Abstract
Objective.
Massive weight loss leads to marked knee pain reduction in persons with knee pain, but the reason for the reduction in pain is unknown. To quantify the contribution of MRI changes in pain sensitive structures, bone marrow lesions (BML), synovitis, and in pain sensitization, or depressive symptoms to knee pain improvement after substantial weight loss.
Methods.
Morbidly obese patients with knee pain on most days were evaluated before bariatric surgery or medical weight management and at 1-year follow-up for BML and synovitis seen on MRI, pressure pain threshold (PPT) at patella and right wrist, depressive symptoms (using CES-D), and WOMAC pain survey. Natural effects models quantified the extent that achieving minimal clinically important difference (MCID) of ≥18% on the WOMAC pain scale could be mediated by weight loss-induced changes in BML, synovitis, PPT, and depressive symptoms.
Results.
Of 75 participants, 53.3% lost ≥20% weight by 1-year; of these, 75% attained an MCID for pain improvement, compared with 34.3% in those who had <20% weight loss. Mediation analyses suggested that, in those with at least 20% weight loss, the odds of pain improvement increased by 62%, 15%, and 22% through changes in patella PPT, wrist PPT, and CES-D, respectively, but pain improvement was not mediated by MRI changes in BMLs or synovitis.
Conclusion.
Weight loss-induced knee pain improvement is partially mediated by changes in pain sensitization and depressive symptoms, but is independent of MRI changes in BML and synovitis.
INTRODUCTION
Osteoarthritis (OA) is the most common cause of arthritis, which is estimated to affect 91.2 million adults in the United States (1), making OA a leading cause of disability. While pain is the most prominent cause of OA-related disability, the association between pain and structural features of joints affected by OA is incompletely understood. These structural features often include bone marrow lesions (BML) and synovitis visualized by magnetic resonance imaging (MRI). Some studies have suggested an association of pain with BML (2–8) and synovitis (4,7–10), while others have suggested no association (11–14). In addition to structural features, there are reports of other factors affecting knee pain. For example, pain sensitization is associated with pain severity in knee OA (15), and a recent study suggested an improvement in pain sensitization following bariatric surgery (16). Further, sustained depressive symptoms that often co-occur with OA (17) have been reported to be associated with pain severity (18).
Obesity is a major risk factor for OA (19), and is itself also associated with pain and depressive symptoms. Several studies have reported considerable improvement in knee pain following bariatric surgery (16,20,21), which causes substantial weight loss. However, no study to our knowledge has evaluated to what extent changes in BML, synovitis, pain sensitization, or depressive symptoms contribute to pain improvement in persons who experience substantial weight loss. A cause-specific assessment for the pain improvement would provide insights into causes of knee pain in obese persons and might provide clues to effective pain-relieving treatments.
Our study focuses on BML and synovitis assessed by longitudinal MRI evaluations, pain sensitization, and depressive symptoms, measured pre- and post-intervention, in morbidly obese persons who experienced substantial weight loss after bariatric surgery or medical weight management. Specifically, we quantified the extent to which changes in BML, synovitis, pain sensitization, or depressive symptoms that may occur with substantial weight loss could mediate the effect of weight loss on knee pain improvement.
METHODS
Setting.
Study participants were recruited from the Nutrition and Weight Management Center at Boston Medical Center in Boston, Massachusetts. The Institutional Review Board at the Boston University Medical Campus approved the study protocol. All study participants provided written informed consent.
The eligible participants included obese patients between 25–60 years of age and a body mass index (BMI) ≥35 kg/m2 with a weight-related comorbidity or BMI of ≥40 kg/m2. To be eligible for our study, subjects additionally had to have knee pain on most days of the past month and had to be able to undergo a knee MRI evaluation. If both knees were affected, the more painful knee was selected for the MRI. Patients with a history of knee surgery or inflammatory arthritis were not eligible.
Following the baseline assessment, study participants received either medical weight management or bariatric surgery treatments. The medical management group received dietary recommendations with or without prescription medications that included phentermine with or without topiramate, lorcaserin, bupropion with or without naltrexone, or liraglutide. The dietary guidance consisted a 1200–1500 and 1500–1800 kilocalories/day high-protein low-fat diets with or without meal replacements for women and men, respectively. The bariatric surgery treatment was either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy. Both treatment groups received the exercise recommendation of a minimum 30-minute walk per day in addition to resistance exercise twice weekly. The follow-up study visit occurred approximately one year after bariatric surgery or the baseline visit in the medical weight management group.
Measurements.
All study participants underwent 3T MRI by Philips Achieva and pulse sequences including a sagittal WATSc and 3D fat saturation SPAIR sequences. Semi-quantitative MRI assessments consisted of baseline and follow-up image paired readings by an experienced musculoskeletal radiologist using MRI Osteoarthritis Knee Score (MOAKS) for BML and synovitis. The reader was blinded to treatment received and weight loss experienced. For BML and synovitis in a knee, we tested both maximum (i.e., worst) and sum of scores for BML and synovitis, as described in previous work (22).
Pain sensitization was assessed by measuring pressure pain thresholds (PPT) with a hand-held algometer applied at a rate of 0.5kg/second as the point at which the pressure first changed to slight pain. PPT was obtained at the index patella and right wrist. Three consecutive PPT measurements were averaged. Low patella and wrist PPT are suggestive of greater pain sensitization. Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression (CES-D) scale (23).
Knee-specific pain severity was assessed with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale (24). We defined the minimal clinically important difference (MCID) for WOMAC pain as a reduction of 18% or more in the WOMAC pain subscale score, as described in Angst et al (25).
Analytic approach.
Characteristics of the study sample were represented by frequencies for binary and categorical covariates and distribution summaries for continuous covariates. As in prior reports (22) and based on a cutoff that represented approximately the median distribution of weight loss, these descriptive statistics were stratified based on whether participant lost ≥20% weight by the 1-year follow-up visit. Changes in mean PPT for the patella and wrist, and mean CES-D changes between baseline and one-year follow-up were evaluated by linear mixed-effects models with random-effects specified for subjects to account for the correlation due to repeated measurements on the same knees across visits (26).
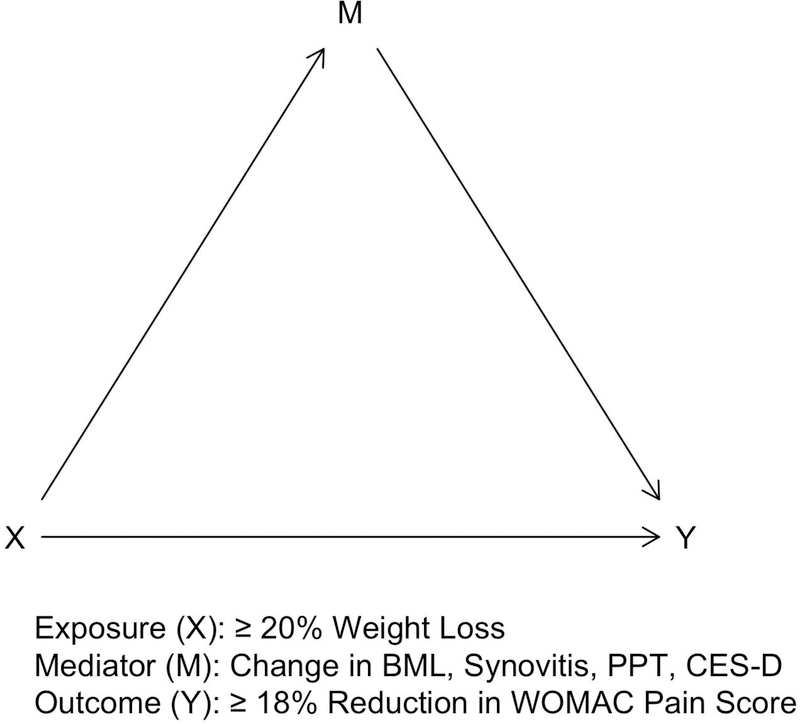
We performed causal mediation analysis to quantify the extent that specific causal pathways allow weight loss to exert its effects on pain improvement. These potential causal pathways included an indirect effect, that is the extent of pain improvement attributable to weight loss-induced changes in BML, synovitis, patella and wrist PPT, and CES-D score (Figure 1). The direct effect quantified any remaining effect not mediated by changes in BML, synovitis, patella and wrist PPT, and CES-D score (Figure 1).
Figure 1.
Directed acyclic graph (DAG) representing the natural indirect effect (that is mediated by changes in bone marrow lesion, synovitis, patella and wrist sensitization, or depressive symptoms; X to Y pathway through M) and the natural direct effect (that is not mediated; X to Y pathway not passing through M) of weight loss on knee pain improvement.
BML, bone marrow lesion; PPT, pressure pain threshold; CES-D, Center for Epidemiologic Studies Depression scale
To estimate indirect and direct effects, we fit a natural-effects model (27), which is a flexible and robust method to decompose the overall total effect into specific potential causal pathways, without reliance on a restrictive linear parametric statistical model (28). These indirect and direct effects are defined based on the counterfactual framework for causal inference, that is, the effect that would have been observed if the exposure or mediator were absent in contrast to the observed data (29).
Mediated interaction.
We assessed mediated interaction, that is, the effect modification of natural effects between the exposure, weight loss, and the mediators such that we assessed whether weight loss and BML, synovitis, patella or wrist PPT, or CES-D score interact in their effect on pain improvement. In this effect decomposition, both indirect and direct effects are decomposed into pure indirect and pure direct effects in addition to the interactive effect as described in VanderWeele (30).
Interpretation of natural effects.
Modern causal mediation analysis approaches rely on modeling techniques to estimate mediated/indirect effects under the counterfactual framework (for technical details see Shpitser and Tchetgen (31)). These sets of parametric models or non-parametric (i.e. model-free) approaches allow an estimate of effect by changing treatment assignment (e.g., exposure level of ≥20% and <20% weight loss) along specific pathways, but not along other pathways. A natural-effects model is one such approach that allows for model-free decomposition of exposure effect into indirect and direct effects. Identifying indirect and direct effects depends on three causal assumptions, exchangeability (e.g.., those with ≥20% weight loss, had they lost <20% weight, would have experienced the same average outcome as those with <20% weight loss), consistency (e.g., the observed outcome for each subject with ≥20% weight loss equals the counterfactual outcome if the subject lost ≥20% weight, and same for those with <20% weight loss), and positivity (e.g.., there is a non-zero positive probability that each subject could lose ≥20% or <20% weight). Consequently, this counterfactual framework under which causal mediating effects are estimated does not rely on other assumptions such as testing the statistical significance of the association between mediators and exposure (i.e., weight loss).
The natural direct effect was defined by Robins and Greenland (32) as the expected effect of an exposure on an outcome, while retaining the mediator constant at the level that would have naturally occurred in the absence of the exposure. The natural indirect effect provides an estimate for the effect of an exposure on an outcome if all subjects were exposed by setting the mediator to a level that would have occurred in the absence of the exposure. Note that in estimating the natural indirect effect, the exposure status remains constant, whereas the mediator level is held constant in the natural direct effect estimate (28).
Computation.
We used the imputation-based approach developed by Vansteelandt et al (33) to estimate the parameters of the natural-effects model. In this approach, no model is specified for the distribution of mediator; thus, unlike a competing approach using weighting, it is not sensitive to the potential misspecification of the mediator model especially if the mediator is continuous (34). Nonetheless, in a sensitivity analysis, we calculated our effect measures using both imputation- and weighting-based approaches. Effects measures for our binary outcome were expressed as odds ratios (OR) and the corresponding confidence intervals (CI) that were calculated by bootstrap techniques. All analyses were implemented in R software version 3.4.0 (35) and the medflex library version 0.6–1 (36).
Sensitivity analyses.
We tested weight loss of ≥20% vs. <20% in our primary analyses. We additionally carried out sensitivity analyses using a threshold of ≥10% vs. <10% for weight loss and another analysis in which we limited the analyses to those with BML or synovitis at baseline as well as a sensitivity analysis where we compared mediating effects in bariatric surgery patients vs. those in medical weight management group regardless of percent weight loss.
RESULTS
Baseline data.
Baseline characteristics of the study participants are presented in Table 1, and consisted of 75 participants, 63% (47/75) from the bariatric surgery group and the remaining 37% (28/75) in the medical weight management group. Nearly half of the study participants (40/75; 53%) lost ≥20% bodyweight by the 1-year follow-up visit, where 97.5% (39/40) of those were from the bariatric surgery group.
TABLE 1.
Baseline characteristics of the study population stratified by weight-loss at 1-year follow-up visit.
| Characteristic at Baseline N = 75 |
< 20% Weight Loss at 1-Year N = 35 |
≥ 20% Weight Loss at 1-Year N = 40 |
|---|---|---|
| Age [mean (SD), median] | 47.3 (8.3), 49.0 | 42.5 (9.6), 49.0 |
| Female (%) | 30 (85.7) | 39 (97.5) |
| African-American (%) | 27 (77.1) | 19 (47.5) |
| Bariatric surgery (%) | 8 (22.9) | 39 (97.5) |
| BMI [mean (SD), median] | 40.9 (4.5), 40.0 | 42.3 (4.5), 41.6 |
| College/graduate education | 15 (42.9) | 14 (35.0) |
| Employment (%) | 9 (25.7) | 9 (22.5) |
| KL grade (%) | ||
| 0 | 6 (17.1) | 8 (20.0) |
| 1 | 11 (31.4) | 11 (27.5) |
| 2 | 8 (22.9) | 14 (35.0) |
| 3 | 9 (25.7) | 7 (17.5) |
| 4 | 1 (0.0) | 0 (0.0) |
| WOMAC pain (0–24 scale) [mean (SD), median] | 12.5 (4.9), 13.0 | 11.8 (4.2), 12.0 |
| BML worst score > 0 (%) | 24 (68.6) | 24 (60.0) |
| Synovitis worst score > 0 (%) | 18 (51.4) | 24 (60.0) |
| Patella PPT [mean (SD), median] | 429.6 (198.1), 412.2 | 345.4 (158.0), 318.1 |
| Wrist PPT [mean (SD), median] | 346.4 (150.0), 299.5 | 351.7 (144.9), 326.0 |
| CES-D scale | 19.3 (11.6), 17.5 | 14.4 (9.2); 11.5 |
Continuous variables are summarized by mean (standard deviation) and median. Categorical variables are summarized by frequency and percentages.
BMI, body mass index; BML, bone marrow lesion; KL, Kellgren-Lawrence; PPT, pressure pain threshold; SD, standard deviation; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
On baseline MRI assessment, 64% (48/75) and 56% (42/75) of the participants had BML and synovitis, respectively (Table 1) based on MOAKS scores of 1 or greater. Among patients who eventually experienced ≥20% weight loss over the follow-up, 60.0% (24/40) had baseline BML as well as baseline synovitis, compared with 68.6% (24/35) and 51.4% (18/35) who had baseline BML and synovitis, respectively, among patients who did not achieve ≥20% weight loss by the 1-year follow-up.
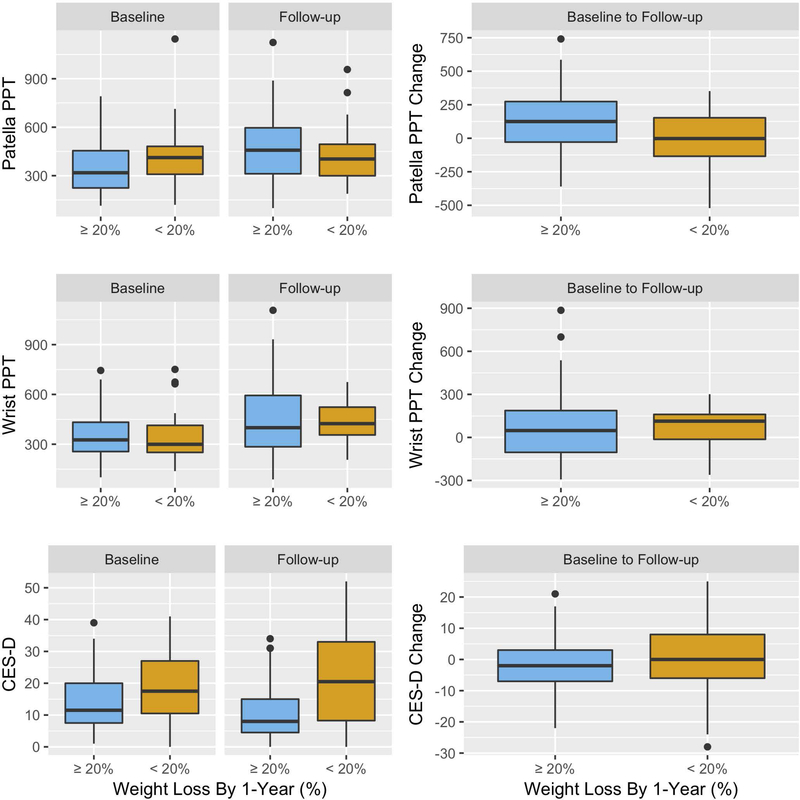
At baseline, neither patella PPT (mean difference = −84.2, 95% confidence interval [CI]: −2.9, 171.2) nor wrist PPT (mean difference = 5.4, 95% CI: −75.4, 64.6) were significantly different among participants who lost ≥20% weight by the follow-up, compared with those with <20% weight loss (Table 1, Figure 3). At baseline, mean CES-D was lower (mean difference = −4.9, 95% CI: 0.0, 9.8) in those with ≥20% weight loss by 1-year, compared to those without (Table 1, Figure 3).
Figure 3.
Boxplots of baseline and follow-up measures of the pressure pain threshold (PPT) for index patella and right wrist, and Center for Epidemiologic Studies Depression (CES-D) scale stratified by weight loss at 1-year.
The middle bold horizontal lines are the median (i.e. 50th percentile). The lower and upper hinges are the first (i.e. 25th percentile) and third (i.e. 75th percentile) quartiles. Dots along the whiskers are beyond the 1.5 times of the interquartile range.
Change in Mediators: BML, synovitis, sensitization, and depressive symptoms.
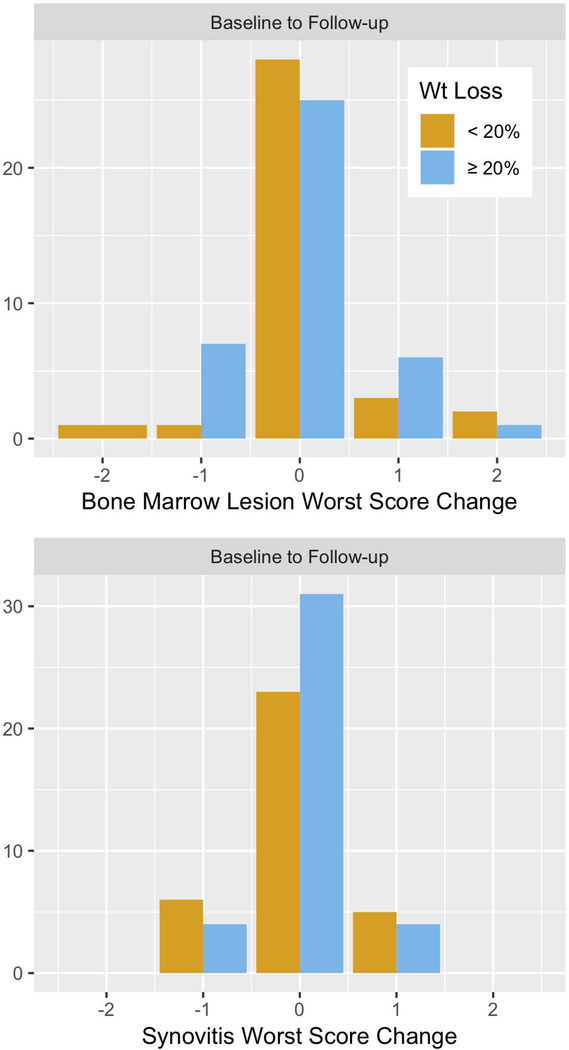
Change in the worst score from baseline to follow-up for an individual patient for BML (coefficient for mean difference [coef.] = 0.1; 95% CI: −0.2, 0.4) and synovitis (coef. = 0.1, 95% CI: - 0.3, 0.2), scores were not significantly different between the group of patients with ≥20% weight loss, compared with those who lost <20% weight (Figure 2). Similarly, when the sum of MRI scores was considered for each feature, changes in BML (coef. = 0.2, 95% CI: −0.7, 1.2) and synovitis (coef. = 0.1, 95% CI: −0.5, 0.2) were not significantly different among patients with ≥20% weight loss than those without (Figure 2).
Figure 2.
Longitudinal changes in bone marrow lesion (BML) and synovitis worst scores, measured semi- quantitatively by magnetic resonance imaging, stratified by weight loss at 1-year follow-up.
The mean PPT increased significantly from baseline to follow-up for both the patella (coef. = 68.4, 95% CI: 10.8, 125.9) and the wrist (coef. = 88.5, 95% CI: 38.1, 138.0) for all subjects, suggesting less sensitization across visits. However, the change in mean PPT for patella (coef. = −11.3, 95% CI: −84.6, 62.6) and wrist (coef. = 18.0, 95% CI: −48.0, 87.6) did not differ significantly between those with and without ≥20% weight loss (Figure 3). Mean CES-D score decreased from baseline to follow-up for all subjects (coef. = −0.8, 95% CI: −3.1, 1.67), and was significantly lower (coef. = −6.2, 95% CI: −11.0, −1.4) in those who lost ≥20% weight than those who did not (Figure 3).
Pain Improvement.
Among participants who lost ≥20% bodyweight, 75% (30/40) experienced the MCID improvement in WOMAC pain, compared with 35% (12/35) of those who experienced <20% weight loss.
Mediated effects.
Estimates for the natural indirect (i.e., mediating) effect suggested that the effect of weight loss on pain improvement was not mediated by BML score (for worst BML, odds ratio [OR] = 1.01; 95% CI: 0.78, 1.28) or synovitis (OR = 1.03; 95% CI: 0.73, 1.45). Results were similar for the sum of BML or synovitis score (Table 2). However, there was a 62% and 15% increased odds of attaining the MCID in pain improvement by changes in patella PPT (OR = 1.62-fold, 95% CI: 0.89-, 2.75-fold change) and wrist PPT (OR = 1.15-fold, 95% CI: 0.77-, 1.77-fold change), respectively, in participants who lost ≥20% body weight compared with those who did not (Table 2). Further, the estimate of the natural indirect effect for CES-D score suggested 22% increased odds (OR = 1.22-fold, 95% CI: 0.77-, 1.87-fold) of pain improvement in those with ≥20% weight loss than those without.
TABLE 2.
Estimates of the natural indirect and direct effects of massive (≥20%) weight loss on knee pain improvement.
| Feature | Odds Ratio (95% Bootstrap CI) | |
|---|---|---|
| Natural Indirect Effect (Mediated) | Natural Direct Effect (Not Mediated) |
|
| BML | ||
| Worst score | 1.01 (0.78, 1.28) | 5.39 (1.43, 16.99) |
| Sum of scores | 1.01 (0.72, 1.30) | 4.70 (1.35, 15.22) |
| Synovitis | ||
| Worst score | 1.03 (0.73, 1.45) | 5.07 (1.38, 15.33) |
| Sum of scores | 0.98 (0.73, 1.33) | 4.87 (1.44, 13.82) |
| PPT | ||
| Patella | 1.62 (0.89, 2.75) | 2.47 (0.73, 7.71) |
| Wrist | 1.15 (0.77, 1.77) | 3.82 (1.10, 11.30) |
| CES-D | 1.22 (0.77, 1.87) | 4.08 (1.08, 13.17) |
BML, bone marrow lesion; CI, confidence interval; PPT, pressure pain threshold; CES-D, Center for Epidemiologic Studies Depression scale
Unmediated effects.
Estimates for the natural direct effect, not mediated through changes in the worst scores for BML and synovitis, suggested 5.39-fold (95% CI: 1.43-, 16.99-fold) and 5.07-fold (95% CI: 1.38-, 15.33- fold) increase in the odds of pain improvement for patients with ≥20% weight loss compared with those without such weight loss, respectively (Table 2). Natural direct effect estimates not mediated through patella PPT and wrist PPT also suggested 2.47-fold (95% CI: 0.73-, 7.71-fold) and 3.82-fold (95% CI: 1.10-, 11.30-fold) increase in the odds of pain improvement for subjects who lost ≥20% bodyweight compared with those who did not, respectively (Table 2). The estimate for the natural direct effect not mediated through changes in CES-D score suggested a 4.08-fold (95% CI: 1.08-, 13.17-fold) higher odds of pain improvement in those with ≥20% weight loss than for those without (Table 2).
Mediated interaction.
Estimates for the mediated interaction between weight loss and worst BML or synovitis scores suggested no evidence of effect modification between the effect of weight loss and BML (mediated interaction OR = 0.95-fold; 95 CI: 0.32-, 3.75-fold) or synovitis (mediated interaction OR = 0.88-fold, 95% CI: 0.50-, 1.40-fold) on pain improvement. Similar results were seen for the sum of BML or synovitis scores. Mediated interaction effect measures for patella PPT (mediated interaction OR = 0.81-fold, 95% CI: 0.44-, 1.35-fold), wrist PPT (mediated interaction OR = 0.89-fold, 95% CI: 0.51-, 1.86-fold), and CES-D score (mediated interaction OR = 1.05-fold, 95% CI: 0.59-, 1.95-fold) also suggested no effect modification between the effect of weight loss and patella or wrist PPT and CES-D score on pain improvement.
Sensitivity analyses.
The effect sizes for the natural effects were similar in sensitivity analyses when the analysis was restricted to those with BML and synovitis at baseline, or when a weight loss of ≥10%, rather than ≥20%, was considered. They were also similar when persons who had bariatric surgery were compared with those who underwent medical weight management regardless of weight loss percentage at follow-up visit, although all, except one of the patients who lost ≥20% bodyweight by the 1-year follow-up were in the bariatric surgery group (Supplementary Table 1).
DISCUSSION
We found that the clinically meaningful improvement in knee pain that occurred in morbidly obese patients following ≥20% weight loss over a one-year follow-up period was not explained by changes in BML and synovitis. However, changes in pain sensitization assessed as patella and wrist PPT, and depressive symptoms quantified by CES-D partially explained the effects of weight loss on pain reduction. No effect modification was found between these mediated effects and weight loss levels on knee pain improvement.
As expected, persons with knee pain undergoing bariatric surgery experienced a marked reduction in pain. To our knowledge, there have been no studies examining factors potentially mediating the effect of weight loss on pain. The inclusion of persons undergoing substantial weight loss and knee pain reduction offered us a unique opportunity to explore potential mechanisms of pain reduction. While our goal was to report the effect sizes for natural direct and indirect effects, the uncertainty around our reported effect sizes, expressed by bootstrap confidence intervals, slightly overlapped the null, partially attributable to the study sample size (37,38). Our inference focused on effect sizes more than the results of hypothesis testing and corresponding p-values. While we hoped that we could achieve a narrower confidence interval around our calculated estimates, power analysis for the main study outcome (i.e., pain improvement) assumed satisfactory sample size. However, power analysis for a mediation analysis is more complex and much less developed in statistical literature, and no statistical development exists for imputation-based (and non-parametric) natural-effects model to the best of our knowledge. However, the width of confidence bounds that we calculated by bootstrap resampling (for increased accuracy) appear reasonable. As we noted earlier in the interpretation of natural direct and indirect effects and assessing mediating roles, the status of BML or synovitis have to stay at a level that naturally occurs for a given level of weight loss. This means that the natural levels of BML and synovitis scores could be the same across exposure levels (i.e., ≥20% and <20% weight loss levels), regardless of the significance of their mediating role.
Beyond our initial study aims, we also tested a mediation hypothesis to examine how much pain improvement could be due to bariatric surgery potentially mediated by weight loss. The results of this new mediation hypothesis suggested that compared to persons undergoing medical weight management, the direct (unmediated) effect of bariatric surgery on pain improvement was 39% greater, (odds ratio for natural direct effect = 1.39; 95% CI: 0.14, 12.13). Further, there was a 2.75-fold increase in the odds of pain improvement by bariatric surgery that was mediated by weight loss (odds ratio for natural indirect effect = 2.75; 95% CI: 0.42, 16.98). These effect sizes suggest that bariatric surgery effect on pain improvement are mostly mediated by weight loss; however, the sample size for this secondary mediation hypothesis was small.
In an earlier work, we assessed cartilage both semi-quantitatively and quantitatively in this study sample (22) and reported no effect of weight loss on this measure over a year, although morphologic change in cartilage would be unusual in this time frame. BMLs and synovitis have both been shown to change within a few weeks (2,5,39). Our longitudinal MRI assessments revealed statistically insignificant changes in BML and synovitis scores within one year despite massive weight loss. Similarly, Gudbergsen, et al. (14) reported lack of improvement in BML in response to rapidly decreasing bodyweight following a weight loss intervention, and also concluded that there was a lack of association between changes in BML and clinical symptoms including pain; however, no formal assessment of a potential mediating role (i.e., an estimate for indirect effect) was presented (14). Further, results of the CAROT trial on the influence of weight loss therapy on cartilage in obese knee OA subjects have similarly suggested insignificant changes in synovitis at 1-year follow-up after the intervention, but reported increased BML only in those who lost weight by exercise compared with those who experienced weight loss by diet or a no-attention group (40). Moreover, in the Intensive Diet and Exercise for Arthritis (IDEA) trial in which 10% weight loss by the 18-month follow-up through diet and exercise, solely by diet, and solely through exercise were studied, no significant changes in structural features such as BML and synovitis were found, despite improvement in knee pain (41).
While previous studies have both supported (2,4–8,10) and not supported (11–14) the association between BML and synovitis with pain in OA, few studies have explored the underlying causal mechanisms by which these structural features could be related to pain. Exceptions were studies (15,42) that attributed chronic pain in OA to changes in central sensitization during OA development and progression. A strength of our study was the assessment of these mediating effects in a population losing a lot of weight (i.e., ≥20%) in contrast to previous studies that assessed lesser degrees of weight loss (14,40,41). Another strength was the use of modern approaches to causal mediation that allow assessment of intermediate variables on a causal pathway to quantify knee pain reduction mediated by these factors as part of the effect of weight loss.
There are some limitations to our study as well. Our study participants were almost entirely women despite our efforts to recruit men. Our sample size was relatively small, resulting in limited precision manifested by relatively wide confidence intervals. While MRI is an ideal instrument for quantifying the changes in structural features of OA compared with plain radiography, the semi-quantitative nature of MOAKS scoring method does not allow a volumetric measurement for quantifying the size of these structural features. Future studies should assess other potential causal pathways through which substantial weight loss could exert its effect on knee pain improvement, such as changes in biomechanical or inflammatory factors.
In conclusion, pain is a complex phenomenon in knee OA. Our findings suggest that changes in pain sensitization and in depressive symptoms mediate in part the knee pain improvement experienced by those undergoing substantial weight loss, especially following bariatric surgery. This suggests that pain sensitization and depressive symptoms could be a promising target for future intervention studies in those with chronic knee pain.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health (grants NIAMS P60AR047785, NIAMS K24AR070892, NIA R03AG060272). Dr. Felson was also supported by the NIHR BRC to the University of Manchester.
Footnotes
Study conception and design. Jafarzadeh, Felson, Neogi, Stefanik.
Acquisition of data. Felson, Neogi, Stefanik, Guermazi, Li, Apovian.
Analysis and interpretation of data. Jafarzadeh, Felson, Neogi.
REFERENCES
- 1.Jafarzadeh SR, Felson DT. Updated estimates suggest a much higher prevalence of arthritis in United States adults than previous ones. Arthritis Rheumatol Hoboken NJ 2018;70:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 2001;134:541–549. [DOI] [PubMed] [Google Scholar]
- 3.Zhai G, Blizzard L, Srikanth V, Ding C, Cooley H, Cicuttini F, et al. Correlates of knee pain in older adults: Tasmanian Older Adult Cohort Study. Arthritis Rheum 2006;55:264–271. [DOI] [PubMed] [Google Scholar]
- 4.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage 2006;14:1033–1040. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 2007;56:2986–2992. [DOI] [PubMed] [Google Scholar]
- 6.Davies-Tuck ML, Wluka AE, Wang Y, English DR, Giles GG, Cicuttini F. The natural history of bone marrow lesions in community-based adults with no clinical knee osteoarthritis. Ann Rheum Dis 2009;68:904–908. [DOI] [PubMed] [Google Scholar]
- 7.Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2009;17:1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum 2011;63:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol 2001;28:1330–1337. [PubMed] [Google Scholar]
- 10.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 2007;66:1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sowers MF, Hayes C, Jamadar D, Capul D, Lachance L, Jannausch M, et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthritis Cartilage 2003;11:387–393. [DOI] [PubMed] [Google Scholar]
- 12.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology 2003;226:373–381. [DOI] [PubMed] [Google Scholar]
- 13.Kornaat PR, Kloppenburg M, Sharma R, Botha-Scheepers SA, Le Graverand M-PH, Coene LNJEM, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis; associations with clinical features. Eur Radiol 2007;17:3073–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudbergsen H, Boesen M, Christensen R, Bartels EM, Henriksen M, Danneskiold-Samsøe B, et al. Changes in bone marrow lesions in response to weight-loss in obese knee osteoarthritis patients: a prospective cohort study. BMC Musculoskelet Disord 2013;14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Rheumatol Hoboken NJ 2016;68:654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanik JJ, Felson DT, Apovian CM, Niu J, Margaret Clancy M, LaValley MP, et al. Changes in pain sensitization after bariatric surgery. Arthritis Care Res 2018;70:1525–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stubbs B, Aluko Y, Myint PK, Smith TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing 2016;45:228–235. [DOI] [PubMed] [Google Scholar]
- 18.Rathbun AM, Stuart EA, Shardell M, Yau MS, Baumgarten M, Hochberg MC. Dynamic effects of depressive symptoms on osteoarthritis knee pain. Arthritis Care Res 2018;70:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med 1988;109:18–24. [DOI] [PubMed] [Google Scholar]
- 20.Speck RM, Bond DS, Sarwer DB, Farrar JT. A systematic review of musculoskeletal pain among bariatric surgery patients: implications for physical activity and exercise. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 2014;10:161–170. [DOI] [PubMed] [Google Scholar]
- 21.King WC, Chen J-Y, Belle SH, Courcoulas AP, Dakin GF, Elder KA, et al. Change in pain and physical function following bariatric surgery for severe obesity. JAMA 2016;315:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafarzadeh SR, Clancy M, Li J-S, Apovian CM, Guermazi A, Eckstein F, et al. Changes in the structural features of osteoarthritis in a year of weight loss. Osteoarthritis Cartilage 2018;26:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840. [PubMed] [Google Scholar]
- 25.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol 2002;29:131–138. [PubMed] [Google Scholar]
- 26.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- 27.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol 2012;176:190–195. [DOI] [PubMed] [Google Scholar]
- 28.Loeys T, Moerkerke B, De Smet O, Buysse A, Steen J, Vansteelandt S. Flexible mediation analysis in the presence of nonlinear relations: beyond the mediation formula. Multivar Behav Res 2013;48:871–894. [DOI] [PubMed] [Google Scholar]
- 29.Vansteelandt S. Understanding counterfactual-based mediation analysis approaches and their differences. Epidemiol Camb Mass 2012;23:889–891. [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele TJ. A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiol Camb Mass 2013;24:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shpitser I, Tchetgen ET. Causal inference with a graphical hierarchy of interventions. Ann Stat 2016;44:2433–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiol Camb Mass 1992;3:143–155. [DOI] [PubMed] [Google Scholar]
- 33.Vansteelandt S, Bekaert M, Lange T. Imputation strategies for the estimation of natural direct and indirect effects. Epidemiol Methods 2012;1:131–158. [DOI] [PubMed] [Google Scholar]
- 34.Steen J, Loeys T, Moerkerke B, Vansteelandt S. medflex: an R package for flexible mediation analysis using natural effect models. J Stat Softw 2017;76:1–46. [Google Scholar]
- 35.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available at: http://www.r-project.org. [Google Scholar]
- 36.Steen J, Loeys T, Moerkerke B, Vansteelandt S, Meys J, Lange T, et al. medflex: flexible mediation analysis using natural effect models.; 2018. Available at: https://cran.r-project.org/package=medflex. Accessed May 8, 2017.
- 37.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat 2016;70:129–133. [Google Scholar]
- 38.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 2016;31:337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neill TW, Parkes MJ, Maricar N, Marjanovic EJ, Hodgson R, Gait AD, et al. Synovial tissue volume: a treatment target in knee osteoarthritis (OA). Ann Rheum Dis 2016;75:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henriksen M, Christensen R, Hunter DJ, Gudbergsen H, Boesen M, Lohmander LS, et al. Structural changes in the knee during weight loss maintenance after a significant weight loss in obese patients with osteoarthritis: a report of secondary outcome analyses from a randomized controlled trial. Osteoarthritis Cartilage 2014;22:639–646. [DOI] [PubMed] [Google Scholar]
- 41.Hunter DJ, Beavers DP, Eckstein F, Guermazi A, Loeser RF, Nicklas BJ, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage 2015;23:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum 2009;61:1226–1234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.