
| ≥65 y | 27 (9.4) | 10 (6.9) |
| Race, no. (%) | ||
| White | 192 (66.9) | 108 (75.0) |
| Asian | 31 (10.8) | 16 (11.1) |
| Black or African American | 12 (4.2) | 1 (0.7) |
| Other | 5 (1.7) | 1 (0.7) |
| Not reported | 47 (16.4) | 18 (12.5) |
| Ethnicity, no. (%) | ||
| Not Hispanic or Latino | 210 (73.2) | 111 (77.1) |
| Hispanic or Latino | 31 (10.8) | 15 (10.4) |
| Not reported | 46 (16.0) | 18 (12.5) |
| ECOG PS, no. (%) | ||
| 0 | 153 (53.3) | 84 (58.3) |
| 1 | 127 (44.3) | 57 (39.6) |
| 2 | 6 (2.1) | 2 (1.4) |
| Stage of breast cancer, no. (%) | ||
| Locally advanced* | 15 (5.2) | 9 (6.3) |
| Metastatic disease | 271 (94.4) | 135 (93.8) |
| Number of metastatic sites, no. (%)† | ||
| 1 | 68 (23.7) | 41 (28.5) |
| 2 | 88 (30.7) | 43 (29.9) |
| ≥3 | 131 (45.6) | 60 (41.7) |
| Measurable disease by investigator, no. (%) | ||
| Measurable | 219 (76.3) | 114 (79.2) |
| Nonmeasurable disease only | 68 (23.7) | 30 (20.8) |
| History of CNS metastases, no. (%) | 42 (14.6) | 20 (13.9) |
| Sites of disease, no. (%) | ||
| Visceral disease‡ | 200 (69.7) | 103 (71.5) |
| Bone-only disease | 25 (8.7) | 16 (11.1) |
| Hormone receptor status, no. (%) | ||
| TNBC | 130 (45.3) | 60 (41.7) |
| BRCA1+ and TNBC | 100 (76.9) | 43 (71.7) |
| BRCA2+ and TNBC | 30 (23.1) | 17 (28.3) |
| HR+ | 157 (54.7) | 84 (58.3) |
| BRCA1+ and HR+ | 33 (21.0) | 20 (23.8) |
| BRCA2+ and HR+ | 124 (79.0) | 64 (76.2) |
| BRCA status by central laboratory, no. (%)§ | 270 (94.1) | 138 (95.8) |
| BRCA1+ | 123 (45.6) | 60 (43.5) |
| BRCA2+ | 147 (54.4) | 78 (56.5) |
| BRCA status by local laboratory, no. (%)§ | 17 (5.9) | 6 (4.2) |
| BRCA1+ | 10 (58.8) | 3 (50.0) |
| BRCA2+ | 7 (41.2) | 3 (50.0) |
| Tumor histology, no. (%) | ||
| Ductal | 246 (85.7) | 131 (91.0) |
| Lobular | 22 (7.7) | 5 (3.5) |
| Other/unknown | 19 (6.6) | 8 (5.6) |
| De novo breast cancer (stage IV at initial diagnosis), no. (%) | 53 (18.5) | 22 (15.3) |
| Disease-free interval (initial diagnosis to ABC) | ||
| <12 months, no. (%) | 108 (37.6) | 42 (29.2) |
| ≥12 months, no. (%) | 178 (62.0) | 102 (70.8) |
| Time from initial diagnosis of breast cancer to randomization, median (range), y | 3.9 (0–35) | 5.0 (0–28) |
| Prior Therapies | ||
| Prior neoadjuvant/ adjuvant therapy, no. (%) | 238 (82.9) | 121 (84.0) |
| Prior HT, no. (%) | 161 (56.1) | 77 (53.5) |
| Prior HT regimens (ABC), no. | 157 | 84 |
| HT regimens, median (range) | 1.0 (0–6) | 1.0 (0–5) |
| ≥1 HT regimen, no. (%) | 92 (58.6) | 49 (58.3) |
| Prior HT regimens (any setting), median (range) | 2.0 (0–6) | 2.0 (0–6) |
| ≥1 HT regimen | 142 (90.4) | 70 (83.3) |
| Prior cytotoxic chemotherapies (ABC), median (range) | 1.0 (0–10) | 1.0 (0–3) |
| Prior anthracycline therapy, no. (%) | 243 (84.7) | 115 (79.9) |
| Prior taxane therapy, no. (%) | 262 (91.3) | 130 (90.3) |
| Prior capecitabine therapy, no. (%) | 73 (25.4) | 43 (29.9) |
| Prior eribulin therapy, no. (%) | 11 (3.8) | 7 (4.9) |
| Prior platinum therapy, no. (%) | 46 (16.0) | 30 (20.8) |
| Prior cytotoxic chemotherapy regimens for ABC, no. (%) | ||
| 0 | 111 (38.7) | 54 (37.5) |
| 1 | 107 (37.3) | 54 (37.5) |
| 2 | 57 (19.9) | 28 (19.4) |
| 3 | 11 (3.8) | 8 (5.6) |
| ≥4 | 1 (0.3) | 0 |
Patients were considered to have locally advanced disease if the date of first distant metastasis was missing from case report form. ABC = advanced breast cancer; BRCA1/2 = breast cancer susceptibility gene 1 or 2; CNS = central nervous system; ECOG PS = Eastern Cooperative Oncology Group performance status; ITT = intent-to-treat; HR+ = hormone receptor-positive; HT = hormone therapy; max = maximum; min = minimum; PCT = physician’s choice of chemotherapy; TNBC = triple-negative breast cancer; y = years.
The number of metastatic sites was derived from target and nontarget lesions assessed by the investigator at baseline. These included breast (only) lesions and counting the organ once if there were more lesions in the same location.
Visceral disease was defined as non-nodal target or nontarget lesions identified as lung, liver, kidney, heart, stomach, small intestine, colon, rectum, ovary, uterus/endometrium, pancreas, thyroid, adrenal, or spleen at baseline.
Determined by either central or local laboratory. If central and local laboratory results were both available, the central result was used if positive; if both results were entered and the central result was negative, the local result was used.
Efficacy
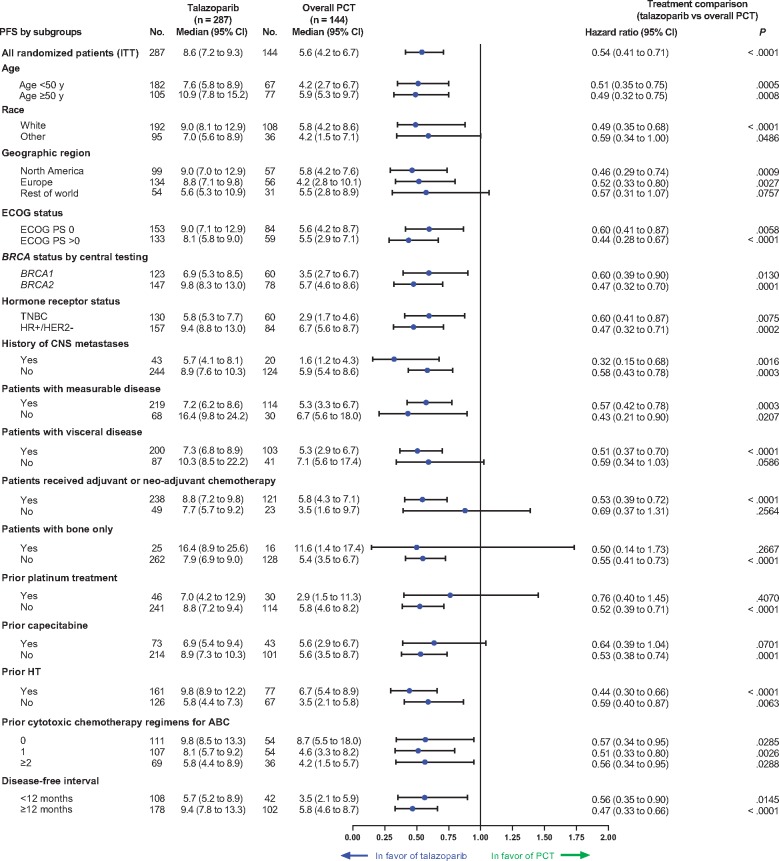
All subgroups demonstrated superior PFS with talazoparib vs PCT (Figure 1; HR = 0.32 to 0.76 across subgroups). For the two breast cancer subgroups, treatment effect for PFS was HR = 0.47, 95% CI = 0.32 to 0.71 for HR+/HER2− and HR = 0.60, 95% CI = 0.41 to 0.87 for TNBC. Patients with measurable or nonmeasurable disease, visceral or nonvisceral disease, DFI less than 12 or ≥ 12 months, or a history of CNS metastases (yes and no) all benefited from talazoparib treatment compared with PCT (Figure 1). Of the prespecified subgroups, use of prior platinum therapy, prior capecitabine, rest of world region, bone-only disease, race (other than white), no prior neoadjuvant or adjuvant chemotherapy, and nonvisceral disease were the only subgroups in which the 95% CI upper bound exceeded 1.0.
Figure 1.
Progression-free survival (PFS) by blinded independent central review (BICR): talazoparib vs physician’s choice of chemotherapy (PCT; subgroup analysis). ABC = advanced breast cancer; BRCA1/BRCA2 = breast cancer susceptibility gene 1 or 2; CI = confidence interval; CNS = central nervous system; ECOG PS = Eastern Cooperative Oncology Group performance status; HER2− = human epidermal growth factor receptor 2-negative; HR+ = hormone receptor-positive; HT = hormone therapy; ITT = intent-to-treat; TNBC = triple-negative breast cancer; y = years.
Similarly, the objective response rate (ORR) for talazoparib was greater across all subgroups receiving talazoparib vs PCT (odds ratio [OR] = 1.97 to 11.89; Table 2). In several of the more difficult-to-treat subgroups, such as visceral disease and prior platinum therapy, a higher ORR was observed with talazoparib (visceral disease: talazoparib, 62.2% vs PCT, 25.5%; prior platinum therapy: talazoparib, 50.0% vs PCT, 24.0%). For patients without prior platinum therapy, ORR was also higher with talazoparib than PCT (65.2% vs 28.1%) (Table 2).
Table 2.
Investigator-assessed unconfirmed and confirmed* objective response by subgroup (ITT with measurable disease)
| Objective response by subgroup | Talazoparib (n = 219) | Overall PCT (n = 114) |
|---|---|---|
| Overall objective response, No. (% [95% CI]) | 137 (62.6 [55.78 to 68.99]) | 31 (27.2 [19.28 to 36.33]) |
| OR (95% CI) | 4.99 (2.93 to 8.83) | |
| Age <50 y, No. | 142 | 49 |
| Objective response, No. (% [95% CI]) | 88 (62.0 [53.45 to 69.98]) | 11 (22.4 [11.77 to 36.62]) |
| OR (95% CI) | 5.77 (2.54 to 13.67) | |
| Age ≥50 to <65 y, No. | 54 | 57 |
| Objective response, No. (% [95% CI]) | 35 (64.8 [50.62 to 77.32]) | 19 (33.3 [21.40 to 47.06]) |
| OR (95% CI) | 5.82 (2.05 to 15.11) | |
| Age ≥65 y, No. | 23 | 8 |
| Objective response, No. (% [95% CI]) | 14 (60.9 [38.54 to 80.29]) | 1 (12.5 [0.32 to 52.65]) |
| OR (95% CI) | NA (1.67 to NA) | |
| Race | ||
| White, No. | 143 | 86 |
| Objective response, No. (% [95% CI]) | 93 (65.0 [56.62 to 72.81]) | 27 (31.4 [21.81 to 42.30]) |
| OR (95% CI) | 4.52 (2.41 to 8.72) | |
| Asian, No. (% [95% CI]) | 23 | 13 |
| Objective response, No. (% [95% CI]) | 11 (47.8 [26.82 to 69.41]) | 2 (15.4 [1.92 to 45.45]) |
| OR (95% CI) | NA | |
| Geographic region | ||
| North America,† No. | 81 | 45 |
| Objective response, No. (% [95% CI]) | 51 (63.0 [51.51 to 73.44]) | 11 (24.4 [12.88 to 39.54]) |
| OR (95% CI) | 5.54 (2.40 to 16.09) | |
| Europe,† No. | 97 | 45 |
| Objective response, No. (% [95% CI]) | 57 (58.8 [48.31 to 68.67]) | 13 (28.9 [16.37 to 44.31]) |
| OR (95% CI) | 3.75 (1.57 to 9.87) | |
| Rest of world,† No. | 41 | 24 |
| Objective response, No. (% [95% CI]) | 29 (70.7 [54.46 to 83.87]) | 7 (29.2 [12.62 to 51.09]) |
| OR (95% CI) | 6.70 (1.64 to 28.39) | |
| ECOG PS = 0, No. | 120 | 64 |
| Objective response, No. (% [95% CI]) | 77 (64.2 [54.90 to 72.71]) | 14 (21.9 [12.51 to 33.97]) |
| OR (95% CI) | 6.06 (3.08 to 15.07) | |
| ECOG PS > 0, No. | 98 | 49 |
| Objective response, No. (% [95% CI]) | 60 (61.2 [50.85 to 70.90]) | 17 (34.7 [21.67 to 49.64]) |
| OR (95% CI) | 3.32 (1.47 to 7.37) | |
| BRCA1, No. | 92 | 50 |
| Objective response, No. (% [95% CI]) | 59 (64.1 [53.46 to 73.87]) | 11 (22.0 [11.53 to 35.96]) |
| OR (95% CI) | 7.01 (2.99 to 19.54) | |
| BRCA2, No. | 114 | 60 |
| Objective response, No. (% [95% CI]) | 71 (62.3 [52.72 to 71.19]) | 18 (30.0 [18.85 to 43.21]) |
| OR (95% CI) | 4.15 (1.90 to 8.52) | |
| TNBC based on most recent biopsy, No. | 102 | 48 |
| Objective response, No. (% [95% CI]) | 63 (61.8 [51.61 to 71.21]) | 6 (12.5 [4.73 to 25.25]) |
| OR (95% CI) | 11.89 (4.54 to 41.37) | |
| HR+/HER2−, No. | 117 | 66 |
| Objective response, No. (% [95% CI]) | 74 (63.2 [53.84 to 71.97]) | 25 (37.9 [26.22 to 50.66]) |
| OR (95% CI) | 2.89 (1.43 to 5.83) | |
| History of CNS metastasis, No. | 38 | 19 |
| Objective response, No. (% [95% CI]) | 24 (63.2 [45.99 to 78.19]) | 3 (15.8 [3.38 to 39.58]) |
| OR (95% CI) | 8.95 (1.86 to 52.26) | |
| No history of CNS metastasis, No. | 181 | 95 |
| Objective response, No. (% [95% CI]) | 113 (62.4 [54.94 to 69.51]) | 28 (29.5 [20.56 to 39.71]) |
| OR (95% CI) | 4.48 (2.53 to 8.43) | |
| Patients with visceral disease by investigator, No. | 180 | 98 |
| Objective response, No. (% [95% CI]) | 112 (62.2 [54.71 to 69.33]) | 25 (25.5 [17.24 to 35.31]) |
| OR (95% CI) | 5.27 (2.87 to 9.74) | |
| Patients with nonvisceral disease by investigator, No. | 39 | 16 |
| Objective response, No. (% [95% CI]) | 25 (64.1 [47.18 to 78.80]) | 6 (37.5 [15.20 to 64.57]) |
| OR (95% CI) | 2.93 (0.85 to 15.10) | |
| Prior neoadjuvant/adjuvant therapy, No. | 183 | 96 |
| Objective response, No. (% [95% CI]) | 119 (65.0 [57.64 to 71.91]) | 25 (26.0 [17.62 to 36.00]) |
| OR (95% CI) | 6.40 (3.41 to 11.98) | |
| No prior neoadjuvant/adjuvant therapy, No. | 36 | 18 |
| Objective response, No. (% [95% CI]) | 18 (50.0 [32.92 to 67.08]) | 6 (33.3 [13.34 to 59.01]) |
| OR (95% CI) | 1.97 (0.50 to 8.54) | |
| Prior platinum therapy, No. | 38 | 25 |
| Objective response, No. (% [95% CI]) | 19 (50.0 [33.38 to 66.62]) | 6 (24.0 [9.36 to 45.13]) |
| OR (95% CI) | 3.16 (0.88 to 15.67) | |
| No prior platinum therapy, No. | 181 | 89 |
| Objective response, No. (% [95% CI]) | 118 (65.2 [57.77 to 72.11]) | 25 (28.1 [19.07 to 38.62]) |
| OR (95% CI) | 5.36 (2.89 to 9.89) | |
| DFI‡ <12 months, No. | 90 | 32 |
| Objective response, No. (% [95% CI]) | 45 (50.0 [39.27 to 60.73]) | 6 (18.8 [7.21 to 36.44]) |
| OR (95% CI) | 4.86 (1.85 to 19.71) | |
| DFI‡ ≥12 months, No. | 129 | 82 |
| Objective response, No. (% [95% CI]) | 92 (71.3 [62.70 to 78.93]) | 25 (30.5 [20.80 to 41.64]) |
| OR (95% CI) | 6.33 (3.19 to 12.49) | |
Unconfirmed objective response contains the confirmed as well as unconfirmed responses. BRCA1/BRCA2 = breast cancer susceptibility gene 1 or 2; CI = confidence interval; CNS = central nervous system; DFI = disease-free interval; ECOG PS = Eastern Cooperative Oncology Group performance status; HR+ = hormone receptor-positive; ITT = intent-to-treat; OR = odds ratio; NA = not available; PCT = physician’s choice of chemotherapy; TNBC = triple-negative breast cancer.
Europe (Belgium, France, Germany, Ireland, Israel, Italy, Poland, Russia, Spain, Ukraine, United Kingdom); North America (United States); Rest of world (Australia, Brazil, Korea, Taiwan).
Time from initial diagnosis of breast cancer to initial diagnosis of locally advanced or metastatic breast cancer.
Median DOR was longer across subgroups receiving talazoparib than PCT, with more patients showing a continued objective response at month 12 (Supplementary Table 2, available online). For example, patients with visceral disease receiving talazoparib had a DOR approximately 2 months longer than those receiving PCT (talazoparib, 5.3 months vs PCT, 3.1 months). Results were similar with nonvisceral disease (talazoparib, 6.2 months vs PCT, 3.2 months). Median DOR for talazoparib was longer for patients who had not received any prior platinum therapy (5.4 months) than for patients who had prior platinum therapy (4.2 months).
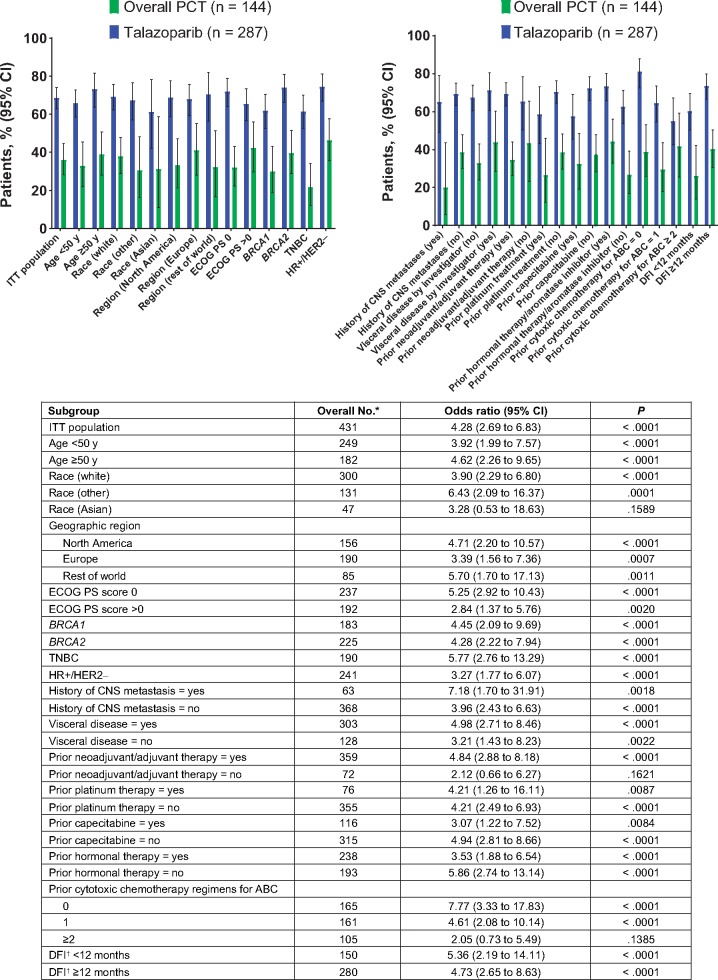
Talazoparib nearly doubled the clinical benefit rate (CBR) compared with PCT in most subgroups and tripled the CBR in TNBC patients and those with a history of CNS metastases. The CBR was statistically significantly greater with talazoparib compared with PCT in all subgroups (OR = 2.05 to 7.77) except for those subgroups in which the lower bound of the 95% CI crossed below 1.0 (two or more prior chemotherapies, no prior neoadjuvant or adjuvant therapy, and Asian patients) (Figure 2).
Figure 2.
Investigator-assessed clinical benefit rate at week 24 by subgroup (intent-to-treat [ITT] population). *No. includes both treatment arms together. †Time from initial diagnosis of breast cancer to initial diagnosis of locally advanced or metastatic breast cancer. ABC = advanced breast cancer; BRCA1/BRCA2 = breast cancer susceptibility gene 1 or 2; CI = confidence interval; CNS = central nervous system; DFI = disease-free interval; ECOG PS = Eastern Cooperative Oncology Group performance status; HER2− = human epidermal growth factor receptor 2-negative; HR+ = hormone receptor-positive; PCT = physician’s choice of chemotherapy; TNBC = triple-negative breast cancer; y = years.
Safety
In general, the safety profile of talazoparib was similar across subgroups and was comparable with that seen in the safety population in the EMBRACA and ABRAZO studies (10,11). Common adverse events (AEs) included anemia, fatigue, and nausea with talazoparib and nausea, fatigue, and neutropenia with PCT (10,11). Permanent discontinuations due to AEs were infrequent (talazoparib, 5.9%; PCT, 8.7%). Although hematologic AEs were common with talazoparib (all grade, 68.2%), only two (0.7%) patients permanently discontinued talazoparib for anemia, and only one (0.3%) patient discontinued each for thrombocytopenia and neutropenia. Notably, patients discontinuing talazoparib because of hematologic AEs were younger than 50 years; no discontinuations occurred in older age groups.
For the two breast cancer subgroups, the primary toxicities for talazoparib were hematologic, with at least a grade III anemia (HR+/HER2−, 38.5%; TNBC, 40.0%), neutropenia (HR+/HER2−, 16.7%; TNBC, 26.2%), and thrombocytopenia (HR+/HER2−, 12.8%; TNBC, 16.9%). Fewer HR+/HER2− patients had all-grade neutropenia with talazoparib (talazoparib, 31.4%; PCT, 45.9%) than TNBC patients (talazoparib, 38.5%; PCT, 38.5%) (Table 3). Eribulin is known to cause more neutropenia, and only 2.5% of HR+/HER2− patients received eribulin as a prior therapy compared with 5.4% of TNBC patients; differences in the extent of prior eribulin might thus account for incidence of neutropenia. In the HR+/HER2− population, no patients experienced febrile neutropenia compared with two patients in the TNBC population. Incidence of serious AEs with talazoparib was similar between HR+/HER2− (30.8%) and TNBC (33.1%) patients (Table 3). Few patients experienced an AE that resulted in permanent discontinuation of talazoparib (HR+/HER2−, 5.8%; TNBC, 6.2%).
Table 3.
AEs, all grades: TNBC and HR+/HER2− subgroups (safety population*)
| TNBC, No. (%) |
HR+/HER2−, No. (%) |
||||
|---|---|---|---|---|---|
| Adverse events, all grades | Talazoparib (n = 130) | Overall PCT (n = 52) | Talazoparib (n = 156) | Overall PCT (n = 74) | |
| Any AE | 128 (98.5) | 51 (98.1) | 154 (98.7) | 72 (97.3) | |
| Serious† | 43 (33.1) | 16 (30.8) | 48 (30.8) | 21 (28.4) | |
| Resulting in permanent drug discontinuation | 8 (6.2) | 4 (7.7) | 9 (5.8) | 7 (9.5) | |
| Hematologic | |||||
| Patients with ≥1 hematologic AE, any grade, no. (%) | 95 (73.1) | 24 (46.2) | 99 (63.5) | 39 (52.7) | |
| Anemia‡ | 73 (56.2) | 11 (21.2) | 78 (50.0) | 12 (16.2) | |
| Neutropenia§ | 50 (38.5) | 20 (38.5) | 49 (31.4) | 34 (45.9) | |
| Thrombocytopenia‖ | 40 (30.8) | 5 (9.6) | 37 (23.7) | 4 (5.4) | |
| Leukopenia | 24 (18.5) | 10 (19.2) | 25 (16.0) | 7 (9.5) | |
| Lymphopenia | 11 (8.5) | 2 (3.8) | 10 (6.4) | 2 (2.7) | |
| Nonhematologic ≥20%¶ | |||||
| Nausea | 68 (52.3) | 22 (42.3) | Fatigue | 79 (50.6) | 30 (40.5) |
| Fatigue | 65 (50.0) | 24 (46.2) | Nausea | 71 (45.5) | 37 (50.0) |
| Headache | 43 (33.1) | 13 (25.0) | Headache | 50 (32.1) | 15 (20.3) |
| Alopecia | 39 (30.0)# | 11 (21.2) | Vomiting | 45 (28.8) | 18 (24.3) |
| Decreased appetite | 33 (25.4) | 13 (25.0) | Diarrhea | 41 (26.3) | 16 (21.6) |
| Back pain | 26 (20.0) | 11 (21.2) | Constipation | 38 (24.4) | 14 (18.9) |
| Vomiting | 26 (20.0) | 11 (21.2) | Back pain | 34 (21.8) | 9 (12.2) |
| Cough | 26 (20.0) | 6 (11.5) | Alopecia | 33 (21.2)# | 24 (32.4) |
Safety population included patients who received talazoparib. AE grades are evaluated based on National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Patients with multiple AEs were counted once for each preferred term, system organ class, and overall. AEs with action taken of permanent discontinuation were taken from the AE electronic case report form. AE = adverse event; HER2− = human epidermal growth factor receptor 2-negative; HR+ = hormone receptor-positive; PCT = physician’s choice of chemotherapy; TNBC = triple-negative breast cancer.
Serious defined as any AE that results in death, is considered life-threatening or medically important, results in hospitalization/prolonged hospitalization or persistent/significant disability/incapacity or is a congenital anomaly/birth defect.
The category of anemia includes reports of anemia and decreased hemoglobin. No cases of acute myeloid leukemia/myelodysplastic syndrome were reported in the talazoparib arm; one case was reported for a patient receiving capecitabine.
The category of neutropenia includes reports of neutropenia, decreased neutrophil count, and neutropenic sepsis.
The category of thrombocytopenia incudes reports of thrombocytopenia and decreased platelet count.
All AEs in at least 20% of patients or grade III–IV AEs in at least 2.4% of patients. For these selected toxicities, no grade IV AEs were reported in either arm.
72 patients (25.2%) overall (ie, TNBC and HR+ populations) experienced alopecia, with 65 patients (22.7%) having grade I and seven patients (2.4%) having grade II.
In the overall population, grade II alopecia was reported in 2.4% (n = 7) of patients receiving talazoparib vs 7.9% (n = 10) for PCT, and grade I alopecia was reported in 22.7% (n = 65) of patients receiving talazoparib vs 19.8% (n = 25) for PCT.
Among patients with alopecia both in the HR+/HER2− and TNBC populations, the majority in the PCT arm were receiving eribulin (HR+/HER2−, 19 [59.4%]; TNBC, 9 [50.0%]), which is known to cause alopecia. In patients younger than 50 years, any grade alopecia was experienced by approximately 21% and 26% in the talazoparib and PCT arms, respectively. Similarly, in patients aged 50 to 64 years, approximately 26% and 29.5% of patients experienced alopecia in the talazoparib- and PCT-treated groups, respectively. In patients aged greater than or equal to 65 years, 51.9% of patients had alopecia in the talazoparib arm, whereas only 25% had alopecia in the PCT group. The higher proportion of older patients with alopecia should be considered with caution because other factors might be at play, and patient numbers in this group were much smaller.
PROs
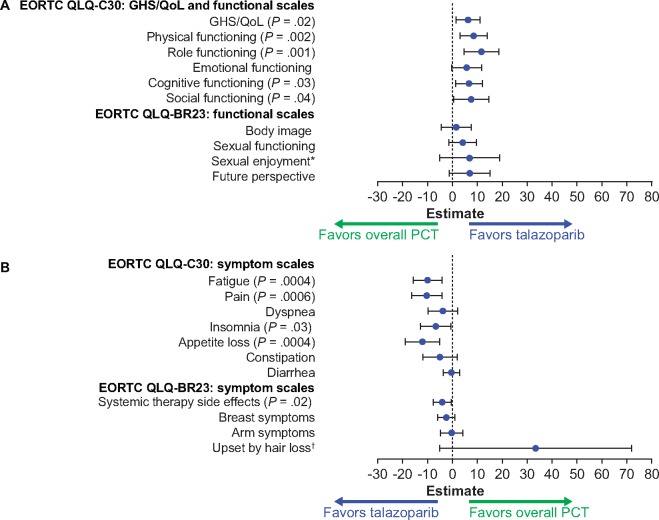
HR+/HER2−Subpopulation. For patients with HR+/HER2− disease, a statistically significant overall change from baseline in GHS/QoL favored talazoparib over PCT (5.8, 95% CI = 0.9 to 10.7, P = .02 vs PCT). A statistically significant overall change from baseline also favored talazoparib over PCT in the physical, role, cognitive, and social functions and the fatigue, pain, insomnia, appetite loss, and systemic therapy side effects symptoms (Figure 3). No statistically significant overall between-arm difference was observed for the emotional, body image, sexual enjoyment, sexual functioning, and future perspective functions or the dyspnea, constipation, diarrhea, breast, arm, and upset by hair loss symptoms. Baseline PRO data in patients with HR+/HER2− disease are provided in the Results section of the Supplementary Materials (available online).
Figure 3.
Patient-reported outcomes (PROs) for the HR+/HER2− population. A) European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30): global health status or quality of life (GHS/QoL) and functional scales; EORTC Quality of Life Questionnaire-Breast Cancer Module (QLQ-BR23): functional scales. B) EORTC QLQ-C30: symptom scales; EORTC QLQ-BR23: symptom scales. Forest plot model of estimated difference (talazoparib and overall PCT) in overall change from baseline (repeated measures mixed-effect model) in PRO-evaluable population (P values are shown only if significant between-arm differences, P < .05, were observed). Nausea/vomiting symptom scale not calculated. HR+ = hormone receptor-positive; HER2− = human epidermal growth factor receptor 2-negative; PCT = physician’s choice chemotherapy treatment. *The sample sizes for the “sexual enjoyment” functional scale were smaller vs other functional scales because patients were asked to respond to this question only if they responded that they were sexually active in a previous question. †The sample sizes for the “upset by hair loss” symptom scale were smaller vs other symptom scales because patients were asked to respond to this question only if they responded to experiencing hair loss in a previous question. PRO-evaluable population defined as all patients who completed one or more PRO question at baseline and one or more PRO question postbaseline.
A statistically significant delay in TTD favoring talazoparib over PCT was observed in GHS/QoL (median = 21.1 vs 12.2 months; HR = 0.41, 95% CI = 0.24 to 0.70, P = .0007), pain (median = 21.8 vs 10.4 months; HR = 0.45, 95% CI = 0.25 to 0.79, P = .004), and systemic therapy side effects (median = 21.1 vs 7.9 months; HR = 0.26, 95% CI = 0.15 to 0.46, P < .0001) (Supplementary Figure 2, available online). Likewise, statistically significant delay in TTD favoring talazoparib was observed in the physical, role, emotional, cognitive, and social functions and in the fatigue, nausea/vomiting, dyspnea, insomnia, appetite loss, constipation, and breast symptoms.
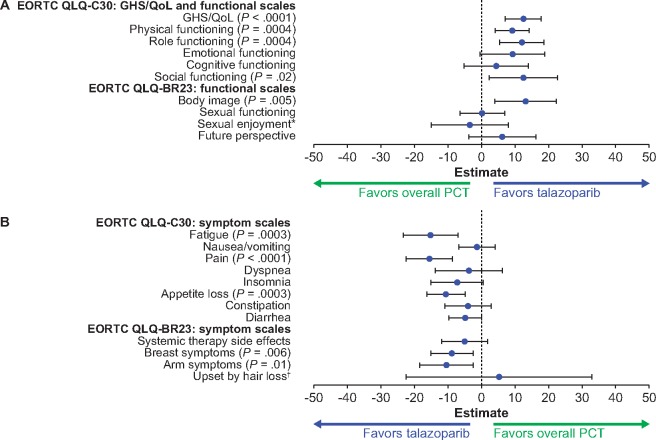
TNBC Subpopulation. For the TNBC population, a statistically significant overall change from baseline in GHS/QoL favored talazoparib over PCT (12.5, 95% CI = 7.1 to 17.8, P < .0001). A statistically significant overall change from baseline also favored talazoparib over PCT in the physical, role, social, and body image functions and the fatigue, pain, appetite loss, breast, and arm symptoms (Figure 4). Baseline PRO data in patients with TNBC are provided in the Results section of the Supplementary Materials (available online). A statistically significant delay in TTD favoring talazoparib over PCT was observed in GHS/QoL (median = 24.3 vs 4.5 months; HR = 0.33, 95% CI = 0.19 to 0.57, P < .0001), pain (median = 22.7 vs 5.6 months; HR = 0.25, 95% CI = 0.14 to 0.45, P < .0001), and systemic therapy side effects (median = 25.6 vs 10.3 months; HR = 0.45, 95% CI = 0.24 to 0.86, P = .01) (Supplementary Figure 3, available online). Likewise, a statistically significant delay in TTD favoring talazoparib over PCT was observed in physical, role, emotional, cognitive, social functions, and body image, and in fatigue, insomnia, appetite loss, and arm symptoms.
Figure 4.
PROs for the TNBC population. A) EORTC QLQ-C30: global health status or quality of life (GHS/QoL) and functional scales; EORTC QLQ-BR23: functional scales. B) EORTC QLQ-C30: symptom scales; EORTC QLQ-BR23: symptom scales. Forest plot model of estimated difference (talazoparib and overall PCT) in overall change from baseline (repeated measures mixed-effect model) in PRO-evaluable population (P values are shown only if significant between-arm differences, P < .05, were observed). *The sample sizes for the “sexual enjoyment” functional scale were smaller vs other functional scales because patients were asked to respond to this question only if they responded that they were sexually active in a previous question. EORTC = European Organisation for Research and Treatment of Cancer; HR+ = hormone receptor-positive; HER2− = human epidermal growth factor receptor 2-negative; PCT = physician’s choice of chemotherapy treatment; PRO = patient-reported outcomes; QLQ-BR23 = Quality of Life Questionnaire-Breast Cancer Module; QLQ-C30 = Quality of Life Questionnaire-Core 30. †The sample sizes for the “upset by hair loss” symptom scale were smaller vs other symptom scales because patients were asked to respond to this question only if they responded to experiencing hair loss in a previous question. PRO-evaluable population defined as all patients who completed one or more PRO question at baseline and one or more PRO question postbaseline.
Discussion
In patients with gBRCA1/2-mutated locally advanced or metastatic breast cancer, treatment with talazoparib was statistically significantly superior to PCT in efficacy and PROs across multiple subgroups. A broad spectrum of subgroups was explored, but the focus fell for the large part on two breast cancer patient subgroups: HR+/HER2− and TNBC. Patients with HR+/HER2− breast cancer or TNBC both experienced better outcomes with talazoparib vs PCT.
Regardless of whether the mutation was gBRCA1 or gBRCA2, talazoparib resulted in superior clinical outcomes (PFS, objective response, DOR, and clinical benefit) vs PCT. The duration of talazoparib therapy was longer, and more patients received talazoparib in a 12-month period. A larger number of patients achieved clinical benefit in the BRCA2 subgroup than in the BRCA1 subgroup. This difference in clinical benefit is consistent with differing biology and historical differences in outcomes between patients with gBRCA1 vs gBRCA2 mutations and metastatic breast cancer (15–17).
Among patients with TNBC, PFS, objective response, and clinical benefit were higher in those receiving talazoparib than those receiving PCT (HR for PFS = 0.60, 95% CI = 0.41 to 0.87, ORR = 61.8% vs 12.5%, and CBR = 61.5% vs 21.7%, respectively) and included a subset of patients with a prolonged response to talazoparib (17% at month 12 in TNBC patients). Similar outcomes were observed with talazoparib over PCT for patients with HR+/HER2− disease (HR for PFS = 0.47, 95% CI = 0.32 to 0.71, ORR = 63.2% vs 37.9%, and CBR = 74.5% vs 46.4%, respectively) and also included a subset of patients with prolonged response to talazoparib (28% at month 12 in HR+/HER2− patients).
These findings suggest that talazoparib should be considered as a possible alternative to chemotherapies like eribulin, capecitabine, gemcitabine, and vinorelbine both for TNBC and HR+/HER2− patients. Similarly, when considering these findings while recognizing that approximately 70% of patients in both arms had visceral disease and approximately 60% had at least one line of prior cytotoxic chemotherapy regimens for advanced breast cancer, the data suggest that talazoparib provides an important treatment option in this population. The latest ESO–ESMO ABC4 2018 guidelines support this conclusion with the recommendation that PARP inhibitors (olaparib or talazoparib) should be considered as “reasonable treatment options for patients with BRCA-associated metastatic TNBC or luminal (after progression on endocrine therapy)/metastatic breast cancer previously treated with an anthracycline with or without a taxane (in the adjuvant and/or metastatic setting), since their use is associated with a PFS benefit, improvement in QoL, and a favorable toxicity profile” (18). Direct comparison of platinum-based chemotherapy and PARP inhibitors is warranted to shed light on the optimal treatment sequence. In addition, optimal treatment sequencing for gBRCA-mutated HR+/HER2− breast cancer is unknown, and current guidelines suggest hormonal therapy and cyclin-dependent kinase 4/6 inhibitor therapy as the primary option for treatment of HR+/HER2− disease (15), although these guidelines also recommend the use of a PARP inhibitor for HR+/HER2− cancers (18).
The safety profile of talazoparib in the HR+/HER2− and TNBC subgroups was comparable and similar to that for the ITT population. The tolerable safety profile was complemented by findings from PROs. Notably, across all EORTC QLQ-C30 and QLQ-BR23 PRO scales, none of the overall change from baseline and TTD analyses statistically significantly favored PCT over talazoparib. These findings demonstrate that talazoparib has superior efficacy and PROs over PCT both in HR+/HER2− and TNBC subgroups.
While chemotherapy is often the primary choice for patients with metastatic breast cancer and visceral disease, our data indicate that talazoparib has a better PFS (HR = 0.51, 95% CI = 0.37 to 0.70) and ORR compared with PCT (62.2% vs 25.5%, respectively) with a longer DOR (5.3 vs 3.1 months, respectively). In addition, talazoparib was efficacious regardless of prior use of chemotherapy regimens for advanced breast cancer, disease site, and/or measurable disease (bone only and nonvisceral disease had HRs of 0.50 and 0.51, respectively).
Large, phase III, randomized, controlled clinical trials comparing platinum monotherapy with other PCT drugs in patients with locally advanced or metastatic breast cancer and gBRCA mutations are lacking, although a recent phase III trial (the Triple-Negative Breast Cancer Trial) included a small subset of patients with gBRCA mutations (n = 43) and concluded that platinum agents were superior in terms of PFS and OR compared with docetaxel (19). However, PRO data from platinum-based chemotherapy trials are limited, which may be a consideration when deciding on treatment and sequence for individual patients.
In the ABRAZO study, a longer platinum-free interval was associated with increased talazoparib efficacy, suggesting that patients who progress on platinum therapy or shortly after stopping platinum therapy may have reduced disease sensitivity to PARP inhibitors (11). Patients enrolled in EMBRACA were permitted to have received platinum therapy in the neoadjuvant or adjuvant setting as long as they did not relapse within 6 months from the last dose of prior platinum therapy and were excluded if they had objective disease progression while receiving platinum therapy for locally advanced or metastatic disease (12). Greater improvements in clinical outcomes were seen for patients who received talazoparib compared with those who received PCT although the benefit was greater in patients not treated with prior platinum therapy (20), similar to ABRAZO (11). Even though some key baseline characteristics (eg, aged less than 50 years; ECOG PS) were associated with a better prognosis for patients in the chemotherapy arm (both prior platinum therapy and no prior platinum therapy groups), efficacy benefits of talazoparib over PCT were still observed in these subgroups, with better prognosis. The percentage of patients permanently discontinuing treatment because of AEs was lower with talazoparib vs PCT regardless of prior platinum therapy, although the percentage of individual AEs reported was higher with talazoparib (20).
This study has some limitations because not all subgroups could be analyzed for the full range of clinical outcomes because some were small in size and response rates were low (especially in the PCT arm), for example, ECOG PS greater than 0 and history of CNS metastases. Furthermore, the study population was largely white with limited enrollment of other races. However, the subgroups considered to be most meaningful (ie, HR+/HER2− vs TNBC) were fully characterized. A further limitation of this study was the omission of platinum-based chemotherapy as an option in the PCT arm, mainly because single-agent, non platinum–based chemotherapy was a global standard of care (18) for this population when the study was initially designed in 2013 and doublets or triplets (with or without platinum therapy) were not considered appropriate for this patient population at the time. A head-to-head comparison of a PARP inhibitor to platinum-based chemotherapy is needed to understand the relative efficacy, toxicity, and effect on PROs. Finally, subgroup analyses can result in false-positive results, and therefore CIs were included in the manuscript so the reader could better evaluate the effects in the different subgroups.
In conclusion, our data highlight that patients with gBRCA1/2-mutated locally advanced or metastatic breast cancer across most subgroups showed statistically significantly greater PFS, ORR, and CBR with talazoparib vs PCT. This includes many prespecified subgroups (eg, HR+/HER2− or TNBC; BRCA1 or BRCA2; history or no history of CNS metastases; poor or good ECOG PS; younger or older age; measurable disease or nonmeasurable disease; visceral or nonvisceral disease), although sample sizes for some of these subgroups were small. Statistically significant overall improvement and statistically significant delay in TTD in PROs favored talazoparib vs PCT both in the HR+/HER2− and TNBC subgroups. The findings from this subgroup analysis are consistent with those for the overall population. Analysis of these additional prespecified subgroups will further aid clinicians in making informed decisions regarding their treatment options for a variety of patients with gBRCA-mutated advanced breast cancer.
Funding
This study was sponsored by Medivation, which was acquired by Pfizer, Inc. in September 2016.
Notes
Affiliations of authors: University of California San Francisco Helen Diller Family Comprehensive Cancer Center, San Francisco, CA (HSR); Department of Obstetrics and Gynecology, Klinikum rechts der Isar, Technische Universität München, Munich, Germany (JE); University of California, Los Angeles/Jonsson, Jonsson Comprehensive Cancer Center (UCLA/JCCC), Los Angeles, CA (SAH); Institut Paoli-Calmettes, Marseille, France (AG); Seoul National University Hospital, Seoul, South Korea (K-HL); Kaiser Permanente, Northern California, Vallejo, CA (LF); MD Anderson Cancer Center, Gilbert, AZ (LAM); Rocky Mountain Cancer Centers, Littleton, CO (SD); Mater Cancer Care Centre–Mater Health Services/Mater Research Institute, University of Queensland, South Brisbane, Queensland, Australia (NEW); Rabin Medical Center, Beilinson Hospital, Petah Tikva, Israel (RY); Concord Repatriation General Hospital, Concord, New South Wales, Australia (AG); Baylor Sammons Cancer Center, Texas Oncology, US Oncology, Dallas, TX (JLB); Instituto de Investigación Sanitaria Gregorio Marañón, CIBERONC, GEICAM, Universidad Complutense, Madrid, Spain (MM); Pfizer Inc., San Francisco, CA (RGWQ, EG); Former employee of Pfizer Inc. (ICT); Pfizer Inc., New York, NY (HB); The University of Texas MD Anderson Cancer Center, Houston, TX (JKL); Interdisziplinäres Onkologisches Zentrum München, Munich, Germany (WE).
All authors received editorial and medical writing support for the drafting of this manuscript, funded by Pfizer, Inc. HSR reports research support to the University of California San Francisco from Eisai, Roche/Genentech, Eli Lilly, MacroGenics, Merck, Novartis, OBI Pharma, Odonate, Immunomedics, Daiichi-Sankyo, and Pfizer; and travel support from Mylan, Pfizer, and Novartis. JE reports consulting fees from Lilly, Novartis, Pfizer, Roche, and Eisai and contracted research from Celgene; honoraria from Pfizer, Roche, Teva, and Pierre Fabre; and travel support from Celgene, Novartis, and Pfizer. SAH reports contracted research support from Ambrx, Amgen, Bayer, Daiichi-Sankyo, Genentech/Roche, GSK, Immunomedics, Lilly, MacroGenics, Novartis, Pfizer, OBI Pharma, Pieris, PUMA, Radius, Sanofi, Seattle Genetics, and Dignitana; and medical writing assistance (listed as consulting fees in CMS) from Pfizer and Roche. AG reports travel, accommodation, and meeting registration fees from Pfizer, AstraZeneca, Roche, Novartis, Amgen, Celgene, and Boehringer Ingelheim. K-HL, LF, LAM, and WE have nothing to disclose. SD is a speaker and advisor for Pfizer, Novartis, Puma, Eli Lilly, Clovis, Genentech, AstraZeneca, Genomic Heath, and Agendia. NEW reports stock and ownership in CSL Behring, research funding (institution) from Medivation, honoraria from advisory boards from Pfizer and Novartis, and consultancy fees, honoraria from advisory board and travel and accommodation fees from Roche. RY reports a grant from Roche; consulting fees from Roche, Pfizer, Novartis, and Eli Lilly; speaker fees from Roche, Teva, Medison, MSD, AstraZeneca, Novartis, and Pfizer. JLB reports consulting fees from Medivation, Pfizer, and Novartis, and honoraria from Dava Oncology, Genomic Health, and Research to Practice. MM reports consulting fees from Pfizer and fees for non–continuing medical education services received directly from commercial interests or their agents from Pfizer. RGWQ, HB, and EG are employees of Pfizer and report ownership interest in Pfizer. ICT is a former employee of Pfizer Inc. RGWQ and HB also report ownership interest in Amgen. JKL reports institutional-contracted research funding from Pfizer, Novartis, EMD Serono, AstraZeneca, GlaxoSmithKline, and Genentech; and advisory board participation with AstraZeneca and Pfizer, both uncompensated, and royalties from UptoDate and speakers bureau for MedLearning Group and Physician’s Education Resource.
The authors would like to thank the patients and their families and caregivers for their participation as well as the study centers and patient advocacy groups that supported this study. Editorial and medical writing support was provided to all authors by Edwin Thrower, PhD, Gautam Bijur, PhD, and Mary Kacillas of Ashfield Healthcare Communications and Zaavan Baildon of CMC AFFINITY, a division of McCann Health Medical Communications Ltd, Macclesfield, UK, and funded by Pfizer.
Pfizer, Inc. was involved in the study design, data collection, analysis, interpretation, and funding for editorial and medical writing support. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
HSR, SAH, JLB, and JKL designed this phase III study in collaboration with the sponsor (Biomarin and Medivation; Medivation was acquired by Pfizer, Inc.). Site investigators recruited patients, contributed to patient care, and collected patient data. HSR guided the initial drafting of the manuscript, with input from all other authors. All authors contributed to review of the data and the manuscript. RGWQ, EG, ICT, and HB contributed to the data analysis and reporting and review of the data and the manuscript. All authors had full access to the study data, contributed to the revision and approval of the manuscript, and participated in the decision to submit the manuscript for publication.
On request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supplementary Material
References
- 1. Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5(4):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Javle M, Curtin NJ.. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3(6):257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lord CJ, Ashworth A.. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murai J, Huang SY, Renaud A, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13(2):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG.. PARP inhibition: PARP1 and beyond. Nat Rev Cancer . 2010;10(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen Y, Rehman FL, Feng Y, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19(18):5003–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Medicines Agency. Talzenna: Summary of Product Characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/talzenna-epar-product-information_en.pdf. Accessed October 3, 2019.
- 9.Pfizer Inc. TALZENNA™ (talazoparib) [prescribing information]. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=11046. Accessed November 13, 2019.
- 10. de Bono J, Ramanathan RK, Mina L, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov . 2017;7(6):620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner NC, Telli ML, Rugo HR, et al. A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations (ABRAZO). Clin Cancer Res. 2019;25(9):2717–2724. [DOI] [PubMed] [Google Scholar]
- 12. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med . 2018;379(8):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ettl J, Quek RGW, Lee K-H, et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutations: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol. 2018;29(9):1939–1947. [DOI] [PubMed] [Google Scholar]
- 14. Osoba D, Rodrigues G, Myles J, Zee B, Pater J.. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 15. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. [DOI] [PubMed] [Google Scholar]
- 16. Turner NC, Lord CJ, Iorns E, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27(9):1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Breastcancer.org. Women With Abnormal BRCA1 Gene Have Worse Prognosis Than Women With Abnormal BRCA2 Gene https://www.breastcancer.org/research-news/20070907. Accessed October 3, 2019.
- 18. Cardoso F, Senkus E, Costa A, et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24(5):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin M, Eiermann W, Rugo HS, et al. EMBRACA: comparison of efficacy and safety of talazoparib and physician’s choice of therapy in patients with advanced breast cancer, a germline BRCA1/2 mutation, and prior platinum treatment. Presented at European Society for Medical Oncology; October 19–23, 2018; Munich, Germany. Poster 303P.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.