Abstract
Objective:
Although there are several evidence-based treatments available to increase Bone Mineral Density (BMD) and reduce fracture risk in aging men and women, there are still uncertainties regarding which treatments are efficacious in reducing lifetime fracture risk in women with Anorexia Nervosa (AN).
Methods:
Medline, PsychInfo, Embase and the Cochrane Database were searched for English Language Studies. Inclusion criteria were studies of females of any age with AN who received pharmacological treatment with the primary aim to increase BMD or reduce fracture risk. Data were extracted from each study regarding pharmacological treatment and dosage used, BMD and bone formation marker outcomes; and participant characteristics including age, Body Mass Index (BMI), duration of AN, and duration of amenorrhea.
Results:
675 studies were reviewed, of which 19 fit the inclusion criteria and were included in the final review, investigating a total of 1119 participants; 10 of the 19 included studies were double-blind RCTs. The remaining studies consisted of prospective observational studies, a retrospective cohort study, a case-control study and five non-randomised control trials. Bisphosphonates were effective in increasing BMD in adult women with AN, while estrogen administered transdermally resulted in significant increases in BMD in mature adolescents with AN. Administration of oral contraceptives (OC) did not significantly increase BMD in randomised or controlled trials, however, lifetime OC use was associated with higher spinal BMD.
Conclusion:
Future research should clarify the safety of long-term bisphosphonate use in adult women with AN, and verify that transdermal estrogen replacement increases BMD in women with AN.
1. Introduction
1.1. Eating disorders
Eating disorders (ED) are mental health conditions associated with negative outcomes and the highest mortality rate among psychiatric disorders [1]. Anorexia Nervosa (AN) and Bulimia Nervosa (BN) are the most studied EDs [2] and are associated with both physical and mental co-morbid conditions [3]. An important physical co-morbidity is low Bone Mineral Density (BMD), and increased incidence of secondary osteoporosis [1,4].
AN has been associated with low BMD, impaired bone structure and an increased risk of bone fractures [3,5–8]. Fractures are associated with significant pain, disability and loss of work days, and AN patients are 7 times more likely to have bone fractures than age-matched healthy women [9]. BMD in AN has been found to be reduced by at least 1.0 SD at one or more skeletal sites in 92% of patients and by at least 2.5 SD in 38% of adult women with AN in a community sample [10]. Adolescents with AN are also at risk for low BMD, with approximately 30% of adolescent girls reporting sustaining a fracture [5,7] and 50% reported to have BMD 1 SD below the mean at one or more skeletal sites [6]. Both restricting sub-type (AN-R) and the binge-purge subtype (AN-BP) of AN are associated with reductions in spine, hip and limb BMD [11] and increased fracture risk [12]. A recent meta-analysis found normal weight women with BN to have spinal BMD significantly lower than healthy control women [13], suggesting that weight loss alone may not account for the deleterious effects of an ED on BMD.
Osteoporosis is a condition characterised by skeletal fragility from a decrease in both bone quality and quantity. Excessive food restriction and malnutrition in individuals with AN can lead to low BMD and secondary osteoporosis at a young age [14]. Weight gain and recovery from AN are known to be the strongest predictor of an increase in BMD at all sites, resulting in mean annual increases in BMD of 3.1% at the spine and 1.8% at the hip [15]. However, the onset of AN during adolescence can impair the acquisition of peak bone mass, resulting in lower BMD throughout adult life [14] and may limit increases in BMD following recovery [16]. Of concern, studies in adolescents with AN demonstrate that weight gain and menstrual recovery allow for some improvement in bone accrual rates at the spine and whole body, but not to the extent observed in normal-weight controls [17].
BMD and secondary osteoporosis are influenced indirectly by factors such as age, sex, ethnicity, BMI, physical activity, muscle function, and calcium and vitamin D intake. Weight-bearing exercise has been suggested to have positive effects on BMD, whereas vigorous exercise may have deleterious effects on BMD in AN [14]. Smoking and alcohol consumption are known to have a negative effect on both directly and indirectly on BMD [18]. Although the risk of fracture cannot be accurately predicted, fracture risk of an individual can be inferred through the measurement of BMD using DXA (dual energy x-ray absorptiometry) or pQCT (peripheral quantitative computed tomography) in combination with clinical features, for example using tools such as the WHO (World Health Organisation) FRAX calculator (https://www.shef.ac.uk/FRAX/) in older women.
Biochemical bone turnover markers can indicate the efficacy of a treatment in decreasing bone resorption or increasing bone formation over time in women with AN in comparison to healthy women. These include bone formation markers: osteocalcin (OCN), serum procollagen type I, N-terminal propeptide (tP1NP); and, bone resorption markers: serum collagen type I cross-linked C-telopeptide (CrossL) and the soluble receptor activator of nuclear factors-κB ligand (sRANKL). The receptor activator of nuclear factors-κB ligand (RANKL) is a potent stimulator of bone resorption, operating by binding the receptor activator of nuclear factors-κB (RANK) in the cell membrane of osteoclast precursors [19]. Adolescents with AN are known to have decreases in biochemical markers of both bone formation and resorption, indicative of reduced bone turnover [20,21], adults with AN have decreased bone formation and increased bone resorption markers, indicative of an uncoupling of bone turnover, both of which lead to reductions in BMD [22,23]. In preliminary treatment trials, markers of bone turnover can be a useful measurement of the potential efficacy of treatment in increasing BMD and reducing fracture risk [24].
The UK-based NICE (National Institute for Health and Care Excellence) guidelines recommend bisphosphonates for prevention of fractures in postmenopausal women, but there are no formal guidelines to treat low BMD and to prevent fractures in pre-menopausal women with AN [25]. Weight gain and the resumption of menstrual function are strong predictors of increased BMD in women with AN [24]; however early intervention to prevent the loss of BMD and pharmacological interventions to preserve and increase BMD in chronic AN are necessary when low weight and amenorrhea persists throughout an ED. For this reason, this review will focus on potential pharmacological treatment options for osteoporosis in AN.
1.2. Aim
The specific aim of the review was to systematically review pharmacological bone active agents that increase BMD in women with AN.
2. Method
We followed the analytical methods and standards established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) group for systematic reviews and meta-analyses [26]. The PICO Framework for using evidence from patient groups (P), the intervention process (I), comparison groups (C) and outcomes of an intervention (O) to answer a clinical question was used [27].
2.1. Study identification
The search strategy for this review was designed by a University of London librarian and the primary reviewer (LR). We searched electronic databases MEDLINE (1), PSYCHInfo (2), EMBASE (3) and Web of Science (4). Broadly, the search terms were categorised into three primary areas: (1) Eating Disorders, (2) Risk for Secondary Osteoporosis, and (3) Treatment options. Additional hand searches were conducted and reference lists of eligible studies and previous systematic reviews and meta-analyses in this area were reviewed. The final literature search was conducted on 3rd March 2017, and this search is reported in a PRISMA diagram in Fig. 1 and the review was registered with PROSPERO under registration number: CRD42015026503.
Fig. 1.
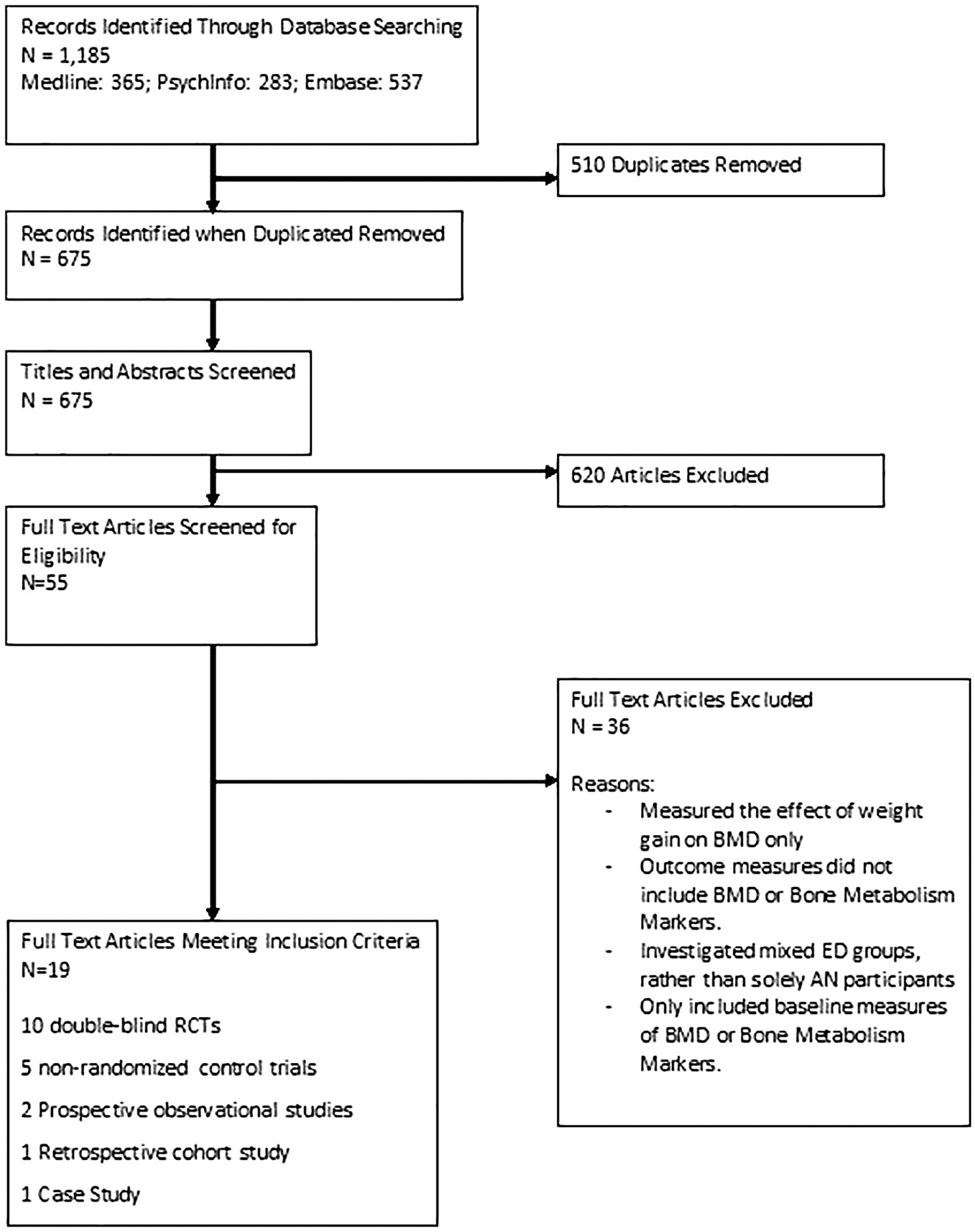
Flow diagram of primary study selection.
The search strategy used terms for eating disorders (Anorexia Nervosa* and Eating Disorders*), Osteoporosis (Osteoporosis*, Bone Loss*, Bone Density, Bone Mineral Density, Bone Mineral Content, Bone Mass, Fracture), and ‘Treatment(s)’. MeSH was used to explore related terms where available (*). Related terms explored by MeSH include: ‘Feeding and Eating Disorders’ (1,3), ‘Feeding and Eating Disorders of Childhood’ (1), ‘Bone and Bones’ (1,2,3), ‘Bone Resorption’ (1,3) and ‘Bone Density’ (1,2,3), ‘Musculoskeletal system’ (2,3). The search was limited to publications in English.
Published articles were eligible if they investigated a treatment to increase BMD in participants with AN. Patients with a diagnosis of EDNOS (eating disorder not otherwise specified) or BN were excluded due to the lack of available data. Only studies on female participants were included. We did not limit inclusion by study type; RCTs (Randomised Control Trials), Controlled Trials, Longitudinal Cohort Published studies and one Case Study, with both independent and overlapping samples of participants were included in the initial review. Study eligibility was then assessed by two authors (LR and VA) who discussed the inclusion criteria and reached a consensus based on the a priori criteria that studies report independent samples (no sample is used in multiple reports) of participants with AN and a corresponding healthy control group.
The study selection process included initial screening of titles and abstracts against the inclusion criteria using EndNote, and screening of full papers against the inclusion criteria. Certain studies that otherwise met inclusion criteria were excluded for reasons such as participants with AN being grouped with other ED participants.
2.2. Inclusion criteria
2.2.1. Participants
Only women with AN who received pharmacological treatment with the primary aim to increase BMD or reduce fracture risk were included. There were no restrictions on the current age of participants or duration or age of onset of AN. AN was defined according to the DSM-III, DSM-IV or ICD-10 diagnostic criteria in 15 of 19 studies, with no specified diagnostic criteria in the remaining 4 studies.
2.2.2. Treatments
Pharmacological treatments with the aim of increasing BMD and reducing fracture risk in participants with AN were included.
2.2.3. Outcome measures
Data on BMD, measured by DXA scanning, or fractures were included in this review and any data regarding biological markers (described as markers of bone formation, bone metabolism or bone regeneration and remodelling markers) were also included as outcome measures in this review.
2.3. Exclusion criteria
Studies that solely used psychological or weight-gain oriented interventions were excluded from this review, and also those studies which did not include BMD or Bone Metabolism Markers as outcome measures were excluded. Studies which investigated mixed ED groups, rather than solely AN participants were also excluded.
2.4. Study selection
Two reviewers (LR and VA) independently screened titles and abstracts of eligible studies selected using the pre-planned search strategy. The full text of all seemingly eligible studies was evaluated and reviewers worked independently to determine their eligibility for the review. Disagreements were discussed and resolved.
2.5. Data collection
Using a data extraction form designed by the reviewers for the purpose of this study, the reviewers extracted data on each of the studies regarding the population studied, the treatment used, and the methodology. The BMD data were extracted for pre- and post-treatment where available, and the method of measurement was recorded. Authors were contacted if relevant data were missing.
2.6. Covariates
This review also investigated BMI, age, duration of disorder, comorbid physical conditions, comorbid mental health conditions, and duration of amenorrhea in the women in each of the reviewed studies. Qualitative assessment investigated trends in the data across the included studies.
2.7. Quality assessment
Both reviewers independently assessed the quality of the studies using the EPHPP (Effective Public Health Practice Project) quality assessment tool [28], which assesses selection bias, study design, confounders, blinding, data collection method, and withdrawals and dropouts. Each study is assigned a global rating of strong = 1, moderate = 2 or weak = 3 on Table 1. The two reviewers (LR) (VA) highlighted discrepancies in quality rating and ultimately reached an agreement on all assessments.
Table 1.
Study characteristics.
| Study | Pharmacological agent | Dose | Type of study | Follow-up | Outcome measures | Sig. change in outcome measure(s) | Quality assessment | Grade of evidence |
|---|---|---|---|---|---|---|---|---|
| Bloch 2012 | DHEA | 100 mg/day | Double-blind placebo controlled RCT | 6 months | Total body Spinal Hip Femoral Neck |
NS | 1 | B |
| DiVasta 2012 | DHEA OC: EE/levonorgestrel |
50 mg 20 μg/0.1 mg |
Double-blind placebo controlled RCT | 6, 12 and 18 months | Lumbar spine Hip Whole body |
Stabilising effect | 2 | B |
| Fazeli 2014 | Teriparatide (TPt) | 20 μg/day SC | Double Blind RCT | 3 and 6 months | Posteroanterior spine Lateral Spine Hip Femoral neck |
Sig. (spinal only) | 1 | A |
| Golden 2002 | OC: EE/progestin | 20–35 μg EE/day | Prospective observational study | 11.4–23.1 months | Lumbar spine Femoral neck |
NS | 2 | C |
| Golden 2005 | Alendronate | 10 mg/day | Double-blind placebo controlled RCT | 3, 6, 9 months and 1 yr | Lumbar spine Femoral neck |
NS | 2 | B |
| Gordon 1999 | DHEA | 50, 100, or 200 mg/day | Controlled trial | 3 month | Lumbar spine Total body Femoral neck |
NS | 2 | C |
| Grinspoon 1996 | rhIGF1 | 100 or 30 μg/kg/day | Controlled trial | 6 days | OC PICP PYRX DPYRX NTX |
Sig increase in all markers (100 μg/day) Sig, increase in PICP only (30 μg/day) | 2 | B |
| Grinspoon 2002 | rhIGF-1 EE with rhIGF-EE+ placebo |
30 μg/kg SC twice/day 35 μg/day + 30 μg/kg SC twice/day 35 μg/day |
Double-blind placebo controlled RCT | 12 weeks | Lateral spine BMD Hip BMD Femoral neck BMD Total body BMD |
NS | 2 | B |
| Iketani 2003 | MED | 45 μg/day | Controlled trial | 0.9 yr | Lumbar spine | Stabilising effect |
2 | C |
| Karlsson 2000 | Estrogen replacement therapy | Various | Retrospective cohort study | 4.3 yr (1–16 yr range) | Lumbar Spine Femoral neck |
NS | 2 | C |
| Misra 2009 | RhIGF1 | 30–40 mcg/k twice daily | Double-blind placebo controlled RCT | 7–9 days | IGF1 P1NP CTX |
Sig. increase in IGF1 and P1NP | 1 | A |
| Misra 2011 | Transdermal: 17b-estradiol with cyclic progesterone Oral: EE + progesterone | 100 mg/day 3.75 mg/7.5 mg/11.25 mg/day | Double-blind placebo controlled RCT | 18 months | Lumbar spine Hip |
Sig. | 1 | A |
| Miller 2011 | Risedronate (R) Transdermal testosterone (T) Combination |
35 mg/week 150 μg/day R:35 mg/week T: 150 μg/day |
Double-blind placebo controlled RCT | 12 months | Spinal BMD Hip, and radius BMD Body composition |
Sig. (R) | 1 | A |
| Miller 2004 | Risedronate | 5 mg/day | Controlled trial | 6 months, 9 months | Spinal BMD | Sig. | 1 | A |
| Miller 2005 | Transdermal testosterone | 150 μg/300 μg/day | Double-blind placebo controlled RCT | 3 weeks | Spinal BMD | NS | 1 | B |
| Munoz 2002 | OC: EE | 30 μg/day | Controlled trial | 12 months | Spinal BMD | NS | 2 | C |
| Seeman 1992 | OC: Various | Various | Prospective observational study | 61 months ± 9% duration of illness | Spinal BMD Femoral Neck BMD |
Sig. (spinal) | 2 | C2 |
| Shibli-Rahhal 2012 | Teriparatide (TPt) | 20 mcg/day | Case study | 2 yr | Lumbar spine Femoral neck |
Sig. | 2 | D |
| Strokosch 2006 | OC: Norgestimate (NGM) OC: EE |
180–250 μg/day 35 μg/day |
Double-blind placebo controlled RCT | 13 consecutive 28 day cycles | Lumbar spine BMD |
NS | 1 | B |
Study characteristics of included studies in the systematic review. ‘Spinal’ refers to measurement of BMD at the lumbar spine. BMD = Bone Mineral Density, aBMD = areal Bone Mineral Density. (n) = number, SC = subcutaneous injection, NS = non-significant, Sig. = statistically significant (p < 0.05), AN = Anorexia Nervosa, HC = Healthy Controls, BMI = Body Mass Index, Tpt = teriparatide, EE = ethinyl estradiol, ERT = estrogen replacement therapy. NGM = Norgestimate, OC = oral contraceptives, MED = Menatetrenone.
Eight of the included studies received a global assessment of ‘strong’, which indicated that they did not receive a ‘weak’ rating on any of the dimensions assessed. The remaining ten studies received a ‘moderate’ rating, which indicates that they received a ‘weak’ rating on only one of the six study dimensions. Quality assessment scores are displayed in Table 1.
2.8. Data extraction and qualitative assessment
Data was extracted using a pre-designed data extraction form by two reviewers, first piloted using five studies to ensure comparable results. Data extracted included age, gender, BMI, menstrual status, comorbidities, ethnicity, duration of AN, duration of amenorrhea (both pre- and post-treatment), treatment focus and comparator (including type of pharmacological treatment and characteristics of comparator treatment), and outcomes (BMD and bone metabolism markers).
The studies were qualitatively compared based on the effect on BMD or biological markers of bone metabolism of the treatment investigated. A meta-analysis was not possible due to the heterogeneity in the methods used and the treatments investigated across the included studies.
2.9. Grade of evidence
Evidence obtained from each study was graded using NICE Guidelines for Anxiety Disorders [29] with the WFSBP Guidelines system [30], described by Bandelow et al. (Table 1).
3. Results
Authors reviewed 675 studies and 19 met the inclusion criteria and were included in the final review. These included 1119 participants, of which 792 had AN at the time of the treatment and the remainder were healthy controls. Table 1 reports the study characteristics for all included studies. Ten of the nineteen included studies were double-blind RCTs [31–40]. The remaining studies consisted of prospective observational studies [14,41], a retrospective cohort study [42], a case-control study [43] and five non-randomised control trials [23,44–47]. One study was conducted in Japan [46], one in Spain [45], one in Israel [37] and two in Australia [14,42] and all other studies were conducted in the USA. The mean length of follow-up ranged from 3 months [31] to 34.5 months [41], with the majority of studies following participants for at least one year.
3.1. Baseline characteristics
A total of 399 participants with AN received a treatment aiming to increase BMD, and 379 participants with AN received placebo treatment. There were 316 healthy control participants across the studies who received no treatment and had no history of an ED. Participants in this review ranged from 11 to 37 years of age, with a duration of AN ranging from 6.1 months to 6.6 years, and duration of amenorrhea ranging from 9.7–33 months, although amenorrhea duration was not recorded in all studies. All included studies investigated women or adolescent girls. The primary source of recruitment was through healthcare referrals and advertisements. Baseline characteristics of all participant groups are reported in Table 2.
Table 2.
Participant characteristics.
| Study | Groups | N | Age (mean baseline) | M BMI Pre (kg/m2) | Duration of amenorrhea (months) | Duration of AN (months) | Diagnostic criteria | Weight gain strategies | Change in BMI or Weight |
|---|---|---|---|---|---|---|---|---|---|
| Bloch 2012 | AN - DHEA AN - Placebo |
15 11 |
26.6 | 17.75 | DSM-IV | Ongoing psychotherapy which included weekly individual dynamic psychotherapy and group cognitive/supportive psychotherapy + nutritional assessment | NS change in BMI | ||
| DiVasta 2012 | AN - DHEA + OC AN - Placebo |
31 29 |
18 18.3 |
18.1 17.8 |
11 | 12 | Participants were enrolled in outpatient therapy for an ED at Boston children’s hospital. | Among trial completers, 5 lost weight (1.4–5.3 kg), 1 subject showed no change, and the remainder gained up to 21.7 kg. | |
| Fazeli 2014 | AN - TPT AN - Placebo |
10 11 |
47 47 |
17.6 16.6 |
N = 8 N = 7 |
292.8 216 |
|||
| Golden 2002 | AN - EE/progestin AN - Treatment controls |
22 28 |
17.5 16.3 |
17.1 16.7 |
16.5 | 29.8 | DSM-IV | All subjects were in outpatient treatment for EDs. | |
| Golden 2005 | AN - Alendronate AN - Placebo |
15 17 |
16.9 16.9 |
16.3 16.4 |
20.1 19.9 |
25.7 34.7 |
DSM-IV | 15 of 29 subjects achieved a weight 85% of standard body weight | |
| Gordon 1999 | AN - DHEA | 15 | 17.3 | 17.3 | 20.9 | 29.1 | DSM-IV | Subjects enrolled in ED program in Boston Children’s Hospital | NS change in BMI |
| Grinspoon 1996 | AN - rhIGF1 100 μg AN - rhIGF1 30 μg |
23 | 23 | 16.3 | 22 | 60 | DSM-IV | NS change in BMI | |
| Grinspoon 2002 | AN - rhIGF-1 AN - Placebo |
29 30 |
25.6 | 16.9 16.3 |
DSM-IV | NS change in BMI | |||
| Iketani 2003 | AN - MED + AN - MED− HC |
10 11 12 |
22.1 21.3 22.2 |
13.6 13.7 19.6 |
3.6 yr 3.5 yr |
3.9 yr 4.1 yr |
DSM-IV | Sig. increase in BMI in both groups, no differences between groups | |
| Karlsson 2000 | AN - ERT AN - No treatment AN - Recovered HC |
58 77 26 205 |
28.4 25.9 27.3 27.3 |
15.4 15.6 20.3 23.1 |
4.5 yr 5.4 yr 3.5 yr |
ICD-10 | |||
| Misra 2009 | AN - rhIGF1 AN - Placebo |
10 10 |
16.2 16.3 |
17.2 17.5 |
6.1 9 |
DSM-IV | NS change in BMI | ||
| Misra 2011 | AN - E + AN - E− HC |
31 30 29 |
16.5 15.6 |
17.4 21.4 |
0.9 yr | DSM-IV | All girls with AN were under the care of multidisciplinary treatment teams organized by their primary providers. | ||
| Miller 2011 | AN - Risedronate-Testosterone AN - Risedronate AN - Testosterone AN - Placebo |
20 20 19 18 |
25.2 25.3 27.1 26.9 |
17.8 17.6 17.5 17.9 |
6.3 5.1 6.6 5.2 |
DSM-IV | Sig increases in weight in both groups, no differences across groups. | ||
| Miller 2004 | AN - Risedronate AN - control |
10 14 |
28.6 26.9 |
44 kg 42 kg |
33 23 |
DSM-IV | No sig increase in BMI in exp. group, but control group BMI sig. Increased at 9 months (p < 0.01) | ||
| Miller 2005 | AN - testosterone AN - Placebo |
24 9 |
25 22 |
16.9 16.6 |
DSM-IV | NS change in BMI | |||
| Munoz - Cavalo 2007 | AN-E + AN - E− HC |
10 10 19 |
15.4 | Sig. increase in BMI in Group 2 (patients with AN for < 1 yr) | |||||
| Seeman 1992 | AN OC AN OC-HC Primary amenorrhea |
37 16 52 12 |
24.4 | 17.2 ± 0.8 | 66.5 ± 15.5 | DSM- III- R | NS difference in BMI across groups (cross-sectional) | ||
| Shibli-Rahhal 2012 | AN TPt | 1 | 52 | 14.3 | 4 yr | 72 | Weight restored prior to treatment. NS change in BMI following TPt. | ||
| Strokosch 2006 | AN - NGM/EE AN - Placebo |
53 59 |
15.2 15.1 |
17.94 | 9.7 (m) | DSM-IV | NS change in BMI |
Baseline participant characteristics. AN = Anorexia Nervosa, HC= Healthy Controls, BMI =Body Mass Index, Tpt= teriparatide, EE =ethinyl estradiol, ERT =estrogen replacement therapy. NGM = Norgestimate, OC = oral contraceptives, MED= Menatetrenone.
3.2. Weight change and BMI
Psychological or weight gain interventions reported and conducted alongside those investigated in this study are presented in Table 2. Five studies reported significant increases in BMI in participants following the study intervention [32,35,45,46,48], with the remaining studies reporting non-significant differences or failing to disclose a change in BMI.
3.3. Outcome measures
The primary outcome measure was spinal BMD in all but three studies, which measured markers of bone metabolism [23,40]. Secondary outcome measures included hip, femoral neck, radius and total body BMD, measures of body composition, and biological markers of bone turnover and fracture incidence. All of the included studies used DXA scanning for BMD measures. All studies controlled for change in body weight, BMI or body composition when reporting outcome measures.
3.4. Bisphosphonates
Three of the included studies investigated the effect of bisphosphonates on BMD in women with AN; two of which investigated the bisphosphonate risedronate in adult women with AN [35,47] and one which investigated alendronate in adolescents with AN [32]. Adolescents with AN failed to demonstrate an increase in spinal BMD following 10 mg orally administered alendronate treatment, the site most significantly affected in AN [32,35,47], though femoral neck BMD did significantly increase following one year of treatment.
Risedronate was administered at a dose of either 5 mg orally each day for 9 months [47] or 35 mg orally weekly for 12 months and either combined with, or compared to transdermal testosterone [35] in adult women with AN. Both studies using risedronate saw a significant increase in spinal BMD (4.9 ± 1% and 3.8 ± 1.8%) in participants with AN. When risedronate was compared to transdermal testosterone, only risedronate increased BMD and suppressed the bone turnover markers serum c-terminal telopeptide (CTX) and procollagen type 1 amino-terminal propeptide (P1NP).
3.5. Hormones
3.5.1. Estrogen
Of the seven included studies that measured the effect of estrogen on BMD, one cross-sectional study found that AN women who took OCs had on average a higher BMD than those who had never taken OCs [14]. One study found significant increases in hip and femoral neck BMD following OC treatment (35 μg EE; 180–250 μg Norgestimate) for 13 consecutive 28-day cycles, however, these increases were no longer significant after controlling for weight changes [34]. Another study found that when oral contraceptives containing EE (20 μg/day) were combined with dehydroepiandrosterone (DHEA (50 mg/day)), subjects with AN had a stabilisation of femoral neck BMD, compared to a decrease in BMD in placebo treated AN women [38]. Two RCTs have shown that EE administered orally in high doses (up to 35 μg) for over one year is not effective in increasing BMD in AN [31,34].
In contrast to orally administered ethinyl estradiol, physiologic replacement doses of transdermal 17-β estradiol, increased spinal and hip BMD in adolescent women with AN [36], although complete ‘catch up’ to a comparable BMD in healthy controls did not occur, likely because other hormonal deficits persisted [49]. In this RCT, 110 adolescents with AN were randomised to 100 μg of 17-β estradiol (with cyclic progesterone), low incremental physiological doses of oral EE (3.75 μg daily from 0 to 6 months, 7.5 μg from 6 to 12 months, 11.25 μg from 12 to 18 months) or placebo for 18 months. These physiological doses of estrogen significantly increased spinal and hip BMD in adolescents with AN to approximate bone accrual rates in normal-weight healthy controls. The effect was primarily driven by the group receiving transdermal 17-β estradiol.
3.5.2. DHEA
Three of the included studies measured the effect of DHEA (dehydroepiandrosterone) on BMD, with no significant increases in BMD found at any skeletal sites. Two reported an increase in biological markers including deoxypiridinolyne (DPD) and osteocalcin following administration of 100 mg of oral DHEA daily [37]. Decreases in NTx (N-terminal telopeptide) in response to 50 mg/day of oral DHEA in another study [44] indicate a decrease in bone remodelling. DiVasta (2012) combined an oral contraceptive with oral DHEA (50 mg + 20 μg EE/ day) and reported a stabilisation of femoral neck BMD in the experimental group in comparison to a trend for a decrease in all BMD sites in the AN placebo controls [38].
3.5.3. Testosterone
Although at baseline, levels of testosterone were highly correlated with BMD [35,50], BMD did not increase following transdermal treatment with replacement doses of testosterone (to attain testosterone levels in the upper half of the normal range for women) over 3 weeks or 12 months compared to placebo, and markers of bone metabolism did not significantly change either [35,50].
3.5.4. Teriparatide
Shibli-Rahhal et al. found a 21% increase in BMD and no reoccurrence of fractures following 2 years or teriparatide in a 52 yr old AN patient [43]. Fazeli et al. investigated the effect of teriparatide (TPT; human PTH1–34) (20 μg/day SC) vs. placebo on BMD, markers of bone metabolism, and IGF-1 in older mature women with AN in a randomised controlled trial. They confirmed the findings of Shibi-Rahhal et al. and found a 6–10% increase in spine BMD after 6 months of teriparatide and an increase in serum P1NP levels in the TPT group [39].
3.5.5. Recombinant human (rh) IGF-1
RhIGF-1 replacement results in an increase in bone formation markers in both adolescents and adults with AN [23,31,40]. Furthermore, increases in both spine and hip BMD are found when rhIGF1 is combined with an estrogen–progesterone combination pill in a placebo-controlled trial (1.8% ± 0.8% vs. −1.0% ± 1.3% at the spine, P < 0.05) [31].
3.5.6. Nutritional treatments
One study investigated the effect of 45 μg/day of Menatetrenone (MED) (vitamin K2) on lumbar spine BMD over a 9 month follow-up period [46]. BMD decreased in women with AN, but MED slowed the decrease in BMD in comparison to placebo controls.
4. Discussion
Despite there being a well-established treatment regimen to protect and increase BMD and reduce fracture incidence in post-menopausal women and older adult males, only nineteen studies investigating pharmacological treatments to increase BMD in women with AN were identified for this review. It was not possible to conduct a meta-analysis of results due to the heterogeneity in the research methods and methods of treatment, and it was not possible to investigate treatment efficacy in any other EDs due to the lack of available data. In order to utilise the available data, the primary outcome measures investigated were BMD values measured by DXA scanning, rather than fracture incidence, which may be a more clinically relevant measure. We uncovered no RCTs or controlled trials aiming to increase BMD in males with AN, despite a new, more gender neutral DSM-5 diagnostic criteria and increasing independent data on socioeconomic factors, prenatal influences, clinical characteristics, assessment, and mortality for EDs in males [51]. Furthermore, very little evidence of the limitations and potential harm caused by long-term use of the treatments investigated in women with AN exists.
A recent meta-analysis suggested that women with AN are likely to have an increased fracture risk of 150–300% based on significantly lower BMD values than healthy control women [13]. These women present unique challenges in treatment of low bone density as we cannot assume that treatments designed to treat the decline in BMD resulting from aging will be efficacious in this group. Furthermore, it is established that weight gain and restoration of menstrual function should be the first line of management when treating women with AN [49] although due to the enduring nature of an ED, alternative therapies should be available while in pursuit of full recovery.
In adult women, the most significant increases in BMD identified in this review were from bisphosphonate therapy [35,47]. No significant increase in BMD was observed following administration of oral contraceptives, transdermal testosterone or oral DHEA alone. Conversely, 100 μg of 17-β estradiol (with cyclic progesterone) administered transdermally did increase spinal and hip BMD in mature adolescents with AN [36]. Nutritional treatment, including Vitamin K2 had a beneficial effect in reducing the loss of BMD in AN participants, but this effect was not strong enough to entirely prevent BMD loss in AN participants over the 9 month trial [46].
4.1. Pharmacological treatments investigated in this review
4.1.1. Bisphosphonates
Bisphosphonates are prescribed to reduce fracture risk in post-menopausal women but have been found to have inconsistent benefits in women with AN [32,35,47].
Both risedronate and alendronate administered orally resulted in significant increases in BMD at the hip [32] and at the spine [35,47] in adults and adolescents with AN. However, adolescents receiving alendronate failed to show BMD increases at the spine [47].
Furthermore, the long-term effects of bisphosphonates are currently unknown and an inadequate number of trials exist to confirm their safety. These drugs decrease the activity of the bone-remodelling unit, and have the potential to reduce bone modelling and shaping when used over time [32], although data to date are reassuring. Oral bisphosphonates have been associated with gastrointestinal distress and musculoskeletal pain, which could be exaggerated in AN due to their poor nutritional state and low BMI [52].
Although bisphosphonates are effective in increasing BMD, fractures can still occur due to reduced tensile strength, possibly resulting from low bone turnover in the restructured bone [53]. For this reason, bisphosphonates have been associated with both atypical fractures at the femoral neck with both oral and IV administration in adult women [54,55] and also osteonecrosis of the jaw [56–58], suggesting that close monitoring of patients on bisphosphonates is necessary.
Other adverse symptoms include a potential risk to the fetus when bisphosphonates are administered to adolescent girls and young adult women of reproductive age, given their long half-life [59], and uveitis, thrombocytopenia, or esophageal or oral ulcerations in adults [60]. Although adolescents and adult with AN are less likely to become pregnant because of associated hypogonadism, women with a history of AN do become pregnant [61]. Bisphosphonates are currently prescribed on a case-by-case basis for premenopausal women, and the US Drug and Food Administration (FDA) has currently only approved bisphosphonates for premenopausal women on glucocorticoids [62].
4.1.2. Hormones
4.1.2.1. Estrogen.
The revised DSM-5 criteria for AN removed the requirement of amenorrhea, although hypothalamic amenorrhea remains to be common among adolescent and adult women with AN. Suppression of the hypothamamic-pituitary gonadal axis (HPG) prevents energy from being invested in reproduction in a state of semi-starvation [63], and results in estrogen deficiency and varying degrees of menstrual dysfunction, the most severe of which is a state of amenorrhea. Despite a similar loss of BMD in AN compared to post-menopausal women and a shared feature of increased bone resorption, a significant decrease in bone formation is also present in AN [64] suggesting that factors other than estrogen deficiency may also be contributing to low BMD in this condition. These other factors may include deficiencies of hormones such as testosterone, DHEA and leptin, and increases in hormones such as cortisol and peptide YY [65].
Estrogen receptors are present on both osteoblasts and osteoclasts, and the reduction of circulating estrogen resulting from an ED can lead to rapid loss of BMD. Estrogen supplementation has antiresorptive effects, increases BMD and reverses the negative calcium balance observed in postmenopausal women.
In the included studies, OCs administered in pharmacological doses (20–35 μg EE) resulted in, at best, a stabilising effect on bone in RCTs, and on average greater spinal BMD in women with AN who had taken OCs compared to those who had not in a retrospective cohort study [14]. However, transdermal estradiol administration in physiological doses resulted in an increase in spinal and hip BMD Z-scores over 18 months in mature adolescents with AN [36].
Although the reasons for the inconsistent benefit on BMD from OCs remain speculative, one hypothesis is the suppression of systemic IGF-1 secretion following oral estrogen administration as is seen in post-menopausal women due to a first-pass hepatic effect [66,67]. Low incremental doses of EE administered orally to mimic the early pubertal rise in estrogen may be effective in preserving bone mass in young girls who are still growing, as very low oral estrogen doses do not suppress IGF-1 [68]. Transdermal estrogen, which also does not suppress IGF-1, does increase bone density in adolescents with AN [69], although complete ‘catch up’ to a comparable BMD in healthy controls does not occur given that other hormonal deficits persist [49].
4.1.2.2. Testosterone.
Older males, and adolescent males with AN [70] have reduced bone density, which is likely from a decline in testosterone rather than estrogen [71]. Women with AN also have low testosterone levels, which correlate with lower spinal BMD [33]; however, transdermal testosterone administration in women with AN resulted in no significant change in osteocalcin and bone-specific alkaline phosphatase [33], which are markers of bone formation, and there was no increase in spinal BMD when transdermal testosterone was administered without risedronate [35].
Testosterone can be converted to 17 β-estradiol by the P450 aromatase enzyme within bone, and subsequently can exert its effects on bone through estrogen receptors: (ER alpha) or P(ERP) [72]. Testosterone may also have direct bone anabolic effects and may be beneficial in AN as part of a wider hormonal rehabilitation treatment.
4.1.2.3. DHEA.
Suppression of the HPG axis in AN may lead to reduced ovarian DHEA production. Based on available studies, this review found no significant effect of DHEA on BMD when administered alone, but a stabilisation of femoral neck BMD was observed when it was combined with OCs [73]. DHEA is aromatised to weak estrogens by aromatase, and may also have weak bone anabolic effects.
4.1.2.4. Teriparatide.
Parathyroid Hormone (PTH) is secreted almost exclusively from the parathyroid gland and is a single chain, 84 amino acid polypeptide hormone. Vitamin D deficiency leads to increased production of PTH due to its close links with calcium homeostasis, and PTH is also regulated by estrogen [74].
PTH increases osteoblast proliferation and differentiation, and decreases osteoblast apoptosis. Teriparatide (TPT; human PTH) has been found to have positive effects on BMD in post-menopausal women, and in one pilot RCT, older women with AN showed a significant increase in BMD following 2 years of teriparatide (TPT; human PTH1–37) administration [39], later supported by a 6 month RCT (TPT; human PTH1–34) resulting in significant increases in spinal BMD in adults women with AN [39].
4.1.3. rhIGF-1
rhIGF-1 has been associated with increased bone formation markers in both adults and adolescents with AN [23,40], an increase in bone formation markers, but not BMD when combined with an estrogenprogesterone combination pill [31]. An ongoing trial at Massachusetts General Hospital (MGH) aims to both compare and combine rhIGF1 and transdermal E2 replacement to determine if this treatment can enable a ‘catch up’ effect in increasing BMD.
4.1.4. Vitamin K
One study in this review investigated Vitamin K-2 supplementation to increase BMD in Japanese women with AN and found that Vitamin K-2 supplementation (Menatetrenone) reduced the rate of bone loss in AN over a 9 month period, although it was insufficient to cause any increase in BMD [46]. Furthermore, vitamin K deficiency was found to be more pronounced in AN binge-purge subtype than AN restricting subtype, suggesting that vitamin K deficiency should be compared in other ED subtypes and investigated further [75].
4.2. Alternative pharmacological treatments outside of this review and recommendations for future research
4.2.1. Oxytocin and vasopressin
Oxytocin (OT) and arginine vasopressin (AVP) systems are typically associated with social behaviours. However, they are also tightly regulated by estrogen receptors and associated with skeletal homeostasis. OT and AVP may be efficacious in increasing BMD in women with AN, but have yet to be investigated in an RCT.
4.2.2. Leptin
Leptin is a cytokine secreted by adipose tissue that suppresses appetite. It also has a positive impact on GnRH pulsatility and may play a role in the onset of puberty. Leptin administration increases levels of osteocalcin and bone-specific alkaline phosphatase, which are markers of bone formation, in women with hypothalamic amenorrhea. However, this treatment was associated with significant weight loss [76], and so would not be a potential treatment for women with AN.
4.2.3. Selective estrogen receptor modulators
The FDA has cautioned against use of Selective Estrogen Receptor Modulators (SERMs) in pre-menopausal women due to a risk to reproductive health, and so no studies have investigated the effect of SERMs on bone health in women with AN. However, in postmenopausal women vertebral fracture risk was reduced by 30–50% when receiving raloxifene [77], suggesting a promising avenue to investigate in women with AN.
4.2.4. Strontium ranelate
Strontium Ranelate is effective in increasing BMD in post-menopausal women [78], however restrictions have been placed by the European Medical Agency regarding administration to patients with existing heart disease [79]. Based on the association with AN and cardiac and nervous system disturbances [80], this review would not recommend Strontium Ranelate to increase BMD in adults or adolescents with AN.
4.2.5. Nutritional supplementation
4.2.5.1. Calcium.
In women with AN the homeostasis of calcium supply and renewal may be disrupted due to long-term malnutrition and disruption of homeostatic functions resulting from a state of semi-starvation [81]. No increase in BMD was observed in adolescent girls with AN receiving calcium supplementation [82], and a recent meta-analysis has found no association between calcium intake and fracture risk in a health population [83]. However, there have been no trials to investigate the effect of calcium food sources on BMD rather than calcium supplementation alone [84].
4.2.5.2. Vitamin D.
A positive association has been found between serum 25-hydroxy-vitamin D below 50 nmol/l and spinal BMD Z-scores in women with AN, and also with increased alkaline phosphatase levels, suggesting an adaptive osteoblastic reserve [85]. However, there is no evidence to suggest that calcium or vitamin D supplementation increase BMD in AN, and no correlation between calcium or vitamin D intake and BMD in adolescents with AN [86].
4.2.5.3. Combination therapies.
Bisphosphonates, estrogen and estrogen agonists/antagonists are antiresorptive medications that slow the bone loss that occurs in the breakdown part of the remodelling cycle. Teriparatide (PTH1–34) and Natpara (PTH1–84) are anabolic therapies that stimulate bone formation and are currently the only drugs of their kind approved for use by the FDA. Combined therapies include combination antiresorptives, antiresorptive therapies followed by anabolic therapies, anabolic therapies followed by antiresorptive therapies (that take advantage of the bone anabolic window), or combined antiresorptive and anabolic therapies. Ettinger et al. found that post-menopausal women treated with alendronate who had previously been administered raloxifene had a rapid increase in BMD, whereas women who had been on alendronate and switched to raloxifene showed delays in BMD increase in the spine and a reduction in BMD at the hip [77].
4.2.5.4. Strengths of this review.
This comprehensive systematic review summarises the evidence available to date on pharmacological treatment of bone loss in subjects with AN, and reviews potential therapeutic strategies to increase BMD that have not yet been trialled in AN. Although there will surely be differences in these population groups, understanding common mechanisms and responses to treatment can inform both future research and clinical practice. Women with AN are often in a state of severe malnutrition, and a greater understanding of the mechanisms associated with both antiresorptive and anabolic therapies, as well as supplementation of vitamin D, calcium and vitamin K is vital to ensuring that treatments administered to women with EDs are both safe and efficacious.
4.2.5.5. Limitations of the review.
The limitations of the current review centre around the restricted number of studies that have been conducted on adolescents and adults with AN with the aim of increasing BMD.
Firstly, women with AN have very low lean and fat mass, which may result in a lower estimate of bone mass in a DXA scan. Future research should consider using both DXA scanning to estimate bone mass and pQCT (peripheral quantitative CT) scanning to estimate bone size parameters and compartmental volumetric BMD. The research investigating the relationship between pharmacological intervention and bone health in EDs has focused on DXA scanning results or bone metabolism markers, however there is not a direct relationship between biomarkers of bone turnover, BMD and fracture incidence [87]. Future research should focus on both BMD change and fracture risk as an outcome measure to provide a more clinically relevant estimate of the efficacy of treatment.
Too few studies have investigated pharmacological agents to promote an increase in BMD in women with AN to accurately predict response to treatments, thus, each patient should be treated on a case-by-case basis. Furthermore, this review did not find any studies investigating treatments to increase BMD in males with ED; therefore, we cannot predict how male ED patients will respond to possible treatments.
Several recommendations for future research made in this review are based on research conducted on post-menopausal women, but BMD loss in AN is known to be in response to starvation, associated estrogen depletion and changes in other hormones that impact bone such as IGF-1 and cortisol. Therefore, post-menopausal women may not serve as an appropriate model to predict treatment outcomes in AN [88].
5. Conclusions
Although weight restoration may be the most efficacious treatment to regain BMD in AN, the majority of the included studies observed non-significant changes in BMI throughout the study duration, providing validation for the need of alternative pharmacological interventions for this population.
Evidence suggests that BMD in AN is under tight endocrine control; however, studies in AN (and ED in general) targeting specific endocrine hormones that are disrupted are limited. The most common form of intervention included estrogen therapy, and the oral route of administration rather than transdermal, which has recently been found to be more effective in improving bone density than the oral route. Although bisphosphonates are an efficacious treatment in adults with AN, their potential side effects in adolescents and pre-menopausal women remain unknown given their long half-life. Therefore, treatments to restore hormonal imbalances should be developed in order to both increase bone mass and encourage the proper development of bone structure and mechanical strength.
The positive results derived from preliminary trials administering the 100 mcg transdermal 17-beta estradiol patch suggest a promising avenue of future treatment, and warrant further investigation. Furthermore, no studies have investigated combination treatments in AN, which may have a greater effect on BMD than a single treatment alone.
References
- [1].Fairburn CG, Harrison PJ, Eating disorders, Lancet 361 (9355) (2003) 407–416. [DOI] [PubMed] [Google Scholar]
- [2].Association AP, Diagnostic and Statistical Manual of Mental Disorders: DSM-5, ManMag, 2003. [Google Scholar]
- [3].Johnson JG, et al. , Eating disorders during adolescence and the risk for physical and mental disorders during early adulthood, Arch. Gen. Psychiatry 59 (6) (2002) 545–552. [DOI] [PubMed] [Google Scholar]
- [4].Rigotti NA, et al. , Osteoporosis in women with anorexia nervosa, N. Engl. J. Med 311 (25) (1984) 1601–1606. [DOI] [PubMed] [Google Scholar]
- [5].Bredella MA, et al. , Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women, J. Clin. Endocrinol. Metab 97 (4) (2012) E584–E590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bredella MA, et al. , Increased bone marrow fat in anorexia nervosa, J. Clin. Endocrinol. Metab 94 (6) (2009) 2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bredella MA, et al. , Trabecular structure analysis of the distal radius in adolescent patients with anorexia nervosa using ultra high resolution flat panel based volume CT, J. Musculoskelet. Neuronal Interact 8 (4) (2008) 315. [PubMed] [Google Scholar]
- [8].Davies KM, et al. , Reduced bone mineral in patients with eating disorders, Bone 11(3) (1990) 143–147. [DOI] [PubMed] [Google Scholar]
- [9].Rigotti NA, et al. , The clinical course of osteoporosis in anorexia nervosa: a longitudinal study of cortical bone mass, JAMA 265 (9) (1991) 1133–1138. [PubMed] [Google Scholar]
- [10].Grinspoon S, et al. , Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa, Ann. Intern. Med 133 (10) (2000) 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Robinson L, Aldridge V, Clark EM, Misra M, Micali N, A Longitudinal Investigation Into the Association Between Eating Disorders, Eating Disorder Behaviours and Bone Mineral Density in Adult Women, University College London, London, 2017. [Google Scholar]
- [12].Nakahara T, et al. , The effects of bone therapy on tibial bone loss in young women with anorexia nervosa, Int. J. Eat. Disord 39 (1) (2006) 20–26. [DOI] [PubMed] [Google Scholar]
- [13].Robinson L, et al. , A systematic review and meta-analysis of the association between eating disorders and bone density, Osteoporos. Int 27 (6) (2016) 1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seeman E, et al. , Osteoporosis in anorexia nervosa: the influence of peak bone density, bone loss, oral contraceptive use, and exercise, J. Bone Miner. Res 7 (12) (1992) 1467–1474. [DOI] [PubMed] [Google Scholar]
- [15].Miller KK, et al. , Determinants of skeletal loss and recovery in anorexia nervosa, J. Clin. Endocrinol. Metab 91 (8) (2006) 2931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heaney R, et al. , Peak bone mass, Osteoporos. Int 11 (12) (2000) 985–1009. [DOI] [PubMed] [Google Scholar]
- [17].Misra M, et al. , Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1, J. Clin. Endocrinol. Metab 93 (4) (2008) 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eleftheriou KI, et al. , Bone structure and geometry in young men: the influence of smoking, alcohol intake and physical activity, Bone 52 (1) (2013) 17–26. [DOI] [PubMed] [Google Scholar]
- [19].Szulc P, Seeman E, Delmas P, Biochemical measurements of bone turnover in children and adolescents, Osteoporos. Int 11 (4) (2000) 281–294. [DOI] [PubMed] [Google Scholar]
- [20].Soyka LA, et al. , Abnormal bone mineral accrual in adolescent girls with anorexia nervosa, J. Clin. Endocrinol. Metab 87 (9) (2002) 4177–4185. [DOI] [PubMed] [Google Scholar]
- [21].Misra M, et al. , Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls, Pediatrics 114 (6) (2004) 1574–1583. [DOI] [PubMed] [Google Scholar]
- [22].Hotta M, et al. , The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa 1, J. Clin. Endocrinol. Metab 85 (1) (2000) 200–206. [DOI] [PubMed] [Google Scholar]
- [23].Grinspoon S, et al. , Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa, J. Clin. Endocrinol. Metab 81 (11) (1996) 3864–3870. [DOI] [PubMed] [Google Scholar]
- [24].Garnero P, Bone markers in osteoporosis, Curr. Osteoporos. Rep 7 (3) (2009) 84–90. [DOI] [PubMed] [Google Scholar]
- [25].NICE Alendronate, Etidronate, Risedronate, Raloxifene, Strontium Ranelate and Teriparatide for the Secondary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women. Technology Appraisal Guidance, vol. 161, (2011). [Google Scholar]
- [26].Moher D, et al. , Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, Int. J. Surg 8 (5) (2010) 336–341. [DOI] [PubMed] [Google Scholar]
- [27].Huang X, Lin J, Demner-Fushman D, PICO as a knowledge representation for clinical questions, AMIA 2006 Symposium Proceedings, 2006. [PMC free article] [PubMed] [Google Scholar]
- [28].Thomas B, et al. , A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions, Worldviews Evid.-Based Nurs 1 (3) (2004) 176–184. [DOI] [PubMed] [Google Scholar]
- [29].Eccles M, Mason J, How to Develop Cost-conscious Guidelines, Core Research, 2001. [DOI] [PubMed] [Google Scholar]
- [30].Bandelow B, et al. , World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders–first revision, World J. Biol. Psychiatry 9 (4) (2008) 248–312. [DOI] [PubMed] [Google Scholar]
- [31].Grinspoon S, et al. , Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa, J. Clin. Endocrinol. Metab 87(6) (2002) 2883–2891. [DOI] [PubMed] [Google Scholar]
- [32].Golden NH, et al. , Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial, J. Clin. Endocrinol. Metab 90 (6) (2005) 3179–3185. [DOI] [PubMed] [Google Scholar]
- [33].Miller KK, et al. , Medical findings in outpatients with anorexia nervosa, Arch. Intern. Med 165 (5) (2005) 561–566. [DOI] [PubMed] [Google Scholar]
- [34].Strokosch GR, et al. , Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double-blind, placebo-controlled study, J. Adolesc. Health 39 (6) (2006) 819–827. [DOI] [PubMed] [Google Scholar]
- [35].Miller KK, et al. , Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study, J. Clin. Endocrinol. Metab 96 (7) (2011) 2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Misra M, et al. , Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa, J. Bone Miner. Res 26 (10) (2011) 2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bloch M, et al. , Dehydroepiandrosterone treatment effects on weight, bone density, bone metabolism and mood in women suffering from anorexia nervosa-a pilot study, Psychiatry Res 200 (2–3) (2012) 544–549. [DOI] [PubMed] [Google Scholar]
- [38].Divasta AD, et al. , The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa, Metab. Clin. Exp 61 (7) (2012) 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fazeli PK, et al. , Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa, J. Clin. Endocrinol. Metab 99 (4) (2014) 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Misra M, et al. , Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa, Bone 45 (3) (2009) 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Golden NH, et al. , The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa, J. Pediatr. Adolesc. Gynecol 15 (3) (2002) 135–143. [DOI] [PubMed] [Google Scholar]
- [42].Karlsson MK, et al. , Bone size and volumetric density in women with anorexia nervosa receiving estrogen replacement therapy and in women recovered from anorexia nervosa, J. Clin. Endocrinol. Metab 85 (9) (2000) 3177–3182. [DOI] [PubMed] [Google Scholar]
- [43].Shibli-Rahhal A, McCormick L, Teriparatide treatment of osteoporosis in a patient with anorexia nervosa, Eat. Weight Disord 18 (2) (2013) 229–231. [DOI] [PubMed] [Google Scholar]
- [44].Gordon CM, et al. , Changes in bone turnover markers and menstrual function after short-term oral DHEA in young women with anorexia nervosa, J. Bone Miner. Res 14 (1) (1999) 136–145. [DOI] [PubMed] [Google Scholar]
- [45].Muñoz-Calvo M, et al. , Maintained malnutrition produces a progressive decrease in (OPG)/RANKL ratio and leptin levels in patients with anorexia nervosa, Scand. J. Clin. Lab. Invest 67 (4) (2007) 387–393. [DOI] [PubMed] [Google Scholar]
- [46].Iketani T, et al. , Effect of menatetrenone (vitamin K2) treatment on bone loss in patients with anorexia nervosa, Psychiatry Res. 117 (3) (2003) 259–269. [DOI] [PubMed] [Google Scholar]
- [47].Miller KK, et al. , Effects of risedronate on bone density in anorexia nervosa, J. Clin. Endocrinol. Metab 89 (8) (2004) 3903–3906. [DOI] [PubMed] [Google Scholar]
- [48].DiVasta AD, et al. , The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa, Metabolism 61 (7) (2012) 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Misra M, Klibanski A, Anorexia nervosa and bone, J. Endocrinol 221 (3) (2014) R163–R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Miller K, Grieco K, Klibanski A, Testosterone administration in women with anorexia nervosa, J. Clin. Endocrinol. Metab 90 (3) (2005) 1428–1433. [DOI] [PubMed] [Google Scholar]
- [51].Raevuori A, Keski-Rahkonen A, Hoek HW, A review of eating disorders in males, Curr. Opin. Psychiatry 27 (6) (2014) 426–430. [DOI] [PubMed] [Google Scholar]
- [52].Black DM, et al. , Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis, N. Engl. J. Med 356 (18) (2007) 1809–1822. [DOI] [PubMed] [Google Scholar]
- [53].Uchiyama S, et al. , The skeletal muscle cross sectional area in long-term bisphosphonate users is smaller than that of bone mineral density-matched controls with increased serum pentosidine concentrations, Bone 75 (2015) 84–87. [DOI] [PubMed] [Google Scholar]
- [54].Meier RP, et al. , Increasing occurrence of atypical femoral fractures associated with bisphosphonate use, Arch. Intern. Med 172 (12) (2012) 930–936. [DOI] [PubMed] [Google Scholar]
- [55].Chang ST, et al. , Atypical femur fractures among breast cancer and multiple myeloma patients receiving intravenous bisphosphonate therapy, Bone 51 (3) (2012) 524–527. [DOI] [PubMed] [Google Scholar]
- [56].Rizzoli R, et al. , Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis, Bone 42 (5) (2008) 841–847. [DOI] [PubMed] [Google Scholar]
- [57].Schilcher J, Michaëlsson K, Aspenberg P, Bisphosphonate use and atypical fractures of the femoral shaft, N. Engl. J. Med 364 (18) (2011) 1728–1737. [DOI] [PubMed] [Google Scholar]
- [58].Marx RE, Oral and Intravenous Bisphosphonate-induced Osteonecrosis of the Jaws. Chicago, Ill, USA: Quintessence, (2007). [Google Scholar]
- [59].Marini JC, Do bisphosphonates make children’s bones better or brittle? N. Engl. J. Med 349 (5) (2003) 423–426. [DOI] [PubMed] [Google Scholar]
- [60].Bachrach LK, Ward LM, Clinical review: bisphosphonate use in childhood osteoporosis, J. Clin. Endocrinol. Metab 94 (2) (2009) 400–409. [DOI] [PubMed] [Google Scholar]
- [61].Larsen PS, et al. , What’s in a self-report? A comparison of pregnant women with self-reported and hospital diagnosed eating disorder, Eur. Eat. Disord. Rev 24 (6) (2016) 460–465. [DOI] [PubMed] [Google Scholar]
- [62].Food, U.S., Drug Administration, Center for Food Safety & Apllied Nutrition. Foodborne Pathogenic Microorganisms and Natural Toxins Handbook. “Bad Bug Book; ”. http://vm.cfsan.fda.gov/~mow 2010 (2001). [Google Scholar]
- [63].Misra M, et al. , Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa, Pediatr. Res 59 (2006) 598–603. [DOI] [PubMed] [Google Scholar]
- [64].Legroux-Gerot I, et al. , Bone loss associated with anorexia nervosa, Joint Bone Spine 72 (6) (2005) 489–495. [DOI] [PubMed] [Google Scholar]
- [65].Misra M, Klibanski A, Endocrine consequences of anorexia nervosa, Lancet Diabetes Endocrinol. 2 (7) (2014) 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Misra M, Klibanski A, The neuroendocrine basis of anorexia nervosa and its impact on bone metabolism, Neuroendocrinology 93 (2) (2011) 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Weissberger AJ, Ho KK, Lazarus L, Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women*, J. Clin. Endocrinol. Metab 72 (2) (1991) 374–381. [DOI] [PubMed] [Google Scholar]
- [68].Moll GW Jr., Rosenfield RL, Fang VS, Administration of low-dose estrogen rapidly and directly stimulates growth hormone production, Arch. Pediatr. Adolesc. Med 140 (2) (1986) 124. [DOI] [PubMed] [Google Scholar]
- [69].Misra M, Klibanski A, Bone metabolism in adolescents with anorexia nervosa, J. Endocrinol. Investig 34 (4) (2011) 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Misra M, et al. , Bone metabolism in adolescent boys with anorexia nervosa, J. Clin. Endocrinol. Metab 93 (8) (2008) 3029–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Orwoll ES, Klein RF, Osteoporosis in men, Endocr. Rev 16 (1) (1995) 87–116. [DOI] [PubMed] [Google Scholar]
- [72].Khosla S, et al. , Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men, J. Clin. Endocrinol. Metab 86 (8) (2001) 3555–3561. [DOI] [PubMed] [Google Scholar]
- [73].DiVasta AD, et al. , Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J. Bone Miner. Res 29 (1) (2014) 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hunter D, et al. , Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation, J. Bone Miner. Res 16 (2) (2001) 371–378. [DOI] [PubMed] [Google Scholar]
- [75].Urano A, et al. , Vitamin K deficiency evaluated by serum levels of under-carboxylated osteocalcin in patients with anorexia nervosa with bone loss, Clin. Nutr 34 (3) (2015) 443–448. [DOI] [PubMed] [Google Scholar]
- [76].Welt CK, et al. , Recombinant human leptin in women with hypothalamic amenorrhea, N. Engl. J. Med 351 (10) (2004) 987–997. [DOI] [PubMed] [Google Scholar]
- [77].Ettinger B, et al. , Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial, JAMA 282 (7) (1999) 637–645. [DOI] [PubMed] [Google Scholar]
- [78].Reginster J-Y, et al. , Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study, J. Clin. Endocrinol. Metab 90 (5) (2005) 2816–2822. [DOI] [PubMed] [Google Scholar]
- [79].Reginster J-Y, et al. , The position of strontium ranelate in today’s management of osteoporosis, Osteoporos. Int 26 (6) (2015) 1667–1671. [DOI] [PubMed] [Google Scholar]
- [80].Petretta M, et al. , Heart rate variability as a measure of autonomic nervous system function in anorexia nervosa, Clin. Cardiol 20 (3) (1997) 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dawson-Hughes B, Calcium D Primer on the Metabolic Bone Diseases and Disorders of Bone Metabolism, (2009), pp. 231–233. [Google Scholar]
- [82].Bachrach LK, et al. , Decreased bone density in adolescent girls with anorexia nervosa, Pediatrics 86 (3) (1990) 440–447. [PubMed] [Google Scholar]
- [83].Tai V, et al. , Calcium intake and bone mineral density: systematic review and meta-analysis, BMJ 351 (2015) h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bolland MJ, et al. , Calcium intake and risk of fracture: systematic review, BMJ 351 (2015) h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Eriksen SA, et al. , Bone and vitamin D status in patients with anorexia nervosa, Dan. Med. J 61 (11) (2014) A4940. [PubMed] [Google Scholar]
- [86].Gatti D, et al. , Strong relationship between vitamin D status and bone mineral density in anorexia nervosa, Bone 78 (2015) 212–215. [DOI] [PubMed] [Google Scholar]
- [87].Sarkar S, et al. , Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk, J. Bone Miner. Res 19 (3) (2004) 394–401. [DOI] [PubMed] [Google Scholar]
- [88].Milos G, et al. , Are patterns of bone loss in anorexic and postmenopausal women similar? Preliminary results using high resolution peripheral computed tomography, Bone 58 (2014) 146–150. [DOI] [PubMed] [Google Scholar]


