Abstract
Background:
Inflamation is widely known as an adaptive pathophysiological response in a variety of cancers. There is an expanding body of research on the key role of diet in inflammation, a risk factor for all types of cancer. Dietary inflammatory index (DII) was recently develpoed to evalute the inflammatory potential of a diet either as anti-inflammatory or pro-inflammatory. In fact, several studies have shown the association of DII and risk of different cancer types. The aim of this meta-analysis was to investigate the association of DII with risk of incidence and mortality of any cancer types.
Methods:
We searched PubMed-Medline, Scopus, and Web of Science databases for pertient studies util January, 2017. All studies conducted to investigate the association of DII and incidence, mortality, and hospitalization of all cancer types were included. According to degree of heterogeneity, fixed- or random-effect model was employed by STATA software.
Results:
Total 38 studies were eligible for the meta-analysis. The results show that a higher level of DII increases the risk for all cancer types incidence by 32% (OR: 1.32; 95% CI: 1.22-1.42) including digestive tract cancers (OR: 1.55; 95% CI: 1.33-1.78), hormone-dependent cancers (OR: 1.14; 95% CI: 1.04-1.24), respiratory tract cancers (OR: 1.64; 95% CI: 1.11-2.17), and urothelial cancers (OR: 1.36; 95% CI: 1.01-1.73). Moreover, a higher level of DII is in association with a higher risk for mortality caused by all types of cancer by 16% (OR: 1.16; 95% CI: 1.01-1.32). In addition, meta-regression analysis reveals that the design of study can have a significant effect on the association of DII and incidence of all cancer types (slope: 0.54; P= 0.05). The stratified meta-analysis shows that the association of DII and incidence of all cancer types in case-control studies (OR: 1.53; 95% CI: 1.36-1.71) were more prominent than cohort studies (OR: 1.18; 95% CI: 1.07-1.30).
Conclusions:
This study shows that a higher level of DII is associated with a higher risk of incidence and mortality of all cancer types. The findings of the present study suggest that modifying inflammatory properties of dietary patterns can reduce the risk of incidence and mortality of all cancer types.
Keywords: Cancer, diet, dietary inflammatory index, inflammation
Background
Inflammation is now widely known as an adaptive pathophysiological response underlying various chronic diseases including type 2 diabetes mellitus, cardiovascular disease, obesity, metabolic diseases, and specific types of cancer.[1,2,3] Several factors are associated with inflammation such as sex, age, and lifestyle. Lifestyle such as diet, physical activity, and smoking as malleable factors can reduce inflammation and thereby contributing to health.
Diet plays a contributing role in the regulation of inflammatory process. Various biomarkers have used to evaluate the association of nutrition and low-grade inflammatory status.[4] Consequently, it may be beneficial to identify dietary patterns related to their inflammatory properties.[5] Dietary inflammatory index (DII) is a new approach used to evaluate the inflammatory potential of a diet as either anti-inflammatory or pro-inflammatory.[6] In fact, some of the dietary patterns such as western pattern diet rich in red meat and refined grains is associated with a higher level of CRP, TNF- α, IL-1β, IL-2, and IL-6, which is often referred to as pro-inflammatory biomarkers. In contrast, there is an inverse association between Mediterranean diet including high amounts of fruits, whole grains, extra-virgin olive oil, and pro-inflammatory status.[7,8]
Nowadays, the inflammatory properties of diet and its role in preventing chronic diseases have attracted much attention from health sciences researchers. Although in recent years several studies have shown the association of DII and risk of different cancer types, the findings of these studies are heterogeneous according to the type of study and cancer. However, according to our knowledge, pooled estimate of association of DII and all cancers is unclear and have not been investigated yet by systematic review. The aim of this meta-analysis was to investigate the association of DII with risk of incidence and mortality of any cancer types.
Methods
To evaluate the maximum level of sensitivity, we simultaneously searched main international electronic data sources; PubMed and NLM Gateway (for MEDLINE), Institute of Scientific Information (ISI), and SCOPUS for studies until January, 2017. Further, a hand-search of all references included in the identified articles. We did not limit our research by the publication date and language.
Our strategy for searching relevant studies was using the following key words “Index-based dietary patterns,” “dietary inflammatory Index or DII,” and all related domains to neoplasm,” “cancer,” “Malignancy,” and “tumor”.
Any observational epidemiologic study, either cross-sectional, case-control, or cohort, which had used DII, and the estimation of a adjusted effect size measure [odds ratio (OR), relative risk (RR), and hazard ratio (HR)] and 95% confidence interval (CI) comparing level and score of the DII with respect to the risk of incidence, mortality, and length of hospitalization of all cancer types were eligible to include in this systematic review. We excluded all papers with duplicate entries. In case of multiple publications on the same population, only the largest study or the main source of data was included.
The quality of studies was assessed using the Newcastle-Ottawa scale designing for cohort and case-control studies. According to this scale, 9 points can be allocated to each study including four scores for selection, two scores for comparability, and three scores for assessment of outcomes. The process of quality assessment and data extraction was carried out independently by two research experts. Quality assessment agreement on quality assessment between raters was established using Cohen's kappa statistic. The Kappa statistic for agreement on quality assessment was 0.92, which shows perfect agreement. The discrepancy between the raters was resolved by an auditor. Data were extracted according to a checklist. The items on the checklist included (a) the number of citation; (b) demographic characteristics of population such as age, target population, and type of cancers; (c) methodological information of study such as study design, food assessment questionnaire, duration of follow-up, sample size, type of effect size measure (OR, RR, and HR), and adjusted covariates.
Statistical analysis
We examined the association of DII and cancers in terms of morbidity (incidence), mortality, and length of hospitalization. For meta-analysis, we classified cancers into four main categories: (a) digestive tract cancers; (b) hormone-dependent cancers; (c) respiratory tract cancers; and (d) urothelial cancers. However, for those studies that reported several adjusted models, we included only the multivariate model. Although in this systematic review we included all studies with reported DII as continuous (score) or categorical variable (tertile/quartile/quintile), we performed meta-analysis only for DII as categorical variable. In meta-analysis, risk of incidence and mortality of cancer in the highest level of DII (last tertile/quartile/quintile) was compared with lowest level of DII (last tertile/quartile/quintile). Although a number of studies have reported cancer subsites, meta-analysis have not performed according to subsites of cancer.[9,10,11,12,13,14] The meta-analysis on the association between DII and risk of cancer mortality has been conducted only for all cancer mortality. Because there was only one study on the association between length of hospitalization and DII, we did perform mate-analysis for the association of DII and length of hospitalization of cancer.
The results reported as adjusted effect size measure and 95% CI. The Chi-square based Q test and I square statistics used to assess the heterogeneity between studies. The results of Q test were statistically significant at P < 0.1. Because of severe heterogeneity among studies on the reported values, pooled estimate was estimated using random-effect meta-analysis model (using the Dersimonian and Laird method). The forest plot also was used to present the results of meta-analysis schematically. A random-effects meta-regression was performed using unrestricted maximum likelihood method to evaluate the association of estimated effect size measure and potential confounders such as design of study, type of cancer, food assessment questionnaire, and publication year. Potential publication bias was assessed using Egger's weighted regression tests, and the results of Egger's test were statistically significant at P < 0.1. The funnel plot also was used to present the results of publication bias schematically. “Trim and fill” method was used to adjust the analysis for the effects of publication bias. All statistical analysis was performed using STATA 11 software.
Ethical considerations
The protocol of study was approved by the ethical committee of Alborz University of Medical Science. All reviewed studies were properly cited. For more information about a certain study, we contacted the corresponding authors.
Results
The literature search strategy yielded a total of 575 publications. Further, 148 duplicated articles were excluded. After screening titles and abstracts, 345 irrelevant publications were excluded. Then, 82 remained articles and 6 retrieved articles through reference checking were carefully assessed and reviewed for eligibility; of which, 50 studies were excluded according to inclusion criteria. Finally, 38 studies met the inclusion criteria [Figure 1]. The main results of the selected articles were discussed in terms of incidence (n = 29), mortality (n = 7), both of them (n = 1), and length of hospitalization (n = 1) in patients with different types of cancers.
Figure 1.
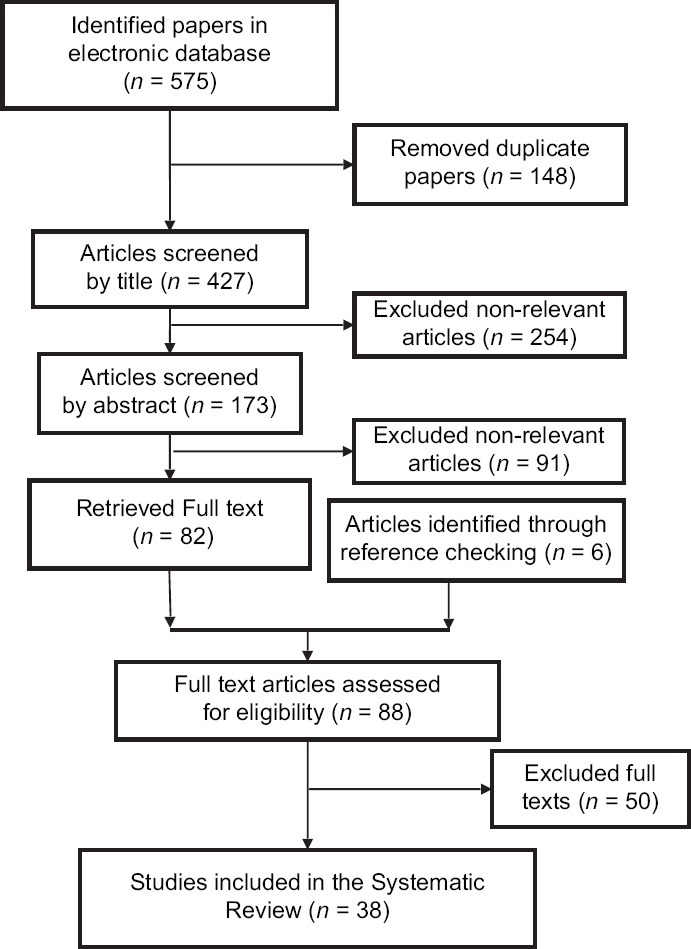
Papers search and review flowchart for selection of primary studies
We found 30 articles (i.e. 20 case- controls and 10 cohorts) on the association of DII and incidence of different cancer types [Table 1]. Twenty-eight articles used food frequency questionnaire (FFQ), and the rest used 24 hour dietary recall (24HR) and dietary history questionnaire as dietary assessment instruments. The highest and lowest effect size measures (95% CI) were observed for esophageal squamous cell carcinoma (OR: 8.24; 95% CI: 2.03-33.47) and breast cancer (HR: 0.85; 95% CI: 0.52-1.41), respectively.
Table 1.
Association between DII and risk of cancer incidence
| Study number | First author (year) | Design | Follow- -up (years) | Food assessment questionnaire | Type/site of cancer | Total sample size (incident cases) | Groups | Type of effect size measure | Effect size measure (95% CI) | Covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Samuel O. Antwi (2016)[30] | Case-control | NA | 144 -item FFQ | Pancreatic cancer | 2573 (817) | Quintile 5 (>-0.03, 4.47) vs. Quintile 1 (-5.33,-3.07) | OR | 2.54 (1.87-3.46) | Age, sex, race, diabetes, BMI, pack-years of smoking, education |
| 2 | Young Ae Cho (2016)[9] | Case-control | NA | 106-item semi-quantitative FFQ | Colorectal cancer Colon cancer Proximal colon cancer Distal colon cancer Rectal cancer | 2769 (923) 2306 (460) 2011 (165) 2141 (295) 2290 (444) | Tertile 3 (≥2.30) vs. Tertile 1 (<0.30) | OR | 2.16 (1.71-2.73) 2.05 (1.53-2.74) 1.68 (1.08-2.61) 2.28 (1.61-3.21) 2.23 (1.66-3.00) | age, sex, BMI, education, family history of colorectal cancer, physical activity, and total calorie intake |
| 3-1 | Pierre-Antoine Dugue (2016)[31] | cohort | 21.3 | 121-item FFQ | Urothelial cell carcinoma | 41514 (379) | Quintile 5 vs. Quintile 1* | HR | 1.24 (0.90-1.70) | sex, country of birth, smoking, alcohol consumption, body mass index physical activity, education, and socioeconomic status |
| 3-2 | Pierre-Antoine Dugue (2016)[31] | cohort | 21.3 | 121-item FFQ | Urothelial cell carcinoma | 41514 (379) | Continuous DII (per one unit increment) | HR | 1.07 (0.97-1.19) | sex, country of birth, smoking, alcohol consumption, body mass index physical activity, education, and socio-economic status |
| 4 | Isabell Ge (2015)[32] | case-control | NA | 176-items FFQ | Breast cancer | 8300 (2887) | Quintile 5 (1.922, 5.504) vs. Quintile 1 (-4.604, -0.213) | OR | 1.01 (0.86-1.17) | age, study region, lifestyle confounders (total physical activity after 50 years, energy intake), breast cancer risk factors (age of menarche, number of pregnancies, breastfeeding history, induction of menopause, first-degree family history of breast cancer, history of benign breast disease, number of mammograms, hormone use) |
| 5 | Laurie Graffouille`re (2016)a[33] | cohort | 12.6 | 24 HR | Breast cancer | 3771 (158) | Quartile 4 vs.Quartile 1* | HR | 0.85 (0.52-1.41) | Age, sex, intervention group of the initial SU.VI.MAX trial, number of 24-h dietary records, BMI, height, physical activity, smoking status, educational level, energy intake, and family history in addition to menopausal status |
| Prostate cancer | 2771 (123) | 2.08 (1.06-4.09) | ||||||||
| vnon-prostate cancer (other cancers) | 6542 (278) | 1.34 (0.92-1.95) | ||||||||
| All cancers | 6542 (559) | 1.23 (0.94-1.62) | ||||||||
| 6 | A. M. Hodge (2016)[26] | cohort | 18 | 121-item FFQ | Lung cancer | 35,303 (403) | Quartile 4 (0.39,4.86) vs. Quartile 1 (-4.91,-2.15) | HR | 1.31 (0.91-1.89) | pack-years, years since quit smoking, smoking status, country of birth, education, BMI, alcohol intake, physical activity, sex, SEIFA quintile, energy (includes an interaction between smoking status and country of birth) |
| 7 | Yunxia Lu (2016)[34] | Case-control | NA | 63-item FFQ | Esophageal squamous cell carcinoma | 946 (158) | Quartile 4 (≥1.46) vs. Quartile 1 (<−1.04) | OR | 4.35 (2.24-8.43) | age, sex, energy, education, tobacco smoking, alcohol intake, and physical activity in addition to reflux, and Helicobacter pylori infection (for oesophageal adenocarcinoma and gastroesophageal junctional adenocarcinoma) |
| Esophageal adenocarcinoma | 987 (181) | 3.59 (1.87-6.89) | ||||||||
| Gastroesophageal junctional adenocarcinoma | 1061 (255) | 2.04 (1.24-3.36) | ||||||||
| Esophageal or gastroesophageal junction adenocarcinoma | 1242 (436) | 2.42 (1.57-3.73) | ||||||||
| 8 | Patrick Maisonneuve (2016)[35] | Cohort | 8.5 | 45-item FFQ | Lung cancer | 4336 (200) | Quartile 4 vs.Quartile 1* | HR | 1.54 (0.93-2.55) | baseline risk probability (age, sex, smoking duration, smoking intensity, years of smoking cessation, and asbestos exposure) and total energy |
| 9-1 | Lauren C. Peres (2017)[36] | case-control | NA | 110-item FFQ | Epithelial ovarian cancer | 1155 (493) | Quartile 4 (-0.32, 3.19) vs. Quartile 1 (-5.57, -3.64) | OR | 1.72 (1.18-2.51) | study design variables, age, and study site, family history of breast or ovarian cancer in a first degree relative, parity, OC use, education, BMI, tubal ligation, menopausal status, smoking status, and endometriosis |
| 9-2 | Lauren C.Peres (2017)[36] | case-control | NA | 110-item FFQ | Epithelial ovarian cancer | 1155 (493) | Continuous DII (per one unit increment) | OR | 1.10 (1.03-1.17) | study design variables, age and study site, family history of breast or ovarian cancer in a first degree relative, parity, OC use, education, BMI, tubal ligation, menopausal status, smoking status, and endometriosis |
| 10-1 | Nitin Shivappa (2016)a[37] | Cohort | 25 | 121-item FFQ | Breast cancer | 34700 (2934) | Tertile 3 (> -0.05) vs.Tertile 1 (<-2.08) | HR | 1.11 (1.00-1.22) | Age, energy and BMI, smoking status, pack-years of smoking, education, HRT use, oral contraceptive use, number of live births, education, age at menarche, age at menopause and history of hysterectomy |
| 10-2 | Nitin Shivappa (2016)a[37] | Cohort | 25 | 121-item FFQ | Breast cancer | 34700 (2934) | Continuous DII (per one unit increment) | HR | 1.01 (0.99-1.04) | Age, energy and BMI, smoking status, pack-years of smoking, education, HRT use, oral contraceptive use, number of live births, education, age at menarche, age at menopause and history of hysterectomy |
| 11 | Nitin Shivappa (2015)c[38] | case-control | NA | 78-item FFQ | Prostate cancer | 2754 (1294) | Quartile 4 (>0.49) vs. Quartile 1 (<-1.98) | OR | 1.33 (1.01-1.76) | Age, study center, BMI, years of education, social class, smoking status, family history of prostate cancer, and total energy intake |
| 12 | Nitin Shivappa (2015)d[39] | case-control | NA | 78-item FFQ | Pancreatic cancer | 978 (326) | Quintile 5 (≥ 1.27) vs.Quintile 1 (< - 1.28) | OR | 2·48 (1.50-4.10) | Age, sex, study center, year of interview, education, BMI, smoking status, alcohol drinking, and history of diabetes |
| 13-1 | Nitin Shivappa (2016)f[40] | case-control | NA | 78-item FFQ | Gastric cancer | 777 (230) | Quartile 4 (>1.49) vs. Quartile 1 (≤1.47) | OR | 2.35 (1.32-4.20) | study center, age, education, year of interview, BMI, smoking and total energy intake |
| 13-2 | Nitin Shivappa (2016)f[40] | case-control | NA | 78-item FFQ | Gastric cancer | 777 (230) | Continuous DII (per one unit increment) | OR | 1.19 (1.06-1.34) | study center, age, education, year of interview, BMI, smoking, and total energy intake |
| 14-1 | Nitin Shivappa (2015)g[41] | case-control | NA | 125-item FFQ | Esophageal squamous cell carcinoma | 143 (47) | High (>1.20) vs. Low (≤120) | OR | 8.24 (2.03-33.47) | age, energy, sex, BMI, years of education, physical activity, smoking, and gastro-oesophageal reflux |
| 14-2 | Nitin Shivappa (2015)g[41] | case-control | NA | 125-item FFQ | Esophageal squamous cell carcinoma | 143 (47) | Continuous DII (per one unit increment) | OR | 3.58 (1.76-7.26) | age, energy, sex, BMI, years of education, physical activity, smoking, and gastro-oesophageal reflux |
| 15-1 | Nitin Shivappa (2016)h[42] | case-control | NA | 78-item FFQ | Breast cancer | 5157 (2569) | Quintile 5 (1.28, 5.14) vs.Quintile 1 (-6.18,-2.13) | OR | 1.75 (1.39-2.21) | age, study center, and energy intake, education, body mass index, parity, menopausal status, and family history of hormone-related cancers |
| 15-2 | Nitin Shivappa (2016)h[42] | case-control | NA | 78-item FFQ | Breast cancer | 5157 (2569) | Continuous DII (per one unit increment) | OR | 1.09 (1.05-1.14) | age, study center, and energy intake, education, body mass index, parity, menopausal status, and family history of hormone-related cancers |
| 16-1 | Nitin Shivappa (2016)i[43] | case-control | NA | 80-item FFQ | Bladder Cancer | 1355 (690) | Quartile 4 (0.42, 4.58) vs.Quartile 1 (-5.94,-2.41) | OR | 1.97 (1.28-3.03) | age, sex, year of interview, study center, total energy intake, education, and tobacco smoking |
| 16-2 | Nitin Shivappa (2016)i[43] | case-control | NA | 80-item FFQ | Bladder Cancer | 1355 (690) | Continuous DII (per one unit increment) | OR | 1.11 (1.03-1.20) | age, sex, year of interview, study center, total energy intake, education, and tobacco smoking |
| 17-1 | Nitin Shivappa (2016)j[27] | case-control | NA | 78-item FFQ | ovarian cancer | 3442 (1031) | Quartile 4 (>1.35) vs. Quartile 1 (≤1.63) | OR | 1.47 (1.07-2.01) | age, energy intake, year of interview, study center, education, body mass index, parity, oral contraceptive use, menopausal status, and family history of ovarian and/or breast cancer in first-degree relatives |
| 17-2 | Nitin Shivappa (2016)j[27] | case-control | NA | 78-item FFQ | Ovarian cancer | 3442 (1031) | Continuous DII (per one unit increment) | OR | 1.08 (1.02-1.14) | age, energy intake, year of interview, study center, education, body mass index, parity, oral contraceptive use, menopausal status, and family history of ovarian and/or breast cancer in first-degree relatives |
| 18-1 | Nitin Shivappa (2016)k[44] | case-control | NA | 78-item FFQ | Laryngeal cancer | 1548 (460) | Quartile 4 (0.27, 5.00) vs.Quartile 1 (-5.48,-2.19) | OR | 3.30 (2.06-5.28) | age, sex, center, education, body mass index, tobacco smoking, alcohol consumption, and non-alcohol energy intake |
| 18-2 | Nitin Shivappa (2016)k[44] | case-control | NA | 78-item FFQ | Laryngeal cancer | 1548 (460) | Continuous DII (per one unit increment) | OR | 1.27 (1.15, 1.40) | age, sex, center, education, body mass index, tobacco smoking, alcohol consumption, and non-alcohol energy intake |
| 19-1 | Nitin Shivappa (2016)l[45] | case-control | NA | 78-item FFQ | Nasopharyngeal cancer | 792 (198) | Tertile 3 (men: >0.59; women: >−0.19) vs. Tertile1 (men: ≤−0.64; women: ≤−1.06) | OR | 1.64 (1.06-2.55) | place of living, sex, age, year of interview, education, smoking, alcohol drinking, and energy intake according to the residual method |
| 19-2 | Nitin Shivappa (2016)l[45] | case-control | NA | 78-item FFQ | Nasopharyngeal cancer | 792 (198) | Continuous DII (per one unit increment) | OR | 1.19 (1.05, 1.36) | place of living, sex, age, year of interview, education, smoking, alcohol drinking, and energy intake according to the residual method |
| 20-1 | Nitin Shivappa (2016)m[46] | case-control | NA | 78-item FFQ | Endometrial cancer | 1362 (454) | Quartile 4 ( >1·04) vs. Quartile 1 (<−1·07) | OR | 1·46 (1·02-2·11) | age, energy, year of interview, education, BMI, age at menarche, menopausal status and age at menopause, parity, history of diabetes, family history of cancers, oral contraceptive use and hormone replacement therapy use |
| 20-2 | Nitin Shivappa (2016)m[46] | case-control | NA | 78-item FFQ | Endometrial cancer | 1362 (454) | Continuous DII (per one unit increment) | OR | 1.07 (0.98-1.17) | age, energy, year of interview, education, BMI, age at menarche, menopausal status and age at menopause, parity, history of diabetes, family history of cancers, oral contraceptive use and hormone replacement therapy use |
| 21-1 | Nitin Shivappa (2015)n[47] | case-control | NA | 21-item FFQ | Prostate cancer | 479 (229) | Quartile 4 vs. Quartile 1* | OR | 2.39 (1.14-5.04) | age, BMI, smoking status, education, physical activity, energy intake, family history of prostate cancer |
| 21-2 | Nitin Shivappa (2015)n[47] | case-control | NA | 21-item FFQ | Prostate cancer | 479 (229) | Continuous DII (per one unit increment) | OR | 1.27 (0.98-1.50) | age, BMI, smoking status, education, physical activity, energy intake, and family history of prostate cancer |
| 22-1 | Nitin Shivappa (2014)o[10] | Cohort | 19.6±7.0 | 121-item FFQ | Colorectal cancer | 34703 (1636) | Quintile 5 (>1.10) vs. Quintile 1 (<-2.75) | HR | 1.20 (1.01-1.43) | age, BMI, smoking status, pack-years of smoking, HRT use, education, diabetes, and total energy intake |
| Colon cancer | 34703 (1329) | 1.19 (0.98-1.45) | ||||||||
| Rectal cancer | 34703 (325) | 1.21 (0.81-1.79) | ||||||||
| 22-2 | Nitin Shivappa (2014)o[10] | Cohort | 19.6±7.0 | 121-item FFQ | Colorectal cancer | 34703 (1636) | Continuous DII (per one unit increment) | HR | 1.07 (1.01-1.13) | age, BMI, smoking status, pack-years of smoking, HRT use, education, diabetes, and total energy intake |
| Colon cancer | 34703 (1329) | 1.05 (0.99-1.12) | ||||||||
| Rectal cancer | 34703 (325) | 1.11 (0.98-1.25) | ||||||||
| 23-1 | Nitin Shivappa (2015)p[48] | Cohort | 20 | 80-item FFQ | Breast cancer | 45257 (1895) | Quartile 4 (>3.77) vs. Quartile 1 (<1.87) | HR | 1.18 (1.00-1.39) | age, energy, age at first birth and number of children, age at menarche, BMI, height, multivitamin use, education, smoking status, oral contraceptive use, and family history of breast cancer in the model |
| 23-2 | Nitin Shivappa (2015)p[48] | Cohort | 20 | 80-item FFQ | Breast cancer | 45257 (1895) | Continuous DII (per one unit increment) | HR | 1.04 (1.01-1.09) | age, energy, age at first birth and number of children, age at menarche, BMI, height, multivitamin use, education, smoking status, oral contraceptive use, and family history of breast cancer in the model |
| 24-1 | Nitin Shivappa (2015)r [11] | case-control | NA | 78-item FFQ | Colorectal cancer | 6107 (1953) | Quintile 5 (>1.22) vs. Quintile 1 (≤ -1·05) | OR | 1.55 (1.29-1.85) | age, sex, study center, education, BMI, alcohol drinking, physical activity, and history of colorectal cancer and energy intake (using the residual method) |
| Colon cancer | 5379 (1225) | 1·39 (1.13-1.71) | ||||||||
| Rectal cancer | 4882 (728) | 1·47 (1.14-1.90) | ||||||||
| 24-2 | Nitin Shivappa (2015)r [11] | Case-control | NA | 78-item FFQ | Colorectal cancer | 6107 (1953) | Continuous DII (per one unit increment) | OR | 1·13 (1·09-1·18) | age, sex, study center, education, BMI, alcohol drinking, physical activity, and history of colorectal cancer and energy intake (using the residual method) |
| Colon cancer | 5379 (1225) | 1·09 (1·04, 1·14) | ||||||||
| Rectal cancer | 4882 (728) | 1·12 (1·06, 1·19) | ||||||||
| 25-1 | Nitin Shivappa (2015)s [49] | Case-control | NA | 78-item FFQ | Esophageal squamous cell cancer | 1047 (304) | Quintile 5 (>1.28) vs. Quintile 1 (<-1.20) | OR | 2.47 (1.40-4.36) | age, sex, year of interview, and area of residence and adjusted for education, alcohol drinking, tobacco smoking, BMI, physical activity, aspirin use, and energy (using the residual method) |
| 25-2 | Nitin Shivappa (2015)s [49] | Case-control | NA | 78-item FFQ | Esophageal squamous cell cancer | 1047 (304) | Continuous DII ( per one unit increment) | OR | 1.23 (1.10-1.38) | age, sex, year of interview, and area of residence and adjusted for education, alcohol drinking, tobacco smoking, BMI, physical activity, aspirin use, and energy (using the residual method) |
| 26 | Fred K Tabung (2016)a [50] | Cohort | 16.02 | 122-item FFQ | Breast cancer | 122788 (7495) | Quintile 5 (1.898,5.519) vs. Quintile 1 (-7.055,< -3.142) | HR | 0.99 (0.91-1.07) | age, energy intake, race/ethnicity, income, education, smoking status, mammography within 2 years of baseline, age at menarche, number of live births, oophorectomy status, hormone therapy use, nonsteroidal anti-inflammatory drug (NSAID) use, dietary modification trial arm, hormone therapy trial arm, body mass index, and physical activity |
| 27 | Fred K Tabung (2015)b [12] | Cohort | 11.3 | 122-item FFQ | Colorectal cancer | 152,536 (1920) | Quintile 5 (1.953, 5.636) vs. Quintile 1 (-7.055, < -3.136) | HR | 1.22 (1.05-1.43) | |
| Colon cancer | 152,536 (1559) | 1.23 (1.03-1.46) | ||||||||
| Proximal colon cancer | 152,536 (1034) | 1.35 (1.09-1.67) | ||||||||
| Distal colon cancer | 152,536 (428) | 0.84 (0.61-1.18) | ||||||||
| Rectal cancer | 152,536 (361) | 1.20 (0.84-1.72) | ||||||||
| 28-1 | Ruth A. Vázquez-Salas (2016)[51] | Case-control | NA | 127-item semi-quantitative FFQ | Prostate cancer | 1188 (394) | Tertile 1 (ref) (<−0·12) vs. Tertile 3 (≥1·28) Continuous DII (per…) | OR | 1.18 (0.85-1.63) | age, educational level, history of PC in first-degree relatives, BMI 2 years before the interview, physical activity throughout life, smoking status 5 years before the interview,history of chronic diseases |
| 28-2 | Ruth A. Vázquez-Salas (2016)[51] | Case-control | NA | 127-itemsemi - quantitative FFQ | Prostate cancer | 1188 (394) | Continuous DII (per one unit increment) | OR | 1·02 (0·94, 1·11) | age, educational level, history of PC in first-degree relatives, BMI 2 years before the interview, physical activity throughout life, smoking status 5 years before the interview, history of chronic diseases |
| 29-1 | Michael D. Wirth (2015)[13] | Cohort | 9.1±2.9 | 124-item FFQ | Colorectal cancer | 489,442 (6225) | Quartile 4 (3.25, 6.97) vs. Quartile 1 ( -7.33,-0.59) | HR | 1.40 (1.28-1.53) | age, smoking status, BMI, self-reported diabetes, and energy intake - for 1:physical activity, marital status, education and age (STRATA statement) - for 2:age (STRATA statement) - For 3:race and age - For 4:marital status, education, perceived health, census-based income and age (STRATA statement) - for 5:self-reported polyps, education, age and census-based income |
| Ascending/Cecum | 489,442 (2060) | 1.27 (1.09-1.49) | ||||||||
| Transverse/Hepatic and Splenic Flexure | 489,442 (802) | 1.58 (1.23-2.03) | ||||||||
| Descending/Sigmoid | 489,442 (1614) | 1.61 (1.35-1.91) | ||||||||
| Rectum/Recto sigmoid | 489,442 (1680) | 1.45 (1.22-1.73) | ||||||||
| 29-2 | Michael D. Wirth (2015)[13] | Cohort | 9.1±2.9 | 124-item FFQ | Colorectal cancer | 489,442 (6225) | Continuous DII ( per one unit increment) | HR | 1.06 (1.05-1.08) | age, smoking status, BMI, self-reported diabetes, and energy intake - for 1:physical activity, marital status, education and age (STRATA statement) - for 2:age (STRATA statement) - For 3:race and age - For 4:marital status, education, perceived health, census-based income and age (STRATA statement) -for 5:self-reported polyps, education, age and census-based income |
| Ascending/Cecum | 489,442 (2060) | 1.05 (1.02-1.07) | ||||||||
| Transverse/Hepatic and Splenic Flexure | 489,442 (802) | 1.06 (1.02-1.10) | ||||||||
| Descending/Sigmoid | 489,442 (1614) | 1.08 (1.05-1.11) | ||||||||
| Rectum/Recto sigmoid | 489,442 (1680) | 1.08 (1.05-1.10) | ||||||||
| 30-1 | Raul Zamora-Ros (2015)[14] | Case-control | NA | dietary history questionnaire | Colorectal cancer | 825 (424) | Quartile 4 (>3.05) vs. Quartile 1 (<-0.73) | OR | 1.65 (1.05-2.60) | sex, age, total energy intake, BMI, first-degree family history of colorectal cancer, physical activity, tobacco consumption, and medication use (aspirin and non-steroidal anti-inflammatory drug) |
| Colon cancer | 666 (265) | 2.24 (1.33-3.77) | ||||||||
| Rectal cancer | 560 (159) | 1.12 (0.61-2.06) | ||||||||
| 30-2 | Raul Zamora-Ros (2015)[14] | Case-control | NA | dietary history questionnaire | Colorectal cancer | 825 (424) | Continuous DII ( per one unit increment) | OR | 1.08 (1.01-1.15) | sex, age, total energy intake, BMI, first-degree family history of colorectal cancer, physical activity, tobacco consumption, and medication use (aspirin and non-steroidal anti-inflammatory drug |
| Colon cancer | 666 (265) | 1.12 (1.04-1.21) | ||||||||
| Rectal cancer | 560 (159) | 1.03 (0.95-1.12) |
Abbreviation: FFQ: food frequency questionnaire, 24HR: 24 hour recall, HR: hazard ratio, OR: odds ratio; DII: dietary inflammatory index; NA: not applicable
Table 2 summarizes 8 cohort studies on the association of DII and mortality of different cancer types. Dietary intake was measured using FFQ and 24HR in the five and three articles, respectively.
Table 2.
Association of DII and risk of cancer mortality
| Study number | First author (year) | design | Follow up (years) | Food assessment questionnaire | Study subjects | Type of cancer mortality | Total sample size (death number) | Groups | Type of effect size measure | Effect size measure (95% CI) | Covariates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | Fang Emily Deng (2016)[52] | cohort | 135 and 168 person - months | 24 HR | Normal | Allcancers | 9631 (385) | Tertile 1 (ref) (<−0.20) vs. Tertile 3 (>2.0) | HR | 1.23 (0.84-1.79) | age, sex, race, HgbA1C, current smoking,physical activity, BMI, SBP |
| Lung cancer | 9631 (99) | 1.4 (0.79-2.47) | |||||||||
| Digestive-tract cancer | 9631 (99) | 1.38 (0.69-2.76) | |||||||||
| 1-2 | Fang Emily Deng (2016)[52] | cohort | 135 and 168 person - months | 24 HR | Pre - diabetic | All cancers | 2681 (208) | Tertile 1 (ref) (<−0.20) vs. Tertile 3 (>2.0) | HR | 2.02 (1.27-3.21) | age, sex, race, HgbA1C, current smoking, physical activity, BMI, SBP |
| Lung cancer | 2681 (66) | 2.01 (0.93-4.34) | |||||||||
| Digestive-tract cancer | 2681 (50) | 2.89 (1.08-7.71) | |||||||||
| 1-3 | Fang Emily Deng (2016)[52] | cohort | 135 and 168 person - months | 24 HR | Diabetic | All cancers | 968 (83) | Tertile 1 (ref) (<−0.20) vs. Tertile 3 (>2.0) | HR | 1.00 (0.49-2.04) | age, sex, race, HgbA1C, current smoking, physical activity, BMI, SBP |
| Lung cancer | 968 (27) | 0.55 (0.09-3.36) | |||||||||
| Digestive-tract cancer | 968 (27) | 1.30 (0.40-4.28) | |||||||||
| 2-1 | Aleksander Galas (2014)a[53] | cohort | 3,180.31 person - years | 148 item semi - quantitative FFQ | Patients without distant metastases | Colorectal cancer | 511 (150) | High (>- 2.27) vs. low (≤ -2.27) | HR | 0.76 (0.55-1.08) | Age, smoking, marital status, overweight or obesity, calendar year when surgery was performed, surgery type, cancer site, chemotherapy after surgery, radiotherapy after surgery |
| Patients with distant metastases | 178 (159) | 1.06 (0.76-1.48) | |||||||||
| 2-2 | Aleksander Galas (2014)a[53] | cohort | 3,180.31 person - years | 148 itemsemi - quantitative FFQ | Patients without distant metastases | Colorectal cancer | 511 (150) | Continuous DII (per one unit increment) | HR | 0.98 (0.92-1.05) | Age, smoking, marital status, overweight or obesity, calendar year when surgery was performed, surgery type, cancer site, chemotherapy after surgery, radiotherapy after surgery |
| Patients with distant metastases | 178 (159) | 1.003 (0.93-1.08) | |||||||||
| 3-1 | Laurie Graffouille`re (2016)b[54] | cohort | 12.4 | 24 HR | Healthy subjects | All cancers | 7994 (123) | Tertile 3 vs. Tertile 1* | HR | 1.83 (1.12-2.99) | Age, sex, intervention group of the initial SU.VI.MAX trial, number of 24-h dietary records, BMI, physical activity, smoking status, educational level, family history of cancer in first-degree relatives, family history of CVD in first-degree relatives, energy intake without alcohol, and alcohol intake |
| 3-2 | Laurie Graffouille`re (2016)b[54] | cohort | 12.4 | 24 HR | Healthy subjects | All cancers | 7994 (123) | Continuous DII (per one unit increment) | HR | 1.18 (1.04-1.34) | age, sex, intervention group of the initial SU.VI.MAX trial, number of 24-h dietary records, BMI, physical activity, smoking status, educational level, family history of cancer in first-degree relatives, family history of CVD in first-degree relatives, energy intake without alcohol, and alcohol intake |
| 4-1 | Nitin Shivappa (2016)b[55] | Cohort | 25 | 121-item FFQ | postmeno-pausal women | All cancers | 37525 (5044) | Quartile 4 (0.6469 to 4.6598) vs. Quartile 1(−5.7509 to−2.5041) | HR | 1.08 (0.99-1.18) | age, BMI, smoking status, pack-years of smoking, HRT use, education, prevalent diabetes, prevalent hypertension, prevalent heart disease, prevalent cancer, total energy intake |
| Digestive tract cancers | 37525 (1240) | 1.19 (1.00-1.43) | |||||||||
| 4-2 | Nitin Shivappa (2016)b[55] | Cohort | 25 | 121-item FFQ | postmeno-pausal women | All cancers | 37525 (5044) | Continuous DII (per one unit increment) | HR | 1.04 (1.01-1.07) | age, BMI, smoking status, pack-years of smoking, HRT use, education, prevalent diabetes, prevalent hypertension, prevalent heart disease,prevalent cancer, total energy intake |
| Digestive tract cancers | 37525 (1240) | 1.07 (1.01-1.14) | |||||||||
| 5-1 | Nitin Shivappa (2016)e[56] | Cohort | 15 | 96-item FFQ | Healthy women | All cancers | 33747 (1996) | Quintile 5 (> 5.10) vs. Quintile 1 (<−4.19) | HR | 1.25 (0.96-1.64) | Age, energy, BMI, education, smoking status, physical activity, alcohol intake |
| Digestive tract cancers | 33747 (602) | 1.42 (0.82-2.49) | |||||||||
| 5-2 | Nitin Shivappa (2016)e[56] | Cohort | 15 | 96-item FFQ | Healthy women | All cancers | 33747 (1996) | Continuous DII (per one unit increment) | HR | 1.04 (0.99-1.11) | Age, energy, BMI, education, smoking status, physical activity, alcohol intake |
| Digestive cancer | 33747 (602) | 1.15 (1.02-1.29) | |||||||||
| 6-1 | Nitin Shivappa (2015)q[57] | Cohort | 13.5±4.0 | 24 HR | Healthy subjects | All cancers | 12366 (615) | Tertile 3 (2.03 to 4.83) vs. Tertile 1 (−5.60 to−0.22) | HR | 1.46 (1.10-1.96) | age, sex, race, diabetes status, hypertension, physical activity, BMI, poverty index, and smoking |
| Digestive tract cancers | 12,366 (158) | ||||||||||
| 2.10 (1.15-3.84) | |||||||||||
| 6-2 | Nitin Shivappa (2015)q[57] | Cohort | 13.5±4.0 | 24 HR | Healthy subjects | All cancers | 12,366 (615) | Continuous DII (per one unit increment) | HR | 1.04 (0.97-1.11) | age, sex, race, diabetes status, hypertension, physical activity, BMI, poverty index, and smoking |
| Digestive tract cancers | |||||||||||
| 12,366 (158) | 1.08 (0.95-1.22) | ||||||||||
| 7 | Fred K Tabung (2016) a[50] | Cohort | 16.02 | 122-item FFQ | Postmeno-pausal women | Breast cancer | 122788 (667) | Quintile 5 (1.874 to 5.519) vs. Quintile 1 (−7.055 to <−3.162) | HR | 1.33 (1.01-1.76) | age, energy intake, race/ethnicity, income, education, smoking status, mammography within 2 years of baseline, age at menarche, number of live births, oophorectomy status, hormone therapy use, nonsteroidal anti-inflammatory drug (NSAID) use, dietary modification trial arm, hormone therapy trial arm, body mass index, and physical activity |
| 8 | Antonella Zucchetto (2016)[58] | Cohort | 12.7 | 78-item FFQ | Patients with prostate cancer | Prostate cancer | 726 (76) | Tertile 3 vs. Tertile 1* | HR | 1.42 (0.73-2.76) | area of residence, calendar period of diagnosis, age at diagnosis, education, smoking habits, abdominal obesity, alcohol intake, energy intake |
FFQ: Food frequency questionnaire, 24HR: 24 hour recall, HR: Hazard ratio, DII: Dietary inflammatory index
We found only one cohort study Table 3 on the association between DII and length of hospitalization. There was no significant association exists between DII and length of hospitalization in surgical patients treated for colorectal cancer.
Table 3.
Association between DII and length of hospitalization
| Study number | First author (year) | design | Follow up (years) | Food assessment questionnaire | Study subjects | Type of cancer mortality | Total sample size (death number) | Groups | Type of effect size measure | Effect size measure (95% CI) | Covariates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aleksander Galas (2014)b[59] | Cohort | 11 days | 148 itemsemi - quantitative FFQ | Surgical patients treated for colorectal cancer | Colorectal cancer | 689 | Over the first tertile (> -3.41) vs. tertile 1 (≤ -3.41) | OR | 0.76 (0.53-1.09) | Age, smoking, marital status, overweight or obesity, calendar year when surgery was performed, surgery type, cancer site, chemotherapy after surgery, radiotherapy after surgery |
| Over the first quartile (> -3.91) vs. quartile 1 (≤ -3.91) | 0.69 (0.46-1.03) | ||||||||||
| Over the first quintile (> -4.25) vs. quintile 1 (≤ -4.25) | 0.69 (0.45-1.07) |
FFQ: Food frequency questionnaire, OR: Odds ratio
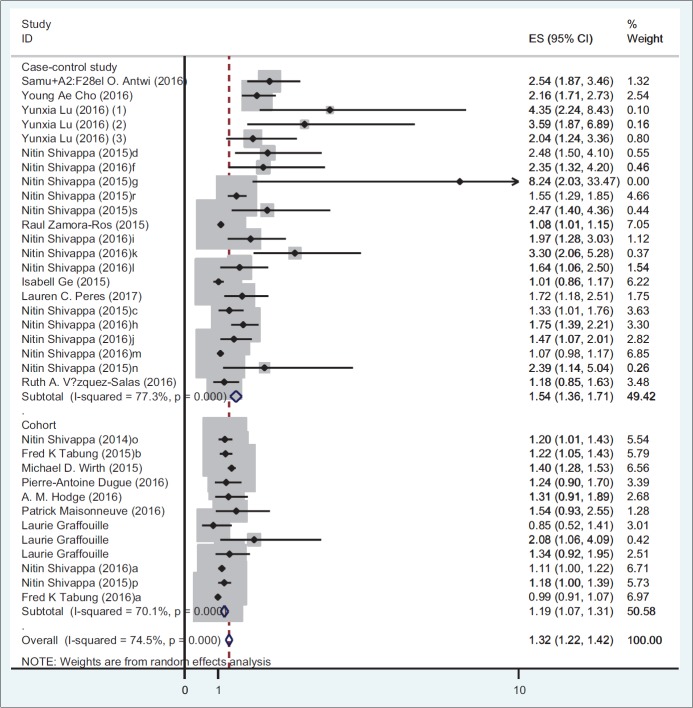
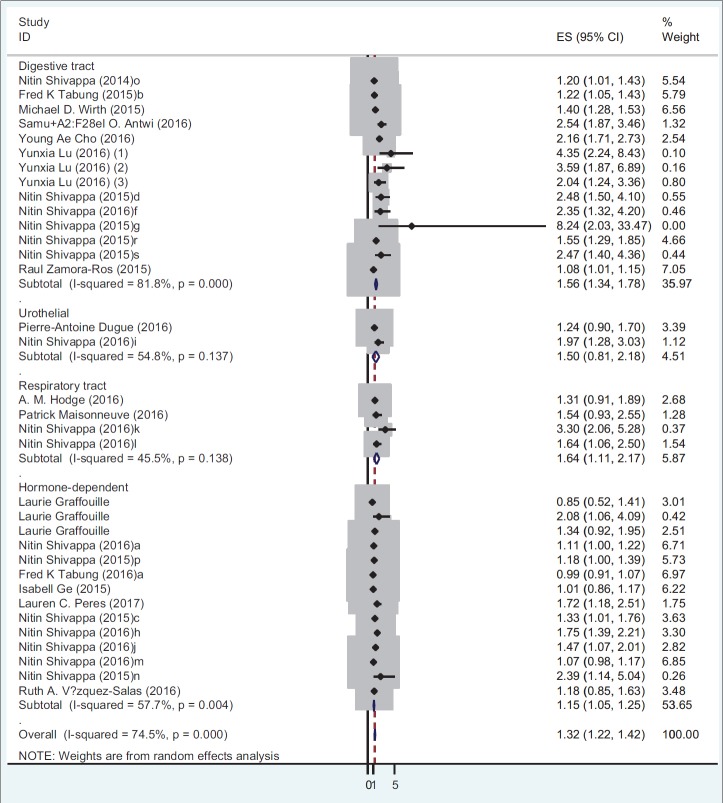
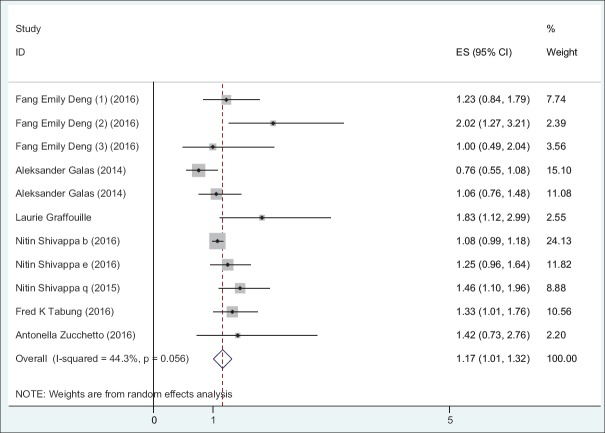
Table 4 presents the results of meta-analysis for the association of DII and incidence and mortality of different cancer types. There is a significant association between DII and incidence for all cancer types (OR: 1.32; 95% CI: 1.22-1.42; P < 0.001). A stratified meta-analysis by types of cancer shows that the highest and lowest effect size measures were observed for respiratory tract cancers and hormone-dependent cancers, respectively (OR: 1.64; 95% CI: 1.10-2.17 vs. OR: 1.14; 95% CI: 1.04-1.24). A stratified meta-analysis according to study design shows that the association of DII and incidence of all cancer types in case-control studies (OR: 1.53; 95% CI: 1.36-1.71) were more prominent than cohort studies (OR: 1.18; 95% CI: 1.07-1.30). Figures 2 and 3 report the forest plot of association between DII and cancer incidence according to the design of study and type of cancers, respectively. Moreover, there is a significant association between DII and mortality for all cancer types (HR: 1.16; 95% CI: 1.01-1.32) [Figure 4].
Table 4.
Meta-analysis of association between DII and mortality/morbidity of cancer
| Type of outcome (Mortality/morbidity) | subgroup | Type of cancer | Number of studies | Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect size measure | 95%CI | P | Model | I2 | Q test | P | ||||
| Morbidity | Type of cancer | Digestive tract cancers | 14 | 1.55 | 1.33-1.78 | < 0.001 | Random | 81.8 | 71.27 | < 0.001 |
| Hormone-dependent cancers | 13 | 1.14 | 1.04-1.24 | < 0.001 | Random | 59.6 | 29.72 | 0.003 | ||
| Respiratory tract cancers | 4 | 1.64 | 1.11-2.17 | < 0.001 | Fixed | 45.5 | 5.51 | 0.13 | ||
| Urothelial cancers | 2 | 1.36 | 1.00-1.73 | < 0.001 | Fixed | 54.8 | 2.21 | 0.13 | ||
| Type of study | Case-control | 22 | 1.53 | 1.36-1.71 | < 0.001 | Random | 77.3 | 92.51 | < 0.001 | |
| Cohort | 12 | 1.18 | 1.07-1.30 | < 0.001 | Random | 70.1 | 36.81 | < 0.001 | ||
| Overall | 34* | 1.32 | 1.22-1.42 | < 0.001 | Random | 74.5 | 129.39 | < 0.001 | ||
| Mortality | All cancers | 11 | 1.16 | 1.01-1.32 | < 0.001 | Random | 44.3 | 17.96 | 0.056 | |
*The sum of number of studies for all cancers (34 studies) is more than the sum of digestive, hormone-dependent, respiratory and urothelial cancers because in one study, type of cancer was not reported
Figure 2.
Odds ratio and 95% CI of individual studies and pooled data for the association between DII and incidence of cancer according to the type of study using random-effect model. OR: Odds of ratios
Figure 3.
Odds ratio and 95% CI of individual studies and pooled data for the association between DII and incidence of cancer according to the type of cancer using random-effect model. OR: Odds of ratios
Figure 4.
Odds ratio and 95% CI of individual studies and pooled data for the association between DII and mortality of cancer according to the type of cancer using random-effect model. OR: Odds of ratios
Meta-regression
A meta-regression analysis suggests that design of study can have a significant effect on the association between DII and cancer incidence (slope: 0.54; P = 0.05), whereas meta-regression does not show any significant associations between DII and type of food assessment questionnaire (slope:-0.33;p = 0.21), type of cancer (slope:-0.22; P = 0.22), and publication year (slope: 0.24;p = 0.31). The result of meta-regression analysis for the association of DII and cancer mortality shows no significant association between DII and type of food assessment questionnaire (slope: 0.43; P = 0.47), type of cancer (slope:-0.54; P = 0.81), and publication year (slope: 0.21; P = 0.59).
Publication bias
The results of Egger test for association of DII and all cancer incidence show that publication bias exists (coefficient: 2.87; P < 0.001) and funnel plot was asymmetric [Figure 5]. “Trim and fill” correction suggested some potentially missing study on the right side of funnel plot [Figure 5]. Imputation for this potentially missing study yielded an effect size of 1.23 (95% CI: 1.12-1.33). In addition, the results of Egger test for association between DII and all cancer mortality show that publication bias does not exist (coefficient: 1.06; P = 0.15) and funnel plot was symmetric [Figure 6].
Figure 5.
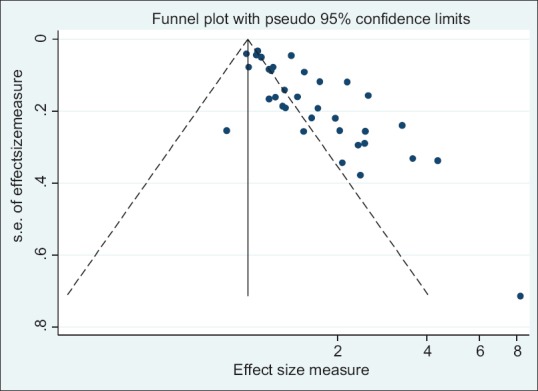
Funnel plot detailing publication bias in the studies reporting the association between DII and all cancer morbidity
Figure 6.
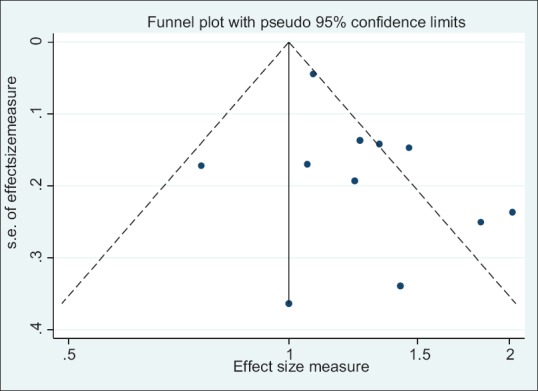
Funnel plot detailing publication bias in the studies reporting the association between DII and cancer mortality
Discussion
To the best of our knowledge, the present study is the first comprehensive systematic review and meta-analysis on the association of DII and cancer incidence and mortality. This meta-analysis shows a significant association between DII and risk of incidence and mortality of all cancer types. The results of the present study shows that a higher level of DII increases the risk of cancers incidence by 32% (95% CI: 1.22-1.42) including digestive tract cancers (OR: 1.55; 95% CI: 1.33-1.78), hormone-dependent cancers (OR = 1.14; 95% CI: 1.04-1.24), respiratory tract cancers (OR: 1.64; 95% CI: 1.11-2.17), and urothelial cancers (OR: 1.36; 95% CI: 1.00-1.73). Moreover, a higher level of DII in association with a higher risk of mortality caused by all type of cancer by 16% (95% CI: 1.01-1.32).
Our findings were consistent with previous studies showing that a higher DII was associated with mortality. Moreover, some studies have documented a direct association between DII and a higher risk for metabolic syndrome and cardiovascular diseases (CVD).[4] One of the study reported different mechanisms by which inflammatory markers used for DII calculation can predict most prevalent diseases including cancers, CVD, and diabetes.[6]
Results of present study show that the association of DII and incidence of all cancer types in case-control studies were more prominent than cohort studies, which was consistent with previous studies.[15,16] It has been suggested that dietary recall bias may justify the discrepant results between case-control and cohort studies on diet and the risk of cancers.
Dietary patterns analysis is one of the most appropriate approaches to understand the relationship between diet and risk for various diseases including diabetes, cancers, and CVD.[17] All of the healthy dietary patterns (e.g. Dietary Approaches to Stop Hypertension and Mediterranean diet) can play a key role in preventing major chronic diseases, especially cancers.[18,19,20] In contrast, there was an inverse relationship between DII and dietary quality indices (e.g. Healthy Eating Index).[21] This was in line with the number of studies showing an inverse correlation between C-reactive protein, one of the inflammatory biomarkers used to calculate the DII, and higher consumption of vegetables, fruits,[22] legumes,[23] and nuts.[24]
To define the inflammatory capacity of diet as a main determining factor for vast majority of chronic diseases, we developed DII from peer-reviewed literature by investigating the association between dietary components and inflammation. However, in contrast to the other dietary patterns, DII focuses on specific biological pathways modulating the impact of dietary factors on inflammation.[21] In fact, in comparison to other dietary pattern, DII can provide more comprehensive information on additional variables affecting inflammation.[25,26,27,28,29]
The present meta-analysis has some strengths and limitations. The main strength is that the study includes all indices of incidence, mortality, and length of hospitalization of cancers in relation with a categorical and continuous score of DII. In addition, we carried out the meta-analysis on all types of cancer. The limitations of the study were as follows: (a) reviewed studies were heterogeneous in terms of population characteristics, design, and duration of follow-up periods; and (b) the questionnaires used for food assessment were different. However, we tried to reduce the effect of heterogeneity on estimated effect sizes by using a random-effect model of analysis.
Conclusions
In conclusion, the present meta-analysis suggested a significant association between DII and incidence, mortality, and hospitalization in patients with different types of cancers. DII, which is used for evaluating inflammatory properties of diets, can be used as an appropriate tool to predict the incidence and mortality of all cancer types. According to the results of the study, we recommend that changing dietary patterns as malleable factors can substantially reduce both incidence and mortality risks in cancer patients.
Financial support and sponsorship
The study was funded by Alborz University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to appreciate Emam Ali Hospital Clinical Research Development Unit, Alborz University of Medical Sciences for their comprehensive cooperation in this study.
References
- 1.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules. 2015;20:9183–213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montecucco F, Liberale L, Bonaventura A, Vecchie A, Dallegri F, Carbone F. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep. 2017;19:11–21. doi: 10.1007/s11883-017-0646-1. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Canela M, Bes-Rastrollo M, Martinez-Gonzalez MA. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci. 2016;17:23–33. doi: 10.3390/ijms17081265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz-Canela M, Zazpe I, Shivappa N, Hebert JR, Sanchez-Tainta A, Corella D, et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br J Nutr. 2015;11:984–95. doi: 10.1017/S0007114514004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. PublicHealth Nutr. 2014;17:1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr. 2013;109:S1–34. doi: 10.1017/S0007114512005119. [DOI] [PubMed] [Google Scholar]
- 8.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–72. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YA, Lee J, Oh JH, Shin A, Kim J. Dietary inflammatory index and risk of colorectal cancer: A case-control study in Korea. Nutr. 2016;8:25–37. doi: 10.3390/nu8080469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivappa N, Prizment AE, Blair CK, Jacobs DR, Jr, Steck SE, Hebert JR Dietary inflammatory index and risk of colorectal cancer in the Iowa Women's Health Study. Cancer epidemiology, biomarkers and prevention: Apublication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Cancer EpidemiolBiomarkers Prev. 2014;23:2383–92. doi: 10.1158/1055-9965.EPI-14-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, et al. Inflammatory potential of diet and risk of colorectal cancer: Acase-control study from Italy. Br J Nutr. 2015;114:152–8. doi: 10.1017/S0007114515001828. [DOI] [PubMed] [Google Scholar]
- 12.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: Results from the Women's Health Initiative. Cancer Causes Control. 2015;26:399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hebert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br J Nutr. 2015;113:1819–27. doi: 10.1017/S000711451500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamora-Ros R, Shivappa N, Steck SE, Canzian F, Landi S, Alonso MH, et al. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case-control study. GenesNutr. 2015;10:447. doi: 10.1007/s12263-014-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannisto S, Pietinen P, Virtanen M, Kataja V, Uusitupa M. Diet and the risk of breast cancer in a case-control study: Does the threat of disease have an influence on recall bias? J Clin Epidemiol. 1999;52:429–39. doi: 10.1016/s0895-4356(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 16.Boyd NF, Martin LJ, Noffel M, Lockwood GA, Trichler DL. A meta-analysis of studies of dietary fat and breast cancer. Br J Cancer. 1993;68:627–36. doi: 10.1038/bjc.1993.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu FB. Dietary pattern analysis: Anew direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Miller PE, Cross AJ, Subar AF, Krebs-Smith SM, Park Y, Powell-Wiley T, et al. Comparison of 4 established DASH diet indexes: Examining associations of index scores and colorectal cancer. Am J Clin Nutr. 2013;98:794–803. doi: 10.3945/ajcn.113.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 20.Schwingshackl L, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: Asystematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2015;115:780–800e5. doi: 10.1016/j.jand.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Wirth MD, Hebert JR, Shivappa N, Hand GA, Hurley TG, Drenowatz C, et al. Anti-inflammatory dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr Res. 2016;36:214–9. doi: 10.1016/j.nutres.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermsdorff HH, Zulet MA, Puchau B, Martinez JA. Fruit and vegetable consumption and proinflammatory gene expression from peripheral blood mononuclear cells in young adults: Atranslational study. NutrMetab. 2010;7:42–58. doi: 10.1186/1743-7075-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermsdorff HH, Zulet MA, Abete I, Martinez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50:61–9. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- 24.Casas-Agustench P, Lopez-Uriarte P, Bullo M, Ros E, Cabre-Vila JJ, Salas-Salvado J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2011;21:126–35. doi: 10.1016/j.numecd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146:1560–70. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodge AM, Bassett JK, Shivappa N, Hebert JR, English DR, Giles GG, et al. Dietary inflammatory index, Mediterranean diet score, and lung cancer: Aprospective study. Cancer Causes Control. 2016;27:907–17. doi: 10.1007/s10552-016-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shivappa N, Hebert JR, Rosato V, Rossi M, Montella M, Serraino D, et al. Dietary inflammatory index and ovarian cancer risk in a large Italian case-control study. Cancer Causes Control. 2016;27:897–906. doi: 10.1007/s10552-016-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirkarimi K, Mansourian M, Kabir MJ, Ozouni-Davaji RB, Eri M, Hosseini SG, et al. Fast food consumption behaviors in high-school students based on the theory of planned behavior (TPB) Int J Pediatr. 2016;4:2131–42. [Google Scholar]
- 29.Azizi-Soleiman F, Motlagh ME, Qorbani M, Heshmat R, Ardalan G, Mansourian M, et al. Dietary habits and health related behaviors in Iranian children and adolescents: The CASPIAN- IV study. Int J Pediatr. 2016;4:2087–97. [Google Scholar]
- 30.Antwi SO, Oberg AL, Shivappa N, Bamlet WR, Chaffee KG, Steck SE, et al. Pancreatic cancer: Associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinogenesis. 2016;37:481–90. doi: 10.1093/carcin/bgw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dugue PA, Hodge AM, Brinkman MT, Bassett JK, Shivappa N, Hebert JR, et al. Association between selected dietary scores and the risk of urothelial cell carcinoma: A prospective cohort study. Int J Cancer. 2016;139:1251–60. doi: 10.1002/ijc.30175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge I, Rudolph A, Shivappa N, Flesch-Janys D, Hebert JR, Chang-Claude J. Dietary inflammation potential and postmenopausal breast cancer risk in a German case-control study. Breast. 2015;24:491–6. doi: 10.1016/j.breast.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Graffouillere L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hebert JR, et al. The dietary inflammatory index is associated with prostate cancer risk in French middle-aged adults in a prospective study. J Nutr. 2016;146:785–91. doi: 10.3945/jn.115.225623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, Shivappa N, Lin Y, Lagergren J, Hebert JR. Diet-related inflammation and oesophageal cancer by histological type: Anationwide case-control study in Sweden. J Nutr. 2016;55:1683–94. doi: 10.1007/s00394-015-0987-x. [DOI] [PubMed] [Google Scholar]
- 35.Maisonneuve P, Shivappa N, Hebert JR, Bellomi M, Rampinelli C, Bertolotti R, et al. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. J Nutr. 2016;55:1069–79. doi: 10.1007/s00394-015-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peres LC, Bandera EV, Qin B, Guertin KA, Shivappa N, Hebert JR, et al. Dietary inflammatory index and risk of epithelial ovarian cancer in African American women. Int J Cancer. 2017;140:535–43. doi: 10.1002/ijc.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hebert JR. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Br J Cancer. 2017;61:23–365. doi: 10.1002/mnfr.201600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, et al. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr. 2015;113:278–83. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br J Nutr. 2015;113:292–8. doi: 10.1017/S0007114514003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivappa N, Hebert JR, Ferraroni M, La Vecchia C, Rossi M. Association between dietary inflammatory index and gastric cancer risk in an Italian case-control study. Nutr Cancer. 2016;68:1262–8. doi: 10.1080/01635581.2016.1224367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivappa N, Hebert JR, Rashidkhani B. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Iran. Nutr Cancer. 2015;67:1253–9. doi: 10.1080/01635581.2015.1082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shivappa N, Hebert JR, Rosato V, Montella M, Serraino D, La Vecchia C. Association between the dietary inflammatory index and breast cancer in a large Italian case-control study. MolNutr Food Res. 2016;61:123–32. doi: 10.1002/mnfr.201600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shivappa N, Hebert JR, Rosato V, Rossi M, Libra M, Montella M, et al. Dietary inflammatory index and risk of bladder cancer in a large Italian case-control study. Urology. 2017;100:84–9. doi: 10.1016/j.urology.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shivappa N, Hebert JR, Rosato V, Serraino D, La Vecchia C. Inflammatory potential of diet and risk of laryngeal cancer in a case-control study from Italy. Cancer Causes Control. 2016;27:1027–34. doi: 10.1007/s10552-016-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shivappa N, Hebert JR, Zucchetto A, Montella M, Libra M, Garavello W, et al. Increased risk of nasopharyngeal carcinoma with increasing levels of diet-associated inflammation in an Italian case-control study. Nutr Cancer. 2016;68:1123–30. doi: 10.1080/01635581.2016.1216137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shivappa N, Hebert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, et al. Dietary inflammatory index and endometrial cancer risk in an Italian case-control study. Br J Nutr. 2016;115:138–46. doi: 10.1017/S0007114515004171. [DOI] [PubMed] [Google Scholar]
- 47.Shivappa N, Jackson MD, Bennett F, Hebert JR. Increased dietary inflammatory index (DII) is associated with increased risk of prostate cancer in Jamaican men. Nutr Cancer. 2015;67:941–8. doi: 10.1080/01635581.2015.1062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivappa N, Sandin S, Lof M, Hebert JR, Adami HO, Weiderpass E. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Nutr Cancer. 2015;113:1099–103. doi: 10.1038/bjc.2015.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer Causes Control. 2015;26:1439–47. doi: 10.1007/s10552-015-0636-y. [DOI] [PubMed] [Google Scholar]
- 50.Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, et al. Association between dietary inflammatory potential and breast cancer incidence and death: Results from the Women's Health Initiative. Nutr Cancer. 2016;114:1277–85. doi: 10.1038/bjc.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazquez-Salas RA, Shivappa N, Galvan-Portillo M, Lopez-Carrillo L, Hebert JR, Torres-Sanchez L. Dietary inflammatory index and prostate cancer risk in a case-control study in Mexico. Br J Nutr. 2016;116:1945–53. doi: 10.1017/S0007114516003986. [DOI] [PubMed] [Google Scholar]
- 52.Deng FE, Shivappa N, Tang Y, Mann JR, Hebert JR. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: Findings from NHANES III. Eur J Nutr. 2016;6:1–9. doi: 10.1007/s00394-016-1158-4. [DOI] [PubMed] [Google Scholar]
- 53.Galas A, Kulig J. Low-grade dietary-related inflammation and survival after colorectal cancer surgery. J Cancer Res Clin Oncol. 2014;140:1517–25. doi: 10.1007/s00432-014-1711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graffouillere L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hebert JR, et al. Prospective association between the dietary inflammatory index and mortality: Modulation by antioxidant supplementation in the SUVIMAX randomized controlled trial. AmJClinNutr. 2016;103:878–85. doi: 10.3945/ajcn.115.126243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivappa N, Blair CK, Prizment AE, Jacobs DR, Jr, Steck SE, Hebert JR. Association between inflammatory potential of diet and mortality in the Iowa Women's Health study. Eur J Nutr. 2016;55:1491–502. doi: 10.1007/s00394-015-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shivappa N, Harris H, Wolk A, Hebert JR. Association between inflammatory potential of diet and mortality among women in the Swedish mammography cohort. Eur J Nutr. 2016;55:1891–900. doi: 10.1007/s00394-015-1005-z. [DOI] [PubMed] [Google Scholar]
- 57.Shivappa N, Steck SE, Hussey JR, Ma Y, Hebert JR. Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in National Health and Nutrition Examination Survey III study. Eur J Nutr. 2015;3:1–10. doi: 10.1007/s00394-015-1112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zucchetto A, Gini A, Shivappa N, Hebert JR, Stocco C, Dal Maso L, et al. Dietary inflammatory index and prostate cancer survival. IntJCancer. 2016;139:2398–404. doi: 10.1002/ijc.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galas A, Kulig P, Kulig J. Dietary inflammatory index as a potential determinant of a length of hospitalization among surgical patients treated for colorectal cancer. Eur J Nutr. 2014;68:1168–74. doi: 10.1038/ejcn.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]