Abstract
Background:
Overproduction of reactive oxygen species and free radicals is the main mechanism beyond gentamicin-induced nephrotoxicity. Irbesartan and other angiotensin II blockers offer significant nephroprotective effect through improvement of renal function and reduction of renal inflammation. Therefore, the objective of this study was to illustrate the nephroprotective effect of irbesartan in rats regarding the oxidative stress of irbesartan biomarkers.
Methods:
Thirty male Sprague–Dawley rats were used; they were divided into three groups: Group I (10 rats) treated with distilled water, Group II (10 rats) treated with gentamicin, and Group III (10 rats) treated with gentamicin plus irbesartan for 12 days. Blood urea, serum creatinine, serum malondialdehyde (MDA), superoxide dismutase (SOD), glutathione reductase (GSH), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule (KIM-1), and cystatin-c were measured in each group.
Results:
Irbesartan significantly reduced blood urea, serum creatinine, serum MDA, NGAL, KIM-1, and cystatin-c [P < 0.05]. Irbesartan significantly increases SOD [P < 0.05] without significant effect in elevation of GSH serum levels.
Conclusions:
This study concluded that irbesartan has a nephroprotective effect in attenuation of acute nephrotoxicity through modulation of oxidative stress and antioxidant capacity in rats.
Keywords: Antioxidant capacity, gentamicin, irbesartan, nephrotoxicity, oxidative stress
Introduction
Nephrotoxicity is a renal-specific situation in which excretions of toxic metabolites are significantly reduced due to toxic agents and drugs. Notably, about 20% of nephrotoxicity is induced and caused by drugs; this percentage is augmented in the elderly due to the increase in the life span and poly-medications. In addition, anticancer medications and other chemotherapeutic agents are omitted and their uses are limited due to risk of drug-induced nephrotoxicity.[1]
Gentamicin is an antibiotic belonging to aminoglycoside group, used in treatment of different bacterial infections; it is bactericidal which acts as a protein synthesis inhibitor through binding to the 30s subunit of bacterial ribosome.[2]
In addition, 90% of administrated gentamicin is not metabolized by the liver and it is mainly excreted unchanged through proximal renal tubules leading to an extensive necrosis at a higher dose, thus long-term gentamicin therapy may cause renal damage.[3]
Moreover, gentamicin and other aminoglycosides enter the cells by specific ion channels and by endocytosis. Most of the subjected cells clear gentamicin deposition by efflux of this drug, but it remains concentrated in renal cells.[4]
Overproduction of reactive oxygen species and free radicals is the main mechanism beyond the gentamicin-induced nephrotoxicity. Indeed, gentamicin induces the expression of cation transporter protein (megalin and cubilin) at proximal renal tubules which augment the accumulation of gentamicin and free radical generations.[5]
Irbesartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II (Ang II) by selective blocking of Ang II receptors. Irbesartan is a specific competitive antagonist of AT1 receptors with a greater affinity for the AT1 receptor than for the AT2 receptor. Blockade of the AT1 receptor removes the negative feedback of Ang II on renin secretion, but succeeding increase in plasma renin activity and circulating Ang II do not overcome the effects of irbesartan on blood pressure.[6] Ang II is the principal presser agent of the renin–angiotensin system (RAS) which encourages aldosterone synthesis and secretion by adrenal cortex, cardiac contraction, renal reabsorbtion of sodium, activation of sympathetic nervous system, and smooth muscle cell growth.[7] Chida et al. reported a significant effect of irbesartan in reduction of serum uric acid with potential antioxidant effect through reduction of oxidative stress in high-risk hypertensive patients.[8] While Hsu et al. illustrated that irbesartan and other Ang II blockers are inferior in nephroprotective effect compared with angiotensin-converting enzyme inhibitors (ACEIs).[9]
Irbesartan and other Ang II blockers offer significant nephroprotective effect through improvement of podocyte function and reduction of renal inflammation. In addition, the nephroprotective effect of Ang II blockers is mediated through attenuation of renal tissue damage that is induced by diabetes mellitus or nephrotoxic agents through regulation of renal hemodynamic and downregulation of cytokine-induced glomerular injury.[10] It has been accounted that Ang II blocker slows the progression of diabetic nephropathy through regulation of intraglomerular pressure since intrarenal Ang II induces glomerular hyperfiltration and renal damage.[11]
Thus, the rational of this study depends on an idea that anti-inflammatory agents might play a potential role in attenuation of gentamicin-induced nephrotoxicity since gentamicin-induced acute kidney injury is partially mediated by activation of Ang II receptors.[12]
Therefore, objective of this study was to illustrate the nephroprotective effect of irbesartan on gentamicin-induced nephrotoxicity in rats regarding the oxidative stress biomarkers.
Methods
Animals
Thirty male Sprague–Dawley rats were used, which were obtained from the National Center for Drug Control and Research; the age of the rats ranged from 3 to 4 months and their body weight ranged from 200 to 400 g. The animals were isolated as three rats in each sterilized cage and placed at suitable temperature (22°C–25°C) and artificial 12/12 light-dark cycle. They were left for 1 week for adaptation without any intervention with free access to normal chow pellets and water. Humane care for animals was according to the Guide for the Care and Use of Laboratory Animals under ethical approval permission 34AH/22-1-2018.
Drugs
All drugs were purchased from private pharmacy, gentamicin ampule (Garamycin 80 mg; Schering-Plough, USA) and irbesartan tablet (Aproval 150 mg tablet; Macleods Pharma USA, Inc., Plainsboro, NJ).
Study design
After acclimatization period, the weight of the rats was taken, and then the rats were randomly divided into three groups, 10 rats in each group. The study protocol and method for induction of nephrotoxicity were according to Singh et al.'s method.[13]
Group I (n = 10): Rats were treated with distilled water (5 mL/kg) orally for 12 days, and on days 6–12 they received intraperitoneal (i.p.) injection of normal saline daily (5 mL/kg).
Group II (n = 10): Rats were treated with distilled water (5 mL/kg) orally for 12 days, and on days 6–12 they received i.p. injection of gentamicin 100 mg/kg.
Group III (n = 10): Rats were treated with irbesartan (10 mg/kg) for 12 days, and on days 6–12 they received i.p. injection of gentamicin 100 mg/kg.
Sample collection
On the 13th day, chloroform was used to anesthetize the rats and sharp scissors was used for rat decapitation. This method gives a large volume of blood, approximately 3–4 mL. The blood sample was allowed to drain into the gel tube and then centrifuged for 10 min at 5000 rpm at room temperature. The formed supernatant layer was isolated as serum sample and kept at −20°C to be used later.
Assessment of biochemical variables
Blood urea and serum creatinine (both expressed as mg/dL) were estimated using an autoanalyzer (ILab 300; bioMérieux Diagnostic, Milano, Italy). Serum malondialdehyde (MDA), superoxide dismutase (SOD), glutathione reductase (GSH), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule (KIM-1), and cystatin C were measured by ELISA kit methods according to the instruction of the manufacturer (MyoBioSource, USA).
Assessment of anthropometric variables
The length was measured by graduated tape measure from nose to the anus (nasoanal length in cm). Rat body weight was measured by specific digital balance in gram. Body mass index (BMI) equals body weight in grams over the square of length in cm, BMI = BW (g)/length (cm)2.[14]
Estimated glomerular filtration rate (eGFR) was measured according to Schwartz formula, eGFR = k × height (cm)/serum creatinine (mg/dL), K = 0.55.[15]
Assessment of histopathological changes
Tissue sample collection
The animals were scarified and the kidney was separated and stored in normal saline solution. The isolated kidneys were fixed in 10% formalin buffer to preserve the tissue structure according to the paraffin methods.[16] The staining of the tissue sections was done using hematoxylin and eosin stains. Hematoxylin stained the negatively charged components with blue color such as DNA, while other cellular structures were stained by eosin red color.
Scoring system for histopathological changes
Scoring system for histopathological changes during nephrotoxicity was according to the methods of Toprak O et al. and Ayla S et al.[17,18]
Statistical analysis
Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, version 20.0; 2014; IBM, Corp., Armonk, NY, USA) software was used for data analysis. Data of this study were presented as mean ± standard deviation, and the variables were tested using unpaired Student's t-test between control and treated groups. One-way analysis of variance test with post hoc test was used to investigate the significance of differences among different groups. Correlation coefficient was applied to detect the correlation of the study parameters. Mann–Whitney–Wilcoxon test was applied for detecting significance of differences regarding the histopathological scoring. The level of significance was regarded when P < 0.05.
Results
The characteristics of this study illustrated that 28 of 30 Sprague–Dawley rats were used in the final analysis due to 6.67% death rate; other characteristics are presented in Table 1.
Table 1.
Characteristics of the present study
| Characteristics | Mean±SD, n (%), other |
|---|---|
| Type of the study | Experimental, animal model study |
| Animal used | |
| Type | Male Sprague-Dawley rats |
| Number of rats | 30 |
| Age (months) | 3-4 |
| Body weight (g) | 268.00±25.01 |
| Death rate, % | 2 (6.67%) |
| Agents used | |
| Normal saline (9%) + distilled water | 10 (33.33) |
| Gentamicin 100 mg/kg + normal saline (9%) | 8 (26.66) |
| Gentamicin 100 mg/kg + irbesartan 10 mg/kg | 10 (33.33) |
| Tissue used | Kidney |
| Biomarkers | Inflammatory and antioxidant biomarkers |
SD: Standard deviation
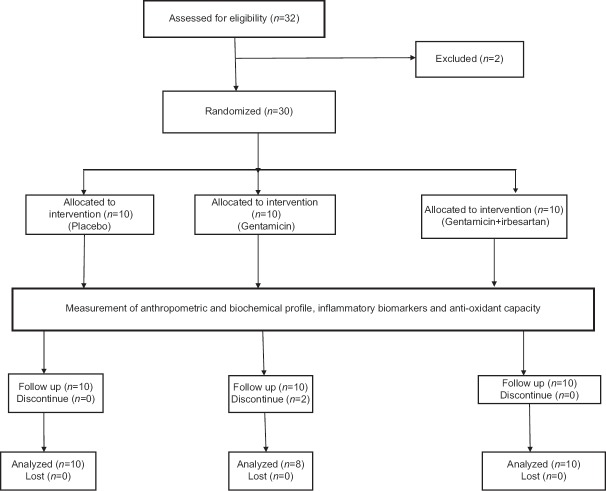
The steps of the study illustrated that 30 of 32 rats were used due to exclusion of 2 rats, thus 28 continued the experimental study as presented in the consort flow diagram of this study [Figure 1].
Figure 1.
Consort flow diagram of this study
Effect of gentamicin on renal and oxidative stress biomarkers
During gentamicin-induced nephrotoxicity, rat body weight was increased to 298.37 ± 25.02 g compared with the control group, 268.00 ± 25.01 g, P = 0.04. While the height of experimental rats was not significantly affected, P > 0.05. The BMI was increased significantly in gentamicin group compared with the control, P = 0.001. Blood urea was increased significantly in gentamicin group compared with the control group, P = 0.001, while serum creatinine of gentamicin group increased significantly (1.08 ± 0.20 mg/dL) compared with the control group, P = 0.001. The eGFR was reduced significantly in gentamicin (10.95 ± 2.16 mL/min/1.73) compared with the control, P = 0.001. Regarding the oxidative stress and endogenous antioxidant capacity, there was significant increase in the MDA serum levels in gentamicin group (408.11 ± 22.8 ng/mL) compared with the control group, P = 0.001; SOD, but not GSH sera levels, was reduced in gentamicin group compared with the control group, P = 0.001 and P = 0.49, respectively. Moreover, KIM-1 was significantly increased in gentamicin group (154.98 ± 16.38 pg/mL) compared with the control group, P = 0.0001. Also, serum levels of NGAL were increased significantly to 23.04 ± 5.88 pg/mL compared with the control group, P = 0.003 [Table 2].
Table 2.
Effect of irbesartan on the anthropometric variables, biochemical, and inflammatory biomarkers in gentamicin-induced nephrotoxicity
| Variables | Control (n=10) | G + S (n=8) | G + I (n=10) | Post hoc test A B C | ANOVA | ||
|---|---|---|---|---|---|---|---|
| Weight (g) | 268.00±25.01 | 298.37±25.02 | 269.54±26.21 | 0.04¶ | NS | 0.04¶ | 0.024¶ |
| Height (cm) | 21.50±0.83 | 21.51±0.84 | 21.51±0.81 | NS | NS | NS | 0.99 |
| BMI (g/cm2) | 0.58±0.02 | 0.64±0.04 | 0.58±0.03 | 0.001* | NS | 0.001* | 0.001* |
| Blood urea (mg/dL) | 41.83±6.46 | 56.87±9.45 | 42.56±7.89 | 0.001* | NS | 0.001* | 0.0005* |
| Serum creatinine (mg/dL) | 0.70±0.14 | 1.08±0.20 | 0.86±0.22 | 0.001* | NS | 0.04 | 0.01¶ |
| E-GFR (mL/min/1.73) | 16.89±4.21 | 10.95±2.16 | 13.75±2.45 | 0.002* | NS | NS | 0.003* |
| MDA (ng/mL) | 289.85±14.18 | 408.11±22.8 | 290.52±12.87 | 0.001* | NS | 0.001* | 0.0001* |
| SOD (pg/mL) | 48.12±7.92 | 26.39±5.86 | 39.98±6.39 | 0.001* | 0.04¶ | 0.001* | 0.0001* |
| GSH (µg/mL) | 15.94±2.39 | 13.89±2.94 | 14.77±2.86 | NS | NS | NS | NS |
| KIM-1 (pg/mL) | 73.78±6.29 | 154.98±16.38 | 77.56±6.98 | 0.001* | NS | 0.001* | 0.0001* |
| NGAL (pg/mL) | 15.78±3.07 | 23.04±5.88 | 16.67±3.98 | 0.006* | NS | 0.01¶ | 0.003* |
G + S: gentamicin + saline; G + I: gentamicin + irbesartan; ANOVA; analysis of variance; NS: not significant; A: control vs G + S; B: control vs G + I; C: G + I vs G + S; BMI: body mass index; E-GFR: estimated glomerular filtration rate; MDA: malondialdehyde; SOD: superoxide dismutase; GSH: glutathione reductase; KIM-1: kidney injury molecule-1; NGAL: neutrophil gelatinase-associated lipocalin *P<0.01; ¶P<0.05, unpaired t-test
Effect of irbesartan on renal and oxidative stress biomarkers during gentamicin-induced nephrotoxicity
Irbesartan significantly reduced rat body weight to 269.54 ± 26.21 g and BMI to 0.58 ± 0.03 g/cm2 compared with gentamicin group, P = 0.001. Also, it reduced blood urea and serum creatinine to 42.56 ± 7.89 mg/dL and 0.86 ± 0.22 mg/dL, respectively, compared with high levels in gentamicin group, P = 0.001. Coadministration of irbesartan with gentamicin improved renal function through improvement of eGFR significantly compared with gentamicin group, P = 0.002. Regarding the oxidative stress and antioxidant capacity, irbesartan significantly reduced MDA serum levels with significant elevation of SOD serum levels compared with gentamicin group, P = 0.001, without significant elevation of GSH serum levels. Notably, irbesartan significantly reduced the biomarkers of renal tubular injury through reduction in both KIM-1 and NGAL sera levels compared with gentamicin group [Table 2].
Concerning the glomerular injury, cystatin C serum level was elevated significantly during gentamicin-induced nephrotoxicity up to 1.7 ± 0.2 mg/dL compared with the control group (0.69 ± 0.03 mg/dL), but irbesartan administration led to significant reduction in cystatin C serum level to 0.8 ± 0.01 mg/dL compared with gentamicin group, P < 0.01 [Figure 2].
Figure 2.
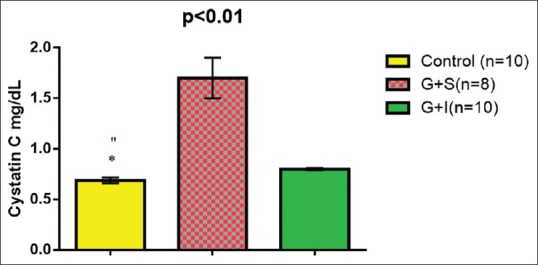
Effect of irbesartan on cystatin C serum levels compared with the control and gentamicin groups. *P < 0.01 (control vs gentamicin), P > 0.05 (control vs irbesartan)
Blood urea insignificantly correlated with the biomarkers of nephrotoxicity in the control group, P > 0.05. During gentamicin-induced nephrotoxicity, high blood urea levels were positively correlated with serum creatinine, MDA, KIM-1, and cystatin C (P < 0.05), but it negatively correlated with eGFR and SOD (P < 0.05). Indeed, reduction in blood urea level through irbesartan coadministration was significantly correlated with reduction in serum creatinine, MDA, KIM-1, and cystatin C as well as elevation of SOD serum levels and the improvement of eGFR [Table 3].
Table 3.
Correlation of blood urea with the anthropometric, biochemical, and renal biomarkers in gentamicin-induced nephrotoxicity regarding the effect of irbesartan compared with the control
| Variables | Control | G + S | G + I | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Serum cr. (mg/dL) | 0.62 | 0.33 | 0.99 | 0.01¶ | 0.96 | 0.01¶ |
| GFR (mL/min/1.73) | 0.33 | 0.50 | −0.91 | 0.04¶ | 0.89 | 0.04¶ |
| MDA (ng/mL) | 0.57 | 0.23 | 0.99 | 0.008* | 0.99 | 0.01¶ |
| SOD (pg/mL) | 0.72 | 0.09 | −0.99 | 0.001* | −0.98 | 0.02¶ |
| GSH (µg/mL) | 0.45 | 0.43 | −0.89 | 0.09 | −0.77 | 0.08 |
| KIM-1 (pg/mL) | 0.86 | 0.06 | 0.89 | 0.02¶ | 0.85 | 0.01¶ |
| Cys-C (mg/dL) | 0.66 | 0.15 | 0.98 | 0.015¶ | 0.96 | 0.01¶ |
r: correlation level; P: level of significance; G + S: gentamicin + saline; G + I: gentamicin + irbesartan; A: control vs G + S; B: control vs G + I; C: G + I vs G + S BMI: body mass index; E-GFR: estimated glomerular filtration rate; MDA: malondialdehyde; SOD: superoxide dismutase; GSH: glutathione reductase; KIM-1: kidney injury molecule-1; NGAL: neutrophil gelatinase-associated lipocalin *P<0.01; ¶P<0.05, unpaired t test
Effect of irbesartan on renal histopathological changes during gentamicin-induced nephrotoxicity
Control group showed normal renal tissue; both glomeruli and tubules look normal; whereas gentamicin produced significant histopathological changes including sloughing of renal tubular epithelium, dilatation of tubules with atrophy of epithelium, and formation of proteinaceous cast inside the tubules. Irbesartan led to significant amelioration of renal histological architecture, it reduced medullary congestion and renal tubular necrosis, and it reduced tubular necrosis from score 4 to score 2 compared with gentamicin group, so it lead to moderate tubular necrosis [Figure 3].
Figure 3.
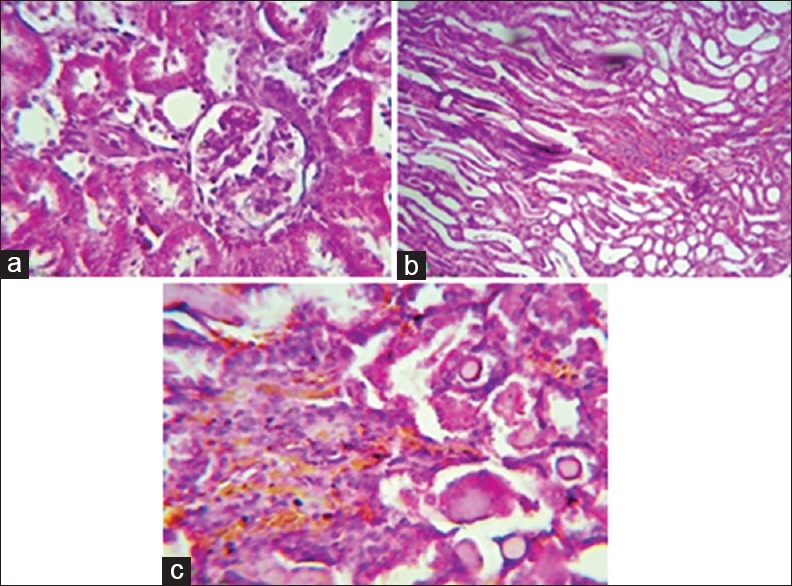
Effect of irbesartan on the renal histopathological changes induced by gentamicin nephrotoxicity. (a) Histological picture showing normal looking renal tissue (glomeruli and tubules), H and E, ×400 reflecting the effect of normal saline. (b) Histological section showing severe medullary congestion (score 4), detected on power of magnification × 100, H and E, reflecting the effect of gentamicin. (c) Histological section showing moderate medullary congestion (score 2), detected on power of magnification × 200, H and E, reflecting the effect of irbesarta
Gentamicin produced significant tubular injury (3.6 ± 0.4) compared with the control (0.7 ± 0.01); this damage was reduced when combined with irbesartan (2.5 ± 0.6) [Figure 4].
Figure 4.
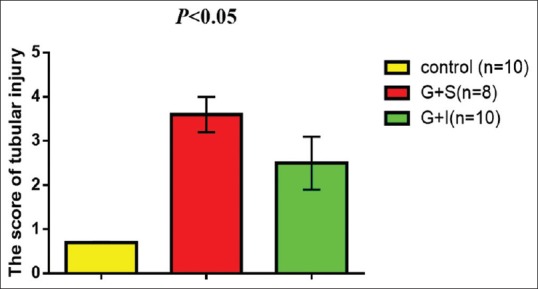
irbesartan reduces the scoring of renal tubular injury during gentamicin induced-nephrotoxicity
Therefore, irbesartan improved the outcome of gentamicin-induced nephrotoxicity through modulation of oxidative stress and augmentation of endogenous antioxidant capacity with significant reduction in tubular and glomerular injury biomarkers compared with gentamicin effects.
Discussion
This study certainly illustrated that gentamicin was proficient in inducing experimental nephrotoxicity in rats through significant elevation of blood urea and serum creatinine with significant reduction in the eGFR as supported by Helal et al.'s study.[19]
Indeed, Druml et al. confirmed that acute kidney injury is associated with distant organ damage with subsequent body fluid accumulations causing peripheral edema.[20] These findings might explain high BMI in gentamicin group as many rats developed ascites due to reduction in GFR.
It has been established that the production of free radicals and induction of oxidative stress are the most important pathways of gentamicin-induced nephrotoxicity. Overproduction of reactive oxygen species is linked with depletion of proximal renal tubules' antioxidant potential which causes lipid peroxidation and tubular damages.[21]
Therefore, serum level of MDA is elevated while SOD and GSH are reduced in different models of gentamicin-induced nephrotoxicity as illustrated by Hajihashemi et al.'s study which confirmed the protective effect of hydroalcoholic extract of Zataria multiflora in reduction in MDA with significant antioxidant effect.[22] In addition, other antioxidants may play a role in attenuation of lipid peroxidation during acute cardiotoxicity as disclosed by Al-kuraishy and Al-Gareeb's study.[23]
Despite these findings, gentamicin in this study elevates MDA serum levels and reduced SOD but not GSH serum levels which might due to insufficient gentamicin dose, small sample size, or short duration of the experimental study.
This study also illustrated significant effect of gentamicin in elevation of KIM-1 and NGAL sera levels similar to Luo et al.'s study which showed that KIM-1 and NGAL sera levels are sensitive and specific biomarkers and correlated with renal histopathological changes during gentamicin-induced nephrotoxicity within seven days.[24]
Normally, renal tissues express a low level of KIM-1; but subsequent to renal injury, KIM-1 gene expression dramatically upregulates at renal proximal convoluted tubules. Moreover, KIM-1 serum levels or urinary KIM-1 is regarded as a predictor and more sensitive than NGAL for development of acute renal injury,[25] as demonstrated in this study.
Cystatin C serum levels were significantly increased in gentamicin group compared with the control as supported by Kader et al.'s study which confirmed significant elevation in cystatin C serum level during gentamicin-induced nephrotoxicity.[26]
Cystatin C is a surrogate biomarker of GFR, not affected by muscle mass, age, gender, and food type; it is superior to serum creatinine since it detects earlier renal injury two days prior to the elevation of blood urea and serum creatinine. Normally, cystatin C is filtered by glomeruli and reabsorbed by proximal convoluted tubules, and thus elevation of serum cystatin C indicates a glomerular damage, whereas elevation of urinary cystatin C indicates renal tubular damage.[27]
On the other hand, RAS plays an important role in the progression of acute renal injury. RAS agonist is regarded as a growth factor which participates in the acceleration of renal damage due to activation of different cytokines including IL-6, TNF-α, and MCP-1 which cause interstitial and glomerular inflammation. Intrarenal angiotensin II is activated by toxic nephropathy or by ischemic-reperfusion injury, and thus Ang II antagonist revealed a nephroprotective effect through reduction of renal inflammation.[28]
These findings corresponded with the results of this study which illustrated that Ang II receptor blocker irbesartan leads to a significant nephroprotective effect through significant reduction in blood urea and improvement of eGFR as supported by Kusunoki et al.'s study which showed significant nephroprotective effect of irbesartan through modulation of Ang II receptors.[29]
Conversely, many studies illustrated deleterious effect of renin–angiotensin blockers on renal function since they may advance renal injury. Alabdan et al.'s study confined that RAS blockers may lead to progression of acute renal failure in hospitalized patients,[30] while a recent study by Mansfield et al. showed that treatment with ACEIs or RAS blockers causes minimal reduction in the renal functions which is a patient-dependent factor.[31]
In addition, this study showed that irbesartan leads to improvement of endogenous antioxidant capacity through significant increase in GSH levels with significant reduction in MDA serum level. The study by Miloradovic et al. shows the antioxidant effect of RAS blockers, such as losartan, which lead to significant nephroprotective effect through acceleration of antioxidant capacity and reduction in reactive oxidative stress during ischemic renal injury.[32]
Besides, irbesartan leads to a significant anti-inflammatory and nephroprotective effect through significant reduction in NGAL and KIM-1 sera levels during gentamicin-induced nephrotoxicity as illustrated in different studies.[33]
Moreover, the nephroprotective effect of irbesartan is through reduction in renal tubular biomarkers (KIM-1, NGAL) in patients with diabetic nephropathy. Besides, losartan and other ARB blockers attenuate acute kidney injury through inhibition of Ang II and IL-18 which are linked in activation of renal oxidative stress.[34]
Regarding the histopathological changes during gentamicin-induced nephrotoxicity, gentamicin led to medullary congestion, interstitial infiltration, tubular epithelium sloughing, tubular necrosis, and glomerular damage compared with the control as revealed in Ogundipe et al.'s study. These histopathological changes were correlated with the elevation of KIM-1 and NGAL sera levels as confirmed by different studies which showed that high levels of KIM-1 and NGAL are correlated with severe renal tissue injury.[35]
Irbesartan when coadministrated with gentamicin led to significant reduction in gentamicin-induced extensive tubular and glomerular damage to mild patchy tubular damage with a score of 2 out of 4 scoring system as revealed by different studies which showed that irbesartan leads to a significant nephroprotective effect. This protective effect was linked to the reduction in oxidative stress or augmentation of antioxidant activity since irbesartan illustrates significant effect on the biomarkers of antioxidant profile and reduction in KIM-1 serum levels.[36]
However, a therapy with reduced glutathione failed in prevention of high-dose gentamicin-induced nephrotoxicity despite the reduction in lipid peroxidation and increment in the renal glutathione activity, and thus, gentamicin produced dose-dependent effects in the progression of nephrotoxicity.[37] Therefore, gentamicin-induced oxidation is not only the sole mechanism since endoplasmic stress, membrane phospholiposis and/or destabilization, activation of calcium-sensing receptors, and disturbance of cellular energy should be regarded as other proposed mechanisms.[38]
Interestingly, large clinical trials including Irbesartan T2DM in Hypertensive Patients (IRMA) and Irbesartan T2DM Diabetic Nephropathy Trial (IDNT) study illustrated that irbesartan revealed potent nephroprotective effect through modulation of peroxisome proliferator-activator receptor γ (PPAR-γ).[39] Irbesartan shows nephroprotective effect independent of Ang II blocking effect which is most likely through PPAR-γ since blocking of PPAR-γ abolishes the nephroprotective effect of irbesartan. In addition, irbesartan inhibits renal tumor growth factor-β1, a mediator of renal damage, through upregulation of PPAR-γ which also improves renal antioxidant status.[40]
These findings may explain the potent nephroprotective effect through reduction in renal injury biomarkers with minimal effect on the marker of lipid peroxidation marker, but unfortunately serum levels of PPAR-γ were not assessed in this study.
Conclusions
Irbesartan has a nephroprotective effect in attenuation of acute nephrotoxicity through modulation of oxidative stress and antioxidant capacity in rats.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the teaching staff in the Department of Clinical Pharmacology for their cooperation and support.
References
- 1.Alkuraishy HM, Al-Gareeb AI, Al-Naimi MS. Pomegranate protects renal proximal tubules during gentamicin induced-nephrotoxicity in rats. J Contemp Med Sci. 2019;5:26. [Google Scholar]
- 2.Al-Kuraishy HM, Al-Gareeb AI, Rasheed HA. Antioxidant and anti-inflammatory effects of curcumin contribute into attenuation of acute gentamicin-induced nephrotoxicity in rats. Asian Journal of Pharmaceutical and Clinical Research. 2019;12:466–8. [Google Scholar]
- 3.Hu JG, Fu Y, Xu JJ, Ding XP, Xie HQ, Li-Ling J. Altered gene expression profile in a rat model of gentamicin-induced ototoxicity and nephrotoxicity, and the potential role of upregulated Ifi44 expression. Mol Med Rep. 2017;16:4650–8. doi: 10.3892/mmr.2017.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge S, Beechinor RJ, Hornik CP, Standing JF, Zimmerman K, Cohen-Wolkowiez M, et al. External evaluation of a gentamicin infant population pharmacokinetic model using data from a national electronic health record database. Antimicrob Agents Chemother. 2018;18:669–18. doi: 10.1128/AAC.00669-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Kuraishy HM, Al-Gareeb AI, Al-Nami MS. Pomegranate attenuates acute gentamicin-induced nephrotoxicity in Sprague-Dawley rats: The potential antioxidant and anti-inflammatory effects. Asian J Pharm Clin Res. 2019;12:1–3. [Google Scholar]
- 6.Jiang S, Hsu YH, Venners SA, Zhang Y, Xing H, Wang X, et al. Interactive effect of angiotensin II type 1 receptor (AGT1R) polymorphisms and plasma irbesartan concentration on antihypertensive therapeutic responses to irbesartan. J Hypertens. 2011;29:890–5. doi: 10.1097/HJH.0b013e32834494f6. [DOI] [PubMed] [Google Scholar]
- 7.Ceotto Freitas-Lima L, Merlo E, Campos Zicker M, Navia-Pelaez JM, de Oliveira M, Dos Santos Aggum Capettini L, et al. Tributyltin impacts in metabolic syndrome development through disruption of angiotensin II receptor signaling pathways in white adipose tissue from adult female rats. Toxicol Lett. 2018;29:31826–5. doi: 10.1016/j.toxlet.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Chida R, Hisauchi I, Toyoda S, Kikuchi M, Komatsu T, Hori Y, et al. Impact of irbesartan, an angiotensin receptor blocker, on uric acid level and oxidative stress in high-risk hypertension patients. Hypertens Res. 2015;38:765–9. doi: 10.1038/hr.2015.82. [DOI] [PubMed] [Google Scholar]
- 9.Hsu FY, Lin FJ, Ou HT, Huang SH, Wang CC. Renoprotective effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in diabetic patients with proteinuria. Kidney Blood Press Res. 2017;42:358–68. doi: 10.1159/000477946. [DOI] [PubMed] [Google Scholar]
- 10.Hartner A, Cordasic N, Klanke B. Renal protection by low dose irbesartan in diabetic nephropathy is paralleled by a reduction of inflammation, not of endoplasmic reticulum stress. Biochimica et Biophysica Acta. 2014;1842:558–65. doi: 10.1016/j.bbadis.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Sasso FC, Carbonara O, Persico M, Iafusco D, Salvatore T, D'Ambrosio R, et al. Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension: A randomized double-blind placebo controlled crossover study. Diabetes Care. 2002;25:1909–13. doi: 10.2337/diacare.25.11.1909. [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi Y, Ichiki T, Takeda K, Tokuno T, Iino N, Takeshita A. Critical role of cAMP-response element-binding protein for angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 2002;277:18710–7. doi: 10.1074/jbc.M110430200. [DOI] [PubMed] [Google Scholar]
- 13.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, et al. Animal models of acute renal failure. Pharmacol Rep. 2012;64:31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 14.Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41:111–9. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 15.Gacka E, Życzkowski M, Bogacki R, Paradysz A, Hyla-Klekot L. The usefulness of determining neutrophil gelatinase associated lipocalin concentration excreted in the urine in the evaluation of cyclosporine a nephrotoxicity in children with nephrotic syndrome. Dis Markers. 2016;2016:6872149. doi: 10.1155/2016/6872149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson SM, Craven RA, Nirmalan NJ, Harnden P, Selby PJ, Banks RE. Impact of pre-analytical factors on the proteomic analysis of formalin-fixed paraffin-embedded tissue. Proteomics Clin Appl. 2013;7:241–51. doi: 10.1002/prca.201200086. [DOI] [PubMed] [Google Scholar]
- 17.Toprak O, Cirit M, Tanrisev M, Yazici C, Canoz O, Sipahioglu M, et al. Preventive effect of nebivolol on contrast-induced nephropathy in rats. Nephrol Dial Transplant. 2008;23:853–9. doi: 10.1093/ndt/gfm691. [DOI] [PubMed] [Google Scholar]
- 18.Ayla S, Seckin I, Tanriverdi G, Cengiz M, Eser M, Soner BC, et al. Doxorubicin induced nephrotoxicity: Protective effect of nicotinamide. Int J Cell Biol. 2011;2011:390238. doi: 10.1155/2011/390238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helal MG, Zaki MMAF, Said E. Nephroprotective effect of saxagliptin against gentamicin-induced nephrotoxicity, emphasis on anti-oxidant, anti-inflammatory and anti-apoptic effects. Life Sci. 2018;208:64–71. doi: 10.1016/j.lfs.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Druml W. Systemic consequences of acute kidney injury. Curr Opin Crit Care. 2014;20:613–9. doi: 10.1097/MCC.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 21.Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. J Laboratory Physicians. 2018;10:276. doi: 10.4103/JLP.JLP_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajihashemi S, Jafarian T, Ahmadi M, Rahbari A, Ghanbari F. Ameliorative effects of Zataria multiflora hydro-alcoholic extract on gentamicin induced nephrotoxicity in rats. Drug Res (Stuttg) 2018;68:387–94. doi: 10.1055/s-0043-124968. [DOI] [PubMed] [Google Scholar]
- 23.Al-Kuraishy HM, Al-Gareeb AI. Potential effects of pomegranate on lipid peroxidation and pro-inflammatory changes in daunorubicin-induced cardiotoxicity in rats. Int J Prev Med. 2016:7. doi: 10.4103/2008-7802.184314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo QH, Chen ML, Sun FJ, Chen ZL, Li MY, Zeng W, et al. KIM-1 and NGAL as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem. 2014;397:53–60. doi: 10.1007/s11010-014-2171-7. [DOI] [PubMed] [Google Scholar]
- 25.Szeto CC, Kwan BC, Lai KB, Lai FM, Chow KM, Wang G, et al. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol. 2010;5:2329–37. doi: 10.2215/CJN.01910310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kader C, Sunbul M, Das YK, Yarim M, Bedir A, Karaca E, et al. Telbivudine attenuates gentamicin-induced kidney injury in rats. Int J Antimicrob Agents. 2017;49:595–602. doi: 10.1016/j.ijantimicag.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Conti M, Moutereau S, Zater M, Lallali K, Durrbach A, Manivet P, et al. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med. 2006;44:288–91. doi: 10.1515/CCLM.2006.050. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Liu Y, Han Y, Guan W, Kou X, Fu Jet al. Protective effects of aliskiren on ischemia-reperfusion-induced renal injury in rats. Eur J Pharmacol. 2013;718:160–6. doi: 10.1016/j.ejphar.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 29.Kusunoki H, Taniyama Y, Rakugi H, Morishita R. Cardiac and renal protective effects of irbesartan via peroxisome proliferator-activated receptorγ hepatocyte growth factor pathway independent of angiotensin II Type 1a receptor blockade in mouse model of salt-sensitive hypertension. J Am Heart Assoc. 2013;2:e000103. doi: 10.1161/JAHA.113.000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alabdan N, Gosmanova EO, Tran NQ, Oliphant CS, Pan H, Broyles JE, et al. Acute kidney injury in patients continued on renin-angiotensin system blockers during hospitalization. Am J Med Sci. 2017;353:172–7. doi: 10.1016/j.amjms.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Mansfield KE, Nitsch D, Smeeth L, Bhaskaran K, Tomlinson LA. Prescription of renin-angiotensin system blockers and risk of acute kidney injury: A population based cohort study. BMJ Open. 2016;6:e012690. doi: 10.1136/bmjopen-2016-012690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miloradović Z, Ivanov M, Jovović Đ, Karanović D, Vajić UJ, Marković-Lipkovski J, et al. Angiotensin 2 type 1 receptor blockade different affects post ischemic kidney injury in normotensive and hypertensive rats. J Physiol Biochem. 2016;72:813–20. doi: 10.1007/s13105-016-0514-4. [DOI] [PubMed] [Google Scholar]
- 33.Hartner A, Cordasic N, Klanke B, Menendez-Castro C, Veelken R, Schmieder RE, et al. Renal protection by low dose irbesartan in diabetic nephropathy is paralleled by a reduction of inflammation, not of endoplasmic reticulum stress. Biochim Biophys Acta. 2014;1842:558–65. doi: 10.1016/j.bbadis.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Valente AJ, Yoshida T, Murthy SN, Sakamuri SS, Katsuyama M, Clark RA, et al. Angiotensin II enhances AT1-Nox 1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox 1, and interleukin-18. Am J Physiol Heart Circ Physiol. 2012;303:H282–96. doi: 10.1152/ajpheart.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogundipe DJ, Akomolafe RO, Sanusi AA, Imafidon CE, Olukiran OS, Oladele AA. Ocimum gratissimum ameliorates gentamicin-induced kidney injury but decreases creatinine clearance following sub-chronic administration in rats. J Evid Based Complementary Altern Med. 2017;22:592–602. doi: 10.1177/2156587217691891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chander V, Singh D, Tirkey N, Chander H, Chopra K. Amelioration of cyclosporine nephrotoxicity by irbesartan, a selective AT1 receptor antagonist. Ren Fail. 2004;26:467–77. doi: 10.1081/jdi-200031731. [DOI] [PubMed] [Google Scholar]
- 37.Wong HS, Chen JH, Leong PK, Leung HY, Chan WM, Ko KM. β-Sitosterol protects against carbon tetrachloride hepatotoxicity but not gentamicin nephrotoxicity in rats via the induction of mitochondrial glutathione redox cycling. Molecules. 2014;19:17649–62. doi: 10.3390/molecules191117649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randjelovic P, Veljkovic S, Stojiljkovic N, Sokolovic D, Ilic I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017;16:388. doi: 10.17179/excli2017-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parving HH Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]