Abstract
Background:
Renal ischemia-reperfusion disturbs both the function and the histology of this organ. Acacetin (Aca) is a natural flavonoid that is effective for relief of many diseases. The aim of this study was to determine the impacts of Aca on renal ischemia-reperfusion process in mice.
Methods:
In total, 84 male Balb/cmice divided into 12 groups and were administrated intraperitoneally for 4 days with or without surgery to dimethyl sulfoxide 0.01% or Aca (10, 25, and 50 mg/kg) as Control, control Acas, sham, sham Acas groups. Ischemia-reperfusion without or with Aca (10, 25, and 50 mg/kg) treatments were the other groups. Parameters related to the function and the histology of the kidneys were evaluated and statistically analyzed from kidney and blood serum samples in the respect of the groups.
Results:
In ischemia-reperfusion and ischemia-reperfusion + Aca (10 mg/kg) groups, there were significantly increased in urea, creatinine, malondialdehyde (MDA), and apoptosis rate, whereas total antioxidant capacity decreased compared to the control and sham and ischemia-reperfusion + Aca (25 and 50 mg/kg) (P < 0.05). The histopathology alteration was seen in the ischemia-reperfusion group than the others (P < 0.01). Moreover, there was a significant difference between ischemia-reperfusion + Aca (25 and50 mg/kg) groups than ischemia-reperfusion + Aca (10 mg/kg) one (P < 0.05).
Conclusions:
The recovery effect of Aca was offered on renal ischemia-reperfusion damage in a dose-dependent manner in mice, showing by kidney histopathology and functional criteria improvements. The attributed mechanism for this impression would be the antioxidant property of Aca, decreasing both MDA levels and apoptosis rate in kidney tissue.
Keywords: Acacetin, antioxidants, apoptosis, malondialdehyde, reperfusion injury
Introduction
Ischemia-reperfusion causes lack of oxygen and nutrients in the tissues, conducting to induce anaerobic conditions as a consequence of decreasing both intracellular PH and ATP levels simultaneous with raised oxidative agents.[1] Although either many reliable diagnostic criteria or management strategies have been developed, induction of renal failure after these operations has remained as a medical conflict. Qua for resolving this challenge, exploiting natural originated compounds has offered many eligible medicinal benefits in accompany with their safety, approachability, and cost-effectiveness.
Three mechanisms have been documented for the onset of acute renal failure following renal ischemia-reperfusion; as, vascular endothelial cells continuous contractions inducing abnormal compensated responses, tubular epithelial cells residua obstruction declining glomerular filtration rate, and reperfusion by itself.[2] Reperfusion increases the production of oxygen free radicals, cytotoxins, chemokines, and leukocyte activators that they motivate inflammatory cascades and damage to the kidney tissue. Hence, anti-oxidant activation would be essential for relieving renal failure-induced by ischemia-reperfusion.[3] Moreover, research demonstrate that the level of malondialdehyde (MDA) increases during kidney ischemia-reperfusion process as a result of rupturing kidneys cell membranes. Thus, measurement of the amount of this parameter is a remarkable criterion for diagnosis of ischemia-reperfusion, either its followed induced-renal failure.[4,5]
Owing to damage to the renal tubules that occurs by ischemia-perfusion, urea and creatinine re-enter to the bloodstream, provoking serum nitrogen levels boosting. Thus, evaluation of urea and creatinine in the serum should be a relevant indicator of renal failure.[6]
Some drugs and procedures are used to prevent cell damage against ischemia-reperfusion defect. For example, thermal shock leading to decline in L-arginine/nitric oxide path; dipyridamole that increases endogenes adenosine; and nicotine that promotes inhibitors of cholinergic anti-inflammatory pathway, calcium channel blockers or antagonists, and chlorpromazine.[7]
Acacetin (Aca) as natural plant-derived flavonoid compound, has many medicinal benefits properties such as anti-oxidant, anti-inflammatory, anti-cancer, anti-fibrillation, analgesic, anti-peroxidation, and anti-aromatase.[8,9] In the case of Ischemia-reperfusion, studies indicate a healing effect of Aca. Ha and colleagues pointed that neuroinflammation in lipopolysaccharide-stimulated BV-2 cells and in male mice were reduced by Aca. As well, Yang and colleagues offered advantageous of Aca on cultured (ex vivo) cardiac muscle cells induced-ischemia of rat pups.[10,11]
Although Aca has its own medical beneficial efficacy, there is a few data about the impact of Aca on the renal ischemia-reperfusion outcomes. In this regard, the present study was aimed to investigate the effectiveness of Aca against renal ischemia-reperfusion in mice and the histological, functional, and biochemical related criteria were evaluated.
Methods
Animals
This experimental study was done on 84 male inbred Balb/c mice (30 ± 2 g) at Kermanshah University of Medical Sciences. All animals were treated in accordance with guidelines of National Institute of Health for the Care and Use of Laboratory Animals approved by Research Deputy at Kermanshah University of Medical Sciences according to WMA Declaration Ethic of Helsinki (Ethic number; IR.KUMS.REC.1396.124). The mice were maintained on a regular diet and water ad libitum with a 12:12 h light/dark cycle at 23°C ± 2°C in animal room of medical school of Kermanshah University of Medical Sciences by considering 1-week adaptation prior to the experiments.
Experimental protocol
The mice were randomly divided into 12 groups (n = 7) that are brought classified as follows:
The mice in sham operation, control, and six groups of sham Aca and control Aca groups, following laparotomy and suturing in the anterior abdominal wall (shams) or not (controls), were injected intraperitoneally (I.P) to Dimethyl sulfoxide (DMSO) (0.01%) or Aca (10, 25, and 50 mg/kg) dissolved in DMSO (0.01%) once a day for 4 consequent days, respectively. In Ischemia-reperfusion involved groups, the laparotomies were carried out as long as bilateral ligating of renal arteries for 60 min.[12,13] Then, the reperfusion was done on left renal arteries followed by suturing the anterior abdominal walls of the mice. Afterward, the ischemia-reperfusion mice did not treated with any reagent and were kept alive for 5 days, whereas the ischemia-reperfusion + Aca involved mice were treated with Aca (10, 25, and 50 mg/kg) (I.P and daily for 4 days) dissolved in DMSO (0.01%), respectively. Finally, the sampling was conducted 24 h after the last injections or fifth day on those remained in non-treated condition, so all mice were sacrificed at the day 5; at 10 A.M. [Table 1]. The sampling included blood from the hearts (at least 1 ml per animal) for evaluating the urea and creatinine. The left kidneys were removed for histological and TUNEL assay examinations and the right ones for the MDA level estimations, in the respect of the groups. All the ischemia-reperfusion, laparotomy, and sacrificing operations were done when the mice were maintained in deep anesthesia that was accomplished by I.P injection of ketamine HCl (100 mg/kg) and xylazine (10 mg/kg), at 10 A.M.
Table 1.
Grouping of the mice for determining, ischemia or different doses of Aca. The treatments were daily intraperitoneally (I.P)
| Groups | Treatment | Laparotomy | Ischemia |
|---|---|---|---|
| Sham operation | No | No | No |
| Control | Dimethyl sulfoxide (DMSO) | yes | No |
| Sham Aca1 | Aca- 10 mg/kg | No | No |
| Sham Aca2 | Aca- 25 mg/kg | No | No |
| Sham Aca3 | Aca- 500 mg/kg | No | No |
| control Aca1 | Aca- 10 mg/kg | Yes | No |
| control Aca2 | Aca- 25 mg/kg | Yes | No |
| control Aca3 | Aca- 50 mg/kg | Yes | No |
| Isc-rep | No | Yes | Yes |
| Isc-rep+ Aca1 | Aca- 10 mg/kg | Yes | Yes |
| Isc-rep+ Aca2 | Aca- 25 mg/kg | Yes | Yes |
| Isc-rep+ Aca1 | Aca- 50 mg/kg | Yes | Yes |
The tissue preparing and staining
The non-parenchymal tissues (fat, fascia, and vessels) of removed left kidneys were dissected and preparing paraffin embedded blocks were gotten using automatic tissue processor. The steps of this process was consequently included fixation with 10% formal saline (for 72 h), washing thoroughly under running water, dehydrating by raised a doses of ethanol (50, 60, 70, 80, 90, and 100%, which included 3 min for each step, and 100% ethanol step was repeated for three times), clearing by xylene (three times and 10 min in each), and embedding in soft paraffin (three times and 15 min in each). At this stage, 5 μm coronal histological thin sections were cut from paraffin-embedded blocks, undertaken by a microtome instrument (Leica RM 2125, Leica Microsystems Nussloch GmbH; Germany), and 5 sections per animal were chosen. For the unification of the section selection, the first section was the 4th and the last was the 24th (5 sections interval), and finally, the routine protocol for hematoxylin and eosin staining was implemented.
At the end of tissue processing, the stained sections were mounted by entalan glue and assessed under microscope Olympus BX-51T-32E01 research microscope connected to a DP12 Camera with a 3.34-million pixel resolution and Olesya Bio software (Olympus Optical Co. LTD, Tokyo, Japan). Randomly selected sections stained by the TUNEL method that was applied in the evaluation of apoptosis as follows.
Histological quantification
In this study, both qualitative and quantitative histological parameters were evaluated, respectively. Qualitative histological involved scoring of the sections by monitoring intra-cellular vacuolization, tubular dilatation, vascular congestion, intra-tubular proteinaceous casts, and tubular cell detachments. Quantitative renal tissue changes included estimation of the number and the diameter of renal corpuscles as long as urinary space (Bowman's space) enhancement. For this reason, 5 sections/animal and 5 random fields for each section (25 fields totally) were captured at 100 × and 400 × magnifications, respectively by the connected camera to the microscope. The field's selection was done by a zigzag form of monitoring of the round or nearly rounded renal corpuscles by a blind observer using a specialized software package (AE-3; Motic S.L.U., Barcelona, Catalonia, Spain), respectively. Briefly, the diameter of each renal corpuscle was estimated as the mean length of two drawing lines, vertical to each other, that connected the distance between opposed basement membranes of the outer cell layer. The Bowman's space, the distance between the outer and the inner cell layers, was estimated by drawing at least 4 lines (in opposed directions) that connected these two layers, and the mean measured amount of these lines was considered as the space volume.[14,15]
Evaluation of BUN and creatinine
Blood serum was collected by centrifuging of the samples, separately and stored at −80°C until analysis of blood serum urea nitrogen (BUN) and urine creatinine as two functional universal biomarkers of the kidney. The concentrations of BUN and creatinine were analyzed in triplicated with a commercially available assay kit (Bioassay System, USA) in accordance with the instructions.
Measurement of renal malondialdehyde
Malondialdehyde (MDA) levels in right renal tissues were evaluated as an index of lipid peroxidation. In this regard, homogenizing of the samples were carried out by homogenization buffer containing 1.15% KCl solution and the specimens centrifuged at 1,500 g for 10 min, respectively. Then, the homogenated subjects were added to a reaction mixture containing SDS, acetic acid (pH 3.5), thiobar-bituric acid, and distilled water. Following boiling the mixture for 1 h at 95°C and centrifuging at 3000 g for 10 min, the absorbency of the supernatant was measured by spectrophotometry at 550 nm light length.
Estimation of renal total antioxidant capacity
To measure the total antioxidant capacity (TAC), an acquisition kit (Cat No: TAC-96A) ZellBio GmbH-Germany was purchased, which was the basis for the oxidation colorimetry resuscitation. The kit contains 1 reagent ready to use, buffer ×100, dye powder, reaction suspension solution, standard, and a microplate of 96 wells. In this assay, the TAC was equivalent to some antioxidant in the sample that was compared with ascorbic acid as standard. The kit's sensitivity was equal to 0.1 mM and the diagnostic range was mM 2-125/0, and final absorbance was read at 490 nm and unit conversion was performed.
TUNEL assay
TUNEL assay was carried out using in situ Cell Death Detection Kit, AP (Roche Diagnostics, Germany) according to the manufacturer's instructions. Briefly, the sections were washed in PBS, permeabilized with 0.1% Triton X-100 (Sigma, USA) for 5 min on ice, followed by incubation with spilled 50 μl of terminal deoxynucleotidyl transferase end-labeling solution for 60 min at 37°C in a humidified chamber in dark. Then, the contra-staining was performed by methylene green (1%).
Statistical analyses
The data were analyzed by SPSS software for windows (version 20) using one-way ANOVA postulation followed by Tukey's post hoc test, and P < 0.05 was considered significant. The variables were represented as mean ± standard error of mean.
Results
Qualitative histopathology changes in treated groups
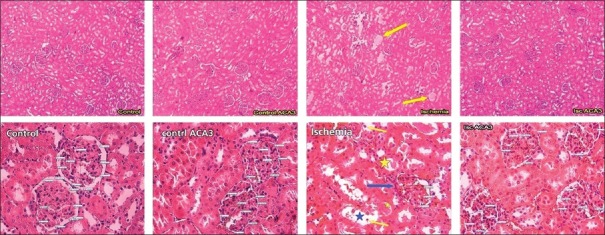
Qualitative histopathology evaluation of renal tissue in the studied groups showed that the control group, sham, Acas (shams and controls) as well as recurrent ischemia-reperfusion + Aca (50 mg/kg) group possess the same tissue indices. In ischemia-reperfusion and ischemia-reperfusion + Aca (10 mg/kg) groups, a significant increase was observed in all histopatological compared to other groups and scored 31 and 15 (P < 0.01). A significant increase of these indices was also observed in ischemia-perfusion + Aca (25 mg/kg) group (with score 6) compared to sham and control groups (P < 0.05). Furthermore, there was a dose-dependent significant difference between the Aca + ischemia-reperfusion groups [Table 2 and Figure 1].
Table 2.
Renal histological qualitative parameters affected by ischemia (ISC) and acacetin (Aca) treatment or both in male mice
| Histopathology Indices | ||||||
|---|---|---|---|---|---|---|
| Groups | Intra-cellular vacuolization | Tubular dilatation | Vascular congestion | Intra-tubular proteinaceous casts | Tubular cell detachment | Total |
| Control | 0 | 0 | I | 0 | 0 | 1 |
| Sham operation | 0 | 0 | I | 0 | 0 | 1 |
| Ischemia | IV | IV | IV | XII | VII | 31** |
| Sham Aca 10 | 0 | 0 | I | 0 | 0 | 1aab≠≠ |
| Sham Aca 25 | 0 | 0 | I | 0 | 0 | 1aab≠≠ |
| Sham Aca 50 | 0 | 0 | I | 0 | 0 | 1aa≠≠ |
| Control Aca 10 | 0 | 0 | I | 0 | 0 | 1*≠≠ |
| Control Aca 25 | 0 | 0 | I | 0 | 0 | 1aab≠≠ |
| Control Aca 50 | 0 | 0 | I | 0 | 0 | 1aab≠≠ |
| ISC+Aca 10 | II | III | IV | III | III | 15**≠ |
| ISC+Aca 25 | 0 | II | II | 0 | II | 6a*≠≠ |
| ISC+Aca 50 | 0 | 0 | I | 0 | I | 2aab≠≠ |
*p<0.05, **p<0.01 compared to control and sham groups; ap<0.05, aap<0.01 compared to isc+Aca10 mg/kg group; bp<0.05 compared to isc+ Aca25 mg/kg group; ≠p<0.05, ≠≠p<0.01 compared to ischemia-reperfusion group
Figure 1.
Showing histological features in the kidney following ischemia-reperfusion and acacetin (Aca) (50 mg/kg) or both by H and E staining. In ischemia-reperfusion group, the yellow arrows in above picture and yellow stars in below picture show intra-tubular proteinaceous casts, whereas in below picture the yellow arrows depict tubular cell detachments, and arrow and star in blue demonstrate the shrinkage of renal glomerulus and tubular dilation, respectively (Above pictures are 100×, and below ones are 400 × captured, respectively). The originating sites of the glomerular vessels are shown by circles
Number of renal corpuscles
The results of the number of renal corpuscles between the groups showed a significant decrease in renal corpuscles number in the ischemic/reperfusion group compared to the control group (P < 0.01), but there was no significant difference in other groups compared to the control group. In all groups, a significant increase in the number of renal corpuscles was observed in comparison to ischemic/reperfusion group (P < 0.05). Although the number of renal corpuscles in the ischemia-reperfusion + Aca groups was increased with an increase in dose, statistical analysis showed no significant difference. A number of renal corpuscles in the sham operation and receiving Aca at doses of 50 mg/kg groups was significantly increased compared to Aca at doses of 10 mg/kg group (P < 0.05) [Table 3].
Table 3.
Renal histological quantitative parameters affected by ischemia (ISC) and acacetin (Aca) treatment or both in male mice
| Groups | Mean±standard error of means | ||
|---|---|---|---|
| Number of renalcorpuscles | Diameter of renal corpuscles (µm) | Urinary space (µm) | |
| Isc+ Aca 50 mg/kg | 8.20±0.37≠≠ | 67.92±4.01≠≠ | 14.20±0.92≠≠,a |
| Isc+ Aca 25 mg/kg | 7.88±0.45≠≠ | 63.17±3.58*,≠≠ | 14.78±0.46≠≠ |
| Isc+ Aca 10 mg/kg | 7.70±0.40≠≠ | 57.05±6.21**,≠≠ | 16.35±1.16*,≠≠ |
| Sham Aca 50 mg/kg | 9.35±0.34≠≠ | 90.95±4.17≠≠,aa,b | 9.05±0.49≠≠,aa,bb,cc |
| Sham Aca 25 mg/kg | 8.91±0.45≠≠ | 88.77±5.18≠≠,aa | 10.05±0.42≠≠,aa,bb,cc |
| Sham Aca 10 mg/kg | 8.78±0.37≠≠ | 84.95±4.09≠≠,aa | 11.04±0.47≠≠,aa,bb,c |
| Sham operation | 8.47±0.37≠≠ | 83.26±3.30≠≠,aa | 13.74±0.56≠≠,aa,b |
| Ischemia | 3.68±0.51** | 49.26±3.05** | 22.56±1.31** |
| Control Aca 50 mg/kg | 9.45±0.44≠≠ | 90.21±5.18≠≠,aa,b | 10.06±0.53≠≠,aa,bb,c |
| Control Aca 25 mg/kg | 9.00±0.60≠≠ | 87.36±5.09≠≠,aa,bb,c | 10.54±0.45≠≠,aa,bb,c |
| Control Aca 10 mg/kg | 8.67±0.48≠≠ | 84.70±5.08≠≠,aa,b | 11.13±0.43≠≠,aa,bb,c |
| Control | 8.57±0.57 | 83.36±3.49 | 13.58±0.73 |
*p<0.05, **p<0.01 compared to control/sham groups; ap<0.05, aap<0.01 compared to isc+ Aca10 mg/kg group; bp<0.05, bbp<0.01 compared to isc+ Aca25 mg/kg group; cp<0.05, ccp<0.01 compared to isc+Aca50 mg/kg group; ≠≠compared to ischemia/reperfusion group
Diameter of renal corpuscles
The diameter of renal corpuscles significantly decreased in the ischemia-reperfusion group compared to the control group (P < 0.01), but there were no significant difference in other groups compared to the control group. A significant increase in diameter of renal corpuscles was observed in groups receiving Aca at doses of 10 and 25 mg/kg and sham operation groups compared to ischemia-reperfusion + Aca at a dose of 10 mg/kg group (P < 0.01). The diameter of renal corpuscles in the group receiving Aca at doses of 50 mg/kg showed a significant increase compared to ischemia-reperfusion + Aca at a dose of 25 mg/kg (P < 0.05).
The diameter of renal corpuscles was increased in the ischemia-reperfusion + Aca groups with a dose-dependent manner, but without showing any significant differences [Table 3].
Urinary (Bowman's) space
The urinary space was increased significantly in ischemic/reperfusion group compared to the control group (P < 0.01).A significant increase was observed also in ischemia-reperfusion + Aca at a dose of 10 mg/kg group. In all groups, a significant increase was found in urinary space compared to the ischemia-reperfusion group (P < 0.01). Further, there was a significant decrease in Aca receiving group than ischemia-reperfusion + Aca groups (P < 0.05). Sham operation group showed a significant decrease compared to ischemia-reperfusion + Aca at doses of 10 and 25 mg/kg group (P < 0.05). There was a significant difference between ischemia-reperfusion groups (P < 0.05) [Table 3].
Measurement of Urea (BUN) levels
The serum levels of BUN were significantly increased in the ischemia-reperfusion and ischemia-reperfusion + Aca at doses of 10 and 25 mg/kg groups in comparison with the control group (P < 0.01). A significant decrease in BUN levels was showed in all experimental groups compared to the ischemia-reperfusion group (P < 0.01). Sham operation and Aca receiving groups (with or without surgery) had a significant reduction in BUN compared with ischemia-reperfusion + Aca at doses of 10 and 25 mg/kg group (P < 0.01), however, in ischemia-reperfusion + Aca at a dose of 50 mg/kg group, there was no significant difference. Furthermore, there was a significant difference in BUN in the ischemia-reperfusion + Aca at doses of 25 mg/kg and 50 mg/kg groups compared with ischemia-reperfusion + Aca at a dose of 10 mg/kg group (P < 0.01) [Table 4].
Table 4.
The serum levels Urea (BUN) and Creatinine following Ischemia (ISC) and Acacetin (Aca) treatment or both in male mice
| Groups | Mean±standard error of means | |
|---|---|---|
| Urea (BUN) | Creatinine | |
| Isc+ Aca 50 mg/kg | 6.25±0.21≠≠,aa | 6.29±0.94≠≠,aa |
| Isc+ Aca 25 mg/kg | 8.23±0.24**,≠≠,aa | 9.25±0.78*,≠≠,aa |
| Isc+ Aca 10 mg/kg | 15.16±0.07**,≠≠ | 11.77±0.34**,≠≠ |
| Sham Aca 50 mg/kg | 4.91±0.23≠≠,aa,bb | 4.78±0.23≠≠,aa,bb |
| Sham Aca 25 mg/kg | 4.22±0.16≠≠,aa,bb | 5.24±0.40≠≠,aa,bb |
| Sham Aca 10 mg/kg | 4.53±0.18≠≠,aa,bb | 5.54±0.13≠≠,aa,bb |
| Sham operation | 28.78±1.48≠≠,aa,bb | 5.48±0.12≠≠,aa,bb |
| Ischemia | 4.55±0.14** | 69.18±0.14** |
| Control Aca 50 mg/kg | 4.07±0.07≠≠,aa,bb | 5.22±0.12≠≠,aa,bb |
| Control Aca 25 mg/kg | 4.33±0.18≠≠,aa,bb | 5.30±0.15≠≠,aa,bb |
| Control Aca 10 mg/kg | 4.55±0.12≠≠,aa,bb | 5.34±0.18≠≠,aa,bb |
| Control | 4.73±0.18 | 5.08±0.18 |
*p<0.05, **p<0.01 compared to control and sham groups; aap<0.01 compared to Isc+ Aca 10 mg/kg group; bbp<0.01 compared to Isc+ Aca 25 mg/kg group; ≠≠compared to ischemia-reperfusion group
Measurement of serum creatinine levels
The serum levels of creatinine were significantly raised in ischemia-reperfusion (P < 0.01), two groups of ischemia-reperfusion + Aca 10 and 25 mg/kg (P < 0.05) compared to the control group. However, there were no significant differences in this parameter between ischemia-reperfusion + Aca (50 mg/kg) and control group. In all experimental groups, a significant reduction was found in serum levels of creatinine compared to the ischemia-reperfusion group (P < 0.01). Sham operation and Aca receiving groups (with or without surgery) had a significant reduction in serum creatinine levels compared with ischemia-reperfusion + Aca at doses of 10 and 25 mg/kg group (P < 0.01), however, in ischemia-reperfusion + Aca at a dose of 50 mg/kg group, there was no significant difference. Moreover, there was a significant difference in serum levels of creatinine in the ischemia-reperfusion + Aca at doses of 25 mg/kg and 50 mg/kg groups compared with ischemia-reperfusion + Aca at a dose of 10 mg/kg group (P < 0.01) [Table 4].
Effect of acacetin and renal ischemia-reperfusion on malondialdehyde levels
The serum levels of MDA significantly boosted in the ischemia-reperfusion (P < 0.01), ischemia-reperfusion + Aca at doses of 10 mg/kg and 25 mg/kg groups compared to the control group (P < 0.01 and P < 0.05 respectively). In comparison with ischemic/reperfusion, MDA levels were significantly reduced in all experimental groups (P < 0.01). In addition, a significant reduction was observed in sham operation and Aca receiving groups (with or without surgery) in serum MDA levels compared with ischemia-reperfusion + Aca at doses of 10 and 25 mg/kg group (P < 0.01), however, in ischemia-reperfusion + Aca at a dose of 50 mg/kg group, there was no significant difference. Furthermore, there was a significant decrease in serum levels of MDA in the ischemia-reperfusion + Aca at doses of 10 mg/kg and 25 mg/kg groups compared with ischemia-reperfusion + Aca at a dose of 50 mg/kg group (P < 0.01) [Figure 2a].
Figure 2.
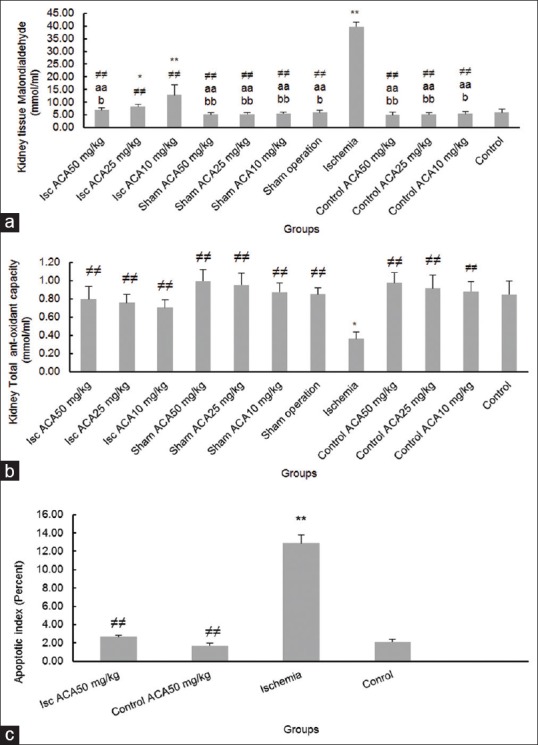
Effect of ischemia- reperfusion (ISC) and Acacetin (Aca) or both on (a) malondialdehyde, (b) Total anti-oxidant capacity, (c) Apoptotic index in mice. *P < 0.05, **P < 0.01 compared to control and sham groups; a = P < 0.05, aa = P < 0.01 compared to Isc + Aca10 mg/kg group; b = P < 0.05, bb = P < 0.01 compared to Isc + Aca 25 mg/kg group; ≠≠: compared to ischemia-reperfusion group
Effect of acacetin and renal ischemia-reperfusion on total antioxidant capacity levels
The TAC levels were decreased significantly in the ischemia-reperfusion group rather than the control group (P < 0.01). In all experimental groups, a significant rise was found in serum levels of TAC compared to the ischemia-reperfusion group (P < 0.01). In addition, Aca receiving groups (with or without surgery) had a significant increase in TAC levels compared with ischemia-reperfusion + Aca groups (P < 0.01). In spite the decline in serum levels of TAC in ischemia-reperfusion + Aca groups compared with each other, no statistically significant difference was found [Figure 2b].
Effect of Aca and renal ischemia-reperfusion on TUNEL positive cells
Evaluation of apoptotic cells showed a significant increase in ischemia-reperfusion than control, Aca at a dose of 50 mg/kg and ischemia-reperfusion + Aca at a dose of 50 mg/kg groups (P < 0.01). However, there was no significant difference in the apoptotic index between the two groups of Aca at a dose of 50 mg/kg and ischemia-reperfusion + Aca at a dose of 50 mg/kg [Figures 2c and 3].
Figure 3.
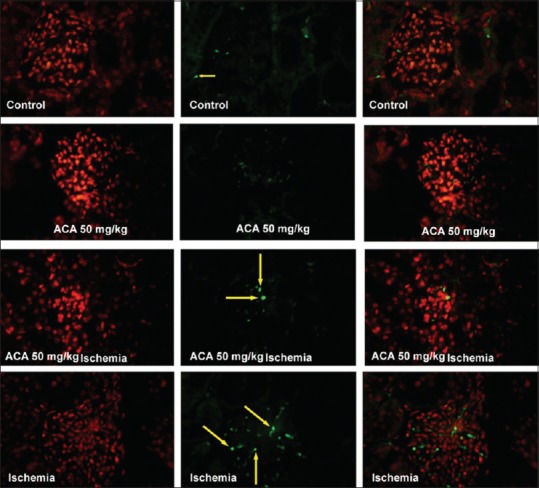
Apoptosis induction in the kidney followed by ischemia-reperfusion and acacetin (Aca) (50 mg/kg) or both by TUNEL staining (×400); Aca = acacetin. The yellow arrows referred to the emerald green nuclei of apoptotic cells. The left pictures are stained cytoplasm, the middle figures show stained nuclei, and the right ones are the merges
Discussion
Overall, the current study represented that ischemia-reperfusion exacerbated histopathology and functional indices of the kidneys, such as Bowman's space, diameter of renal corpuscle, serum levels of BUN and creatinine, oxidant-antioxidant balance, promoting apoptosis, whereas Aca relief these diverse effects in a dose-dependent manner.
The histopathology changes following ischemia-reperfusion on renal tissue have been approved by many authors, and our results in this regard are parallel with them showing enlargement of Bowman's space, injury in tubules of the cortex, and occurrence of dramatically change in whole tissue qualitative indices.[2,3,16]
Studies about the effect of Aca on renal tissue are rare. Ávila-Villarreal and colleagues demonstrated that high doses of plant extract of Brickellia Cavanillesii (Cass.),[17] which contains Aca, was safe and its LD50 is higher than 2000 mg/kg in mice. Further, 100 mg/Kg of Aca did not alter the function of the liver, kidney, and heart in mice.[17] These data are in parallel with our results that showed a high dose of Aca (50 mg/kg) does not induce harmful effects, both histologically and functionally, on kidney of mice, even in chromic usage. The impact of Aca (150 mg/kg) on renal tissue damage induced by bacteria (Staphylococcus aureus) was approved,[18] however, there was not found any evidence for showing the effect of Aca on renal tissue after ischemia-reperfusion, and in this case, our data are unique. Furthermore, the present study indicated that there were no significant differences for the efficacy of Aca with 25 mg/kg and 50 mg/kg in all evaluated parameters; however, the higher dose was more efficient. Thus, in the case of ischemia-reperfusion than bacterial infected kidney, we can say that lower doses of Aca should be considered as retrieve dose.
These differences are may be owing to the doses of Aca, which in the later study was lower than the former one and as we saw in the present study, at the dose of 10 mg/kg, the efficacy of Aca was not complete. Nevertheless, it has been shown an increase in the serum levels of MDA following renal ischemia-reperfusion,[4,5] we conducted the measurement of MDA and TAC in the kidney tissue to omit any bias.
The renal ischemia-reperfusion-induced tubular epithelial damage is either lethal, expressed as necrosis and apoptosis, or sub-lethal, including loss of tight junction integrity and cell polarity, shedding of brush borders and exfoliation of viable cells.[19,20] In the present study, the percentage of apoptotic cells was increased after ischemia-reperfusion treatment in accompany with previous reports; however, the necrosis was not observed.
Recently, several in vitro studies have approved AC therapeutic properties on human cancerous cells such as lung, prostate, and breast cancer cell lines, in which apoptosis induction suggested as the main pharmacokinetic of Aca on cancers.[8,21,22] Thus, we suggest that Aca has dual effects; anti- apoptotic activity for the treatment of degenerative diseases like ischemia-reperfusion and the second, inducing apoptosis (the opposed action), in cancers that we need apoptosis as a treatment trend. Somehow, our data reinforce the anti-cancer capability of Aca that does not exert any side effects.
Conclusions
The results of this study showed that arterial renal ischemia for 60 min and subsequent their reperfusion, even after 5 days, impress dangerous histological and physiological outcomes in the kidney. The study approves that eliminated renal oxidant-antioxidant balance as molecular advocator during the ischemia-reperfusion period would supervise cellular chain reaction. Aca, with a dose-dependent manner, upregulates oxidant system as long as down regulates lipid peroxidation and apoptosis following renal ischemia-reperfusion. Finally, this study emphasizes that suppressing oxidative stress is the major priority for a drug prescription in the case of organ transplantation like kidney. Hence, contemplating of this trend would diminish the destructive sequels of this surgical procedure.
Financial support and sponsorship
This study was fully supported by the Kermanshah University of Medical Sciences as a PhD thesis [Project No: 96348].
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe A, Blute ML, Ficarra V, Gill IS, Kutikov A, Porpiglia F, et al. Renal ischemia and function after partial nephrectomy: A collaborative review of the literature. Eur Urol. 2015;68:61–74. doi: 10.1016/j.eururo.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1 99;;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp Biochem PhysiolC: Toxicol Pharmacol. 2006;142:317–27. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Tucci Junior S, Carvalho RM, Celini FM, Cologna AJ, Suaid HJ, Tirapelli LF, et al. Renal ischemia and reperfusion injury: Influence of chorpromazine on renal function and lipid peroxidation. Acta Cir Bras. 2008;23:42–6. doi: 10.1590/s0102-86502008000700008. [DOI] [PubMed] [Google Scholar]
- 7.Kim SB, Lee T, Lee HS, Song CK, Cho HJ, Kim DD, et al. Development and validation of a highly sensitive LC–MS/MS method for the determination of acacetin in human plasma and its application to a protein binding study. Arch pharm Res. 2006;39:213–20. doi: 10.1007/s12272-015-0697-1. [DOI] [PubMed] [Google Scholar]
- 8.Shim HY, Park JH, Paik HD, Nah SY, Kim DS, Han YS. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. MolCells. 2007;24:95–104. [PubMed] [Google Scholar]
- 9.Li GR, Wang HB, Qin GW, Jin MW, Tang Q, Sun HY, et al. Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation. 2008;117:2449–57. doi: 10.1161/CIRCULATIONAHA.108.769554. [DOI] [PubMed] [Google Scholar]
- 10.Ha SK, Moon E, Lee P, Ryu JH, Oh MS, Kim SY. Acacetin attenuates neuroinflammation via regulation the response to LPS stimuli in vitro and in vivo. Neurochem Res. 2012;37:1560–7. doi: 10.1007/s11064-012-0751-z. [DOI] [PubMed] [Google Scholar]
- 11.Yang WJ, Liu C, Gu ZY, Zhang XY, Cheng B, Mao Y, et al. Protective effects of acacetin isolated from Z iziphora clinopodioides Lam.(Xintahua) on neonatal rat cardiomyocytes. Chin Med. 2014;9:28. doi: 10.1186/s13020-014-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Yang L, Wu HJ, Chen KH, Lin F, Li G, et al. Water-soluble acacetin prodrug confers significant cardioprotection against ischemia/reperfusion injury. Sci Rep. 2016;6:36435. doi: 10.1038/srep36435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HI, Park JH, Choi HS, Kwak JH, Lee DU, Lee SK, et al. Protectivemechanisms of acacetin against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. J Nat Prod. 2016;77:2497–503. doi: 10.1021/np500537x. [DOI] [PubMed] [Google Scholar]
- 14.Venditti CC, Casselman R, Young I, Karumanchi SA, Smith GN. Carbon monoxide prevents hypertension and proteinuria in an adenovirus sFlt-1 preeclampsia-like. PLoS One. 2014;9:e106502. doi: 10.1371/journal.pone.0106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalili C, Salahshoor MR, Hoseini M, Roshankhah S, Sohrabi M, Shabanizadeh A. Protective effect of thymoquinone against morphine injuries to kidneys of mice. Iran J Kidney Dis. 2017;11:142–50. [PubMed] [Google Scholar]
- 16.Altuner D, Cetin N, Suleyman B, Aslan Z, Hacimuftuoglu A, Gulaboglu M, et al. Effect of thiamine pyrophosphate on ischemia-reperfusion induced oxidative damage in rat kidney. Indian J Pharmacol. 2013;45:339–43. doi: 10.4103/0253-7613.115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ávila-Villarreal G, González-Trujano ME, Carballo-Villalobos AI, Aguilar-Guadarrama B, García-Jiménez S, Giles-Rivas DE, et al. Anxiolytic-like effects and toxicological studies of Brickelliacavanillesii (Cass) A Gray in experimental mice models. J Ethnopharmacol. 2016;192:90–8. doi: 10.1016/j.jep.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Bi C, Dong X, Zhong X, Cai H, Wang D, Wang L. Acacetin protects mice from Staphylococcus aureus bloodstream infection by inhibiting the activity of sortase A. Molecules. 2016;21:1285. doi: 10.3390/molecules21101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 20.Moosavi SMS, Bayat GR, Owjie SM, Panjehshahin MR. The early renal post-ischemic tissue damage and dysfunction with contribution of A1-adenosine receptor activation in rat. Nephrology. 2009;14:179–88. doi: 10.1111/j.1440-1797.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- 21.Chien ST, Lin SS, Wang CK, Lee YB, Chen KS, Fong Y, et al. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38α MAPK signaling pathway. Mol Cell Biochem. 2011;350:135–48. doi: 10.1007/s11010-010-0692-2. [DOI] [PubMed] [Google Scholar]
- 22.Shen KH, Hung SH, Yin LT, Huang CS, Chao CH, Liu CL, et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem. 2010;333:279–91. doi: 10.1007/s11010-009-0229-8. [DOI] [PubMed] [Google Scholar]