Abstract
The neonatal gut microbiome undergoes dynamic changes in response to many nutritional and environmental variables. A recent study by Singer et al. in Nature Medicine elucidates several mechanisms to inhibit the expansion of gut-derived pathobionts in a dysbiotic neonatal gut and prevent these pathobionts from disseminating systemically and causing sepsis in neonatal mice.
Progressive population of the neonatal gut with different bacterial species in early life is intricately associated with the maturation of the gut, development of the immune system, and host metabolism through host-microbe crosstalk (Milani et al., 2017; Bäckhed et al., 2015). Emerging evidence in recent years has underscored the importance of the gut microbiome in conferring colonization resistance against pathogens or opportunistic gut-derived pathobionts. This particular function of the gut microbiome is even more critical in early life, given that infection is a major cause of mortality and morbidity among neonates. Passive immunity, through acquisition of maternal protective antibodies, is critical to protect infants whose immune system is not fully developed yet (Melville and Moss, 2013). The incidence of infections, however, increases drastically among infants born prematurely (< 37 weeks of gestation) or with very low birth weight (VLBW) (< 1,500 g). The high susceptibility of this population of infants to infection is likely in part because of the inability of severely preterm or VLBW infants to breastfeed and acquire maternal antibodies (Melville and Moss, 2013). Furthermore, dysbiosis of the gut microbiome of these infants, which has been widely reported, could be another major factor in the predisposition of preterm or VLBW infants to infection by gut-derived pathobionts.
Neonatal sepsis typically can be categorized into two types: early onset sepsis (EOS) that occurs within 3 days after birth and late onset sepsis (LOS) that occurs between day 7 and day 10 after birth (Stoll et al., 2011). Infection by group B Streptococcus (GBS) from GBS+ mothers is the leading cause of EOS and meningitis. Another common causal bacterium for EOS is E. coli, presumably acquired during the birth process. The incidence of EOS in recent years has dropped significantly, largely due to intrapartum antibiotic prophylaxis treatment of women who are positive for GBS or experience complications such as pre-eclampsia and chorioamnionitis. However, GBS and E. coli remain among the common pathogens in neonatal infection, particularly among preterm or VLBW infants (Stoll et al., 2011). LOS can be manifested when commensal skin or gut bacteria disseminate systemically, resist host killing, and finally trigger detrimental sepsis (Stewart et al., 2017). Paradoxically, empiric antibiotics given to pregnant women or preterm infants to rule out EOS might disrupt the gut microbiome and allow opportunistic pathobionts to expand and dominate, ultimately leading to LOS.
A study recently published by Singer et al., (2019) in Nature Medicine demonstrates elegantly how a dysbiotic state of the gut microbiome leads to LOS in neonatal mice. This study utilized a clinically relevant pathobiont, Klebsiella pneumoniae (K. pneumoniae), which expanded quickly in the gut and translocated systemically after inoculation into 5-day-old (P5) neonatal mice. Interestingly, both a virulent strain (Kp-43816) and an avirulent strain (Kp-39) exhibited a similar capability to outgrow commensals in the gut of a P5 pup and disseminate systemically. However, although the virulent strain Kp-43816 caused sepsis because of a protective polysaccharide capsule that resisted phagocytosis, the disseminated avirulent Kp-39 strain was easily eliminated by host phagocytes.
The authors further elucidated three major approaches that were effective in mitigating LOS by Kp-43816 (Figure 1). The first approach was giving vancomycin, which normally kills gram-positive bacteria, to dams 1–2 days prior to delivery through P4 (Singer et al., 2019). In this case, P5 neonates from these dams had enriched Lactobacillus species, particularly L. murinus, that were shown to confer colonization resistance to Kp-43816 and improve the survival of neonates. Second, the authors demonstrated almost complete resistance to LOS by Kp-43816 in P14 neonates. The gut in P14 neonates is considered “mature” with increased diversity and richness of its gut bacterial communities, and—as indicated in this study and others—a distinct shift from dominance by facultative anaerobes to dominance by obligate anaerobes. Obligate anaerobes, particularly Clostridium species, expand substantially around two weeks of life in neonates, coinciding with the reduction in luminal oxygen. However, the authors did not go further to identify candidate obligate anaerobes that could prevent expansion of Kp-43816 in the neonatal gut. In the last approach, fecal transplantation of P19 fecal bacteria (which were deemed “adult gut microbiome”) significantly reduced the mortality of neonates as well. Interestingly, this was accomplished through selective enrichment of Enterobacteriaceae such as E. coli in the P5 gut, instead of obligate anaerobes, because of higher oxygen content in the lumen of the neonatal than in the P5 gut that is not conducive to the growth of obligate anaerobes.
Figure 1. Approaches to Prevent LOS Caused by Expansion of Gut Pathobiont K. pneumoniae in Neonatal Mice.
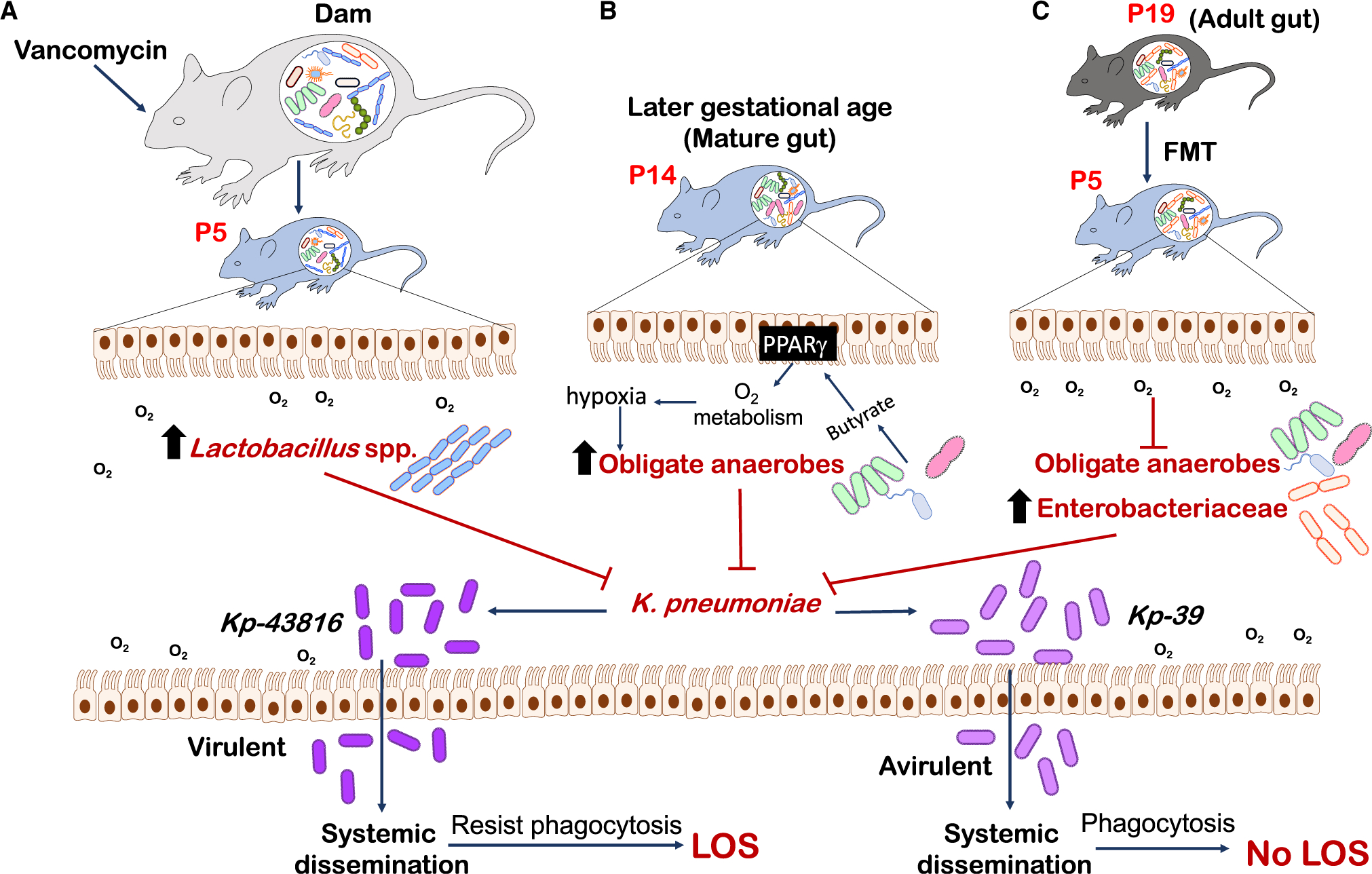
Singer et al., (2019) demonstrated three approaches to K.pneumoniae expansion in the neonatal gut that prevent LOS: (A) administration of the antibiotic vancomycin to maternal dams to increase the expansion of Lactobacillus species in the neonatal gut; (B) a hypoxic environment to support growth of obligate anaerobes in a mature gut (P14); and (C) expansion of Enterobacteriaceae species facilitated by higher oxygen levels in the neonatal than in the mature gut after FMT of adult gut microbiome (P19). Approaches (A)–(C) allow for the expansion of specific gut bacteria (Lactobacillus murinus, obligate anaerobes, and Enterobacteriaceae, respectively) that exert colonization resistance against the pathobiont K. pneumoniae to diminish LOS in neonates.
By attempting to colonize the neonatal gut (P5) with bacteria from donors that were manipulated in different ways (e.g., vancomycin-treated dams or P19 adults), the studies revealed a unique neonatal gut microenvironment with high oxygen content that is not conducive to the colonization of candidate probiotics (including obligate anaerobes) with known colonization resistance to pathobionts such as K. pneumoniae. This underscores the limitation of what we know about the neonatal gut and the challenge to implement probiotics as an approach to mitigate overgrowth of gut pathobionts or pathogens. In the adult gut, a previous study showed that Clostridia-derived butyrate activates the peroxisome proliferator-activated receptor gamma (PPARγ) pathway, an important gut-microbiome-mediated pathway that induces epithelial oxygen metabolism and lowers luminal oxygen levels (Byndloss et al., 2017). However, as demonstrated in this study (Singer et al., 2019), neither butyrate supplementation nor administration of the PPARγ agonist rosiglitazone led to epithelial PPARγ activation in the P5 gut, thus suggesting either immaturity of the neonatal gut or differential mechanisms to regulate oxygen content in the neonatal gut.
Beyond the regulation of oxygen, many other factors are likely differentially regulated or not fully developed in the neonatal gut, such as pH; host digestion of complex carbohydrates, proteins, and lipids; availability of digestive enzymes; and gut motility (Dallas et al., 2012). All these factors shape the neonatal gut environment and select for bacterial species/strains that survive and thrive in such an environment. Furthermore, preterm or VLBW infants frequently have gastrointestinal complications such as abdominal distention, bloating, indigestion, malabsorption, and subsequent inflammation. It is still unclear whether these GI-related issues are consequential to altered gut microbiome in these infants, or whether the gut microbiome changes in such a “hostile” environment. In fact, preterm infants have been reported to have a higher abundance of gram-negative Enterobacteriaceae than term infants, which triggers and propagates gut inflammation in these vulnerable infants by releasing pro-inflammatory lipopolysaccharide (LPS). This has been suggested to contribute to the high incidence of necrotizing enterocolitis (NEC) in severely premature infants (Denning and Prince 2018). Gut damage in NEC might lead to perforation of the gut and, as a result, dissemination of gut bacteria. Systemic dissemination of virulent pathobionts such as Kp-43618 (which is within the Enterobacteriaceae family) in NEC infants would be fatal.
Singer et al., (2019) unraveled at least two gut commensal species, L. murinus and E. coli, in inhibiting the expansion of Kp-43618. However, the mechanistic underpinnings of colonization resistance conferred by these two bacterial species against Kp-43618 are still undefined. Given E. coli and K. pneumoniae are both Enterobacteriaceae, nutritional competition by E. coli might be one mechanism to outgrow K. pneumoniae. However, gram-positive lactic-acid-producing L. murinus vastly differs from K. pneumoniae or E. coli. Common denominators between L. murinus and E. coli for their effective inhibition of K. pneumoniae could possibly be identified by analyzing luminal metabolites in a L.-murinus-enriched (Figure 1A) or Enterobacteriaceae-enriched (Figure 1C) neonatal gut. In addition, an interesting observation from this study is that the virulence of K. pneumoniae appeared irrelevant for its ability to expand and disseminate from a dysbiotic neonatal gut, but only the virulent strain Kp-43618 caused sepsis. Does gut dysbiosis affect intra-species selection of virulent strains of the same species that ultimately become pathobionts? It is still poorly understood how opportunistic pathobionts such as Kp-43618 emerge from a dysbiotic gut. Virulence factors could be acquired through horizontal gene transfer. Another possibility would be better adaptability of virulent strains to a dysbiotic state of the gut that these strains thrive and expand whereas others succumb to the “hostile” environment (Zeng et al., 2017). Understanding and identifying factors that favor the emergence of virulent pathobionts is critical for prevention of potentially fatal sepsis caused by gut pathobionts in vulnerable infants.
In conclusion, vaccination in pregnancy against pathogens such as GBS appears to be rational and have seen some degree of protection against EOS. However, gut pathobionts are essentially “commensals” in an unperturbed gut; over immune-reaction to gut bacteria through vaccination would cause unwanted collateral damage to the gut. Strategies such as probiotics given to the mother either before or after labor, or given directly to vulnerable infants, seem promising. However, the findings by Singer et al. (2019) provide critical insights into the fragility and complexity of the neonatal gut that is influenced by many variables (e.g., antibiotic use, breastmilk versus formula feeding, prematurity, immature immune system, and/or lack of digestive enzymes) (Dallas et al., 2012). Unraveling mechanisms to suppress pathobionts in the neonatal gut, either with or without damage due to prematurity or dysbiosis, would be of paramount importance in the effort to prevent neonatal sepsis caused by gut pathobionts. A better understanding of the complex and unique gut environment as well as dynamically changing gut microbiome in neonates is needed in order to design “precision probiotics” that colonize and persist in the gut long enough to “reverse” the dysbiotic gut. Successful and sustained colonization of probiotics is critical for probiotics to confer benefits to facilitate gut maturation and minimize the likelihood of sepsis caused by opportunistic gut pathobionts in neonates.
ACKNOWLEDGMENTS
MYZ was supported by NIH grant 5 K01 DK114376 and startup funds from the Gale and Ira Drukier Institute for Children’s Health and Children’s Health Council at Weill Cornell Medicine.
REFERENCES
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703. [DOI] [PubMed] [Google Scholar]
- Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, et al. (2017). Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas DC, Underwood MA, Zivkovic AM, and German JB (2012). Digestion of Protein in Premature and Term Infants. J. Nutr. Disord. Ther 2, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning NL, and Prince JM (2018). Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol. Med 24, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville JM, and Moss TJ (2013). The immune consequences of preterm birth. Front. Neurosci 7, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, et al. (2017). The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JR, Blosser EG, Zindl CL, Silberger DJ, Conlan S, Laufer VA, DiToro D, Deming C, Kumar R, Morrow CD, et al. (2019). Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat. Med 25, 1772–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Embleton ND, Marrs ECL, Smith DP, Fofanova T, Nelson A, Skeath T, Perry JD, Petrosino JF, Berrington JE, and Cummings SP (2017). Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID 3rd, Hale EC, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (2011). Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 127, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng MY, Inohara N, and Nuñez G (2017). Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 10, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


