Abstract
Aim
To develop and validate a predictive model for moderate-to-severe periodontitis in the adult USA population, with data from the 2011–2012 National Health and Nutrition Examination Survey (NHANES) cycle.
Material and Methods
A subset of 3017 subjects aged >30 years, with >14 teeth present and having received a periodontal examination in addition to data collected on cardio-metabolic risk measures (smoking habit, body mass index [BMI], blood pressure, total cholesterol and glycated haemoglobin [HbA1c]) were used for model development by multivariable logistic regression.
Results
The prevalence of moderate and severe periodontitis using CDC/AAP classification was 37.1% and 13.2%, respectively. A multivariable logistic regression model revealed that HbA1c ≥5.7% was significantly associated with moderate-to-severe periodontitis (odds ratio, OR = 1.29; p < 0.01). A predictive model including age, gender, ethnicity, HbA1c and smoking habit as variables had 70.0% sensitivity and 67.6% specificity in detecting moderate-to-severe periodontitis in US adults.
Conclusions
Periodontitis is a common disease in North American adults, and its prevalence is significantly higher in individuals with pre-diabetes or diabetes. The present study demonstrates that a model including age, gender, ethnicity, HbA1c and smoking habit could be used as a reliable screening tool for periodontitis in primary medical care settings to facilitate referral of patients at risk for periodontal examination and diagnosis.
Keywords: diabetes, endocrinology, glycated haemoglobin, HbA1c, periodontitis, predictive modelling
1 |. INTRODUCTION
Periodontitis is a chronic inflammatory disease associated with oral biofilm dysbiosis and unresolved inflammation leading to destruction of tooth supporting structures. Severe periodontitis is estimated to affect 11% of world population what implies a significant deterioration of oral health-related quality of life (OHrQL) (Cunha-Cruz, Hujoel, & Kressin, 2007; Gerritsen, Allen, Witter, Bronkhorst, & Creugers, 2010) and heavy economic burdens on healthcare systems (Kassebaum et al., 2014).
While the role of genetics has been associated with up to 50% of susceptibility to periodontitis, there is ample evidence on the impact of modifiable risk factors. In fact, poor oral hygiene, smoking and uncontrolled diabetes increase the odds of developing periodontitis up to 5-fold (Michalowicz et al., 2000; Borgnakke, Ylostalo, Taylor, & Genco, 2013; Chapple & Genco, 2013). Hyperglycaemia is known to favour pro-inflammatory priming of periodontal tissues increasing the risk for gingivitis in patients with diabetes (Salvi, Kandylaki, Troendle, Persson, & Lang, 2005; Sima, Rhourida, Van Dyke, & Gyurko, 2010). Epidemiological evidence also demonstrated that poor control of glycaemia correlated with higher prevalence, severity and progression rate of periodontitis compared to normo-glycemic individuals (Borgnakke et al., 2013).
Severe periodontitis contributes to the systemic inflammatory burden, hence affecting the overall health, and may impact other chronic diseases such as diabetes mellitus and atherosclerotic cardiovascular diseases (Tonetti, 2009; Chapple & Genco, 2013; Tonetti & Van Dyke, 2013). This relationship between diabetes and periodontitis is clearly bidirectional, since significant improvements in glycemic control, measured by the percentage of glycated haemoglobin (HbA1c) have been observed after periodontal therapy (Chapple & Genco, 2013). Central to these associations seems to be the unresolved systemic inflammation indicated by high-sensitivity C-reactive protein measurements and white blood cell counts (Genco & Van Dyke, 2010; Demmer et al., 2013).
Frequently associated with both diabetes and periodontitis are overweight and obesity, conditions affecting around 35% of US adults (Flegal, Carroll, Kit, & Ogden, 2012). Numerous studies have reported a positive association between body mass index (BMI) ≥25 and periodontitis, although the magnitude of this association has varied in different populations (Suvan, D’Aiuto, Moles, Petrie, & Donos, 2011; Suvan et al., 2015). The odds of having periodontitis, adjusted for age, gender, smoking, alcohol consumption and frequency of tooth brushing, seem to increase with BMI in different populations (Suvan et al., 2011). Similarly, there has been a positive association between the metabolic syndrome (increased blood pressure, elevated plasma glucose, excess body fat around the waist and abdominal area and altered cholesterol levels) and periodontitis (Shimazaki et al., 2007; Saxlin et al., 2008; Nesbitt et al., 2010; Gomes-Filho et al., 2016). In the third NHANES survey, individuals ≥45 years of age suffering from severe periodontitis were 2.3 times (95% confidence interval [CI]: 1.13–4.47) more likely to have metabolic syndrome compared with unaffected individuals (D’Aiuto et al., 2008). Unresolved inflammation is the most plausible biological explanation for these associations. Therefore, reinforcement of preventive strategies aimed at reducing periodontitis-associated burden through integrative approaches to pro-inflammatory conditions including obesity, pre-diabetes and diabetes is necessary.
The aim of this study was to assess the associations between cardio-metabolic risk measures and moderate-to-severe periodontitis using the National Health and Nutrition Examination Survey 2011–2012 data set (NHANES 2011–2012), which is a sample representative of US non-institutionalized adult population. A predictive model using a combination of cardio-metabolic and socio-demographic variables was created and validated for predicting moderate-to-severe periodontitis to be used as screening tool by physicians in primary care settings.
2 |. MATERIAL AND METHODS
2.1 |. Study design and sample
NHANES 2011–2012 was a cross-sectional study conducted by the National Center for Oral Health Statistics (NCHS) which is part of the Center for Disease Control and Prevention. NHANES 2011–2012 was designed to evaluate the health and nutritional status of adults and children in the United States using a multistage, stratified, clustered probability sample of the US civilian, non-institutionalized population ≥2 years old. The protocols for NHANES 2011–2012 were approved by the institutional review board of the NCHS. Informed consent was obtained from all participants (Johnson, Dohrmann, Burt, & Mohadjer, 2014).
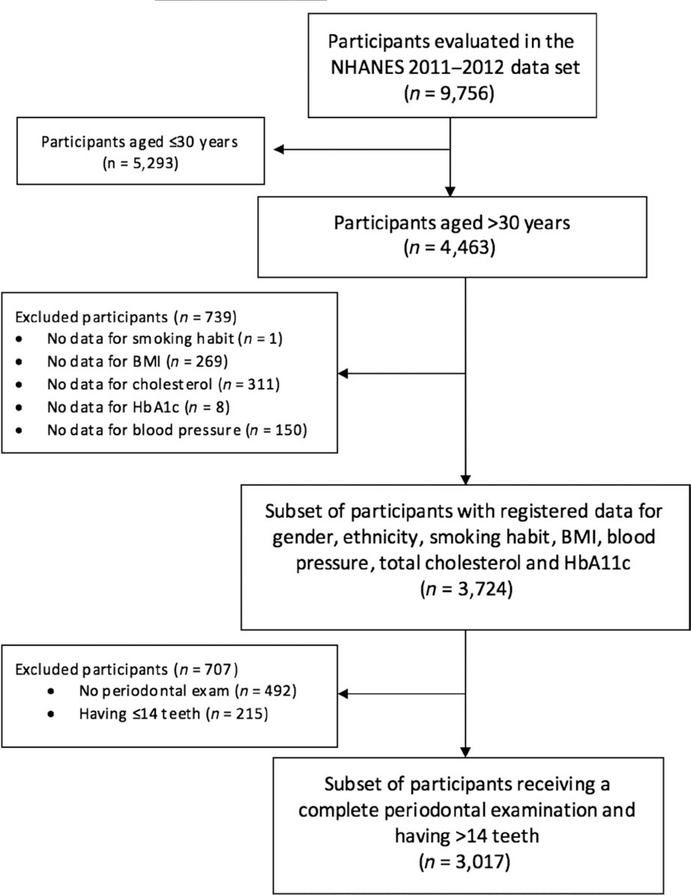
Among the 9,756 subjects evaluated in NHANES 2011–2012, the present study has focused on a subset of participants aged >30 years with the following registered data: age, gender, ethnicity, smoking habit, BMI, blood pressure, total cholesterol and HbA1c. Among these stratified samples, 3,017 subjects were identified as having >14 teeth and having received a periodontal examination. This study conforms with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting cross-sectional studies. Moreover, this manuscript also conforms with the TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis) guidelines for reporting predictive models (Moons et al., 2015). Additional information on the studied sample can be accessed at Supplemental Methods.
2.2 |. Clinical periodontal outcomes
Clinical attachment level (CAL) and probing pocket depth (PPD) measurements were recorded at 6 sites/tooth. CAL was measured from the cement-enamel junction to the base of the sulcus/pocket as a composite measurement of recession (distance from free gingival margin to cement-enamel junction) and PPD (distance from free gingival margin to the base of the sulcus).
Subjects were categorized in one of the three case definitions reported by Page and Eke (Page & Eke, 2007) for their use in population-based studies:
Severe periodontitis, if the patient presented ≥2 inter-proximal sites with CAL ≥6 mm (not on the same tooth) and ≥1 inter-proximal site with PPD ≥5 mm.
Moderate periodontitis, if the patient presented ≥2 inter-proximal sites with CAL≥4 mm (not on the same tooth) or ≥2 inter-proximal sites with PPD ≥5 mm.
No or mild periodontitis, neither “moderate” nor “severe” periodontitis.
Moderate and severe periodontitis were grouped into the same category, moderate-to-severe periodontitis, for the statistical analysis.
2.3 |. Data analysis
All data analyses were performed with a software package (STATA v.13 with SVY package, StataCorp, College Station, TX, USA), which accounts and weights the multistage stratified, clustered sampling method of NHANES III. Means and standard deviations (SDs) were used to describe the demographic, cardio-metabolic and periodontal characteristics of participants.
Candidate predictors were categorized in order to facilitate clinicians’ use of the prediction model. Educational level was categorized into: (a) secondary school, (b) high school graduate, (c) college degree and (d) college graduate or above. Annual household income was presented as follows: (a) <20,000$, (b) 20,000–100,000$ and (c) >100,000$. The definition of smoking was based on participants’ answers to the following questions: (a) “Have you smoked >100 cigarettes in life?” and (b) “Do you smoke cigarettes now?”. If participants answered “no” to both questions, they were coded as non-smokers; if participants answered “yes” to question (a) but “no” to question (b) they were coded as past smokers; if participants answered “yes” to both questions, they were coded as current smokers. Glucose regulation was classified as normal (HbA1<5.7%) or abnormal (HbA1c ≥5.7%). Usual categories for BMI were employed (underweight-normal weight/overweight/obese). Blood pressure and total cholesterol values were dichotomized on the basis of the thresholds established for high blood pressure and hypercholesterolaemia.
Odds ratios (ORs), along with the associated 95% CI, for both periodontal status (either no/mild periodontitis or moderate/severe periodontitis) and mean CAL (after transforming it into a categorical variable; 0–3 mm, 4–6 mm, >6 mm) were estimated separately for each potential risk indicator using multivariate logistic regression with adjustment for potential confounding factors such as age, gender, ethnicity, smoking habit, educational level or annual household income. In the case of mean CAL ordinal logistic regression analysis was used as a consequence of the hierarchical relation between categories.
For the predictive model, first-order interaction terms were evaluated through a global signification test (chunk test). As long as the result was non-significant, all interactions were excluded from the candidate model. Candidate socio-demographic and cardio-metabolic measures were included in a multivariable logistic regression analysis following a backward elimination approach for removal of variables. The best model was selected using the “all possible equations” strategy and both the area under the curve (AUC) and the Akaike’s information criterion (AIC) criteria. Use of the AIC for selection has been considered as an attractive option, as it accounts for model fit while penalizing for the number of parameters being estimated (Sauerbrei, Boulesteix, & Binder, 2011). Candidate models were compared using the receiver operating characteristic (ROC) curves to determine each model’s ability to discriminate between those with no/mild periodontitis and those with moderate-to-severe periodontitis. We assessed internal validity with a bootstrapping procedure for a realistic estimate of the performance of the candidate models. We repeated the entire modelling process, including variable selection, in 600 subjects drawn with replacement from the original sample. This approach allows to calculate the loss of prediction (shrinkage), and although presents several weaknesses, it is the recommended internal validation strategy in prediction model studies, as large sample sizes (like the one derived from the NHANES 2011–2012 data set) make this approach reasonable (Steyerberg et al., 2001; Moons et al., 2015). Finally, a prognostic index table was created in order to facilitate the evaluation of any subject in their risk for suffering moderate-to-severe periodontitis.
3 |. RESULTS
The sample selected from the NHANES 2011–2012 data set included a total of 3,017 subject records, representing 30.92% of the original national sample. Sample characteristics are presented in Table 1. Briefly, 1,516 males (50.25%) and 1,501 females (49.75%) were included in this study. Mean age was 51.62 ± 14.28 for males and 51.82 ± 14.00 for females. The most frequent ethnicity was non-Hispanic whites (38.18%) followed by African American (24.49%) and Hispanics (21.18%). Mean blood pressure values were 123.92 ± 17.75 mmHg for systolic blood pressure (SBP) and 72.17 ± 12.16 mmHg for diastolic blood pressure (DBP). The mean values for other cardio-metabolic measures were borderline high or high (HbA1c, 5.85 ± 1.17% and total cholesterol, 197.99 ± 41.32 mg/dl). The number of subjects by BMI category were 853 (28.27%) for underweight/normal body weight, 1,056 (35.00%) for overweight and 1,108 (36.73%) for obese.
TABLE 1.
Characteristics of the subset of NHANES 2011–2012 sample and prevalence of no/mild, moderate and severe periodontitis
| NHANES 2011–2012 Sample % (n) | Periodontal status category |
|||
|---|---|---|---|---|
| No/Mild periodontitis | Moderate periodontitis | Severe periodontitis | ||
| Overall | 100% (3017) | 49.75% (1501) | 37.06% (1118) | 13.19% (398) |
| Age | 100% (3017) | |||
| 30–39 | 24.76% (747) | 71.22% (532) | 23.96% (179) | 4.82% (36) |
| 40–49 | 22.41% (676) | 54.44%b (368) | 32.84%b (222) | 12.72%b (86) |
| 50–59 | 20.88% (630) | 44.60%b (281) | 36.35%b (229) | 19.05%b (120) |
| 60–69 | 19.22% (580) | 34.31%b (199) | 45.34%b (263) | 20.34%b (118) |
| 70–80 | 12.73% (384) | 31.51%b (121) | 58.59%b (225) | 9.90%b (38) |
| Gender | 100% (3017) | |||
| Male | 50.25% (1516) | 41.09% (623) | 39.51% (599) | 19.39% (294) |
| Female | 49.75% (1501) | 58.49%b (878) | 34.58%b (519) | 6.93%b (104) |
| Race/Ethnicity | 100% (3017) | |||
| Non-Hispanic whitea | 38.18% (1152) | 59.64% (687) | 32.12% (370) | 8.25% (95) |
| Hispanic | 21.18% (639) | 42.41% (271) c | 43.04% (275)c | 14.55% (93)c |
| African American | 24.49% (739) | 38.97% (288) c | 41.41% (306)c | 19.62% (145) c |
| Asian American | 13.52% (408) | 51.47% (210)c | 34.56% (141) | 13.97% (57)c |
| Other or multiracial | 2.62% (79) | 56.96% (45) | 32.91% (26) | 10.13% (8) |
| Education | 79.54% (2400) | |||
| Secondary schoola | 21.58% (518) | 29.92% (155) | 49.03% (254) | 21.04% (109) |
| High school graduate | 21.50% (516) | 38.57% (199) | 42.25% (218) | 19.19% (99) |
| College degree | 28.08% (674) | 52.52% (354)c | 35.76% (241)c | 11.72% (79)c |
| College graduate or above | 28.83% (692) | 66.91% (463)c | 26.73% (185)c | 6.36% (44)c |
| Annual household income | 77.32% (2333) | |||
| <$20,000 | 20.02% (467) | 34.90% (163) | 47.32% (221) | 17.77% (83) |
| $20,000–45,000 | 28.03% (668) | 40.57% (271) | 43.56% (291) | 15.87% (106) |
| $45,000–75,000 | 17.32% (404) | 54.21% (219)c | 32.92% (133)c | 12.87% (52) |
| $75,000–100,000 | 9.73% (227) | 63.44% (144)c | 27.31% (62)c | 9.25% (21)c |
| >$100,000 | 19.46% (454) | 67.40% (306)c | 25.33% (115)c | 7.27 (33)c |
| Smoking habit | 100% (3017) | |||
| Non-smoker | 56.94% (1718) | 57.39% (986) | 33.70% (579) | 8.91% (153) |
| Former smoker | 24.66% (744) | 45.70%b (340) | 39.38%b (293) | 14.92%b (111) |
| Smoker | 18.40% (555) | 31.53%b (175) | 44.32%b (246) | 24.14%b (134) |
| BMI (kg/m2) | 100% (3017) | |||
| Underweight/Normal | 28.27% (853) | 52.52% (448) | 33.29% (284) | 14.19% (121) |
| Overweight | 35.00% (1056) | 49.15% (519) | 37.22% (393) | 13.64% (144) |
| Obese | 36.73% (1108) | 48.19% (534) | 39.80% (441) | 12% (133) |
| HbA1c | 100% (3017) | |||
| min–5.6% | 55.72% (1681) | 58.66% (986) | 31.23% (525) | 10.11% (170) |
| 5.7–6.4% | 31.69% (956) | 41.00%b (392) | 43.51%b (416) | 15.48%b (148) |
| 6.5–8% | 8.02% (242) | 37.19%b (90) | 45.87%b (111) | 16.94%b (41) |
| >8% | 4.57% (138) | 23.91%b (33) | 47.83%b (66) | 28.26%b (39) |
| Blood pressure and total cholesterol | Mean ± SD | |||
| Systolic blood pressure (mmHg) | 123.92 ± 17.75 | 120.70 ± 16.30 | 126.53 ±18.02 | 128.73 ± 19.86 |
| Diastolic blood pressure (mmHg) | 72.17 ± 12.16 | 72.71 ± 11.18 | 71.00 ±12.99 | 73.49 ± 13.02 |
| Total cholesterol (mg/dl) | 197.99 ± 41.33 | 198.31 ± 39.34 | 196.89 ± 42.35 | 199.91 ± 45.54 |
Notes. SD, standard deviation.
Reference category.
Statistically significant difference when comparing with the immediate upper category (p < 0.01).
Statistically significant difference when comparing with the reference category (p < 0.01).
The prevalence of moderate and severe periodontitis was 37.06% and 13.19%, respectively. Moderate-to-severe periodontitis prevalence was progressively higher by decade of age and HbA1c levels, and higher in males and smokers (Table 1). Moderate-to-severe periodontitis prevalence was the highest in African Americans (61.0%) and Hispanics (57.6%), followed by Asian Americans (48.5%), and the lowest in Non-Hispanic Whites (40.4%).
Tables 2 and 3 list the socio-demographic variables considered, along with their OR and their CI, obtained from the multiple logistic regression analysis adjusted for the effect of age, gender, educational level, household income, ethnicity and smoking, when using periodontal status and mean CAL as independent variables.
TABLE 2.
Adjusted associationsb, expressed as odds ratio (95% confidence interval), between demographics and diagnosis of moderate-to-severe periodontitis
| Demographic risk factor | Odds ratio (95% confidence interval) | |
|---|---|---|
| Age | <50 yearsb | |
| ≥50 years | 2.61 (2.12–3.21)d | |
| Gender | Femaleb | |
| Male | 2.21 (1.81–2.69)d | |
| Ethnicity | Non-Hispanic whiteb | |
| Hispanic | 1.58 (1.21–2.06)d | |
| African American | 1.91 (1.50–2.44)d | |
| Asian American | 2.20 (1.61–3.01)d | |
| Educational level | Secondary schoolb | |
| High school graduate | 0.80 (0.60–1.08) | |
| College degree | 0.54 (0.41–0.72)d | |
| College graduate | 0.40 (0.30–0.55)d | |
| Household income | <$20,000b | |
| $20,000–$100,000 | 0.74 (0.57–0.94)c | |
| >$100,000 | 0.44 (0.33–0.59)d | |
Considering as confounders the rest of the demographic, socio-economic and lifestyle variables, named: age, gender, smoking habit, ethnicity, educational level and household income.
Reference category, Odds ratio = 1.
p < 0.05.
p < 0.01.
TABLE 3.
Adjusted associationsb, expressed as odds ratio (95% confidence interval), between demographics and mean CAL (CAL<4 mm serve as category of reference)
| Demographic risk factor | Mean CAL 4–6 mm | Mean CAL ≥6 mm | |
|---|---|---|---|
| Age | <50 yearsb | ||
| ≥50 years | 2.83 (2.04–3.90)d | 8.21 (2.99–22.54)d | |
| Gender | Femaleb | ||
| Male | 2.58 (1.90–3.50)d | 5.98 (2.48–14.38)c | |
| Ethnicity | Non-Hispanic whiteb | ||
| Hispanic | 1.17 (0.79–1.74) | 2.48 (0.86–7.19) | |
| African American | 1.73 (1.22–2.45)d | 4.27 (1.73–10.57)d | |
| Asian American | 1.65 (0.99–2.73) | 1.55 (0.36–6.72) | |
| Educational level | Secondary schoolb | ||
| High School graduate | 0.70 (0.49–0.99)c | 1.16 (0.53–2.56) | |
| College degree | 0.40 (0.27–0.58)d | 0.27 (0.09–0.79)c | |
| College graduate | 0.19 (0.11–0.32)c | 0.52 (0.16–1.64) | |
| Household income | <$20,000b | ||
| $20,000–$100,000 | 0.69 (0.50–0.94)c | 0.91 (0.44–1.90) | |
| >$100,000 | 0.44 (0.28–0.69)d | 0.34 (0.10–1.16) | |
Considering as confounders the rest of the demographic, socio-economic and lifestyle variables, named: age, gender, smoking habit, ethnicity, educational level and household income.
Reference category, Odds ratio = 1.
p < 0.05.
p < 0.01.
Among the socio-demographic determinants, age was the strongest indicator for having moderate-to-severe periodontitis, as well as for mean CAL 4–6 mm or mean CAL >6 mm (OR = 2.61, 95% CI 2.123.21, p < 0.01; OR = 2.83, 95% CI 2.04–3.90, p < 0.01; OR = 8.21, 95% CI 2.99–22.54, p < 0.01; respectively), followed by male gender. Hispanic, African American and Asian American ethnicity were statistically associated with periodontal status according to AAP-CDC case definition (OR = 1.58, 95% CI 1.21–2.06, p < 0.01; OR = 1.91, 95% CI 1.50–2.44, p < 0.01; OR = 2.20, 95% CI 1.61–3.01, p < 0.01; respectively) but only African American ethnicity was associated with mean CAL (OR = 1.73, 95% CI 1.22–2.45, p < 0.01, for CAL 4–6 mm; OR = 4.27, 95% CI 1.73–10.57, p < 0.01, for CAL ≥6 mm). Higher educational level and household income were identified as negatively associated with moderate-to-severe periodontitis.
Regarding the cardio-metabolic risk indicators, and after adjusting for confounders, smoking habit was the strongest indicator, followed by HbA1c. Smoker’s OR for having moderate-to-severe periodontitis were 2.91 (95% CI 2.23–3.80) when compared with non-smokers (Table 4). Also, in subjects with HbA1c ≥5.7% (pre-diabetes or diabetes), OR for having moderate-to-severe periodontitis were 1.29 (95% CI 1.07–1.57), when compared with those with normal HbA1c values. In subjects with <50 years, HbA1c values ≥5.7% were associated with an increased OR for suffering moderate-to-se- vere periodontitis (OR = 1.42, 95% CI 1.05–1.94, p < 0.05), and the magnitude of this association increased in the subgroup of smokers (OR = 2.43, 95% CI 1.47–4.01, p < 0.01). Obese young adults (<50 years) exhibited an OR of 1.56 (95% CI 1.08–2.26) for presenting moderate-to-severe periodontitis. No significant associations were found for blood pressure (SBP or DBP) or total cholesterol and periodontal status.
TABLE 4.
Adjusted associationsb, expressed as odds ratios (95% confidence interval), between individual cardio-metabolic risk factors and diagnosis of moderate-to-severe periodontitis
| Cardio-metabolic risk factor | All sample (n = 3017) | <50 years (n = 1423) | Smokers (n = 555) | |
|---|---|---|---|---|
| Smoking habit | Non-smokerb | |||
| Former smoker | 1.22 (0.97–1.54) | 1.38 (0.92–2.08) | – | |
| Smoker | 2.91 (2.23–3.80)d | 2.57 (1.78–3.69)d | – | |
| HbA1c | <5.7%b | |||
| ≥5.7% | 1.29 (1.07–1.57)d | 1.42 (1.05–1.94)c | 2.43 (1.47–4.01)d | |
| BMI | <25 kg/m2 | |||
| Overweight | 1.10 (0.86–1.40) | 1.14 (0.78–1.66) | 1.26 (0.71–2.24) | |
| Obesity | 1.26 (0.99–1.60) | 1.56 (1.08–2.26)d | 1.37 (0.77–2.44) | |
| SBP | <140 mm Hgb | |||
| ≥140 mm Hg | 1.16 (0.89–1.50) | 1.48 (0.88–2.50) | 1.26 (0.61–2.59) | |
| DBP | <90 mm Hgb | |||
| ≥90 mm Hg | 1.15 (0.79–1.67) | 1.36 (0.82–2.26) | 1.08 (0.47–2.49) | |
| Total cholesterol | <200 mg/dlb | |||
| ≥200 mg/dl | 1.02 (0.85–1.23) | 0.82 (0.62–1.10) | 1.12 (0.70–1.79) |
Notes. BMI, body mass index; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; SBP, systolic blood pressure.
Considering as confounders the rest of the demographic, socio-economic and lifestyle variables, named: age, gender, smoking habit, ethnicity, educational level and household income.
Reference category, Odds ratio = 1.
p < 0.05.
p < 0.01.
For the continuous measure of periodontitis (mean CAL) in fully adjusted models, smoking was again the strongest indicator for mean CAL ≥6 mm (OR = 6.79, 95% CI 2.89–15.98, p < 0.01; Table 5). HbA1c ≥5.7% was the only cardio-metabolic parameter significantly associated with mean CAL ≥ 6 mm (OR = 1.43, 95% CI 1.10–1.87, p<0.01), as neither BMI, blood pressure nor total cholesterol presented significant associations.
TABLE 5.
Adjusted associationsb between individual cardio-metabolic risk factors and mean CAL≥6 mm
| Cardio-metabolic risk factor | All sample (n = 3017) | <50 years (n = 1423) | Smokers (n = 555) | |
|---|---|---|---|---|
| Smoking habit | Non-smokerb | |||
| Former smoker | 1.83 (0.76–4.45) | 0.97 (0.25–3.76) | – | |
| Smoker | 6.79 (2.89–15.98)d | 2.73 (1.18–6.35)c | – | |
| HbA1c | <5.7%c | |||
| ≥5.7% | 1.43 (1.10–1.87)d | 2.08 (0.87–4.93) | 1.46 (0.84–2.15) | |
| BMI | <25 kg/m2 | |||
| Overweight | 0.84 (0.61–1.15) | 0.71 (0.37–1.35) | 0.68 (0.39–1.17) | |
| Obesity | 0.81 (0.59–1.13) | 0.71 (0.37–1.38) | 0.72 (0.40–1.28) | |
| SBP | <140 mm Hgb | |||
| ≥140 mm Hg | 0.90 (0.58–1.40) | 0.73 (0.31–1.73) | 0.92 (0.42–2.05) | |
| DBP | <90 mm Hgb | |||
| ≥90 mm Hg | 1.43 (0.73–2.42) | 1.54 (0.71–3.30) | 1.43 (0.66–3.11) | |
| Total cholesterol | <200 mg/dlb | |||
| ≥200 mg/dl | 1.16 (0.90–1.51) | 1.36 (0.68–1.90) | 1.60 (0.99–2.58) |
Notes. BMI, body mass index; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; SBP, systolic blood pressure.
Considering as confounders the rest of the demographic, socio-economic and lifestyle variables, named: age, gender, smoking habit, ethnicity, educational level and household income.
Reference category, OR = 1.
p < 0.05.
p < 0.01.
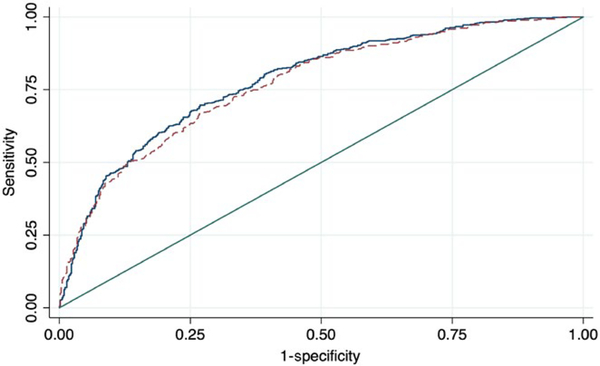
The predictive model with the highest AUC (AUC = 0.801) is always the one comprising all variables: age, gender, ethnicity, HbA1c, BMI, SBP, DBP, total cholesterol, smoking status, educational level and household income. However, this model did not consider the number of variables of the model (11), neither presented the best fit according to the AIC criteria. In order to solve these issues, both AUC and AIC criteria were considered, with a model comprising just five variables (age, gender, ethnicity, HbA1c and smoking habit) presenting a similar AUC, sensitivity and specificity (0.801 vs. 0.773, 73.2% versus 70.0%, 71.2% vs. 67.6%, for the model with 11 variables versus the model with five variables, respectively) but with a lower number of variables involved. The analysis comparing the ROC curves of the maximum and candidate models showed that they were very similar (Figure 1) and using chi-squared tests it was demonstrated that differences between them were not statistically significant (χ2 = 0.228). The proposed predictive model was tested and validated through a bootstrap validation approach. This analysis found that the model’s predictability was reliable, as long as the loss of prediction/shrinkage was 1.00% (result of the difference between the AUC in the entire sample [0.801] and the bootstrap sample [0.791]). The full prediction model is presented in Supporting information Table S2.
FIGURE 1.
Flow chart indicating the subset of participants included for the analysis from the NHANES 2011–2012 data set
Finally, a prognostic index table with the relative risks (RR), for all the possible combinations of the predictive variables, was built in order to facilitate the determination of the risk for suffering moderate-to-advance periodontitis (Appendix S1). The reference pattern (RR = 1) corresponds to a non-Hispanic white non-smoker female between 30–40 years with HbA1c ≤5.7%. The highest RR (RR = 9.91) corresponds to a Hispanic, male, smoker, between 70–80 years old and presenting HbA1c ≥6.5%.
4 |. DISCUSSION
The results from this cross-sectional study provide evidence of the significant relationship between cardio-metabolic risk measures and the prevalence of moderate-to-severe periodontitis in the U S adult population. It further provides the basis for using algorithms integrating demographic, lifestyle and cardio-metabolic measures to screen for periodontitis in patients examined in primary medical care settings.
The prevalence of moderate-to-severe periodontitis in this subset of the 2011–2012 NHANES data set (participants aged >30 years with >14 teeth having received a periodontal examination, as well as having age, gender, ethnicity, smoking habit, BMI, blood pressure, total cholesterol and HbA1c registered) was ≈50%, with 13.19% having severe periodontitis and 37.06% moderate periodontitis. These findings are similar to those reported by Eke et al. (Eke et al., 2015) when combining NHANES 2009 to 2012 data. They reported that ≈46% of US dentate adults had periodontitis, with 8.9% having severe periodontitis and 37.1% having less severe forms (Eke et al., 2015).
These results also confirm the reported disparities in the burden of periodontitis according to the different socio-demographic segments of the population. Among ethnic groups, Hispanic and African American populations showed the highest prevalence of periodontitis, while Asian Americans and Non-Hispanic Whites had the lowest. The prevalence of moderate-to-severe periodontitis also increased with the decrease in educational levels and annual household income. These socio-economic and demographic patterns together with the identification of current smoking as the most important risk indicator were consistent with previous findings from NHANES, and reinforce the need to adjust for confounders when evaluating the association between cardio-metabolic risk factors and periodontitis (Albandar, Brunelle, & Kingman, 1999; Tomar & Asma, 2000; Eke, Dye, Wei, Thornton-Evans, & Genco, 2012).
Mean values for cardio-metabolic risk measures were higher (although non-statistically significant) in the subset of individuals included in this study, when compared with the NHANES data set, which may be in part explained by the inclusion of only >30 years of age in the study sample (Supporting information Table S1). In this subset, there was a significant association between HbA1c levels and moderate-to-severe periodontitis, as well as between HbA1c levels and attachment levels in adult individuals. It has been long known that there is a two-way relationship between periodontitis and diabetes, as inflammation is a central feature of both diseases. Recent evidence indicates that high levels of IL-1β, TNF-α and IL-6 are present in gingival tissues in poorly controlled diabetes subjects and that periodontitis worsens glycaemic control and leads to circulating elevated levels of these and other systemic inflammatory mediators such as C-reactive protein (Polak & Shapira, 2018). The results of this study highlight the importance of collecting information on blood glucose levels during periodontal diagnosis, which may result in identification of undiagnosed pre-diabetes or diabetes and better individualized treatment regimens for patients with diabetes and periodontitis (Lalla, Kunzel, Burkett, Cheng, & Lamster, 2011; Dye & Genco, 2012; Lalla, Cheng, Kunzel, Burkett, & Lamster, 2013). Moreover, in the light of mounting evidence indicating that periodontal therapy can improve HbA1c levels by up to 0.40% (Engebretson & Kocher, 2013), these findings are of particular importance. It is estimated that 1% reduction in HbA1c levels in diabetic patients results in 35% reduction in the risk of cardiovascular complications (Stratton et al., 2000) and that a 0.2% HbA1c reduction is associated with a 10% reduction in mortality in the general population (Khaw et al., 2004).
Although BMI within the multivariate analyses was not an important predictor for periodontitis, the ORs for obese young adults to present moderate-to-severe periodontitis were significantly higher after adjustment for all confounders. This fact is in agreement with the evidence derived from numerous studies showing an association between obesity and periodontitis (Suvan et al., 2011; Chaffee & Weston, 2010). In a long-term longitudinal study (30 years), on the progression of periodontitis and body adiposity in men, Gorman et al. found that both subcutaneous and visceral adiposity increases were associated with periodontitis progression (Gorman, Kaye, Nunn, & Garcia, 2012). In the present study, a significant association was found only for subjects <50 years, supporting that a stronger association between periodontitis and obesity may occur on younger individuals, mainly in women and non-smokers (Chaffee & Weston, 2010). Other studies have reported that waist circumference and waist-to-hip ratio correlated stronger than BMI with periodontitis (Al-Zahrani, Bissada, & Borawskit, 2003; Wood, Johnson, & Streckfus, 2003; Kim, Jin, & Bae, 2011). This is partly explained by BMI not distinguishing between visceral and subcutaneous adiposity, and the former is the main source of pro-inflammatory adipokines that prime for increased systemic inflammatory tone.
Using a representative national US sample of adults >30 years old, we have developed a predictive risk model for moderate-to-se- vere periodontitis. However, due to the cross-sectional nature of this study, periodontitis disease activity or progression as well as the history of exposure to cardio-metabolic risk factors could not be assessed and, hence, the possible inferences about the direction of the reported relationships cannot be made. It is also important to consider that the predictive model is applicable mainly to those subjects not visiting a dentist regularly. Unfortunately, this a frequent finding in the study population, as 42.09% of the participants did not attend to the dentist in the last year. Moreover, just attending the dentist does not necessarily imply that proper periodontal diagnosis has been made, as non-recognition of periodontitis is a common cause of professional litigation (Zinman, 2001). Another limitation is that the predictive model is applicable to the US population and cannot be extrapolated to other nations with different socio-economic demographics and healthcare systems. For these reasons, the proposed predictive model, or similar ones based on socio-demographic and cardio-metabolic risk factors, needs future validation in others, if possible, prospective cohorts such as the Study of Health in Pomerania (SHIP-Trend), or other data sets from NHANES.
In conclusion, our findings support the concept of personalized integrative approaches by physicians and periodontists in the management of cardio-metabolic disorders and periodontitis. The predictive model developed and validated to screen for existing undiagnosed and untreated moderate-to-severe periodontitis, using a combination of cardio-metabolic (HbA1c), demographic (age, gender and ethnicity) and lifestyle variables (smoking habit), may be used by physicians in primary medical care settings. However, further validation of this model across different populations is needed for development of guidelines and applicability in non-US populations. This study further reinforces the need for guidelines to screen for periodontitis, pre-diabetes and diabetes in dental and medical care settings. Measures of modifiable risk factors for periodontitis and diabetes, such as glycaemic control and adiposity, seem to be universally applicable screening parameters.
Supplementary Material
FIGURE 2.
Graphic representation of the receiver operating characteristic (ROC) curves of the maximum (straight blue line) and proposed (dash maroon line) predictive models
Clinical Relevance.
Scientific rationale for study
Periodontitis has been associated with several cardio-metabolic risk factors, diabetes and cardiovascular disease. A model comprising commonly registered risk factors for these diseases would be useful for their co-management by primary care physicians and periodontists, to control the associated systemic inflammatory burden.
Principal findings
A predictive model including age, gender, ethnicity, HbA1c and smoking habit as variables presented appropriate sensitivity and specificity to be used as screening tool for moderate-to-severe periodontitis in primary medical care settings. In any case, the absence of risk factors/determinants included in the model should be interpreted as an indicator of periodontal health.
Practical implications
The results from this study support the concept of integrative approaches by physicians and periodontists in the management of cardio-metabolic disorders and periodontitis. The predictive model presented may be used by physicians to integrate oral screening in the patient management workflow and reinforces the need for guidelines to screen for periodontitis in primary medical care settings. This will further facilitate inter-disciplinary co-management of pre-diabetes/diabetes and periodontitis.
Acknowledgments
Funding information
This study was supported by a pre-doctoral research grant from the Complutense University of Madrid (E.M) and by the U.S.A. National Institute of Dental and Craniofacial Research grants DE25020 (T.VD) and K99/R00DE024575 (C.S).
Footnotes
CONFLICT OF INTEREST
Authors declare no conflicts of interest in relation to this study.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Albandar JM, Brunelle JA, & Kingman A (1999). Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. Journal of Periodontology, 70, 13–29. 10.1902/jop.1999.70.1.13 [DOI] [PubMed] [Google Scholar]
- Al-Zahrani MS, Bissada NF, & Borawskit EA (2003). Obesity and periodontal disease in young, middle-aged, and older adults. Journal of Periodontology, 74, 610–615. 10.1902/jop.2003.74.5.610 [DOI] [PubMed] [Google Scholar]
- Borgnakke WS, Ylostalo PV, Taylor GW, & Genco RJ (2013). Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. Journal of Periodontology, 84, S135–S152. 10.1902/jop.2013.1340013 [DOI] [PubMed] [Google Scholar]
- Chaffee BW, & Weston SJ (2010). Association between chronic periodontal disease and obesity: A systematic review and meta-analysis. Journal of Periodontology, 81, 1708–1724. 10.1902/jop.2010.100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple IL, Genco R; Working group 2 of the joint EFP/AAP workshop. (2013). Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Clinical Periodontology, 40(Suppl 14), S106–S112. 10.1111/jcpe.12077 [DOI] [PubMed] [Google Scholar]
- Cunha-Cruz J, Hujoel PP, & Kressin NR (2007). Oral health-related quality of life of periodontal patients. Journal of Periodontal Research, 42, 169–176. 10.1111/jM600-0765.2006.00930.x [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani AD, Deanfield J, & Tsakos G (2008). Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. Journal of Clinical Endocrinology and Metabolism, 93, 3989–3994. 10.1210/jc.2007-2522 [DOI] [PubMed] [Google Scholar]
- Demmer RT, Trinquart L, Zuk A, Fu BC, Blomkvist J, Michalowicz BS, … Desvarieux M (2013). The influence of anti-infective periodontal treatment on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE, 8, e77441 10.1371/journal.pone.0077441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BA, & Genco RJ (2012). Tooth loss, pocket depth, and HbA1c information collected in a dental care setting may improve the identification of undiagnosed diabetes. Journal of Evidence Based Dental Practice, 12, 99–102. 10.1016/j.jebdp.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, … Genco RJ (2015). Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. Journal of Periodontology, 86, 611–622. 10.1902/jop.2015.140520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, & Genco RJ; CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washington) (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research, 91, 914–920. 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- Engebretson S, & Kocher T (2013). Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. Journal of Clinical Periodontology, 40(Suppl 14), S153–S163. 10.1111/jcpe.12084 [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, & Ogden CL (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA, 307, 491–497. 10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- Genco RJ, & Van Dyke TE (2010). Prevention: Reducing the risk of CVD in patients with periodontitis. Nature Reviews Cardiology, 7, 479–480. 10.1038/nrcardio.2010.120 [DOI] [PubMed] [Google Scholar]
- Gerritsen AE, Allen PF, Witter DJ, Bronkhorst EM, & Creugers NH (2010). Tooth loss and oral health-related quality of life: A systematic review and meta-analysis. Health and Quality of Life Outcomes, 8, 126 10.1186/1477-7525-8-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Filho IS, das Merces MC, de Santana Passos-Soares J, Seixas da Cruz S, Teixeira Ladeia AM, Trindade SC, … Scannapieco FA (2016). Severity of Periodontitis and Metabolic Syndrome: Is There an Association? Journal of Periodontology, 87, 357–366. 10.1902/jop.2015.150367 [DOI] [PubMed] [Google Scholar]
- Gorman A, Kaye EK, Nunn M, & Garcia RI (2012). Changes in body weight and adiposity predict periodontitis progression in men. Journal of Dental Research, 91, 921–926. 10.1177/0022034512457372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Dohrmann SM, Burt VL, & Mohadjer LK (2014). National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Statistics, 2, 1–33. [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, & Marcenes W (2014). Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. Journal of Dental Research, 93, 1045–1053. 10.1177/0022034514552491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, & Day N (2004). Association of hemoglobin A1c with cardiovascular disease and mortality in adults: The European prospective investigation into cancer in Norfolk. Annals of Internal Medicine, 141, 413–420. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Jin BH, & Bae KH (2011). Periodontitis and obesity: A study of the Fourth Korean National Health and Nutrition Examination Survey. Journal of Periodontology, 82, 533–542. 10.1902/jop.2010.100274 [DOI] [PubMed] [Google Scholar]
- Lalla E, Cheng B, Kunzel C, Burkett S, & Lamster IB (2013). Dental findings and identification of undiagnosed hyperglycemia. Journal of Dental Research, 92, 888–892. 10.1177/0022034513502791 [DOI] [PubMed] [Google Scholar]
- Lalla E, Kunzel C, Burkett S, Cheng B, & Lamster IB (2011). Identification of unrecognized diabetes and pre-diabetes in a dental setting. Journal of Dental Research, 90, 855–860. 10.1177/0022034511407069 [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, Brooks CN, Koertge TE, … Schenkein HA (2000). Evidence of a substantial genetic basis for risk of adult periodontitis. Journal of Periodontology, 71, 1699–1707. 10.1902/jop.2000.71.11.1699 [DOI] [PubMed] [Google Scholar]
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, … Collins GS (2015). Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Annals of Internal Medicine, 162, W1–W73. 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- Nesbitt MJ, Reynolds MA, Shiau H, Choe K, Simonsick EM, & Ferrucci L (2010). Association of periodontitis and metabolic syndrome in the Baltimore Longitudinal Study of Aging. Aging Clinical and Experimental Research, 22, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, & Eke PI (2007). Case definitions for use in population-based surveillance of periodontitis. Journal of Periodontology, 78, 1387–1399. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- Polak D, & Shapira L (2018). An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. Journal of Clinical Periodontology, 45, 150–166. 10.1111/jcpe.12803 [DOI] [PubMed] [Google Scholar]
- Salvi GE, Kandylaki M, Troendle A, Persson GR, & Lang NP (2005). Experimental gingivitis in type 1 diabetics: A controlled clinical and microbiological study. Journal of Clinical Periodontology, 32, 310–316. 10.1111/j.1600-051X.2005.00682.x [DOI] [PubMed] [Google Scholar]
- Sauerbrei W, Boulesteix AL, & Binder H (2011). Stability investigations of multivariable regression models derived from low- and high-dimensional data. Journal of Biopharmaceutical Statistics, 21, 1206–1231. 10.1080/10543406.2011.629890 [DOI] [PubMed] [Google Scholar]
- Saxlin T, Suominen-Taipale L, Kattainen A, Marniemi J, Knuuttila M, & Ylostalo P (2008). Association between serum lipid levels and periodontal infection. Journal of Clinical Periodontology, 35, 1040–1047. 10.1111/j.1600-051X.2008.01331.x [DOI] [PubMed] [Google Scholar]
- Shimazaki Y, Saito T, Yonemoto K, Kiyohara Y, lida M, & Yamashita Y (2007). Relationship of metabolic syndrome to periodontal disease in Japanese women: The Hisayama Study. Journal of Dental Research, 86, 271–275. 10.1177/154405910708600314 [DOI] [PubMed] [Google Scholar]
- Sima C, Rhourida K, Van Dyke TE, & Gyurko R (2010). Type 1 diabetes predisposes to enhanced gingival leukocyte margination and macromolecule extravasation in vivo. Journal of Periodontal Research, 45, 748–756. 10.1111/j.1600-0765.2010.01295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, & Habbema JD (2001). Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. Journal of Clinical Epidemiology, 54, 774–781. [DOI] [PubMed] [Google Scholar]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, … Holman RR (2000). Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ, 321, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvan J, D’Aiuto F, Moles DR, Petrie A, & Donos N (2011). Association between overweight/obesity and periodontitis in adults. A systematic review. Obesity Reviews, 12, e381–e404. 10.1111/j.1467-789X.2010.00808.x [DOI] [PubMed] [Google Scholar]
- Suvan JE, Petrie A, Nibali L, Darbar U, Rakmanee T, Donos N, & D’Aiuto F (2015). Association between overweight/obesity and increased risk of periodontitis. Journal of Clinical Periodontology, 42(8), 733–739. 10.1111/jcpe.12421 [DOI] [PubMed] [Google Scholar]
- Tomar SL, & Asma S (2000). Smoking-attributable periodontitis in the United States: Findings from NHANES III. National Health and Nutrition Examination Survey. Journal of Periodontology, 71, 743–751. 10.1902/jop.2000.71.5.743 [DOI] [PubMed] [Google Scholar]
- Tonetti MS (2009). Periodontitis and risk for atherosclerosis: An update on intervention trials. Journal of Clinical Periodontology, 36(Suppl 10), 15–19. 10.1111/j.1600-051X.2009.01417.x [DOI] [PubMed] [Google Scholar]
- Tonetti MS, & Van Dyke TE; Working group 1 of the joint EFP/AAP workshop. (2013). Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Clinical Periodontology, 40(Suppl 14), S24–S29. 10.1111/jcpe.12089 [DOI] [PubMed] [Google Scholar]
- Wood N, Johnson RB, & Streckfus CF (2003). Comparison of body composition and periodontal disease using nutritional assessment techniques: Third National Health and Nutrition Examination Survey (NHANES Ill). Journal of Clinical Periodontology, 30, 321–327. [DOI] [PubMed] [Google Scholar]
- Zinman E (2001). Dental and legal considerations in periodontal therapy. Periodontology, 2000(25), 114–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.