Abstract
Background:
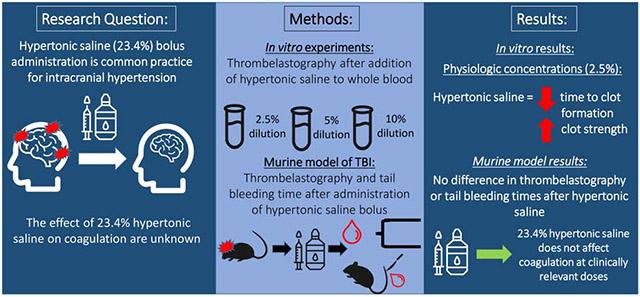
Hypertonic saline (23.4%, HTS) bolus administration is common practice for refractory intracranial hypertension, but its effects on coagulation are unknown. We hypothesize that 23.4% HTS in whole blood results in progressive impairment of coagulation in vitro and in vivo in a murine model of traumatic brain injury (TBI).
Study Design:
For the in vitro study, whole blood was collected from 10 healthy volunteers, and citrated native thrombelastography (TEG) was performed with normal saline (0.9%, NS) and 23.4% HTS in serial dilutions (2.5%, 5%, 10%). For the in vivo experiment, we assessed the effects of 23.4% HTS bolus versus NS on serial TEGs and tail bleeding times in a TBI murine model (n=10 rats with TBI and 10 controls).
Results:
For the in vitro work, clinically relevant concentrations of HTS (2.5% dilution) shortened time to clot formation (R) and increased clot strength (maximum amplitude, MA) as compared to control and NS. With higher HTS dosing (5% and 10% blood dilution), there was progressive R prolongation, decreased angle and decreased MA. In the in vivo study, there was no significant difference in TEG measurements or tail bleeding times after bolus administration of 23.4% HTS compared to NS at 2.5% blood volume.
Conclusion:
At clinically relevant dilutions of HTS, there is a paradoxical shortening of time to clot formation and increase in clot strength in vitro and no significant effects in a murine TBI model. However, with excess dilution, caution should be exercised when employing serial HTS boluses in TBI patients at risk for trauma induced coagulopathy.
Graphical Abstract
Precis
The effect of 23.4% hypertonic saline (HTS) on coagulation is unknown. This study includes thrombelastographic evaluation of blood from healthy volunteers diluted with HTS and from a murine traumatic brain injury model. Hypertonic saline shortens clot formation time and increases clot strength in vitro but has no coagulation effects in vivo.
Introduction
Hypertonic saline (HTS) resuscitation has been investigated as a low volume resuscitative agent in trauma to rapidly increase intravascular volume. Pre-clinical trial studies examining 7.5% HTS as a resuscitative fluid in trauma-related hemorrhagic shock and mortality have been inconclusive, with some studies reporting no adverse effect or reduced hypocoagulability with HTS(1-10) and others describing increased hypocoagulability with HTS(11-17). However, a multicenter randomized, blinded, placebo-controlled clinical trial in which hypotensive patients were randomized to resuscitation with 7.5% HTS or normal saline (0.9%) was terminated early due to safety concerns with increased mortality in the HTS group(18). One of the proposed mechanisms of increased mortality with HTS resuscitation has been aggravation of trauma induced coagulopathy (TIC). Both in vitro dilution work with 3% HTS and 7.5% HTS and in vivo animal models of HTS-based resuscitation of hemorrhagic shock report an anticoagulant, antiplatelet effect with HTS(13, 17, 19, 20). In clinical studies of trauma and surgical patients who receive 7.5% HTS, hypertonic saline provokes a hypocoagulable and hyperfibrinolytic profile with increased bleeding events, prolonged international normalized ratio (INR), and increased ratios of pro- to anti-plasmin proteins(16, 21).
Despite the adverse effects of HTS on coagulation in trauma patients, boluses of 23.4% HTS remain accepted as a standard of care in traumatic brain injury (TBI) due to its ability to rapidly decrease intracranial hypertension(9, 22, 23). However, despite its widespread use in the setting of intracranial hypertension, the effects of 23.4% HTS on coagulation on unknown, as studies exploring the effects of hypertonic saline on coagulation have been limited to 3% HTS or 7.5% HTS. Some reports cite concern for increased hemorrhagic contusion areas in TBI patients after receiving 20% HTS(24), but, to our knowledge, the effects of 23.4% HTS on coagulation have not been investigated . Understanding the effects of HTS on hemostatic capacity is crucial given the potential consequences of increased bleeding in patients with intracranial hemorrhage and associated polytrauma.
The objective of this study is to examine the effects of 23.4% HTS in whole blood in vitro through thrombelastographic analysis and in vivo in a murine TBI model. We hypothesize that 23.4% HTS in whole blood results in progressive impairment of coagulation in vitro and in vivo in a murine model of traumatic brain injury (TBI).
Methods
In vitro thrombelastography
We examined the effects of 23.4% HTS in vitro to create a dose-response curve, using clinically relevant sodium concentrations in a variety of blood dilutions (osmolalities). Healthy adult (≥18 years) volunteers were recruited from an urban hospital, the Ernest E Moore Shock Trauma Center, and screened via a questionnaire (eDocument 1) to determine eligibility. Subjects were excluded if they had any acute or chronic health problems or were taking medication known to affect coagulation. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB #18-0625), and all subjects provided informed consent.
Blood was collected in citrated tubes (3.2% sodium citrate, Greiner Bio-One, Monroe, North Carolina). Citrated native thrombelastography (TEG) was performed using the TEG 5000® Thrombelastography Hemostasis Analyzer (Haemonetics Inc., Niles, IL) as described by manufacturer guidelines(25). While the average time from venipuncture to assay run was short (15 minutes), citrate was required to prevent coagulation prior to the calcified assay; use of citrate is beneficial in that coagulation can be initiated within the TEG apparatus at the same time for each individual, mitigating the variability of contact activation and coagulation which occurs in native tubes during the various lengths of time from venipuncture to assay run between individuals.
CN-TEG yields the following variables: reaction time (R, time to clot formation), angle (rate of clot propagation), maximum amplitude (MA, maximal clot strength) and percent clot lysis 30 after reaching MA (LY30). Whole blood was diluted with either normal saline (0.9%, NS) or hypertonic saline (23.4%, HTS) in at 2.5% whole blood dilution (resembles up to three boluses of hypertonic saline, as would be administered clinically). A dose-response curve was created escalating doses to 5% and 10% whole blood dilution.
Friedman test with Dunn's multiple comparisons adjustment was performed within the blood sample results of each healthy volunteer to compare the effects of whole blood, NS, and HTS, accounting for repeated measures (escalating doses). R statistical software was used(26) for all statistical analyses with significance defined as p < 0.05. Based on the mean and standard deviation of previously published R times for saline dilution studies in humans(12, 13, 21), with a sample size of 10, we are powered at 80% to detect a minimum difference in R of 1.4 seconds.
Murine model
We examined the 23.4% HTS effects in adult male Sprague-Dawley rats weighing 350 to 490 grams (Harlan Labs, Indianapolis, IN). Female rats were not tested because they are relatively hypercoagulable(27-29). Experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver (IACUC #00781). All animals were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, including barrier-sustained conditions with 12-hour light-dark cycles and free access to food and water before experimentation. All materials were purchased from Sigma-Aldrich Corp (St. Louis, MO) unless otherwise specified.
Rats were anesthetized with intraperitoneal pentobarbital (50 mg/kg) and re-dosed intravenously (1 mg/kg) as necessary during the procedure. Lidocaine injection was administered at all incision sites for analgesia. A tracheostomy was performed, and the femoral artery and internal jugular vein were cannulated (for vitals monitoring, blood draws, and saline administration respectively). Femoral artery cannulas were connected to Pro-Paq devices (Protocol Systems, Beaverton, OR) for invasive vitals monitoring. Core body temperature was monitored rectally, and normothermia (> 36°C) was maintained with a heat lamp and heated operative table. After cannulation, baseline blood samples were obtained, then a bolus of normal saline (NS, control) or 23.4% HTS was administered at a dose equivalent to 2.5% blood dilution (0.4 mL) via the internal jugular vein. NS or HTS was administered on alternating experiment days (ten male rats were included, five receiving an NS bolus and five receiving a HTS bolus).
Immediately following the bolus, the tail was sharply transected 0.5 cm from the tip in order to measure tail bleeding time, as previously described(30). In brief, after tail tip transection, the tail was gently blotted with filter paper every 30 seconds and the time in minutes to cessation of bleeding was noted, ensuring no pressure was exerted on the tail tip which could affect hemostasis. At five, 20, and 40 minutes from bolus, blood samples were collected. Rats were then euthanized by lethal pentobarbital injection.
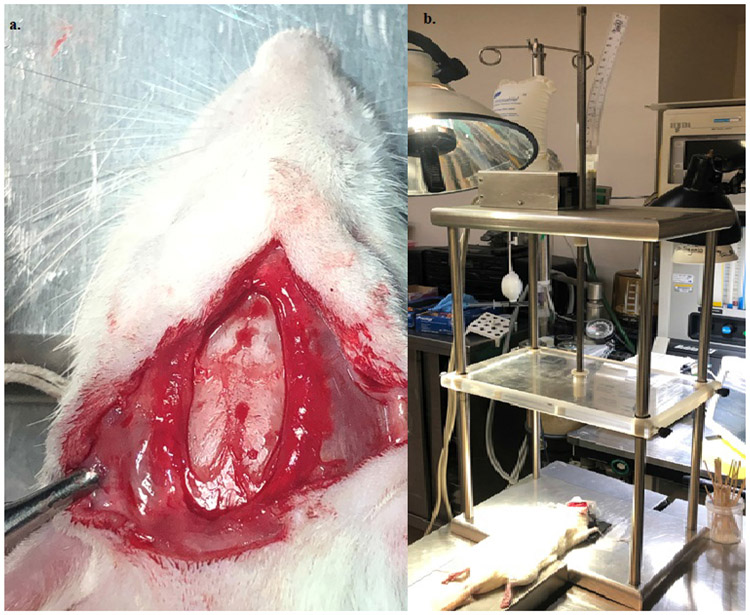
The results in the murine model without injury were compared to a TBI murine model. In the TBI model, following induction and cannulation, a weight drop injury was performed as previously described(31, 32). In brief, rats were positioned on a foam platform, a 0.5 cm scalp incision over midline of the frontal head area was made, the skull was exposed, and the area of impact was marked 2 mm anterior to the bregma over the midline. A 20 g stainless steel rod (total length of 100 cm, diameter 1 cm, with a pointed silicon tip of diameter 1 mm and length 1 cm) was dropped from a height of 20 cm to the point of marked impact (Figure 1, eFigure 1). After cranial impact, baseline blood samples were drawn, rats were randomized to receive the bolus of NS or HTS, and tail tip transection was performed. As in the uninjured animals, tail bleeding time was measured, and blood was drawn over 40 minutes before sacrifice. TEG measurements and tail bleeding time were compared
Figure 1.
(A) Cranial exposure and (B) weight drop apparatus for controlled traumatic brain injury in a murine model.
Statistical analysis
Friedman test with Dunn’s multiple comparisons adjustment was performed to compare the effects of whole blood, NS, and HTS, accounting for repeated measures. Comparisons between groups were done with the Mann-Whitney two-tailed test with significance defined as p<0.05. Based on the mean and standard deviation of previously published R times for saline dilution studies in humans(12, 13, 21), with a sample size of 10, we are powered at 80% to detect a minimum difference in R of 1.4 seconds. As described above, R statistical software was used(14) for all statistical analyses with significance defined as p<0.05.
Results
In vitro experiment
Of the10 healthy volunteers, half were female and the average age was 32.6 years (range 28-50). At a 2.5% blood volume replacement, there were no significant differences in angle or fibrinolysis (LY30) with NS or HTS (Table 1). However, as compared to NS and control, this HTS dose resulted in shortening of R from 11.4 min (9.9-14.0 interquartile range [IQR]) to 8.6 min (7.4-10.4 IQR) (p=0.01) and increase MA from 49.5 mm (48.5-57.9 IQR) to 58.2 mm (56.9-60.6 IQR) (p=0.005). There were no significant changes in R or MA with NS.
Table 1.
Changes in Thrombelastography with 0.9% Normal Saline and 23.4% Hypertonic Saline at Various In Vitro Dilutions in Blood of Healthy Volunteers
| Variable | Whole blood, median (IQR) |
0.9% normal saline, median (IQR) |
23.4% hypertonic saline, median (IQR) |
p value |
|---|---|---|---|---|
| 2.5% blood dilution | ||||
| Reaction time, min | 11.4 (9.9-14.0)* | 10.7 (9.4-13.4)† | 8.6 (7.4-10.4)*† | 0.01 |
| Angle, degrees | 33.2 (25.6-39.7) | 40.3 (28.7-49.3) | 38.4 (32.6-43.0) | 0.60 |
| MA, mm | 49.5 (48.5-57.9)* | 51.5 (48.9-56.4)† | 58.2 (56.9-60.6)*† | 0.001 |
| LY30, % | 2.4 (1.5-5.2) | 3.2 (1.4-8.1) | 0.6 (0.2-1.9) | 0.07 |
| 5% blood dilution | ||||
| Reaction time, min | 11.4 (9.9-14.0)* | 10.7 (8.6-12.0)† | 20.2 (17.6-23.3)*† | <0.0001 |
| Angle, degrees | 33.2 (25.6-39.7)* | 36.8 (33.9-43.4)† | 10.6 (7.4-13.8)*† | <0.0001 |
| MA, mm | 49.5 (48.5-57.9)* | 50.8 (47.6-52.6) | 30.2 (23.5-35.8)* | 0.01 |
| LY30, % | 2.4 (1.5-5.2) | 3.8 (1.8-5.2) | 1.0 (0.0-1.9) | 0.09 |
| 10% blood dilution | ||||
| Reaction time, min | 11.4 (9.9-14.0)* | 8.7 (7.1-11.3) | 0.0 (0.0-0.0)* | 0.0002 |
| Angle, degrees | 33.2 (25.6-39.7)* | 49.8 (42.9-55.0) | 0.0 (0.0-0.0)* | <0.0001 |
| MA, mm | 49.5 (48.5-57.9)* | 52.5 (49.6-59.4)† | 0.0 (0.0-0.0)*† | <0.0001 |
| LY30, % | 2.4 (1.5-5.2)* | 2.7 (0.9-5.3)† | 0.0 (0.0-0.0)*† | <0.0001 |
MA, maximum amplitude; LY30, fibrinolysis 30 minutes after MA
pairwise comparison < 0.05
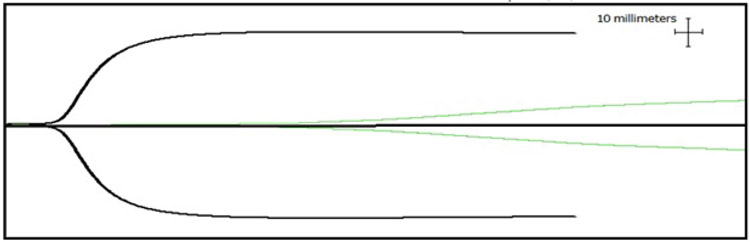
At replacement of 5% blood volume (Table 1), HTS significantly prolonged R and decreased angle and MA as compared to NS and control. No significant changes in TEG measurements were seen with NS. At 10% blood volume replacement, the anti-coagulant and anti-platelet effects were so profound that there was a “flat line” effect in the TEG with no sign of blood clot formation (>4 hours for clot formation/reaction time) (Table 1, Figure 2).
Figure 2.
Thrombelastography tracing of blood dilution with hypertonic saline at physiologic dilutions (2.5%, black line) and supraphysiologic dilutions (10%, green line).
Murine Model
In the murine model without TBI, there was no significant difference in TEG baseline measurements between the NS and HTS groups. While there was a decrease in R, increase in angle, increase in MA, and decrease in LY30 over time with both HTS and NS rats, these values were not significantly different compared to baseline (Friedman test, p=0.01). There was no significant difference in any TEG measurement between the NS and HTS groups at 5, 20, or 40 minutes (Table 2) as well as in tail bleeding time (29.5 min [21.2-35.5 IQR] versus 37.5 min [29.5-42.5 IQR] p=0.15), however type II error is possible. The serum sodium increased significantly in the rats receiving HTS boluses, as compared to the NS rats, from 141.0 mEq/L (140.0-141.0) at baseline to a peak of 153.0 mEq/L (151.5-153.5 IQR) at five minutes (whereas there was no change in sodium in NS group over time, p=0.008) (Table 3).
Table 2.
Changes in Thrombelastography over Time in Rats Receiving Normal Saline or Hypertonic Saline
| Variable | Normal saline, median (IQR) |
Hypertonic saline, median (IQR) |
p value |
|---|---|---|---|
| Reaction time, min | |||
| Baseline | 5.9 (3.6-9.1) | 4.1 (3.5-8.6) | 0.68 |
| 5 minutes | 3.4 (2.1-11.8) | 2.5 (1.6-3.0) | 0.39 |
| 20 minutes | 2.8 (1.4-5.8) | 2.8 (1.8-3.9) | 0.90 |
| 40 minutes | 3.4 (1.2-5.2) | 2.2 (1.6-2.9) | 0.41 |
| Angle, degrees | |||
| Baseline | 56.4 (50.6-73.5) | 69.8 (53.6-71.3) | 0.86 |
| 5 minutes | 75.7 (40.0-79.2) | 76.5 (75.1-80.7) | 0.79 |
| 20 minutes | 76.6 (61.4-80.2) | 70.9 (67.6-76.2) | 0.79 |
| 40 minutes | 68.7 (65.3-71.4) | 66.6 (65.5-74.0) | 0.98 |
| Maximum amplitude, mm | |||
| Baseline | 61.8 (51.0-68.0) | 61.5 (60.1-65.0) | 0.94 |
| 5 minutes | 67.0 (47.3-71.6) | 68.0 (66.5-73.0) | 0.62 |
| 20 minutes | 66.5 (57.0-68.8) | 60.5 (55.5-69.5) | 0.79 |
| 40 minutes | 64.5 (61.5-70.1) | 66.6 (65.5-74.0) | 0.29 |
| LY30, % | |||
| Baseline | 1.0 (0.7-1.5) | 0.3 (0.1-1.5) | 0.29 |
| 5 minutes | 0.7 (0.4-1.3) | 0.4 (0.1-1.4) | 0.56 |
| 20 minutes | 1.2 (0.8-2.5) | 0.4 (0.2-2.2) | 0.39 |
| 40 minutes | 0.8 (0.2-1.4) | 1.2 (0.7-2.4) | 0.39 |
p value reflects Friedman test
NS, normal saline; HTS, hypertonic saline
Table 3.
Changes in Sodium over Time in Rats Receiving Normal Saline or Hypertonic Saline
| Time, min | Normal saline, median (IQR) |
Hypertonic saline, median (IQR) |
p value |
|---|---|---|---|
| 0 | 141.0 (140.0-143.0) | 141.0 (140.0-141.0) | 0.68 |
| 5 | 141.0 (140.5-142.5) | 153.0 (151.5-153.5) | 0.008 |
| 20 | 142.0 (140.3-143.0) | 150.5 (150.0-152.5) | 0.03 |
| 40 | 142.0 (139.0-143.0) | 149.0 (149.0-151.3) | 0.03 |
In the murine model with TBI, there was no significant difference in TEG measurements at baseline between the NS and HTS groups, except for a trend towards increased LY30 in the HTS group at baseline (0.4% versus 0.1% in NS group, p=0.05). As seen in the non-TBI model, while there was a decrease in R, increase in angle, and increase in MA over time with both HTS and NS rats after bolus administration, these values were not significantly different within individual rats. There was no significant difference in reaction time, angle, or MA between the NS and HTS groups at 5, 20, or 40 minutes (Table 4). There was no difference in the fold change in LY30 between HTS and NS groups (p=0.67 at 5 minutes, 0.10 at 20 minutes, 0.52 at 40 minutes). There was no difference in tail bleeding time between NS versus HTS groups (25.5 min [22.0-31.0 IQR] versus 27.0 min [21.5-33.8 IQR] p=0.80). The serum sodium increased significantly in the rats which received HTS boluses, as compared to the NS rats (Table 5). There was no difference in baseline sodium levels between HTS and NS groups, there was a significant difference in sodium levels by five minutes after bolus (149.0 [148.0-150.0 IQR] versus 139.0 [137.5-139.5 IQR], p=0.008) which persisted at 20 and 40 minutes.
Table 4.
Changes in Thrombelastography over Time in Rats with Traumatic Brain Injury Receiving Normal Saline or Hypertonic Saline
| Variable | Normal saline, median (IQR) |
Hypertonic saline, median (IQR) |
p value |
|---|---|---|---|
| Reaction time, min | |||
| Baseline | 5.4 (4.7-6.1) | 6.0 (4.2-7.6) | 0.73 |
| 5 minutes | 6.5 (5.0-8.9) | 5.3 (4.0-6.2) | 0.22 |
| 20 minutes | 5.9 (5.1-9.0) | 5.9 (4.9-6.9) | 0.51 |
| 40 minutes | 5.4 (4.3-8.0) | 4.5 (4.2-6.1) | 0.55 |
| Angle, degrees | |||
| Baseline | 65.3 (50.1-68.4) | 67.8 (59.3-72.1) | 0.69 |
| 5 minutes | 65.2 (45.8-68.4) | 69.4 (60.2-73.4) | 0.31 |
| 20 minutes | 57.7 (45.2-68.3) | 65.7 (59.9-68.2) | 0.39 |
| 40 minutes | 69.0 (45.2-72.4) | 66.4 (63.6-71.6) | 0.99 |
| Maximum amplitude, mm | |||
| Baseline | 71.0 (63.5-73.2) | 67.0 (66.2-69.5) | 0.22 |
| 5 minutes | 70.5 (64.0-72.0) | 68.5 (64.5-70.2) | 0.34 |
| 20 minutes | 66.5 (62.8-68.0) | 64.5 (64.2-70.8) | 0.95 |
| 40 minutes | 70.5 (61.8-72.0) | 69.5 (68.0-70.8) | 0.85 |
| LY30, % | |||
| Baseline | 0.1 (0.0-0.3) | 0.4 (0.2-1.0) | 0.05 |
| 5 minutes | 0.3 (0.1-0.6) | 0.9 (0.6-1.2) | 0.02 |
| 20 minutes | 0.2 (0.1-0.4) | 0.8 (0.4-1.5) | 0.02 |
| 40 minutes | 0.3 (0.1-0.4) | 0.4 (0.2-0.7) | 0.27 |
Table 5.
Changes in Sodium over Time in Rats with Traumatic Brain Injury After Bolus of Normal Saline or Hypertonic Saline
| Time (min) | Normal saline, median (IQR) | Hypertonic saline, median (IQR) | p value |
|---|---|---|---|
| 0 | 139.0 (138.5-140.0) | 138.0 (137.5-139.5) | 0.38 |
| 5 | 139.0 (137.5-139.5) | 149.0 (148.0-150.0) | 0.008 |
| 20 | 138.0 (136.5-138.5) | 146.0 (146.0-148.0) | 0.008 |
| 40 | 137.0 (136.0-138.0) | 145.0 (144.0-145.5) | 0.008 |
Discussion
In this study examining the effects of 23.4% HTS on hemostatic capacity, we identified that at clinically relevant dilutions in vitro, there was a shortening of time to clot formation and increase in clot strength, but no changes in clot propagation or fibrinolysis. At supraphysiologic dilutions in vitro, there were increasing anti-coagulant and anti-platelet effects with HTS with a “flat line” effect on TEG at 10% dilution. However, in our murine model, both in the uninjured and TBI subjects, there were no changes in hemostatic capacity in the form of coagulation on TEG or tail bleeding time. Thus, these results are reassuring that 23.4% HTS in clinically standard dosing does not have adverse effects on hemostatic capacity.
While there have been studies exploring the effects of hypertonic saline on coagulation, it has been limited to 3% HTS or 7.5% HTS(2, 4, 33). In our examination of 23.4% HTS bolus administration in a murine TBI model, we did not identify any significant changes in hemostatic capacity. This supports conclusions from a recently published prospective, randomized, double-blind trial of 40 patients undergoing elective craniotomy, in which patients were randomized to receive 5 mL/kg of 3% HTS (equivalent to 7% whole blood dilution) or placebo (6). There were no abnormalities in ROTEM variables, except for an increase in EXTEM and decrease in INTEM as compared to baseline (which were still within normal limits), suggesting safety of use in the context of coagulation profile(6). In a similar prospective, randomized double-blind study with moderate TBI patients and 3% HTS (dose of 3.0 mL/kg, 4% blood dilution)(4), there were no significant changes in coagulation or platelet function on ROTEM following administration. This has also been described in 7.5% HTS, including work by Yang et. al. in which 34 TBI patients were bolused 7.5% HTS, and there were no changes in consequent prothrombin time, PTT, or fibrinogen levels thereafter(2). Collectively, these data and our study with 23.4% HTS indicate that HTS for intracranial hypertension is safe in terms of coagulation. Considering other literature which describe the ability of HTS to decrease endotheliopathy and the proinflammatory response to TBI(2, 11, 34, 35), HTS may not only be safe, but beneficial for a myriad of reasons.
In our in vitro model, at 2.5% blood dilution, there was a paradoxical increase in angle and MA. This procoagulant effect at smaller blood dilutions has been described in other studies with 7.5% HTS. In an in vitro study of whole blood from healthy volunteers diluted with 7.5% HTS/6% dextran 70, there was a procoagulant effect up to 11% dilution, followed by a highly anticoagulant effect at higher dilutions (36). In a swine model of hemorrhagic shock and resuscitation with 4 mL/kg of a 7.2% HTS/6% hydroxyethyl starch 200, swine demonstrated increased fibrinogen polymerization on ROTEM and decreased liver bleeding time as compared to pigs resuscitated with 4% gelatin or 6% hydroxyethyl starch 200(37). Increased hypercoagulability has also been observed clinically and reported in TBI patients bolused with HTS. Webster et. al. describe increased thrombotic risk in pediatric TBI patients after administration of 3.0% HTS infusion(38). This hypercoagulability noted in experimental models and TBI patients may be due to the hypernatremia resultant from HTS. Hypernatremia stimulates antidiuretic hormone (ADH) release from the posterior pituitary gland, resulting in endothelial release of factor VIII and promotion of coagulation (39).
Our study offers a novel description of a dose response curve for 23.4% HTS. The anticoagulant, antiplatelet effect observed with excessive blood dilutions of 23.4% HTS is consistent with previous human studies with 7.5% HTS and similar animal work with 3% HTS(12, 13, 20, 21). While the anticoagulant effect observed is likely due to a dilutional effect, the antiplatelet effect may be due to the hypertonic solution itself, as previous animal work indicates that hypertonic fluid dilution results in platelet dysfunction and excessive osmolarity-induced platelet death(15, 40). The murine model described here provides insight into the pharmacodynamics of HTS after bolus administration of 23.4% HTS as well. Our data suggest that HTS causes a rapid increase in sodium, with an increase of 10 meq/L in ten minutes and enduring hypernatremia at 40 minutes. Persistent hypernatremia lasting up to several days has been reported in several human studies with less concentrated hypertonic solutions(36). This persistent hypernatremia may be cause for concern, as hypernatremia has been linked to acute renal failure, thrombocytopenia, neutropenia, anemia, central pontine myelinolysis, and acute respiratory distress syndrome(41, 42). While this study did not look at repeat doses, it highlights reason for potential caution with increasing blood dilution with HTS. For example, in a retrospective review of patients with repeat dosing of HTS (23.4% HTS) for intracranial hypertension, patients received an average of 8.9 doses (with a range of 2-61 doses)(43). Hypernatremic effects persist up to 12 hours after repeat boluses of 14.6% HTS for ICH and 72 hours after infusion of 3% HTS (7, 44), highlighting potential risk with repeat doses and cumulative anticoagulant effects.
This study has several limitations. While these data describe paradoxical hypercoagulability, followed by an anticoagulant, antiplatelet effect induced by HTS in vitro, a mechanistic investigation was not pursued. Future work is required to further understand and delineate the mechanistic effects of HTS on enzymatic and cell-based hemostasis. Another limitation of this work is we did not include blood from TBI patients in our in vitro experiments; our experiment was designed to collect blood from healthy volunteers only, in which we could manipulate blood dilutions to ensure any changes in coagulation observed with HTS were due to the saline itself versus TBI (as neurotrauma is known to be associated with unique, dynamic changes in coagulation(45)). Future work with blood from TBI patients will be interesting and valuable, but this was beyond the scope of this project. It is worth mentioning the lack of differences identified by LY30 (p=0.07) may be type II error due to small sample size. Another limitation is we did not measure intracranial pressure in the murine TBI model (to detect the accepted indication for HTS bolus administration), however we conducted several pilot studies prior to formal experimentation in which we ensured the mechanism of TBI was adequate to cause significant brain edema and impending herniation (this was confirmed by necropsy after each experiment, titrating the height of weight drop). Lastly, our animal model is lacking serial doses of 23.4% HTS to assess the dynamic, cumulative effects of HTS on coagulation. However, given the 2.5% blood dilution is equivalent to two to three clinical boluses and in clinical practice boluses are typically administered close to one another, we believe this model has clinical relevance.
Conclusions
In this study examining the effects of 23.4% HTS on hemostatic capacity, we identified that at clinically relevant dilutions, there is a paradoxical shortening of time to clot formation and increase in clot strength in vitro but no effects in an animal model of TBI. At higher dilutions of HTS in vitro, there were anti-coagulant and anti-platelet effects with virtual arrest of coagulation at 10% dilution in vitro. These data support previous literature on 3% HTS and 7.5% HTS and its effects on coagulation and suggest safety of 23.4% HTS use. Excessive dilution data suggest, however, that caution should be exercised when employing serial 23.4% HTS boluses to reduce intracranial hypertension in patients with acute TBI.
Supplementary Material
eFigure 1. Necropsy after traumatic brain injury by weight drop, with evidence of (A) epidural hematoma and (B) left cerebral contusion.
Acknowledgments
Support: Research reported in this publication was supported by the National Institute of General Medical Sciences (RM1 grant under review) of the National Institutes of Health (T32 GM008315 and P50 GM049222), the National Heart, Lung, and Blood Institute (TACTIC - 5 UM 1HL120877), and the Department of Defense (USAMRAA W81XWH-12-2-0028).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors of the project.
Disclosure Information: This research was supported with materials from Haemonetics Inc, and other research conducted by this group is supported by Instrumentation Laboratory and Stago. Dr Silliman is a scientific advisory board member of Hemanext, and Dr Moore is founder of ThromboTherapeutics Inc.
Footnotes
Presented at the American College of Surgeons Committee on Trauma State Resident Paper Competition in Colorado Springs, Colorado, August 2017 and at the American College of Surgeons Committee on Trauma National Resident Paper Competition in San Antonio, Texas, March 2018.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Crescenzo C, Gorouhi F, Salcedo ES, Galante JM. Prehospital hypertonic fluid resuscitation for trauma patients: A systematic review and meta-analysis. The Journal of Trauma and Acute Care Surgery. 2017;82(5):956–62. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Chen Y, Li J, et al. Hypertonic saline maintains coagulofibrinolytic homeostasis following moderatetosevere traumatic brain injury by regulating monocyte phenotype via expression of lncRNAs. Molecular Medicine Reports. 2019;19(2):1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yozova ID, Howard J, Henke D, et al. Comparison of the effects of 7.2% hypertonic saline and 20% mannitol on whole blood coagulation and platelet function in dogs with suspected intracranial hypertension - a pilot study. BMC Veterinary Research. 2017;13(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Cao H, Zhang X, et al. The effect of hypertonic saline and mannitol on coagulation in moderate traumatic brain injury patients. The American Journal of Emergency Medicine. 2017;35(10):1404–7. [DOI] [PubMed] [Google Scholar]
- 5.Kheirabadi BS, Miranda N, Terrazas IB, et al. Does small-volume resuscitation with crystalloids or colloids influence hemostasis and survival of rabbits subjected to lethal uncontrolled hemorrhage? The Journal of Trauma and Acute Care Surgery. 2017;82(1):156–64. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Palazon J, Fuentes-Garcia D, Domenech-Asensi P, et al. Equiosmolar Solutions of Hypertonic Saline and Mannitol Do Not Impair Blood Coagulation During Elective Intracranial Surgery. Journal of Neurosurgical Anesthesiology. 2017;29(1):8–13. [DOI] [PubMed] [Google Scholar]
- 7.Joseph B, Aziz H, Snell M, et al. The physiological effects of hyperosmolar resuscitation: 5% vs 3% hypertonic saline. American Journal of Surgery. 2014;208(5):697–702. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JJ, Kade AM, Sheehan KM, Wilson TJ. A case-cohort study with propensity score matching to evaluate the effects of mannitol on venous thromboembolism. Journal of Clinical Neuroscience. 2014;21(8):1323–8. [DOI] [PubMed] [Google Scholar]
- 9.Major EH, O'Connor P, Mullan B. Single bolus 30% hypertonic saline for refractory intracranial hypertension. Irish Journal of Medical Science. 2015;184(1):159–65. [DOI] [PubMed] [Google Scholar]
- 10.Luostarinen T, Niiya T, Schramko A, et al. Comparison of hypertonic saline and mannitol on whole blood coagulation in vitro assessed by thromboelastometry. Neurocritical Care. 2011;14(2):238–43. [DOI] [PubMed] [Google Scholar]
- 11.Rhind SG, Crnko NT, Baker AJ, et al. Prehospital resuscitation with hypertonic salinedextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. Journal of Neuroinflammation. 2010;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seshia S, Casey Gaunt M, Kidney BA, Jackson ML. The effect of 3 resuscitative fluid therapies on hemostasis as measured by rotational thromboelastometry in dogs. Veterinary Clinical Pathology. 2018;47(1):38–44. [DOI] [PubMed] [Google Scholar]
- 13.Ali A, Sencan B, Sabanci PA, et al. A Comparison of the Effects of 20% Mannitol and 3% NaCl on Coagulation Parameters In Vitro using ROTEM: A Prospective Randomized Crossover Study. Turkish Journal of Anaesthesiology and Reanimation. 2017;45(2):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamik KN, Butty E, Howard J. In vitro effects of 3% hypertonic saline and 20% mannitol on canine whole blood coagulation and platelet function. BMC Veterinary Research. 2015;11:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wurlod VA, Howard J, Francey T, et al. Comparison of the in vitro effects of saline, hypertonic hydroxyethyl starch, hypertonic saline, and two forms of hydroxyethyl starch on whole blood coagulation and platelet function in dogs. Journal of Veterinary Emergency and Critical Care. 2015;25(4):474–87. [DOI] [PubMed] [Google Scholar]
- 16.Delano MJ, Rizoli SB, Rhind SG, et al. Prehospital Resuscitation of Traumatic Hemorrhagic Shock with Hypertonic Solutions Worsens Hypocoagulation and Hyperfibrinolysis. Shock. 2015;44(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke AA, Maschler S, Schochl H, et al. In vitro impairment of whole blood coagulation and platelet function by hypertonic saline hydroxyethyl starch. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2011;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Annals of Surgery. 2011;253(3):431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess JR, Dubick MA, Summary JJ, et al. The effects of 7.5% NaCl/6% dextran 70 on coagulation and platelet aggregation in humans. The Journal of Trauma. 1992;32(1):40–4. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovici R, Yue TL, Krausz MM, et al. Hemodynamic, hematologic and eicosanoid mediated mechanisms in 7.5 percent sodium chloride treatment of uncontrolled hemorrhagic shock. Surgery, Gynecology & Obstetrics. 1992;175(4):341–54. [PubMed] [Google Scholar]
- 21.Scherer R, Giebler R, Kampe S, Kox WJ. Effects of hypertonic saline hydroxyethyl starch solution on collagen-induced platelet aggregation and ATP secretion. Infusions Therapy und Transfusion Medicine. 1994;21(5):310–4. [DOI] [PubMed] [Google Scholar]
- 22.Dunham CM, Malik RJ, Huang GS, et al. Hypertonic saline administration and complex traumatic brain injury outcomes: a retrospective study. International Journal of Burns and Trauma. 2018;8(3):40–53. [PMC free article] [PubMed] [Google Scholar]
- 23.Wu AG, Samadani U, Slusher TM, et al. 23.4% Hypertonic Saline and Intracranial Pressure in Severe Traumatic Brain Injury Among Children: A 10-Year Retrospective Analysis. Pediatric Critical Care Medicine. 2019;20(5):466–73. [DOI] [PubMed] [Google Scholar]
- 24.Lescot T, Degos V, Zouaoui A, et al. Opposed effects of hypertonic saline on contusions and noncontused brain tissue in patients with severe traumatic brain injury. Critical Care Medicine. 2006;34(12):3029–33. [DOI] [PubMed] [Google Scholar]
- 25.Haemonetics. TEG 5000 System User Manual. P/N 06-510-US, Manual revision: AC. Niles, IL: Haemonetics Corporation, Haemoscope Division; 2010. [Google Scholar]
- 26.dplyr: Hadley Wickham, Romain François, Lionel Henry and Kirill Müller (2018). dplyr: A Grammar of Data Manipulation. R package version 0.7.7. https://CRAN.R-project.org/package=dplyr. [Google Scholar]
- 27.Kawasaki T, Chaudry IH. The effects of estrogen on various organs: therapeutic approach for sepsis, trauma, and reperfusion injury. Part 2: liver, intestine, spleen, and kidney. Journal of Anesthesia. 2012;26(6):892–9. [DOI] [PubMed] [Google Scholar]
- 28.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14(2):81–90. [DOI] [PubMed] [Google Scholar]
- 29.Knoferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Annals of Surgery. 2002;235(1):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry CN, Girard D, Lochot S, Lecoffre C. Antithrombotic actions of argatroban in rat models of venous, 'mixed' and arterial thrombosis, and its effects on the tail transection bleeding time. British Journal of Pharmacology. 1994;113(4):1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchele F, Morawska MM, Schreglmann SR, et al. Novel Rat Model of Weight Drop-Induced Closed Diffuse Traumatic Brain Injury Compatible with Electrophysiological Recordings of Vigilance States. Journal of Neurotrauma. 2016;33(13):1171–80. [DOI] [PubMed] [Google Scholar]
- 32.Flierl MA, Stahel PF, Beauchamp KM, et al. Mouse closed head injury model induced by a weight-drop device. Nature Protocols. 2009;4(9):1328–37. [DOI] [PubMed] [Google Scholar]
- 33.Zoran DL, Jergens AE, Riedesel DH, et al. Evaluation of hemostatic analytes after use of hypertonic saline solution combined with colloids for resuscitation of dogs with hypovolemia. American Journal of Veterinary Research. 1992;53(10):1791–6. [PubMed] [Google Scholar]
- 34.Junger WG, Rhind SG, Rizoli SB, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline-without dextran-inhibits neutrophil and endothelial cell activation. Shock. 2012;38(4):341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sgarbi MWM, Silva Junior BA, Pires DA, Velasco IT. Comparison of the effects of volemic reposition with 7.5% NaCl or blood in an experimental model of muscular compression and hemorrhagic shock. Revista Brasileira de Ortopedia. 2018;53(5):614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coats TJ, Heron M. The effect of hypertonic saline dextran on whole blood coagulation. Resuscitation. 2004;60(1):101–4. [DOI] [PubMed] [Google Scholar]
- 37.Haas T, Fries D, Holz C, et al. Less impairment of hemostasis and reduced blood loss in pigs after resuscitation from hemorrhagic shock using the small-volume concept with hypertonic saline/hydroxyethyl starch as compared to administration of 4% gelatin or 6% hydroxyethyl starch solution. Anesthesia and Analgesia. 2008;106(4):1078–86. [DOI] [PubMed] [Google Scholar]
- 38.Webster DL, Fei L, Falcone RA, Kaplan JM. Higher-volume hypertonic saline and increased thrombotic risk in pediatric traumatic brain injury. Journal of Critical Care. 2015;30(6):1267–71. [DOI] [PubMed] [Google Scholar]
- 39.Grant PJ, Tate GM, Hughes JR, et al. Does hypernatraemia promote thrombosis? Thrombosis Research. 1985;40(3):393–9. [DOI] [PubMed] [Google Scholar]
- 40.Watters JM, Tieu BH, Differding JA, et al. A single bolus of 3% hypertonic saline with 6% dextran provides optimal initial resuscitation after uncontrolled hemorrhagic shock. The Journal of Trauma. 2006;61(1):75–81. [DOI] [PubMed] [Google Scholar]
- 41.Gonda DD, Meltzer HS, Crawford JR, et al. Complications associated with prolonged hypertonic saline therapy in children with elevated intracranial pressure. Pediatric Critical Care Medicine. 2013;14(6):610–20. [DOI] [PubMed] [Google Scholar]
- 42.Rabinstein AA. Treatment of brain edema in acute liver failure. Current Treatment Options in Neurology. 2010;12(2):129–41. [DOI] [PubMed] [Google Scholar]
- 43.Lewandowski-Belfer JJ, Patel AV, Darracott RM, et al. Safety and efficacy of repeated doses of 14.6 or 23.4 % hypertonic saline for refractory intracranial hypertension. Neurocritical Care. 2014;20(3):436–42. [DOI] [PubMed] [Google Scholar]
- 44.Eskandari R, Filtz MR, Davis GE, Hoesch RE. Effective treatment of refractory intracranial hypertension after traumatic brain injury with repeated boluses of 14.6% hypertonic saline. Journal of Neurosurgery. 2013;119(2):338–46. [DOI] [PubMed] [Google Scholar]
- 45.Samuels JM, Moore EE, Silliman CC, et al. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. The Journal of Trauma and Acute Care Surgery. 2019;86(4):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Necropsy after traumatic brain injury by weight drop, with evidence of (A) epidural hematoma and (B) left cerebral contusion.