Abstract
Background
Hot flashes (HFs) negatively affect quality of life among perimenopausal and postmenopausal women. This study investigated the efficacy of oxybutynin vs placebo in decreasing HFs.
Methods
In this randomized, multicenter, double-blind study, women with and without breast cancer with 28 or more HFs per week, lasting longer than 30 days, who were not candidates for estrogen-based therapy, were assigned to oral oxybutynin (2.5 mg twice a day or 5 mg twice a day) or placebo for 6 weeks. The primary endpoint was the intrapatient change from baseline in weekly HF score between each oxybutynin dose and placebo using a repeated-measures mixed model. Secondary endpoints included changes in weekly HF frequency, HF-related daily interference scale questionnaires, and self-reported symptoms.
Results
We enrolled 150 women. Baseline characteristics were well balanced. Mean (SD) age was 57 (8.2) years. Two-thirds (65%) were taking tamoxifen or an aromatase inhibitor. Patients on both oxybutynin doses reported greater reductions in the weekly HF score (5 mg twice a day: −16.9 [SD 15.6], 2.5 mg twice a day: −10.6 [SD 7.7]), placebo −5.7 (SD 10.2); P < .005 for both oxybutynin doses vs placebo), HF frequency (5 mg twice a day: −7.5 [SD 6.6], 2.5 mg twice a day: −4.8 [SD 3.2], placebo: −2.6 [SD 4.3]; P < .003 for both oxybutynin doses vs placebo), and improvement in most HF-related daily interference scale measures and in overall quality of life. Patients on both oxybutynin arms reported more side effects than patients on placebo, particularly dry mouth, difficulty urinating, and abdominal pain. Most side effects were grade 1 or 2. There were no differences in study discontinuation because of adverse effects.
Conclusion
Oxybutynin is an effective and relatively well-tolerated treatment option for women with HFs.
Hot flashes (HFs) occur in about 75% of women at midlife, interfering with many spheres of life and overall quality of life (QoL) (1,2). Breast cancer survivors are at higher risk for long-term and more severe HFs as a consequence of chemotherapy-induced menopause, ovarian function suppression, and the use of tamoxifen or aromatase inhibitors (3). Development of HFs can be associated with premature discontinuation of adjuvant endocrine therapy and lead to worse breast cancer outcomes (4–6).
The most established treatment for HFs is estrogen-based therapy (7); however, it is usually avoided in women with a history of, or at increased risk for, breast cancer. Several randomized trials have identified non estrogen medications that are effective for HFs treatment, such as serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors (8–21), and anticonvulsants (10,22–27). Unfortunately, these may not be effective for all women, and they may have limiting side effects, and/or women may be reluctant to take them. Moreover, some antidepressants inhibit CYP2D6, which has been associated with decreased tamoxifen efficacy (28), although data on this are mixed (29). Therefore, additional nonestrogen treatment options for women with breast cancer and HFs are needed.
Although the pathophysiology of HFs is not fully understood, multiple neurotransmitters have been implicated, including norepinephrine, serotonin, and acetylcholine (30–32). Oxybutynin is an anticholinergic drug approved by the US Food and Drug Administration for treatment of overactive bladder symptoms. Decreased sweating is a common side effect of oxybutynin, which has led to its successful use in the treatment of generalized hyperhidrosis (33). Anecdotal and retrospective data suggest that oxybutynin could also be effective in the treatment of refractory HFs (34). In a prospective, double-blind, clinical trial evaluating an extended-release formulation of oxybutynin for HFs, at a dose of 15 mg daily, patients experienced significant reductions in the frequency and severity of HFs at 12 weeks (35). Unfortunately, this dose was associated with excess toxicity and treatment discontinuation because of side effects.
The present trial evaluated the hypothesis that oxybutynin, at lower doses of 2.5 mg twice a day (Oxy2.5) or 5 mg twice a day (Oxy5), would be more effective than placebo in treating HFs and in improving QoL with an acceptable toxicity profile.
Patients and Methods
Participants
In this randomized, double-blind, placebo-controlled clinical trial, we recruited premenopausal and postmenopausal women with HFs from 10 centers in the United States, all members of the Academic and Community Cancer Research United Network. Eligible patients were adult women who, over a period greater than 30 days, experienced 28 or more HFs per week of sufficient severity to prompt them to seek treatment. Patients with or without a history of breast cancer were eligible, as long as they did not have evidence of active disease. Additional inclusion criteria were: Eastern Cooperative Oncology Group performance status of 0–1 and the ability of the participant to provide informed written consent and to complete study questionnaires.
Patients were excluded if they were receiving cytotoxic chemotherapy, estrogen, progestogens, androgens, or potent anticholinergics. Human epidermal growth factor receptor 2 (HER2)–directed therapy was allowed. Ongoing treatment with tamoxifen, raloxifene, or an aromatase inhibitor was allowed, as long as the dose had been stable for at least 28 days and there was a plan to continue treatment during the study period. Additional exclusion criteria were prior use of oxybutynin (during the period in which the patient experienced HFs), pregnancy, breastfeeding, or contraindications to the use of oxybutynin.
Written informed consent was obtained from each participant, and the study protocol was reviewed and approved by the appropriate local institutional review boards of each study center.
Random Assignment and Masking
Women were randomly assigned to receive either 2.5 or 5 mL twice a day of a liquid, oral formulation containing 2.5 mg oxybutynin/placebo or 5 mg oxybutynin/placebo, resulting in a 2:1 chance of receiving oxybutynin, compared with placebo. Web-based randomization was used, using the Pocock and Simon dynamic allocation procedure (36). Stratification factors included age (18–49 years vs 50 years or older), concurrent tamoxifen use, concurrent aromatase inhibitor use, HF duration (< 9 vs > 9 months), and average baseline HFs frequency per day (4–9 vs ≥10).
Procedures
During the first week of the study, no medication was administered, and questionnaires (HF diary, a symptom experience questionnaire, and the Hot Flash Related Daily Interference Scale (HFRDIS)) were completed to establish baseline symptoms. Following this, patients received their assigned treatment for 6 weeks. All patients started at a dose of 2.5 mL twice a day (2.5 mg oxybutynin/placebo) and received their target dose on the second week. Patients continued to complete a daily HF diary and a weekly symptom experience questionnaire during their 6 weeks on the study and the HFRDIS at the end of the study.
Outcomes
The primary endpoint was the intrapatient change from baseline to 6 weeks in the weekly HF score. HFs were measured by a prospective, self-reported HF diary (37). The daily HF score (37) (a composite of both frequency and severity) was computed by multiplying the frequency of each HF grade by the severity of the HF (grade 1 = mild; 2 = moderate; 3 = severe; and 4 = very severe) and subsequently summing all of the numbers.
Secondary endpoints included change from baseline of HF frequency; change from baseline of daily HF interference between oxybutynin and placebo, as measured by the HFRDIS (39), summarized by descriptive statistics, and then compared using Wilcoxon rank sum tests or two sample t-tests; and adverse effects, evaluated using the National Cancer Institute Common Terminology for Adverse Events (CTCAE) version 4.0, as well as a weekly self-reported symptom experience questionnaire. The HFRDIS and adverse effects were rated on a 0- to 10-point scale, where 0 is as bad as it can be and 10 is as good as it can be.
Statistical Analysis
Sample-size calculations were based on a time-averaged repeated-measures model, comparing oxybutynin to placebo. Model assumptions include a moderate correlation of 0.5 between repeated HF scores and a minimal meaningful difference in changes from the baseline of half an SD, which is considered a moderate effect size and clinically meaningful (39). Using a two-sided 5% significance level, 42 patients per arm were required to provide 85% power to detect this half SD effect size. Accrual goals were adjusted for an expected dropout rate of 15%, resulting in an accrual goal of 50 patients per arm.
The time-averaged intrapatient changes in HF activity from baseline during the treatment period were compared between treatment and placebo arms using a repeated-measures mixed model of weekly HF scores and frequency. Patient baseline characteristics, including age, concurrent use of tamoxifen or aromatase inhibitor, and baseline HF duration and frequency, were used as covariates in the model. Estimates from this model were used to construct a 95% confidence interval (CI) for the mean difference in intrapatient change of HFs between the treatment and placebo arms. Sensitivity analyses, using 10 different methods of imputing missing values, were conducted to provide evidence that missing data did not unduly influence the results of the study.
Because the two treatment arms represented different doses of the same drug, a fixed sequence of up to two hypotheses tests was performed, rather than two simultaneous tests, as would generally be done for studies in which the primary analysis involves two independent hypotheses. This is based on the belief that the treatment effect of oxybutynin, if one existed, would change monotonically with respect to dose. To control the overall type-I error for the primary analysis, a gatekeeping procedure, a method recommended by the Food and Drug Administration and the National Cancer Institute for adjusting for multiple testing (40), was employed. In particular, a time-averaged longitudinal model to test the higher-dose oxybutynin arm vs placebo was first used at the level 0.05, using a two-sided alternative. Plans to test the lower-dose oxybutynin arm vs placebo was to be carried out only if the higher-dose arm–vs-placebo test was statistically significant, again at the level 0.05 and using a two-sided alternative.
An intention-to-treat analysis (ITT) was also performed to account for missing data. For this analysis, we chose as endpoints the percentage of patients who reported a 50% or greater reduction from baseline in their HF score and HF frequency during the study period. Both oxybutynin doses were compared against placebo using the Fisher exact test. Additionally, a logistic regression model was used, adjusting for age, concomitant tamoxifen or aromatase inhibitor use, baseline HF duration, and baseline number of HFs per day. In all ITT analyses, patients with missing values were assumed to not have a 50% or greater reduction from baseline in either HF score or frequency.
This study was registered in Clinicaltrials.gov as NCT02961790.
Results
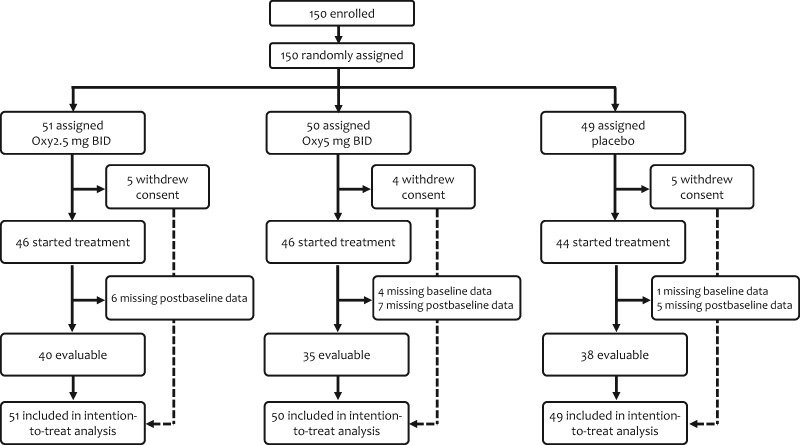
In total, 150 women were accrued between February 23, 2017, and March 5, 2018. A Consolidated Standards of Reporting Trials diagram is illustrated in Figure 1. Of the 150 patients randomly assigned, 14 (9%) withdrew consent before treatment initiation, and 23 (15%) did not submit either baseline or postbaseline questionnaires, leaving 113 patients evaluable for the primary endpoint. Mean (SD) patient age was 57 (8.2) years. Baseline characteristics were well balanced and are detailed in Table 1. Seventy-three evaluable patients (65%) were receiving active endocrine therapy for breast cancer, either tamoxifen or an aromatase inhibitor, during the conduct of the study.
Figure 1.
Consolidated Standards of Reporting Trials diagram. Oxy = oxybutynin.
Table 1.
Baseline characteristics (N = 113)*
| Placebo (n = 38) | 2.5 mg twice a day (n = 40) | 5 mg twice a day (n = 35) | |
|---|---|---|---|
| Variable | No. (%) | No. (%) | No. (%) |
| Mean (SD) age, y | 58.2 (8.4) | 55.6 (8.0) | 57.6 (8.4) |
| Age group | |||
| 18–49 | 6 (15.8) | 9 (22.5) | 6 (17.1) |
| >49 | 32 (84.2) | 31 (77.5) | 29 (82.9) |
| Concurrent treatment | |||
| Concurrent AI | 13 (34.2) | 15 (37.5) | 11 (31.4) |
| Concurrent tamoxifen | 12 (31.6) | 9 (22.5) | 13 (37.1) |
| HF frequency at enrollment, HF/day | |||
| 4-9 | 22 (57.9) | 20 (50.0) | 19 (54.3) |
| ≥10 | 16 (42.1) | 20 (50.0) | 16 (45.7) |
| HF duration, months | |||
| <9 | 7 (18.4) | 9 (22.5) | 8 (22.9) |
| ≥9 | 31 (81.6) | 31 (77.5) | 27 (77.7) |
| Average (SD) HF score per day during baseline week | 19.7 (12.2) | 15.6 (9.7) | 19.5 (17.4) |
| Average (SD) HF frequency during baseline week, HF/day | 9.6 (5.3) | 8.0 (4.3) | 9.7 (7.6) |
*AI = aromatase inhibitor; HF = hot flash.
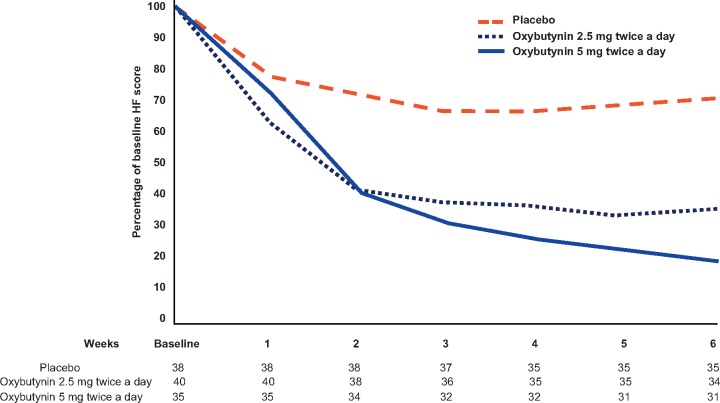
Patients on each of the oxybutynin arms, compared with those on placebo, achieved greater reductions in both HF score and HF frequency (Figure 2 and Table 2). The observed reduction in HF score was −16.9 (SD 15.6) with Oxy5, −10.6 (SD 7.7) with Oxy2.5, and −5.7 (SD 10.2) with placebo (P < .005 for both oxybutynin doses vs placebo). The observed reduction in HF frequency was −7.5 (SD 6.6) with Oxy5, −4.8 (SD 3.2) with Oxy 2.5, and −2.6 (SD 4.3) with placebo (P < .003 for both oxybutynin doses vs placebo). This was confirmed with repeated-measures mixed models, adjusting for baseline variables (P < .001). A decrease in the HF score was seen as early as 1 week after initiation of oxybutynin, and it reached the maximum decrease after 4 weeks with both doses (Figure 2).
Figure 2.
Mean hot flash (HF) score percentage of baseline. Numbers under each week of treatment represent the number of evaluable patients at each week.
Table 2.
Reductions in hot flash (HF) score and frequency from baseline to week 6
| HF measure | Placebo (n = 38) | Oxy2.5 (n = 40) | P * | Oxy5 (n = 35) | P † | P ‡ |
|---|---|---|---|---|---|---|
| HF score | .0368 | |||||
| Mean (SD) reduction | 5.7 (10.2) | 10.6 (7.7) | .004 | 16.9 (15.6) | < .001 | |
| Percentage reduction | 29% | 70% | 86% | |||
| HF frequency | .0355 | |||||
| Mean (SD) reduction | 2.6 (4.3) | 4.8 (3.2) | .002 | 7.5 (6.6) | < .001 | |
| Percentage reduction | 27% | 60% | 77% |
Placebo vs oxybutynin 2.5 mg twice a day.
Placebo vs oxybutynin 5 mg twice a day.
Oxybutynin 2.5 mg twice a day vs oxybutynin 5 mg twice a day.
In addition to the effect on HF score and frequency, patients on Oxy5 had larger improvements in most of the HFRDIS interference measures, including work, social activities, leisure activities, sleep, mood, relationships, life enjoyment, and overall QoL (P < .008 for all, Table 3). The only interference measures not improved by Oxy5 were concentration and sexuality. Similarly, most interference measures were statistically significantly improved with Oxy2.5, with the exception of concentration, sexuality, mood, and life enjoyment. Self-reported satisfaction with HF control and improvement in HF distress were higher with Oxy5 vs placebo (P = .002 and .005, respectively), but not with Oxy2.5 vs placebo (P = .669 and .066, respectively).
Table 3.
Changes in hot flash daily interference scales from baseline to week 6
| Placebo (n = 38) |
2.5 mg twice a day (n = 40) |
5 mg twice a day (n = 35) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Interference measure* | Mean | SD | Mean | SD | P (placebo vs Oxy2.5) | Mean | SD | P (placebo vs Oxy5) | P (Oxy2.5 vs Oxy5) |
| Work | 0.2 | 3.2 | −2.9 | 3.2 | .001 | −2.3 | 3.4 | .003 | .754 |
| Social activities | −0.1 | 3.4 | −2.3 | 2.8 | .005 | −2.6 | 2.8 | .002 | .823 |
| Leisure activities | −0.5 | 3.0 | −2.5 | 2.5 | .007 | −3.1 | 2.5 | <.001 | .323 |
| Sleep | −1.2 | 3.7 | −3.7 | 3.0 | .003 | −4.9 | 3.7 | <.001 | .141 |
| Mood | −1.3 | 3.6 | −2.3 | 2.6 | .092 | −3.4 | 2.5 | .007 | .076 |
| Concentration | −1.1 | 3.0 | −1.9 | 2.5 | .301 | −2.2 | 2.2 | .115 | .398 |
| Relationships | 0.0 | 2.3 | −1.9 | 2.6 | .013 | −2.4 | 2.3 | <.001 | .189 |
| Sexuality | −0.4 | 3.4 | −2.3 | 3.2 | .064 | −2.4 | 3.3 | .06 | .987 |
| Life enjoyment | −1.0 | 2.8 | −2.1 | 2.7 | .052 | −3.1 | 2.8 | .005 | .256 |
| Overall quality of life | −0.5 | 3.2 | −2.5 | 2.8 | .009 | −3.2 | 2.7 | <.001 | .471 |
Interference scores run from 0 to 10, with 0 being no interference, and 10 being complete interference. Changes reported are comparing end of study (week 6 of treatment) to baseline. A negative value indicates improvement, whereas a positive value indicates interference is worse than at baseline.
To evaluate whether study results could have been influenced by the missing data on 37 patients, an ITT analysis including data for all 150 randomly assigned patients was conducted. Thirteen patients (26.5%) on placebo reported a 50% or greater reduction in HF score, compared with 26 patients (51%) on Oxy2.5 (P = .015) and 30 patients (60%) on Oxy5 (P = .001). Similarly, 10 patients (20.4%) on placebo reported a 50% or greater reduction in HF frequency, compared with 25 patients (49%) on Oxy2.5 (P = .003) and 28 patients (56%) on Oxy5 (P < .001). These differences remained after adjusting for baseline factors using a logistic regression model. Patients on either oxybutynin dose were more likely than patients on placebo to have a 50% or greater reduction in HF score (OR = 3.2, 95% CI = 1.3 to 7.7 for Oxy2.5 vs placebo; OR = 5.8, 95% CI = 2.2 to 15.2 for Oxy5 vs placebo).
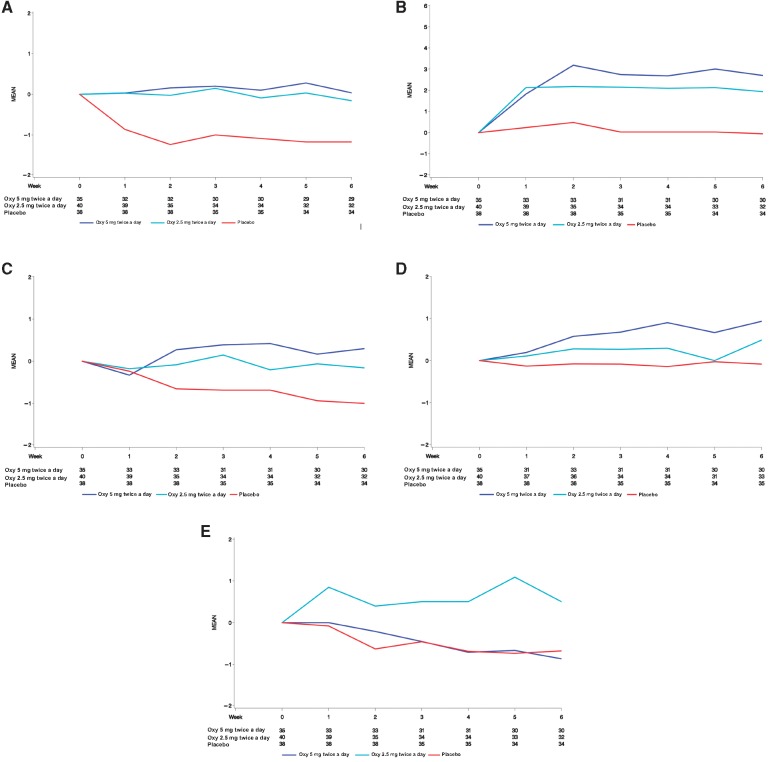
Oxybutynin was well tolerated at both doses. When assessing symptoms as reported by study staff using CTCAE 4.0, dry mouth was the only symptom more frequent with oxybutynin than with placebo. Dry mouth was reported by study staff in 14 patients (33%) on Oxy5, 10 patients (21%) on Oxy2.5, and 3 patients (7%) on placebo (Oxy5 vs placebo P = .004, Oxy2.5 vs placebo P = .06). Diarrhea was reported less frequently with Oxy2.5 compared to placebo (P = .049), and there was no difference between Oxy5 and placebo (.173). Most of the other CTCAE 4.0 toxicities reported by study staff were grade 1, reported in fewer than 5% of patients, and not different from placebo. Grade 2 dry mouth was reported in five patients on oxybutynin 5 mg twice a day and one patient on 2.5 mg twice a day. In addition, grade 3 urinary tract pain was reported in one patient on oxybutynin 5 mg twice a day, and grade 3 headache was reported in one patient on 2.5 mg twice a day. Self-reported changes in baseline symptoms after the initiation of oxybutynin, which are probably a better measure of toxicity than are obtained with CTCAE criteria, are provided in Table 4 and Figure 3.
Table 4.
Changes in self-reported adverse events from baseline to week 6
| Placebo (n = 38) |
2.5 mg twice a day (n = 40) |
5 mg twice a day (n = 35) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom* | Mean | SD | Mean | SD | P (placebo vs Oxy2.5) | Mean | SD | P (placebo vs Oxy5) | P (Oxy2.5 vs Oxy5) |
| Dry mouth | −0.1 | 2.1 | 1.9 | 3.0 | .003 | 2.9 | 3.4 | .001 | .268 |
| Difficulty urinating | −0.1 | 1.3 | 0.5 | 1.6 | .048 | 0.9 | 1.8 | .002 | .245 |
| Constipation | −0.9 | 1.8 | −0.2 | 1.4 | .057 | 0.3 | 2.1 | .004 | .186 |
| Abdominal pain | −1.4 | 2.5 | −0.3 | 1.5 | .017 | 0.0 | 1.3 | .028 | .861 |
| Vomiting | −0.2 | 1.0 | 0.0 | 0.2 | .086 | 0.0 | 0.2 | .091 | .949 |
| Decrease in appetite | −0.6 | 2.1 | −0.1 | 0.8 | .995 | 0.6 | 2.2 | .115 | .090 |
| Rash | 0.1 | 0.5 | 0.5 | 1.9 | .612 | 0.0 | 1.4 | .204 | .173 |
| Dry eyes | −0.6 | 2.5 | 0.8 | 2.1 | .025 | 0.2 | 1.9 | .281 | .250 |
| Insomnia | −2.0 | 3.2 | −1.8 | 2.4 | .780 | −2.5 | 3.2 | .369 | .346 |
| Diarrhea | −0.7 | 1.9 | 0.5 | 1.6 | .004 | −0.8 | 2.5 | .456 | .020 |
| Episodes of confusion | −0.4 | 1.2 | 0.2 | 1.0 | .031 | −0.1 | 0.8 | .469 | .163 |
| Nausea | −1.1 | 2.3 | −0.2 | 0.7 | .122 | −0.7 | 1.8 | .521 | .430 |
| Blurry vision | −0.4 | 1.7 | 0.2 | 1.1 | .211 | 0.1 | 1.4 | .646 | .563 |
| Headaches | −1.1 | 2.5 | −0.3 | 1.8 | .049 | −1.3 | 2.6 | .699 | .147 |
| Difficulty concentrating | −1.1 | 2.1 | −0.4 | 1.2 | .099 | −1.0 | 2.3 | .723 | .246 |
| Dizziness | −0.6 | 2.3 | 0.0 | 1.6 | .464 | −0.1 | 1.9 | .754 | .754 |
| Myalgias or arthralgias | −0.9 | 2.7 | −0.6 | 1.7 | .824 | −1.0 | 2.2 | .757 | .758 |
| Excessive somnolence | −0.4 | 2.4 | −0.3 | 1.7 | .842 | −0.5 | 1.7 | .874 | .940 |
| Urinary incontinence | −0.4 | 1.2 | −0.3 | 1.1 | .537 | −0.4 | 1.4 | .955 | .539 |
| Fatigue | −1.7 | 3.0 | −0.6 | 1.9 | .197 | −1.7 | 2.4 | .980 | .163 |
Symptoms were scored from 0 to 10, with higher values representing higher severity. Changes reported are comparing end of study (week 6 of treatment) to baseline. A negative value indicates improvement, whereas a positive value indicates the symptom is worse than at baseline.
Figure 3.
Mean change from baseline in select adverse events. Numbers under each week of treatment represent the number of evaluable patients at each week. Oxy = oxybutynin. A) Mean change from baseline in abdominal pain, B) Mean change from baseline in dry mouth, C) Mean change from baseline in constipation, D) Mean change from baseline in difficulty urinating, and E) Mean change from baseline in diarrhea.
Among patients who started treatment, study discontinuation per treatment arm were as follows: 2 of 44 (5%) in the placebo arm, 5 of 46 (11%) in the Oxy2.5 arm, and 4 of 46 (9%) in the Oxy5 arm. There were no statistically significant differences in reasons for study discontinuation between the oxybutynin arms and placebo.
Discussion
The results from this study support the prestudy hypothesis that oxybutynin would improve HF frequency and severity. The positive effect of treatment with oxybutynin on several HF interference measures and QoL supports that the magnitude of the effect was clinically meaningful. The degree of HF improvement with oxybutynin compares favorably with other agents that have been evaluated in prospective trials (8,9,18,27,41–43), with greater reduction in HF than has been observed with antidepressants and gabapentinoids, and similar to what has been reported with progesterone analogues.
The toxicity profile seen in the present trial contrasts with the toxicity profile seen in the study by Simon et al. (35), which used a higher dose (15 mg) of an extended-release formulation of oxybutynin. In that study, in addition to similar rates of dry mouth, patients on oxybutynin also reported more dyspepsia (12% vs 1%, P = .009) and diarrhea (10% vs 0%, P = .006) than did patients on placebo. Though some of the self-reported adverse effects (Table 4) were slightly worse on the Oxy2.5 arm compared with the Oxy5 arm, these differences are most likely due to random chance. The magnitude of the effect on HF on the trial by Simon et al. appears similar to what is reported in the present trial. As such, routine escalation of the dose beyond 10 mg daily may not be beneficial.
The treatment duration in this study was 6 weeks. As such, this trial demonstrated that oxybutynin provided short-term relief and that its short-term use was safe. By 6 weeks, HF score and frequency were reduced by 29% and 27%, respectively, with placebo, consistent with the placebo effect observed in other HF trials (8,9,18,27,41–43). HF trials have commonly been conducted for periods ranging between 4 and 12 weeks, without good scientific evidence that a particular study duration is superior. A joint analysis of five HF trials (44) and other HF trials lasting longer than 4 weeks (12,13,42) have consistently shown that the effect of evaluated drugs on HF plateaus at 4 weeks, suggesting that this is a reasonable period to assess short-term efficacy. Furthermore, there are no data to suggest that therapeutic efficacy on HF diminishes over time. However, long-term safety may be a different issue.
Anticholinergic drugs can be associated with acute mentation changes, delirium, electroencephalogram changes, and other negative cognitive effects (45–51). There are also reports linking anticholinergic drugs and dementia (52), although causality has not been established. The link with negative cognitive effects has been mostly reported in the elderly and in those with preexisting neurologic conditions. However, there are no good data to support or disprove that similar effects may occur in healthy younger women. The present trial did not conduct formal cognitive or psychometric testing. Patients in the Oxy2.5 arm did report slight worsening of episodes of confusion compared to placebo. It is also important to note that the population in this study was relatively young (mean [SD] = age 57 [8.2] years), and those taking other potent anticholinergic drugs were not allowed to participate. Patients and clinicians need to be aware of these concerns, particularly because cognitive impairment may be a problem among breast cancer survivors.
A possible advantage of oxybutynin for HF management over most antidepressants is the lack of interference with CYP2D6. This enzyme is important in the metabolic activation of tamoxifen, and it has been shown that concurrent use of CYP2D6 inhibitors leads to decreased plasma concentrations of endoxifen (the most potent tamoxifen metabolite). Whether this effect can affect the anticancer efficacy of this agent continues to be a matter of debate, with different studies showing mixed results (29,53–56). However, given this potential, patients and physicians may have concerns about using potent CYP2D6 inhibitors for HF treatment in women taking tamoxifen. Thus, oxybutynin may be an attractive choice for this patient population.
Although this study included only women, in a recent letter to the editor (57), Smith et al. reported a case of a male patient with severe and intrusive, drenching HF secondary to androgen- deprivation therapy for prostate cancer; these HFs were refractory to gabapentin and venlafaxine. The addition of oxybutynin 5 mg twice a day resulted in significant HF relief, a benefit that persisted after discontinuation of gabapentin and venlafaxine. After discontinuation of oxybutynin because of insomnia, dry mouth, and restless legs, HFs recurred but improved again within hours of restarting it, at a dose of 2.5 mg twice a day. Further prospective evaluation of oxybutynin in men with androgen deprivation–related HFs is planned.
Strengths of this study are the inclusion of breast cancer patients on active antiestrogen therapy, the use of standardized HF metric tools and questionnaires, and its prospective, randomized design. Limitations include the short duration, which precludes the demonstration of long-term safety (especially on cognition), and the missing data points in 15% of patients who started treatment. Despite these limitations, this study supports the short-term use of oxybutynin in patients with HFs refractory to other agents.
Funding
This work was supported by a grant to CLL from the Breast Cancer Research Foundation. The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Notes
The authors declare the following potential conflicts of interest: RALF reports nonfinancial support from Immunomedics, outside the submitted work. SSF reports personal fees from Mithra Pharmaceuticals, personal fees from AMAG, and personal fees from Procter & Gamble, outside the submitted work. KJR reports previous stock ownership of Merck and Pfizer, outside the submitted work. CLL reports grants from Breast Cancer Research Foundation during the conduct of the study; personal fees from Pled Pharma, Asahi Kasei, Disarm Therapeutics, and Metys, outside the submitted work. PJN, EGW, DF, CSRD, KMR, MLG, NLL, and TJS declare no competing interests.
The authors wish to acknowledge Mr Jason Ellis for reviewing the manuscript for style.
This study was reported as an oral presentation at the 2018 San Antonio Breast Cancer Symposium, on December 7, 2018.
Affiliations of authors: Medical Oncology, Mayo Clinic, Rochester, MN (RALF, KJR, CLL); Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN (PJN, EGW); Mayo Clinic Center for Women’s Health, Mayo Clinic, Rochester, MN (SSF); Oncology/Hematology, St. Elizabeth Physicians, Crestview Hills, KY (DF); Medical Oncology/Hematology, Cancer Center of Kansas, Wichita, KS (CSRD); Medical Oncology, Carle Cancer Center, Urbana, IL (KMR); Medical Oncology, Waverly Hematology/Oncology, Cary, NC (MLG); Medical Oncology, Illinois Cancer Care, Peoria, IL (NLL); Medical Oncology, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD (TJS).
References
- 1. Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360(9348):1851–1861. [DOI] [PubMed] [Google Scholar]
- 2. Gracia C, Freeman E. Acute consequences for the menopausal transition: the rise of common menopausal symptoms. Endocrinol Metab Clin North Am. 2004;33(4):675–689. [DOI] [PubMed] [Google Scholar]
- 3. Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29(3):E16–E25. [DOI] [PubMed] [Google Scholar]
- 4. Kuba S, Ishida M, Nakamura Y, Taguchi K, Ohno S. Persistence and discontinuation of adjuvant endocrine therapy in women with breast cancer. Breast Cancer. 2016;23(1):128–133. [DOI] [PubMed] [Google Scholar]
- 5. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCowan C, Shearer J, Donnan P, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;4:CD002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356(9247):2059–2063. [DOI] [PubMed] [Google Scholar]
- 9. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578–1583. [DOI] [PubMed] [Google Scholar]
- 10. Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J Clin Oncol. 2009;27(17):2831–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2011;29(29):3862–3868. [DOI] [PubMed] [Google Scholar]
- 12. Evans ML, Pritts E, Vittinghoff E, McClish K, Morgan KS, Jaffe RB. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105(1):161–166. [DOI] [PubMed] [Google Scholar]
- 13. Speroff L, Gass M, Constantine G, Olivier S, Study I. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol. 2008;111(1):77–87. [DOI] [PubMed] [Google Scholar]
- 14. Archer DF, Seidman L, Constantine GD, Pickar JH, Olivier S. A double-blind, randomly assigned, placebo-controlled study of desvenlafaxine efficacy and safety for the treatment of vasomotor symptoms associated with menopause. Am J Obstet Gynecol. 2009;200(2):172.e1–e10. [DOI] [PubMed] [Google Scholar]
- 15. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289(21):2827–2834. [DOI] [PubMed] [Google Scholar]
- 16. Suvanto-Luukkonen E, Koivunen R, Sundstrom H, et al. Citalopram and fluoxetine in the treatment of postmenopausal symptoms: a prospective, randomized, 9-month, placebo-controlled, double-blind study. Menopause. 2005;12(1):18–26. [DOI] [PubMed] [Google Scholar]
- 17. Gordon PR, Kerwin JP, Boesen KG, Senf J. Sertraline to treat hot flashes: a randomized controlled, double-blind, crossover trial in a general population. Menopause. 2006;13(4):568–575. [DOI] [PubMed] [Google Scholar]
- 18. Barton DL, LaVasseur BI, Sloan JA, et al. Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG trial N05C9. J Clin Oncol. 2010;28(20):3278–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109(4):823–830. [DOI] [PubMed] [Google Scholar]
- 21. Kimmick GG, Lovato J, McQuellon R, Robinson E, Muss HB. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006;12(2):114–122. [DOI] [PubMed] [Google Scholar]
- 22. Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101(2):337–345. [DOI] [PubMed] [Google Scholar]
- 23. Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005;366(9488):818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reddy SY, Warner H, Guttuso T, Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108(1):41–48. [DOI] [PubMed] [Google Scholar]
- 25. Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25(3):308–312. [DOI] [PubMed] [Google Scholar]
- 26. Bordeleau L, Jugovic O, Ennis M, et al. A randomized crossover trial of venlafaxine (V) versus gabapentin (G) for hot flashes (HF) in breast cancer survivors. J Clin Oncol. 2010;28(15 suppl):9023.. [DOI] [PubMed] [Google Scholar]
- 27. Loprinzi CL, Qin R, Baclueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1. J Clin Oncol. 2010;28(4):641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764. [DOI] [PubMed] [Google Scholar]
- 29. Sanchez-Spitman A, Dezentjé V, Swen J, et al. Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J Clin Oncol. 2019;37(8):636–646. [DOI] [PubMed] [Google Scholar]
- 30. Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181(1):66–70. [DOI] [PubMed] [Google Scholar]
- 31. Blum I, Vered Y, Lifshitz A, et al. The effect of estrogen replacement therapy on plasma serotonin and catecholamines of postmenopausal women. Isr J Med Sci. 1996;32(12):1158–1162. [PubMed] [Google Scholar]
- 32. Low DA, Hubing KA, Del Coso J, Crandall CG. Mechanisms of cutaneous vasodilation during the postmenopausal hot flash. Menopause. 2011;18(4):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696–1700. [DOI] [PubMed] [Google Scholar]
- 34. Sexton T, Younus J, Perera F, Kligman L, Lock M. Oxybutynin for refractory hot flashes in cancer patients. Menopause. 2007;14(3):505–509. [DOI] [PubMed] [Google Scholar]
- 35. Simon JA, Gaines T, LaGuardia KD. Extended-release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial. Menopause. 2016;23(11):1214–1221. [DOI] [PubMed] [Google Scholar]
- 36. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 37. Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19(23):4280–4290. [DOI] [PubMed] [Google Scholar]
- 38. Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22(6):979–989. [DOI] [PubMed] [Google Scholar]
- 39. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 40. Dmitrienko A, Tamhane AC, Wang X, Chen X. Stepwise gatekeeping procedures in clinical trial applications. Biom J. 2006;48(6):984–991. [DOI] [PubMed] [Google Scholar]
- 41. Goldberg RM, Loprinzi CL, O'Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12(1):155–158. [DOI] [PubMed] [Google Scholar]
- 42. Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depomedroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24(9):1409–1414. [DOI] [PubMed] [Google Scholar]
- 43. Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331(6):347–352. [DOI] [PubMed] [Google Scholar]
- 44. Loprinzi CL, Diekmann B, Novotny PJ, Stearns V, Sloan JA. Newer antidepressants and gabapentin for hot flashes: a discussion of trial duration. Menopause. 2009;16(5):883–887. [DOI] [PubMed] [Google Scholar]
- 45. Pagoria D, O’Connor RC, Guralnick ML. Antimuscarinic drugs: review of the cognitive impact when used to treat overactive bladder in elderly patients. Curr Urol Rep. 2011;12(5):351–357. [DOI] [PubMed] [Google Scholar]
- 46. Kay G, Ebinger U. Preserving cognitive function for patients with overactive bladder: evidence for a differential effect with darifenacin. Int J Clin Pract. 2008;62(11):1792–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morrow SA, Rosehart H, Sener A, Welk B. Anti-cholinergic medications for bladder dysfunction worsen cognition in persons with multiple sclerosis. J Neurol Sci. 2018;385:39–44. [DOI] [PubMed] [Google Scholar]
- 48. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639–658. [DOI] [PubMed] [Google Scholar]
- 49. Yang Y-W, Liu H-H, Lin T-H, Chuang H-Y, Hsieh T. Association between different anticholinergic drugs and subsequent dementia risk in patients with diabetes mellitus. PloS One. 2017;12(4):e0175335.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Todorova A, Vonderheid-Guth B, Dimpfel W. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J Clin Pharmacol. 2001;41(6):636–644. [DOI] [PubMed] [Google Scholar]
- 51. Pietzko A, Dimpfel W, Schwantes U, Topfmeier P. Influences of trospium chloride and oxybutynin on quantitative EEG in healthy volunteers. Eur J Clin Pharmacol. 1994;47(4):337–343. [DOI] [PubMed] [Google Scholar]
- 52. Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:k1315.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drug Metab. 2002;3(1):13–37. [DOI] [PubMed] [Google Scholar]
- 54. Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30–39. [DOI] [PubMed] [Google Scholar]
- 55. Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74. [DOI] [PubMed] [Google Scholar]
- 56. Goetz MP, Sangkuhl K, Guchelaar HJ, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther. 2018;103(5):770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith TJ, Loprinzi CL, Deville C. Oxybutynin for hot flashes due to androgen deprivation in men. N Engl J Med. 2018;378(18):1745–1746. [DOI] [PubMed] [Google Scholar]