ABSTRACT
Purpose
Poor sleep quality in intensive care unit (ICU) can be associated with poor outcome. Excessive noise and lights in ICU are known to disrupt patients’ sleep by causing arousals.
Study design
A prospective randomized controlled study.
Materials and methods
The patients admitted to the medical ICU were prospectively included and randomized to receive earplugs and eye masks or no intervention during their first 5 nights in ICU. Their arousal index and other sleep parameters were measured during the first night by polysomnography. Secondary outcomes including wrist actigraphy profiles and subjective sleep quality were recorded during all study nights.
Results
Seventeen patients were enrolled. Eight patients were randomized to earplugs and eye masks group and nine patients were randomized to control group during their first 5 nights in the ICU. The use of earplugs and eye masks demonstrated the trend toward lower arousal index during the first night (21.15 (14.60) vs 42.10 (18.20) events per hour, p = 0.086) and increased activity index (activity count/hour) (16.12 (7.99) vs 10.84 (10.39) count/hour, p = 0.059) compared to control group. Polysomnography and actigraphy did not demonstrate good agreement.
Conclusion
The use of earplugs and eye masks has a trend toward reduction in arousal index and increased activity in patients admitted to ICU. Limited sample size most likely explained insignificant difference in outcomes. Wrist actigraphy did not accurately measure sleep parameters in ICU patients.
Trial registration
www.clinicaltrials.in.th, TCTR20170727003.
How to cite this article
Arttawejkul P, Reutrakul S, Muntham D, Chirakalwasan N. Effect of Nighttime Earplugs and Eye Masks on Sleep Quality in Intensive Care Unit Patients. Indian J Crit Care Med 2020;24(1):6–10.
Keywords: Delirium, Earplugs, Eye masks, Intensive care unit, Light and noise control, Sleep quality
INTRODUCTION
Sleep during critical illness is known to be poor1 secondary to several factors including pain, anxiety, medication side effect, ventilator dyssynchrony, and nurse's intervention.2 Noises and light disturbances are known to disrupt sleep in intensive care unit (ICU).3 The noise in the ICU was demonstrated to be in part responsible for sleep–wake abnormalities.1 Theoretically, poor sleep quality may reduce muscle endurance resulting in prolonged weaning.4 Delirium can also be observed with sleep deprivation in ICU associated with increased mortality and long-term cognitive function impairment.5 Furthermore, sleep deprivation may also reduce immune response and increase risk of nosocomial infection.6
Intervention to improve sleep quality in ICU includes medical and nonmedical interventions.7 However, sedation should be used cautiously due to potential prolongation of mechanical ventilation.8 Environmental modifications were demonstrated to improve perceived sleep quality and cognition.9 Ambient light and noise reduction were proven to improve sleep quality.10 However, these modalities may require complicated hospital environmental and work schedule modification.
Simple interventions including earplugs and eye masks have been shown to be beneficial in postoperative patients in surgical ICU. In this study, the intervention group was observed to have better sleep quality, required less hypnotics and analgesics, and less delirium.11 Currently, there have been no studies using these simple interventions in medical ICU. Given different patient characteristics and environmental factors between surgical and medical ICU, we aimed to study the effect of earplugs and eye masks on sleep quality and related ICU outcomes in medical ICU setting.
MATERIALS AND METHODS
The study was a randomized controlled trial. The patients admitted to medical ICU (single unit, total of 16 beds) and expected to remain in ICU for at least 24 hours who were at least 18 years of age, able to understand Thai, and can communicate with investigator, and Glasgow coma score ≥13, Richmond agitation–sedation score was −1 to +1, and did not require medication or intervention to facilitate sleep were enrolled in our study within 24 hours of their ICU admission. Exclusion criteria were bilateral deafness, bilateral blindness, severe encephalopathy, severe dementia, hepatic encephalopathy, uremic encephalopathy, encephalitis, increased intracranial pressure, metabolic derangements, severe hemodynamic instability, high vasopressor requirement (dopamine > 15 μg/kg/minute, epinephrine > 0.1 μg/kg/minute, and norepinephrine > 0.1 μg/kg/minute for at least 1 hour), and severe respiratory failure (PaO2/FiO2 < 100). Our medical ICU work environment includes nurse to patient ratio of 1:1 (8-hour shift) and standard hospital environment including 24-hour hospital lightings and no sound level control. The patients were randomized by stratified block randomization to receive earplugs and eye masks or to be in control group.
Intervention
In the study group, the patients were given earplugs (noise reduction rating of 32 dB) and cloth eye masks by physician or nurse during sleep at nighttime according to their habitual home bedtime but not after 22:00 hours. Earplugs and eye masks were removed at 07:00 hours on the following morning. Earplugs and eye masks were allowed to be removed for no longer than 10 minutes for communication if needed. If the patients did not wish to continue with the use of these interventions, they were allowed to inform the nurse (verbal communication if not intubated and bell ringing if intubated) and the inventions can be stopped upon patient request. The patients were informed to use earplugs and eye masks every night during the ICU stay. We also obtained therapeutic intervention scoring system (TISS-28 score) in order to monitor the nurse's activity involving in medical care for each patient.12
Polysomnography
During the first night of the admission to the ICU, both study group and control group underwent type I polysomnography conducted in medical ICU. The polysomnography was conducted using standard EEG including frontal leads (F1, F2), central leads (C3, C4), occipital leads (O1, O2), and reference leads at mastoids (M1, M2), electromyography, and electrooculography methodologies. The polysomnography was conducted at the patient's habitual home bedtime but not after 22:00 hours and the study was concluded at 07:00 hours in the following morning. Sleep stages were scored by board certified sleep medicine specialist blinded to the randomization using 30-second epoch window according to the standard criteria from the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events, 2016.13
Actigraphy
Actiwatch® 2 (Respironics) was worn on patient's nondominant wrist throughout study duration. These monitors use highly sensitive omnidirectional accelerometers to count the number of wrist movements in 30-second epochs. This device also has light sensor, which records the light intensity and report this data in lux. The software scores each 30-second epoch as sleep or wake based on a threshold of activity counts that is estimated using activity within the epoch being scored as well as the epochs 2 minutes before and after that epoch. Total sleep time was defined as the amount of actual sleep obtained at night. Sleep efficiency was the percentage of time in bed spent sleeping. Sleep latency was defined as the time period from bedtime to the first epoch of sleep. Wake after sleep onset was defined as the time spent awake after the patient was asleep and before the patient was awake. All parameters were calculated using Actiware 6.0 software, supplied by the manufacturer. The investigator who scored the Actiwatch's data was blinded to the group allocation and the polysomnography data. The patients wore actigraphy during the entire duration in the study while they were in the ICU.
Questionnaires
Sleep history and sleep quality were obtained using Pittsburgh sleep quality index questionnaire (PSQI)14 as baseline at the enrollment period. In order to accurately monitor sleep quality in the ICU, both Richard-Campbell sleep questionnaire15 (RCSQ) and Verran/Snyder-Halpern sleep scale16 were utilized the following morning after the polysomnography and every morning for 5 days during the ICU stay or terminated earlier if the patient was discharged from the ICU before 5 days. In order to assess for the presence of delirium, CAM-ICU was utilized during the study for 5 days during the ICU stay or terminated earlier if the patient was discharged from the ICU before 5 days.
Primary Outcome of the Study
We aimed to determine the difference in arousal index compared between the group using earplugs and eye masks as primary outcome. We also compared other polysomnographic parameters, actigraphy parameters, sleep quality, the prevalence of delirium, the sedation requirement, duration of mechanical ventilation, rate of nosocomial infection, and duration of ICU stay.
Statistical Analysis
The study was analyzed as intention to treat analysis. Sample size was calculated from arousal index using mean difference between two independent samples derived from Huang et al. paper.17 We used α = 0.05, and β = 0.20, and calculated to have 6 patients in each arm (total of 12 patients). We also added 30% for dropout rate and another 30% for potential uninterpretable data. Eventually, a total of 20 patients were planned. Quantitative variables were compared between two groups using t test or Mann–Whitney U test and linear regression analysis. Chi-squared or Fisher's exact test was used to compare proportion between groups. Correlation analysis between polysomnography and Actiwatch parameters was analyzed using Lin's concordance correlation coefficient (0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, and 0.81–1.00 = almost perfect). STATA v12.1 software was utilized. Descriptive analysis was used for analysis. This study was approved by the Ethics Committee. The study was registered at www.clinicaltrials.in.th (#TCTR20170727003).
RESULTS
During the period of June 2017 to May 2018, a total of 20 subjects were enrolled in the study. Ten patients were randomized to control group and 10 patients were randomized to intervention group (earplugs and eye masks). Two patients in the intervention group were excluded (poor polysomnographic quality and ICU discharge prior to the conduct of polysomnography). One patient in control group was excluded due to uninterpretable polysomnography data. A total of 17 patients were analyzed. Baseline clinical characteristics were similar between two groups. Most of the patients had poor sleep quality as baseline according to PSQI. Clinical characteristic and information upon admission are listed in Table 1. The most common primary diagnosis for ICU admission was pneumonia.
Table 1.
Clinical characteristic and information upon admission of control and intervention groups
| Clinical characteristics | Control (n = 9) | Intervention (n = 8) |
|---|---|---|
| Sex | ||
| Male, n (%) | 5 (56%) | 6 (75%) |
| Female, n (%) | 4 (44%) | 2 (25%) |
| Age (years) | 76 (32)* | 67 (25)* |
| BMI | 21.23 (5.42)* | 21.35 (4.74)* |
| Sedation use, n (%) | 1 (11%) | 1 (13%) |
| Smoking (pack-year) | 0 (10)* | 14 (20)* |
| Alcohol use, n (%) | 2 (22%) | 1 (13%) |
| Primary diagnosis, pneumonia (%) | 4 (44.4%) | 3 (37.5%) |
| Direct admission to ICU, n (%) | 5 (56%) | 5 (63%) |
| APACHE II | 14 (1)* | 15 (7)* |
| SOFA | 2 (2)* | 4 (6)* |
| Pittsburgh sleep quality index | 7 (2)* | 6 (6)* |
| Nights in the study | 2 (1)* | 4 (2)* |
| TISS-28 score during the first night | 19 (3)* | 24 (7)* |
| Light exposure | 27.77 (16.93)* | 52.68 (48.44)* |
Data was demonstrated in median (IQR)
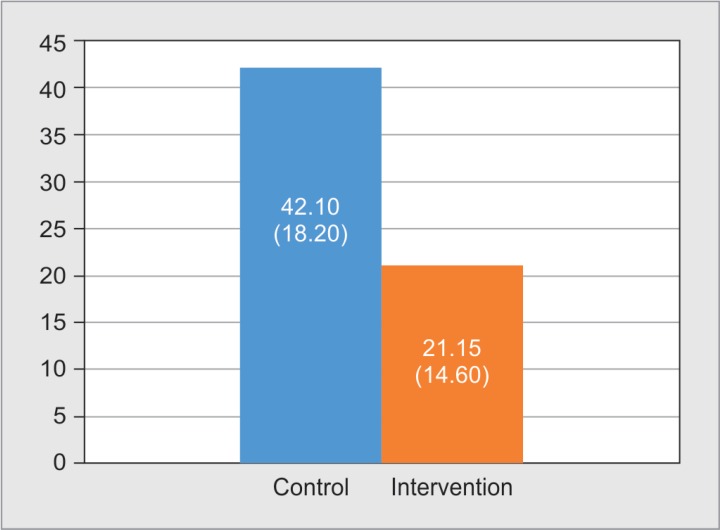
Arousal index during the first night of the study demonstrated a trend toward lower value in the intervention group compared to control group after adjustment for overnight nursing interventions (TISS-28) (p = 0.086) (Fig. 1).
Fig. 1.
Arousal index during the first night of the study
Other polysomnographic parameters including total sleep time, sleep efficiency, wake after sleep onset, sleep latency, % rapid eye movement (REM) sleep, and % N3 sleep were similar between two groups (Table 2).
Table 2.
Polysomnography data of control and intervention groups
| PSG parameter | Control (n = 9) | Intervention (n = 8) | p value§ |
|---|---|---|---|
| Total sleep time | 333 (112)* | 319 (174)* | 0.452 |
| Sleep efficiency | 77.10 (9.70)* | 65.2 (32.85)* | 0.891 |
| Wake after sleep onset | 114 (107)* | 190 (175.5)* | 0.698 |
| Sleep latency | 14 (21)* | 0 (22)* | 0.368 |
| REM sleep percentage | 6.4 (12.5)* | 4.75 (6.45)* | 0.608 |
| Slow wave sleep percentage | 1.3 (22.1)* | 3 (31.6)* | 0.961 |
Data was demonstrated in median (IQR) which was adjusted for TISS-28
value was adjusted for overnight nursing interventions (TISS-28)
Subjective sleep quality according to RCSQ score did not demonstrate the difference between the intervention group and the control group (p = 0.236). The prevalence of delirium, the use of sedation, duration of ICU stay, and duration of mechanical ventilation were not different between two groups (Table 3).
Table 3.
Clinical outcomes of control and intervention groups
| Clinical parameter | Control (n = 9) | Intervention (n = 8) | p value |
|---|---|---|---|
| RCSQ score | 56.4 (5.17)§ | 58.5 (5.26)§ | 0.236 |
| Delirium | 1 (11.1%) | 1 (12.5%) | 1.000 |
| Sedation use | 0 (0%) | 1 (12.5%) | 0.471 |
| ICU duration (hours) | 68 (20)* | 96 (66.5)* | 0.572 |
| Mechanical ventilation (hours) | 63.5 (58)* | 72.5 (47.5)* | 0.925 |
| Nosocomial infection | 0 (0%) | 0 (0%) | – |
Data was demonstrated in median (IQR)
Data was demonstrated in mean (SD)
Actiwatch data demonstrates a trend toward more activity in the intervention group (Table 4).
Table 4.
Activity data from Actiwatch of control and intervention groups
| Actiwatch parameter | Control (n = 9) | Intervention (n = 8) | p value |
|---|---|---|---|
| Activity count | 2529.25 (887.23)* | 5872.95 (2611.53)* | 0.093 |
| Activity index** | 10.84 (10.39)* | 16.12 (7.99)* | 0.059 |
Data was demonstrated in median (IQR)
Activity index = activity count per hour
Overall correlation between polysomnography and actigraphy was poor to fair. Polysomnography and wrist actigraphy data showed fair agreement on sleep latency (correlation coefficient = 0.389) and sleep efficiency (correlation coefficient = 0.223) but poor agreement on total sleep time (correlation coefficient = 0.188) and wake after sleep onset (correlation coefficient = 0.031). Wake after sleep onset appeared to have the poorest correlation with polysomnography (Table 5).
Table 5.
Correlation of actigraphy parameters and polysomnography parameters
| Polysomnography parameter | Actigraphy parameter | Correlation coefficient |
|---|---|---|
| Total sleep time | Actual sleep time | 0.188 |
| Sleep efficiency | Sleep efficiency | 0.223 |
| Wake after sleep onset | Wake after sleep onset | 0.031 |
| Sleep latency | Sleep latency | 0.389 |
DISCUSSION
Our study conducted as a randomized controlled trial on the efficiency of the use of earplugs and eye masks and sleep quality in real medical ICU using polysomnography. The previous studies measured sleep quality only by subjective questionnaire.18–20 Three previous studies using polysomnography were conducted in healthy subjects in simulated ICU environment.17,21,22 One study was conducted with earplugs21 and two studies were conducted with earplugs and eye masks.17,22 All previous studies using objective polysomnography to measure the efficacy of earplugs and eye masks on sleep quality in ICU were done only in simulated ICU environment and may not represent the full auditory and visual experience of the ICU.
Our study demonstrated a trend toward lower arousal index 21.15 (14.60) in intervention group compared to 42.10 (18.20) in control group (p = 0.086). This finding supports the benefit of noise and light reduction in promoting continuity of sleep in ICU setting. Similar to our finding, Hu et al. studied 14 healthy subjects exposed to recorded ICU noise and light and demonstrated that the use of earplugs and eye masks was associated with reduction in arousal index.22 Reduction in arousal index may clinically benefit since prior studies demonstrated detrimental effect of arousal.23–25 Prior study conducted in healthy volunteers demonstrated that arousal index is a single strongest polysomnographic predictor of daytime sympathetic discharge which could pose risk of increased blood pressure.23 In studies conducted in obstructive sleep apnea population, arousal index was demonstrated to be marker of carotid artery atherosclerosis,24 risk of acute coronary syndrome, transient ischemic attack, and stroke or death.25
Furthermore, our study also demonstrated a trend toward increase in activity with the use of earplugs and eye masks compared to control group (p = 0.059). Winkelman et al. studied ventilated subjects in medical and surgical ICU using actigraphy and demonstrated that activity appeared to be associated with a decrease in IL-6 level.26 The same group also conducted another study in medical ICU and step-down unit on ventilated subjects participated in an early mobility program.27 The study demonstrated that lower activity count from actigraphy has the negative impact with potential toward alteration in inflammatory profiles.27 Clinical importance of increased activity of ICU patients was also previously demonstrated. Early immobilization was shown to reduce ICU and hospital lengths of stay,28 increase ventilator free days,29 reduce ICU-acquired weakness,29,30 increase proportion to return to independent function at discharge,31 reduce proportion of time in ICU with delirium,31 and improve glycemic control.30
Insignificant difference in the outcomes can be explained by several reasons. First, the limited sample size; the calculated sample size was from the study of healthy volunteers wearing earplugs and eye masks in simulated ICU setting with large difference between the intervention group and control group. Statistically, this large difference resulted in small calculated sample. This calculated sample size could have been inadequate since the difference between intervention group and control group in real ICU setting with more disturbing environment could have been less. In fact, larger sample size may be needed to detect the difference in expected outcomes. Second, inhomogeneous baseline characteristics between two groups could be potential explanation. Despite the randomization, the data indicated a trend toward less clinical severity (lower sequential organ failure assessment (SOFA) score) and lower light exposure during sleep observed in the control group compared to the intervention group. These differences may have promoted overall better sleep quality in the control group compared to the intervention group and masked the expected difference in the outcome. Finally, there are potential occult factors, which may have affected the outcome including various medication, procedures, or mode of medical ventilation that were not controlled in our study.
Although wrist actigraphy has long been used to measure sleep in ambulatory patients with great reliability, poor correlation between polysomnography and actigraphy in sleep measurement was in agreement with current evidence in the literature. Actigraphy was observed to be valid and reliable for detecting sleep in healthy adult populations but less reliable for detecting sleep in disturbed setting.32 Actigraphy may incorrectly score wakefulness as sleeping in ICU patients as they were awake but immobile due to restraints, sedation, or severe illness.33 Recent systematic review of 13 eligible studies also concluded that since actigraphy only measures gross motor activity, it is not recommended to be used to measure sleep in ICU.34 Algorithm of software used to analyze this data in actigraphy may have to be corrected for other variables before this device can be used accurately in ICU setting.
Our study suggests that polysomnography is still a gold standard for sleep measurement in ICU setting. Knauert et al. conducted polysomnography in 29 patients in medical ICU and demonstrated sufficient data to determine sleep stage, sleep efficiency, and arousal indices in their studies with 93% interpretable quality.35 Similarly, our study demonstrated interpretable quality of polysomnography data to be 94%.
CONCLUSION
Earplugs and eye masks insignificantly reduced arousal index and increased activity in patients admitted to ICU. Limited sample size most likely explained insignificant difference in outcomes in our study and larger sample size is needed. Wrist actigraphy did not accurately measure sleep parameters in ICU patients. To date, polysomnography is still a valid and reliable method to measure sleep parameters in ICU setting.
ACKNOWLEDGMENT
The study was conducted at King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand. This research was supported by the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University.
Footnotes
Source of support: This research has been supported by the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University
Conflict of interest: None
REFERENCES
- 1.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451–457. doi: 10.1164/ajrccm.163.2.9912128. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Bihari S, Doug McEvoy R, Matheson E, Kim S, Woodman RJ, Bersten AD. Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012;8(3):301–307. doi: 10.5664/jcsm.1920. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–715. doi: 10.1164/rccm.2201090. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. Am Rev Respir Dis. 1989;140(4):907–909. doi: 10.1164/ajrccm/140.4.907. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P, et al. Bench-to-bedside review: delirium in ICU patients - importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288(12):1471–1472. doi: 10.1001/jama.288.12.1469. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41(3):800–809. doi: 10.1097/CCM.0b013e3182746442. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114(2):541–548. doi: 10.1378/chest.114.2.541. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Hu RF, Jiang XY, Chen J, Zeng Z, Chen XY, Li Y, et al. Non-pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. 2015;10:CD008808. doi: 10.1002/14651858.CD008808.pub2. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuBose JR, Hadi K. Improving inpatient environments to support patient sleep. Int J Qual Health Care. 2016;28(5):540–553. doi: 10.1093/intqhc/mzw079. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112(1):89–95. doi: 10.1093/bja/aet304. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Miranda DR, de Rijk A, Schaufeli W. Simplified therapeutic intervention scoring system: the TISS-28 items–results from a multicenter study. Crit Care Med. 1996;24(1):64–73. doi: 10.1097/00003246-199601000-00012. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Berry BB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. The ASSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, Illinois: American Academy of Sleep Medicine; 2016. For the American Academy of Sleep Medicine. Version 2.3. [Google Scholar]
- 14.Sitasuwan T, Bussaratid S, Ruttanaumpawan P, Chotinaiwattarakul W. Reliability and validity of the Thai version of the Pittsburgh Sleep Quality Index. J Med Assoc Thai. 2014;97(Suppl 3:):S57–S67. [PubMed] [Google Scholar]
- 15.Richards KC, O'Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8(2):131–144. doi: 10.1891/1061-3749.8.2.131. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Snyder-Halpern R, Verran JA. Instrumentation to describe subjective sleep characteristics in healthy subjects. Res Nurs Health. 1987;10(3):155–163. doi: 10.1002/nur.4770100307. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Huang HW, Zheng BL, Jiang L, Lin ZT, Zhang GB, Shen L, et al. Effect of oral melatonin and wearing earplugs and eye masks on nocturnal sleep in healthy subjects in a simulated intensive care unit environment: which might be a more promising strategy for ICU sleep deprivation? Crit Care. 2015;19:124. doi: 10.1186/s13054-015-0842-8. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16(3):R73. doi: 10.1186/cc11330. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneshmandi M, Neiseh F, SadeghiShermeh M, Ebadi A. Effect of eye mask on sleep quality in patients with acute coronary syndrome. J Caring Sci. 2012;1(3):135–143. doi: 10.5681/jcs.2012.020. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babaii A, Adib-Hajbaghery M, Hajibagheri A. Effect of using eye mask on sleep quality in cardiac patients: a randomized controlled trial. Nurs Midwifery Stud. 2015;4(4):e28332. doi: 10.17795/nmsjournal28332. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace CJ, Robins J, Alvord LS, Walker JM. The effect of earplugs on sleep measures during exposure to simulated intensive care unit noise. Am J Crit Care. 1999;8(4):210–219. doi: 10.4037/ajcc1999.8.4.210. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 2010;14(2):R66. doi: 10.1186/cc8965. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor KS, Murai H, Millar PJ, Haruki N, Kimmerly DS, Morris BL, et al. Arousal from sleep and sympathetic excitation during wakefulness. Hypertension. 2016;68(6):1467–1474. doi: 10.1161/HYPERTENSIONAHA.116.08212. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M, Shimamoto K, Sekiguchi H, Harada T, Satoya N, Inoue Y, et al. Arousal index as a marker of carotid artery atherosclerosis in patients with obstructive sleep apnea syndrome. Sleep Breath. 2019;23(1):87–94. doi: 10.1007/s11325-018-1664-0. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73(5):472–480. doi: 10.1136/thoraxjnl-2017-210431. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkelman C, Higgins PA, Chen YJ, Levine AD. Cytokines in chronically critically ill patients after activity and rest. Biol Res Nurs. 2007;8(4):261–271. doi: 10.1177/1099800406298168. DOI: [DOI] [PubMed] [Google Scholar]
- 27.Winkelman C. Investigating activity in hospitalized patients with chronic obstructive pulmonary disease: a pilot study. Heart Lung. 2010;39(4):319–330. doi: 10.1016/j.hrtlng.2009.09.004. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. DOI: [DOI] [PubMed] [Google Scholar]
- 29.Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, Tripodaki E, et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14(2):R74. doi: 10.1186/cc8987. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel BK, Pohlman AS, Hall JB, Kress JP. Impact of early mobilization on glycemic control and ICU-acquired weakness in critically ill patients who are mechanically ventilated. Chest. 2014;146(3):583–589. doi: 10.1378/chest.13-2046. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. DOI: [DOI] [PubMed] [Google Scholar]
- 33.Kamdar BB, Kadden DJ, Vangala S, Elashoff DA, Ong MK, Martin JL, et al. Feasibility of continuous actigraphy in patients in a medical intensive care unit. Am J Crit Care. 2017;26(4):329–335. doi: 10.4037/ajcc2017660. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab KE, Ronish B, Needham DM, To AQ, Martin JL, Kamdar BB. Actigraphy to evaluate sleep in the intensive care unit. A systematic review. Ann Am Thorac Soc. 2018;15(9):1075–1082. doi: 10.1513/AnnalsATS.201801-004OC. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43(5):445–452. doi: 10.1016/j.hrtlng.2014.06.049. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]