Abstract
Purpose:
The use of radiation therapy (RT) in consolidating oligometastatic prostate cancer (OPCa) is a rapidly evolving treatment paradigm. We review our institutional experience using metastasis-directed therapy in the definitive management of men with OPCa.
Methods and Materials:
Patients with OPCa treated with definitive RT were included. The Kaplan-Meier method and multivariable Cox regression analysis were performed to assess biochemical progression-free survival (bPFS) and time to next intervention. Cumulative incidence functions were used to calculate rates of local failure. Toxicity was assessed using Common Terminology Criteria for Adverse Events (version 4).
Results:
This study analyzed 156 patients with OPCa and 354 metastatic lesions with median follow-up of 24.6 months. Of 150 patients with toxicity data, 53 (35%) experienced acute grade 1 toxicity, 8 (5%) had grade 2, and none had grade 3 toxicity. Only 13 patients (9%) had late toxicities. At 24 months, the cumulative incidence of local failure was 7.4%. Median bPFS for the entire cohort was 12.9 months and 52% at 1 year. On multivariable analysis, factors associated with prolonged bPFS were periRT androgen deprivation therapy (ADT), lower gross tumor volume, and hormone-sensitive (HS) OPCa. Median time to next intervention, including repeat RT, was 21.6 months. Median bPFS for men with HS prostate cancer was 17.2 months compared with 7.2 months in men with castrate-resistant OPCa (P < .0001), and cumulative incidence of local failure at 24 months was lower with HS OPCa (4.8% vs 12.1%; P = .034). We analyzed 28 men with HS OPCa treated with a course of peri-RT ADT (median, 4.3 months) with recovery of testosterone. At a median follow-up of 33.5 months, 20 patients had not developed bPFS, median bPFS had not been reached, and 24-month bPFS was 77%.
Conclusions:
Metastasis-directed therapy can be effective across a wide range of OPCa subtypes, but with differential efficacy. Further study is warranted to investigate the use of RT across the wide range of patients with OPCa.
Summary
Local consolidation of oligometastatic disease is a rapidly emerging treatment paradigm. This article reviews our institutional experience treating oligometastatic prostate cancer with definitive intent radiation therapy. We demonstrate that metastasis-directed therapy with stereotactic ablative radiation therapy to oligometastatic lesions can be effective across a wide range of oligometastatic prostate cancer subtypes, but with differential efficacy. Continued study is warranted to investigate the use of radiation therapy over the wide range of patients with oligometastatic prostate cancer.
Introduction
The oligometastatic hypothesis, whereby tumors occupy an intermediate state between localized primary disease and widely metastatic lesions, was first proposed in the 1990s by Hellman and Weichselbaum.1 If true, this state implies that individuals with few metastatic lesions might have extended periods of disease-free survival2 or potentially be cured with locally directed therapy to the metastatic site. Traditionally, surgical resection was the means of treatment; however, the advent of stereotactic ablative radiation therapy (SABR) provided a form of noninvasive therapy in which high-dose radiation therapy (RT) could be delivered in a highly conformal manner. The excellent control rates in combination with the minimal adverse effect profile of SABR has resulted in an increasing trend toward treating oligometastatic lesions in an attempt to delay the initiation of potentially toxic systemic therapies, provide treatment breaks for those with accumulating side effects, or prolong progression-free survival (PFS).3–9
As the experience treating oligometastatic disease has evolved, accumulating evidence suggests that the proposed benefits of metastasis-directed therapy (MDT) have merit. Prospectively performed trials demonstrate improvements in PFS10,11 in non-small-cell lung cancer and overall survival in a variety of primaries, including breast, lung, colorectal, and prostate cancer (PCa).12 In PCa-specific cohorts, MDT is associated with sustained periods of disease-free survival, and it prolongs the time to initiation of androgen deprivation therapy (ADT).13,14
At this time, the definition of oligometastatic PCa (OPCa) is based on clinical characteristics such as number of lesions,15 although biologic parameters might soon supplement or replace this numerical definition.16,17 Nonetheless, individuals with OPCa remain a heterogeneous population, and most available studies consist of small cohorts of homogeneous patients treated for a small number of lesions. Thus, further elucidation of the benefit of MDT in OPCa is needed. Herein, we provide a descriptive report of the largest single-institution experience in the treatment of OPCa, comprising a cohort of 156 consecutively treated men with 354 total lesions.
Methods and Materials
Patient population
After institutional review board approval, we reviewed our retrospectively collected database of patients with OPCa consecutively treated with SABR at the Johns Hopkins Hospital between August 21, 2013, and September 11, 2018. The vast majority (92.3%) were treated by a single physician (PTT). Inclusion criteria included men with histologically confirmed PCa with imaging features consistent with metastatic disease and who received definitive-intent RT to the metastatic lesions. Typically, men with fewer than 5 lesions seen on imaging were considered oligometastatic and appropriate for MDT. Four patients initially thought to have 5 or fewer lesions were noted to have 6 or 7 during treatment planning; all lesions were treated and thus included in this analysis. Individuals without any follow-up data, either in the form of prostate-specific antigen (PSA) or repeat imaging, were excluded from analysis.
Patients were typically seen every 3 to 6 months after SABR with repeat history, physical examination, PSA, and testosterone analysis. Imaging was often repeated at 6- to 12-month intervals or sooner if warranted by symptoms or change in PSA dynamics. The decision regarding changes to a patient’s treatment paradigm and the use of ADT after SABR was typically made in a multidisciplinary manner. The majority of patients were treated by a limited number of Johns Hopkins medical oncologists with similar practice patterns. Broadly, the indication for the next intervention was determined by objective evidence of disease progression on PSA, radiographic testing, or symptomatic progression.
SABR technique
Custom immobilization was created at the time of computed tomography (CT) simulation using an Alpha Cradle (Smithers Medical Products, North Canton, OH) or an equivalent device. Gross tumor volume (GTV) was delineated during the planning process on CT images with the help of fused magnetic resonance imaging, bone scan, sodium fluoride, choline, or DCFPyL prostate-specific membrane antigen positron emission tomography/CT when available. Motion management was used when applicable; at our institution, patients with ≥3 mm breathing motion on 4dimensionalCTweremanaged with active breathing control. Those with <3 mm of motion were treated with free breathing and an internal target volume based on the 0% and 60% phases of the breathing cycle.
The clinical tumor volume was typically equal to that of the GTV. The planning target volume (PTV) usually was a 3- to 5-mm expansion on the GTV (or internal target volume). Plans were prescribed to the PTV. Before treatment each day, a cone-beam CT scan was coregistered (spine) with the free breathing or active breathing control simulation scan, and patients were shifted as needed for alignment. A minority of patients were treated with CyberKnife Accuray (Sunnyvale, CA), for which image guidance was per the instructions for that device.
Statistical analysis
Summary statistics were calculated for patients and lesions. Survival analysis was conducted for biochemical PFS (bPFS) and time to next intervention (TTNI). Events of interest for bPFS included PSA failure, local or distant failure (assessed by follow up imaging), start of systemic therapy (ie, ADT), or death. PSA failure was defined as PSA nadir plus 2 ng/mL for those treated with RT to the prostate primary or castrate-resistant PCa. A PSA of 0.2 ng/mL or the first increase from nadir was a considered failure for those who underwent prostatectomy. Events for TTNI were a change in therapy after SABR (including repeat SABR to oligometastatic lesions). Median bPFS and TTNI were calculated using the Kaplan-Meier method and, when stratified by clinical characteristics, differences were compared using the log rank test. Univariable Cox regression analysis was conducted to identify variables associated with bPFS and TTNI and log transformed when applicable. Variables found to be associated on univariable analysis (P < .05) were included in multivariable models, allowing approximately 1 variable per 10 events. Rates of local failure after SABR were calculated using cumulative incidence function curves and were assessed on an individual lesion basis. Local failure was defined as the growth of a lesion within the PTV on imaging scans, in conjunction with rising PSA, or clinically significant events (eg, bone fractures) in a previously stable lesion. Toxicity was assessed at each follow-up using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. All statistical analyses were conducted using R.
Results
Patient, disease, and treatment characteristics
One hundred fifty-six patients were identified and analyzed. Table 1 describes baseline characteristics of the population. The median age of the population was 65.5 years. The majority of patients had either pT2 (24.4%) or pT3 (51.9%) disease. Gleason groups were as follows: (1) 8 patients (5.2%), (2) 23 patients (14.7%), (3) 40 patients (25.6%), (4) 28 patients (17.9%), and (5) 57 patients (36.6%). Further Gleason breakdown can be seen in Table 1. Median initial PSA was 8.85 ng/mL (range, 2–1255 ng/mL) and median PSA doubling time was 4.8 months (0.3–61.7 months). A large fraction of men had node-positive (24.4%) or metastatic disease (26.3%) at the time of initial diagnosis; 23.7% of patients were treated when de novo metastatic disease was diagnosed. The majority (81.4%) had hormone-sensitive PCa (HSPC) at the time of first MDT, and 59.6% received peri-RT (neoadjuvant, concurrent, or adjuvant) ADT for a median duration of 12.2 months after treatment.
Table 1.
Patient baseline characteristics
| Characteristic (n = 156) | Value |
|---|---|
| Age (y) at treatment, median (range) | 65.5 (46–84) |
| Initial PSA (ng/mL), median (range) | 8.85 (2.0–1255.0) |
| Pre-RT PSA (ng/mL), median (range) | 1.90 (0–95.8) |
| PSA-DT (mo) | 4.8 (0.3–61.7) |
| PSA nadir (ng/mL), median (range) | 0.20 (0–65.0) |
| Peri-RT ADT (%) | |
| Yes | 93 (59.6) |
| No | 63 (40.4) |
| Hormone status (%) | |
| HSPC | 127 (81.4) |
| CRPC | 29 (18.6) |
| Synchronous node positive (%) | |
| N0 | 111 (71.2) |
| N1 | 38 (24.4) |
| Nx | 6 (3.8) |
| N/A | 1 (0.6) |
| Metastatic disease at diagnosis (%) | |
| M0 | 65 (41.7) |
| M1 | 44 (28.2) |
| Mx | 46 (29.5) |
| Unknown | 1 (0.6) |
| Original number metastasis (%) | |
| 1 | 71 (45.5) |
| 2 | 50 (32.1) |
| 3 | 21 (13.5) |
| 4 | 7 (4.5) |
| 5 | 3 (1.9) |
| 6 | 3 (1.9) |
| 7 | 1 (0.6) |
| Total number metastasis treated (%) | |
| 1 | 61 (39.1) |
| 2 | 51 (32.7) |
| 3 | 22 (14.1) |
| 4 | 9 (5.9) |
| 5 | 3 (1.9) |
| 6 | 5 (3.2) |
| 7 | 3 (1.9) |
| 8 | 1 (0.6) |
| 9 | 1 (0.6) |
| Initial treatment | |
| Treated when de novo | 367 (23.7) |
| Treated when oligorecurrent | 119 (76.3) |
| Staging imaging | |
| Enhanced | 86 (55.1) |
| Conventional | 69 (44.2) |
| N/A | 11 (0.7) |
| Follow-up imaging | |
| Enhanced | 66 (42.3) |
| Conventional | 65 (41.7) |
| N/A | 25 (16) |
| Posttreatment PSA change (%) | |
| Decline/stable | 134 (85.9) |
| Increase | 22 (14.1) |
| Treatment site (%) | |
| Node only | 60 (38.5) |
| Bone/visceral/node | 96 (61.5) |
| T stage (%) | |
| Tx | 1 (0.6) |
| Clinical T1 | 8 (5.1) |
| Clinical T2 | 11 (7.1) |
| Clinical T3 | 11 (7.1) |
| Clinical T4 | 5 (3.2) |
| Pathologic T2 | 38 (24.4) |
| Pathologic T3 | 81 (51.9) |
| Unknown | 1 (0.6) |
| Gleason score (%) | |
| Clinical 6 | 2 (1.3) |
| Clinical 7 | 11 (7.0) |
| Clinical 8 | 8 (5.1) |
| Clinical 9 | 11 (7.1) |
| Clinical 10 | 4 (2.6) |
| Pathologic 6 | 6 (3.8) |
| Pathologic 7 | 56 (35.9) |
| Pathologic 8 | 19 (12.2) |
| Pathologic 9 | 37 (23.7) |
| Pathologic 10 | 2 (1.3) |
Abbreviations: ADT = androgen deprivation therapy; CRPC = castration-resistant prostate cancer; HSPC = hormone-sensitive prostate cancer; PSA = prostate-specific antigen; PSA-DT = prostate-specific antigen doubling time; RT = radiation therapy.
Three hundred and fifty-four lesions were treated with RT. At first MDT, the number of lesions treated were as follows: 1 lesion for 71 patients (45.5%), 2 lesions for 50 patients (32.1%), 3 lesions for 21 patients (13.5%), 4 lesions for 7 patients (4.5%), 5 lesions for 3 patients (1.9%), 6 lesions for 3 patients (1.9%), and 7 lesions for 1 patient (0.6%). Several patients underwent repeated SABR with a total final lesion count as follows: 1 lesion for 61 patients (39.1%), 2 lesions for 51 patients (32.7%), 3 lesions for 22 patients (14.1%), 4 lesions for 9 patients (5.9%), 5 lesions for 3 patients (1.9%), 6 lesions for 5 patients (3.2%), 7 lesions for 3 patients (1.9%), 8 lesions for 1 patients (0.6%), and 9 lesions for 1 patient (0.6%).
Baseline lesion characteristics are reported in Table 2. One hundred ninety-two (54.2%) treated lesions were bone metastases, 152 (42.9%) were nodal metastases, and 10 (2.9%) were visceral lesions. Median GTV was 2.9 cm3 (range, 0.06–152.3 cm3). The majority of lesions (n = 344; 97.7%) were treated with SABR. The other 10 lesions were treated with definitive-dose intensity modulated RT, and they were often lesions in men with synchronous metastatic disease integrated into treatment of the prostate primary. The median biologic equivalent dose using and an alpha/beta of 3 was 116.7 (range, 54–450), and the most common fractionation schemes were 8 to 10 Gy for 3 fractions (33.6%), 6 to 8 Gy for 5 fractions (26.3%), and 15 to 20 Gy for 1 fractions (13%).
Table 2.
Baseline lesion characteristics
| Characteristic (n = 354) | Value |
|---|---|
| Median GTV, cm3 | 2.9 (0.06–152.3) |
| Median pre-RT PSA, ng/mL | 2.30 (0–95.80) |
| Median BED3 | 116.67 (54.0–450.0) |
| Site, n (%) | |
| Node | 192 (54.2) |
| Bone | 152 (42.9) |
| Visceral | 10 (2.9) |
Abbreviations: BED = biological dose equivalent; GTV = gross tumor volume; PSA = prostate-specific antigen; RT = radiation therapy.
Clinical outcomes
Entire cohort
Median follow-up time was 24.6 months (range, 0.2–70 months). Of the 150 patients with toxicity data available, 61 (41%) experienced an acute toxicity, of whom 53 had CTCAE grade 1 and 8 had CTCAE grade 2 toxicities. No acute toxicities of grade 3 or greater were observed. The most common adverse effect was fatigue, experienced by 37 patients, followed by gastrointestinal symptoms (constipation, diarrhea or nausea) experienced by 20 patients, and genitourinary (ie, urgency or dysuria) in 6 patients. Only 13 of 142 patients (9%) were noted to have late toxicities at follow-up.
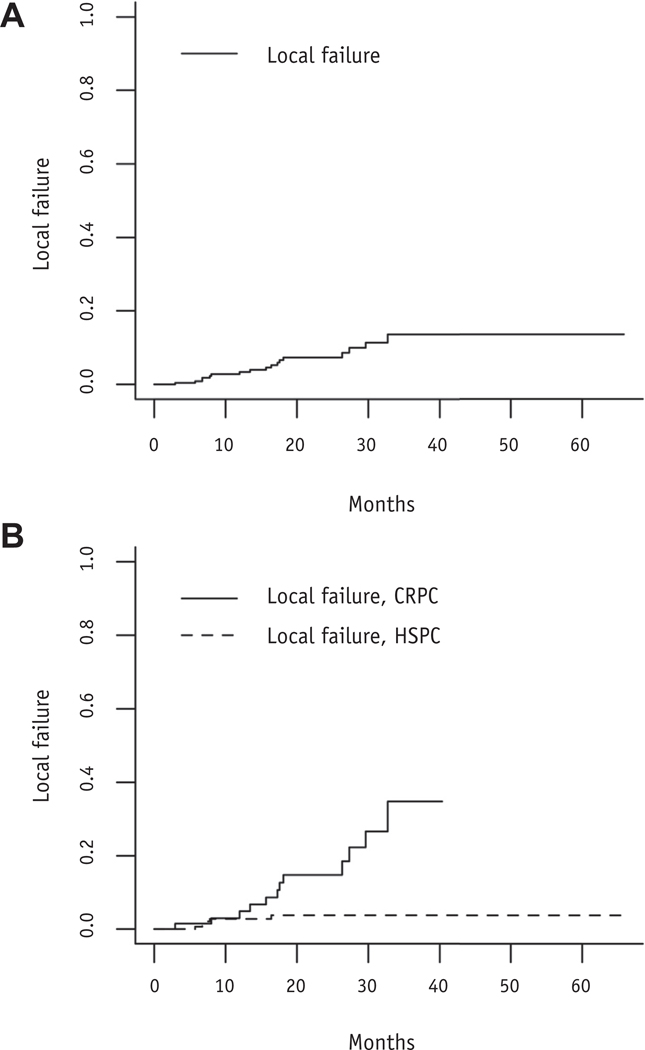
Median PSA before first MDT was 1.9 ng/mL (range, undetectable to 95.8 ng/mL). We found that 134 patients (85.9%) had PSA stability or decline after RT. The rates of local failure at 12 and 24 months were 3.9% and 7.4%, respectively (Fig. 1A). Median bPFS for the whole population was 12.9 months (95% confidence interval [CI], 10.5–17.9 months) and 52% at 1 year (Fig. 2A). On univariable analysis (Table 3), metastatic disease at diagnosis, peri-RT ADT, nonnodal disease, HSPC, lower pre-RT PSA, lower GTV, and treatment when disease was de novo metastatic were associated with improved bPFS. Median bPFS for men with HSPC was 17.2 months compared with 7.2 months in men with castration-resistant PCa (CRPC; P < .0001; Fig. 2B). Those treated with peri-RT ADT had a median bPFS of 19.0 months compared with 7.7 months in those not treated with ADT (P < .0001; Fig. 2C). HSPC, lower GTV, and peri-RT ADT were associated with longer bPFS on multivariable analysis (Table 4).
Fig. 1.
(A) Cumulative incidence curve of local failure after radiation therapy. (B) Cumulative incidence curve of local failure after radiation therapy stratified by hormone status. Abbreviations: CRPC = castration-resistant prostate cancer; HSPC = hormone-sensitive prostate cancer.
Fig. 2.
(A) Biochemical progression-free survival after radiation therapy. (B) Biochemical progression-free survival after radiation therapy stratified by hormone status. (C) Biochemical progression-free survival after radiation therapy stratified by the use of ADT. (D) Time to next intervention after radiation therapy. Abbreviations: ADT = androgen deprivation therapy; bPFS = biochemical progression-free survival; CRPC = castration-resistant prostate cancer; HSPC = hormone-sensitive prostate cancer; RT = radiation therapy.
Table 3.
Univariate analysis for factors associated with bPFS and TTNI
| Characteristic | HR (95% CI) | P value |
|---|---|---|
| bPFS | ||
| GTV | 1.22 (1.05–1.43) | .01 |
| Age | 1.02 (0.99–1.05) | .10 |
| Node status | ||
| N1 | 0.72 (0.44–1.18) | .20 |
| Nx | 1.47 (0.53–4.05) | .46 |
| Met status | ||
| M1 | 0.33 (0.18–0.59) | <.001 |
| Mx | 0.94 (0.60–1.47) | .80 |
| ADT | 0.44 (0.29–0.66) | <.001 |
| Initial PSA | 0.98 (0.80–1.18) | .80 |
| Site | ||
| Node | 1.68 (1.12–2.52) | .01 |
| HSPC | 0.39 (0.25–0.62) | <.001 |
| Pre-RT PSA | 1.01 (1.0–1.03) | .005 |
| T1/T2 | 0.66 (0.43–1.02) | .06 |
| Gleason | ||
| 7 | 082 (0.37–1.82) | .62 |
| 8–10 | 0.56 (0.25–1.25) | .16 |
| No. of metastasis | ||
| ≥2 | 1.50 (0.99–2.25) | .055 |
| PSA-DT | 0.89 (0.72–1.09) | .24 |
| Enhanced imaging | 1.20 (0.80–1.82) | .37 |
| Treatment when de novo | 0.23 (0.12–0.44) | <.001 |
| TTNI | ||
| GTV | 1.25 (1.05–1.49) | .01 |
| Age | 1.01 (0.98–1.04) | .54 |
| Node status | ||
| Nx | 1.68 (0.61–4.65) | .32 |
| N1 | 0.70 (0.40–1.23) | .21 |
| Met status | ||
| M1 | 0.27 (0.14–0.52) | <.0001 |
| Mx | 0.74 (0.45–1.22) | .24 |
| ADT | 0.49 (0.31–0.78) | .002 |
| Initial PSA | 0.98 (0.78–1.21 | .83 |
| Site | ||
| Node | 1.67 (1.07–2.62) | .02 |
| HSPC | 0.37 (0.23–0.60) | <.0001 |
| Pre-RT PSA | 1.02 (1.004–1.03) | .007 |
| T1/T2 | 0.57 (0.35–0.92) | .02 |
| Gleason | ||
| 7 | 1.15 (0.45–2.93) | .78 |
| 8–10 | 0.78 (0.30–1.97) | .59 |
| No. of metastasis | ||
| ≥2 | 1.31 (0.83–2.05) | .25 |
| PSA-DT | 0.75 (0.60–0.95) | .02 |
| Enhanced imaging | 1.33 (0.84–2.10) | .23 |
| Treatment when de novo | 0.20 (0.09–0.44) | <.0001 |
Abbreviations: ADT = androgen deprivation; bPFS = biochemical progression-free survival; CI = confidence interval; GTV = gross tumor volume, HR = ha=ard ratio; HSPC = hormone-sensitive prostate cancer; Met = metastasis; PSA = prostate-specific antigen; PSA-DT = prostate-specific antigen doubling time; RT = radiation therapy; TTNI = time to next intervention.
Table 4.
Multivariate analysis for factors associated with bPFS and TTNI by patient
| Characteristic | HR (95% CI) | P value |
|---|---|---|
| bPFS | ||
| ADT | 0.36 (0.19–0.67) | .001 |
| HSPC | 0.35 (0.18–0.69) | .002 |
| Pre-RT PSA | 1.01 (0.99–1.02) | .48 |
| GTV | 1.23 (1.02–1.45) | .04 |
| status | ||
| M1 | 0.86 (0.33–2.25) | .75 |
| Mx | 1.08 (0.68–1.71) | .74 |
| Nodal disease | 1.34 (0.85–2.12) | .21 |
| Treatment when de novo | 0.56 (0.17–1.85) | .34 |
| TTNI | ||
| GTV | 1.20 (0.96–1.49) | .11 |
| ADT | 0.38 (0.18–0.78) | .009 |
| Site | ||
| Node | 1.44 (0.85–2.44) | .17 |
| HSCP | 0.40 (0.19–0.85) | .02 |
| Pre-RT PSA | 1.008 (0.99–1.03) | .33 |
| T1/T2 | 0.69 (0.40–1.18) | .17 |
| M status | ||
| M1 | 0.99 (0.33–2.92) | .98 |
| Mx | 0.85 (0.51–1.41) | .52 |
| PSA-DT | 0.84 (0.63–1.12) | .24 |
| Treatment when de novo | 0.41 (0.10–1.63) | .21 |
Abbreviations: ADT = androgen deprivation therapy; bPFS = biochemical progression-free survival; CI = confidence interval; GTV = gross tumor volume; HR = ha=ard ratio; HSPC = hormone sensitive prostate cancer; OPC = oligometastatic prostate cancer; PSA = prostate specific antigen; PSA-DT = prostate specific antigen; RT = radiation therapy; TTNI = time to next intervention.
Median TTNI after MDT was 21.6 months (95% CI, 17.332.5 months; Fig. 2D). Factors associated with longer TTNI on univariable analysis included lower GTV, metastatic disease at diagnosis, nonnodal site of metastasis, HSPC, peri-RT ADT, stage T1/2 disease, lower PSA doubling time, and treatment when disease was de novo metastatic (Table 3). On multivariable analysis, only peri-RT ADT and HSPC were associated with longer TTNI (Table 4).
Hormone-sensitive cohort
One hundred twenty-eighty men with HSPC were included in the analysis. Two hundred seventy-nine lesions were treated, with cumulative incidence of local failure of 1% at 12 months and 4.8% at 24 months (Fig. 1B). Median bPFS in the entire group was 17.2 months (95% CI, 11.4–39.0 months), 57% at 1 year, and 40% at 2 years. Median TTNI for the group was 28.6 months (95% CI, 20.6 months to not reached ). In the cohort as a whole, 68 men (53.1%) were treated with peri-RT ADT for a median of 9.7 months. bPFS was significantly longer in those treated with peri-RT ADT (40.8 months; 95% CI, 23.2 months to not reached ) compared with those not treated with peri-RT ADT (7.7 months; 95% CI, 5.3–11.4 months; P < .0001). Similarly, TTNI was significantly longer in those treated with peri-RT ADT, with median TTNI not yet reached (95% CI, 42.9 months to not reached) compared with 14 months (95% CI, 7.1–28.6 months) in those not treated with ADT (P < .0001).
Sixty men did not receive peri-RT ADT, with a median bPFS of 7.7 months and 34% at 1 year. This result corresponded to a median ADT-free survival of 27.8 months (95% CI, 20.6 months to not reached). Median PSA before MDT was 1.6 ng/mL (range, 0–46.9 ng/mL). After MDT, 83.3% of patients had a decline in PSA, whereas 3% had stability. Median PSA nadir was 0.3 ng/mL (range, 0–65 ng/mL), and 19.1% of patients with a prostatectomy had an undetectable PSA after MDT. At the time of this report, several patients continue to have decline in the PSA and have not reached nadir yet. After MDT, a group of 12 men were re-treated with SABR as their next intervention, 10 of whom remain off ADT with a median follow-up of 21.1 months after treatment.
Twenty-eight men with HSPC were treated with peri-RT ADT during MDT, which was subsequently stopped. Median ADT treatment time after MDT was 4.3 months (range, 0.13–25.1 months), and all had recovery of testosterone subsequent to stopping ADT. At last follow-up (median, 33.5 months), 20 patients have not developed bPFS; for the group, median bPFS has not been reached, and 24-month bPFS was 77%. Five patients have restarted ADT; thus, median ADT-free survival has not been met (95% CI, 30.6 months to not reached), and 24-month ADT-free survival in this group is 82%.
Castrate-resistant prostate cancer
Twenty-eight men with CRPC were included in the analysis. This population was heavily pretreated, with 39% having received prior chemotherapy. Seventy-five lesions were treated, and cumulative incidence of local failure was 3.4% at 12 months and 12.1% at 24 months, which was significantly higher than in the HSPC group (P = .034; Fig. 1B). Median bPFS was 7.2 months (95% CI, 5.4–12.6 months), and median TTNI was 12.2 months (95% CI, 9.317.8 months). Median PSA before MDT was 4.35 ng/mL (range, 0–95.8 ng/mL), 67.9% of men had a decrease or stability in their PSA, and median post-MDT PSA nadir was 1.45 ng/mL (range, 0–48.4 ng/mL).
Patterns of failure
In all patients, the most common site of failure after treatment was the bone (57.4%), followed by nodes (29.6%), multiple locations (11.1%), and visceral locations (1.9%). Patients initially treated to nodal lesions had a slight preponderance to recur in another node (52.9%). In this group, 23.5% had the bone as the next site of failure, whereas the remaining recurrences were in multiple locations (bone and node). The vast majority of patients who initially had a bone lesion treated also had recurrence in an osseous site (84%). A small percentage (8%) subsequently failed in a node, whereas the rest failed in a mixture of bone, node, and visceral locations. Those who were initially treated to both a bone and node lesion split their next site of failure, half in the bone and half in a node. These distinctions in patterns of failure were significantly different (P = .01).
Discussion
In this article, we describe a single-institution experience treating OPCa with RT at a median follow-up of 24.6 months. In our cohort of 156 patients, median bPFS and TTNI after RT was 12.9 and 21.6 months, respectively. Treatment was well tolerated, with 53 CTCAE grade 1 and 8 CTCAE grade 2 acute toxicities. During follow-up, only 13 of 142 patients (9%) were noted to have late toxicities.
Prospective high-level evidence demonstrates that local therapy to oligometastatic lesions improves PFS10,11 and overall survival12,18 in a multitude of malignancies. Within the realm of OPCa, the Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP) trial demonstrated local therapy, delivered primarily in the form of SABR, and prolonged time to initiation of ADT (21 vs 13 months) compared with surveillance.13 The rationale of MDT in OPCa is also supported by a plethora of retrospective reports documenting its safety and efficacy in prolonging PFS, delaying initiation of systemic therapy, and establishing oncologic control of locally treated lesions.3–7 Our report adds to the literature surrounding OPCa in several important ways.
This study represents, to our knowledge, the largest single institutional series of men with OPCa treated with definitive-intent RT to oligometastatic lesions. We included 156 men treated to a total of 354 lesions, triple the size of most series, which report on populations on the order of 40 to 50 patients. Our large series allowed for a more in-depth analysis of factors that can help to select who might benefit most from MDT. On MVA peri-RT ADT, smaller GTV, and HSPC were associated with improved bPFS. These findings appear to indicate the optimal time for intervention is with lower-volume disease. PCa is unique compared with most malignancies in that it has a highly sensitive biomarker, PSA, which can identify disease recurrence early. However, conventional imaging, such as bone or CT scans, has poor sensitivity to detect recurrence at PSA values less than 10 ng/mL.19–22 As our experience with molecular imaging (eg, prostate-specific membrane antigen positron emission tomography/CT scan) improves, disease will be detected at lower thresholds, resulting in the identification of metastatic lesions when present at lower quantities and aiding in the stratification of who might most benefit from MDT or identifying those with higher subclinical disease burden than expected, who might best be treated with systemic therapies alone.23 The large benefit of using peri-RT ADT also brings into question how MDT should be incorporated with ADT. A combination of ADT with MDT might improve local control rates of treated lesions and decrease the risk of distant failure.24 In addition, although ADT remains the standard of care in men with metastatic disease, a cohort of patients in our study treated with a course of ADT and MDT appear to have sustained disease response after testosterone recovery; therefore, indefinite ADT might not be necessary in all cases, although it is currently not clear how to identify this population, if it exists.
Our study also provides a large cohort of patients to identify patterns of failure after MDT. Similar to previous reports,25 failure patterns appear to favor recurrence in the bone. This finding has several important implications for future advances in MDT in OPCa. First, it allows for investigation of future MDT in combination with radium223, which will be at the center of investigation for our institution’s soon-to-open phase 2 randomized RAdium223 and SABR Versus SABR foroligomEtastatic prostate caNcerS (RAVENS) trial, which treats men with oligometastatic bone lesions with SABR to the macroscopic deposits followed by randomization to radium-223 or SABR alone. Second, given that we observed that half of the men treated for nodal lesions had subsequent nodal recurrence, questions remain regarding the best management for pelvic recurrences. This is the topic being studied in the currently opened Salvage Treatment of OligoRecurrent Nodal Prostate Cancer Metastases (STORM) trial (NCT03569241), which randomizes individuals with pelvic nodal recurrence to MDT (SABR to the node or lymphadenectomy) with or without whole pelvis radiation. The results of this trial will provide clarity surrounding the topic, but it remains an open question.
In addition, we were able to include several subgroups of OPCa that are less reported in the literature. We found that those with CRPC experienced a median bPFS of 7.2 months and a median TTNI of 12.2 months after MDT. This finding could have important clinical significance for those with oligoprogressive disease by allowing a delay before the switch of systemic therapy in a group whose systemic options can become limited after initial chemotherapy and enhanced ADT. In addition, we report on a group of patients treated with a course of ADT and MDT with subsequent testosterone recovery. At a median follow-up of 33.5 months, median bPFS has not been met, signifying a sustained treatment response. The majority of these patients had 1 (42.9%) or 2 (32.1%) metastases, thus advocating for the aggressive management of those with limited OPCa.
MDT using SABR is extremely well tolerated; 41% of patients experienced an acute toxicity during treatment, the vast majority of which (87%) were mild CTCAE grade 1 toxicities, such as fatigue. Only 9% of patients had a late toxicity after treatment. Therefore, MDT using SABR appears feasible in delaying the initiation of systemic therapies that often have an unfavorable adverse effect profile, especially ADT,26 while itself minimizing adverse effects. In our cohort, bPFS for men with HSPC treated with RT without ADT was 7.7 months, and median time to initiation of ADT was 27.8 months, in line with the findings of STOMP.13
This study has several limitations. First, it is retrospective nature, which inherently makes it open to biases. Although we attempted to account for them through multivariable analyses, confounding may remain. For example, treating in a non-controlled environment such as this can lead to bias toward treating those who might intrinsically have better oncologic outcomes, thus making a study like this a hypothesis-generating one at best. In addition, we performed variable selection for the multivariable Cox models and the association analysis using the same data set. Doing so could lead to selection bias in the results; therefore, this model should be validated on an external data set in the future. Finally, our median follow-up time was only 24 months. This report adds to a growing literature surrounding MDT, and further elucidation will come from prospectively run trials.
Conclusion
MDT can be effective across a wide range of OPCa subtypes, but with differential efficacy. Continued study investigating the use of RT over the wide range of patients with OPCa is warranted.
Disclosures:
R.P. was supported by the RSNA and RefleXion. P.T.T was supported by the Nesbitt-McMaster Foundation, Ronald Rose & Joan Lazar, Movember Foundation, Prostate Cancer Foundation, Commonwealth Foundation, and the National Institutes of Health and National Cancer Institute (grants R01CA166348, U01CA212007, U01CA231776, and 1R21CA223403).
References
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995; 13:8–10. [DOI] [PubMed] [Google Scholar]
- 2.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: A multi-institutional study of patterns of recurrence. Surgery 1986;100:278–284. [PubMed] [Google Scholar]
- 3.Decaestecker K, De Meerleer G, Lambert B, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014;9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muacevic A, Kufeld M, Rist C, et al. Safety and feasibility of imageguided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol 2013;31:455–460. [DOI] [PubMed] [Google Scholar]
- 5.Muldermans JL, Romak LB, Kwon ED, et al. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys 2016;95:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: A multi-institutional analysis. Eur Urol 2016;69:9–12. [DOI] [PubMed] [Google Scholar]
- 7.Moyer CL, Phillips R, Deek MP, et al. Stereotactic ablative radiationtherapy for oligometastatic prostate cancer delays time-to-next systemic treatment [e-pub ahead of print. World J Urol 2018. September 6. doi: 10.1007/s00345-018-2477-2. [Epub ahead of print]. PMID: 30191396. PMCID: PMC6401357 [Available on 2020–03-06]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase ii trial. Radiother Oncol 2018;126:177–180. [DOI] [PubMed] [Google Scholar]
- 9.Osti MF, Agolli L, Valeriani M, et al. 30 Gy single dose stereotactic body radiation therapy (SBRT): Report on outcome in a large series of patients with lung oligometastatic disease. Lung Cancer 2018;122: 165–170. [DOI] [PubMed] [Google Scholar]
- 10.Gomez DR, Blumenschein GR Jr., Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol 2018;4:e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palma DA, Rao S, Harrow S, et al. Stereotactic ablative radiation therapy for the comprehensive treatment of oligometastatic tumors (SABR-COMET): Results of a randomized trial. Int J Radiat Oncol Biol Phys 2018;102:S3–4. [Google Scholar]
- 13.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase ii trial. J Clin Oncol 2018; 36:446–453. [DOI] [PubMed] [Google Scholar]
- 14.Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol 2018;1:531–537. [DOI] [PubMed] [Google Scholar]
- 15.Tosoian JJ, Gorin MA, Ross AE, et al. Oligometastatic prostate cancer: Definitions, clinical outcomes, and treatment considerations. Nat Rev Urol 2017;14:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong AC, Watson SP, Pitroda SP, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 2016;122:2242–2250. [DOI] [PubMed] [Google Scholar]
- 17.Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun 2018;9:1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol 2016;70:926–937. [DOI] [PubMed] [Google Scholar]
- 20.Smith CP, Laucis A, Harmon S, et al. Novel imaging in detection of metastatic prostate cancer. Curr Oncol Rep 2019;21:31. [DOI] [PubMed] [Google Scholar]
- 21.Kane CJ, Amling CL, Johnstone PA, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 2003;61:607–611. [DOI] [PubMed] [Google Scholar]
- 22.Partin AW, Pearson JD, Landis PK, et al. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology 1994; 43:649–659. [DOI] [PubMed] [Google Scholar]
- 23.Hope TA, Aggarwal R, Chee B, et al. Impact of (68)Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]
- 24.Steuber T, Jilg C, Tennstedt P, et al. Standard of care versus metastases-directed therapy for pet-detected nodal oligorecurrent prostate cancer following multimodality treatment: A multiinstitutional case-control study [e-pub ahead of print]. Eur Urol Focus. 10.1016/j.euf.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Soldatov A, von Klot CAJ, Walacides D, et al. Patterns of progression after (68)Ga-PSMA-ligand PET/CT-guided radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys 2019;103:95104. [DOI] [PubMed] [Google Scholar]
- 26.Cornford P, Bellmunt J, Bolla M, et al. EAU-Estro-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 2017;71:630–642. [DOI] [PubMed] [Google Scholar]