Abstract
Background
Abnormal hair growth is a defining feature of RASopathies, syndromes caused by germline mutations in the RAS pathway. However, detailed hair manifestations and the mechanisms of altered hair growth in RASopathies are poorly delineated.
Objectives
To identify distinguishing clinical features and investigate how the RAS pathway influences hair growth by performing a systematic and detailed side-by-side comparison of hair manifestations in Cardio-facio-cutaneous syndrome (CFCS) and Costello syndrome (CS), two RASopathies caused by mutations in the downstream and upstream elements of the RAS pathway, respectively.
Methods
Sixteen individuals with CFCS and 23 individuals with CS were enrolled. Mutation data was recorded. Scalp hair, eyebrows and eyelashes of individuals with CFCS or CS were examined for texture, colour, density and morphology. Scalp hairs were examined by light microscopy.
Results
While both syndromes displayed abnormal hair, striking differences were observed, including darker and thicker scalp hair and sparse eyebrows and eyelashes in CFCS. By contrast, synophrys, trichomegaly and abnormalities of the scalp hair shafts were observed in CS. Possible correlation with straight hair and genotype was observed in CS.
Conclusion
The results emphasize the role of the RAS pathway in hair growth, improve accuracy of clinical diagnosis of CFCS and CS and provide a foundation for identification of therapeutic targets.
Keywords: RASopathy, Cardio-facio-cutaneous syndrome, Costello syndrome, hair follicle, BRAF, alopecia
Introduction
Hair disorders are common and can significantly affect a patient’s quality of life 1, 2 yet targeted therapeutic options remain limited. Understanding the regulation of hair growth leads to greater knowledge of the mechanisms of hair diseases and ultimately to novel targeted therapies.
The RAS/mitogen activated protein kinase pathway (RAS/MAPK pathway) serves a critical role in development and cancer but also plays a role in regulating hair growth.3 In humans, germline mutations in the RAS pathway result in developmental syndromes termed RASopathies.4, 5 Together, the RASopathies represent one of the most prevalent groups of developmental syndromes affecting approximately 1 in 1,000 individuals and include neurofibromatosis type 1, Noonan syndrome, Noonan syndrome with multiple lentigines, Legius syndrome, Costello syndrome (CS), Cardio-facio-cutaneous syndrome (CFCS) and capillary malformation-arteriovenous malformation.6 The RASopathies show overlapping phenotypic features including characteristic craniofacial morphology, cardiac defects, developmental delay and ectodermal abnormalities.7–9 Notably, abnormal hair growth, including slow-growing, sparse and curly hair, is a defining feature of many RASopathies, including CFCS and CS.10–12 CFCS is most commonly caused by mutations in the downstream elements of the RAS pathway, including BRAF, MAP2K1 and MAP2K2, or rarely KRAS genes.13, 14 In contrast, CS is caused by mutations in an upstream core component of the pathway, HRAS.7, 15
This study aims to identify distinguishing clinical features and investigate how the RAS pathway influences hair growth by performing a systematic comparison of hair manifestations in Cardio-facio-cutaneous syndrome (CFCS) and Costello syndrome (CS), two RASopathies caused by mutations in the downstream and upstream elements of the RAS pathway, respectively. This effort will improve accuracy of clinical diagnosis of RASopathies and increase understanding of the role of the RAS pathway in hair growth in humans, providing a foundation for the identification of much needed novel therapeutic targets for hair diseases.
Materials and methods
Study subjects
Approval for the study was obtained through the Institutional Review Board at University of California Davis. Study subjects with CFCS were recruited at the 9th International CFCS Family Conference in Houston, Texas and study subjects with CS at the 10th International Costello Syndrome Family Conference in Orlando, Florida. Individuals were included in the study if they volunteered, were six years of age or older and had a diagnosis of CFCS or CS. Knowledge of mutation status was not an inclusion criterion. However, if the mutation status was unknown, the individual was excluded from the data analysis. All ethnicities were included. Consent was obtained for all participants. For minors, consent was obtained from the parent and assent from the minor. Surveys for demographic information (age, sex) and mutation status were distributed to the study participants. Mutation data was recorded based on the patient reported survey results.
Hair exam and photographs
An examination of the photographs of the scalp, eyebrow and eyelash hair was performed by the authors (JU, MK). Photographs of the hair (frontal, lateral and posterior view of the head) were obtained by a medical photographer (Canfield Scientific) enabling a standardized lighting, background, distance and photography technique. The photographs were examined independently and without knowledge of the mutation status by the authors (JU, MK). The definition of each variable was obtained from prior literature on periorbital morphology in genetic diseases and are described in detail in Table 2.16, 17 A consensus agreement by the authors (JU, MK) was obtained for any discrepant observations.
Table 2.
Variables and definitions used for examination of the scalp, eyebrow and eyelash hair.16
| Variable | Definition | Additional remarks |
|---|---|---|
| Scalp hair colour | ||
| Black/dark brown | ||
| Brown | ||
| Sandy/blonde | ||
| Red | ||
| Scalp hair texture | ||
| Straight | Forms a straight line or “I” shape | |
| Wavy | Forms a semi-circle, crescent or “S” shape | |
| Curly | Forms a full circle, ring or “O” shape | |
| Eyebrow density | ||
| Absent | No eyebrows present | |
| Sparse | Decreased density/number and/or decreased diameter of eyebrow hairs | Can be regional (medial, lateral) or total |
| Eyebrow morphology | ||
| Broad | Regional increase in width of the eyebrow | Can be regional (medial, lateral) or total |
| Flared | Widening with a change in direction of the eyebrow hairs | |
| Highly arched | Increased height of the central portion of the eyebrow forming a crescent, semi-circular or inverted U-shape | |
| Horizontal | An eyebrow that extends straight across the brow without a curve | |
| Synophrys | Meeting of the medial eyebrows in the midline | |
| Eyebrow skin | ||
| Erythema | Redness of skin | |
| Keratosis pilaris | Discrete follicular papules with scale | |
| Eyelash density | ||
| Absent | No eyelashes are present | |
| Sparse | Decreased density/number of eyelashes | Can be regional (medial, lateral, upper, lower) or total |
| Eyelash morphology | ||
| Trichomegaly | Increased length of the eyelashes | |
| Prominent | Eyelashes that draw attention of the viewer due to increased density and/or length and/or curl without meeting the criteria of trichomegaly | |
| Distichiasis | An extra row of eyelashes emerges from the ducts of meibomian glands | |
| Trichiasis | Eyelashes curved inward toward the cornea | |
| Scalp hair light microscopy | ||
| Hair diameter | Measured in mm | |
| Pigmentation | Light (translucent) Medium/dark (near opaque/opaque) | |
| Medullation | A dark line due to air spaces within the medulla in the center of the hair shaft | Can be continuous or discontinuous Independent of the degree of pigmentation but correlates with the hair diameter |
| Hair cycle | Anagen (pigmented triangular bulb or bent bulb) Catagen/telogen (depigmented club-shaped bulb, not bent) |
Light microscopy of hair shafts
Hair pluck specimens were obtained by a firm pull of approximately 20 hairs from the vertex scalp and evaluated by light microscopy. The diameter, colour, medullation pattern, stage of hair cycle and possible hair shaft abnormalities (trichoschisis, trichorrhexis nodosum, trichoptilosis, trichorrhexis invaginata, light and dark bands, pili annulati, beaded hair, pili torti, pili trianguli et canaliculi, pencil point hairs and hair casts) were examined. A representative hair from each specimen was measured for diameter, colour and medullation pattern.
Statistical analysis
For the continuous age variable and the hair shaft diameter, a two sample t-test was implemented to examine whether the means are significantly different between CFCS and CS. For all categorical factors, contingency tables (frequency and %) were generated and the Chi-squared test was used to investigate their associations with the disease status (CFCS or CS). The Fisher’s exact test was used for factors with any frequency < 5 in the CFCS or the CS group. Statistical analysis was conducted using SAS, version 9.4 (SAS Institute, Cary, NC). All tests were two sided and P<0.05 was considered statistically significant.
Results
A total of 39 individuals, 16 individuals with CFCS (11 female and 5 male) and 23 individuals with CS (15 female and 8 male), were included in the study. To ensure that all individuals met diagnostic criteria, two CS individuals with unknown mutation status were excluded. The mean age for individuals with CFCS was 15.1 years (range 6-35 years) and for individuals with CS 15.1 years (range 6-31 years; see Table 1). Similar to prior reports 13, 14, the majority of individuals with CFCS reported mutations in BRAF (13/16 or 81.3%, see Table 1). One individual reported a mutation in MAP2K1 and one in MAP2K2. By definition, all reported mutations in individuals with CS were in HRAS (Table 1). As expected, the most common HRAS mutant was p.G12S (16/23 or 69.6%). Additionally, rarer mutants, including p.G12A, p.G12C, p.G13C, p.G13D, p.A146V and p.K117R, were reported.
Table 1.
Demographic information on study participants.
| CFCS | CS | |||
|---|---|---|---|---|
| Number of participants | 16 | 23 | ||
| Gender | ||||
| Female | 11 | 68.8% | 15 | 65.2% |
| Male | 5 | 31.3% | 8 | 34.8% |
| Age | ||||
| Range (years) | 6-35 | 6-31 | ||
| Mean | 15.1 | 15.1 | ||
| Mutation | ||||
| BRAF (Unknown) | 8 | 50.0% | 0 | |
| BRAF (Exon 6, includes Q257R) | 2 | 12.5% | 0 | |
| BRAF (Exon 15, includes G596V) | 2 | 12.5% | 0 | |
| BRAF (Exon 11) | 1 | 6.3% | 0 | |
| MAP2K1 | 1 | 6.3% | 0 | |
| MAP2K2 | 1 | 6.3% | 0 | |
| HRAS p.G12S | 0 | 16 | 69.6% | |
| HRAS p.G12C | 0 | 2 | 8.7% | |
| HRAS p.G12A | 0 | 1 | 4.3% | |
| HRAS p.G13C | 0 | 1 | 4.3% | |
| HRAS p.G13D | 0 | 1 | 4.3% | |
| HRAS p.A146V | 0 | 1 | 4.3% | |
| HRAS p.K117R | 0 | 1 | 4.3% | |
| Other | 11 | 6.3% | N/A | N/A |
One individual with CFCS reported that the mutation was known, but did not recall the exact mutation
Scalp hair
Photography of hair and analysis of photographs was performed on 16 individuals with CFCS and 22 individuals with CS. The definitions for each analysed variable are described in Table 2. A difference in scalp hair colour was evident in CFCS and CS (see Table 3). The majority of individuals with CFCS had black/dark brown hair (11/16 or 68.8% in CFCS vs 6/22 or 27.3% in CS, p=0.03). Sandy or blonde scalp hair was more common in CS (2/16 or 12.5% in CFCS vs 11/22 or 50.0% in CS). Wavy or curly hair was characteristic in both groups (12/13 or 92.3% in CFCS vs 16/20 or 80.0% in CS), though curly hair was more commonly seen in CFCS. Straight hair was observed in a small subset of individuals, mainly in CS. Notably in CS, these included the individuals with HRAS p.A146V, p.G13C and p.K117R (3/6 or 50% of individuals with HRAS mutant other than p.G12S) compared with only one of 15 individuals with HRAS p.G12S (6.7%; p=0.05).
Table 3.
Characteristics of scalp, eyebrow and eyelash hair in CFCS and CS.
| Variable | CFCS | CS | |||
|---|---|---|---|---|---|
| n | % | n | % | P value1 | |
| Clinical variables Number of individuals examined | 16 | 22 | |||
| Scalp hair colour | 0.03 | ||||
| Black/dark brown | 11 | 68.8 | 6 | 27.3 | |
| Brown | 3 | 18.8 | 5 | 22.7 | |
| Sandy or blonde | 2 | 12.5 | 11 | 50.0 | |
| Red | 0 | 0 | 0 | 0 | |
| Scalp hair texture3 | 0.06 | ||||
| Straight | 1 | 7.7 | 4 | 20.0 | |
| Wavy | 3 | 23.1 | 6 | 30.0 | |
| Curly | 9 | 69.2 | 10 | 50.0 | |
| Eyebrow: density | 0.02 | ||||
| Absent | 7 | 43.8 | 0 | 0.0 | |
| Sparse, total | 5 | 31.3 | 3 | 13.6 | |
| Sparse, medial | 0 | 0 | 1 | 4.5 | |
| Sparse, lateral | 1 | 6.3 | 5 | 22.7 | |
| Absent or sparse | 13 | 81.3 | 9 | 40.9 | |
| Normal density | 3 | 18.8 | 13 | 59.1 | |
| Eyebrow: broad4 | 0.18 | ||||
| Broad, total | 0 | 0 | 4 | 18.2 | |
| Broad, medial | 0 | 0 | 0 | 0.0 | |
| Broad, lateral | 5 | 62.5 | 14 | 63.6 | |
| Not broad | 3 | 37.5 | 4 | 18.2 | |
| Eyebrow: flared4 | 1.00 | ||||
| Flared | 2 | 25.0 | 6 | 28.6 | |
| Not flared | 6 | 75.0 | 15 | 71.4 | |
| Eyebrow: highly arched4 | |||||
| Highly arched | 0 | 0 | 0 | 0 | |
| Not highly arched | 8 | 100.0 | 22 | 100.0 | |
| Eyebrow: horizontal4 | 0.36 | ||||
| Horizontal | 3 | 37.5 | 4 | 19.0 | |
| Not horizontal | 5 | 62.5 | 17 | 81.0 | |
| Eyebrow: synophrys4 | 0.01 | ||||
| Synophrys | 0 | 10 | 45.5 | ||
| No synophrys | 13 | 100.0 | 12 | 54.5 | |
| Eyebrow: erythema | 0.002 | ||||
| Erythema | 8 | 50.0 | 1 | 4.5 | |
| No erythema | 8 | 50.0 | 21 | 95.5 | |
| Eyebrow: keratosis pilaris | 0.01 | ||||
| Keratosis pilaris | 7 | 43.8 | 1 | 4.5 | |
| No keratosis pilaris | 9 | 56.3 | 21 | 95.5 | |
| Eyelash: density | 0.02 | ||||
| Absent, total | 4 | 25.0 | 0 | 0.0 | |
| Absent, upper | 0 | 0 | 0 | 0.0 | |
| Absent, lower | 2 | 12.5 | 3 | 13.6 | |
| Sparse, total | 0 | 0 | 3 | 13.6 | |
| Sparse, medial | 1 | 6.3 | 0 | 0.0 | |
| Sparse, lateral | 0 | 0 | 0 | 0.0 | |
| Normal | 9 | 56.3 | 16 | 72.7 | |
| Eyelash: trichomegaly5 | 0.03 | ||||
| Trichomegaly | 0 | 0 | 8 | 36.4 | |
| No trichomegaly | 11 | 100.0 | 14 | 63.6 | |
| Eyelash: prominence5 | 0.21 | ||||
| Prominent | 1 | 9.1 | 8 | 36.4 | |
| Not prominent | 10 | 90.9 | 14 | 63.6 | |
| Eyelash: distichiasis5 | 0.22 | ||||
| Distichiasis | 1 | 9.1 | 7 | 31.8 | |
| No distichiasis | 10 | 90.9 | 15 | 68.2 | |
| Eyelash: trichiasis5 | 0.53 | ||||
| Trichiasis | 0 | 0 | 3 | 13.6 | |
| No trichiasis | 11 | 100.0 | 19 | 86.4 | |
| Microscopic variables Number of individuals examined | 15 | 22 | |||
| Scalp hair pigmentation | 0.03 | ||||
| Light | 7 | 46.7 | 19 | 86.4 | |
| Medium/dark | 8 | 53.3 | 3 | 13.6 | |
| Medullation | 0.005 | ||||
| Non-medullated | 6 | 40.0 | 19 | 86.4 | |
| Medullated | 9 | 60.0 | 3 | 13.6 | |
P values were obtained from the Chi-squared test and the Fisher’s exact test was used when any cell frequency was less than 5
P value shown for combined absent or sparse versus normal
For statistical analyses, the following adjustments were made:
Excluded “Indeterminate (due to very short hair)”
Excluded “Indeterminate (due to absence of eyebrows)”
Excluded “Indeterminate (due to absence of eyelashes)”
Eyebrow hair
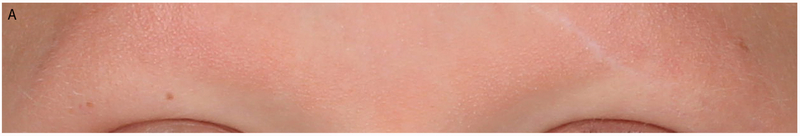
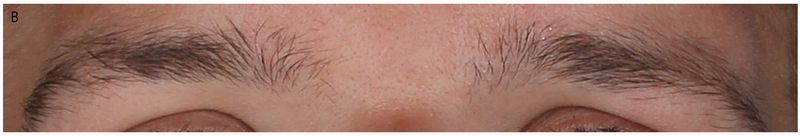
A striking difference was observed in the density of eyebrows between CFCS and CS. Individuals with CFCS commonly showed very sparse (for definitions of variables, see Table 2) or entirely absent eyebrows while eyebrow density was typically relatively normal in CS (13/16 or 81.3% in CFCS vs 9/22 or 40.9% in CS, p=0.02; Table 3, Fig. 1). Consistent with prior studies reporting ulerythema ophryogenes in CFCS, individuals with CFCS typically showed erythema and keratosis pilaris of the eyebrows (erythema in 8/16 or 50.0% in CFCS vs 1/22 or 4.5% in CS, p=0.002; keratosis pilaris in 7/16 or 43.8% in CFCS vs 1/22 or 4.5% in CS, p=0.01). Synophrys (for definition, see Table 2) was only seen in CS individuals (0/13 or 0% in CFCS vs 10/22 or 45.5% in CS, p=0.01). The density of eyebrows was high enough in only eight of 16 individuals with CFCS to allow assessment of eyebrow breadth and shape. Although eyebrows in CS appeared slightly disorganized, no statistically significant differences were observed in the shape of the eyebrows between CFCS and CS (Table 3).
Figure 1.
Eyebrow morphology in CFCS and CS. (a) CFCS patient with BRAF mutation and absence of eyebrow hair. (b) CS patient with HRAS p.G12S and full eyebrows.
Eyelash hair
The density of eyelashes was also significantly associated with CFCS and CS status (p=0.02, Table 3). Eyelashes tended to be more commonly absent or sparse in CFCS compared with CS (7/16 or 43.8% in CFCS vs 6/22 or 27.3% in CS). Long eyelashes (trichomegaly) were only observed in CS (0/11 or 0% in CFCS vs 8/22or 36.4% in CS, p=0.03). Eyelash abnormalities including distichiasis and trichiasis (for definitions, see Table 2) were both more prevalent in individuals with CS although not statistically significant (distichiasis in 1/11 or 9.1% in CFCS vs 7/22 or 31.8% in CS; trichiasis in 0/11 or 0% in CFCS vs 3/22 or 13.6% in CS).
Microscopic features of hair shafts
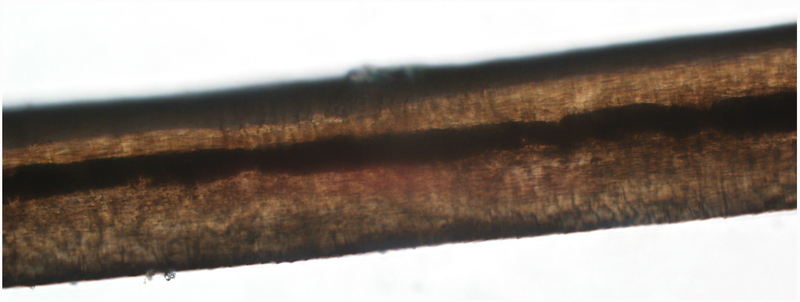
Scalp hairs from 15 individuals with CFCS and 22 individuals with CS were available for light microscopic evaluation. Scalp hair shafts in CFCS were thicker than in CS (mean 0.145 mm, SEM 0.011 mm in CFCS vs 0.095 mm, SEM 0.004 mm, in CS, p<0.0001; Fig. 2). Additionally, they were darker in pigmentation and medullated when compared to CS hairs (medium/dark pigmentation in 8/15 or 53.3% in CFCS vs 3/22 or 13.6% in CS, p=0.03; medullation in 9/15 or 60.0% in CFCS vs 3/22 or 13.6% in CS, p=0.005; Table 3). Collectively, there were no differences in the stage of the hair cycle between CFCS and CS, but abnormal anagen hairs showing aberrantly shaped anagen bulbs, loss of the inner root sheath and a ruffled cuticle were present in both CFCS and CS. Hair casts were noted in one individual with CFCS. Structural abnormalities of the hair shafts were only observed in CS and included trichorrhexis nodosum, trichoptilosis and fractures (0/15 or 0.0% in CFCS vs 5/22 or 22.7% in CS). No other structural abnormalities were observed.
Figure 2.
Microscopic features of scalp hair in CFCS and CS. (a) Thick, pigmented and medullated hair in CFCS. (b) Thin, lightly pigmented and non-medullated hair in CS. Magnification 400x.
Discussion
Our study shows distinct hair manifestations in CFCS and CS, two RASopathies caused by aberrant activation of the RAS pathway. In a systematic side-by-side phenotypic comparison of the two syndromes, we validate prior observations of wavy or curly scalp hair in both CFCS and CS and absent or sparse eyebrows in CFCS.10–12 Moreover, we demonstrate that while the eyebrows and eyelashes are sparse in CFCS, hair growth of eyebrows and eyelashes is often increased in CS, as exemplified by the observed synophrys and trichomegaly, respectively. Additionally, scalp hair shafts in CS are lighter and thinner in comparison to CFCS and show structural abnormalities. As CFCS and CS model aberrant activation of the downstream and upstream elements of the RAS pathway, respectively, the results highlight the complex role of the RAS pathway in regulating hair growth in humans and establish a foundation for further investigations on the specific mechanisms underlying these phenotypic differences as well as for identification of novel therapeutic targets.
In our study, wavy or curly hair was characteristic of both CFCS and CS, though slightly more prevalent in CFCS, present in 92.3% of individuals with CFCS and 81.8% of individuals with CS. Previously, wavy or curly hair was reported in 69-93.4% in CFCS and 95.7% in CS.10–12 Curiously, a subset of individuals in our study had straight scalp hair and in CS, this appeared associated with mutants other than HRAS p.G12S. Although the numbers of individuals with these mutations were low, this finding suggests possible correlation between the genotype and hair texture in CS. Previously, a potential correlation between the disease severity, malignancy risk and genotype has been suggested in CS.18, 19
One of the most characteristic findings of CFCS was absent or very sparse eyebrows and eyelashes. In many cases, absent eyebrows were associated with erythema and keratosis pilaris, validating prior observations of ulerythema ophryogenes in a systematic fashion.9–12 By contrast, none of the individuals with CS had entirely absent eyebrows and most had a normal density of eyebrows and eyelashes. Moreover, in a subset of cases, increased hair growth of eyebrows and eyelashes was observed in CS, as exemplified by synophrys and trichomegaly, and in some cases by distichiasis and trichiasis of the eyelashes. Supporting these findings are prior anecdotal reports describing individuals with CS needing to intermittently trim their eyelashes.10
The aetiology of trichomegaly is broad and includes congenital disorders, acquired diseases, drugs and rarely infections or malignancy. Higgins and co-workers 20 reported a family with trichomegaly caused by homozygous mutation in FGF5, a known regulator of hair length in non-human species. Interestingly, fibroblast growth factor family ligands interact with their signalling receptors activating the RAS, PI3K/AKT, PLCγ and JAK/STAT intracellular signalling pathways. Further investigation is needed to examine whether a mechanistic link exists between FGF5 and HRAS in trichomegaly. Finally, lengthening of eyelashes may also be a therapeutic goal for patients with hypotrichosis. In fact, a prostaglandin inhibitor bimatoprost is approved by the US Food and Drug Administration for treatment of hypotrichosis of the eyelashes. Prostaglandins activate the RAS pathway among many others.21 Whether prostaglandins are involved in trichomegaly associated with HRAS mutations awaits further studies.
In vitro and murine models have demonstrated that cyclical on/off switching of the RAS pathway is required for the onset of catagen, hair cycle progression and maintenance of hair follicles.3 In a murine model with activated Kras this lead to the development of hair loss, disorderly oriented hair follicles and wavy coat and curly whiskers.3, 22–24 Abnormal anagen hairs were common in both CFCS and CS, suggesting abnormal hair cycling. One clinical example of abnormal hair cycling is loose anagen, where hairs typically demonstrate a deformed anagen bulb, an absent inner root sheath and a ruffled cuticle accompanied with a complete absence of telogen hairs.25 Loose anagen hairs have been described in one of the RASopathies, Noonan-like syndrome with loose anagen hair, caused by mutations in SHOC2 and PPP1CB. Our light microscopic examination also showed that scalp hairs were typically darker and thicker in CFCS than CS, possibly secondary to a difference in hair cycling, as normal anagen hairs are typically darker and larger in diameter.25
Successful treatment of hair disorders is often challenging, as therapeutic options are limited or suboptimal. Increased understanding of the mechanisms of hair diseases is needed to identify novel therapeutic targets, as demonstrated by the successful introduction of JAK inhibitors for treatment of alopecia areata.26–30 RASopathies offer an insight into the role of the RAS pathway in the regulation of human hair growth. Understanding the mechanisms involved may lead to identification of much needed novel therapies for hair diseases.
In summary, our study demonstrates distinct hair manifestations in CFCS and CS, two human diseases that also model aberrant activation of the RAS pathway. While wavy or curly scalp hair were common in both syndromes, CFCS was associated with darker and thicker scalp hair, sparse eyebrows combined with keratosis pilaris and sparse eyelashes and CS with increased hair growth of the eyebrows and eyelashes, including synophrys and trichomegaly. Differences in the hair phenotype between CFCS and CS emphasize the complex role of the RAS pathway in regulating hair growth, facilitate improved accuracy of clinical diagnosis and set a foundation for identification of novel therapeutic targets.
Acknowledgments
We are grateful to patients and families for participating in this study. We also thank Jed Smith, Lan Yu, Negar Foolad and Farzam Gorouhi for assistance in this study. The study was approved by the Institutional Review Board of the University of California Davis. A part of this project was presented at the International Investigative Dermatology meeting in 2018 in Orlando, FL.
Funding sources: Dermatology Foundation, through Career Development Award in Dermatopathology (MK); National Cancer Institute, National Institutes of Health, through grant #K12CA138464 (MK); University of California Davis Comprehensive Cancer Center Support Grant P30CA093373-04 (LQ); National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health through grant #R01AR062165 (KAR)
Footnotes
Conflict of interest: The authors state no conflicts of interest.
References
- 1.Garcia-Hernandez MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, Camacho F. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26(10):625–32. [DOI] [PubMed] [Google Scholar]
- 2.Liu LY, King BA, Craiglow BG. Alopecia areata is associated with impaired health-related quality of life: A survey of affected adults and children and their families. J Am Acad Dermatol. 2018;79(3):556–8 e1. [DOI] [PubMed] [Google Scholar]
- 3.Drosten M, Lechuga CG, Barbacid M. Ras signaling is essential for skin development. Oncogene. 2014;33(22):2857–65. [DOI] [PubMed] [Google Scholar]
- 4.Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet. 2013;14:355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19(3):230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tidyman WE, Rauen KA. Expansion of the RASopathies. Curr Genet Med Rep. 2016;4(3):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estep AL, Tidyman WE, Teitell MA, Cotter PD, Rauen KA. HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am J Med Genet A. 2006;140(1):8–16. [DOI] [PubMed] [Google Scholar]
- 8.Tajan M, Paccoud R, Branka S, Edouard T, Yart A. The Rasopathy family: Consequences of germline activation of the RAS/MAPK pathway. Endocr Rev. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Pierpont ME, Magoulas PL, Adi S, Kavamura MI, Neri G, Noonan J, et al. Cardio-facio-cutaneous syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2014;134(4):e1149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel DH, Mann JA, Krol AL, Rauen KA. Dermatological phenotype in Costello syndrome: consequences of Ras dysregulation in development. Br J Dermatol. 2012;166(3):601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel DH, McKenzie J, Frieden IJ, Rauen KA. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br J Dermatol. 2011;164(3):521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bessis D, Morice-Picard F, Bourrat E, Abadie C, Aouinti S, Baumann C, et al. Dermatological manifestations in cardiofaciocutaneous syndrome: a prospective multicentric study of 45 mutation-positive patients. Br J Dermatol. 2018. [DOI] [PubMed] [Google Scholar]
- 13.Niihori T, Aoki Y, Narumi Y, Neri G, Cave H, Verloes A, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38(3):294–6. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311(5765):1287–90. [DOI] [PubMed] [Google Scholar]
- 15.Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–40. [DOI] [PubMed] [Google Scholar]
- 16.Hall BD, Graham JM Jr., Cassidy SB, Opitz JM. Elements of morphology: standard terminology for the periorbital region. Am J Med Genet A. 2009;149A(1):29–39. [DOI] [PubMed] [Google Scholar]
- 17.Silengo M, Belligni E, Molinatto C, Baldassarre G, Biamino E, Chiesa N, et al. Eyebrow anomalies as a diagnostic sign of genomic disorders. Clin Genet. 2010;77(1):28–31. [DOI] [PubMed] [Google Scholar]
- 18.Kerr B, Delrue MA, Sigaudy S, Perveen R, Marche M, Burgelin I, et al. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet. 2006;43(5):401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick EM, Hopkins E, Conway L, Catalano S, Hossain J, Sol-Church K, et al. Assessing genotype-phenotype correlation in Costello syndrome using a severity score. Genet Med. 2013;15(7):554–7. [DOI] [PubMed] [Google Scholar]
- 20.Higgins CA, Petukhova L, Harel S, Ho YY, Drill E, Shapiro L, et al. FGF5 is a crucial regulator of hair length in humans. Proc Natl Acad Sci U S A. 2014;111(29):10648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansari KM, Rundhaug JE, Fischer SM. Multiple signaling pathways are responsible for prostaglandin E2-induced murine keratinocyte proliferation. Mol Cancer Res. 2008;6(6):1003–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doma E, Rupp C, Baccarini M. EGFR-ras-raf signaling in epidermal stem cells: roles in hair follicle development, regeneration, tissue remodeling and epidermal cancers. Int J Mol Sci. 2013;14(10):19361–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay A, Krishnaswami SR, Yu BD. Activated Kras alters epidermal homeostasis of mouse skin, resulting in redundant skin and defective hair cycling. J Invest Dermatol. 2011;131(2):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay A, Krishnaswami SR, Cowing-Zitron C, Hung NJ, Reilly-Rhoten H, Burns J, et al. Negative regulation of Shh levels by Kras and Fgfr2 during hair follicle development. Dev Biol. 2013;373(2):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling LC, Cowper SE, Knopp EA. An atlas of hair pathology with clinical correlations2012.
- 26.Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy Crispin M, Ko JM, Craiglow BG, Li S, Shankar G, Urban JR, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134(12):2988–90. [DOI] [PubMed] [Google Scholar]
- 29.Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang EHC, Sallee BN, Tejeda CI, Christiano AM. JAK Inhibitors for Treatment of Alopecia Areata. J Invest Dermatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]