Abstract
Gene therapy has become an important treatment option for a variety of hematological diseases. The biggest advances have been made with CAR T cells and many of those studies are now FDA approved as a routine treatment for some hematologic malignancies. Hematopoietic stem cell (HSC) gene therapy is not far behind with treatment approvals granted for beta-hemoglobinopathies and adenosine deaminase severe combined immune deficiency (ADA-SCID), and additional approbations currently being sought. With the current pace of research, the significant investment of biotech companies, and the continuously growing toolbox of viral as well as non-viral gene delivery methods, the development of new ex vivo and in vivo gene therapy approaches is at an all-time high.
Research in the field of gene therapy has been ongoing for more than 4 decades with big success stories as well as devastating drawbacks along the way. In particular, the damaging effect of uncontrolled viral vector integration observed in the initial gene therapy applications in the 90s led to a more comprehensive upfront safety assessment of treatment strategies. Since the late 90s, an important read-out to comprehensively assess the quality and safety of cell products has come forward with the mouse xenograft model. Here, we review the use of mouse models across the different stages of basic, pre-clinical and translational research towards the clinical application of HSC-mediated gene therapy and editing approaches.
Keywords: Gene Therapy, Hematopoiesis, Stem Cells, Mouse Models, Review
Graphical Abstract

Introduction - The use of mouse models for gene therapy
Development and clinical translation of HSC-mediated gene therapy and editing approaches requires the comprehensive assessment of cell products in order to guarantee quality and safety. This assessment includes detailed analysis of cells regarding 1) their maintenance of multilineage differentiation potentials after ex vivo modification and culture, 2) the capability of human hematopoietic stem and progenitor cells (HSPCs) to efficiently engraft into the bone marrow (BM) stem cell niche, and 3) the safety of cell products by longitudinal monitoring for potential side/off-target effects due to the gene modification. All three requirements are nowadays routinely addressed in the mouse xenograft model throughout the different phases of basic, translational, and pre-clinical development of gene therapy approaches (Table 1).
Table 1:
Overview of mouse models and examples of their use in gene therapy
| Mouse model | Genotype | Field of research |
|---|---|---|
| BNX | NIH-Beige-Nude-XID | Adenosine deaminase (ADA)- severe combined immunodeficiencies (SCID) |
| C57BL/6 | B6(Cg)-Tyrc-2J/J | Human HSC research, Immunotherapy |
| NBSGW | NOD.Cg-KitW−41J Tyr+ Prkdcscid Il2rgtm1Wji/ThomJ | β-thalassaemia/sickle cell disease |
| NOD/SCID | NOD.CB17- Prkdcscid/J | Human HSC research |
| NOG | NOD/SCID/IL-2Rγ-nuN | Human HSC research |
| NSG | (NOD.Cg-B2mtm1Unc Prkdcscid Il2rgtm1Wjl/SzJ) | Human HSC research X-linked SCID (X-SCID) β-thalassaemia/sickle cell disease Fanconi anemia (FA) |
| SCID | B6.CB17-Prkdcscid/SzJ | Human HSC research |
| Humanized strains | ||
| BLT | NOD/SCID with human bone marrow, liver, thymus | Immunotherapy |
| MISTRG | C;129S4-Rag2tm1.1Flv Csf1tm1(CSF1)FlvCsf2/Il3tm1.1(CSF2,IL3)Flv Thpotm1.1(TPO)Flv Il2rgtm1.1FlvTg(SIRPA)1Flv/J | Human HSC research NHP HSC research |
| NSG-S | NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3, CSF2, KITLG)1Eav/MloySzJ | Human HSC research |
| NSG-W41 | NOD.Cg-KitW−41J Prkdcscid Il2rgtm1Wjl/WaskJ | Human HSC research |
| Hu-SCID | Humanized with fetal liver and thymus tissue fragments | Human HSC research Immunotherapy |
| Disease-specific models | ||
| FANCA knockout | 129S4 and C57/BL | Fanconi anemia (FA) |
| ADA knockout | NIH-Beige-Nude-XID ADA−/− | Adenosine deaminase (ADA)- severe combined immunodeficiencies (SCID) |
| HBB deficient | C57BL/6 Hbbth3−/+ | β-thalassaemia/sickle cell disease |
| WASP knockout | BL6-wasnull | Wiskott-Aldrich syndrome (WAS) |
| X-SCID knockout | gC−/−;Rag2−/− | X-linked SCID (X-SCID) |
Here we review key literature that involves the mouse model to address fundamental questions on basic stem cell biology as well as genetically-engineered and humanized mouse strains to model ex vivo and in vivo HSC gene therapy-based strategies. The review has been organized in 4 main chapters. Chapter 1: Immunocompromised mice have been incredibly valuable for many gene therapy studies to demonstrate the maintenance of long-term multilineage engraftment potentials, confirm the therapeutic benefits brought by the gene modification, and validate the safety of ex vivo generated infusion products. Chapter 2: Humanized mouse strains have been developed to overcome limitations of the current models that have entered the field and are expected to replace the “classically” cross-bread strains providing improved multilineage differentiation of human HSCs. Chapter 3: A great variety of genetically-engineered murine disease models have also been generated to demonstrate efficacy of treatment and lay the foundation for clinical translation of novel HSC-based therapeutic strategies. Chapter 4: Lastly, the engraftment of neonatal mice with human HSCs enables the development of in vivo matured human T cells for the evaluation of HSC-mediated immunotherapy strategies as well as gene therapy approaches directly targeting human stem cells in vivo.
“Classical” mouse models as a read-out for HSCs
The first use of mouse xenograft models for the assessment of human HSCs started in the 1980s. Research in this field continuously increased over the last four decades as comprehensively reviewed by others [1–3]. Here, we focus on some key literature from the last 40 years specifically relevant to the field of HSC gene therapy.
First studies engrafting human fetal liver (FL) CD34+ cells into immunodeficient SCID (severe combined immunodeficiency) mice were performed in the late 80s by McCune et al. demonstrating successful development into functional human T and B cells [4]. However, the level of human engraftment in these SCID mice was low, differentiation restricted to these two lymphoid lineages, and administration of human cytokines required throughout the entire follow-up. A few years later, Shultz et al. described the non-obese diabetic (NOD)/SCID mouse [5]. Lack of an adaptive as well as innate immune system permitted human multilineage engraftment without external administration of cytokines. In the following two decades, several groups continued to improve the mouse model by cross-breeding new strains (e.g. the NSG mouse) to accommodate higher levels of human chimerism, increase the immune-tolerance of the graft, and enhance the support for multilineage differentiation [1, 2].
Availability of the mouse xenograft model triggered the idea to model human gene therapy protocols in vivo and pre-evaluate experimental strategies for clinical translation [6]. Particularly with the serious setback encountered by HSC gene therapy in the treatment of X-linked SCID patients in the 90s [7], countless publications utilized various mouse strains to test safety and efficacy of gene therapy approaches in order to avoid more adverse events [8, 9]. Ever since, the NOD/SCID and NSG mouse model developed during this time period became by far the most frequently used strains and widely accepted gold standard read-out to determine the multilineage engraftment potential and safety of candidate human HSCs from different stem cell sources [10, 11] after ex vivo expansion [12], undergoing gene-modification [13–15], as well as for ESC (embryonic stem cells)-/IPSC (induced pluripotent stem cell)-derived human HSPCs [16–18].
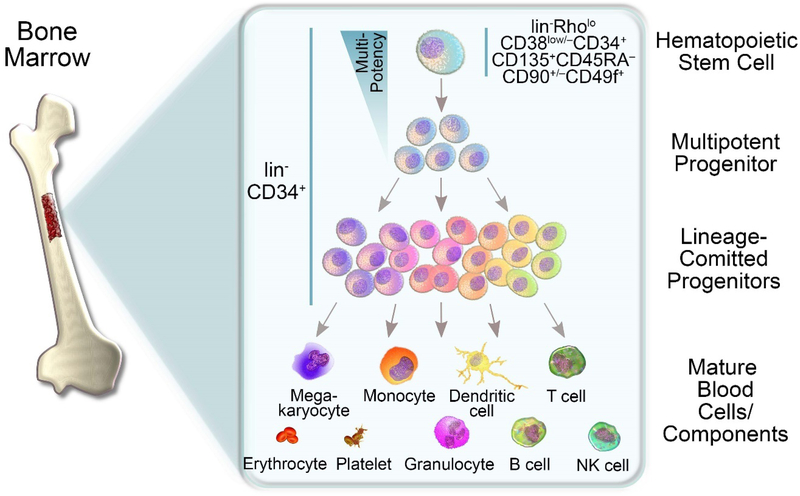
Attempts to model and improve gene therapy in these early mouse models (here SCID and NOD/SCID) was accompanied by the discovery of new cell surface antigens for the purification of human HSCs. Of special interest for HSC gene therapy, the identification of cell surface marker for human HSCs would allow improved targeting and at the same time potentially reduce unwanted side-effects. In 1992, enrichment of human HSCs with SCID engraftment potential in the Lin-CD34+CD90+ subset was reported [19]. Bhatia et al. 5 years later associated the lack of CD38 expression (CD34+CD38- cells) with primitive human HSCs capable of multilineage repopulation potential in NOD/SCID mice [20]. Majeti et al. combined previous marker and refined the HSC-enriched subset in umbilical cord blood (UCB) and BM as Lin- CD34+CD38-CD90+CD45RA- using newborn NOG mice [21]. Setting the current standard for the identification of highly purified human HSCs, Notta et al. reported human HSCs in UCB as lin- RholoCD38low/−CD34+CD135+CD45RA−CD90+/−CD49f+ [22] (Figure 1). Intra-femoral transplantation of only a single cell from this phenotype was sufficient to reconstitute hematopoiesis in sublethally irradiated NSG mice and display multi-lineage chimerism [22].
Figure 1: Development of hematopoietic lineages.
The formation of blood cells originates in the bone marrow containing lin-CD34+ hematopoietic stem and progenitor cells (HSPCs) with the most primitive human hematopoietic stem cells (HSCs) enriched in the lin-RholoCD38low/− CD34+CD135+ CD45RA−CD90+/−CD49f+ phenotype [22]. HSCs gradually lose their multipotency giving rise to multipotent progenitors (MPPs) followed by a variety of lineage committed progenitor cells and ultimately mature blood cells.
While the analysis of such complex phenotypes has become the standard for gene-modified cell products in basic and pre-clinical research, isolation and gene-modification of these highly-defined subsets in the clinical routine is technically challenging and hard to translate. Due to these mostly technical limitations, most if not all currently available HSC gene therapy approaches still modify CD34+ cells, a heterogeneous mix of >99% committed progenitor cells and only very few “true” HSCs with long-term multilineage engraftment potential (Figure 1). As a result, current gene therapy strategies inefficiently target true long-term engrafting HSCs [23–26], are costly [27–29], and may cause unwanted side effects [30–36]. Attempts to reduce the target cell number, improve targeting efficiency, and enhance feasibility are currently ongoing [37]. Approaches with translational potential currently aim to purify HSC-enriched CD34 subset using only one additional cell surface marker. Examples are the CD34+CD38low/− [14, 38], CD34+CD133+ [39], or CD34+CD90+ [40, 41] cell fractions. The potency of the different subsets identified in these studies relies on mouse xenograft experiments to evaluate stem cell features such as homing, multilineage differentiation, and long-term reconstitution in serial transplants of gene-modified cells.
First attempts to enrich for a phenotypically defined, HSC-enriched CD34-subpopulation for stem cell transplantation in humans date back into the late 1990s. Myeloma, breast cancer, and Non-Hodgkin lymphoma patients received autologous flow-sorted lin−CD34+CD90+ or CD34+CD90+ cell fractions which are enriched for primitive long-term engrafting HSCs and phenotypically depleted for malignant cells [42–45]. Rapid and sustained hematopoietic recovery was seen in patients with myeloma and breast cancer [43–45]. These initial studies showed that the purification of HSC-enriched CD34-subpopulations for transplantations and consequently for HSC-mediated gene therapy is technically possible and at the same time safe. While the very first purification and gene-modification of an HSC-enriched CD34+ subset in SCID patients is currently in a phase-1 trial at Stanford University (trial identifier: NCT02963064), proof-of-concept studies in the NSG mouse [14] and the nonhuman primate (NHP) [46, 47] have already demonstrated improved efficiency and feasibility of HSC gene therapy with this purified CD34 subset. Enrichment of HSCs reduced the target cell number, improved the efficiency of gene-modification in long-term engrafting HSCs, and significantly reduced expenses making HSC gene therapy a more accessible treatment option for patients.
Another hurdle currently limiting the efficiency of gene therapy has been associated with the quiescence and therefore inherent protection of long-term engrafting HSCs from gene modification [48, 49]. Particularly problematic for gene editing approaches, the low activity of the homology-directed repair (HDR) pathway in HSCs due to the quiescent state limits the ability to stably integrate genetic material at precise genomic loci [25, 50, 51]. Current attempts therefore focus on the short-term exposure of HSCs to small molecules or other chemical compounds to temporarily stimulate them, make them permissive to the gene modification, and shortly after either set them back to a primitive state or expand them without exhaustion or differentiation. Reported approaches include the use of compounds such as UM171 [52], PGE2 [14, 53], rapamycin [54], cyclosporine [54], or inhibitors such as i53 to favor HDR after CRISPR/Cas9 cutting [55]. In the majority of studies, assessment of ex vivo gene-modified and expanded cells was performed in the NSG mouse model [14, 52, 53] to demonstrate multilineage long-term engraftment of human HSPCs after the exposure of HSCs to these novel compounds.
“Humanized” mouse models for improved multilineage engraftment of HSCs
Although classically cross-bred mouse strains (NOD/SCID, NSG, etc.) can harbor human B cells, T cells, and granulocytic/myeloid CD33+ cells [56, 57], the maturation of some lineages is only partly supported. Furthermore, the frequency of most lineages is not representative of human blood composition. For example, the frequency of fully mature and functional monocytes and macrophages is typically low [58, 59], long-term erythropoiesis, full erythroid maturation, and megakaryopoiesis barely supported [11, 56, 60], and the function as well as homeostasis of NK cells defective [61, 62]. In addition, detailed discrimination of granulocyte subsets (basophils, eosinophils, neutrophils), monocyte/macrophage subtypes (M1, M2), detection of human mast cells, or the assessment of dendritic cells (DCs) in mouse xenografts is rarely performed [56, 63].
In an effort to overcome these restrictions and improve the hematopoietic lineage output, various groups have genetically engineered and humanized existing mouse strains to overexpress human cytokines, increase their immune tolerance, and reduce the rejection of human blood cells [64–66]. These novel humanized mouse strains (e.g. NSG-S, NSG-W41, MISTRG) demonstrate significantly improved levels of human cell engraftment in the peripheral blood (PB) and BM, a more realistic lympho-myeloid composition of WBCs, and better development of functional monocyte subsets as well as NK cells [64, 67–69].
Even though these genetically modified mice demonstrate improved support for human engraftment, most research labs still rely on older stains such as NOD/SCID and NSG while not taking advantage of the novel humanized models. To promote this transition, we recently compared the ability of next-generation humanized mouse models regarding their ability to support the BM engraftment of phenotypically and functionally primitive human HSPCs [67]. Comparison of multiple mouse strains showed high levels of human chimerism in the PB as well as HSCs in the BM of NSGW41 and MISTRG mice, whereas HSC exhaustion was observed in NSG mice. Most importantly, MISTRG mice supported the development of lymphoid (B, T, NK cells) as well as myeloid (granulocyte, monocyte) lineages providing an improved multilineage read-out for transplanted human HSCs over the classical mouse strains.
Designed to support improved engraftment of human cells, the MISTRG mouse was further shown to accommodate multilineage engraftment of nonhuman primate (NHP) HSPCs [41]. While NOD/SCID and NSG mice do not support engraftment of NHP HSPCs [41, 70], knocked-in human SIRPA along with other human cytokines in MISTRG mice enabled monocytes, granulocytes, NK cells, B cells, and CD4+ and CD8+ T cells to engraft in the spleen, BM, thymus, and PB. Most importantly, only CD34+CD90+CD45RA- NHP HSPCs were capable of engrafting, consistent with our recent findings using autologous NHP transplantation [46, 47]. Availability and similarity of this monkey-mouse xenograft model with the autologous transplant setting in the NHP transplantation and gene therapy model is closing a gap in between basic and translational stem cell research. Virtually all HSC gene therapy studies currently evaluated in the NHP can be pre-tested in this new ‘monkeynized’ MISTRG mouse, hopefully enhancing the thorough testing of new gene therapy approaches.
The use of these next-generation mouse strains will likely gain traction with the currently growing field of in vivo gene therapy, where HSPC transduction takes place in situ, thus bypassing the need to purify and manipulate cells ex vivo. Robust levels of BM CD34 HSPCs in MISTRG mice and the generation of more complete and mature human hematopoiesis makes this model highly attractive for the modelling of in vivo HSC gene therapy. Proof-of-concept studies in C57BL/6 mice stably overexpressing the human CD46 receptor have shown successful mobilization of BM-resident HSPCs into the PB to make them accessible to the modifying vehicle that is ideally delivered intravenously. GCSF/AMD3100 efficiently mobilized the murine HSCs into PB to enable in vivo transduction with an adenoviral vector targeting the CD46 transgenic murine HSCs [71]. This approach has also been more recently used in the context of beta-thalassemia where in vivo delivery of the hemoglobin transgene via adenoviral vectors resulted in a near complete phenotypic correction of the disorder [72].
Despite the advantages we highlighted of these new mouse strains, their implementation in pre-clinical and translational research is not guaranteed and may take significantly longer since this field requires more time to adapt new tools replacing old standards. Until then and due to the ease of use and availability, NOD/SCID and NSG mice will very likely remain the in vivo model of choice for many researchers.
HSC-mediated gene-therapy in the mouse xenograft model
After facing an initial setback in the 90s, the field of stem cell gene therapy incorporated more stringent regulations and additional safety features in its pre-clinical development. The mouse xenograft model has been instrumental to establish new guidelines and assess engraftment of gene-modified HSPCs. In addition, transgenic mouse models were created to recapitulate human disorders by the knockout of the disease-causing genes. These murine disease models have proved extremely valuable to demonstrate correction by gene therapies employing viral vectors or more recently by gene-editing-based technologies. As a result, major milestones have been reached with several of these approaches now being approved for use in patients. Below, we provide a few examples of studies that built upon the mouse model for pre-clinical testing of new therapies specifically focusing on the treatment of primary immunodeficiencies, hematopoietic and hemoglobin disorders.
Stem cell gene therapy for primary immunodeficiencies.
Primary immunodeficiencies (PIDs) define rare monogenic disorders that cause a severe impairment in the development of a normal immune system, resulting in immune dysregulation, autoimmunity and susceptibility to opportunistic infections. PIDs include a large number of distinct genetic disorders [73], which are estimated to occur only in 1:10,000 birth [74] but can be fatal in infants, particularly in SCID. The introduction of newborn screening [75] and advances in gene therapy, now allow for earlier detection and treatment to improve the prognosis of these diseases.
The first clinical trials of stem cell gene therapy for PIDs began in the late 1990s with adenosine deaminase (ADA)-SCID patients using gamma-retroviral vectors [76]. This clinical trial was based on comprehensive upfront studies demonstrating successful engraftment of gene-modified human T lymphocytes in immunocompromised BNX mice [77]. Despite the limitations of this mouse model at that time, the researchers were able to demonstrate successful restoration of enzyme activity, full maturation, and long-term engraftment of functional human T cells from ADA-SCID patients in the spleen and PB of xenotransplanted mice [78]. To further improve safety, lentiviral vectors for ADA-SCID were later generated and tested in vivo in ADA-deficient (−/−) mice. Since ADA−/− mice die perinatally, further genetic engineering was necessary to restore ADA expression in trophoblast cells, to prolong survival [79]. The resulting mice retained many features associated with ADA deficiency in humans, including a combined immunodeficiency, severe pulmonary insufficiency, as well as bone and kidney abnormalities, leading to postnatal death within the first 3 weeks [80, 81]. This model allowed the validation of ADA activity restoration leading to normal immune function after ex vivo transduction and transplantation of BM cells modified with an ADA-encoding lentiviral vector.
Gamma-retroviral vectors were also used initially for the treatment of X-linked SCID (X-SCID), a disease caused by deficiency of the common gamma chain (γc), also known as interleukin 2 receptor subunit gamma or IL-2RG, resulting in a failure of both cellular and humoral immune responses. As discussed earlier, gamma-retroviral vector gene therapy demonstrated clear clinical benefits but also resulted in leukemogenesis with monoclonal blast expansion due to the activation of a proto-oncogene following viral vector integration [82, 83]. Safer vectors were subsequently generated and tested including self-inactivating (SIN) gamma retroviral and lentiviral vectors. For X-SCID, SIN lentiviral vectors were optimized and tested by transduction and transplantation of BM cells in the X-SCID (γC−/−; Rag2−/−) mouse model to evaluate reconstitution of a functional immune system [84]. While restoration of the disease phenotype was established in this model, its relevance to assess vector safety is however limited since it lacks the sensitivity necessary to measure vector-mediated oncogenesis. Tumor-prone mouse models generated by knocking out of Cdkn2a, a major regulator of cell proliferation, senescence and apoptosis, could alternatively be used with the drawback that they show high background rate of tumor formation independent of insertion events [85, 86]. More recently, the NSG model was employed to assess CRISPR/Cas9-based gene correction strategies of CD34+ HSPCs obtained from multiple human donors carrying different types of X-SCID-causing mutations [87]. Beyond verifying the adequate engraftment of HSPCs edited by the HDR repair pathway for correction of the mutations, this model was also useful to demonstrate rescue of lymphopoiesis and thus validated this novel and precise gene correction strategy as treatment of X-SCID. In an alternative approach to assess safety and efficacy of gene editing based treatment for X-SCID, the Naldini group developed a new X-SCID mouse model derived from NSG mice by substituting the murine Il2rg locus with the human IL2RG counterpart that contained a disease-causing mutation [88]. These animals showed comparable immunophenotypical and histological phenotypes with NSG mice and enabled the validation of gene editing strategies that are directly translatable to the correction of human HSPCs. Notably, in this study, mouse HSPCs that were corrected for the human IL2RG gene rescued the mouse X-SCID defect indicating cross reactivity of the human γc chain function with other subunits of the mouse pathway.
Similar to ADA-SCID and X-SCID, gammaretroviral vectors were initially used and shown to be effective in 10 patients suffering from Wiskott-Aldrich syndrome (WAS), a primary immunodeficiency characterized by eczema, thrombocytopenia, infections, and a high risk of developing autoimmunity and cancer. However, long-term follow up studies showed expansion of clones with insertions in proto-oncogenes, some of which progressed to leukemias [89]. SIN vectors were subsequently generated for WAS and safety was assessed in primary transplantation experiments in WASP-deficient mice (BL6-wasnull) and also in secondary transplantation using a different background, the 129-wasnull mouse model, which has a shorter lifespan due to colitis exacerbation [90]. Additional preclinical data was also later generated from engraftment studies of lentiviral vector-modified human CD34+ from healthy and WAS patients in sublethally irradiated Rag2−/−/γc−/− neonate mice generated in the BALB/c background [91], which demonstrated a safe and polyclonal distribution of vector integration profile.
Hematopoietic stem cell gene therapy for hematological disorders.
Fanconi anemia (FA) is a hereditary disease characterized by cellular hypersensitivity to DNA crosslinking agents, resulting in BM failure and aplastic anemia during early childhood. Since more than half of FA patients demonstrate nucleotide mutations in the FANCA gene, therapies that deliver a corrected copy of the FANCA cDNA to HSCs have been developed and tested in a FANCA knockout model developed by Noll et al. [92], which was generated in both the 129S4 and C57/BL syngeneic background. This model recapitulates certain phenotypes of the human disease, such as sensitivity to genotoxic agents that cause DNA double-stranded cross-links such as mitomycin C, a potent DNA-damaging agent used to assess functionality of the DNA damage repair pathway. Other phenotypes such as anemia or tumor development have however not been reported in these mice. This model proved instrumental for the validation of viral vectors used for delivery of the corrected gene [93, 94] as well as for the establishment of short transduction protocols that promote engraftment of corrected HSPCs [95]. Complementary to these studies, NSG mice were used to test engraftment of transduced CD34+ cells obtained from Fanconi anemia patients and to determine if the gene therapy could restore in vivo repopulating activity as well as mitomycin C resistance in these cells [96]. This work ultimately provided protocols to successfully treat several FA patients using autologous lentiviral gene therapy in HSPCs with no prior conditioning [97].
Hematopoietic stem cell gene therapy for β-thalassemia/sickle cell disease.
Efforts to develop gene therapy treatments for hemoglobinopathies were initiated over 3 decades ago. β-hemoglobinopathies are the most common monogenic disorder worldwide that affect the normal production of adult hemoglobin due to mutations in the β-globin gene. The two most common diseases are β-thalassemia with low or absent β-globin production and sickle cell disease (SCD) with the production of a mutant form of β-globin causing polymerization of globin molecules and sickling of red blood cells. Pioneering work used a gamma-retroviral vector with an intact copy of the β-globin gene that was validated in transduction and transplantation of mouse BM cells [98]. The subsequent use of the newly discovered locus control region (LCR) for expression of the β-globin transgene [99] in conjunction with the development of safer lentiviral vector platforms helped overcome many of the initial limitations of retroviral vectors. Consequently, the first cell gene therapy trial in humans was performed in the early 2000s with results made public in 2010 [100].
This long journey towards clinical translation would have not been possible without decades of research in the mouse model. A number of models have been created over the years to closely recapitulate the human disease phenotype, which have been the subject of a recent review [101]. Transgenic methodologies permitted the introduction of the entire human β-globin locus while replacing the murine counterpart to mimic human globin gene expression. SCD models including the so-called Berkeley, Birmingham or San Francisco models were constructed and exhibited faithful sickle cell pathology. Fully humanized β-thalassemia strains were produced with different degrees of β-thalassemia intermedia named th1, th2 and th3. The latest model involving deletion of both the βmajor and βminor genes was particularly useful for the validation of lentiviral vectors used in the first human clinical trial. Transduction and primary/secondary transplantation of gene-modified BM cells in syngeneic, lethally irradiated C57BL/6 Hbbth3−/+ mice rescued anemia, abnormal red cell morphology and splenomegaly that characterize these animals [102]. Rescue of the disease phenotypes was also confirmed in homozygote Hbbth3−/−/th3 animals suffering from severe thalassemia [103]. Recently, non-viral gene editing strategies for the correction of the underlying disease-causing mutation or for the reactivation of fetal hemoglobin have been investigated in mouse models. In the majority of studies, NSG or NBSGW animals were employed to assess engraftment of HSPCs engineered using a strategy aimed at the correction of the SCD mutation [51, 104, 105], at inactivating the repressor of fetal hemoglobin BCL11A [106, 107], or at recapitulating mutations associated with hereditary persistence of hemoglobin [108]. In particular, Wu and colleagues demonstrated the engraftment and in vivo persistence of CD34+ HSPCs obtained from SCD patients and corrected for the mutation using CRISPR/Cas9-mediated HDR [105]. Together, these studies served as launching platform for several clinical trials such as CTX001, which started to enroll patients suffering from SCD and β-thalassemia in February 2019.
HSC-mediated gene therapy in humanized mice
While most HSC-mediated gene therapy protocols successfully transplant gene-modified cell products into adult mice to assess multilineage engraftment and safety, immunotherapy-centered strategies frequently face severe graft-versus-host disease (GvHD) symptoms in the existing mouse models due to HLA incompatibility between infused donor T cells and recipient cells. Maturation of either human T cells from gene-modified HSPCs or the direct infusion of human CAR T cells into immunocompromised and conditioned adult mice often results in GvHD-mediated death anywhere in between 14 to 60 days limiting the ability of this model to follow the cells long-term or even test their response to their target [109, 110].
The idea to mature human T cells in mice and increase tolerance in the host originates in the 1980s with McCune et al. surgically transplanting human fetal liver and thymus tissue fragments into SCID mice (hu-SCID). The human tissue supports the engraftment of human fetal liver HSPCs and the generation of functional T cells [4]. While this early model has been successfully used in multiple studies with the primary focus on HIV [111], hu-SCID mice lack the support for the development of normal adaptive immune responses of human T cells in vivo. To solve this problem, Lan et al. performed identical human tissue transplants in NOD/SCID mice demonstrating engraftment of a fully functional human immune system [112]. So-called BLT (BM, liver, thymus) mice and their derivatives were successfully used in countless studies predominantly associated with viral infections [111, 113–115]. However, BLT mice still developed GvHD symptoms and generation of this model is labor-intensive. A recent report replacing human tissues with biomaterial-based scaffolds, so called BM cryogel (BMC), was able to mitigate GvHD symptoms, enhanced the seeding of the murine thymus, and promoted a greater T cell repertoire diversity in the murine model [116].
Another promising strategy to circumvent limitations of classical mouse strains is to engraft human HSPCs into the fetal liver of neonatal NSG mice within the first 3 days post-birth. Human HSPCs home together with murine HSCs into the BM, human T cells develop and mature with constant exposure to the foreign environment, thus building tolerance and reducing GvHD symptoms [117]. Making this animal model especially attractive to HSC-mediated immunotherapy approaches, human HSPCs can be gene-modified before transplantation and HSC-derived CAR-T cells are stably produced throughout the lifetime of the animal inducing tolerance without causing unspecific and deadly side-effects. Once engrafted, these mice can be followed long-term, challenged with target cells (e.g. tumor cell lines, primary cancerous tissue) to verify efficacy. Mimicking autologous immunotherapy approaches in vivo matured CAR-T cells can even be transplanted into other syngeneic mice engrafted with human HSPCs from the same donor [117].
HSC-mediated immunotherapy approaches are currently far less frequently performed than regular T cell-based strategies and in most cases performed ex vivo [118, 119]. In comparison to T cell-based strategies, successful engraftment of gene-modified HSCs can provide a potentially life-long supply of T cell receptor (TCR)- or CAR-modified T lymphocytes. Similar to previously discussed gene therapy approaches, pre-evaluation of gene-modified HSPCs and the successful generation of engineered T cells is commonly analyzed in BLT [120, 121] and NSG mice [122]. Humanized mouse strains have not yet entered this field of research either. Instead, classical mouse strains such as the NSG strain are getting modified to achieve an HLA-restricted human immune response of in vivo HSC-derived T cell [123]. Other improvements for T cell function include the expression of human cytokines specifically for the development and maturation as well as the modification of the environment to closely mimic either lymphoid tissues or the tumor microenvironment as comprehensively reviewed before [124, 125].
Despite their promising features, genetically humanized mouse strains such as the NSGW41 and MISTRG are only slowly entering the field of HSC-mediated immunotherapy. Forward-looking, the field of immunotherapy is evolving significantly faster than other HSC-mediated gene therapy approaches discussed above and pioneering work in this field may actually help to facilitate the adaptation of novel humanized mouse models throughout the field of HSC gene therapy.
Limitations of the mouse model of HSC gene therapy
Immunocompromised and genetically engineered mouse strains have been extremely instrumental in the field of HSC gene therapy and editing. Easily outcompeting other USDA-covered species due to the ease of use, accessibility, cost, and availability of reagents, the mouse model has manifested its central role in the field. However, every model has its own limitations and not all questions can be addressed in the mouse. General differences in the physiology of humans and mice, incompatibility of several cytokines, and the relatively short lifespan still dampen the use of this model for some applications. For example, the lack or incomplete disease phenotype in some genetically engineered strains precludes the assessment of clinically-relevant levels of gene-modification required for a functional cure. Similarly, the lack of support for certain human lineages (particularly platelets and erythrocytes) in classical as well as more recently developed strains makes the research on hemoglobinopathies and thrombocytopenia less attractive.
However, a less frequently discussed and obvious limitation of the mouse model is actually disease phenotype-independent and associated with the lack of standardization. As a consequence, interpretation and especially comparison of data obtained from different research groups focusing on similar or even identical approaches is getting increasingly complicated due to the enormous variety of experimental parameters. Variables include different mouse strains (SCID, NOD/SCID, NOG, NSG, NSGS, NSGW41 MISTRG, etc.), modes of donor cell injection (in utero, intrahepatic, intravenous, intrafemoral), age of mice (neonatal, adult), human stem cell source (fetal liver, UCB, BM, granulocyte colony-stimulation factor [GCSF]-mobilized PB stem cells), cell dose, purity/composition/phenotype of the HSPC infusion product (CD34+, CD34+CD38low/- etc.), type/dose of conditioning (total body irradiation, chemical, none), duration of follow-up, mode of PB sampling (orbital sinus, submandibular), frequency of PB draws, engraftment acquisition in PB (staining panel, lineage coverage), tissue harvest for final necropsy (BM, spleen, thymus, liver, gut, lung), and performance of human cells in sequential transplants (secondary, tertiary).
In addition to these experimental parameters, assessment of human engraftment and interpretation of data has significantly changed in the last few decades. Affected by the discovery of novel hematopoietic progenitor cells and the revision of the classical model of human hematopoiesis, multilineage engraftment in transplanted mice seems to be not exclusively limited to HSCs [63, 126]. As demonstrated by Hogan et al., lineage-committed CD34+CD38+ human HSPCs from UCB show high levels of engraftment giving rise to lymphoid (CD19+) and myeloid (CD33+, CD13+, CD14+) cells in the PB [127]. This CD34 subset is further capable of lympho-myeloid, erythroid (CD45-CD71+CD235a+), and CD34+ engraftment in the BM up to 12 weeks post-transplant [127]. Confirming these findings, Majeti et al. reported robust myeloid, lymphoid, erythroid and megakaryocytic engraftment of “short-term” repopulating human multipotent progenitors (MPPs) from UCB (CD34+CD38-CD90-) with reduced but not fully absent secondary repopulation potential [21]. Furthermore, culture-expanded CD133+CD34+ HSPCs lacking erythro-megakaryocytic in vitro differentiation potential demonstrate robust lympho-myeloid differentiation as well as BM CD34-engraftment in sublethally irradiated NSG mice [128].
The described engraftment of human progenitor cells in the mouse model is actually contradictory to findings from autologous transplants performed in the nonhuman primates (NHPs), demonstrating that committed progenitor cells are not contributing to the short-term recovery after transplantation for more than a week [46, 47]. While the reason for this discrepancy remains unknown and research in this particular field is lacking, the support of engraftment for committed progenitor cells is particularly beneficial for studying the maturation of lymphoid and myeloid lineages that are otherwise complicated to generate from culture of human HSPCs ex vivo. Providing a less artificial environment, maturation of functional human blood cells is supported to a certain extend despite the incomplete cross-reactivity of several cytokines and signals between both species.
Closing this gap and complementing the features of the murine xenograft model, many gene therapy approaches are tested in large animal models such as the dog, swine, or NHP. Major advantages of large-animal models include the ability to perform autologous/allogeneic transplants with full multilineage support for HSC differentiation, the ability to track long-term engraftment over several years or even decades post-transplant, and an intact immune system for most models. In addition, closer genetic relatedness, physiology, size and HSC biology relative to humans, as well as the cross-reactivity of reagents offer unique opportunities for the translation of experimental and pre-clinical gene therapy protocols into actual treatment strategies and clinical applications [129, 130]. While these large animal models have successfully been used for the development of novel gene therapy strategies for multiple hematological diseases, they do not permit a high throughput, cost-efficient, and timely assessment of new gene therapy approaches. Financial limitations and lower availability of large animal models make researchers favor the mouse model at least for the initial screening of conditions that will later on advance into more elaborate testing within these models.
Outlook
Mouse models will very likely remain the primary and most frequently used in vivo read-out for the development of HSC-mediated gene therapy approaches. The ease of use, low cost, widespread availability, and multifaceted aspect of this model offer considerable advantages as compared to most large-animal models. Far less predictable is the use of specific strains and their future acceptance in the community. Despite the undisputable advantages of some genetically-humanized mouse strains, the transition proves to be surprisingly slow in the field of HSC gene therapy. While cross-breed strains combining various naturally occurring mutations into new strain backgrounds were easily accepted by researchers in the 90s and early 2000s, mutations generated by means of genetic engineering may require more thorough upfront validation.
Acknowledgments:
We thank Helen Crawford for help in preparing this manuscript and Margaret Cui for designing the figures.
Funding: This work has been supported by the grants R01 HL136135 and AI134953–01 from the NI H, Bethesda, MD. H.-P.K. is a Markey Molecular Medicine Investigator and received support as the inaugural recipient of the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research and the Fred Hutch Endowed Chair for Cell and Gene Therapy.
Footnotes
Competing interests: None
Data and materials availability: N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ito R, Takahashi T, Ito M, Humanized mouse models: Application to human diseases, J Cell Physiol (2017). [DOI] [PubMed] [Google Scholar]
- [2].Shultz LD, Ishikawa F, Greiner DL, Humanized mice in translational biomedical research, Nat Rev Immunol 7(2) (2007) 118–30. [DOI] [PubMed] [Google Scholar]
- [3].Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD, Humanized Mouse Models of Clinical Disease, Annu Rev Pathol 12 (2017) 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL, The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function, Science 241(4873) (1988) 1632–9. [DOI] [PubMed] [Google Scholar]
- [5].Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL, et al. , Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice, J Immunol 154(1) (1995) 180–91. [PubMed] [Google Scholar]
- [6].Larochelle A, Vormoor J, Lapidot T, Sher G, Furukawa T, Li Q, Shultz LD, Olivieri NF, Stamatoyannopoulos G, Dick JE, Engraftment of immune-deficient mice with primitive hematopoietic cells from beta-thalassemia and sickle cell anemia patients: implications for evaluating human gene therapy protocols, Human molecular genetics 4(2) (1995) 163–72. [DOI] [PubMed] [Google Scholar]
- [7].Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, Fischer A, A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency, N Engl J Med 348(3) (2003) 255–6. [DOI] [PubMed] [Google Scholar]
- [8].Miyoshi H, Smith KA, Mosier DE, Verma IM, Torbett BE, Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors, Science 283(5402) (1999) 682–6. [DOI] [PubMed] [Google Scholar]
- [9].Guenechea G, Gan OI, Inamitsu T, Dorrell C, Pereira DS, Kelly M, Naldini L, Dick JE, Transduction of human CD34+ CD38- bone marrow and cord blood-derived SCID-repopulating cells with third-generation lentiviral vectors, Molecular therapy : the journal of the American Society of Gene Therapy 1(6) (2000) 566–73. [DOI] [PubMed] [Google Scholar]
- [10].Radtke S, Haworth KG, Kiem HP, The frequency of multipotent CD133(+)CD45RA(−)CD34(+) hematopoietic stem cells is not increased in fetal liver compared with adult stem cell sources, Exp Hematol 44(6) (2016) 502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, Kaufmann KB, McLeod J, Laurenti E, Dunant CF, McPherson JD, Stein LD, Dror Y, Dick JE, Distinct routes of lineage development reshape the human blood hierarchy across ontogeny, Science (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, Csaszar E, Knapp DJ, Miller P, Ngom M, Imren S, Roy DC, Watts KL, Kiem HP, Herrington R, Iscove NN, Humphries RK, Eaves CJ, Cohen S, Marinier A, Zandstra PW, Sauvageau G, Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal, Science 345(6203) (2014) 1509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Masiuk KE, Brown D, Laborada J, Hollis RP, Urbinati F, Kohn DB, Improving Gene Therapy Efficiency through the Enrichment of Human Hematopoietic Stem Cells, Molecular therapy : the journal of the American Society of Gene Therapy (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zonari E, Desantis G, Petrillo C, Boccalatte FE, Lidonnici MR, Kajaste-Rudnitski A, Aiuti A, Ferrari G, Naldini L, Gentner B, Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy, Stem cell reports 8(4) (2017) 977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Humbert O, Laszlo GS, Sichel S, Ironside C, Haworth KG, Bates OM, Beddoe ME, Carrillo RR, Kiem HP, Walter RB, Engineering resistance to CD33-targeted immunotherapy in normal hematopoiesis by CRISPR/Cas9-deletion of CD33 exon 2, Leukemia 33(3) (2019) 762–808. [DOI] [PubMed] [Google Scholar]
- [16].Sugimura R, Jha DK, Han A, Soria-Valles C, da Rocha EL, Lu YF, Goettel JA, Serrao E, Rowe RG, Malleshaiah M, Wong I, Sousa P, Zhu TN, Ditadi A, Keller G, Engelman AN, Snapper SB, Doulatov S, Daley GQ, Haematopoietic stem and progenitor cells from human pluripotent stem cells, Nature 545(7655) (2017) 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lis R, Karrasch CC, Poulos MG, Kunar B, Redmond D, Duran JGB, Badwe CR, Schachterle W, Ginsberg M, Xiang J, Tabrizi AR, Shido K, Rosenwaks Z, Elemento O, Speck NA, Butler JM, Scandura JM, Rafii S, Conversion of adult endothelium to immunocompetent haematopoietic stem cells, Nature 545(7655) (2017) 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gori JL, Butler JM, Chan YY, Chandrasekaran D, Poulos MG, Ginsberg M, Nolan DJ, Elemento O, Wood BL, Adair JE, Rafii S, Kiem HP, Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells, The Journal of clinical investigation 125(3) (2015) 1243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B, Isolation of a candidate human hematopoietic stem-cell population, Proc Natl Acad Sci U S A 89(7) (1992) 2804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE, Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice, Proc Natl Acad Sci U S A 94(10) (1997) 5320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Majeti R, Park CY, Weissman IL, Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood, Cell Stem Cell 1(6) (2007) 635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE, Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment, Science 333(6039) (2011) 218–21. [DOI] [PubMed] [Google Scholar]
- [23].Peterson CW, Haworth KG, Burke BP, Polacino P, Norman KK, Adair JE, Hu SL, Bartlett JS, Symonds GP, Kiem HP, Multilineage polyclonal engraftment of Cal-1 gene-modified cells and in vivo selection after SHIV infection in a nonhuman primate model of AIDS, Molecular therapy. Methods & clinical development 3 (2016) 16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peterson CW, Wang J, Norman KK, Norgaard ZK, Humbert O, Tse CK, Yan JJ, Trimble RG, Shivak DA, Rebar EJ, Gregory PD, Holmes MC, Kiem HP, Long-term multilineage engraftment of autologous genome-edited hematopoietic stem cells in nonhuman primates, Blood 127(20) (2016) 2416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Exline CM, DeClercq JJ, Llewellyn GN, Hayward SB, Li PW, Shivak DA, Surosky RT, Gregory PD, Holmes MC, Cannon PM, Homology-driven genome editing in hematopoietic stem and progenitor cells using ZFN mRNA and AAV6 donors, Nat Biotechnol 33(12) (2015) 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Genovese P, Schiroli G, Escobar G, Di TT, Firrito C, Calabria A, Moi D, Mazzieri R, Bonini C, Holmes MC, Gregory PD, van der Burg M, Gentner B, Montini E, Lombardo A, Naldini L, Targeted genome editing in human repopulating haematopoietic stem cells, Nature 510(7504) (2014) 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Naldini L, Gene therapy returns to centre stage (Review), Nature 526(7573) (2015) 351–360. [DOI] [PubMed] [Google Scholar]
- [28].Morrison C, $1-million price tag set for Glybera gene therapy, Nat Biotechnol 33(3) (2015) 217–8. [DOI] [PubMed] [Google Scholar]
- [29].Melchiorri D, Pani L, Gasparini P, Cossu G, Ancans J, Borg JJ, Drai C, Fiedor P, Flory E, Hudson I, Leufkens HG, Muller-Berghaus J, Narayanan G, Neugebauer B, Pokrotnieks J, Robert JL, Salmonson T, Schneider CK, Regulatory evaluation of Glybera in Europe - two committees, one mission, Nat. Rev. Drug Discov. 12(9) (2013) 719. [DOI] [PubMed] [Google Scholar]
- [30].Baldo A, van den Akker E, Bergmans HE, Lim F, Pauwels K, General considerations on the biosafety of virus-derived vectors used in gene therapy and vaccination (Review), Current Gene Therapy 13(6) (2013) 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Basner-Tschakarjan E, Mingozzi F, Cell-mediated immunity to AAV vectors, evolving concepts and potential solutions (Review), Frontiers in Immunology 5 (2014) 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML, Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer, Molecular genetics and metabolism 80(1–2) (2003) 148–58. [DOI] [PubMed] [Google Scholar]
- [33].Hacein-Bey-Abina S, von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M, LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 [erratum appears in Science. 2003 Oct 24;302(5645):568], Science 302(5644) (2003) 415–419. [DOI] [PubMed] [Google Scholar]
- [34].Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, Schmidt M, Kramer A, Schwable J, Glimm H, Koehl U, Preiss C, Ball C, Martin H, Gohring G, Schwarzwaelder K, Hofmann WK, Karakaya K, Tchatchou S, Yang R, Reinecke P, Kuhlcke K, Schlegelberger B, Thrasher AJ, Hoelzer D, Seger R, von Kalle C, Grez M, Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease, Nature Medicine 16(2) (2010) 198–204. [DOI] [PubMed] [Google Scholar]
- [35].Braun CJ, Boztug K, Paruzynski A, Witzel M, Schwarzer A, Rothe M, Modlich U, Beier R, Gohring G, Steinemann D, Fronza R, Ball CR, Haemmerle R, Naundorf S, Kuhlcke K, Rose M, Fraser C, Mathias L, Ferrari R, Abboud MR, Al-Herz W, Kondratenko I, Marodi L, Glimm H, Schlegelberger B, Schambach A, Albert MH, Schmidt M, von Kalle C, Klein C, Gene therapy for Wiskott-Aldrich syndrome--long-term efficacy and genotoxicity, Science Translational Medicine 6(227) (2014) 227ra33. [DOI] [PubMed] [Google Scholar]
- [36].Braun CJ, Witzel M, Paruzynski A, Boztug K, von Kalle C, Schmidt M, Klein C, Gene therapy for Wiskott-Aldrich Syndrome-Long-term reconstitution and clinical benefits, but increased risk for leukemogenesis, Rare diseases 2(1) (2014) e947749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Radtke S, Humbert O, Kiem HP, Sorting Out the Best: Enriching Hematopoietic Stem Cells for Gene Therapy and Editing, Molecular therapy : the journal of the American Society of Gene Therapy 26(10) (2018) 2328–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Masiuk KE, Brown D, Laborada J, Hollis RP, Urbinati F, Kohn DB, Improving gene therapy efficiency through the enrichment of human hematopoietic stem cells, Mol Ther 25(9) (2017) 2163–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gordon PR, Leimig T, Babarin-Dorner A, Houston J, Holladay M, Mueller I, Geiger T, Handgretinger R, Large-scale isolation of CD133+ progenitor cells from G-CSF mobilized peripheral blood stem cells, Bone Marrow Transplant 31(1) (2003) 17–22. [DOI] [PubMed] [Google Scholar]
- [40].Radtke S, Adair JE, Giese MA, Chan YY, Norgaard ZK, Enstrom M, Haworth KG, Schefter LE, Kiem HP, A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates, Sci Transl Med 9(414) (2017) [Epub ahead of print; doi: 10.1126/scitranslmed.aan1145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Radtke S, Chan YY, Sippel TR, Kiem HP, Rongvaux A, MISTRG mice support engraftment and assessment of nonhuman primate hematopoietic stem and progenitor cells, Exp Hematol 70 (2019) 31–41 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tricot G, Gazitt Y, Leemhuis T, Jagannath S, Desikan KR, Siegel D, Fassas A, Tindle S, Nelson J, Juttner C, Tsukamoto A, Hallagan J, Atkinson K, Reading C, Hoffman R, Barlogie B, Collection, tumor contamination, and engraftment kinetics of highly purified hematopoietic progenitor cells to support high dose therapy in multiple myeloma, Blood 91(12) (1998) 4489–95. [PubMed] [Google Scholar]
- [43].Michallet M, Philip T, Philip I, Godinot H, Sebban C, Salles G, Thiebaut A, Biron P, Lopez F, Mazars P, Roubi N, Leemhuis T, Hanania E, Reading C, Fine G, Atkinson K, Juttner C, Coiffier B, Fiere D, Archimbaud E, Transplantation with selected autologous peripheral blood CD34+Thy1+ hematopoietic stem cells (HSCs) in multiple myeloma: impact of HSC dose on engraftment, safety, and immune reconstitution, Exp Hematol 28(7) (2000) 858–70. [DOI] [PubMed] [Google Scholar]
- [44].Negrin RS, Atkinson K, Leemhuis T, Hanania E, Juttner C, Tierney K, Hu WW, Johnston LJ, Shizurn JA, Stockerl-Goldstein KE, Blume KG, Weissman IL, Bower S, Baynes R, Dansey R, Karanes C, Peters W, Klein J, Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer, Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 6(3) (2000) 262–71. [DOI] [PubMed] [Google Scholar]
- [45].Vose JM, Bierman PJ, Lynch JC, Atkinson K, Juttner C, Hanania CE, Bociek G, Armitage JO, Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with recurrent indolent non-Hodgkin’s lymphoma, Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 7(12) (2001) 680–7. [DOI] [PubMed] [Google Scholar]
- [46].Humbert O, Radtke S, Samuelson C, Carrillo RR, Perez AM, Reddy SS, Lux C, Pattabhi S, Schefter LE, Negre O, Lee CM, Bao G, Adair JE, Peterson CW, Rawlings DJ, Scharenberg AM, Kiem HP, Therapeutically relevant engraftment of a CRISPR-Cas9-edited HSC-enriched population with HbF reactivation in nonhuman primates, Science translational medicine 11(503) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Radtke S, Adair JE, Giese MA, Chan YY, Norgaard ZK, Enstrom M, Haworth KG, Schefter LE, Kiem HP, A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates, Science translational medicine 9(414) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pietras EM, Warr MR, Passegue E, Cell cycle regulation in hematopoietic stem cells, The Journal of cell biology 195(5) (2011) 709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A, Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair, Cell 135(6) (2008) 1118–29. [DOI] [PubMed] [Google Scholar]
- [50].Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegue E, Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis, Cell Stem Cell 7(2) (2010) 174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].DeWitt MA, Magis W, Bray NL, Wang T, Berman JR, Urbinati F, Heo SJ, Mitros T, Munoz DP, Boffelli D, Kohn DB, Walters MC, Carroll D, Martin DI, Corn JE, Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells, Science translational medicine 8(360) (2016) 360ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ngom M, Imren S, Maetzig T, Adair JE, Knapp D, Chagraoui J, Fares I, Bordeleau ME, Sauvageau G, Leboulch P, Eaves C, Humphries RK, UM171 Enhances Lentiviral Gene Transfer and Recovery of Primitive Human Hematopoietic Cells, Molecular therapy. Methods & clinical development 10 (2018) 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Heffner GC, Bonner M, Christiansen L, Pierciey FJ, Campbell D, Smurnyy Y, Zhang W, Hamel A, Shaw S, Lewis G, Goss KA, Garijo O, Torbett BE, Horton H, Finer MH, Gregory PD, Veres G, Prostaglandin E2 Increases Lentiviral Vector Transduction Efficiency of Adult Human Hematopoietic Stem and Progenitor Cells, Molecular therapy : the journal of the American Society of Gene Therapy 26(1) (2018) 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Petrillo C, Cesana D, Piras F, Bartolaccini S, Naldini L, Montini E, Kajaste-Rudnitski A, Cyclosporin a and rapamycin relieve distinct lentiviral restriction blocks in hematopoietic stem and progenitor cells, Molecular therapy : the journal of the American Society of Gene Therapy 23(2) (2015) 352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Canny MD, Moatti N, Wan LCK, Fradet-Turcotte A, Krasner D, Mateos-Gomez PA, Zimmermann M, Orthwein A, Juang YC, Zhang W, Noordermeer SM, Seclen E, Wilson MD, Vorobyov A, Munro M, Ernst A, Ng TF, Cho T, Cannon PM, Sidhu SS, Sicheri F, Durocher D, Inhibition of 53BP1 favors homology-dependent DNA repair and increases CRISPR-Cas9 genome-editing efficiency, Nat Biotechnol 36(1) (2018) 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Beyer AI, Muench MO, Comparison of Human Hematopoietic Reconstitution in Different Strains of Immunodeficient Mice, Stem Cells Dev 26(2) (2017) 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, Burzenski L, Gott B, Foreman O, Kavirayani A, Herlihy M, Rossini AA, Shultz LD, Greiner DL, Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation, Clin Immunol 135(1) (2010) 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tanaka S, Saito Y, Kunisawa J, Kurashima Y, Wake T, Suzuki N, Shultz LD, Kiyono H, Ishikawa F, Development of mature and functional human myeloid subsets in hematopoietic stem cell-engrafted NOD/SCID/IL2rgammaKO mice, J Immunol 188(12) (2012) 6145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gille C, Orlikowsky TW, Spring B, Hartwig UF, Wilhelm A, Wirth A, Goecke B, Handgretinger R, Poets CF, Andre MC, Monocytes derived from humanized neonatal NOD/SCID/IL2Rgamma(null) mice are phenotypically immature and exhibit functional impairments, Human immunology 73(4) (2012) 346–54. [DOI] [PubMed] [Google Scholar]
- [60].Mazurier F, Doedens M, Gan OI, Dick JE, Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells, Nat Med 9(7) (2003) 959–63. [DOI] [PubMed] [Google Scholar]
- [61].Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP, IL-15 trans-presentation promotes human NK cell development and differentiation in vivo, J Exp Med 206(1) (2009) 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Strowig T, Chijioke O, Carrega P, Arrey F, Meixlsperger S, Ramer PC, Ferlazzo G, Munz C, Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence, Blood 116(20) (2010) 4158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Görgens A, Radtke S, Horn PA, Giebel B, New relationships of human hematopoietic lineages facilitate detection of multipotent hematopoietic stem and progenitor cells, Cell Cycle 12(22) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA, Development and function of human innate immune cells in a humanized mouse model, Nat Biotechnol 32(4) (2014) 364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS, Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells, Nat Immunol 8(12) (2007) 1313–23. [DOI] [PubMed] [Google Scholar]
- [66].Chen Q, Khoury M, Chen J, Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice, Proc Natl Acad Sci U S A 106(51) (2009) 21783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sippel TR, Radtke S, Olsen TM, Kiem HP, Rongvaux A, Human hematopoietic stem cell maintenance and myeloid cell development in next-generation humanized mouse models, Blood Adv 3(3) (2019) 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wunderlich M, Chou FS, Link KA, Mizukawa B, Perry RL, Carroll M, Mulloy JC, AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3, Leukemia 24(10) (2010) 1785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cosgun KN, Rahmig S, Mende N, Reinke S, Hauber I, Schafer C, Petzold A, Weisbach H, Heidkamp G, Purbojo A, Cesnjevar R, Platz A, Bornhauser M, Schmitz M, Dudziak D, Hauber J, Kirberg J, Waskow C, Kit regulates HSC engraftment across the human-mouse species barrier, Cell Stem Cell 15(2) (2014) 227–38. [DOI] [PubMed] [Google Scholar]
- [70].Horn PA, Thomasson BM, Wood BL, Andrews RG, Morris JC, Kiem HP, Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice compared with nonhuman primates, Blood 102(13) (2003) 4329–35. [DOI] [PubMed] [Google Scholar]
- [71].Richter M, Stone D, Miao C, Humbert O, Kiem HP, Papayannopoulou T, Lieber A, In Vivo Hematopoietic Stem Cell Transduction, Hematol Oncol Clin North Am 31(5) (2017) 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang H, Georgakopoulou A, Psatha N, Li C, Capsali C, Samal HB, Anagnostopoulos A, Ehrhardt A, Izsvak Z, Papayannopoulou T, Yannaki E, Lieber A, In vivo hematopoietic stem cell gene therapy ameliorates murine thalassemia intermedia, The Journal of clinical investigation 129(2) (2019) 598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C, Etzioni A, Holland SM, Klein C, Nonoyama S, Ochs HD, Oksenhendler E, Puck JM, Sullivan KE, Tang ML, Franco JL, Gaspar HB, Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015, Journal of clinical immunology 35(8) (2015) 696–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Tang MLK, Tangye SG, Torgerson TR, Casanova JL, Sullivan KE, The 2017 IUIS Phenotypic Classification for Primary Immunodeficiencies, Journal of clinical immunology 38(1) (2018) 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].King JR, Hammarstrom L, Newborn Screening for Primary Immunodeficiency Diseases: History, Current and Future Practice, Journal of clinical immunology 38(1) (2018) 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, Ugazio AG, Mavilio F, Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients, Science 270(5235) (1995) 470–5. [DOI] [PubMed] [Google Scholar]
- [77].Ferrari G, Rossini S, Nobili N, Maggioni D, Garofalo A, Giavazzi R, Mavilio F, Bordignon C, Transfer of the ADA gene into human ADA-deficient T lymphocytes reconstitutes specific immune functions, Blood 80(5) (1992) 1120–4. [PubMed] [Google Scholar]
- [78].Ferrari G, Rossini S, Giavazzi R, Maggioni D, Nobili N, Soldati M, Ungers G, Mavilio F, Gilboa E, Bordignon C, An in vivo model of somatic cell gene therapy for human severe combined immunodeficiency, Science 251(4999) (1991) 1363–6. [DOI] [PubMed] [Google Scholar]
- [79].Blackburn MR, Datta SK, Kellems RE, Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency, The Journal of biological chemistry 273(9) (1998) 5093–100. [DOI] [PubMed] [Google Scholar]
- [80].Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR, A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice, J Immunol 173(2) (2004) 1380–9. [DOI] [PubMed] [Google Scholar]
- [81].Mortellaro A, Hernandez RJ, Guerrini MM, Carlucci F, Tabucchi A, Ponzoni M, Sanvito F, Doglioni C, Di Serio C, Biasco L, Follenzi A, Naldini L, Bordignon C, Roncarolo MG, Aiuti A, Ex vivo gene therapy with lentiviral vectors rescues adenosine deaminase (ADA)-deficient mice and corrects their immune and metabolic defects, Blood 108(9) (2006) 2979–88. [DOI] [PubMed] [Google Scholar]
- [82].Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M, Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1, The Journal of clinical investigation 118(9) (2008) 3132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K, Chatters SJ, de Ridder D, Gilmour KC, Adams S, Thornhill SI, Parsley KL, Staal FJ, Gale RE, Linch DC, Bayford J, Brown L, Quaye M, Kinnon C, Ancliff P, Webb DK, Schmidt M, von Kalle C, Gaspar HB, Thrasher AJ, Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients, The Journal of clinical investigation 118(9) (2008) 3143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhou S, Mody D, DeRavin SS, Hauer J, Lu T, Ma Z, Hacein-Bey Abina S, Gray JT, Greene MR, Cavazzana-Calvo M, Malech HL, Sorrentino BP, A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LMO2 expression in human T cells, Blood 116(6) (2010) 900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A, Di Serio C, Doglioni C, von Kalle C, Naldini L, Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration, Nat Biotechnol 24(6) (2006) 687–96. [DOI] [PubMed] [Google Scholar]
- [86].Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, Benedicenti F, Sergi LS, Ambrosi A, Ponzoni M, Doglioni C, Di Serio C, von Kalle C, Naldini L, The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy, The Journal of clinical investigation 119(4) (2009) 964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pavel-Dinu M, Wiebking V, Dejene BT, Srifa W, Mantri S, Nicolas CE, Lee C, Bao G, Kildebeck EJ, Punjya N, Sindhu C, Inlay MA, Saxena N, DeRavin SS, Malech H, Roncarolo MG, Weinberg KI, Porteus MH, Gene correction for SCID-X1 in long-term hematopoietic stem cells, Nature communications 10(1) (2019) 1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Schiroli G, Ferrari S, Conway A, Jacob A, Capo V, Albano L, Plati T, Castiello MC, Sanvito F, Gennery AR, Bovolenta C, Palchaudhuri R, Scadden DT, Holmes MC, Villa A, Sitia G, Lombardo A, Genovese P, Naldini L, Preclinical modeling highlights the therapeutic potential of hematopoietic stem cell gene editing for correction of SCID-X1, Science translational medicine 9(411) (2017). [DOI] [PubMed] [Google Scholar]
- [89].Braun CJ, Boztug K, Paruzynski A, Witzel M, Schwarzer A, Rothe M, Modlich U, Beier R, Gohring G, Steinemann D, Fronza R, Ball CR, Haemmerle R, Naundorf S, Kuhlcke K, Rose M, Fraser C, Mathias L, Ferrari R, Abboud MR, Al-Herz W, Kondratenko I, Marodi L, Glimm H, Schlegelberger B, Schambach A, Albert MH, Schmidt M, von Kalle C, Klein C, Gene therapy for Wiskott-Aldrich syndrome--long-term efficacy and genotoxicity, Science translational medicine 6(227) (2014) 227ra33. [DOI] [PubMed] [Google Scholar]
- [90].Marangoni F, Bosticardo M, Charrier S, Draghici E, Locci M, Scaramuzza S, Panaroni C, Ponzoni M, Sanvito F, Doglioni C, Liabeuf M, Gjata B, Montus M, Siminovitch K, Aiuti A, Naldini L, Dupre L, Roncarolo MG, Galy A, Villa A, Evidence for long-term efficacy and safety of gene therapy for Wiskott-Aldrich syndrome in preclinical models, Molecular therapy : the journal of the American Society of Gene Therapy 17(6) (2009) 1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Scaramuzza S, Biasco L, Ripamonti A, Castiello MC, Loperfido M, Draghici E, Hernandez RJ, Benedicenti F, Radrizzani M, Salomoni M, Ranzani M, Bartholomae CC, Vicenzi E, Finocchi A, Bredius R, Bosticardo M, Schmidt M, von Kalle C, Montini E, Biffi A, Roncarolo MG, Naldini L, Villa A, Aiuti A, Preclinical safety and efficacy of human CD34(+) cells transduced with lentiviral vector for the treatment of Wiskott-Aldrich syndrome, Molecular therapy : the journal of the American Society of Gene Therapy 21(1) (2013) 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Noll M, Battaile KP, Bateman R, Lax TP, Rathbun K, Reifsteck C, Bagby G, Finegold M, Olson S, Grompe M, Fanconi anemia group A and C double-mutant mice: functional evidence for a multi-protein Fanconi anemia complex, Exp Hematol 30(7) (2002) 679–88. [DOI] [PubMed] [Google Scholar]
- [93].Rio P, Segovia JC, Hanenberg H, Casado JA, Martinez J, Gottsche K, Cheng NC, Van de Vrugt HJ, Arwert F, Joenje H, Bueren JA, In vitro phenotypic correction of hematopoietic progenitors from Fanconi anemia group A knockout mice, Blood 100(6) (2002) 2032–9. [PubMed] [Google Scholar]
- [94].Adair JE, Zhao X, Chien S, Fang M, Wohlfahrt ME, Trobridge GD, Taylor JA, Beard BC, Kiem HP, Becker PS, Cyclophosphamide promotes engraftment of gene-modified cells in a mouse model of Fanconi anemia without causing cytogenetic abnormalities, J Mol Med (Berl) 90(11) (2012) 1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Muller LU, Milsom MD, Kim MO, Schambach A, Schuesler T, Williams DA, Rapid lentiviral transduction preserves the engraftment potential of Fanca(−/−) hematopoietic stem cells, Molecular therapy : the journal of the American Society of Gene Therapy 16(6) (2008) 1154–60. [DOI] [PubMed] [Google Scholar]
- [96].Rio P, Navarro S, Guenechea G, Sanchez-Dominguez R, Lamana ML, Yanez R, Casado JA, Mehta PA, Pujol MR, Surralles J, Charrier S, Galy A, Segovia JC, Diaz de Heredia C, Sevilla J, Bueren JA, Engraftment and in vivo proliferation advantage of gene-corrected mobilized CD34(+) cells from Fanconi anemia patients, Blood 130(13) (2017) 1535–1542. [DOI] [PubMed] [Google Scholar]
- [97].Rio P, Navarro S, Wang W, Sanchez-Dominguez R, Pujol RM, Segovia JC, Bogliolo M, Merino E, Wu N, Salgado R, Lamana ML, Yanez RM, Casado JA, Gimenez Y, Roman-Rodriguez FJ, Alvarez L, Alberquilla O, Raimbault A, Guenechea G, Lozano ML, Cerrato L, Hernando M, Galvez E, Hladun R, Giralt I, Barquinero J, Galy A, Garcia de Andoin N, Lopez R, Catala A, Schwartz JD, Surralles J, Soulier J, Schmidt M, Diaz de Heredia C, Sevilla J, Bueren JA, Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia, Nat Med 25(9) (2019) 1396–1401. [DOI] [PubMed] [Google Scholar]
- [98].Dzierzak EA, Papayannopoulou T, Mulligan RC, Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells, Nature 331(6151) (1988) 35–41. [DOI] [PubMed] [Google Scholar]
- [99].Tuan DY, Solomon WB, London IM, Lee DP, An erythroid-specific, developmental-stage-independent enhancer far upstream of the human “beta-like globin” genes, Proc Natl Acad Sci U S A 86(8) (1989) 2554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R, Maouche-Chretien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ, Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B, Gourmel B, Socie G, Chretien S, Cartier N, Aubourg P, Fischer A, Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F, Hacein-Bey-Abina S, Leboulch P, Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia, Nature 467(7313) (2010) 318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Parker MP, Peterson KR, Mouse Models of Erythropoiesis and Associated Diseases, Methods Mol Biol 1698 (2018) 37–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, Sadelain M, Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin, Nature 406(6791) (2000) 82–6. [DOI] [PubMed] [Google Scholar]
- [103].Rivella S, May C, Chadburn A, Riviere I, Sadelain M, A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer, Blood 101(8) (2003) 2932–9. [DOI] [PubMed] [Google Scholar]
- [104].Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, Nicolas CE, Pavel-Dinu M, Saxena N, Wilkens AB, Mantri S, Uchida N, Hendel A, Narla A, Majeti R, Weinberg KI, Porteus MH, CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells, Nature 539(7629) (2016) 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Pattabhi S, Lotti SN, Berger MP, Singh S, Lux CT, Jacoby K, Lee C, Negre O, Scharenberg AM, Rawlings DJ, In Vivo Outcome of Homology-Directed Repair at the HBB Gene in HSC Using Alternative Donor Template Delivery Methods, Mol Ther Nucleic Acids 17 (2019) 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chang KH, Smith SE, Sullivan T, Chen K, Zhou Q, West JA, Liu M, Liu Y, Vieira BF, Sun C, Hong VP, Zhang M, Yang X, Reik A, Urnov FD, Rebar EJ, Holmes MC, Danos O, Jiang H, Tan S, Long-Term Engraftment and Fetal Globin Induction upon BCL11A Gene Editing in Bone-Marrow-Derived CD34(+) Hematopoietic Stem and Progenitor Cells, Molecular therapy. Methods & clinical development 4 (2017) 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wu Y, Zeng J, Roscoe BP, Liu P, Yao Q, Lazzarotto CR, Clement K, Cole MA, Luk K, Baricordi C, Shen AH, Ren C, Esrick EB, Manis JP, Dorfman DM, Williams DA, Biffi A, Brugnara C, Biasco L, Brendel C, Pinello L, Tsai SQ, Wolfe SA, Bauer DE, Highly efficient therapeutic gene editing of human hematopoietic stem cells, Nat Med 25(5) (2019) 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lux CT, Pattabhi S, Berger M, Nourigat C, Flowers DA, Negre O, Humbert O, Yang JG, Lee C, Jacoby K, Bernstein I, Kiem HP, Scharenberg A, Rawlings DJ, TALEN-Mediated Gene Editing of HBG in Human Hematopoietic Stem Cells Leads to Therapeutic Fetal Hemoglobin Induction, Molecular therapy. Methods & clinical development 12 (2019) 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser AD, Pouw N, Debets R, Kieback E, Uckert W, Song JY, Haanen JB, Schumacher TN, Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy, Nat Med 16(5) (2010) 565–70, 1p following 570. [DOI] [PubMed] [Google Scholar]
- [110].Covassin L, Laning J, Abdi R, Langevin DL, Phillips NE, Shultz LD, Brehm MA, Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rgamma(null) H2-Ab1 (tm1Gru) Tg (human leucocyte antigen D-related 4) mice: a mouse model of human allogeneic graft-versus-host disease, Clinical and experimental immunology 166(2) (2011) 269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Karpel ME, Boutwell CL, Allen TM, BLT humanized mice as a small animal model of HIV infection, Curr Opin Virol 13 (2015) 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lan P, Tonomura N, Shimizu A, Wang S, Yang YG, Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation, Blood 108(2) (2006) 487–92. [DOI] [PubMed] [Google Scholar]
- [113].Smith DJ, Lin LJ, Moon H, Pham AT, Wang X, Liu S, Ji S, Rezek V, Shimizu S, Ruiz M, Lam J, Janzen DM, Memarzadeh S, Kohn DB, Zack JA, Kitchen SG, An DS, Yang L, Propagating Humanized BLT Mice for the Study of Human Immunology and Immunotherapy, Stem Cells Dev 25(24) (2016) 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Frias-Staheli N, Dorner M, Marukian S, Billerbeck E, Labitt RN, Rice CM, Ploss A, Utility of humanized BLT mice for analysis of dengue virus infection and antiviral drug testing, Journal of virology 88(4) (2014) 2205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang LX, Kang G, Kumar P, Lu W, Li Y, Zhou Y, Li Q, Wood C, Humanized-BLT mouse model of Kaposi’s sarcoma-associated herpesvirus infection, Proc Natl Acad Sci U S A 111(8) (2014) 3146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Shah NJ, Mao AS, Shih TY, Kerr MD, Sharda A, Raimondo TM, Weaver JC, Vrbanac VD, Deruaz M, Tager AM, Mooney DJ, Scadden DT, An injectable bone marrow-like scaffold enhances T cell immunity after hematopoietic stem cell transplantation, Nat Biotechnol 37(3) (2019) 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Haworth KG, Ironside C, Norgaard ZK, Obenza WM, Adair JE, Kiem HP, In Vivo Murine-Matured Human CD3(+) Cells as a Preclinical Model for T Cell-Based Immunotherapies, Molecular therapy. Methods & clinical development 6 (2017) 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gschweng E, De Oliveira S, Kohn DB, Hematopoietic stem cells for cancer immunotherapy, Immunological reviews 257(1) (2014) 237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].De La Rochere P, Guil-Luna S, Decaudin D, Azar G, Sidhu SS, Piaggio E, Humanized Mice for the Study of Immuno-Oncology, Trends Immunol 39(9) (2018) 748–763. [DOI] [PubMed] [Google Scholar]
- [120].Kitchen SG, Bennett M, Galic Z, Kim J, Xu Q, Young A, Lieberman A, Joseph A, Goldstein H, Ng H, Yang O, Zack JA, Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice, PLoS One 4(12) (2009) e8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Vatakis DN, Koya RC, Nixon CC, Wei L, Kim SG, Avancena P, Bristol G, Baltimore D, Kohn DB, Ribas A, Radu CG, Galic Z, Zack JA, Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells, Proc Natl Acad Sci U S A 108(51) (2011) E1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kitchen SG, Levin BR, Bristol G, Rezek V, Kim S, Aguilera-Sandoval C, Balamurugan A, Yang OO, Zack JA, In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells, PLoS pathogens 8(4) (2012) e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, Yasukawa M, Ishikawa F, Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice, Proc Natl Acad Sci U S A 107(29) (2010) 13022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Carrillo MA, Zhen A, Kitchen SG, The Use of the Humanized Mouse Model in Gene Therapy and Immunotherapy for HIV and Cancer, Frontiers in immunology 9 (2018) 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Morton JJ, Bird G, Refaeli Y, Jimeno A, Humanized Mouse Xenograft Models: Narrowing the Tumor-Microenvironment Gap, Cancer Res 76(21) (2016) 6153–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Horn PA, Kiem HP, Expansion of SCID repopulating cells does not prove expansion of hematopoietic stem cells, Blood 108(2) (2006) 771; author reply 771–2. [DOI] [PubMed] [Google Scholar]
- [127].Hogan CJ, Shpall EJ, Keller G, Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice, Proc Natl Acad Sci U S A 99(1) (2002) 413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gorgens A, Radtke S, Mollmann M, Cross M, Durig J, Horn PA, Giebel B, Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages, Cell reports 3(5) (2013) 1539–52. [DOI] [PubMed] [Google Scholar]
- [129].Trobridge GD, Kiem HP, Large animal models of hematopoietic stem cell gene therapy, Gene therapy 17(8) (2010) 939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Larochelle A, Dunbar CE, Genetic manipulation of hematopoietic stem cells, Seminars in hematology 41(4) (2004) 257–71. [DOI] [PubMed] [Google Scholar]