Abstract
Synaptic dysfunction has been implicated as an early cause of cognitive decline in neurodegenerative diseases (NDDs) such as Alzheimer’s disease (AD). Methods to slow down or reverse the loss of functional synapses, therefore, represent a promising avenue to explore for treating NDDs. We have previously reported the development of a class of benzothiazole amphiphiles (BAMs) that exhibited the capability to improve memory and learning both in wild‐type mice and in an AD rodent model, putatively through promoting RasGRF1‐associated formation of dendritic spines in hippocampal neurons. While these results represent a good first step in exploring a new approach to treating NDDs, the capability of these compounds to increase spine density has not been previously examined in a human neuronal model. Here, we found that neurons derived from differentiated human induced pluripotent stem cells exhibited both an increase in RasGRF1 expression and a phenotypic increase in the density of postsynaptic density protein 95‐positive puncta (which we use to provide an estimate of dendritic spine density) in BAM‐treated vs. control neurons. These results demonstrate that the previously observed spinogenic effects of BAMs in rodent neurons can be recapitulated in a human neuronal model, which further supports the potential utility of BAM agents for treating human diseases associated with spine deficits such as AD or other NDDs.
Keywords: benzothiazole, dendritic spine, neural stem cells, spinogenesis
Methods to promote dendritic spine formation or prevent dendritic spines loss represent a novel approach to combat memory deficits in neurodegenerative diseases. Using induced pluripotent stem cell‐derived neurons as the first human cell model capable of evaluating dendritic spine dynamics, we show that benzothiazole amphiphiles can activate cell machinery in human neurons to promote the formation of dendritic spines.

Abbreviations
- AD
Alzheimer’s disease
- BAM
benzothiazole amphiphile
- BDNF
brain‐derived neurotrophic factor
- dbcAMP
dibutyryl cyclic adenosine monophosphate
- FGF
fibroblast growth factor
- iPSC
induced pluripotent stem cell
- NDDs
neurodegenerative diseases
- NSC
neuronal stem cell
Synaptic dysfunction is a hallmark feature in many neurodegenerative disorders. Synapse loss was first correlated to Alzheimer’s disease (AD) in 1987 1 and was later suggested to be a major link to memory loss in AD 2. Additionally, synapse dysfunction and loss have been implicated in many other forms of dementia including spongiform encephalopathy 3, vascular dementia 4, and frontal lobe degeneration 5. However, there are no current approved methods to slow or halt the progressive synaptic or neuronal loss in neurodegenerative diseases (NDDs) 6. The functional unit of the synapse consists of both presynaptic and postsynaptic structures 7, which contain the necessary machinery to transmit neurochemical signals across the synaptic cleft 8. The postsynaptic structure, known as the dendritic spine, is actively remodeled and recycled on the surface of the dendrite 9, 10, 11, and the density of dendritic spines in the hippocampus is positively associated with memory and learning 12, 13. Methods to increase the density of dendritic spines, therefore, may represent a novel approach to slow down or counteract the cognitive effects of synapse loss in NDDs.
To date, relatively few compounds have been shown to increase dendritic spine density 14, 15, 16, 17, 18, 19, 20, 21, 22. For instance, we have previously reported that benzothiazole amphiphiles (BAMs) can increase dendritic spine density in primary rat hippocampal neuronal culture 15 as well as in vivo in wild‐type mice and in a 3xTg mouse model for AD 23, 24, 25. These initial reports showed that BAMs are able to improve memory and learning in rodents by a RasGRF1 (a guanine nucleotide release factor involved in regulation of dendritic spine formation)‐associated mechanism for promoting dendritic spine formation. Here, we examine whether the effects of BAM compounds on dendritic spine density found in rodent neurons can also be replicated in human neuronal models. Toward this end, we choose three different human neuronal model systems to evaluate efficacy of three different BAM compounds for their capability to promote RasGRF1‐dependent increases in dendritic spine density: undifferentiated SH‐SY5Y neuroblastoma cells, retinoic acid (RA)‐induced differentiated SH‐SY5Y neuroblastoma cells, and mature neurons derived from human‐induced pluripotent stem cells (iPSCs). We estimated dendritic spine formation by measuring postsynaptic density protein 95 (PSD95)‐positive puncta, a protein that is commonly found in high levels in mature dendritic spines. We found that neurons derived from iPSCs were the best human model system for evaluating spinogenic activity of BAM compounds, as these neurons treated with BAMs 1‐3 (Fig. 1) displayed a significant increase in density of PSD95 puncta compared to vehicle treatment. Additionally, RasGRF1 expression was found to be increased in the BAM‐treated neurons compared to the control neurons. Together, these results indicate that the same machinery found activated in rat primary culture by BAMs can also be activated in human neurons. To our knowledge, this report represents the first example of the use of human iPSC‐derived neurons to examine the biochemical and phenotypic activity of promoters of dendritic spine density, which could be useful for developing such compounds for treating human diseases.
Figure 1.
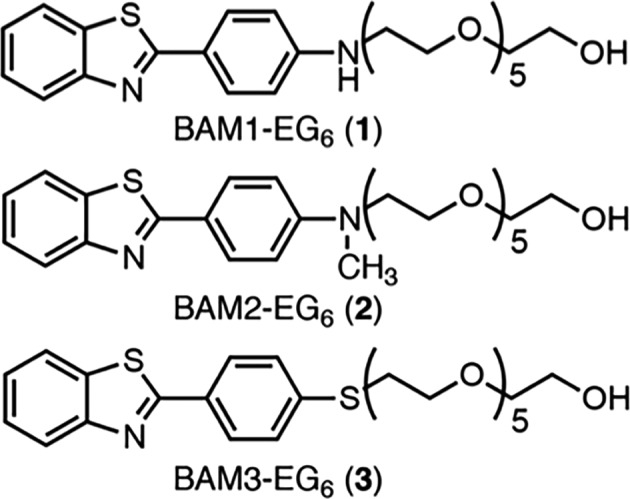
Structures of BAMs 1‐3.
Materials and methods
Materials
Human iPSC‐derived neuronal stem cells (NSCs) were a generous gift from L. Goldstein (Department of Cellular and Molecular Medicine, UCSD). Dulbecco’s modified Eagle’s medium (DMEM)/F12 + Glutamax (#10565‐018), N‐2 supplement (#17502‐048), and B27 supplement (#170504‐044) were purchased from Gibco (Gaithersburg, MD, USA). Human basic fibroblast growth factor (bFGF) (#100‐18B) and Brain‐derived neurotrophic factor (BDNF) (#450‐02B) were purchased from PeproTech (Rocky Hill, NJ, USA). Penicillin–streptomycin (Pen/Strep) (#17‐602E) was purchased from Lonza (Basel, Switzerland). Accutase (AT‐104) was purchased from Innovative Cell Technologies, Inc. (San Diego, CA, USA). Sterile 0.4 μm cell strainers (352340) were purchased from BD Falcon (Franklin Lakes, NJ, USA). Laminin (#L2020) and poly‐l‐ornithine (#P3655) were purchased from Sigma (St. Louis, MO, USA). Coverslips, 25 mm #1 German Glass (#CLS‐1760‐025), were purchased from Chemglass (Vineland, NJ, USA). Six‐well culture plates (#353046) were purchased from Falcon. Human Neural Stem Cell Immunocytochemistry Kit (#A24354) was purchased from Molecular Probes by Life Technologies (Carlsbad, CA, USA). Primary antibodies used were as follows: mouse anti‐Nestin (Life Technologies #A24345), rabbit anti‐SOX2 (Life Technologies #A24339), mouse anti‐MAP2 (Sigma #M1406), rabbit anti‐PSD95 (Invitrogen #51‐6900, Carlsbad, CA, USA), mouse anti‐RasGRF1 (BD 610149), and mouse anti‐GADPH (Sigma G8795). Secondary antibodies used were as follows: Alexa Fluor® 488 donkey anti‐mouse (Life Technologies #A24350), Alexa Fluor® 555 donkey anti‐rabbit (Life Technologies #A24342), TRITC‐conjugated goat anti‐mouse (Jackson ImmunoResearch, West Grove, PA, USA), Alexa Fluor® 488‐conjugated goat anti‐rabbit (Jackson ImmunoResearch), and ECL™ Horseradish Peroxidase (HRP) linked anti‐mouse (GE #NA931, Issaquah, WA, USA). Amersham™ ECL™ Prime western blotting Detection Reagent (RPN2232) was purchased from GE Healthcare. Protease inhibitor tablets (# 05892791001) were purchased from Roche (Basel, Switzerland). Pierce™ bicinchoninic acid Assay Kit (#23225) was purchased from Thermo Scientific (San Diego, CA, USA).
SH‐SY5Y neuroblastoma cultures
SH‐SY5Y human neuroblastoma cells were grown in 1 : 1 Eagle’s minimum essential medium (EMEM) and Ham’s F12 supplemented with 10% FBS. Cells were maintained in a humidified incubator at 37 °C in 95% air and 5% carbon dioxide (CO2). Complete media were exchanged two times per week.
Differentiated SH‐SY5Y neuroblastoma cultures
SH‐SY5Y neuroblastoma cells were differentiated as previously described 26. Briefly, SH‐SY5Y human neuroblastoma cells were grown in complete medium (1 : 1 EMEM and Ham’s F12 supplemented with 10% FBS). After adhering overnight, cells were differentiated by the addition of 10 μm all‐trans‐RA to the complete medium. Medium with RA was replaced every 2 days for 8 days total. Cells were maintained in a humidified incubator at 37 °C in 95% air and 5% CO2. Differentiation of SH‐SY5Y cells by RA was monitored over the course of 9 days, and we observed large changes in cell morphology accompanied by substantial neuritic outgrowth as has been reported previously for this cell line 27.
Human iPSC‐derived neural stem cell culture
Human iPSC‐derived, FACS‐purified, NSCs were maintained in base medium composed of DMEM/F12 medium with Glutamax supplemented with N‐2 (0.5×), B‐27 (0.5×), bFGF (20 ng·mL−1), and Pen/Strep (1×). bFGF was added fresh every week. Cells were plated on 6‐well plates that were coated with laminin (5 μg·mL−1) and poly‐l‐ornithine (20 μg·mL−1) and incubated at 37 °C, 5% CO2, and 95% humidity. Base medium was changed every 2–3 days, and cells were split at a ratio of 1 : 3 at 80–90% confluency with Accutase.
Human iIPSC‐derived neural stem cell differentiation
For the differentiation of human iPSC‐derived NSCs into mature neurons, base medium was used [DMEM/F12 medium with Glutamax supplemented with N‐2 (0.5×), B‐27 (0.5×), bFGF (20 ng·mL−1), and Pen/Strep (1×)] with the addition of BDNF (20 ng·mL−1) and dibutyryl cyclic adenosine monophosphate (dbcAMP; 0.5 mm) (here, no bFGF was added). This differentiation medium was changed every 3–4 days, and cells were maintained at 37 °C, 5% CO2, and 95% humidity. Cells were plated on either 6‐well dishes, MatTeks, or 8‐well chamber slides coated with laminin (5 μg·mL−1) and poly‐l‐ornithine (20 μg·mL−1) and let differentiate for up to 3 months.
Immunocytochemistry of human iPSC‐derived neural stem cells
A Human Neural Stem Cell Immunocytochemistry Kit (A24354) was utilized for the characterization of human NSCs. The kit was used to confirm expression of the common undifferentiated precursor markers Nestin as well as SOX2. Briefly, medium was removed and cells were rinsed with PBS. Cells were fixed with the provided fixative solution [4% PFA in Dulbecco’s phosphate‐buffered saline (DPBS)] for 15 min at room temperature (RT), followed by permeabilization with the provided permeabilization solution (0.5% Triton X‐100 in DPBS) for 15 min at RT. After permeabilization, cells were blocked with the blocking solution (3% BSA/DPBS) for 1 h at RT. After removal of blocking solution, primary antibodies diluted to 1X in blocking solution were added and let incubate at 4 °C overnight. After washing (3×), the appropriate secondary antibodies were added (diluted to 1X in blocking solution) and incubated for 1 h at RT. Cells were washed 3× with two drops per mL of the provided NucBlue® Fixed Cell Stain (DAPI) added in the last wash step and allowed to incubate for 5 min. Slides or coverslips were mounted with Vectashield® and imaged with an FV1000 confocal microscope (Olympus, Waltham, MA, USA).
Immunocytochemistry for PSD95/MAP2 staining
Cells were stained for microtubule‐associated protein 2 (MAP2), a neuron‐specific marker, as well as PSD95, a postsynaptic marker. Briefly, cells were dosed for 24 h with or without BAMs 1‐3 (5 μm in PBS). After dosing, cells were rinsed (3× PBS), fixed with 4% PFA (15 min, RT), permeabilized with 0.3% Triton X‐100/DPBS (10 min RT), blocked with 3% BSA/DPBS (1 h, RT), and incubated overnight at 4 °C with primaries: MAP2 (1 : 500) and PSD95(1 : 1000) in blocking solution. Secondaries were diluted 1 : 250 in blocking solution and let incubate for 1 h at RT. Slides or coverslips were mounted with Vectashield® and imaged with an FV1000 confocal microscope (Olympus). PSD95‐positive puncta were quantified per micron along MAP2‐positive neurons. For each treatment, three separate cultures were used and, among those cultures, a minimum of 9 total neurons were evaluated for counting the density of PSD95‐positive puncta.
Western blot quantification of RasGRF1 expression
We performed western blot analyses to quantify protein expression of either dosed or control cells. Briefly, cells were dosed for 24 h with or without BAMs 1‐3 (0 or 5 μm in PBS). After dosing, cells were rinsed on ice (3× with ice‐cold PBS), lysed with RIPA buffer with protease inhibitors. Lysates were agitated for 30 min at 4 °C, followed by centrifugation at 4 °C (20 min, 13 000 g). Supernatants were aspirated into a new ice‐cold tube, and a bicinchoninic acid assay was performed to determined protein concentration. Lysates were frozen and stored at −80 °C until use for western blot analysis. Briefly, proteins were separated by SDS/PAGE followed by transfer onto nitrocellulose membranes. Membranes were blocked with either 5% BSA or milk in Tris‐buffered saline with Tween‐20, followed by incubation with primary antibodies overnight at 4 °C with shaking. Proteins were visualized using the appropriate HRP‐labeled secondary by ECL, and detection was carried out on film (Freedom Imaging, SRX‐101A). The density of each band was then quantified using imagej (National Institutes of Health, Bethesda, MD, USA) software.
Results and discussion
BAMs marginally increase RasGRF1 levels but do not promote the expression of PSD95 in undifferentiated SH‐SY5Y neuroblastoma cells
In order to initially examine whether the BAM compounds activated the same RasGRF1‐dependent spinogenic pathway in human cells as observed in rat hippocampal neurons, we attempted to utilize the neuroblastoma cell line, SH‐SY5Y, as the simplest of the three human cell model systems tested 28. The SH‐SY5Y neuroblastoma cell line was specifically chosen because of its human origin and because these cells have been reported to exhibit neuron‐like properties, especially upon differentiation 27, 28. We dosed SH‐SY5Y cells with 5 μm concentrations of BAMs 1‐3 or PBS (as vehicle control) for 24 h, followed by lysis and probing by western blot to analyze RasGRF1 expression levels. RasGRF1 was previously found to have the highest change in expression levels in a RasGRF1/Ras/ERK‐dependent pathway for increasing dendritic spines in response to exposure of the BAM compounds in rodent models 14, 15. As such, we sought to use changes in RasGRF1 expression for evaluating the potential activation of the spinogenesis pathway by these compounds. We observed only a slight increase in RasGRF1 expression for cells treated with any of the three BAM compounds, with only BAM2‐EG6 producing a statistically significant increase (Fig. 2A). These results are in contrast to the significant increase in Ras‐GRF1 expression levels found in primary rat hippocampal neurons dosed with the same concentrations of BAMs 1‐3 14. These opposing results could be due to lower abundance of neuronal and synaptic proteins found in undifferentiated neuroblastoma cells compared to mature mammalian neurons 29. This low expression of synaptic proteins in SH‐SY5Y cells was further supported by immunocytochemistry experiments. Dendritic spines (the major site of excitatory synaptic input) appear on mature neurons and comprise a high local concentration of the PSD95 30. Microtubule‐associated protein 2 is typically used as a biomarker to locate dendritic arborization in neurons 31. While concurrent visualization of PSD95 and MAP2 has been used previously to identify spines in neurons, 32 high levels of PSD95 are not a requirement for all mature spines 33. Measuring density of observed PSD95 puncta may, therefore, only serve as an estimate of the density of dendritic spines in these cell studies. The SH‐SY5Y neuroblastoma cells showed undetectable levels of PSD95 (Fig. 2B), with or without exposure of cells to BAM1‐EG6 (as a representative spine‐promoting agent). The data indicate that undifferentiated SH‐SY5Y cells do not function as an adequate model for the evaluation of dendritic spine density.
Figure 2.
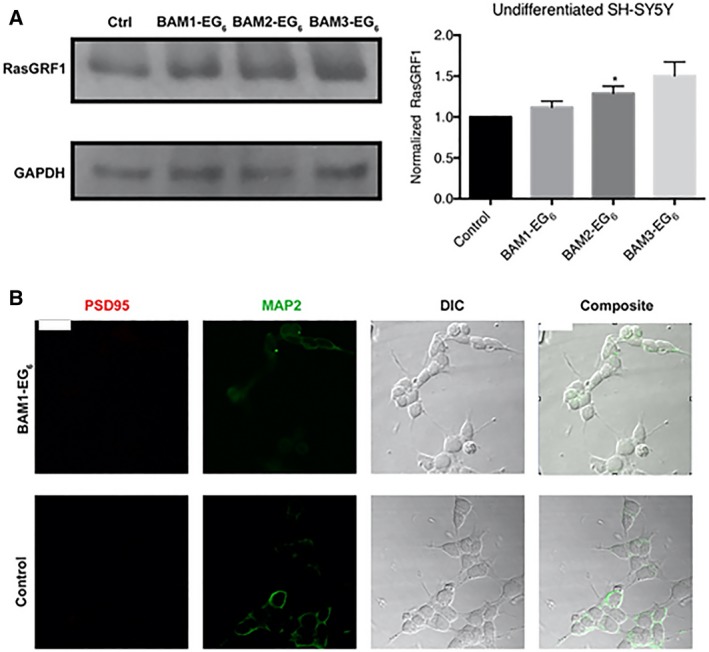
Evaluation of BAM efficacy in SH‐SY5Y neuroblastoma. (A) Comparison of relative expression levels of RasGRF1 between SH‐SY5Y cells dosed with vehicle control (PBS) or BAMs 1‐3 (5 µm). (B) Representative images of cells stained for PSD95 (red) and MAP2 (green). Data are expressed as mean values ± SEM, n ≥ 3, *P < 0.05 as determined by unpaired t‐test compared to control. Scale bar = 20 microns.
BAMs increase RasGRF1 levels but do not promote the formation of PSD95 puncta in differentiated SH‐SY5Y neuroblastoma cells
Since the BAM compounds are known to affect synapse formation, we next turned to differentiated SH‐SY5Y cells. SH‐SY5Y neuroblastoma cells have been shown to differentiate into a more neuron‐like phenotype through the use of RA treatment to increase neuritic outgrowth 27. We observed large morphological changes in SH‐SY5Y cell cultures over 9 days of differentiation with RA. In addition, differentiation of these cells has been reported to increase dendritic arborization and the expression of synaptic proteins 28. The expression of these synaptic proteins is most likely vital for the capability of the BAM agents to promote dendritic spine formation.
Differentiated SH‐SY5Y cells were dosed with BAM compounds, lysed, and probed by western blot to analyze for changes in RasGRF1 expression levels compared to control cells. Here, we observed a more consistent increase in expression of RasGRF1 for cells dosed with BAM compounds compared to cells exposed only to PBS vehicle control (Fig. 3A). The observed ~ 20% increase in expression of RasGRF1 levels in the presence of BAM agents was similar to the increase in RasGRF1 levels observed in rodent models exposed to these compounds 14. For this experiment, we attempted to quantify the number of PSD95‐positive puncta along MAP2‐labeled neurites in treated vs. control cells. While we found intracellular areas with increased PSD95, we did not find any overt puncta containing PSD95 that would be consistent with a dendritic spine in the presence or absence of the BAM agents (Fig. 3B). These results highlight a potential drawback for using neuroblastoma cells for analysis of PSD95‐positive puncta, even when differentiated with RA, as they do not appear to exhibit adequate neuron‐like properties for analysis of the memory‐enhancing phenotypic activity of BAM compounds.
Figure 3.

Evaluation of BAM efficacy in differentiated SH‐SY5Y neuroblastoma. (A) Comparison of relative expression levels of RasGRF1 between differentiated SH‐SY5Y cells dosed with vehicle control (PBS) or BAMs 1‐3 (5 µm). (B) Representative images of cells stained for PSD95 (red) and MAP2 (green). White boxes denote areas of segments shown in higher magnification below either BAM1‐EG6 dosed or control cells, respectively. Data are expressed as mean values ± SEM, n ≥ 3, *P < 0.05 as determined by unpaired t‐test compared to control. Scale bar = 20 microns.
Differentiation of human iPSC‐derived neural stem cells (NSCs)
Induced pluripotent stem cells represent exciting and potentially more reliable models than immortalized cells for evaluating the activity of potential drug candidates for human diseases 34, 35. Several attractive attributes of using human iPSC technology for evaluating efficacy of bioactive compounds include the following: (a) the ability to provide a continuous supply of normal (i.e., nonimmortalized) human cells 36, (b) the capability to differentiate pluripotent cells to generate specific cell types 37, 38, and (c) the unique capacity to generate either disease or healthy patient‐derived cells 39, 40, 41. Importantly for the work presented here, iPSCs have been shown to successfully differentiate to neural stem cells (NSCs) and subsequently into mature neurons 42, 43.
We derived human NSCs from human iPSCs as previously described by Carson and coworkers 44. The isolated NSCs were initially characterized by the undifferentiated precursor markers Nestin (an intermediate filament protein) and SOX2 (a transcription factor essential for pluripotency) (Fig. 4). These results indicate that the iPSCs were successfully differentiated into NSCs 44, 45.
Figure 4.

Immunohistochemistry characterization of NSCs. Undifferentiated precursor‐specific marker staining of (A) Nestin and (B) SOX2. Additional images of the (C) staining of nuclei with DAPI and (D) composite image of A‐C. Scale bar = 25 μm.
After characterization, the NSCs were differentiated into mature neurons using a previously described method of FGF removal and addition of BDNF and dbcAMP 40, 41. The differentiation process was monitored weekly by live‐cell microscopy and maintained until ready for use. Additionally, mature neurons from several different preparations were then stained with the neuronal‐specific marker, MAP2 (images of cells from three such preparations are shown in Fig. 5).
Figure 5.

Immunohistochemistry of the neuronal‐specific marker MAP2 from three different representative preparations of human neurons derived from NSCs. MAP2 staining from preparations 1 (A), 2 (B), and 3 (C–E). DAPI staining (D) and composite (E) of images C and D. Top panel scale bar = 100 μm, bottom panel scale bar = 50 μm.
BAMs increase RasGRF1 levels and increase the density of PSD95 puncta in human iPSC‐derived mature neurons
After differentiating human NSCs into mature neurons, we next sought to examine whether the BAM agents induced increased expression of RasGRF1 in these cells. For this experiment, 3‐month‐old mature iPSC‐derived neurons were exposed to solutions of BAMs 1‐3 in PBS (0 or 5 μm) for 24 h. Subsequently, cells were lysed and western blot analysis was performed in order to quantify any changes in RasGRF1 expression. We observed a significant increase in RasGRF1 expression in iPSC‐derived neurons that were dosed with 5 μm BAMs 1‐3 over the control (PBS) (Fig. 6A,B). This result is consistent with the hypothesis that the machinery activated in rodent models leading to dose‐dependent increases in spine density by the BAM agents is also present and can be activated by these compounds in a human neuronal model.
Figure 6.

Evaluation of the activity of BAM 1‐3 in neurons derived from iPSCs. (A) Blot comparing the expression of RasGRF1 and GAPDH. (B) Comparison of relative expression levels of RasGRF1 between neurons dosed with vehicle control (PBS) or BAMs 1‐3 (5 µm). (C) Quantitative representation of the relative density of PSD95 puncta along MAP2‐positive dendrites on iPSC‐derived neurons dosed with vehicle control or BAMs 1‐3; for clarity, the data for density of PSD95 puncta are normalized to the density on the control neurons. Data are expressed as mean values ± SEM, n ≥ 9 neurons selected among three separate cultures, *P < 0.05 as determined by unpaired t‐test compared to control. Scale bar = 25 microns. (D) Representative images of dendrite segments.
We next sought to examine whether BAMs 1‐3 could elicit the same phenotypic increase in PSD95 puncta in human neurons as was previously observed in rat primary hippocampal neuronal culture 14. For this experiment, we dosed 3‐month‐old neurons derived from iPSCs with 0 or 5 μm BAMs 1‐3 for 24 h. Neurons were then fixed, and immunochemistry was performed to examine the relative density of PSD95‐positive puncta located along MAP2‐labeled segments of dendrites (Fig. 6C,D). Here, a 1.5‐ to 2‐fold increase in the density of PSD95 puncta was observed for neurons treated with BAMs 1‐3 compared to the control neurons (incubated with PBS alone). These data support that BAMs 1‐3 exhibit similar phenotypic activity for promoting dendritic spines as had previously been observed in rat primary hippocampal neurons 14. These data also further support the utility of iPSC‐derived neurons to evaluate both the biochemical and phenotypic activities of promoters of dendritic spine formation in a non‐immortalized human cell model.
Conclusion
We have examined the capability of BAMs to promote the formation of PSD95‐positive puncta in three different human cellular models: (a) undifferentiated, (b) differentiated SH‐SY5Y neuroblastoma cells and (c) neurons derived from human iPSCs. We found that both of the undifferentiated and differentiated neuroblastoma cell preparations were not suitable to examine both the biochemical and phenotypic activities of the BAM compounds as promoters of dendritic spine formation. In neurons derived from differentiating iPSCs, however, we were indeed able to observe both significant increases in RasGRF1 expression and phenotypic increases in dendritic spine density (as estimated by the density of PSD95‐positive puncta along MAP2‐labeled dendrites) when cells were exposed to the BAM compounds. These results demonstrate that iPSC‐derived neurons can serve as an accessible human model for examining the activity of compounds that affect dendritic spine dynamics. Additionally, these results further support that the analogous cellular machinery activated by BAM compounds in rodent hippocampal neurons may also be activated in human neurons, suggesting the possible utility of BAM agents to improve memory and learning through promoting dendritic spine formation for the treatment of human NDDs and other memory impairment disorders.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
JLC and JY conceived the research. JLC and JY designed and JLC performed the experiments. JLC, KRB, and JY analyzed and interpreted the data. JLC, KRB, and JY wrote the manuscript.
Acknowledgements
This manuscript was funded by the National Institute on Aging of the National Institutes of Health under award number R01AG053577. We also thank Jennifer Santini and the UC San Diego School of Medicine Microscopy Core in the Department of Neurosciences (P30 NS047101) for imaging support. We thank Dr. Jessica Young and Prof. Lawrence Goldstein in the Department of Cellular and Molecular Medicine at UCSD for the generous gift of the NSCs used in this work.
Data accessibility
All data generated or analyzed during this study are included in this manuscript.
References
- 1. Bergeron C, Ranalli PJ and Miceli PN (1987) Amyloid angiopathy in Alzheimer’s disease. Can J Neurol Sci 14, 564–569. [PubMed] [Google Scholar]
- 2. Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA and Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30, 572–580. [DOI] [PubMed] [Google Scholar]
- 3. Clinton J, Forsyth C, Royston MC and Roberts GW (1993) Synaptic degeneration is the primary neuropathological feature in prion disease: a preliminary study. NeuroReport 4, 65–68. [DOI] [PubMed] [Google Scholar]
- 4. Sinclair LI, Tayler HM and Love S (2015) Synaptic protein levels altered in vascular dementia. Neuropathol Appl Neurobiol 41, 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brun A, Liu X and Erikson C (1995) Synapse loss and gliosis in the molecular layer of the cerebral cortex in Alzheimer’s disease and in frontal lobe degeneration. Neurodegeneration 4, 171–177. [DOI] [PubMed] [Google Scholar]
- 6. Alzheimer's Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimers Dement 13, 325–373. [Google Scholar]
- 7. Stewart MG, Popov VI, Kraev IV, Medvedev N and Davies HA (2014) Chapter One ‐ Structure and Complexity of the Synapse and Dendritic Spine In The Synapse (Pickel V. and Segal M, eds), pp. 1–20. Academic Press, Boston, MA. [Google Scholar]
- 8. Whittaker VP (1993) Thirty years of synaptosome research. J Neurocytol 22, 735–742. [DOI] [PubMed] [Google Scholar]
- 9. Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M and Hayashi Y (2014) Structural and molecular remodeling of dendritic spine substructures during long‐term potentiation. Neuron 82, 444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bramham CR (2008) Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol 18, 524–531. [DOI] [PubMed] [Google Scholar]
- 11. Meyer D, Bonhoeffer T and Scheuss V (2014) Balance and stability of synaptic structures during synaptic plasticity. Neuron 82, 430–443. [DOI] [PubMed] [Google Scholar]
- 12. Hayashi ML, Choi S‐Y, Rao BSS, Jung H‐Y, Lee H‐K, Zhang D, Chattarji S, Kirkwood A and Tonegawa S (2004) Altered cortical synaptic morphology and impaired memory consolidation in forebrain‐ specific dominant‐negative PAK transgenic mice. Neuron 42, 773–787. [DOI] [PubMed] [Google Scholar]
- 13. Morgado‐Bernal I (2011) Learning and memory consolidation: linking molecular and behavioral data. Neuroscience 176, 12–19. [DOI] [PubMed] [Google Scholar]
- 14. Cifelli JL, Dozier L, Chung TS, Patrick GN and Yang J (2016) Benzothiazole amphiphiles promote the formation of dendritic spines in primary hippocampal neurons. J Biol Chem 291, 11981–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cifelli JL, Chung TS, Liu H, Prangkio P, Mayer M and Yang J (2016) Benzothiazole amphiphiles ameliorate amyloid β‐related cell toxicity and oxidative stress. ACS Chem Neurosci 7, 682–688. [DOI] [PubMed] [Google Scholar]
- 16. Duman RS, Li N, Liu R‐J, Duric V and Aghajanian G (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cansev M, Ulus IH, Wang L, Maher TJ and Wurtman RJ (2008) Restorative effects of uridine plus docosahexaenoic acid in a rat model of Parkinson’s disease. Neurosci Res 62, 206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakamoto T, Cansev M and Wurtman RJ (2007) Oral supplementation with docosahexaenoic acid and uridine‐5′‐monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res 1182, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rozkalne A, Hyman BT and Spires‐Jones TL (2011) Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque‐bearing Alzheimer model mice. Neurobiol Dis 41, 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spires‐Jones TL, Kay K, Matsouka R, Rozkalne A, Betensky RA and Hyman BT (2011) Calcineurin inhibition with systemic FK506 treatment increases dendritic branching and dendritic spine density in healthy adult mouse brain. Neurosci Lett 487, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar P and Baquer N (2018) Estradiol neuroprotective against the neurotoxic effects of β‐amyloid (25–35) in aging rat brains. Alzheimers Dement 14, P1121–P1122. [Google Scholar]
- 22. Zhu Y, Zhang Q, Zhang W, Li N, Dai Y, Tu J, Yang F, Brann DW and Wang R (2017) Protective effect of 17β‐estradiol upon hippocampal spine density and cognitive function in an animal model of vascular dementia. Sci. Rep 7, 42660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Megill A, Lee T, DiBattista AM, Song JM, Spitzer MH, Rubinshtein M, Habib LK, Capule CC, Mayer M, Turner RS et al (2013) A Tetra(Ethylene Glycol) derivative of benzothiazole aniline enhances ras‐mediated spinogenesis. J Neurosci 33, 9306–9318.23719799 [Google Scholar]
- 24. Song JM, DiBattista AM, Sung YM, Ahn JM, Turner RS, Yang J, Pak DTS, Lee H‐K and Hoe H‐S (2014) A tetra(ethylene glycol) derivative of benzothiazole aniline ameliorates dendritic spine density and cognitive function in a mouse model of Alzheimer’s disease. Exp Neurol 252, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee NJ, Song JM, Cho H‐J, Sung YM, Lee T, Chung A, Hong S‐H, Cifelli JL, Rubinshtein M, Habib LK et al (2016) Hexa (ethylene glycol) derivative of benzothiazole aniline promotes dendritic spine formation through the RasGRF1‐Ras dependent pathway. Biochim Biophys Acta 1862, 284–295. [DOI] [PubMed] [Google Scholar]
- 26. Datki Z, Juhász A, Gálfi M, Soós K, Papp R, Zádori D and Penke B (2003) Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma cells. Brain Res Bull 62, 223–229. [DOI] [PubMed] [Google Scholar]
- 27. Påhlman S, Ruusala AI, Abrahamsson L, Mattsson ME and Esscher T (1984) Retinoic acid‐induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester‐induced differentiation. Cell Differ 14, 135–144. [DOI] [PubMed] [Google Scholar]
- 28. Forster JI, Köglsberger S, Trefois C, Boyd O, Baumuratov AS, Buck L, Balling R and Antony PMA (2016) Characterization of differentiated SH‐SY5Y as neuronal screening model reveals increased oxidative vulnerability. J Biomol Screen 21, 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovalevich J and Langford D (2013) Considerations for the use of SH‐SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El‐Husseini AE, Schnell E, Chetkovich DM, Nicoll RA and Bredt DS (2000) PSD‐95 involvement in maturation of excitatory synapses. Science 290, 1364–1368. [PubMed] [Google Scholar]
- 31. Soltani MH, Pichardo R, Song Z, Sangha N, Camacho F, Satyamoorthy K, Sangueza OP and Setaluri V (2005) Microtubule‐associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. Am J Pathol 166, 1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bresler T, Ramati Y, Zamorano PL, Zhai R, Garner CC and Ziv NE (2001) The dynamics of SAP90/PSD‐95 recruitment to new synaptic junctions. Mol Cell Neurosci 18, 149–167. [DOI] [PubMed] [Google Scholar]
- 33. Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC and Sabatini BL (2008) Destabilization of the postsynaptic density by PSD‐95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60, 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Inoue H and Yamanaka S (2011) The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther 89, 655–661. [DOI] [PubMed] [Google Scholar]
- 35. Ebert AD and Svendsen CN (2010) Human stem cells and drug screening: opportunities and challenges. Nat Rev Drug Discov 9, 367–372. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K and Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- 37. Yu J, Vodyanik MA, Smuga‐Otto K, Antosiewicz‐Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- 38. Park I‐H, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW and Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146. [DOI] [PubMed] [Google Scholar]
- 39. Park I‐H, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K and Daley GQ (2008) Disease‐specific induced pluripotent stem cells. Cell 134, 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Young JE, Boulanger‐Weill J, Williams DA, Woodruff G, Buen F, Revilla AC, Herrera C, Israel MA, Yuan SH, Edland SD et al (2015) Elucidating molecular phenotypes caused by the SORL1 Alzheimer’s disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell 16, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA and Svendsen CN (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paşca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Paşca AM, Cord B, Palmer TD, Chikahisa S, Nishino S et al (2011) Using iPSC‐derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med 17, 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS et al (2012) Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA et al (2011) Cell‐surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One 6, e17540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pankratz MT, Li X‐J, LaVaute TM, Lyons EA, Chen X and Zhang S‐C (2007) Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells 25, 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.


