Abstract
The lack of knowledge regarding the ecology of Coccidioides spp. makes both modeling the potential for disease outbreaks and predicting the distribution of the organism in the environment challenging. No single ecological parameter explains the biogeography of the pathogen. Previous investigations suggest an association with desert mammals, but these results should be confirmed with modern molecular techniques. Therefore, we used molecular tools to analyze soils associated with animal activity (i.e. burrows) to better define the ecology and biogeography of Coccidioides spp. in Arizona. Soils were collected from locations predicted to have favorable habitat outside of the established endemic regions to better understand the ecological niche of the organism in this state. Our central hypothesis is that soils taken from within animal burrows will have a higher abundance of Coccidioides spp. when compared to soils not directly associated with animal burrows. Our results show that there is a positive relationship with Coccidioides spp. and animal burrows. The organism was detected in two locations in northern Arizona at sites not known previously to harbor the fungus. Moreover, this fungus is able to grow on keratinized tissues (i.e. horse hair). These results provide additional evidence that there is a relationship between Coccidioides spp. and desert animals, which sheds new light on Coccidioides’ ecological niche. These results also provide evidence that the geographic range of the organism may be larger than previously thought, and the concept of endemicity should be reevaluated for Coccidioides.
Introduction
The disease coccidioidomycosis, which is commonly known as valley fever (VF), is caused by two species of dimorphic fungal pathogens: Coccidioides immitis and C. posadasii [1, 2]. The disease outcome in human patients is highly variable with 60% of cases being asymptomatic after the establishment of infection and the other 40% of cases having symptoms ranging from mild pneumonia to severe disseminating disease involving the spleen, liver brain, bone, and other host tissues [2-4]. However, the infection primarily involves the lungs and is defined by the morphological switch from an inhaled arthroconidia into a large pathogenic endosporulating structure called a spherule. Complications in diagnosis, treatment failure and unusual presentations may result in severe disease progression and even death. The causes for variability in disease outcome are not well understood, but are influenced by genetic factors of the host, environmental conditions, and variation in virulent fungal genotypes [4, 5].
Both species are soil-inhabiting fungi that are endemic to arid and semi-arid regions throughout North and South America; including California, Arizona, New Mexico, Texas, Mexico, and portions of Central and South America [6]. Genetically distinct species appear to have discrete geographic distributions. Genetically distinct populations of C. immitis inhabit Central and Southern California, Northern Mexico as well as a newly identified novel genotype in eastern Washington State. Within C. posadasii, genetically isolated populations are found in Arizona, parts of Mexico, New Mexico, Texas, Central and South America [2, 6-8]. Both species are found in soils that are slightly alkaline, but previous studies revealed that the sporadic distribution of Coccidioides spp. cannot be explained by soil characteristics alone [4, 9-12]. This observation suggests that additional factors play a role in the patchy distribution of the fungus. To date conditions required for growth and maturation in the environment are unknown, but enriched keratin sources, temperature, and precipitation all play a role in the fungal development [13-15].
Coccidioides spp. belong to the fungal phylum Ascomycota in the order Onygenales. Most Ascomycete fungi are saprotrophic (extracellular digestion of decaying organic material) in the environment and many have a direct or indirect connection with plants, but Coccidioides spp. and other Onygenales are associated with animals [13, 16]. During the mycelial phase of the life cycle, Coccidioides spp. are thought to be saprotrophic soil dwelling fungi with sporadic and irregular dispersal driven by abiotic soil factors (e.g. pH and electrical conductivity) and/or by biotic associations with mammals (e.g. rodents, bats and armadillos) [13, 17-20]. Lacy and Swatek investigated C. immitis around California archeological sites and reveled that sandy-textured soils made up of 98% of positive samples and 96.7% of positive soils were alkaline [21, 22]. Fisher et al. analyzed multiple abiotic factors and suggested that soils with low water content are more favorable for filamentous fungi because hyphae may reach water pockets that are unavailable to bacteria and yeast forming fungi. Thus, bacteria and yeast are predicted to be potential competitors for resources in wetter conditions where they outcompete filamentous fungi [20]. It is important to note that the relatively few studies that have examined the role of abiotic factors had small sample sizes and relied on culture-based approaches for fungal identification and, thus, do not provide strong evidentiary support for soil characteristics that can be used to predict the growth pattern and distribution of Coccidioides spp. Studies have also shown a lack of support for a correlation between a high prevalence of the pathogen and an association with certain flora [9, 10, 16, 22, 23].
To understand the ecology of Coccidioides, it is necessary to have a well-validated method to detect the presence of both species in the soil. However, detection in soil via classical microbiological methods is a difficult task as those are shown to be insensitive (thousands of soils with no/few cultured strains) and mouse inoculation is expensive and time consuming with variable results [10, 11, 17, 21, 23, 24]. Greene et al. attempted to isolate the fungus from 535 soil samples from the endemic region of California and only three were culture positive [11]. One difficulty in culturing Coccidioides spp. from soil is overgrowth by fast-growing fungi on antibiotic media plates [11]. Molecular technologies (PCR, DNA sequencing, and real-time qPCR) and more reliable and easy to use soil DNA extraction methods improve detection of the organism in the environment. However, even with sensitive molecular techniques, detection of the organism is still difficult. Previous studies have a detection rate from 0% to 8.42% [11, 24-26]. Some molecular methods require additional amplification and sequencing of the internal transcribed spacer (ITS) regions for identification [17, 24, 27, 28]. A newly developed real-time qPCR method that targets a repetitive sequence of a unique Coccidioides-specific transposon was highly sensitive in soil [29, 30]. The main benefit of using this approach is the ability to screen a large number of soil samples in a relatively short amount of time with high specificity to Coccidioides spp., which improves ability to perform ecological modeling. Using this methodology, it is possible to better understand the ecology of these organisms and make more informed recommendations about possible high exposure areas or activities.
Based on previous research, we hypothesize that burrowing desert mammals play an important role in the distribution and maintenance of Coccidioides. To test this hypothesis, we collected soils from animal burrows and outside of animal burrows from 5 different locations throughout Arizona and determined the presence of Coccidioides spp. in these soils samples using real-time qPCR assays. Our goal was to determine if animal burrows may be the preferred microhabitat for Coccidioides spp. by determining if the fungi were present at higher frequency in soils from burrows compared to nearby soil outside of burrows in Arizona. Moreover, we investigate the ability of this fungus to degrade keratin under “in vitro” conditions, and we defined the animal species that are common at the positive endemic sites.
Materials and Methods
Soil Collection
Soil samples were collected during the spring and summer months, pre- and post-monsoon season at five different locations throughout Arizona to encompass different elevations, life zones, and climatic conditions (Table 1). The site names and locations are as follows: Tucson site is located within Tucson, Arizona city limits, Tom Mix site is located between Florence and Tucson, Arizona, POW site is located in Florence, Arizona city limits, Flagstaff site is located just south of the Flagstaff, Arizona city limits, and Lake Mead is located southeast of Lake Mead National Recreation Area. Soils were collected using a garden trowel and put directly into sterile 50 milliliter collection containers; trowels were sterilized with 10% bleach between soil samples. A 20 × 20-meter plot was established by randomly picking a location at each site and every animal burrow within that transect was sampled. Five soils samples were taken from inside animal burrows and one sample was taken from outside of the animal burrow to compare the prevalence of Coccidioides spp. inside burrows to outer sites. Due to the difficulty in detecting the pathogen and to increase the chance of observing a pattern more soils were taken from inside the burrow. Soils collected from inside animal burrows were taken from approximately 100-1000 centimeters depth (depending on size of burrow) carefully to avoid collecting soil from outside of the burrow. This depth was determined by depth of burrow, soil itself was taken at 10cm depths. Soils taken from outside of the animal burrow were collected approximately 1 meter away from the entrance to the burrow at a depth of approximately 10 centimeters. 456 total soil samples (n=456) were collected from 90 different animal burrows at five different locations in Arizona. A total of 385 soils were collected from within animal burrows and 77 were taken outside of burrows on the soil surface. Soils were stored at room temperature in sealed containers and processed within one to three months after collection.
Table 1:
Description of the 5 Sample Sites.
| n=456 | |||||
|---|---|---|---|---|---|
| Site Name and Location |
UTM Coordinates NAD3 12n |
Elevation | Average Annual Temperature |
Average Annual Precipitation |
Number of Cases
of Coccidioidomycosis Reported in 2017 per county |
| 407-Tucson, Arizona Pima County |
Easting:509428 Northing:3577062 |
865m | 21.6°C | 30.2cm | Pima-1,022 |
| Tom Mix-Between Tucson and Florence,
Arizona Pinal County |
Easting:480961.61 Northing:3631473.45 |
609m | 20.8°C | 25.5cm | Pinal-518 |
| POW-Florence, Arizona Pinal County |
Easting:463757.36 Northing:3658111.77 |
457m | 20.8°C | 25.5cm | Pinal-518 |
| Flagstaff, Arizona Coconino County |
Eastmg:441204 Northing:3882722 |
2,133m | 6,5°C | 58.7cm | Coconino-32 |
| Lake Mead, Arizona Mojave County |
Easting:736014.35 Northing:3982317.18 |
731m | 18.5°C | 17.3cm | Mojave-67 |
Information on the 5 locations in Arizona that soil was collected from. Data was retrieved from National Oceanic and Atmospheric Administration, United States Geological Survey, and Arizona Department of Health Services. Tom Mix site that is located between Florence and Tucson Arizona, lower Sonoran life zone. Florence represents the POW site that is located in Florence Arizona, lower Sonoran life zone. Lake Mead represents the Lake Mead site that is located adjacent to Lake Mead National Recreational Area on the Nevada/Arizona Border. Flagstaff represents the Flagstaff site that is located just south of Flagstaff Arizona, Ponderosa Pine dominated vegetation.
Soil DNA Extraction
DNA from soil samples was extracted using the DNeasy PowerSoil Pro kit (QIAGEN, Valencia, CA). Due to potential infectious propagules in the soil, DNA extractions were performed in a biological safety level two laboratory inside a biological safety cabinet. The manufacturer’s protocol was followed except for a 10-minute heat step at 65° C that was added before the homogenization step [24]. Two DNA extractions per soil sample were performed, and one microliter was used to estimate purity and concentration with FungiQuant real-time qPCR [31]. Although NanoDrop was used as well, estimated concentration measurements were unreliable. Remaining DNA was stored at −20°C until Coccidioides-specific molecular analysis was performed.
Molecular Detection of Coccidioides spp. by qPCR
Two real-time qPCR-based assays were used to detect Coccidioides DNA extracted from the collected soil samples. These TaqMan-based assays are highly sensitive, and target a repetitive region of DNA that is only known to occur in the two species within the Coccidioides genus [29]. The CocciDX assay targets a 106-bp sequence present in multiple copies within a transposable element in the genome of both Coccidioides species and is specific to this genus [30, 32]. In addition, the CocciENV assay was used to refine detection by adding additional target alleles based on newly genomes sequenced isolates of Coccidioides [29]. Both assays were performed on the Applied Biosystems QuantStudio 12K Flex Real-Time PCR System (Thermo Fisher Scientific). Each reaction was performed in 20 μl-volume containing a mixture of 2X TaqMan Environmental Master Mix 2.0 (Thermo Fisher Scientific), 1x of either CocciENV or CocciDX at 100μM concentration of Cocci Assay oligo/probe mix and two μl of DNA template. PCR cycling conditions were performed as followed: Two minutes of activation step at 50° C, 10 minutes at 95° C for denaturation step, followed by 45 cycles of 15 seconds at 95°C and one minute at 60° C. Each sample was performed in triplicate and the average Cycle threshold (Ct) value was taken if amplification occurred. Control samples included purified DNA from Coccidioides posadasii strain Silveira as a positive control and molecular grade sterile water was used as negative no-template DNA control. Soils were considered positive if the Ct value was < 40 and negative for a Ct value >40. A burrow was considered negative when zero out of the five soils samples taken from it did not show detectable real-time qPCR results. A burrow was considered “low positive” when one out of five soils was positive, “moderate positive” two or three out of five were positive, and “high positive” when greater than three out of five soil samples were positive.
Vertebrate DNA Collection and Genotyping
Animal identification was achieved by DNA analysis of fecal and hair samples collected at four of the five previously described study sites. A total of 32 hair and fecal samples were collected during spring and summer months at each location that soil was collected from. Useable DNA was extracted from 24 of the 32 samples. Samples were collected using sterile tweezers or a garden trowel that was sterilized with 70% ethanol in between samples. The fecal and hair material were placed in sterile 50mL collection containers and stored at 4° C until DNA extraction. Samples were processed within 1-2 months of collection. DNA was extracted using the DNeasy Powersoil Pro kit (QIAGEN, Valencia, CA) with the addition of a 10-minute incubation at 65° C. Primers developed by Xie et al. were used to amplify short (211 and 107 bp) highly polymorphic fragments of the 12s rRNA gene of the mitochondrial DNA of mammalian species from degraded samples[33]. The two sets of primers used in a single reaction master-mix L899 (5-GTAAATTTCGTGCCAGCCACCG-3) and H1066 (5- GTGGGGTATCTAATCCCAGTTTG-3) for the longer fragment (12S-Long); L1497 (5- ACACACACCGCCC GTCACCCTC-3) and H1559 (5 -CCAGTATGCTTACCTTGTTACGAC-3) for the shorter fragment (12S-Short) [33]. The amplification was performed in a volume of 25ul containing 5 ng of template DNA, 200nM concentration of each primer and 12.5 μl of lx PCR supermix from ThermoFisher. PCR was carried out with an initial denaturation step at 94° C for 5 minutes and followed by 30 cycles of 94°C for 30 seconds, 62° C for 30 seconds, 72° C for 15 seconds and a final extension at 72° C for 10 minutes. 24 PCR products underwent Sanger-sequencing using the aforementioned primer set. Sequencing was performed on the ABI 3130 genetic analyzer (ThermoFisher Scientific). The sequences were aligned and assembled using Sequencher® version 5.4.6 DNA sequence analysis software, (Gene Codes Corporation, Ann Arbor, MI USA). A Basic Local Alignment Search Tool (BLASTn) search was used to identify the species of each sequence by using the NCBI database [7].
Keratin Degradation
Experiments involving live Coccidioides spp. isolates were performed at the Biosecurity Level 3 (BSL3) laboratory at the Pathogen and Microbiome Institute, Northern Arizona University following pre-approved protocols. In order to determine if C. posadasii can germinate and proliferate on a keratin source we inoculated 0.5 × 0.5 cm mycelial plugs of the strains CPA0001 or CPA00020 into Soil Extract Agar plates [17, 34] containing autoclaved horse hair as keratin source. Strains CPA0001 and CPA00020 are soil isolates that are maintained at the Pathogen and Microbiome Institute in Flagstaff, Arizona, originally isolated from soil using mouse passage [17]. After inoculation, the plates were incubated at 28° C for 8 weeks and the fungus was killed using vapor formaldehyde in a sealed container for 48 hours. The cells were sterility-checked by plating part of the killed mycelial cells onto a fresh 2X-GYE plate for 72hs. The aerial mycelial were mounted on specimen stubs and coated with an ultra-thin layer of gold. Morphology was analyzed by SEM in an UltraDry EDS and MagnaRay WDS spectrometer (Thermo Scientific).
Statistical Analysis
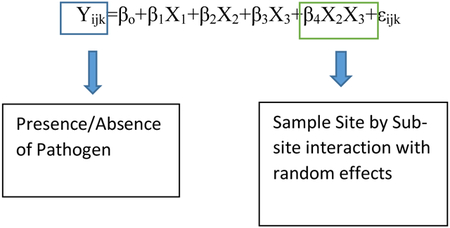
The data that we obtained were binary, meaning that the response variable was coded as either a 1 or 0, corresponding to the presence or absence of Coccidioides spp., respectively. For this reason, the chosen statistical model was a binomial regression model with presence/absence of the fungus in a soil sample being the response variable and burrow/topsoil, sample site, and sub-site all (sample location within sample site) being predictor variables. A model with random effects was chosen because individual soil samples taken from the same burrow can be considered dependent observations and our sampling protocol called for more samples from the burrow than outside the burrow. The random effects in the model addresses these sampling issues. Random effects were also chosen because soil is very heterogeneous and a subsequent experiment will not be able to sample the exact same soil due to destructive sampling. The random effects that were chosen were the sample site (location) and sub-site (burrow). The chosen alpha value was 0.05. The assumptions for binomial regression models are as follows. The outcome should be discrete and dichotomous (e.g. present versus absent), there should be no outliers in the data (z scores below −3.29 or greater than 3.29 should be removed), the predictor variables should not be inter-correlated (correlation coefficients among predictor variables are less than 0.90), and a large sample size is beneficial for this type of model [35]. All of these assumptions for a binomial regression model were met. All statistical analyses were done in R (R Core Team 2013).
Results
qPCR Detection of Coccidioides spp. in Arizona Soils
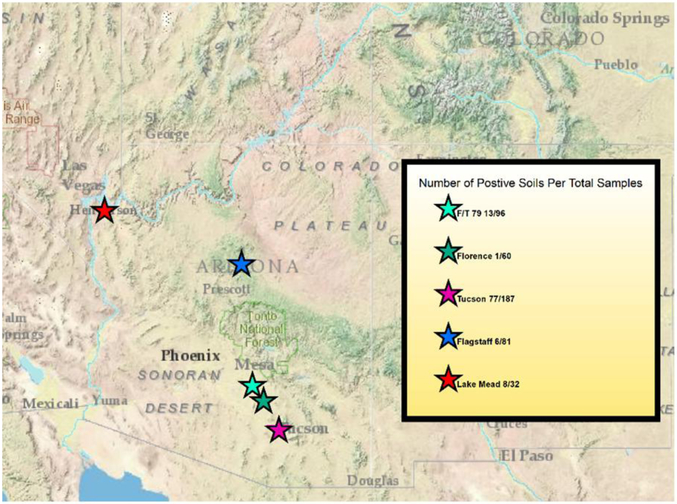
Of a total of 456 soil samples collected, 105 had real-time qPCR amplification for Coccidioides spp. (23% positive) using both assays employed. All five sample locations in Arizona had real-time qPCR amplification for Coccidioides DNA. The Lake Mead location had 8 out of 32 samples test positive (25%), Flagstaff had 6 out of 81 samples test positive (7.4%), Tucson had 77 out of 187 samples test positive (41.1%), Florence had 1 out of 60 samples test positive (1.6%), and the location located between Florence and Tucson Arizona had 13 out of 96 test positive (13.5%) (Figure 1).
Figure 1:
Map of Arizona indicating the locations of each sample site. The total number of samples collected from each site as well as the number of positive samples is in the map legend. F/T represents the Tom Mix site that is located between Florence and Tucson Arizona, lower Sonoran life zone. Florence represents the POW site that is located in Florence Arizona, lower Sonoran life zone. Lake Mead represents the Lake Mead site that is located adjacent to Lake Mead National Recreational Area on the Nevada/Arizona Border. Flagstaff represents the Flagstaff site that is located just south of Flagstaff Arizona, Ponderosa Pine dominated vegetation. This map was created with Environmental Systems Research Institute (ESRI). (2012). ArcGIS ArcMap Release 10.1. Redlands, CA.
qPCR Detection of Coccidioides spp. inside and outside Animal Burrows
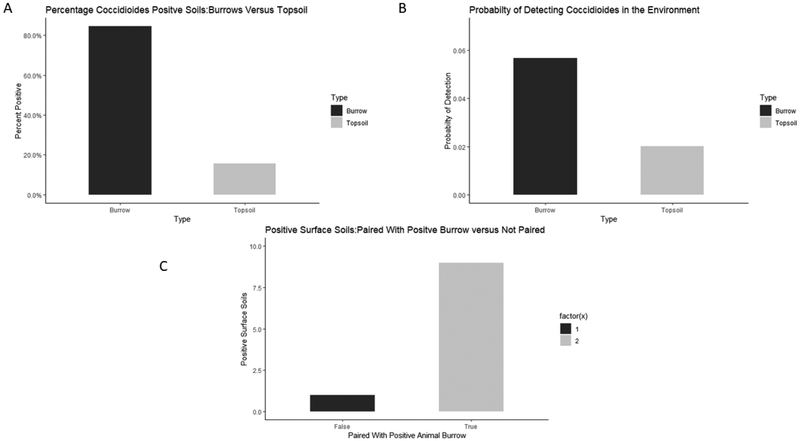
Of the 105 soils that had real-time qPCR amplification for Coccidioides spp., 95 soils were taken from within animal burrows and 10 were taken from outside of animal burrows. Figure 2A shows the percentage of positive soils from within burrows and outside of burrows. Statistical analysis shows that there is a positive relationship between animal burrows and the presence of Coccidioides spp. (p-value=0.0152). The probability of detecting Coccidioides spp. in Arizona soils within animal burrows was calculated in the statistical model and is approximately 5.7% with a 95% confidence interval of 2.5% to 12.5% (Figure 2B). The probability of detecting the fungus in Arizona soils outside of animal burrows is approximately 2.01% with a 95% confidence interval of 0.5% to 6.8% (Figure 2B). The statistical model calculated the odds ratio on a log odds ratio scale. The calculated odds ratio of soils within animal burrows to soils outside of animal burrows was 2.93, meaning that it is approximately 3 times more likely to detect Coccidioides spp. in soils within animal burrows than soils that are located outside of animal burrows. Ten soils amplified Coccidioides spp. DNA outside of animal burrows. Nine of those soils were taken outside of a burrow that detected Coccidioides spp. within the burrow and only one of those soils were taken from a site not near any positive animal burrows (Figure 2C).
Figure 2:
Comparing prevalence of Coccidioides spp. within animal burrows to outside of animal burrows. A) Percentage of the total positive soils and comparing burrows to non-burrow soils. 105 soils that were positive for Coccidioides spp. separated into soils taken from within animal burrows and soils taken outside of animal burrows from the surface of the soil. Greater than 80% of the soils that tested positive for the pathogen came from within an animal burrows. B) Comparing the probability of detecting Coccidioides spp. within animal burrows to outside of animal burrows. . The probability of detecting Coccidioides spp. in Arizona soils within animal burrows is approximately 5.7% with a 95% confidence interval of 2.5% to 12.5%. The probability of detecting the fungus in Arizona soils outside of animal burrows is approximately 2.01% with a 95% confidence interval of 0.5% to 6.8%. This figure suggests that it is more likely to detect the fungus within animal burrows than outside of burrows. C) 10 soils that were taken from the soil surface outside of animal burrows that were positive for Coccidioides. False indicates that the soil was not paired with a positive burrow and true represents that is was paired with a positive burrows. 9 soils were paired with positive burrows and only 1 soil was not paired. This indicates that even though these soils were collected from the soil surface and not inside of animal burrows, the association with animal material still exists and may be a factor on the distribution of the pathogen.
In summary, we detected Coccidioides spp. DNA in all sampled areas in Arizona for animal burrows. Five out of five animal burrows sampled were positive for Coccidioides DNA from Lake Mead (three low positives and two moderate positives). Three out of fourteen animal burrows were positive from Flagstaff with one high positive and two low positives. Nineteen out of thirty-one animal burrows tested positive from Tucson with three low positives, nine moderate positives, and seven high positives. Eight animal burrows out of 16 were positive from Tom Mix wash with three low positives and five moderate positives. One animal burrow out of 10 was a low positive from POW camp, Florence (Table 2). These data provide strong evidence that there is an association between Coccidioides spp. and the animal burrow environment.
Table 2:
Positive burrows from each site and the degree of positivity.
| Location | Burrow | Degree | Species Detected at Positive Burrow |
|---|---|---|---|
| Lake Mead | B1 | Low | ND |
| Lake Mead | B2 | Low | ND |
| Lake Mead | B3 | Low | ND |
| Lake Mead | B9 | Moderate | Xerospermophilus tereticaudus |
| Lake Mead | B10 | Moderate | Cornis Lupus familairis |
| Flagstaff | B1 | High | ND |
| Flagstaff | B6 | Low | ND |
| Flagstaff | B10 | Low | ND |
| Tucson | B1 | Moderate | ND |
| Tucson | B7 | Low | ND |
| Tucson | B9 | High | ND |
| Tucson | B10 | High | ND |
| Tucson | B12 | Moderate | ND |
| Tucson | B14 | Moderate | ND |
| Tucson | B15 | Moderate | ND |
| Tucson | B16 | High | Neotoma mexicana |
| Tucson | B17 | Low | ND |
| Tucson | B19 | Low | ND |
| Tucson | B21 | High | Neotoma mexicana |
| Tucson | B22 | Moderate | ND |
| Tucson | B23 | High | ND |
| Tucson | B24 | Moderate | Lepus Californicus |
| Tucson | B26 | High | Syvilagus audubonii |
| Tucson | B27 | High | ND |
| Tucson | B28 | Moderate | ND |
| Tucson | B30 | Moderate | ND |
| Tucson | B31 | Moderate | ND |
| TomMix | B1 | Low | ND |
| TomMix | B4 | Moderate | Xerospermophilus tereticaudus |
| TomMix | B6 | Moderate | ND |
| TomMix | B7 | Moderate | ND |
| TomMix | B10 | Moderate | Xerospermophilus tereticaudus |
| TomMix | B13 | Moderate | ND |
| TomMix | B14 | Low | ND |
| TomMix | B16 | Low | Neotoma mexicana |
| Florence | B2 | Low | ND |
The burrows that were positive from each location and the degree of positivity. Degree means how many different soil samples that are positive that were taken from each burrow. Positives were determined by Ct value < 40. 1 sample that was positive is considered a low positive, 2-3 moderate, and >3 is considered a high positive burrow. Animal species that were detected at a positive burrow is also included in the last column. This means that hair or fecal samples from these animals were collected at the entrance to a burrow that was positive.
qPCR Detection of Coccidioides spp. throughout Arizona
We compared the detection of the pathogen at each of the 5 locations that soil was collected from throughout Arizona. Lake Mead had 25% soils that were positive, Flagstaff had 7.4% soils that were positive, Tucson had 41.1% soils that were positive, Florence had 1.6% soils that were positive, and the site located between Florence and Tucson had 13.5% soils that were positive. The calculated probabilities of detecting the pathogen at each location as well as a 95% confidence interval are as follows, Lake Mead 8.46% CI 1.87%-3.09%, Flagstaff 1.22% CI 0.21%-6.88%, Tucson 23.9% CI 11.4%-43.4%, Florence 0.3% CI 0.02%-4.2%, Florence/Tucson 4.7% CI 1.2%-16.2%. The probability of detecting the pathogen is greatest in Tucson compared to other areas sampled within Arizona. At all locations the prevalence of the pathogen is greatest within animal burrows.
Molecular Detection of Mammalian Species at Burrows
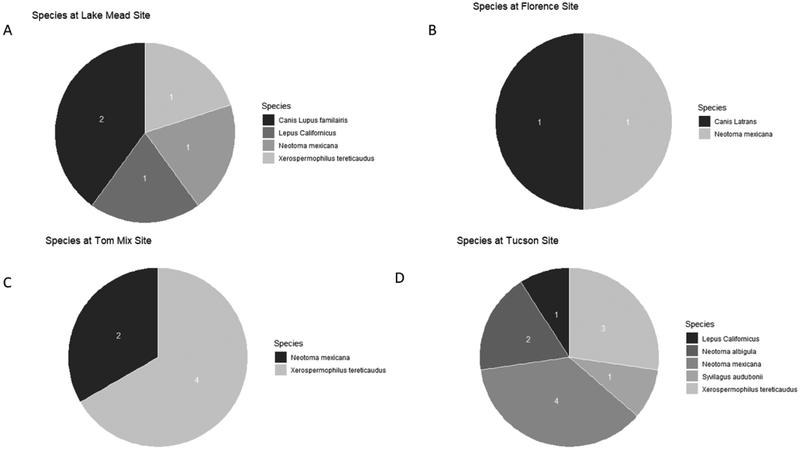
To identify animals that may associate with burrows at our study sites and, which may be reservoir hosts, animal species were molecularly identified from hair and fecal samples. We identified seven different species across four of our study sites (no samples collected from Flagstaff). We identified Canis latrans (coyote), Canis lupus familiaris (domestic dog), Lepus californicus (Black-tailed jackrabbit), Neotoma albigula (White-throated woodrat), Neotoma mexicana (Mexican woodrat), Sylvilagus audubonii (Desert cottontail), and Xerospermophilus tereticaudus (Round-tailed ground squirrel) at our sampling locations. The proportion of each species detected at the four sites is shown in Figure 3. Of the seven species identified at our sites, five species were found at burrows that were positive for Coccidioides spp. (Table 2). The species’ DNA found at each positive burrow are displayed at Figure 3. We cannot determine if each species inhabited a given burrow at the time of soil collection. These data suggest that the fungus may not be specific to one host, but rather may be a generalist pathogen.
Figure 3:
Number of animal species detected at four out of five sample sites. The number in the figure represents the number of that particular species was detected at each location. A) Lake Mead B) POW located in Florence Arizona C) Tom Mix located in between Florence and Tucson Arizona D) Tucson.
C. posadasii Growth in Keratin-Enriched Nutrient Source
In order to explore whether C. posadasii has predilection for growth on animal hair, SEM microscopies were performed of the fungus growing on horsehair. We observed that both C. posadasii CPA0001 and CPA0020 strains are able to grow on keratin-enriched material (Figure 4). The horse hair filaments are completely dominated by C. posadasii mycelial cells and appear to invade the hair follicles (Figure 4B). The period of growth is long enough to produce arthroconidia on standard glucose-enriched 2X-GYE media however, only filamentous mycelia cells were observed suggesting that the growth on abundant keratin-enriched substrate does not induce sporulation in those C. posadasii strains after two months of growth (Figure 4).
Figure 4:
Electron Micrograph of Coccidioides posadasii growing on horse hair. A) Mycelia starting to grow onto the horse hair. B) After 8 weeks of incubation mycelia have proliferated onto the hair. No sign of sporulation. C) Horse Hair D) Mycelia
Discussion
Increased soil disturbance due to land development and natural phenomenon in endemic areas has been hypothesized as the cause of increased morbidities due to VF, but the lack of knowledge about areas of the true prevalence of the organism makes exposure risk management a difficult task [36]. The distribution of the pathogen has been described as irregular and unpredictable, and no one factor has been found that explains the biogeography of the organism [37]. The majority of data on biogeographic distribution of the Coccidioides spp. was gathered indirectly from skin testing done on humans and cattle in the 1950s [38]. Clearly, the metrics used to determine the geographical distribution of the organism are outdated. Many studies have tried to ascertain abiotic conditions, such as soil texture, soil salinity, pH, soil and air temperature, and water concentration that aid in the proliferation of the fungus in the environment but the evidence was inconclusive with weak correlations [12, 17, 20-23].
In this study we detected the presence of Coccidioides spp. at five different locations throughout Arizona using molecular diagnostics. One caveat to molecular testing is the inability to determine pathogen viability. Direct plating and injecting soil solutions in mice have been utilized in several of the previous studies [17] but with limited success. Thus, while we feel that molecular methods allow for higher throughput and greater sampling across low-endemic areas, future ecological modeling will need to consider this variable. Although the pathogen was detected at higher frequencies within the true endemic area (Southern Arizona), Coccidioides spp. is present throughout Arizona, which has public health implications. The three locations in southern Arizona had the highest prevalence, which agrees with previous studies that first described the endemic region of the pathogen [38]. These data are also in agreement with current case reporting data, with the highest reported incidences of disease in southern Arizona counties (Pima, Pinal, and Maricopa). However, we also detected the pathogen in soils in two locations in northern Arizona, an area of low endemicity, and reported cases in this area are thought to be mostly travel-related. Although more work is still needed, our data suggest that Coccidioides spp. have a greater geographic range in Arizona than previously thought and the endemic range needs to be reevaluated. This information can also help to alert medical professionals to test patients with VF symptoms when they have no travel history to the highly endemic regions.
The data from this study also provide evidence that Coccidioides spp. is more prevalent within animal burrows, and the realized niche may be the burrow microhabitat. Burrows are considered to be a stable environment with relatively narrow daily and seasonal shifts in temperature and relative humidity, hold moisture, and are shielded from direct UV radiation [39, 40]. For example, in the peak of summer in the Texas desert ambient temperatures ranged from 23-58°C, whereas animal burrow temperatures stayed within the range of 28-32°C [41]. Even when soil moisture is low, burrows tend to maintain a high vapor pressure (6.1-33.9 mm Hg) which keeps the burrow interior relatively moist [42]. The Sonoran desert in Arizona has a surface temperature that fluctuates throughout the year by more than 80°C, but one meter below the surface (common depth of rodent burrows) the annual temperature fluctuation is only 12°C and the maximum temperature at that depth rarely exceeds 31°C, while the surface temperature easily can reach 50°C in the summer [43, 44]. Although the desert conditions are extreme, the microclimate of animal burrows maintains a more strict homeostasis. Burrows also have associated animal activity, so the amount of keratin is likely higher inside animal burrows than outside. However, no information on keratin concentrations was found in the literature. Because we observed a lack of conidiation on a keratin rich source (hair), it is difficult to assess how growth at keratin-rich locations would affect conidia burden in the environment with other organisms competing for the same resources.
Coccidioides spp., as well as other Onygenales, can degrade animal-derived material and utilize it as a nutrient source [13, 16, 45]. The genome of Coccidioides has a reduced gene family of fungal-cellulose binding domains that aid in plant cell wall destruction and give the ability to digest plant material as a nutrient source [13]. The subtilisin N-domain-containing proteins are expanded in the Coccidioides genome as well as in close relatives and upregulated during the pathogenic stage of this fungus [13]. This gene family contains the peptidase S8 family domain, which encodes several keratinolytic subtilases (keratinases) expanded in Coccidioides when compared to other related taxa [13]. This genomic information suggests that unlike the sister order Eurotiales, which are often associated with plants and plant material, Coccidioides and other Onygenales utilize animal-derived substrates, and may have reduced ability to thrive on a plant-based diet.
An animal reservoir has not been identified for Coccidioides spp. but there is evidence of an association with desert rodents or armadillos, and both pathogenic and non-pathogenic relatives of Coccidioides have been shown to be associated with a variety of different animals [9, 10, 28, 46]. Coccidioidal antibodies were detected in deer mice in the Baja California region of Mexico, and isolation of Coccidioides spp. from wild rodents in Arizona and California [9, 10, 46]. Paracoccidioides brasiliensis, a close pathogenic relative of Coccidioides, has been isolated from bat (Artibeus lituratus) guano, in South America and has been found within internal organs of nine-banded armadillos (Dasypus novemcinctus) [47, 48]. Coccidioides has also been isolated from bat lungs in Brazil [18]. Blastomyces spp., and Histoplasma spp. have also has been isolated from bat guano and various other mammal species [49-52]. Thus, fungi within the order Onygenales are linked with wildlife, either in vivo or in situ, and this close relationship may be a clue to pathogenic potential in humans and other animals.
While our data provide evidence that Coccidioides spp. preferentially occupy animal burrows at our tested sites, there is not enough evidence to say that the fungus is infecting the animals that occupy the burrow. Our data show that the probability of finding the organism in a burrow is greater than outside a burrow. The burrowing desert animals may be the natural reservoirs that host Coccidioides spp., or the burrows may represent a stable microclimate in the environment. The classical definition of a disease reservoir states that the reservoir needs to have the ability to maintain the pathogen in the environment and have a feasible transmission route between the target population (reservoir) and non-target population (human) [53]. We propose an expanded definition of the classical disease reservoir. The Coccidioides reservoir system is likely the development of arthroconidia in soil in the burrows. This would maintain the fungus in the environment, and support transmission to a naive host through airborne arthroconidia from burrows (Figure 5).
Figure 5:
This figure represents our proposed sequence of events that may happen in the Coccidioides spp. reservoir system. A) The fungus is living within the rodent as an endozoan or on the rodent as a dermatophyte. B) The rodent either dies within the burrow or fungus is shed from the exterior of the rodent and then establishes in the soil of burrow. C) A new rodent is inhabited by the fungus and cycle continues. D) Airborne arthroconidia is dispersed from burrow and inhaled by human or another rodent establishing infection.
Taylor and Barker 2019 have proposed a new hypothesis suggesting that Coccidioides spp. is actually living as an endozoan inside mammals, forming a granuloma, and are rendered inactive by the mammalian immune system until which time the animal dies and the fungus transforms into vegetative spore-forming hyphae [16]. There are numerous examples of many species of burrowing animals that are dying naturally within their burrows or dens [54, 55] allowing the fungus to transform into growing hyphae and establish outside of the carcass in the soil. This is a similar system to entophytic plant fungi that live inside of plant tissues without causing apparent disease that then decompose the tissue when the plant dies or the leaf falls off the plant [56].
We detected seven different mammal species at our sampling sites across Arizona. DNA from these animals was found in the vicinity of Coccidioides positive burrows and could possibly be the species that are harboring the fungus as an endozoan. Coccidioides spp. is not restricted to infecting a single species or even closely related group, but rather is a generalist and can take advantage of a wide variety of species. Many closely related human pathogens from the Onygenales order, such as Blastomyces, Emmonsia, Paracoccidioides, and Histoplasma, also have been isolated from a wide variety of animals (wild mice, bats, birds, armadillos, etc.) and have the ability to cause devastating mycoses in humans [47-52, 57]. A generalist pathogen can infect a wide variety of host species with varying severities and the host range of the pathogen is determined by the physiological, behavioral and ecological characteristics of the host that determines the ability of a pathogen to infect the host and complete its lifecycle [58-60]. The data show that a variety of animal species are associated with burrows where Coccidioides posadasii is prevalent in Arizona. These species are excellent candidates for possible Coccidioides reservoirs and targets for further analysis.
Finally, the currently defined endemic region for this pathogen needs to be reexamined. There is a population of Coccidioides in Washington State that was recently discovered, an area well outside of the historic endemic region [32]. This study detected the pathogen in new locations in Northern Arizona suggesting that Coccidioides spp. may have a much broader geographic distribution than previously thought. More studies need to examine new areas that the fungus may located at low concentrations such as New Mexico, Utah, Texas, and areas of Mexico, and South America. The methods from this study (directed sampling in and around animal burrows) may aid in detecting the pathogen in proposed potential habitat and help better understand the distribution of this fungus.
Conclusion
The ecology of Coccidioides spp. is vastly understudied. With disease numbers increasing every year the public health implications of information about the ecology of the organism are becoming progressively more important. Existing data do not accurately predict disease outbreaks and inform the public about specific areas and activities that can put them at high risk for being infected. Furthermore, there are few predictions of how the changing climate will affect the geographical distribution of the organism and the potential naive hosts that are at risk for becoming infected. Ecological studies form the necessary foundational support to establish better predictive models. This ecological modeling may then expand the true endemic region, and sharing this new information with clinicians so that they consider testing for VF could reduce delayed diagnoses and misdiagnoses and improve public health interventions.
Acknowledgments
Thanks to Austin Blackmon and Kaitlyn Parra for technical support. Thanks to Dr. David Wagner and Dr. Matthew Bowker for advisement and consultation throughout this study. This work was supported by an Arizona Biomedical Research Centre grant (ABRC 16-162415) to BMB.
Footnotes
Compliance with ethical standards
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Canteros CE, et al. , [Genetic characterization of the fungus involved in the first case of coccidioidomycosis described by Alejandro Posadas in 1892]. Medicina (B Aires), 2009. 69(2): p. 215–20. [PubMed] [Google Scholar]
- 2.Fisher MC, et al. , Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia, 2002. 94(1): p. 73–84. [PubMed] [Google Scholar]
- 3.Lee CY, et al. , Coccidioides Endospores and Spherules Draw Strong Chemotactic, Adhesive, and Phagocytic Responses by Individual Human Neutrophils. PLoS One, 2015. 10(6): p. e0129522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CE, Beard RR, and et al. , Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health, 1946. 36(12): p. 1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson GR 3rd, et al. , Current Concepts and Future Directions in the Pharmacology and Treatment of Coccidioidomycosis. Med Mycol, 2019. 57(Supplement_1): p. S76–S84. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira MM and Barker BM, Use of Population Genetics to Assess the Ecology, Evolution, and Population Structure of Coccidioides. Emerg Infect Dis, 2016. 22(6): p. 1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altschul SF, et al. , Basic local alignment search tool. Journal of molecular biology, 1990. 215(3): p. 403–410. [DOI] [PubMed] [Google Scholar]
- 8.Engelthaler DM, et al. , Local Population Structure and Patterns of Western Hemisphere Dispersal for Coccidioides spp., the Fungal Cause of Valley Fever. MBio, 2016. 7(2): p. e00550–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmons C and Ashburn L, The isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from wild rodents. Their relationship to coccidioidomycosis. Public Health Rep, 1942. 57(46): p. 1715–1727.19315895 [Google Scholar]
- 10.Emmons CW, Coccidioidomycosis in wild rodents. A method of determining the extent of endemic areas. Public Health Reports (1896-1970), 1943: p. 1–5.19315902 [Google Scholar]
- 11.Greene DR, et al. , Soil isolation and molecular identification of Coccidioides immitis. Mycologia, 2000. 92(3): p. 406–410. [Google Scholar]
- 12.Kolivras KN and Comrie AC, Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int J Biometeorol, 2003. 47(2): p. 87–101. [DOI] [PubMed] [Google Scholar]
- 13.Sharpton TJ, et al. , Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res, 2009. 19(10): p. 1722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamerius JD and Comrie AC, Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PLoS One, 2011. 6(6): p. e21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Untereiner WA, et al. , The Ajellomycetaceae, a new family of vertebrate-associated Onygenales. Mycologia, 2004. 96(4): p. 812–21. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JW and Barker BM, The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med Mycol, 2019. 57(Supplement_1): p. S16–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker BM, et al. , Detection and phylogenetic analysis of Coccidioides posadasii in Arizona soil samples. fungal ecology, 2012. 5(2): p. 163–176. [Google Scholar]
- 18.Cordeiro Rde A, et al. , Coccidioides posadasii infection in bats, Brazil. Emerg Infect Dis, 2012. 18(4): p. 668–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eulalio KD, et al. , Coccidioides immitis isolated from armadillos (Dasypus novemcinctus) in the state of Piaui, northeast Brazil. Mycopathologia, 2001. 149(2): p. 57. [DOI] [PubMed] [Google Scholar]
- 20.Fisher FS, et al. , Coccidioides niches and habitat parameters in the southwestern United States: a matter of scale. Ann N Y Acad Sci, 2007. 1111: p. 47–72. [DOI] [PubMed] [Google Scholar]
- 21.Elconin AF, Egeberg RO, and Egeberg MC, Significance of Soil Salinity on the Ecology of Coccidioides Immitis. J Bacteriol, 1964. 87: p. 500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacy GH and Swatek FE, Soil ecology of Coccidioides immitis at Amerindian middens in California. Appl Microbiol, 1974. 27(2): p. 379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swatek FE and Omieczynski DT, Isolation and identificationof coccidioides immitis from natural sources. Mycopathol Mycol Appl, 1970. 41(1): p. 155–66. [DOI] [PubMed] [Google Scholar]
- 24.Lauer A, et al. , Detection of Coccidioides immitis in Kern County, California, by multiplex PCR. Mycologia, 2012. 104(1): p. 62–9. [DOI] [PubMed] [Google Scholar]
- 25.Chow NA, et al. , Molecular detection of airborne Coccidioides in Tucson, Arizona. Med Mycol, 2016. 54(6): p. 584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson SM, et al. , Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Med Mycol, 2014. 52(6): p. 610–7. [DOI] [PubMed] [Google Scholar]
- 27.Alvarado P, et al. , Detection of Coccidioides posadasii from xerophytic environments in Venezuela reveals risk of naturally acquired coccidioidomycosis infections. Emerg Microbes Infect, 2018. 7(1): p. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baptista-Rosas RC, et al. , Molecular detection of Coccidioides spp. from environmental samples in Baja California: linking Valley Fever to soil and climate conditions. fungal ecology, 2012. 5(2): p. 177–190. [Google Scholar]
- 29.Bowers JR, et al. , Direct detection of Coccidioides from Arizona soils using CocciENV, a highly sensitive and specific real-time PCR assay. Med Mycol, 2019. 57(2): p. 246–255. [DOI] [PubMed] [Google Scholar]
- 30.Saubolle MA, et al. , Multicenter Clinical Validation of a Cartridge-Based Real-Time PCR System for Detection of Coccidioides spp. in Lower Respiratory Specimens. J Clin Microbiol, 2018. 56(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CM, et al. , FungiQuant: a broad-coverage fungal quantitative real-time PCR assay. BMC microbiology, 2012. 12(1): p. 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litvintseva AP, et al. , Valley Fever: finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human Infection. Clinical Infectious Diseases, 2014. 60(1): p. e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J, et al. , Identification of mammalian species using the short and highly variable regions of mitochondrial DNA. Mitochondrial DNA, 2015. 26(4): p. 550–554. [DOI] [PubMed] [Google Scholar]
- 34.Kwon-Chung KJ, Studies on Emmonsiella capsulata. I. Heterothallism and development of the ascocarp. Mycologia, 1973. 65(1): p. 109–21. [PubMed] [Google Scholar]
- 35.Watson JC, Establishing evidence for internal structure using exploratory factor analysis. Measurement and Evaluation in Counseling and Development, 2017. 50(4): p. 232–238. [Google Scholar]
- 36.Colson AJ, et al. , Large-Scale Land Development, Fugitive Dust, and Increased Coccidioidomycosis Incidence in the Antelope Valley of California, 1999-2014. Mycopathologia, 2017. 182(5-6): p. 439–458. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen C, et al. , Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev, 2013. 26(3): p. 505–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards PQ and Palmer CE, Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis Chest, 1957. 31(1): p. 35–60. [DOI] [PubMed] [Google Scholar]
- 39.Bennett N and Jarvis J, The reproductive biology of the Cape mole-rat, Georychus capensis (Rodentia, Bathyergidae). Journal of Zoology, 1988. 214(1): p. 95–106. [Google Scholar]
- 40.Mayer W THE PROTECTIVE VALUE OF THE BURROW SYSTEM TO THE HIBERNATING ARCTIC GROUND SQUIRREL, SPERMOPHILUS-UNDULATUS. in Anatomical Record. 1955. WILEY-LISS DIV JOHN WILEY & SONS INC, 605 THIRD AVE, NEW YORK, NY: 10158–0012. [Google Scholar]
- 41.Kennerly T, Microenvironmental conditions of pocket gopher burrow. Texas Journal of Science, 1964. 16(4): p. 395–&. [Google Scholar]
- 42.Kay FR and Whitford WG, The burrow environment of the banner-tailed kangaroo rat, Dipodomys spectabilis, in southcentral New Mexico. American Midland Naturalist, 1978: p. 270–279. [Google Scholar]
- 43.Turnage W, Nocturnal Surface-Soil Temperatures, Air Temperatures, and Ground Inversions in Southern Arizona. Monthly Weather Review, 1937. 65(5): p. 189–190. [Google Scholar]
- 44.Vorhies CT, Water requirements of desert animals in the southwest. 1945: College of Agriculture, University of Arizona; (Tucson, AZ: ). [Google Scholar]
- 45.Lewis ER, Bowers JR, and Barker BM, Dust devil: the life and times of the fungus that causes valley Fever. PLoS Pathog, 2015. 11(5): p. e1004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catalan-Dibene J, et al. , Detection of coccidioidal antibodies in serum of a small rodent community in Baja California, Mexico. Fungal Biol, 2014. 118(3): p. 330–9. [DOI] [PubMed] [Google Scholar]
- 47.Restrepo A, et al. , Clues to the presence of pathogenic fungi in certain environments. Med Mycol, 2000. 38 Suppl 1: p. 67–77. [PubMed] [Google Scholar]
- 48.Vergara ML and Martinez R, Role of the armadillo Dasypus novemcinctus in the epidemiology of paracoccidioidomycosis. Mycopathologia, 1998. 144(3): p. 131–3. [DOI] [PubMed] [Google Scholar]
- 49.Baumgardner DJ, et al. , Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis, 1992. 15(4): p. 629–35. [DOI] [PubMed] [Google Scholar]
- 50.Baumgardner DJ and Paretsky DP, The in vitro isolation of Blastomyces dermatitidis from a woodpile in north central Wisconsin, USA. Med Mycol, 1999. 37(3): p. 163–8. [PubMed] [Google Scholar]
- 51.Chaturvedi VP, et al. , In vitro interactions between Blastomyces dermatitidis and other zoopathogenic fungi. Can J Microbiol, 1988. 34(7): p. 897–900. [DOI] [PubMed] [Google Scholar]
- 52.DiSalvo AF, The ecology of Blastomyces dermatitidis, in Blastomycosis. 1992, Springer; p. 43–73. [Google Scholar]
- 53.Hallmaier-Wacker LK, Munster VJ, and Knauf S, Disease reservoirs: from conceptual frameworks to applicable criteria: Disease reservoir criteria. Emerging microbes & infections, 2017. 6(1): p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanchard R, Blanchard DC, and Flannelly KJ, Social stress, mortality and aggression in colonies and burrowing habitats. Behavioural processes, 1985. 11(2): p. 209–213. [DOI] [PubMed] [Google Scholar]
- 55.Reichman O and Smith SC, Burrows and burrowing behavior by mammals. Current mammalogy, 1990. 2: p. 197–244. [Google Scholar]
- 56.Petrini O, Fungal endophytes of tree leaves, in Microbial ecology of leaves. 1991, Springer; p. 179–197. [Google Scholar]
- 57.Baumgardner DJ, Soil-related bacterial and fungal infections. J Am Board Fam Med, 2012. 25(5): p. 734–44. [DOI] [PubMed] [Google Scholar]
- 58.Andreou D and Gozlan RE, Associated disease risk from the introduced generalist pathogen Sphaerothecum destruens: management and policy implications. Parasitology, 2016. 143(9): p. 1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes JC, Parasite populations and host community structure Host-parasite interfaces. Academic Press, New York, 1979: p. 27–46. [Google Scholar]
- 60.Solter LF and Maddox JV, Physiological host specificity of microsporidia as an indicator of ecological host specificity. Journal of Invertebrate Pathology, 1998. 71(3): p. 207–216. [DOI] [PubMed] [Google Scholar]